Abstract
Mutations in the gene CHD7 cause CHARGE syndrome, a rare multi-organ syndromic disorder. Gonadal defects are common in individuals with CHARGE syndrome (seen in ~60–80% of cases) and represent the letter “G” in the CHARGE syndrome acronym. The gonadal defect in CHARGE syndrome results from congenital deficiency of the hypothalamic hormone Gonadotropin-releasing hormone (GnRH), which manifests clinically as pubertal failure and infertility, and biochemically as hypogonadotropic hypogonadism (low sex steroid hormone levels with inappropriately normal or low gonadotropin levels). In addition to the gonadal endocrine abnormalities, in a small minority of individuals with CHARGE, additional endocrine defects including growth hormone deficiency, multiple pituitary hormone deficits and primary hypothyroidism may also be seen. CHD7 mutations disrupt the targeting of olfactory axons and the migration of GnRH-synthesizing neurons during embryonic development, resulting in congenital idiopathic hypogonadotropic hypogonadism (IHH) and anosmia (or hyposmia), two features that define human Kallmann syndrome. Since Kallmann syndrome is as one of its constituent phenotypes in individuals with CHARGE, recent studies have investigated the role of CHD7 mutations in individuals with IHH and established that deleterious missense mutations in CHD7 are associated with Kallmann syndrome as well as normosmic form of IHH. These missense mutations affect the ATPase and nucleosome remodeling activities of the CHD7 protein. These observations suggest that CHD7 protein function is critical for the ontogeny of GnRH neurons and neuroendocrine regulation of GnRH secretion.
Keywords: CHARGE syndrome, Kallmann syndrome, Isolated GnRH deficiency, idiopathic hypogonadotropic hypogonadism, CHD7, GnRH
Introduction
CHARGE syndrome (Coloboma, Heart defects, choanal Atresia, Retardation of growth and development, Gonadal defects, and Ear/hearing abnormalities) is a rare multi-organ syndromic disorder in which haploinsufficiency in the gene CHD7 accounts for nearly 70% of cases (Vissers et al. 2004). It is now well established that individuals with CHARGE display a wide spectrum of pleiotropic phenotypes, which have resulted in the definition of ‘major’ and ‘minor’ clinical criteria for clinical diagnosis of this developmental disorder (Hale et al. 2016; Jongmans et al. 2006). The letter “G” in the CHARGE acronym refers to gonadal defects which represents one of the four minor diagnostic criteria that clinically defines CHARGE syndrome (van Ravenswaaij-Arts et al. 2015). The gonadal defects in CHARGE were initially recognized in male infants who displayed genital hypoplasia characterized by micropenis and/or cryptorchidism (Pagon et al. 1981). It is now well established that these gonadal defects result from gonadotropin-releasing hormone (GnRH) deficiency leading to idiopathic hypogonadotropic hypogonadism (IHH) that can manifest in both sexes (Balasubramanian et al. 2014; Kim et al. 2008; Marcos et al. 2014). While gonadal defects are seen in a substantial proportion of individuals with CHARGE, additional endocrine abnormalities such as short stature, growth hormone deficiency, and multiple pituitary hormone deficits have also been infrequently reported in CHARGE (Dorr et al. 2015; Esposito et al. 2014; Gregory et al. 2013; Aramaki et al. 2006; Asakura et al. 2008; Pinto et al. 2005). In addition, several patients with just IHH without the pleotropic spectrum of CHARGE features are also known to harbor presumed pathogenic CHD7 variants (Kim et al. 2008; Balasubramanian et al. 2014). This review will focus on the endocrine phenotypes associated with CHARGE syndrome, the CHD7 mutational spectrum in humans with IHH without full CHARGE features, and the molecular basis for reproductive endocrine phenotypes seen in individuals with CHD7 mutations, and discuss the specific role of CHD7 in GnRH neuronal ontogeny.
Endocrine abnormalities in CHARGE syndrome
Congenital Idiopathic Hypogonadotropic Hypogonadism (IHH), due to GnRH deficiency
The most common endocrine phenotype seen ~60–80 in % of individuals with CHARGE is hypogonadotropic hypogonadism (HH) (Bergman, Janssen, et al. 2011). Since penile growth and descent of the testes are dependent on an intact hypothalamic-pituitary-gonadal (HPG) axis in utero and during the neonatal period (Grumbach 2005), congenital hypogonadism due to hypothalamic/pituitary dysfunction manifests itself readily in boys who display micropenis and/or cryptorchidism. Hence, in male patients, HPG axis defects (micropenis and/or cryptorchidism) were recognized very early in the initial reports of individuals with CHARGE, thus constituting the “G” (genital hypoplasia) in the CHARGE association (Pagon et al. 1981). In contrast to boys with CHARGE in whom these HPG defects are readily evident, girls do not manifest any evident external genitalia phenotypes. Thus, the gonadal defects in females with CHARGE were not recognized initially and only became evident when these patients with features of CHARGE failed to enter normal puberty (Wheeler et al. 2000). In both sexes, the cause of the HPG axis dysfunction and resultant genital/gonadal defects was initially unclear. Subsequently, a series of case reports firmly established that the genital defects in boys with CHARGE as well as the lack of puberty in females with CHARGE were secondary to hypogonadotropic hypogonadism [HH] (Oley, Baraitser, and Grant 1988; Harvey, Leaper, and Bankier 1991; Pinto et al. 2005; Wheeler et al. 2000). HH can result from either GnRH-related defects in the hypothalamus and/or defects in anterior pituitary function and further insights into the precise etiology of HH in CHARGE came from the study of the olfactory phenotypes in CHARGE children. Fetal studies in CHARGE children showed that olfactory bulb agenesis was one of their most consistent morphological features (Teixeira et al. 2010; Sanlaville et al. 2006). Olfactory testing and/or MRI imaging in individuals with CHARGE with gonadal defects revealed defective olfaction/olfactory bulb development in a significant number of patients (Pinto et al. 2005; Chalouhi et al. 2005). This phenotypic combination of HH and anosmia is well recognized as Kallmann syndrome (KS) (also referred as olfactogenital syndrome), a phenotypic form of congenital idiopathic hypogonadotropic hypogonadism (IHH) that results from a shared migratory defect of the GnRH and olfactory axons (Balasubramanian et al. 2010; Schwanzel-Fukuda and Pfaff 1989). Kallmann syndrome results from a pathological sequence during embryonic development wherein olfactory sensory neurons are prematurely interrupted in their migratory journey from the nose to the brain, resulting in concomitant arrest of migration of GnRH-synthesizing neurons that migrate alongside the olfactory fibers (Schwanzel-Fukuda and Pfaff 1989; Teixeira et al. 2010). The KS form of IHH is distinct to the other major phenotypic form of IHH in which individuals exhibit GnRH deficiency but without olfactory deficits, i.e. normosmic IHH [nIHH], which represents a neuroendocrine defect in GnRH secretion once the GnRH neurons reach the hypothalamus (Balasubramanian et al. 2010).Thus, the presence of KS in individuals with CHARGE confirms that HH in CHARGE is due to embryonic GnRH deficiency, indicating a primary defect in GnRH neuronal migration/integrity rather than a primary pituitary defect. In addition, the presence of anosmia has been shown to be highly predictive of HH in CHARGE (Bergman, Bocca, et al. 2011). In keeping with these observations, majority of individuals with CHARGE have otherwise normal anterior pituitary hormone function and anatomy of the pituitary/sella, confirming hypothalamic GnRH deficiency (Aramaki et al. 2006; Asakura et al. 2008; Pinto et al. 2005). In terms of therapy, boys with micropenis require testosterone or gonadotropin therapy during infancy to restore penile growth. Cryptorchidism may require orchidopexy for repositioning the testes in the scrotum, a critical element for subsequent normal gonadal function as well as future fertility. In adolescent individuals with CHARGE who display absence of puberty and HH, induction of puberty in the form of testosterone/gonadotropins in boys and estradiol/progestin therapy in females is indicated. For adult CHARGE patients (both men and women) who seek fertility, induction of fertility requires the use of either exogenous gonadotropin therapy or pulsatile GnRH therapy, monitored and overseen by a specialist reproductive endocrinologist. Patients undergoing such fertility therapy should also be referred to the Clinical Genetics team for appropriate genetic counseling and determination of recurrence risk for CHARGE in the offspring. The precise therapeutic regimens for induction of puberty and fertility are beyond the scope of this review, but are described in detail elsewhere, both by the author’s group (Balasubramanian and Crowley 1993) and others (Boehm et al. 2015; Palmert and Dunkel 2012). In addition to hormone replacement, additional monitoring required for individuals with CHARGE includes bone age estimation and follow-up with serial bone density measurements to diagnose and treat their potential osteopenia/osteoporosis. Although some patients with genetic forms of IHH are known to “reverse” their hypogonadotropism in adulthood (Raivio et al. 2007), to-date, reversal of HH in patients with the full CHARGE spectrum has not been reported (see below for reversal of HH in patients with CHD7 mutations associated with IHH alone but without full CHARGE spectrum).
