Abstract
Background
Mild traumatic brain injuries (mTBI) are an increasing health concern due to persistent behavioral and neurological effects. To better understand these effects, researchers frequently rely on animal injury models. Existing models, however, may not adequately reproduce the mechanism of injury as it occurs in humans.
New Method
Our new model for inducing mTBI in rodents entails acceleration of the animal toward a stationary impact zone to produce rapid rotational movement of the head. The aim of the present experiment was to characterize the effects of this injury in female and male rats on behavior, cognition, and neural plasticity.
Results
mTBI produced the most widespread effects in females: they were more active during recovery within minutes of mTBI and more active in the center of the open field 4 days after mTBI. Spatial learning deficits in the water maze were mild but persistent and accompanied by reduced numbers of immature neurons in the hippocampus along with reductions in sera levels of the neurotrophin, BDNF. By contrast, male mTBI rats mainly exhibited mild spatial learning deficits, with no other observed effects.
Comparison with Existing Methods
Our model induced effects on behavior and biology in rats that aligned with existing models. However, new patterns were observed, particularly when comparing females and males.
Conclusions
Taken together, these findings confirm the validity of this model and point to key differences between females and males in symptom severity and type. Additionally, our model adds a novel injury mechanism that complements existing rodent models.
Keywords: adult hippocampal neurogenesis, biological sex differences, behavioral inhibition, spatial cognition, BDNF
1. Introduction
Traumatic brain injuries (TBIs) are a mounting health concern resulting in millions of emergency department visits annually (NCIPC, 2014) and a growing national economic burden estimated at nearly 80 billion dollars in direct healthcare costs and indirect costs, such as lost productivity at work (Ma et al., 2014). Approximately 75% of TBIs are classified as mild (mTBI), commonly referred to as a concussion (NCIPC, 20003). While media attention towards the occurrence of concussions in contact sports has increased dramatically in recent years, the incidence and consequences of concussions in other domains may be underdiagnosed and overlooked. Whether sustained in sports or other ways, of particular concern is that even mTBI results in serious, long-term consequences (Ling et al., 2015; McKee et al., 2013; Stern et al., 2011) including emotion dysregulation and deficits in cognitive functioning, such as memory loss, learning deficits, and trouble concentrating (Fujimoto et al., 2004; McAllister et al., 2006 Walker & Tesco, 2013). Unfortunately, mTBI is difficult to study as there are a wide range of criteria for diagnosis, many definitions, and a range of symptoms that may not fit into existing standards. Accordingly, developing and validating animal models is also a challenge and at present few experimental models exist that adequately and consistently reproduce the kinds of events, damage, and outcomes that are seen in human patients. Nonetheless, valid models are vital for advancing our understanding of symptoms and their underlying biological basis as well as the efficacy of treatment options and neuroprotective factors (O’Connor et al., 2011). Additionally, these models allow researchers to learn more about the ways in which mild injury may differentially impact an individual based on age, biological sex, and past injury history. The aim of the present experiment was to assess a new animal model of mTBI for use with rodents that addresses limitations with and complements existing models.
Understanding the range and kinds of outcomes following mTBI in humans is challenging due to the difficulties in gathering data. Consequently, there is a dearth of information regarding injury mechanisms in current diagnostic coding, inconsistencies in diagnostic criteria, and a lack of gross anatomical findings using neuroimaging that leave critical gaps in mTBI research (Gao & Chen, 2011; Sharp & Jenkins, 2015; Walker & Tesco, 2013). Another difficulty lies in understanding the complex biomechanical forces that produce a secondary injury cascade that lead to persistent neurological deficits. Researchers rely on animal models to address these issues and advance our knowledge on the mechanisms of injuries (Morales et al., 2005). Animal models enable researchers to control for the injury mechanism, thus allowing characterization of specific components of TBI that include the primary and secondary injury cascades. Many such models have been developed and are used extensively in the field. Current models of non-penetrating impact TBIs, such as weight drop and controlled concussion paradigms, generally have the animal’s head constrained and a projectile or impactor inflicts a brain injury (Cernak et al., 2010; Morales et al., 2005). The acceleration and deceleration forces can be delivered along many axes, resulting in highly variable biomechanical injuries and associated outcomes (Eucker et al., 2011). Absent or minimal in many models, however, is the rotational acceleration of the animal’s head, which results in more frequent concussions (Walker & Tesco, 2013). Further, forces delivered along the sagittal plane lead to worse physiological outcomes, with horizontal and sagittal components leading to the worst brain pathology (Eucker et al., 2011). To incorporate rotational forces in our model, we used a beveled impact block that allows for rotational acceleration and deceleration primarily along the sagittal axis, with components of horizontal motion.
Another limitation of current models is that each attempts to isolate specific aspects of the injury induced, rarely capturing the entire profile of highly variable head injuries (Morganti-Kossman et al., 2010). Controlled cortical impact (CCI) and weight drop (WD) are widely accepted models for focal injuries and such are good replications of the mechanism of injury of that type as it may occur in humans. However, existing models of diffuse brain injury, including modified WD and lateral fluid percussion (LFPI), are not as good at replicating the mechanisms of injury comparably to that as it occurs in humans (DeWitt et al., 2013). In the case of LFPI in which exposed brain tissue is subjected to a stream of fluid, the head is immobilized in a stereotactic device and unable to accelerate or decelerate after impact. Several WD models exist, but most share the feature of head immobilization, or limited motion at the time of impact.
The novel device used in the present study was designed to bring the animal’s head into motion toward a stationary object and allow for recoil on the impact surface after the initial impact until the head came to rest. Accordingly, characterizing the behavioral and neural features arising from our novel model was the goal of the present study. Also examined was the extent to which these effects were sexually dimorphic. Many studies of mTBI only include male subjects and fail to explore the impact of biological sex on mTBI outcomes. Interestingly, little information is available on the differential effects of sex as a biological variable in animal models or gender in humans and what is known remains largely inconclusive (Bazarian et al., 2010; Cancelliere et al., 2016). In some cases of TBI, studies report greater neuroprotection in females possibly due to hormonal differences (Bramlett & Dietrich, 2001; Djebaili et al., 2005). Others report that gender is not a good predictor for mTBI outcome, but females more frequently report post-concussive syndrome associated with pain (Bazarian et al., 2010; Cancelliere et al., 2016). The purpose of our study was to design an apparatus that could deliver reproducible, precise, and equitable injuries across females and males and lay a foundation for future work to address these open questions.
A key component in animal models is assessing the validity of the injury mechanism and the behavioral outcomes, such that the animal’s symptomatology gives some predictive value to the human condition (O’Connor et al., 2011). Immediately following the injury, we measured the latency to right and videotaped recovery to observe post-injury activity levels. In the subsequent days, we assessed locomotor activity and behavior in an open field test and spatial learning and memory using a water maze. These tests are well established in the literature, and provide a sensitive and reliable measure of rat emotion and cognition. We also sought to investigate underlying neural pathology that may align with behavioral findings. To evaluate hippocampal function and plasticity we examined the effects of injury on adult hippocampal neurogenesis. Based on previous reports, neurogenesis may be disordered following traumatic brain injury, though there are mixed findings on the specific nature of the outcome (Ibrahim et al., 2016; Robinson et al., 2016). Using the marker for immature neurons, doublecortin, we tested the hypothesis that mTBI may lead to disrupted neuronal growth and migration. Additionally, we sought evidence that levels of neurotrophic factors may be altered in the injury cascade and specifically focused on brain-derived neurotrophic factor (BDNF), which plays a critical role in neural plasticity especially as it pertains to learning, memory, and emotion. Here too, we tested the hypothesis that neurotrophic response to injury is disrupted following mTBI.
2. Materials & Methods
2.1. Subjects
The subjects were 32 female and male Sprague-Dawley rats (CD strain, Charles River Laboratories, Wilmington, MA; n=16 of each sex). Rats arrived in the colony on postnatal day (PD) 23 and were housed in same-sex pairs in individually-ventilated, clear polycarbonate cages (30.8 × 30.8 × 18.7 cm; Thoren Caging Systems, Hazleton, PA) with ad libitum access to food and water. The colony was maintained on a 12:12 h light-dark cycle with lights on at 07:00 h and all procedures were carried out during the light phase. The colony temperature was 21 ± 2 °C with 40–50% humidity. Experimental procedures, described below, began when rats were PD 102; average weight for females was 288.88 g and the average weight for males was 456.19 g. All procedures were reviewed and approved by Colby College’s Institutional Animal Care and Use Committee and in accordance with federally regulated standards.
