ABSTRACT
Therapies targeting programmed death 1-(PD-1) or its ligand (PD-L1), promoting antitumor T-cell activity have been successfully introduced into clinical practice. Clinical response correlates with PD-L1 expression by tumor cells or immune cells within the tumor microenvironment. The PD-L1/PD-1 axis and tumor microenvironment has been rarely studied in high-grade sarcomas of soft tissue (hSTS), a group of rare, genetically heterogenous and clinically aggressive tumors. We examined PD-L1 protein and CD274/PD-L1 gene copy number variations in 128 primary resected, therapy-naive hSTS using immunohistochemistry and fluorescence-in-situ hybridization. Frequency of tumoral PD-L1 expression varied widely in different disease subentities, with highest rates of positivity (40%) seen in undifferentiated pleomorphic sarcomas (UPS) and rare positivity detected in synovial sarcomas (6%). Amplification of the CD274/PD-L1 gene occurred in 14% of UPS and was rare in other subtypes. PD-L1 protein expression was significantly more frequent in CD274/PD-L1 amplified cases (p = 0.015). The subgroup of UPS was further characterized regarding the interaction between PD-L1 and the immunologic tumor microenvironment. High density of CD3+ and CD8+ tumor infiltrating lymphocytes (TILs) was significantly correlated with the presence of PD-L1 expression and seen more frequently in tumors with lower TNM stage (p = 0.024). Both, PD-L1 expression and high density lymphocytic infiltration were independent prognostic factors for a favorable overall (p = 0.001, HR 6.105 (2.041–8.258)), disease-specific (p = 0.003, HR 10.536 (2.186–50.774)) and disease-free survival (p = 0.020, HR 3.317 (1.209–9.106); values for CD8) in this particular subgroup of hSTS, whereas PD-L1 expression in TILs or CD274/PD-L1 gene amplification were not associated with outcome. These findings represent novel insights into the immune landscape of soft tissue sarcomas, in particular UPS and strengthen the rationale for immunotherapy, including targeting the PD-1/PD-L1 axis in these tumors.
KEYWORDS: High-grade sarcoma, UPS, PD-L1, PD-1, Prognosis, Tumor-microenvironment, Immunohistochemistry, FISH
Introduction
High-grade sarcomas of soft tissue (hSTS) and bone are rare mesenchymal tumors, accounting for approximately 1% of all malignant tumors diagnosed. They comprise a multitude of histological subtypes differing in terms of their tissue of origin, clinical presentation, and genetic alterations. Mainstays of therapy are radical surgery, chemotherapy and radiotherapy,1,2 but overall survival rates are low, with close to 50% of patients dying from their disease.3–5 As there are few effective therapeutic options - especially for patients, who suffer from relapse or present with advanced/metastatic disease - the advent of novel therapeutics is of major importance.
Immune therapy targeting immune checkpoint molecules Programmed Cell Death Protein 1 (PD-1) and its ligand Programmed Death-Ligand 1 (PD-L1) has become a successful therapeutic option for patients with late stage cancer in recent years, with long term remissions seen in melanoma, lung, renal and bladder carcinoma patients. Clinical response correlates with PD-L1 expression by tumor cells (TCs) or tumor-infiltrating lymphocytes (TILs) as well as the presence of tumor specific T-cells within the tumor microenvironment as has been demonstrated in various solid tumor types (reviewed in6–9). In contrast, PD-L1 and PD-1 expression and underlying tumor microenvironment has been rarely studied in hSTS. A thorough analysis has been additionally hampered by the multitude of histological hSTS subtypes.10–16 Interestingly, in a recent phase II study involving patients with advanced hSTS and bone sarcomas treated with the PD-1-inhibitor Pembrolizumab, a subgroup of patients, especially those with undifferentiated pleomorphic sarcomas showed partial response.17
T-cell infiltration into tumor tissue is a strong indicator of the presence of a productive anti-tumor response of the hosts´ immune system.18–20 It is dependent on several factors, including the recognition of tumor-specific antigens (i.e. neoantigens) presented on MHC-molecules leading to TC killing mediated particularly by CD8+ effector T-cells.8,9,21 Based on the presence or absence of T-cells, the tumor microenvironment (TME) is classified as T-cell inflamed or non T-cell inflamed.6,8 In T-cell inflamed TME, presence of CD8+ T-cells leads to upregulation of immune inhibitory mechanisms in TCs – including upregulation of PD-L1.22,23 PD-L1 is bound to PD-1 on TILs, thereby inhibiting T-cell functions and consecutively inducing tumor immune escape. This receptor – ligand interaction can be targeted by therapeutic intervention blocking PD-1- and/or PD-L1 receptor.8,24 In contrast, non-T-cell inflamed TME lacks both immune infiltrate and immunologically induced upregulation of escape mechanisms. Recent studies provide evidence, that these tumors might not be responsive to checkpoint inhibition.6,8,22,25 Underlying mechanisms for this primary immune escape can either be a lack of innate immune sensing –linked for example to activation of β-catenin signaling pathway – or an ineffective T-cell recruitment, due to methylation of chemokine receptors like CXCR3 or alteration of the tumor stroma (reviewed in (6,8)). Interestingly, in a subgroup of non-T-cell inflamed tumors, expression of PD-L1 is observed, most likely due to oncogenic activation mediated by loss of PTEN or amplification of the CD274/PD-L1 gene located on chromosome 9p24.1.6,26–30
PD-L1 and PD-1 expression as well as lymphocytic infiltration into tumor tissue not only have potential predictive impact on immunotherapy response, but are also valuable in prognostic patient stratification. In patients with renal cell, gastric and ovarian cancer, PD-L1 overexpression is linked with poor prognosis, whereas in lung, colorectal cancer, melanoma, as well as in sarcoma patients, prognostic value is controversial (reviewed in,7,31). In contrast, positive prognostic impact of high density T-cell infiltration on patient prognosis is widely accepted especially in epithelial cancers and melanoma.18–20,32 In sarcomas, only few studies have investigated the TME including TILs, and the prognostic impact remains unclear.11,15
The aim of the present study was to analyze PD-L1 expression in TCs and TILs and copy number changes of the CD274/PD-L1 gene in a histomorphologically and molecularly well characterized cohort of 128 primary, therapy-naïve hSTS comprising undifferentiated pleomorphic sarcoma (UPS), synovial sarcoma (SS), angiosarcoma (AS), leiomyosarcoma (LMS), epithelioid sarcoma (ES) and alveolar soft part sarcoma (ASPS). Furthermore, focusing on UPS, the largest subgroup from whom detailed clinic-pathologic data were available, the TME was characterized in more detail and prognostic impact of PD-L1, PD-1 and TILs was evaluated.