Growth defects and other anterior pituitary hormone defects
The “R” in the CHARGE acronym denotes retardation of growth and development. These growth defects in individuals with CHARGE often become evident in the postnatal period and their etiology is often multifactorial, including feeding difficulties, poor nutrition, gastro-esophageal reflux, renal dysfunction, cardiac dysfunction and rarely, Growth Hormone (GH) deficiency (Zentner, Layman, et al. 2010). In reports where GH axis was specifically examined in individuals with CHARGE, ~9% exhibited suboptimal GH levels requiring treatment and these subjects respond favorably to exogenous GH replacement (Shoji et al. 2014; Asakura et al. 2008; Pinto et al. 2005; Dorr et al. 2015; Esposito et al. 2014). Rarely, individuals with CHARGE show evidence of combined pituitary hormone deficiency (combination of GH, LH/FSH, TSH, ACTH) and such cases can demonstrate radiological evidence of anterior pituitary hypoplasia (Pinto et al. 2005) or ectopic posterior pituitary (Gregory et al. 2013) suggesting that rare subsets of patients with CHARGE may manifest structural pituitary abnormalities that result in combined pituitary hormone dysfunction. In terms of therapy, appropriate hormone replacement (thyroxine, growth hormone, steroid replacement) is indicated for these anterior pituitary hormone dysfunctions. Although the majority of individuals with CHARGE display endocrine phenotypes relating to hypothalamic/pituitary defects, primary hypothyroidism has also been reported rarely in CHARGE (Aramaki et al. 2006; Asakura et al. 2008).
Molecular and biologic basis of endocrine defects in CHARGE
CHD7 mutations in patients with full CHARGE syndrome
Nearly 70–90% of patients with suspected CHARGE syndrome harbor pathogenic heterozygous mutations in the CHD7 gene (Zentner, Layman, et al. 2010; Bergman, Janssen, et al. 2012; Janssen et al. 2012), located on chromosome 8q12.1, that encodes the evolutionarily conserved Chromodomain Helicase DNA binding [CHD] protein 7, a member of the larger CHD family that functions as an ATP-dependent chromatin remodeling protein (Manning and Yusufzai 2017; Bouazoune and Kingston 2012). The CHD family of proteins have pleotropic functions: chromatin remodeling, regulation of embryonic stem cell pluripotency, methylated histone binding, DNA binding, transcriptional regulation, cell cycle regulation, and regulation of apoptosis (He et al. 2016; Manning and Yusufzai 2017; Zentner, Hurd, et al. 2010). The vast majority of CHD7 mutations demonstrable in individuals with full CHARGE spectrum are truncating mutations (frameshift; non-sense) that are distributed across all 37 coding exons, suggesting haplo-insufficiency of CHD7 as the major molecular mechanism for this syndrome (Zentner, Layman, et al. 2010). In addition to these truncating mutations, splice variants, missense mutations and structural variants (exonic deletions, whole gene or chromosomal deletions and chromosomal rearrangements) leading to loss of function can also cause CHARGE (Harvey, Leaper, and Bankier 1991). The vast majority of all CHD7 mutations are de novo and hence most patients present as sporadic cases with a small minority of CHD7 mutations showing familial inheritance in an autosomal dominant manner (Zentner, Layman, et al. 2010; Delahaye et al. 2007). Rarely, germline mosaic CHD7 variants have also been reported (Jongmans et al. 2008).
Missense allelic variants in CHD7 can cause IHH in the absence of full CHARGE features
Enrichment of CHD7 missense mutations in IHH patients
Although patients with typical CHARGE features are easily recognized clinically, more recent reports have begun to recognize a growing complexity of this diagnosis with the milder end of the CHARGE spectrum occurring in individuals with CHD7 mutations who exhibit clinical features that do not fulfill the clinical criteria for CHARGE. Since CHARGE syndrome may include Kallmann syndrome as one of its constituent phenotypes, two initial studies used a candidate gene approach to test the hypothesis that IHH may represent the milder end of CHD7-related CHARGE syndrome. By sequencing the CHD7 gene in IHH (either KS or normosmic form of IHH [nIHH]), Jongmans et al identified 3/38 KS patients harboring de novo CHD7 mutations (2 stop-mutations and 1 missense) whereas the normosmic IHH patients were negative for CHD7 variants (Jongmans et al. 2009). However, all 3 KS patients with CHD7 variants, upon additional phenotypic review, universally exhibited major CHARGE features. In contrast, Kim et al, identified 7 IHH patients (3KS, 4 nIHH) harboring CHD7 mutations (2 intronic mutations leading to exon skipping and 5 missense mutations), all of whom lacked major CHARGE phenotypes, thus implicating CHD7 allelic variants as a cause of both KS AND nIHH forms of IHH without CHARGE features (Kim et al. 2008). In view of the conflicting data as to whether CHD7 mutations are capable of causing isolated KS without full CHARGE, Bergman et al, examined 36 KS patients in whom they identified 3 with CHD7 mutations (2 nonsense, and 1 de novo missense) (Bergman, de Ronde, et al. 2012). However, all 3 subjects displayed additional CHARGE features, leading to their conclusion that CHD7 mutations do not cause isolated IHH.