2.2. Novel apparatus to induce mTBI in rats
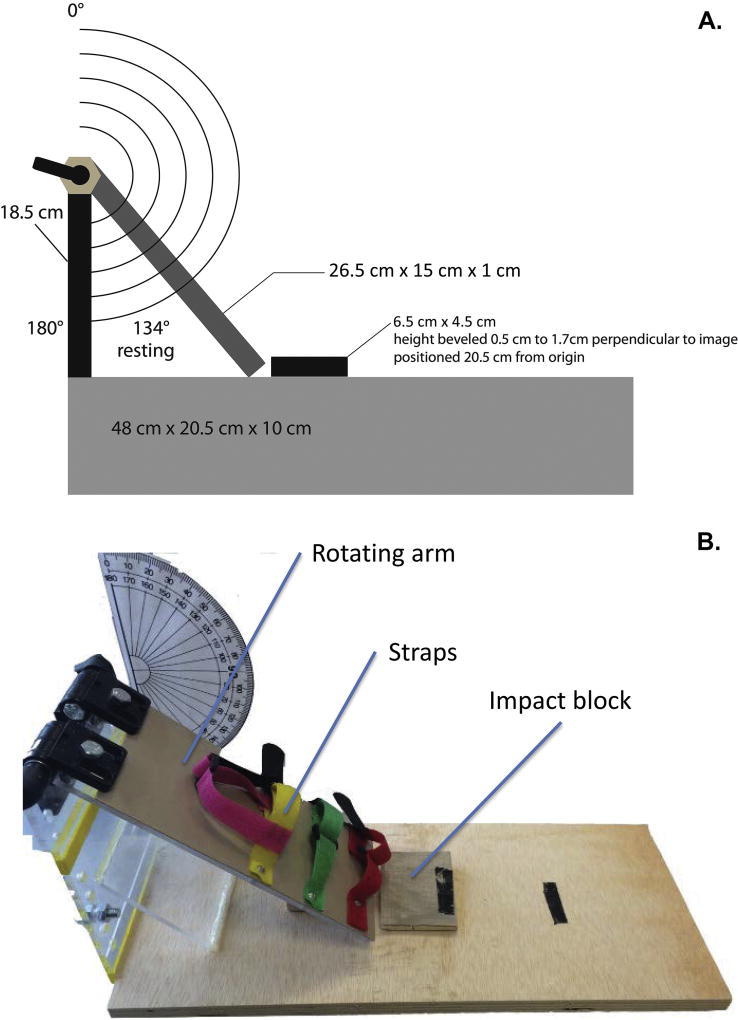
The main goal of the current work was to characterize outcomes following mTBI induced using a novel apparatus, a schematic and image of which is displayed in Fig. 1. This apparatus functions differently from existing models, in which the rat is stationary and an object is propelled toward it; in this case, the rat is in motion and propelled toward a stationary impact zone. To achieve this, rats were first anesthetized with isoflurane (Pirimal Healthcare, Patterson Veterinary Supply) delivered in 1.5% oxygen: induction was 2 and 3 minutes, respectively, for females and males. These differences in exposure times were based on past observations in the lab of the minimum induction durations for females and males, with female rats taking less time than male rats to reach surgical levels of anesthesia as indicated by reflexive reaction to a tail pinch. Once anesthetized, the rat was quickly positioned on its back on the ‘rotating arm platform’ (see Fig. 1) and secured in place with three Velcro straps around its upper torso and abdomen with its head freely moveable off the edge of the rotating arm platform. The rotating arm was then raised to the appropriate angle —175° and 180° for females and males, respectively. We elected to make this modest adjustment to reflect the differing body mass, musculature, and skull thickness of each sex. From the raised position, the rotating arm platform was released and thus swung freely downward allowing the back of the rat’s head to hit the “impact zone” (see Fig. 1). The impact zone was equipped with a resistance pad designed to collect the force of impact. Unfortunately, the sensor was not useable as all impacts exceeded its capacity. Thus, we were unable to calculate the force of the impact of each rat or use force data in our analyses. Future work with our apparatus will focus on mechanisms to collect these kinds of data with improved sensors. Immediately following the impact, the rat was moved to recovery (see 2.3.). This method of inducing mTBI in rats did not in any instance produce skull fractures, as evidenced by visual inspection of the head immediately after and of the skull at sacrifice. Sham rats were subjected to all procedures described with the sole exception of the release of the rotating arm platform and thus sustained no mTBI. Half of the females and males underwent mTBI; the remaining rats made up the sham groups. This resulted in the following experimental conditions: female mTBI; female sham; male mTBI; male sham (n=8 in each condition).
Fig. 1.
Apparatus used to induce mTBI in rats. The top panel (A) shows a schematic diagram of the apparatus with key measurements; the bottom panel (B) shows a photograph of the apparatus with key features identified.
2.3. mTBI recovery
Immediately following the impact procedures described above, each rat, including sham rats, were taken to a quiet room to recover. They were placed individually into a 45-cm2 holding area onto an absorbent pad with one transparent wall through which video could be recorded. Video footage was collected for 10 minutes, at which time all rats were awake and moving and returned to their home cages in the colony. Videos were viewed by an impartial rater unaware of the rats’ injury condition and coded for latency to first movements (emergence from anesthesia) and latency to right, operational defined as full support on all limbs. In addition, for each of 5 minutes following righting, the rater scored rats’ activity levels on a scale from 0 to 5: 0 = stationary; 1 = movements within a localized area using forelimbs only; 2 = intermittent movements within half of the area of the cage; 3 = continuous movement within half of the cage; 4 = intermittent movements within the whole area of the cage; 5 = continuous movements within the whole area of the cage (Andine et al., 1999; Manahan-Vaughan et al., 2008). Analyzing these motor behaviors over a 5-minute period was designed to capture minute-to-minute changes in activity as a function of injury in female and male rats. The expectation that the activity of female and male rats may be differentially affected by mTBI was based on previous findings that females are generally more active than males (e.g. Blizard et al., 1975) and that females emerge more quickly from anesthesia than males (e.g. Myles et al., 2001). Following this observation period, rats were returned to a holding cage in the colony, housed singly overnight, and checked every 4–5 h for signs of any unexpected adverse effects; none were observed. The following day all rats were returned to their original cage pairs and left undisturbed aside from routine husbandry chores for the next 3 days.
2.4. Locomotor activity and anxiety-like behavior in an open field
On the 4th day following the impact procedures, each rat was individually evaluated in an open field for levels of locomotor activity and anxiety-like behavior. The open field was a 100-cm2 wood arena that was painted black. Each rat was placed in the field, facing a corner, and could freely explore it for 5 minutes. A video camera was positioned above the field and rats’ behavior was recorded, tracked, and summarized using ANY-Maze video tracking system (Stoelting Co., Wood Dale, IL). Using the ANY-Maze software, the field was divided into two ‘zones’, a 20-cm wide perimeter corridor and a 60-cm2 center area. This was done to aid in our ability to distinguish between general activity levels and anxiety-like behavior. The primary measure used to gauge activity was the distance traveled in the perimeter and the primary measure used to gauge anxiety-like behavior was the distance traveled in the center. An additional index of anxiety/exploration was the numbers of entries into each of these zones, reflecting rats’ willingness to repeatedly venture away from the more secure perimeter area into the more anxiety-provoking center area.
2.5. Spatial learning and memory in a water maze
On the 5th day following the impact procedures—the day following the open field testing—rats’ ability to learn the spatial location of a stationary, hidden platform in a large pool of water was assessed. The pool itself was devoid of information and thus rats needed to learn the platform’s location relative to available distal visual, auditory, and olfactory cues, including large black and white geometric figures on the walls; the experimenter and a computer; the holding area of the platoon of rats undergoing testing on a counter; and an iPad playing music. The maze was a 1.5-m circular steel tub (Stoelting Co.) that was 70 cm deep and filled approximately half way with cool water (23±1 °C). A clear Plexiglas, 10-cm diameter platform was positioned in the center of one of the four quadrants in the pool approximately 2 cm below the surface of the water. Rats received 4 days of 4 training trials for a total of 16 trials in platoons of 8 rats. Females were always tested before males and all platoons contained both sham and injured rats. On each training trial, rats were released into the pool, facing the wall, from one of four randomly determined release positions, arbitrarily designated N, S, W, and E; each position was used once per day. Rats were given 60 seconds to search the pool for the hidden platform and, if they found and escaped onto it before time was up, the trial was stopped and the rat remained on the platform for 10 seconds before being returned to a holding cage in the same room that concealed its view of the room. If the rat failed to find the platform, they were gently guided to it by the experimenter after 60 seconds, and remained on it for 10 seconds before being removed to its holding cage. All rats in a platoon received a trial before proceeding to the next trial resulting in inter-trial intervals of 7–10 minutes.
The magnitude of rats’ spatial biases toward the quadrant of the pool that contained the platform during training was assessed with two probe trials. These trials were also 60 seconds each and on them the platform was removed from the pool and rats’ search patterns were recorded. The first probe trial (ACQUISITION) was used to assess spatial learning and occurred on training day 4, between trials 2 and 3. By giving rats 2 training trials before the first probe trial, learning could immediately be evaluated in the absence of long-term memory retention. The 2 trials that occurred after the probe trial served to mitigate the impact of the probe, which is an extinction trial, so that rats could be reminded of the platform’s location. This was important for the second probe trial that occurred 6 days later; this probe trial (RETENTION) was used to assess long-term spatial memory for the platform’s location. The primary dependent measure on probe trials was the percentage of the total distance rats traveled in the pool that was in the quadrant that contained the platform during training (TARGET quadrant). These trials were evaluated in 30-second bins to capture any potentially strong biases that emerged only initially and then waned thereafter, typical of training probe trials, or, conversely, evidence of biases that emerged only later after more random exploration of the pool that may sometimes occur after a long absence from the training procedures, typical of retention probe trials.