Pathogenic missense CHD7 alleles are enriched in IHH
Given the uncertainty of the role of CHD7 in IHH in the absence of full CHARGE and to address the functional consequences of CHD7 variants associated with IHH, we examined 783 well-characterized IHH patients for CHD7 variants (Balasubramanian et al. 2014). Functional validation of a representative subset of IHH- and CHARGE- associated CHD7 variants was performed using zebrafish injected with a splice-blocking morpholino against Chd7, in which otolith morphology was used as a surrogate phenotype to assess CHD7 pathogenicity. Overall, 5.2% (n=41 patients) harbored heterozygous CHD7 variants of whom only 9 showed evidence of minor CHARGE features while the rest had no CHARGE features. Strikingly, in contrast to the truncating CHD7 mutations observed typically in patients with CHARGE, patients with IHH harbored predominantly missense variants. Next, a representative spectrum of 20 different non-synonymous missense CHD7 variants were subjected to functional analyses in zebrafish based on their population frequency and associated human phenotypes: (i) Control alleles: n=3; all with population minor allele frequency (MAF) ≥ 0.5%; (ii) 12 IHH alleles with MAF <0.5% associated with IHH without full CHARGE and (iii) 5 CHARGE alleles with MAF <0.5% that were known to cause full CHARGE. Functional studies in zebrafish tested the relative ability of each disease-associated missense allele to rescue the morphant phenotype compared to control alleles or their ability to phenocopy MO-induced otolith defects when over-expressed. While all control missense alleles tested benign on the zebrafish assay, 9/12 IHH-associated missense alleles tested deleterious, displaying either hypomorphic or dominant phenotypes. All CHARGE- associated alleles were also functionally deleterious. In addition, three-dimensional structural modeling of CHD7 using an automated homology modeling [SWISS-MODEL(http://swissmodel.expasy.org/)] or the FoldX protein design algorithm (Balasubramanian et al. 2014) identified 4 CHD7 missense variants that disrupted the three-dimensional protein structure of CHD7, all of which were also determined to be deleterious rare sequence variants from the zebrafish experiments. Thus, this large study confirmed that CHD7 missense mutations can cause IHH without CHARGE.
The above functional studies provided molecular validation that allelic missense CHD7 variants cause IHH and select CHD7 mutations that have been functionally determined to be deleterious and linked to IHH are shown in Figure 1. This significant excess of deleterious CHD7 missense mutations in IHH compared to the CHARGE syndrome was also confirmed by another independent study (Marcos et al. 2014). These observations suggest an exquisite dose-function-responsiveness for CHD7 protein function in relation to the varied developmental processes and organ systems that are critically regulated by CHD7. Hypomorphic/dominant CHD7 alleles may preferentially disrupt only the GnRH/HPG axis when compared to the multi-organ systems affected by null mutations. Taken together, these genetic and phenotypic studies suggest that the ontogeny of GnRH neurogenesis is particularly susceptible to the mildest dysregulation of CHD7 function. In this regard, some of the IHH patients with CHD7 missense alleles also display hearing loss and hence it can be hypothesized that semi-circular canal development/function may also be equally susceptible to these allelic defects, but this requires additional targeted auditory phenotyping studies in CHD7-related IHH subjects.
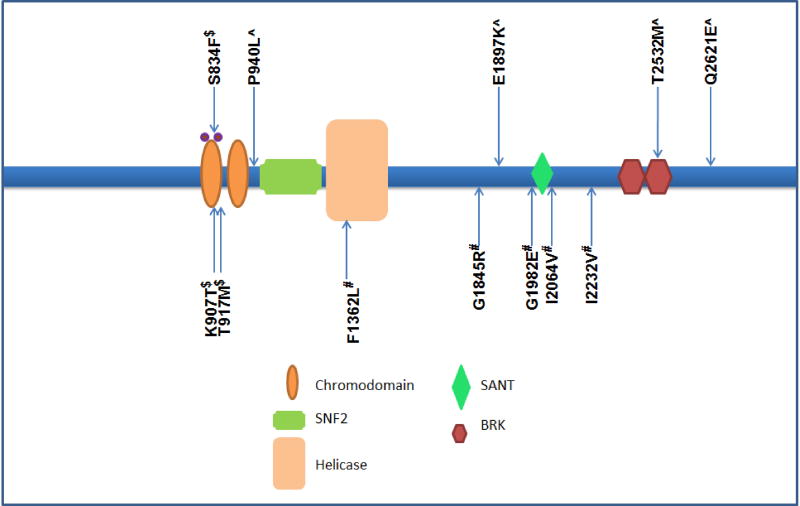
Figure 1.
Schematic of CHD7 protein, its domains and published IHH-associated functionally validated CHD7 mutations, missense and two deleterious splice variants are shown. ^Variants pathogenic and shown to be dominant on zebrafish assays (Balasubramanian and others 2014); #Variants pathogenic and shown to be hypomorphic in zebrafish assays (Balasubramanian and others 2014); $Variants pathogenic and shown to be loss of function in ATPase and nucleosome remodelling in vitro assays (Bouazoune and Kingston 2012).
While these data implicating the missense CHD7 alleles are compelling, given the large size of the CHD7 gene, it is important to recognize the not all rare CHD7 missense variants are necessarily pathogenic. Indeed, the recent expanded population level sequencing data available through the Exome Aggregation Consortium (ExAC) show that CHD7 missense mutations are not constrained in the human genome (Karczewski et al. 2017). Indeed, in our in-house sequencing data, a burden test of CHD7 missense mutations in IHH compared to ExAC shows no statistical enrichment for CHD7 missense mutations with MAF <1%. However, the IHH cohort was statistically enriched for CHD7 missense variants with MAF <0.1% (ultra-rare variants) (R.B unpublished observations) (Table 1). This excess of CHD7 ultra-rare variants suggests that the rarity of the CHD7 variants may be a reliable proxy for likely pathogenicity. Moreover, while the ExAC data does not show constraint for missense mutations across the whole CHD7 gene, exon-specific constraint analysis for CHD7 in the ExAC data reveals significant constraint for missense mutations in exons 10–29 (Samocha et al from ExAC consortium, personal communication). These constrained exons correspond to amino acid positions 952–2065 which encompass the critical CHD7 domains (SNF2, helicase, and SANT domains), all known to impact chromatin remodeling (Bergman, Janssen, et al. 2011). These exon-specific constraint data are largely in keeping with the most recent zebrafish missense mutation modeling data (Balasubramanian et al. 2014), confirming that the IHH-associated pathogenic CHD7 missense alleles disrupt key evolutionarily constrained amino acid residues that are critical for normal function of the CHD7 protein.
Table I.
Rare heterozygous variant burden for CHD7 missense mutations in IHH vs. ExAC database
| Minor Allele Frequency of CHD7 missense variant |
Cases (n=703) |
Controls (n=33,370) |
Odds Ratio (95% CI) |
P-value (Fischer’s Exact) |
|---|---|---|---|---|
| < 1% | 40/703 (5.7%) | 2108/33370 (6.3%) | 0.9 (0.6–1.2) | 0.58 |
| < 0.5% | 32/703 (4.5%) | 1316/33370 (3.9%) | 1.2 (0.8 –2.7) | 0.38 |
| < 0.1% | 32/703 (4.5%) | 1005/33370 (3%) | 1.5 (1.1 –2.2) | 0.03 |
Cases represent 703 IHH patients (Non-Finnish European ancestry) from the MGH Reproductive Endocrine Unit. Controls represent 33,370 subjects of Non-Finnish European ancestry in the ExAC database (Karczewski et al. 2017).
Pathogenic CHD7 alleles cause both KS and nIHH, including reversible forms of GnRH deficiency and contribute to oligogenic inheritance in IHH
In contrast to the highly penetrant olfactory dysfunction in full CHARGE syndrome, missense alleles in CHD7 are associated with both KS and nIHH (Kim et al. 2008; Balasubramanian et al. 2014). These observations suggest distinct neurodevelopmental and neuroendocrine roles for CHD7 function. In addition, whereas IHH has been previously thought to be a life-long disorder, it is now clear that ~10% of IHH patients may experience of spontaneous recovery of HPG axis function later in adulthood (Raivio et al. 2007). While this phenotypic subset has been association with patients harboring specific milder mutational forms of IHH [e.g. mutations in the TAC3/TACR3 genes (Sidhoum et al. 2014)], a recent report identified a patient with IHH and CHD7 truncating mutation who experienced spontaneous reversal of his IHH (Laitinen et al. 2012). Hence, serial clinical examination and intermittent cessation of hormonal therapy and biochemical re-evaluation for hypogonadism may be relevant for patients with CHD7 related IHH who display clinical features that may signal reversal. Features that should prompt consideration of such reversal of GnRH deficiency include: spontaneous growth of testes while off testosterone therapy, eugonadal testosterone levels off therapy, or the appearance of spontaneous menses/fertility in females. Although IHH subjects harboring pathogenic CHD7 missense mutations typically do not fulfill the clinical criteria for CHARGE diagnosis, known CHARGE features such as hearing loss and cleft lip/palate have been shown to be enriched in IHH patients harboring CHD7 variants upon more careful phenotyping (Marcos et al. 2014; Costa-Barbosa et al. 2013). Therefore, the presence of hearing loss and/or cleft lip/palate in IHH patients should alert clinicians to search for CHD7 mutations as this information will be critical for diagnosis as well as genetic counseling given the autosomal dominant mode of transmission associated with these variants.
In contrast to the de novo and paternal origin of mutations associated with severe CHARGE (Pauli et al. 2012), the vast majority of IHH-associated CHD7 variants is inherited, often from unaffected parents, and exhibit no apparent parent of origin bias (Balasubramanian et al. 2014). In addition, in reports where segregation analysis of CHD7 missense alleles is available, there is considerable incomplete penetrance for the IHH phenotype in those harboring CHD7 missense mutations (Marcos et al. 2014; Balasubramanian et al. 2014). Moreover, in two families reported in our study, pathogenic CHD7 variants were found in IHH patients who also carried a known pathogenic mutation in a 2nd gene known to be associated with IHH (e.g. FGFR1 and GNRHR), suggesting that some CHD7 alleles contribute to the known oligogenic architecture of IHH (Balasubramanian et al. 2014). Although the remaining individuals with CHD7 mutations did not harbor any additional known IHH gene variants, it is noteworthy that only 40% of IHH patients have mutations in the genes identified so far and hence some of these individuals with CHD7 mutations with IHH may harbor variants in novel IHH genes that are yet to be identified.
Pathogenic CHD7 missense alleles disrupt nucleosome remodeling activity of CHD7
The precise molecular mechanisms underlying missense mutations causing IHH has been recently examined. Using site-directed mutagenesis, Bouazoune et al engineered CHD7 missense mutations identified in IHH and examined ATPase and nucleosome remodeling activity of mutant and WT CHD7 (Bouazoune and Kingston 2012). Three chromodomain mutations previously linked to IHH were examined: one mutation affecting a highly conserved sequence motif in the first chromodomain (S834F) and two mutations affecting the second chromodomain of CHD7 (K907T; T917M). The mutant S834F protein completely abolished both ATPase and nucleosome remodeling activities by CHD7. In contrast, the mutant K907T protein, and to a lesser extent, T917M protein, showed only reductions in ATPase activity and nucleosome-remodeling capabilities compared with WT. These results suggest the milder missense CHD7 alleles linked to IHH impair CHD7 related nucleosome remodeling function and this defect may underlie the pathologic human phenotypes such as IHH.
Insights into the role of CHD7 gene in the ontogeny of GnRH neurons: Lessons from humans with IHH, CHARGE and from mouse models of CHARGE syndrome
The precise role of CHD7 in the ontogeny of the GnRH neurons is under active investigation. Comprehensive human and mouse embryonic expression analysis shows ubiquitous CHD7 expression during early development (Layman et al. 2009; Sanlaville et al. 2006; Bergman et al. 2010; Layman, Hurd, and Martin 2011). Specifically, in relation to the recognized endocrine phenotypes that primarily affect the neuroendocrine hypothalamus and pituitary, CHD7 is widely expressed in the early in the undifferentiated neuroepithelium, neural crest derived mesenchyme, and later in the nasal epithelia, olfactory bulbs and nerves, and also in the anterior and median lobes of pituitary (Layman et al. 2009; Sanlaville et al. 2006; Bergman et al. 2010; Layman, Hurd, and Martin 2011).This pattern of expression is consistent with the endocrine phenotypes of HH and other rare anterior pituitary hormone defects in CHARGE.
Embryonic GnRH neuronal migration and pubertal timing is disrupted in human CHARGE syndrome and murine CHARGE syndrome models
Given the intricate relationship and joint origin of the olfactory axons and the GnRH neurons, studies in a human fetus with CHARGE syndrome has confirmed that the CHARGE fetus showed arhinencephaly (olfactory bulb agenesis) with absence of GnRH neurons in the forebrain (Teixeira et al. 2010). The migrating olfactory and GnRH neurons accumulated in the fronto-nasal region with the formation of bilateral spherical structures consistent with neuromas near the cribriform plate, implicating a significant migratory defect (Teixeira et al. 2010). In keeping with these observations, Chd7 deficient mice display smaller olfactory bulbs, reduced number of olfactory sensory neurons, defective senses of smell with loss of odor-evoked electro-olfactogram responses, reduced hypothalamic GnRH neuronal numbers, hypoplastic genitalia, hypogonadotropism and impaired pubertal timing (Bergman et al. 2010; Layman, Hurd, and Martin 2011; Layman et al. 2009). In addition, they show significant defects in neural stem proliferation with reductions in production, proliferation and regeneration of olfactory sensory neurons (Layman et al. 2009). These complementary observations in humans and mice provide compelling evidence that the reproductive deficits in CHARGE are secondary to impaired GnRH migration and consequent GnRH deficiency. The GnRH deficiency in CHARGE syndrome, thus results from the olfactogenital pathological sequence, implicating the disruption of olfactory axon migration to the forebrain as the primary cause of the defective migration of GnRH synthesizing cells, which normally migrate along these nerve fibers.