2.6. Doublecortin immunohistochemistry and unbiased stereology
On the same day of the second, retention, probe trial and 14 days following the mTBI procedures, rats were deeply anesthetized using isoflurane anesthesia delivered in 1.5% oxygen, decapitated, and brains were rapidly extracted and post-fixed in 4% paraformaldehyde at 4 °C. At the time of decapitation, approximately 1 ml of trunk blood was collected and placed on ice. These samples were then centrifuged at 1,500 × g for 10 minutes at 4°C, the supernatant reserved and stored at −80°C until processing (see 2.7.).
Post-fixed brains were sectioned through the rostral-caudal extent of the hippocampal formation on a vibratome with every 4th 40-µm section retained for doublecortin (DCX) immunohistochemistry, which was conducted as previously described (Glenn et al., 2007; McCall et al., 2015). Briefly, free floating sections were rinsed in Tris-buffered saline (TBS; pH 7.3) and treated with 0.6% hydrogen peroxide for 30 minutes followed by incubation in 0.1% Triton X-100 (TTX; Sigma-Aldrich Corp., St. Louis, MO) and 3% normal horse serum (NHS; Vector Laboratories, Burlingame, CA) at room temperature to reduce non-specific staining. Sections were then incubated overnight at 4 °C in a primary antibody solution (DCX polyclonal goat antibody; 1:200; Santa Cruz Biotechnology, Santa Cruz, CA) also containing 0.1% TTX and 3% NHS. On the second day, sections were rinsed again in TBS and incubated at room temperature in a secondary antibody solution (biotinylated horse anti-goat; 1:200; Vector) for 2 h. After rinsing sections again with TBS, they were incubated in an avidin-biotin complex (ABC; Vector) for 1 h at room temperature, rinsed again in TBS, and developed with vector grey (Vector SG Peroxidase kit; Vector).
Unbiased stereology was used to estimate the number of new, immature, neurons in the dentate gyrus of the hippocampal formation. DCX-labeled cells in the dentate gyrus in one hemisphere were counted using the optical fractionator method (Mouton et al., 2002; West, 1999). From each brain, five sections through the dentate gyrus in the dorsal hippocampus were used with a sampling region restricted to the granule cell layers of the dorsal and ventral blades of the dentate gyrus. StereoInvestigator software (Microbrightfield Inc., Willison, VT) was used to guide the systematic sampling through this region with a 100 × 100 µm counting frame and generated 30–40 sites per section for analysis, yielding 150–200 frames per rat, consistent with our past work using this technique (Glenn et al., 2007). The optical dissector was set to a height of 20 µm with a 2-µm guard zone; DCX-labeled cells observed in each frame were counted at 400× magnification. Using the same sections, estimates of the volume of dentate gyrus sampled in each rat was obtained based on the boundaries of the contour tracings and final estimates of numbers of DCX-labeled neurons are shown as numbers per volume of dentate gyrus. This method minimizes the contribution of differences in the contour region traced or region size among rats or groups, particularly occurring as a result of brain size differences between females and males (McCall et al., 2015).
2.7. Sera levels of brain-derived neurotrophic factor (BDNF)
The sera collected from a small, randomly-generated sub-sample of rats in each of the 4 experimental conditions (n=3/condition) was assessed for levels of BDNF using a ChemiKine™ BDNF sandwich ELISA kit (Millipore, Temecula, CA; cat. no. CYT306). Sera samples were prepared according to manufacturer’s instructions, processed in duplicate, and are shown in pg/ml.
2.8. Statistical analyses
Means and standard error of the means were calculated for dependent measures and are displayed in figures. Based on the a priori predictions that mTBI would induce deficits on assays used, we tested a finite number of planned comparisons: female sham versus injured rats comprised one set of comparisons and male sham versus injured rats comprised the other set of comparisons. As outlined by Keppel & Wickens (2004), for experimental designs in which a dependent measure is expected to be influenced by a treatment condition, planned comparisons are an appropriate statistical tool. Given the mild nature of the injury the novel methods in this study were designed to produce and the ways in which behavior is variable, this statistical plan was adopted to carefully seek out mild outcomes—particular when the appropriate interaction was not statistically significant with α set at 0.05. That said, the effects of the independent variables of Injury (sham versus mTBI) and biological Sex (female versus male) on the dependent measures were also analyzed using 2×2 completely between analyses of variance (ANOVA). Based on our conservative statistical approach, the risk of inflating type 1 error rates was considered modest and tests with fewer than five comparisons were reported uncorrected with statistical significance set at p < 0.05, though trends in the data were acknowledged if p values were between 0.05 and 0.10. For the analysis across 5 minutes of recovery, a Bonferroni correction was applied. All statistical analyses were conducting use Statistical Package for the Social Sciences (SPSS, IBM ver. 23).
3. Results
3.1. mTBI Recovery
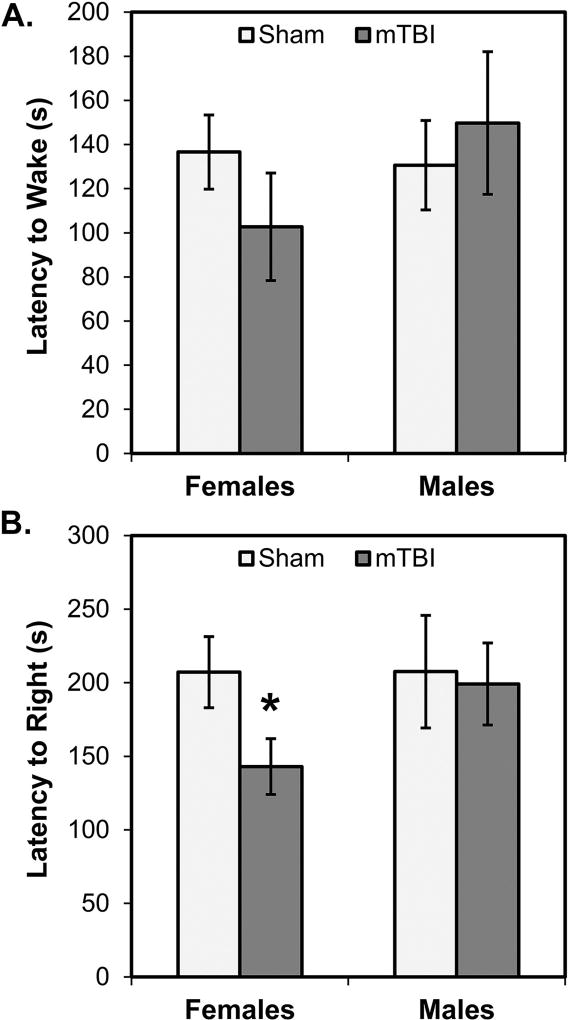
Fig. 2 shows data gathered from the observations of rats from each of our experimental conditions during recovery. Neither female nor male mTBI rats differed from their respective same-sex sham group in latencies to emerge from anesthesia (all p’s>0.10; see Fig. 2A). Additionally, male mTBI rats were not significantly different from sham mTBI rats in their latencies to right (p>0.10). However, female mTBI rats had significantly shorter latencies to right themselves compared to female sham rats (p=0.029; see Fig. 2B). Supporting this pattern, ANOVAs conducted on the latencies of female and male, sham and mTBI rats to emerge from anesthesia and to right themselves revealed no statistically significant main effects of Sex or Injury, and no significant interactions between them (all p’s>0.05).
Fig. 2.