Pathogenic CHD7 mutations in humans cause both KS and nIHH suggesting additional role(s) for CHD7 in GnRH neuronal integrity beyond its migratory defects
KS and nIHH represent the two distinct phenotypic forms of IHH, the former pointing to early neurodevelopmental failure of GnRH neuronal migration during development while nIHH represents neuroendocrine failure of GnRH secretion/action within the hypothalamus, i.e. much later in their developmental history (Balasubramanian et al. 2010). The association of pathogenic CHD7 mutations with both KS and nIHH is thus intriguing and suggests that CHD7 may have temporal and spatially distinct actions on GnRH neurons, i.e. initially during embryonic development and then also later in the regulation of GnRH neuronal function in the hypothalamus. Alternatively, deleterious missense CHD7 alleles associated with IHH may cause subtle olfactory phenotypes that may require detailed olfactory imaging studies to fully assess olfactory function. Another possibility is that olfactory phenotype may be weakly penetrant in the context of CHD7 missense alleles and in this regard, it is notable that in Chd7 mutant mice, the penetrance of olfactory agenesis is significantly lower than that seen in human individuals with CHARGE (Bergman et al. 2010), suggesting that CHD7 disruption can result in variable olfactory defects. However, the precise role of CHD7 mutations in nIHH is still unclear and requires further evaluation.
CHD7 regulates neural crest related genes and CHD7 related CHARGE/IHH represents a likely ‘neurocristopathy’
Cranial neural crest cells are a transient, multi-potent, migratory cellular population (Bronner 2015) arising adjacent to the olfactory placode from where GnRH neurons also emerge. It has been hypothesized that the myriad organ systems affected by CHARGE relate to abnormalities in neural crest development and in their various crest-derived cell types (Siebert, Graham, and MacDonald 1985). Recent studies in humans (Bajpai et al. 2010; Schulz et al. 2014) and Xenopus (Schulz et al. 2014) have shown an evolutionarily conserved role for CHD7 in the formation of multipotent migratory neural crest cells and for activation of their transcriptional circuitry. Genome-wide microarray expression analysis on wild-type and Chd7−/− mouse embryos has identified 98 differentially expressed genes downstream of CHD7, several of which are known to be involved in neural crest cell and axon guidance (Schulz et al. 2014). Accumulating data from mouse and zebrafish suggest that a small (~30%) but significant subset of GnRH neurons may have a neural crest origin (Whitlock et al. 2006; Forni et al. 2011). In addition, biallelic expression of Chd7 has been shown to be essential for maintaining appropriate levels of Fgf8 expression during cerebellar development (Basson et al. 2008; Yu et al. 2013). FGF8 is involved in the maintenance of progenitor status and in fate determination of cranial neural crest cells (Shao et al. 2015). Given that FGF8 mutations are also known to cause Kallmann Syndrome (Falardeau et al. 2008), the emerging evidence of epistatic interactions between Chd7 and Fgf8 provides validation to the relevance of neural crest cells to GnRH neurogenesis. Recently, mutations in SOX10, a key neural crest cell specific transcription factor has also now been shown to be an important genetic cause for the KS form of IHH (Pingault et al. 2013). Chd7 is known to interact with Sox10 (Hebert et al. 2003) and recent zebrafish Chd7 morphant model of CHARGE has also shown Sox10 deregulation is an important driver of the neural crest-derived aspects of CHARGE syndrome . In addition to its interaction with Sox10, Chd7 has also been identified as a Sox2 transcriptional cofactor with direct physical interaction between Sox2 and Chd7 with overlapping genome-wide binding sites (Engelen et al. 2011; Puc and Rosenfeld 2011), again attesting to a critical role for CHD7 during embryonic neurogenesis. Taken together, several of the CHARGE features, including IHH, may represent direct phenotypic consequences of neural crest dysregulation, supporting the hypothesis that CHD7-associated CHARGE and IHH may represent novel neurocristopathies.
Conclusions and future directions
Endocrine abnormalities constitute a significant morbidity to patients with CHARGE syndrome that should be screened for and treated. Disruption of CHD7 in humans causes a spectrum of human phenotypes ranging from severe CHARGE phenotypes at one of the spectrum while allelic phenotypes affecting selected organ systems, such as those leading to IHH lie at the other end of the spectrum. Also, this spectrum of disorders may represent novel neural crest cell related human phenotypes. Although, it is clear that CHD7 protein’s chromatin remodeling activity is critical for the ontogeny of olfactory and GnRH neurons during development, the precise cellular mechanisms that underlie the disruption of this dynamic migratory journey remain to be fully deciphered. In addition, humans with CHD7 mutations, especially missense alleles, display incomplete penetrance and are likely to harbor additional epistatic mutations in other genes that are yet to be fully identified. With increasing availability of next generation sequencing, additional putatively causal CHD7 alleles and mutations in other novel genes governing GnRH ontogeny are likely to be uncovered. However, to fully understand the molecular and biologic basis of CHD7-associated CHARGE and IHH, there is an urgent need for developing high throughput and rapidly accessible assay platforms for ascertaining the functional consequence of these mutations. Such functional studies are imperative for accurate clinical interpretation of identified CHD7 mutations and genetic counseling of patients and their families. This pressing need for understand the systems biology of CHD7-related human disorders can only be met by developing multidisciplinary collaborative teams involving clinicians, physician-scientists, geneticists and basic investigators. Detailed phenotyping of patients harboring CHD7 mutations will uncover the true spectrum of phenotypes associated with these mutations and accurate functional interpretation of these mutations will require development of patient-derived induced pleuripotent cell models.
Acknowledgments
Both RB and WFC are supported by grants from the NICHD: K23 HD077043 (RB) and P50 HD028138 (WFC)
Footnotes
Conflicts of Interest: None
References
- Aramaki M, Udaka T, Kosaki R, Makita Y, Okamoto N, Yoshihashi H, Oki H, Nanao K, Moriyama N, Oku S, Hasegawa T, Takahashi T, Fukushima Y, Kawame H, Kosaki K. Phenotypic spectrum of CHARGE syndrome with CHD7 mutations. J Pediatr. 2006;148:410–4. doi: 10.1016/j.jpeds.2005.10.044. [DOI] [PubMed] [Google Scholar]
- Asakura Y, Toyota Y, Muroya K, Kurosawa K, Fujita K, Aida N, Kawame H, Kosaki K, Adachi M. Endocrine and radiological studies in patients with molecularly confirmed CHARGE syndrome. J Clin Endocrinol Metab. 2008;93:920–4. doi: 10.1210/jc.2007-1419. [DOI] [PubMed] [Google Scholar]
- Bajpai R, Chen DA, Rada-Iglesias A, Zhang J, Xiong Y, Helms J, Chang CP, Zhao Y, Swigut T, Wysocka J. CHD7 cooperates with PBAF to control multipotent neural crest formation. Nature. 2010;463:958–62. doi: 10.1038/nature08733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasubramanian R, Choi JH, Francescatto L, Willer J, Horton ER, Asimacopoulos EP, Stankovic KM, Plummer L, Buck CL, Quinton R, Nebesio TD, Mericq V, Merino PM, Meyer BF, Monies D, Gusella JF, Al Tassan N, Katsanis N, Crowley WF., Jr Functionally compromised CHD7 alleles in patients with isolated GnRH deficiency. Proc Natl Acad Sci U S A. 2014;111:17953–8. doi: 10.1073/pnas.1417438111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasubramanian R, Crowley WF., Jr Isolated Gonadotropin-Releasing Hormone (GnRH) Deficiency 1993 [Google Scholar]
- Balasubramanian R, Dwyer A, Seminara SB, Pitteloud N, Kaiser UB, Crowley WF., Jr Human GnRH deficiency: a unique disease model to unravel the ontogeny of GnRH neurons. Neuroendocrinology. 2010;92:81–99. doi: 10.1159/000314193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basson MA, Echevarria D, Ahn CP, Sudarov A, Joyner AL, Mason IJ, Martinez S, Martin GR. Specific regions within the embryonic midbrain and cerebellum require different levels of FGF signaling during development. Development. 2008;135:889–98. doi: 10.1242/dev.011569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman JE, Bocca G, Hoefsloot LH, Meiners LC, van Ravenswaaij-Arts CM. Anosmia predicts hypogonadotropic hypogonadism in CHARGE syndrome. J Pediatr. 2011;158:474–9. doi: 10.1016/j.jpeds.2010.08.032. [DOI] [PubMed] [Google Scholar]
- Bergman JE, Bosman EA, van Ravenswaaij-Arts CM, Steel KP. Study of smell and reproductive organs in a mouse model for CHARGE syndrome. European journal of human genetics : EJHG. 2010;18:171–7. doi: 10.1038/ejhg.2009.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman JE, de Ronde W, Jongmans MC, Wolffenbuttel BH, Drop SL, Hermus A, Bocca G, Hoefsloot LH, van Ravenswaaij-Arts CM. The results of CHD7 analysis in clinically well-characterized patients with Kallmann syndrome. J Clin Endocrinol Metab. 2012;97:E858–62. doi: 10.1210/jc.2011-2652. [DOI] [PubMed] [Google Scholar]
- Bergman JE, Janssen N, Hoefsloot LH, Jongmans MC, Hofstra RM, van Ravenswaaij-Arts CM. CHD7 mutations and CHARGE syndrome: the clinical implications of an expanding phenotype. Journal of medical genetics. 2011;48:334–42. doi: 10.1136/jmg.2010.087106. [DOI] [PubMed] [Google Scholar]
- Bergman JE, Janssen N, van der Sloot AM, de Walle HE, Schoots J, Rendtorff ND, Tranebjaerg L, Hoefsloot LH, van Ravenswaaij-Arts CM, Hofstra RM. A novel classification system to predict the pathogenic effects of CHD7 missense variants in CHARGE syndrome. Human mutation. 2012;33:1251–60. doi: 10.1002/humu.22106. [DOI] [PubMed] [Google Scholar]
- Boehm U, Bouloux PM, Dattani MT, de Roux N, Dode C, Dunkel L, Dwyer AA, Giacobini P, Hardelin JP, Juul A, Maghnie M, Pitteloud N, Prevot V, Raivio T, Tena-Sempere M, Quinton R, Young J. Expert consensus document: European Consensus Statement on congenital hypogonadotropic hypogonadism--pathogenesis, diagnosis and treatment. Nat Rev Endocrinol. 2015;11:547–64. doi: 10.1038/nrendo.2015.112. [DOI] [PubMed] [Google Scholar]
- Bouazoune K, Kingston RE. Chromatin remodeling by the CHD7 protein is impaired by mutations that cause human developmental disorders. Proc Natl Acad Sci U S A. 2012;109:19238–43. doi: 10.1073/pnas.1213825109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronner ME. Evolution: On the crest of becoming vertebrate. Nature. 2015;527:311–2. doi: 10.1038/nature15645. [DOI] [PubMed] [Google Scholar]
- Chalouhi C, Faulcon P, Le Bihan C, Hertz-Pannier L, Bonfils P, Abadie V. Olfactory evaluation in children: application to the CHARGE syndrome. Pediatrics. 2005;116:e81–8. doi: 10.1542/peds.2004-1970. [DOI] [PubMed] [Google Scholar]
- Costa-Barbosa FA, Balasubramanian R, Keefe KW, Shaw ND, Al-Tassan N, Plummer L, Dwyer AA, Buck CL, Choi JH, Seminara SB, Quinton R, Monies D, Meyer B, Hall JE, Pitteloud N, Crowley WF., Jr Prioritizing genetic testing in patients with Kallmann syndrome using clinical phenotypes. J Clin Endocrinol Metab. 2013;98:E943–53. doi: 10.1210/jc.2012-4116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delahaye A, Sznajer Y, Lyonnet S, Elmaleh-Berges M, Delpierre I, Audollent S, Wiener-Vacher S, Mansbach AL, Amiel J, Baumann C, Bremond-Gignac D, Attie-Bitach T, Verloes A, Sanlaville D. Familial CHARGE syndrome because of CHD7 mutation: clinical intra- and interfamilial variability. Clin Genet. 2007;72:112–21. doi: 10.1111/j.1399-0004.2007.00821.x. [DOI] [PubMed] [Google Scholar]
- Dorr HG, Boguszewski M, Dahlgren J, Dunger D, Geffner ME, Hokken-Koelega AC, Lindberg A, Polak M, Rooman R Kigs International Board. Short Children with CHARGE Syndrome: Do They Benefit from Growth Hormone Therapy? Hormone research in paediatrics. 2015;84:49–53. doi: 10.1159/000382017. [DOI] [PubMed] [Google Scholar]
- Engelen E, Akinci U, Bryne JC, Hou J, Gontan C, Moen M, Szumska D, Kockx C, van Ijcken W, Dekkers DH, Demmers J, Rijkers EJ, Bhattacharya S, Philipsen S, Pevny LH, Grosveld FG, Rottier RJ, Lenhard B, Poot RA. Sox2 cooperates with Chd7 to regulate genes that are mutated in human syndromes. Nat Genet. 2011;43:607–11. doi: 10.1038/ng.825. [DOI] [PubMed] [Google Scholar]