Recovery observations in the 10 minutes following mTBI or sham procedures. There were no significant differences in the latency of rats to wake from anesthesia (A) as a function of biological sex of injury condition. Female mTBI rats had significantly shorter latencies to right themselves (B) compared to female sham rats; the difference between male mTBI and sham rats was not significantly different. *p < 0.05 female mTBI vs. female sham
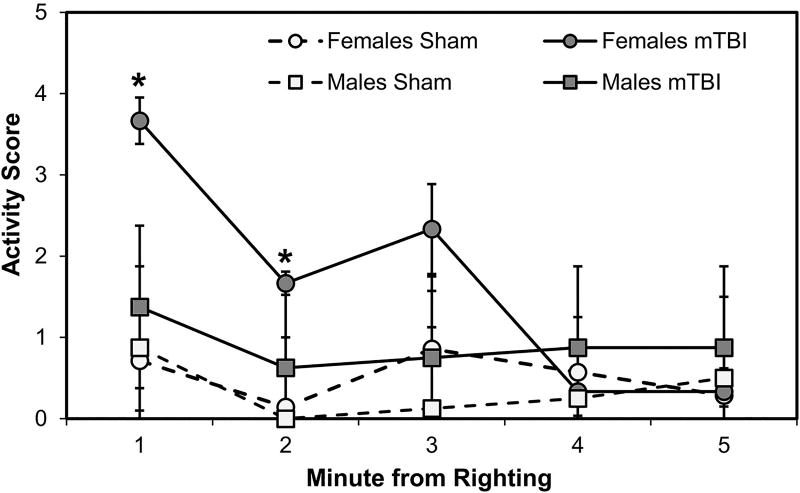
Fitting with the findings reported above, female mTBI, compared to female sham, rats also showed increased activity levels during the subsequent 5-minute observation period after waking. This was statistically significant during minutes 1 (p<0.001) and 2 (p=0.008), but not during minute 3 (p=0.063), or minutes 4 and 5 (p’s>0.10); male mTBI and sham rats showed no significant differences (all p’s>0.10). A 2×2×5 mixed factorial ANOVA, with between factors of Sex and Injury and the repeated measure of Minute (1 through 5), on rats’ activity scores confirmed this pattern with a significant 3-way interaction (F[4,104]=2.465, p=0.050). This indicates that changes in activity level over time were differentially impacted by Sex and Injury status of the rats (see Fig. 3). Accordingly, the interactions between Minute and Injury (F[4,104]=3.099, p=0.019) and Sex and Injury (F[1,26]=27.363, p<0.001) were also statistically significant: female mTBI rats were significantly more active than all other groups in the first 2 minutes after waking. The interaction between Minute and Sex (F[4,104]=1.817, p=0.131) and the main effect of Sex (F[1,26]=3.766, p=0.063) were not statistically significant, though the main effects of Injury (F[1,26]=8.229, p=0.008) and Minute (F[4,104]=5.728, p<0.001) were significant. These effects arose out of the substantial influence of the female mTBI group in the early minutes of the observation (Fig. 3).
Fig. 3.
Activity levels during the 5 minutes after waking. Female mTBI rats had significantly higher activity scores during the first minutes after waking compared to all other groups. *p < 0.05 female mTBI vs. female sham
3.2. Locomotor activity and anxiety-like behavior in an open field
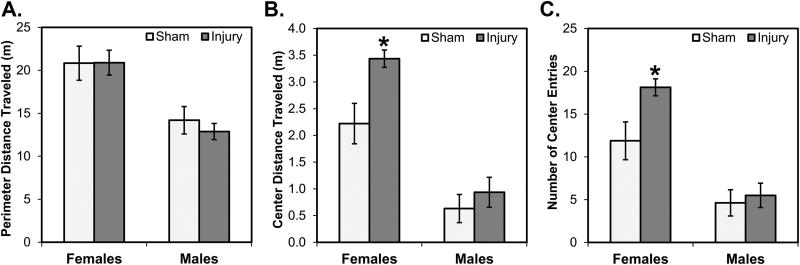
Fig. 4 shows the activity of rats in the open field arena. In terms of general activity levels, indexed by distance traveled in the perimeter region of the open field, there were no significant differences between female mTBI and sham rats or male mTBI and sham rats (all p’s>0.10; see Fig. 4A). A 2×2 ANOVA conducted on this measure revealed a statistically significant main effect of Sex (F[1,28]=37.564, p<0.001) but not a significant main effect of Injury nor a significant interaction between Sex and Injury (p’s>0.10). This reflects the characteristic pattern of female rats, overall, being more active than male rats. In terms of anxiety-like effects, indexed by distance traveled in the center region of the open field, female mTBI rats traveled significantly farther in the center of the field than female sham rats (p=0.005; see Fig. 4B); this was not true for male rats (p=0.221). A 2×2 ANOVA conducted on this measure also revealed significant main effects of Sex (F[1,28]=52.631, p<0.001), but also of Injury (F[1,28]=7.253, p=0.012). The former effect, again reflecting more activity in female rats, overall, when compared to male rats; the latter effect reflecting greater distances traveled in rats with mTBI due to the large effect of mTBI in female, not male, rats. A follow-up analysis of another anxiety-like measure, numbers of entries into the center of the field, revealed similar results: female mTBI rats made significantly more entries into the center of the field than female sham rats (p=0.011; see Fig. 4C); males did not differ (p=0.341). A 2×2 ANOVA on this measure also revealed significant main effects of Sex (F[1,28]=38.563, p<0.001) and Injury (F[1,28]=4.956, p=0.034), but a non-significant interaction (F[1,28]=2.82, p=0.104).
Fig. 4.
Activity and anxiety-like behavior in the open field. Overall, females, compared to males, traveled further distances in the perimeter of the field (A) and in the center of the field (B) and made more entries into the center of the field (C). There were no effects of injury in either sex on perimeter distance traveled, whereas female mTBI rats traveled significant further distances in and made more entries to the center than female sham rats. This injury effect was not observed in male rats. *p < 0.05 female mTBI vs. female sham
3.3. Spatial learning and memory in a water maze
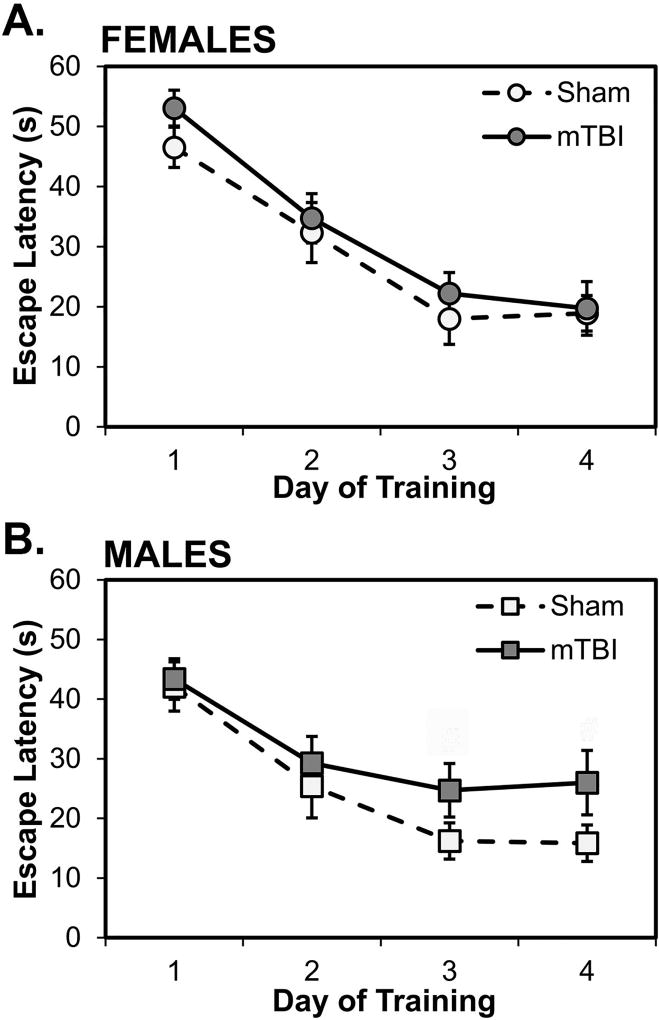
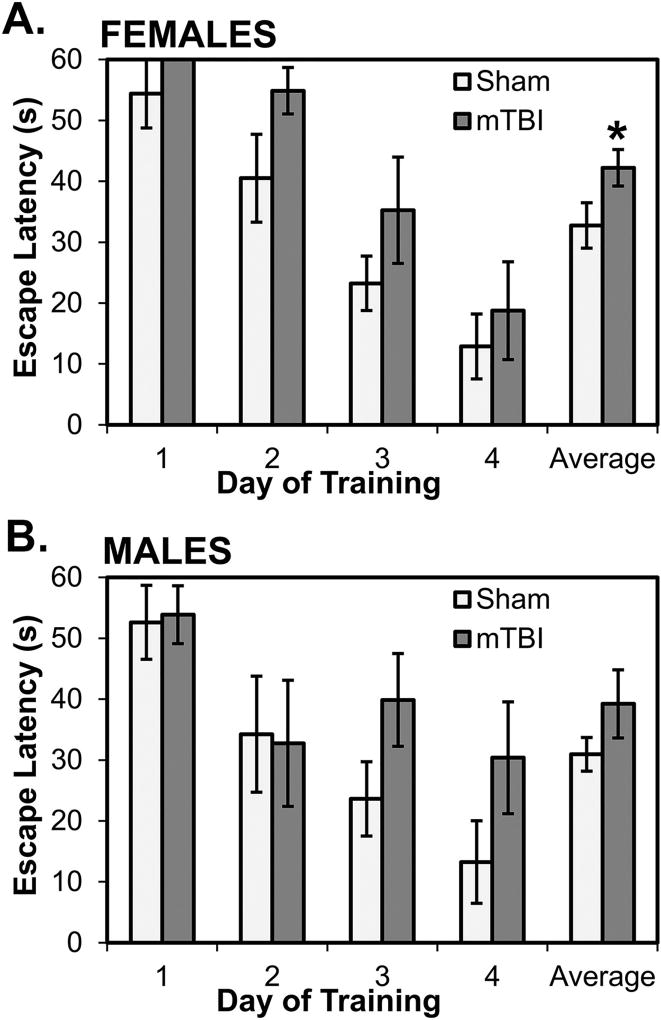
Fig. 5 shows the performance of rats on the stationary platform test of reference memory in the water maze. On latencies averaged over trials for each day of acquisition, there were, overall, no effects in female rats (p’s>0.10; see Fig. 5A) and only mild and statistically non-significant effects in male rats on the last 2 training days (p=0.069 and p=0.062, respectively; see Fig. 5B). A 2×2×4 mixed factorial ANOVA with Sex and Injury as between factors and Day (1–4) as a repeated measure revealed a statistically significant main effect of Day (F[3, 84]=45.767, p<0.001), indicating that, overall, rats showed decreasing latencies to locate the hidden platform over the 4 days of training. There were no interactions between Day and Sex, Day and Injury, or Day, Injury and Sex (all p’s>0.05). Interestingly, however, when examining rats’ latencies on individual trials over each day of training, it was evident that female, but less so male, mTBI rats consistently took longer than sham rats to locate the platform on the first trials of days; these data are displayed in Fig. 6 for females and males. Thus, latencies on the first trial of each day was analyzed using a 2×2×4 ANOVA with Sex and Injury as between factors and Trial 1 from each day (1–4) as a repeated measure. The ANOVA revealed a statistically significant main effect of Trial 1 on each of the 4 days (F[3,84]=21.903, p<0.001); rats’ latencies on the first trial declined with days of training. The interactions between Trial 1 and Sex, Trial 1 and Injury, and Trial 1, Sex, and Injury were not statistically significant (all p’s>0.05). The main effect of Sex and the interaction between Sex and Injury were also not significant (all p’s>0.05). However, the main effect of Injury was statistically significant (F[1,28]=5.090, p=0.032): mTBI rats had, on average, longer latencies on first trials than sham rats and this was particularly marked in females compared to males (p=0.034 and p=0.103, respectively; see Fig. 6A).
Fig. 5.
Spatial learning in the water maze. All groups showed decreasing latencies to locate the hidden platform over days of training. In females (A), there were no significant differences between sham and mTBI; in males (B), mTBI rats had longer latencies than sham rats on days 3 and 4 of training. #p < 0.07 male mTBI vs. male sham
Fig. 6.
Performance of rats on first trials of each training day. Overall, female mTBI rats (A) had longer latencies than female sham rats to locate the hidden platform on the first trial of each day; the average of first trials was significantly longer mTBI rats. In male mTBI rats (B), latencies on first trials tended to be longer than male sham rats on days 3 and 4 but these differences, along with the average of first trials, were not statistically significant. *p < 0.05 female mTBI vs. female sham
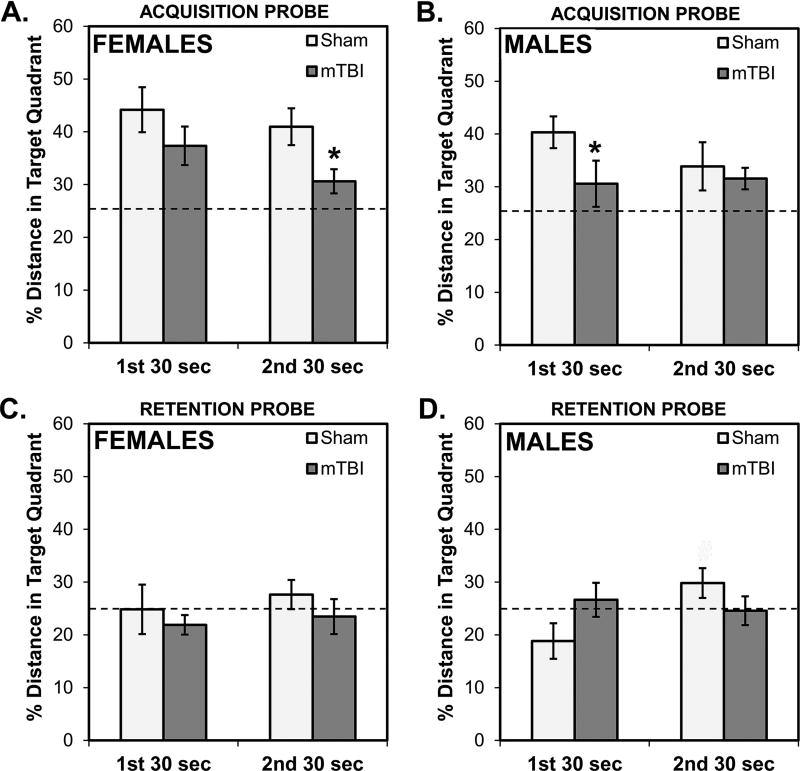
Results of the two probe trials conducted to assess acquisition and retention, respectively, are displayed in Fig. 7. For the ACQUISITION probe, both female groups showed a bias for the quadrant that contained the platform during training that exceeded what would be expected from random searching (25% chance line in the graphs; see Fig. 7A). However, this bias was significantly diminished in female mTBI rats in comparison to their sham comparison groups during the 2nd segment of the probe trial (p=0.013) and overall (p=0.010). mTBI male rats, unlike their sham counterparts, did not show a bias for the target quadrant in the 1st segment (p>0.1; see Fig. 7B) and, during that time, spent a significantly smaller proportion of their time than sham rats in the target quadrant (p=0.044). A 2×2×2 mixed factorial ANOVA with Sex and Injury as between factors and Segment of the probe trial (1st 30 sec; 2nd 30 sec) as the repeated measure revealed that only the main effect of Injury was statistically significant (F[1,28]=7.840, p=0.009); the other main effects and interactions were not significant (all p’s>0.05). This confirms the overall pattern that, on the acquisition probe test, mTBI rats showed diminished biases for the quadrant that contained the platform during training.
Fig. 7.
Performance of rats on probe trials. On the ACQUISITION probe trial (A, B) that occurred on the last day of training, rats with mTBI did not display strong biases toward the quadrant of the pool that contained the platform on training trials. This was most evident in females (A) during the second segment of the trial and in males (B) on the first segment of the trial. On the RETENTION probe trial (C, D) that occurred a week after the last training day, there was little evidence of a quadrant bias in all female groups (C) and, in males (D), sham rats showed a mild bias toward the training quadrant. #p < 0.07 male mTBI vs. male sham
For the RETENTION probe, none of the female groups showed a bias for the target quadrant (all p’s>0.1; Fig. 7C) and only male sham rats showed a non-significant bias and only during the 2nd segment (p=0.066). There were no biases for this quadrant in sham rats in the 1st segment and the mTBI rats, overall, failed to show a bias for the quadrant at any time during the probe trial (see Fig. 7D). Additionally, a 2×2×2 ANOVA revealed that there were no significant main effects or interactions (all p’s>0.1), however the interaction between Segment of the probe trial and Injury approached significance (F[1,28]=3.398, p=0.076). This is consistent with somewhat better performance by Sham rats on the 2nd segment of the test.
3.4. Adult hippocampal neurogenesis and sera levels of brain-derived neurotrophic factor
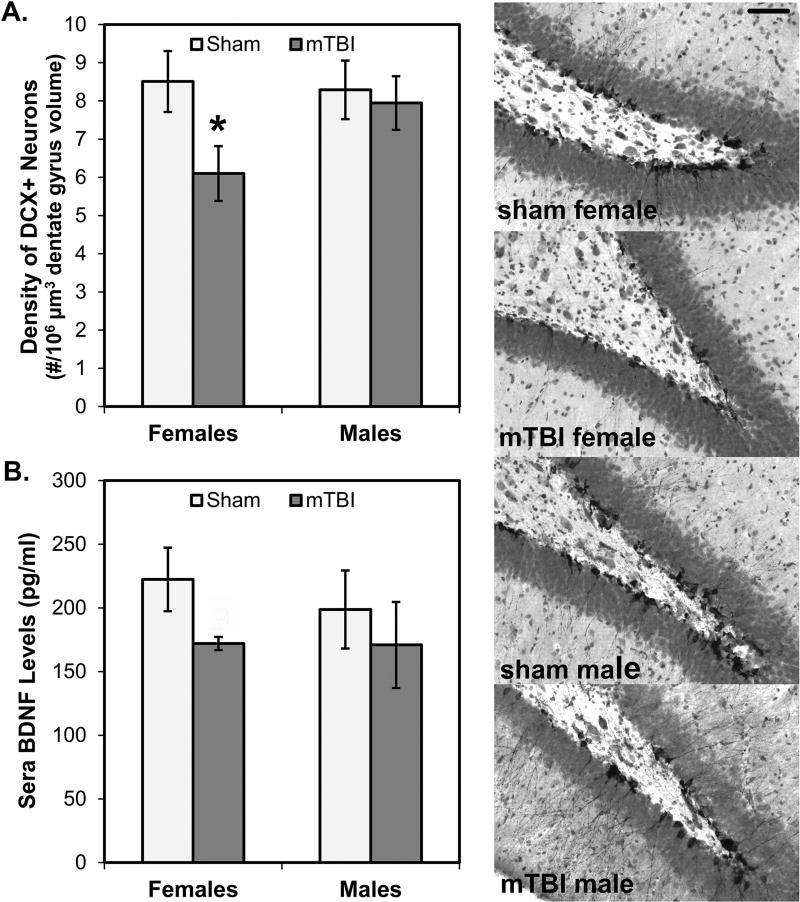
Fig. 8A shows the estimated numbers of neurons in the dentate gyrus of the hippocampal formation that were positive for the immature new neuron marker, doublecortin (DCX). Female mTBI rats had significantly fewer DCX+ neurons than female sham rats (p=0.021) but there was no significant difference in males (p=.372). A 2×2 ANOVA with Sex and Injury as between factors did not reveal a statistically significant main effect of Sex (p>0.05), but the main effect of Injury approached significance (F[1,28]=3.401, p=0.076); mTBI rats exhibited fewer DCX+ neurons per volume of region sampled than sham rats. The interaction between Sex and Injury was also not significant (p>0.05).