- Esposito A, Tufano M, Di Donato I, Rezzuto M, Improda N, Melis D, Salerno M. Effect of long-term GH treatment in a patient with CHARGE association. Ital J Pediatr. 2014;40:51. doi: 10.1186/1824-7288-40-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falardeau J, Chung WC, Beenken A, Raivio T, Plummer L, Sidis Y, Jacobson-Dickman EE, Eliseenkova AV, Ma J, Dwyer A, Quinton R, Na S, Hall JE, Huot C, Alois N, Pearce SH, Cole LW, Hughes V, Mohammadi M, Tsai P, Pitteloud N. Decreased FGF8 signaling causes deficiency of gonadotropin-releasing hormone in humans and mice. J Clin Invest. 2008;118:2822–31. doi: 10.1172/JCI34538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forni PE, Taylor-Burds C, Melvin VS, Williams T, Wray S. Neural crest and ectodermal cells intermix in the nasal placode to give rise to GnRH-1 neurons, sensory neurons, and olfactory ensheathing cells. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:6915–27. doi: 10.1523/JNEUROSCI.6087-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory LC, Gevers EF, Baker J, Kasia T, Chong K, Josifova DJ, Caimari M, Bilan F, McCabe MJ, Dattani MT. Structural pituitary abnormalities associated with CHARGE syndrome. J Clin Endocrinol Metab. 2013;98:E737–43. doi: 10.1210/jc.2012-3467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grumbach MM. A window of opportunity: the diagnosis of gonadotropin deficiency in the male infant. The Journal of clinical endocrinology and metabolism. 2005;90:3122–7. doi: 10.1210/jc.2004-2465. [DOI] [PubMed] [Google Scholar]
- Hale CL, Niederriter AN, Green GE, Martin DM. Atypical phenotypes associated with pathogenic CHD7 variants and a proposal for broadening CHARGE syndrome clinical diagnostic criteria. American journal of medical genetics. Part A. 2016;170A:344–54. doi: 10.1002/ajmg.a.37435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey AS, Leaper PM, Bankier A. CHARGE association: clinical manifestations and developmental outcome. Am J Med Genet. 1991;39:48–55. doi: 10.1002/ajmg.1320390112. [DOI] [PubMed] [Google Scholar]
- He D, Marie C, Zhao C, Kim B, Wang J, Deng Y, Clavairoly A, Frah M, Wang H, He X, Hmidan H, Jones BV, Witte D, Zalc B, Zhou X, Choo DI, Martin DM, Parras C, Lu QR. Chd7 cooperates with Sox10 and regulates the onset of CNS myelination and remyelination. Nat Neurosci. 2016;19:678–89. doi: 10.1038/nn.4258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert JM, Lin M, Partanen J, Rossant J, McConnell SK. FGF signaling through FGFR1 is required for olfactory bulb morphogenesis. Development. 2003;130:1101–11. doi: 10.1242/dev.00334. [DOI] [PubMed] [Google Scholar]
- Janssen N, Bergman JE, Swertz MA, Tranebjaerg L, Lodahl M, Schoots J, Hofstra RM, van Ravenswaaij-Arts CM, Hoefsloot LH. Mutation update on the CHD7 gene involved in CHARGE syndrome. Human mutation. 2012;33:1149–60. doi: 10.1002/humu.22086. [DOI] [PubMed] [Google Scholar]
- Jongmans MC, Admiraal RJ, van der Donk KP, Vissers LE, Baas AF, Kapusta L, van Hagen JM, Donnai D, de Ravel TJ, Veltman JA, Geurts van Kessel A, De Vries BB, Brunner HG, Hoefsloot LH, van Ravenswaaij CM. CHARGE syndrome: the phenotypic spectrum of mutations in the CHD7 gene. Journal of medical genetics. 2006;43:306–14. doi: 10.1136/jmg.2005.036061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jongmans MC, Hoefsloot LH, van der Donk KP, Admiraal RJ, Magee A, van de Laar I, Hendriks Y, Verheij JB, Walpole I, Brunner HG, van Ravenswaaij CM. Familial CHARGE syndrome and the CHD7 gene: a recurrent missense mutation, intrafamilial recurrence and variability. American journal of medical genetics. Part A. 2008;146A:43–50. doi: 10.1002/ajmg.a.31921. [DOI] [PubMed] [Google Scholar]
- Jongmans MC, van Ravenswaaij-Arts CM, Pitteloud N, Ogata T, Sato N, Claahsen-van der Grinten HL, van der Donk K, Seminara S, Bergman JE, Brunner HG, Crowley WF, Jr, Hoefsloot LH. CHD7 mutations in patients initially diagnosed with Kallmann syndrome--the clinical overlap with CHARGE syndrome. Clinical genetics. 2009;75:65–71. doi: 10.1111/j.1399-0004.2008.01107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karczewski KJ, Weisburd B, Thomas B, Solomonson M, Ruderfer DM, Kavanagh D, Hamamsy T, Lek M, Samocha KE, Cummings BB, Birnbaum D, Consortium The Exome Aggregation. Daly MJ, MacArthur DG. The ExAC browser: displaying reference data information from over 60 000 exomes. Nucleic Acids Res. 2017;45:D840–D45. doi: 10.1093/nar/gkw971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HG, Kurth I, Lan F, Meliciani I, Wenzel W, Eom SH, Kang GB, Rosenberger G, Tekin M, Ozata M, Bick DP, Sherins RJ, Walker SL, Shi Y, Gusella JF, Layman LC. Mutations in CHD7, encoding a chromatin-remodeling protein, cause idiopathic hypogonadotropic hypogonadism and Kallmann syndrome. Am J Hum Genet. 2008;83:511–9. doi: 10.1016/j.ajhg.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laitinen EM, Tommiska J, Sane T, Vaaralahti K, Toppari J, Raivio T. Reversible congenital hypogonadotropic hypogonadism in patients with CHD7, FGFR1 or GNRHR mutations. PLoS One. 2012;7:e39450. doi: 10.1371/journal.pone.0039450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layman WS, Hurd EA, Martin DM. Reproductive dysfunction and decreased GnRH neurogenesis in a mouse model of CHARGE syndrome. Human molecular genetics. 2011;20:3138–50. doi: 10.1093/hmg/ddr216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layman WS, McEwen DP, Beyer LA, Lalani SR, Fernbach SD, Oh E, Swaroop A, Hegg CC, Raphael Y, Martens JR, Martin DM. Defects in neural stem cell proliferation and olfaction in Chd7 deficient mice indicate a mechanism for hyposmia in human CHARGE syndrome. Human molecular genetics. 2009;18:1909–23. doi: 10.1093/hmg/ddp112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning BJ, Yusufzai T. The ATP-dependent chromatin remodeling enzymes CHD6, CHD7, and CHD8 exhibit distinct nucleosome binding and remodeling activities. The Journal of biological chemistry. 2017;292:11927–36. doi: 10.1074/jbc.M117.779470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcos S, Sarfati J, Leroy C, Fouveaut C, Parent P, Metz C, Wolczynski S, Gerard M, Bieth E, Kurtz F, Verier-Mine O, Perrin L, Archambeaud F, Cabrol S, Rodien P, Hove H, Prescott T, Lacombe D, Christin-Maitre S, Touraine P, Hieronimus S, Dewailly D, Young J, Pugeat M, Hardelin JP, Dode C. The prevalence of CHD7 missense versus truncating mutations is higher in patients with Kallmann syndrome than in typical CHARGE patients. J Clin Endocrinol Metab. 2014;99:E2138–43. doi: 10.1210/jc.2014-2110. [DOI] [PubMed] [Google Scholar]