Fig. 8.
Adult hippocampal neurogenesis and sera BDNF. Female mTBI rats had significantly fewer numbers of neurons (A) marked by the immature new neuron marker, doublecortin (DCX) than female sham rats; there were no significant differences between male mTBI and sham rats. Shown on the right panel are representative photomicrographs from each of the four conditions taken at 200× magnification; bar in top image represent 25 µm and apply to all images. Consistent with the effects on numbers of new neurons, female mTBI rats also had reduced expression of BDNF (B) than female sham rats; here too, male groups were not significantly different. *p < 0.05; #p < 0.06 female mTBI vs. female sham
Fig. 8B shows the levels of BDNF detected in sera. In this case, there were no statistically significant results. However, fitting well with the consistent pattern of mTBI effects emerging frequently in females, more so than males, there was a non-significant tendency for female mTBI rats to have reduced BDNF levels in comparison to female sham rats (p=0.059). This effect was not observed in male rats and a 2×2 ANOVA failed to reveal any statistically significant effects (all p’s>0.05).
4. Discussion
The present study describes a novel mTBI delivery device to reproduce a mechanism of injury not currently being modeled in the field: rats were in motion and came into contact with a stationary object. Immediately following and within days of the injury, rats displayed changes to their behavior consistent with the kinds of symptoms observed in other animal models along with some new patterns. Overall, the effects of mTBI induced in this novel way were also consistent with observations of the effects of mTBI in humans. In particular, we found clear-cut sexually dimorphic effects with injured females displaying changes to their immediate recovery behavior and, over the next several days, changes to their emotional and cognitive function. Immediate effects were not evident in males and though their learning deficits were more apparent, the outcomes were consistently worse in females and extended to the biological findings. These novel results strongly point to the necessity of evaluating females and males in studies of brain injury outcomes. Additionally, the new methods of inducing mTBI described in this paper addresses a gap in the field and will allow researchers to study more varieties of injuries and have access to a model more comparable to an array of situations in which humans may sustain concussions.
As the novel methods used to induce mTBI in this study led to, overall, a number of behavioral and neural effects in the rats, this new rodent model of concussion has significant potential to increase understanding of this mildest category of head injury. The prototype developed for use in this study has numerous modifiable aspects that can be adjusted according to study goals, including the height and angle from which rats are dropped and the position of the rats’ heads upon impact. In the present study, we elected to have rats land on the dorsal surface of their head; in future studies we intend to compare outcomes following lateral or frontal impacts. In addition to these kinds of modifications, the apparatus may be particularly useful for the study of differential outcomes following mTBI in female and male rats of different ages and injury histories. Our methods and apparatus are well-suited to accommodate the realities of the ways in which differences in weight, bone structure and density, and musculature may affect one’s injury profile. This type of unique injury profile is achieved by allowing the rats’ weights to affect their acceleration towards the impact zone, a component absent in some other models of mTBI. In this study, the angle of descent was modified slightly, by 5°, to additionally compensate for differences between female and male rats; for future studies, there are many possible configurations that can be utilized. Our primary goal in designing the apparatus and selecting the measures used was to attempt to better replicate the kind of scenario in which someone sustains a head injury by accelerating towards a stationary object. This situation is not well-represented in existing models and the present findings offer a solution to this gap in the field.
In addition to characterizing the viability of the new methods and apparatus, a major aim of the present study was to compare the behavioral and biological effects of mTBI, sustained in this new way, in female and male rats. This comparison yielded a number of compelling patterns: overall, there were significantly different effects of the injury in female versus male rats with injury in females being especially profound—affecting them on all measures. By contrast, injury in males produced modest deficits in spatial learning and long-term memory retention without significant outcomes on any other measures collected. Thus, the novel methods used in this report may be an example of a type of injury to which females are vulnerable and it is possible that the females sustained a more severe injury. Alternatively, or additionally, the outcomes studied may better reflect the symptom profile this specific injury induces in female, but not male rats. According to this interpretation, other kinds of tests may have been more sensitive to the effects in males and future research will address this possibility. It should be noted that the female estrous cycle was not tracked in the present study and it is possible that sustaining the injury, or undergoing assessment at specific phases of the cycle could have affected the results. However, we elected to have one rat from each cage undergo injury while its cage-mate served as a sham; given the propensity of animals housed together to have synchronous cycles it seems unlikely, but cannot be ruled out, that all the shams were in one cycle and all injured rats in another during testing. Nonetheless, the results are consistent with other reports in the literature that females and males have differential outcomes following concussion and, in particular, that females show more symptoms (Broshek et al., 2005; Covassin et al., 2007; 2012; Frommer et al., 2011). Whether these differences are rooted in culture or biology is not clear from human studies: are women more likely to report symptoms and more concerned about their future health outcomes? Findings from animal studies, such as those presented here, point to a biological explanation that warrants continued, careful exploration.
The pattern of findings in female rats in the present study, in addition to being distinct from that of males, was novel in several ways. First, the injured females emerged from anesthesia immediately following the injury markedly faster than sham females and both male groups. Then, once awake, they were more quick to right themselves and were very active during the recovery observation period. By contrast, mTBI in other rodent models leads to delays in waking and righting (Mychasiuk et al., 2015; 2016; Schmidt et al., 2000; Stern et al., 2011). Thus, the mechanism of injury in the present study clearly resulted in a unique immediate effect in female rats and no immediate effects in male rats. As females, in general, suffer from concussions at a higher rate (Dick, 2009), it is possible that the pattern observed was due to physical differences between females and males, thereby leading a greater injury in females. Thus, this model may be particularly worthwhile in its sensitivity to differences in body mass and musculature. The females, in this case, may have awoken more rapidly because the impact, for them, was sufficient to accelerate emergence from the anesthesia and increase their overall activity in the minutes following the injury.
Another way that the female data were novel was in the significant reduction to anxiety-like behavior in the open field in the days following injury. Specifically, the injured females entered the center, anxiety-provoking area of the field sooner and spent more time there in comparison to the sham females. This pattern occurred without any significant increase to overall activity levels, suggesting that their increased center activity was not an artifact of a more general effect on locomotion. One way to interpret their increased center activity is that injured females are less anxious and more exploratory. However, the finding is also consistent with a possible deficit in impulse control and disinhibition (Königs et al., 2015; Max et al., 2005; Konrad et al., 2000). Also, it is plausible to attribute the declines in hippocampal neurogenesis to the memory deficit, but that decline was only observed in female rats, though male rats also showed memory deficits. The integrity of the hippocampus is also critical for aspects of emotion regulation, including behavioral inhibition (Gray, 1982; Gray & McNaughton, 2000). Thus, the open field changes in injured females may be linked to the hippocampal changes. This interpretation also points to undetected effects on hippocampal function that are likely to underlie the spatial memory impairments. More detailed analysis of cell structures and neurochemistry may be useful targets for future studies (Gao & Chen, 2011; Ling et al., 2015) Interestingly, Mychasiuk et al. (2015) recently reported secondary ADHD symptoms in male rats following mTBI sustained during the juvenile period; females in this study were more likely to be hypoactive. The present study used adult rats and the mechanism of injury was different, yet the consistency in observing sex differences along this domain strongly suggest that this may be a sexually dimorphic feature to mTBI and the nature of the effect may depend strongly on mechanism of injury and the developmental period in which it occurs.
The likelihood that females were more vulnerable to the novel mechanism of injury used in this study is further supported by the significant decrease in sera levels of the neurotrophin, BDNF. This finding adds to other literature in the field that drops in BDNF may serve as a viable biomarker of concussion. For example, Korley et al. (2016) found that BDNF levels were significantly lower in mild TBI cases compared to moderate and severe TBI when assessed on the day of the injury. Additionally, they reported that lower BDNF levels following mTBI was predictive of poorer outcomes at a 6-month follow-up. That significantly lowered levels of BDNF in sera were still evident 14 days after injury in the present study suggests that this may be a robust and persistent marker of mTBI in our model. Key goals of future studies are to determine the time course to BDNF changes in sera and to seek correlations between the extent of the decline in BDNF with force data during injury and outcomes in behavioral tests at multiple post-injury intervals. These kinds of analyses were not possible in the present study as we only measured BDNF in a small subset of rats from each experimental condition and assessed rats only within 2 weeks of the injury. Additionally, adequate force data were not available for even a preliminary analysis. Interestingly, it is probable that the declines in BDNF were a likely contributor to the decreased levels of neurogenesis detected in the dentate gyrus of the hippocampus in injured female rats.
The present novel methods and mechanism of injury are promising avenues for future work aimed at better replicating, and thus understanding mild closed head injuries in rodents. There were, however, some limitations to the present study. First, injuries in males may not be severe enough to provide an equitable injury across the sexes. While immediate post-recovery data supports this hypothesis, we cannot rule out the possibility that the differential outcomes may be a result of sexually dimorphisms in symptom type that was best captured in female rats with the selected behavioral tests. Secondly, the angle of impact for males (180°) was the highest angle achievable with our present apparatus, thus limiting our ability to produce a larger injury in males. We are continuing to make adjustments to the apparatus for future studies, namely making the rotating arm longer and taller, giving us a larger range of motion and the ability to increase the impact angle for larger impact forces. With a device capable of producing larger impacts and an appropriately calibrated impact sensor, we anticipate varying the injury severity to evaluate the spectrum of cognitive, behavioral, and locomotor deficits to better investigate sex and age difference and the effects of multiple injuries.
5. Conclusion
The new device described here allowed for the induction of a single, mild traumatic brain injury that produced sexually dimorphic locomotor, emotional, and cognitive deficits and resulted in biological differences from sham rats. More research is needed to explore the complex sequelae of mTBI and this model allows for flexibility in the severity, timing, and location of each impact. It is well established in the literature that multiple mTBIs can lead to poorer outcomes (Nichols et al., 2016; Petraglia et al., 2014) and injuries sustained during adolescence may have differential outcomes and alter adult responses to injury. The new method to induce injury described in this paper is well suited for the exploration of these variables and satisfies many features of a valid animal model (Cernak, 2005): the novel approach of accelerating the head towards a stationary object is more akin to some injuries in which a human is in motion at the time of impact; and there were sexually dimorphic and robust behavioral and biochemical changes as a result of inflicting a single, mild TBI. Much more research is needed to explore the complex sequelae of mTBI and this model allows for flexibility in the severity, timing, and location of each impact.
Supplementary Material
Highlights.
New method to produce mTBI propel rats’ head toward stationary impact zone
Female mTBI rats show widespread effects on recovery, activity, and spatial memory
Male rats exhibit only spatial memory deficits in comparison to sham-injured rats
Female, but not male, mTBI rats show reduced hippocampal neurogenesis
Female, but not male, mTBI rats shows reduced BDNF levels in sera
Acknowledgments
The authors would like to thank Edward Yeterian, Bruce Maxwell, and Joseph Atkins for their feedback and assistance on aspects of apparatus development, Amelia Chambers for assisting with data collection, and Stephanie Desrochers and Sarah Steimel for their editorial assistance. This wok was supported by funding to MJG from the National Center for Research Resources (5P20RR016463-12) and the National Institute of General Medical Sciences (8P20GM103423-12) from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andine P, Widermark N, Axelsson R, Nyberg G, Olofsson U, Martensson E. Characterization of MK-801-induced behavior as a putative rat model of psychosis. J Pharmacol Exp Ther. 1999;290:1393–1408. [PubMed] [Google Scholar]
- Bazarian JJ, Blyth B, Mookerjee S, He H, McDermott MP. Sex differences in outcome after mild traumatic brain injury. J. Neurotrauma. 2010;27:527–539. doi: 10.1089/neu.2009.1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blizard DA, Lippman HR, Chen JJ. Sex differences in open-field behavior in the rat: the inductive and activational role of gonadal hormones. Physiol Behav. 14:601–608. doi: 10.1016/0031-9384(75)90188-2. [DOI] [PubMed] [Google Scholar]
- Bramlett HM, Dietrich WD. Neuropathological protection after traumatic brain injury in intact female rats versus males or ovariectomized females. J. Neurotrauma. 2001;18:891–900. doi: 10.1089/089771501750451811. [DOI] [PubMed] [Google Scholar]
- Broshek DK, Kaushik T, Freeman JR, Erlanger D, Webbe F, Barth JT. Sex differences in outcome following sports-related concussion. J Neurosurg. 2005;102:856–863. doi: 10.3171/jns.2005.102.5.0856. [DOI] [PubMed] [Google Scholar]
- Cancelliere C, Donovan J, Cassidy JD. Is sex an indicator of prognosis after mild traumatic brain injury: A systematic analysis of the findings of the World Health Organization Collaborating Centre Task Force on Mild Traumatic Brain Injury and the International Collaboration on Mild Traumatic Brain Injury Prognosis. Arch Phys Med Rehabil. 2016;97:S5–S18. doi: 10.1016/j.apmr.2014.11.028. [DOI] [PubMed] [Google Scholar]
- Cernak I. Animal models of head trauma. NeuroRx. 2005;2:410–422. doi: 10.1602/neurorx.2.3.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cernak I, Chang T, Ahmed FA, Cruz MI, Vink R, Stoica B, Faden Al. Pathophysiological response to experimental diffuse brain trauma differs as a function of developmental age. Dev Neurosci. 2010;32:442–453. doi: 10.1159/000320085. [DOI] [PubMed] [Google Scholar]
- Covassin T, Elbin RJ, Harris W, Parker T, Kontos A. The role of age and sex in symptoms, neurocognitive performance, and postural stability in athletes after concussion. Am J Sports Med. 2012;40:1303–1312. doi: 10.1177/0363546512444554. [DOI] [PubMed] [Google Scholar]
- Covassin T, Schatz P, Swanik CB. Sex differences in neuropsychological function and post-concussion symptoms of concussed collegiate athletes. Neurosurgery. 2007;61:345–350. doi: 10.1227/01.NEU.0000279972.95060.CB. [DOI] [PubMed] [Google Scholar]
- DeWitt D, Perez-Polo R, Hulsebosch C, Dash P, Robertson C. Challenges in the development of rodent models of mild traumatic brain injury. J. Neurotrauma. 2013;30:688–701. doi: 10.1089/neu.2012.2349. [DOI] [PubMed] [Google Scholar]
- Dick RW. Is there a gender difference in concussion incidence and outcomes? Br J Sports Med. 2009;43:46–50. doi: 10.1136/bjsm.2009.058172. [DOI] [PubMed] [Google Scholar]
- Djebaili Myriam, Guo Q, Pettus EH, Hoffman SW, Stein DG. The neurosteroids progesterone and allopregnanolone reduce cell death, gliosis, and functional deficits after traumatic brain injury in rats. J. Neurotrauma. 2005;22:106–118. doi: 10.1089/neu.2005.22.106. [DOI] [PubMed] [Google Scholar]
- Eucker SA, Smith C, Ralston J, Friess SH, Margulies SS. Physiological and histopathological responses following closed rotational head injury depend on direction of head motion. Exp Neurol. 2011;227:79–88. doi: 10.1016/j.expneurol.2010.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frommer LJ, Gurka KK, Cross KM, Ingersoll CD, Comstock RD, Saliba SA. Sex differences in concussion symptoms of high school athletes. J Athl Train. 2011;46:76–84. doi: 10.4085/1062-6050-46.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto ST, Longhi L, Saatman KE, McIntosh TK. Motor and cognitive function evaluation following experimental traumatic brain injury. Neurosci Biobehav Rev. 2004;28:365–378. doi: 10.1016/j.neubiorev.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Gao X, Chen J. Mild traumatic brain injury results in extensive neuronal degeneration in the cerebral cortex. J Neuropathol Exp Neurol. 2011;70:183–191. doi: 10.1097/NEN.0b013e31820c6878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenn MJ, Gibson EM, Kirby ED, Mellott TJ, Blusztajn JK, Williams CL. Prenatal choline availability modulates hippocampal neurogenesis and neurogenic responses to enriching experiences in adult female rats. Eur J Neurosci. 2007;25:2473–2482. doi: 10.1111/j.1460-9568.2007.05505.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JA. The neuropsychology of anxiety: An enquiry in to the functions of the septo-hippocampal system. Oxford: Oxford University Press; 1982. [Google Scholar]