- Oley CA, Baraitser M, Grant DB. A reappraisal of the CHARGE association. Journal of medical genetics. 1988;25:147–56. doi: 10.1136/jmg.25.3.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagon RA, Graham JM, Jr, Zonana J, Yong SL. Coloboma, congenital heart disease, and choanal atresia with multiple anomalies: CHARGE association. J Pediatr. 1981;99:223–7. doi: 10.1016/s0022-3476(81)80454-4. [DOI] [PubMed] [Google Scholar]
- Palmert MR, Dunkel L. Clinical practice. Delayed puberty. N Engl J Med. 2012;366:443–53. doi: 10.1056/NEJMcp1109290. [DOI] [PubMed] [Google Scholar]
- Pauli S, von Velsen N, Burfeind P, Steckel M, Manz J, Buchholz A, Borozdin W, Kohlhase J. CHD7 mutations causing CHARGE syndrome are predominantly of paternal origin. Clin Genet. 2012;81:234–9. doi: 10.1111/j.1399-0004.2011.01701.x. [DOI] [PubMed] [Google Scholar]
- Pingault V, Bodereau V, Baral V, Marcos S, Watanabe Y, Chaoui A, Fouveaut C, Leroy C, Verier-Mine O, Francannet C, Dupin-Deguine D, Archambeaud F, Kurtz FJ, Young J, Bertherat J, Marlin S, Goossens M, Hardelin JP, Dode C, Bondurand N. Loss-of-function mutations in SOX10 cause Kallmann syndrome with deafness. Am J Hum Genet. 2013;92:707–24. doi: 10.1016/j.ajhg.2013.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto G, Abadie V, Mesnage R, Blustajn J, Cabrol S, Amiel J, Hertz-Pannier L, Bertrand AM, Lyonnet S, Rappaport R, Netchine I. CHARGE syndrome includes hypogonadotropic hypogonadism and abnormal olfactory bulb development. J Clin Endocrinol Metab. 2005;90:5621–6. doi: 10.1210/jc.2004-2474. [DOI] [PubMed] [Google Scholar]
- Puc J, Rosenfeld MG. SOX2 and CHD7 cooperatively regulate human disease genes. Nat Genet. 2011;43:505–6. doi: 10.1038/ng.843. [DOI] [PubMed] [Google Scholar]
- Raivio T, Falardeau J, Dwyer A, Quinton R, Hayes FJ, Hughes VA, Cole LW, Pearce SH, Lee H, Boepple P, Crowley WF, Jr, Pitteloud N. Reversal of idiopathic hypogonadotropic hypogonadism. N Engl J Med. 2007;357:863–73. doi: 10.1056/NEJMoa066494. [DOI] [PubMed] [Google Scholar]
- Sanlaville D, Etchevers HC, Gonzales M, Martinovic J, Clement-Ziza M, Delezoide AL, Aubry MC, Pelet A, Chemouny S, Cruaud C, Audollent S, Esculpavit C, Goudefroye G, Ozilou C, Fredouille C, Joye N, Morichon-Delvallez N, Dumez Y, Weissenbach J, Munnich A, Amiel J, Encha-Razavi F, Lyonnet S, Vekemans M, Attie-Bitach T. Phenotypic spectrum of CHARGE syndrome in fetuses with CHD7 truncating mutations correlates with expression during human development. Journal of medical genetics. 2006;43:211–17. doi: 10.1136/jmg.2005.036160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz Y, Wehner P, Opitz L, Salinas-Riester G, Bongers EM, van Ravenswaaij-Arts CM, Wincent J, Schoumans J, Kohlhase J, Borchers A, Pauli S. CHD7, the gene mutated in CHARGE syndrome, regulates genes involved in neural crest cell guidance. Hum Genet. 2014;133:997–1009. doi: 10.1007/s00439-014-1444-2. [DOI] [PubMed] [Google Scholar]
- Schwanzel-Fukuda M, Pfaff DW. Origin of luteinizing hormone-releasing hormone neurons. Nature. 1989;338:161–4. doi: 10.1038/338161a0. [DOI] [PubMed] [Google Scholar]
- Shao M, Liu C, Song Y, Ye W, He W, Yuan G, Gu S, Lin C, Ma L, Zhang Y, Tian W, Hu T, Chen Y. FGF8 signaling sustains progenitor status and multipotency of cranial neural crest-derived mesenchymal cells in vivo and in vitro. J Mol Cell Biol. 2015;7:441–54. doi: 10.1093/jmcb/mjv052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoji Y, Ida S, Etani Y, Yamada H, Kayatani F, Suzuki Y, Kosaki K, Okamoto N. Endocrinological Characteristics of 25 Japanese Patients with CHARGE Syndrome. Clin Pediatr Endocrinol. 2014;23:45–51. doi: 10.1297/cpe.23.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidhoum VF, Chan YM, Lippincott MF, Balasubramanian R, Quinton R, Plummer L, Dwyer A, Pitteloud N, Hayes FJ, Hall JE, Martin KA, Boepple PA, Seminara SB. Reversal and relapse of hypogonadotropic hypogonadism: resilience and fragility of the reproductive neuroendocrine system. J Clin Endocrinol Metab. 2014;99:861–70. doi: 10.1210/jc.2013-2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebert JR, Graham JM, Jr, MacDonald C. Pathologic features of the CHARGE association: support for involvement of the neural crest. Teratology. 1985;31:331–6. doi: 10.1002/tera.1420310303. [DOI] [PubMed] [Google Scholar]
- Teixeira L, Guimiot F, Dode C, Fallet-Bianco C, Millar RP, Delezoide AL, Hardelin JP. Defective migration of neuroendocrine GnRH cells in human arrhinencephalic conditions. J Clin Invest. 2010;120:3668–72. doi: 10.1172/JCI43699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ravenswaaij-Arts CM, Blake K, Hoefsloot L, Verloes A. Clinical utility gene card for: CHARGE syndrome - update 2015. European journal of human genetics : EJHG. 2015;23 doi: 10.1038/ejhg.2015.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vissers LE, van Ravenswaaij CM, Admiraal R, Hurst JA, de Vries BB, Janssen IM, van der Vliet WA, Huys EH, de Jong PJ, Hamel BC, Schoenmakers EF, Brunner HG, Veltman JA, van Kessel AG. Mutations in a new member of the chromodomain gene family cause CHARGE syndrome. Nat Genet. 2004;36:955–7. doi: 10.1038/ng1407. [DOI] [PubMed] [Google Scholar]
- Wheeler PG, Quigley CA, Sadeghi-Nejad A, Weaver DD. Hypogonadism and CHARGE association. Am J Med Genet. 2000;94:228–31. doi: 10.1002/1096-8628(20000918)94:3<228::aid-ajmg8>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Whitlock KE, Illing N, Brideau NJ, Smith KM, Twomey S. Development of GnRH cells: Setting the stage for puberty. Molecular and cellular endocrinology. 2006;254–255:39–50. doi: 10.1016/j.mce.2006.04.038. [DOI] [PubMed] [Google Scholar]
- Yu T, Meiners LC, Danielsen K, Wong MT, Bowler T, Reinberg D, Scambler PJ, van Ravenswaaij-Arts CM, Basson MA. Deregulated FGF and homeotic gene expression underlies cerebellar vermis hypoplasia in CHARGE syndrome. Elife. 2013;2:e01305. doi: 10.7554/eLife.01305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zentner GE, Hurd EA, Schnetz MP, Handoko L, Wang C, Wang Z, Wei C, Tesar PJ, Hatzoglou M, Martin DM, Scacheri PC. CHD7 functions in the nucleolus as a positive regulator of ribosomal RNA biogenesis. Human molecular genetics. 2010;19:3491–501. doi: 10.1093/hmg/ddq265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zentner GE, Layman WS, Martin DM, Scacheri PC. Molecular and phenotypic aspects of CHD7 mutation in CHARGE syndrome. American journal of medical genetics. Part A. 2010;152A:674–86. doi: 10.1002/ajmg.a.33323. [DOI] [PMC free article] [PubMed] [Google Scholar]