- Gray JA, McNaughton N. The neuropsychology of anxiety: An enquiry into the functions of the septo-hippocampal system. Oxford: Oxford University Press; 2000. [Google Scholar]
- Ibrahim S, Hu W, Wang X, Gao X, He C, Chen J. Traumatic brain injury causes aberrant migration of adult-born neurons in the hippocampus. Sci Rep. 2016;6:21793. doi: 10.1038/srep21793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keppel G, Wickens TD. Design and analysis: a researcher's handbook. 4. New Jersey: Pearson-Prentice Hall; 2004. [Google Scholar]
- Königs M, Heij HA, van der Sluijs JA, Vermeulen RJ, Goslings JC, Luitse JS, Poll-Thé BT, Beelen A, van der Wees M, Kemps RJ, Catsman-Berrevoets CE, Oosterlaan J. Pediatric traumatic brain injury and attention deficit. Pediatrics. 2015;136:534. doi: 10.1542/peds.2015-0437. [DOI] [PubMed] [Google Scholar]
- Konrad K, Gauggel S, Manz A, Schöll M. Inhibitory control in children with traumatic brain injury (TBI) and children with attention deficit/hyperactivity disorder (ADHD) Brain Inj. 2000;14:859–875. doi: 10.1080/026990500445691. [DOI] [PubMed] [Google Scholar]
- Korley FK, Diaz-Arrastia R, Wu AH, Yue JK, Manley GT, Sair HI, Van Eyk J, Everett AD, Okonkwo DO, Valadka AB, Gordon WA, Maas AI, Mukherjee P, Yuh EL, Lingsma HF, Puccio AM, Schnyer DM. Circulating brain-derived neurotrophic factor has diagnostic and prognostic value in traumatic brain injury. J. Neurotrauma. 2016;33:215–225. doi: 10.1089/neu.2015.3949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling H, Hardy J, Zetterberg H. Neurological consequences of traumatic brain injuries in sports. Mol Cell Neurosci. 2015;66:114–122. doi: 10.1016/j.mcn.2015.03.012. [DOI] [PubMed] [Google Scholar]
- Ma VY, Chan L, Carruthers KJ. The incidence, prevalence, costs and impact on disability of common conditions requiring rehabilitation in the US: Stroke, spinal cord injury, traumatic brain injury, multiple sclerosis, osteoarthritis, rheumatoid arthritis, limb loss, and back pain. Arch Phys Med Rehabil. 2014;95:986–995. doi: 10.1016/j.apmr.2013.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manahan-Vaughan D, von Haebler D, Winter C, Juckel G, Heinemann U. A single application of MK801 causes symptoms of acute psychosis, deficits in spatial memory, and impairment of synaptic plasticity in rats. Hippocampus. 2008;18:125–134. doi: 10.1002/hipo.20367. [DOI] [PubMed] [Google Scholar]
- Max JE, Schachar RJ, Levin HS, Ewing-Cobbs L, Chapman SB, Dennis M, Saunders A, Landis J. Predictors of secondary attention-deficit/hyperactivity disorder in children and adolescents 6 to 24 months after traumatic brain injury. J Am Acad Child Adolesc Psychiatry. 2005;44:1041–1049. doi: 10.1097/01.chi.0000173292.05817.f8. [DOI] [PubMed] [Google Scholar]
- McAllister TW, Flashman LA, McDonald BC, Saykin AJ. Mechanisms of working memory dysfunction after mild and moderate TBI: Evidence from functional MRI and neurogenetics. J. Neurotrauma. 2006;23:1450–1467. doi: 10.1089/neu.2006.23.1450. [DOI] [PubMed] [Google Scholar]
- McCall N, Mahadevia D, Corriveau JA, Glenn MJ. Adult emotionality and neural plasticity as a function of adolescent nutrient supplementation in male rats. Pharmacol Biochem Behav. 2015;132:125–135. doi: 10.1016/j.pbb.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee AC, Stein TD, Nowinski CJ, Stern RA, Daneshvar DH, Alvarez VE, Lee H, Hall G, Wojtowicz SM, Baugh CM, Riley DO, Kubilus CA, Cormier KA, Jacobs MA, Martin BR, Abraham CR, Ikezu T, Reichard RR, Wolozin BL, Budson AE, Goldstein LE, Kowall NW, Cantu RC. The spectrum of disease in chronic traumatic encephalopathy. Brain. 2013;136:43–64. doi: 10.1093/brain/aws307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myles PS, McLeod ADM, Hunt JO, Fletcher H. Sex differences in speed of emergence and quality of recovery after anaesthesia: cohort study. BMJ : British Medical Journal. 2001;322:710–711. doi: 10.1136/bmj.322.7288.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales DM, Marklund N, Lebold D, Thompson HJ, Pitkanen A, Maxwell WL, Longhi L, Laurer H, Maegele M, Neugebauer E, Graham DI, Stocchetti N, McIntosh TK. Experimental models of traumatic brain injury: Do we really need to build a better mousetrap? Neuroscience. 2005;136:971–989. doi: 10.1016/j.neuroscience.2005.08.030. [DOI] [PubMed] [Google Scholar]
- Morganti-Kossmann MC, Yan E, Bye N. Animal models of traumatic brain injury: Is there an optimal model to reproduce human brain injury in the laboratory? Injury. 2010;41:S10–S13. doi: 10.1016/j.injury.2010.03.032. [DOI] [PubMed] [Google Scholar]
- Mouton PR, Gokhale AM, Ward NL, West MJ. Stereological length estimation using spherical probes. J Microsc. 2002;206:54–64. doi: 10.1046/j.1365-2818.2002.01006.x. [DOI] [PubMed] [Google Scholar]
- Mychasiuk R, Hehar H, Esser MJ. A mild traumatic brain injury (mTBI) induces secondary attention-deficit hyperactivity disorder-like symptomology in young rats. Behav Brain Res. 2015;286:285–292. doi: 10.1016/j.bbr.2015.03.010. [DOI] [PubMed] [Google Scholar]
- Mychasiuk R, Hehar H, Candy S, Ma I, Esser MJ. The direction of acceleration and rotational forces associated with mild traumatic brain injury in rodents effect behavioural and molecular outcomes. J. Neurosci Methods. 2016;257:168–178. doi: 10.1016/j.jneumeth.2015.10.002. [DOI] [PubMed] [Google Scholar]
- National Center for Injury Prevention and Control. Report to Congress on mild traumatic brain injury in the United States: steps to prevent a serious public health problem. Atlanta, GA: Centers for Disease Control and Prevention; 2003. [Google Scholar]
- National Center for Injury Prevention and Control; Division of Unintentional Injury Prevention. Report to Congress on traumatic brain injury in the United States: epidemiology and rehabilitation. Atlanta, GA: Centers for Disease Control and Prevention; 2014. [Google Scholar]
- Nichols JN, Deshane AS, Niedzielko TL, Smith CD, Floyd CL. Greater neurobehavioral deficits occur in adult mice after repeated, as compared to single, mild traumatic brain injury (mTBI) Behav Brain Res. 2016;1:111–124. doi: 10.1016/j.bbr.2015.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor CA, Cernak I, Vink R. Interaction between anesthesia, gender, and functional outcome task following diffuse traumatic brain injury in rats. J. Neurotrauma. 2003;20:533–541. doi: 10.1089/089771503767168465. [DOI] [PubMed] [Google Scholar]
- O'Connor WT, Smyth A, Gilchrist MD. Animal models of traumatic brain injury: A critical evaluation. Pharmacol Ther. 2011;130:106–113. doi: 10.1016/j.pharmthera.2011.01.001. [DOI] [PubMed] [Google Scholar]
- Petraglia AL, Plog BA, Dayawansa S, Chen M, Dashnaw ML, Czerniecka K, Walker CT, Viterise T, Hyrien O, Iliff JJ, Deane R, Nedergaard M, Huang JH. The spectrum of neurobehavioral sequelae after repetitive mild traumatic brain injury: a novel mouse model of chronic traumatic encephalopathy. J. Neurotrauma. 2014;31:1211–1224. doi: 10.1089/neu.2013.3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson C, Apgar C, Shapiro LA. Astrocyte hypertrophy contributes to aberrant neurogenesis after traumatic brain injury. Neural Plast. 2016;2016:1347987. doi: 10.1155/2016/1347987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt RH, Scholten KJ, Maughan PH. Cognitive impairment and synaptosomal choline uptake in rats following impact acceleration injury. J. Neurotrauma. 2000;17:1129–1139. doi: 10.1089/neu.2000.17.1129. [DOI] [PubMed] [Google Scholar]
- Sharp DJ, Jenkins PO. Concussion is confusing us all. Pract Neurol. 2015;15:172–186. doi: 10.1136/practneurol-2015-001087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern RA, Riley DO, Daneshvar DH, Nowinski CJ, Cantu RC, McKee AC. Long-term consequences of repetitive brain trauma: Chronic traumatic encephalopathy. PM R. 2011;3:S460–S467. doi: 10.1016/j.pmrj.2011.08.008. [DOI] [PubMed] [Google Scholar]
- Walker KR, Tesco G. Molecular mechanisms of cognitive dysfunction following traumatic brain injury. Front Aging Neurosci. 2013;9:29. doi: 10.3389/fnagi.2013.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West MJ. Stereological methods for estimating the total number of neurons and synapses: Issues of precision and bias. Trends Neurosci. 1999;22:51–61. doi: 10.1016/s0166-2236(98)01362-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.