Results
Expression pattern of PD-L1 on TCs and TILs and alterations of the CD274/PD-L1 gene locus in high-grade sarcomas of soft tissue
PD-L1 expression and CD274/PD-L1 copy number status was first analyzed in a large histologically and molecularly well-characterized cohort of 128 primary resected, therapy-naïve hSTS, including 57 UPS, 30 SS, 23 AS, 8 LMS, 5 ASPS and 5 ES. PD-L1 expression in sarcoma cells (defined as positive PD-L1 staining of any percentage) was observed in 36/128 (28.1%) cases. Intensity of staining varied between weak (3/36; 8.3%), intermediate (23/36; 63.9%) and strong (10/36; 27.8%). PD-L1 staining results were not influenced by the age of the paraffin tissue blocks used (Supplementary Table 2). Staining intensity correlated positively with the percentage of positive TCs (p<0.001). Distribution of PD-L1 expression using different cut-off values for definition of positivity (1%; 5%; 10%; 50%) is detailed in Supplementary Table 1. In TILs, PD-L1 expression was present in 15/128 (11.7%) cases.
Amplification of the CD274/PD-L1 gene locus was present in 9/128 (7.1%) cases, including 8 cases with high-level and one case with low-level amplification. Information regarding the PD-L1/CEP9 ratio is given in Supplementary Table 3. In 32/128 (25.0%) cases, polysomy of the gene locus was detected, whereas 81/128 (63.3%) cases were disomic. In 2/128 (1.6%) cases, one allele of the CD274/PD-L1 gene was deleted. In amplified cases, concordant PD-L1 protein expression assessed by immunohistochemistry, was present in 6/9 (66.7%) cases, and was significantly more frequent in comparison to cases with PD-L1 polysomy, disomy or deletion (p = 0.015). PD-L1 expression assessed by immunohistochemistry did not show a correlation with the presence of a copy number variation (CNV) of the CD274/PD-L1 gene locus. To exclude any possible bias due to tissue heterogeneity, staining results based on TMAs were confirmed by staining of whole slide sections in CD274/PD-L1 amplified cases (correlation coefficient r = 0.991; p = 0.001). The gene locus for PD-L1 is in close proximity to the gene locus for PD-L2. We therefore also analyzed expression of PD-L2 by immunohistochemistry in these 9 amplified cases by staining whole slides of the respective cases. PD-L2 was not expressed in any of these cases (0/9) (data not shown).
PD-L1 expression and CD274/PD-L1 gene locus alterations in different subtypes of hSTS
The number of PD-L1 positive cases was highest in UPS (23/57; 40.4%) compared to AS (8/23; 34.8%), ASPS (1/5; 20.0%), ES (1/5; 20%), LMS (1/8; 12.5%) and SS (2/30; 6.7%); p = 0.022; Fig. 1a). PD-L1 positive TILs were most frequently detected in ASPS (1/5; 20.0%), ES (1/5; 20.0%) and UPS (10/57; 17.5%) compared to the other hSTS subtypes (p = 0.050; Fig. 1b). Amplification of the CD274/PD-L1 gene locus was exclusively observed in UPS (8/57; 14.0%) and AS (1/23; 4.3%). (p<0.001; Table 1).
Figure 1.
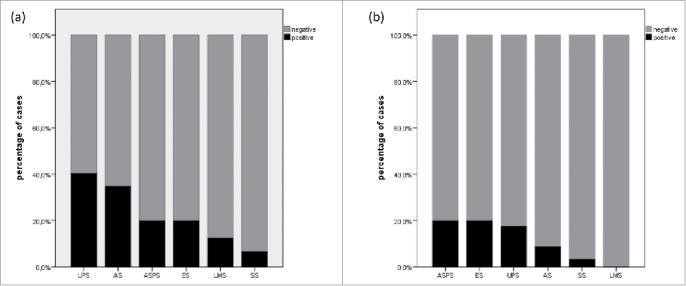
Distribution of PD-L1 expression in tumor cells (a) and tumor-infiltrating lymphocytes (b) in high-grade sarcomas of soft tissue depending on histopathologic entity.
Table 1.
PD-L1 expression in tumor cells and tumor-infiltrating lymphocytes and CD274/PD-L1 copy number status in high-grade sarcomas of soft-tissue; Mean and median value of cases with PD-L1 positive TCs respectively TILs are shown. TC Tumor cell; TIL Tumor-infiltrating lymphocyte; UPS Undifferentiated pleomorphic sarcoma; ASPS Alveolar soft part sarcoma; ES Epithelioid sarcoma AS Angiosarcoma; LMS Leiomyosarcoma; SS Synovial sarcoma.
| Number of Cases |
PD-L1 positive TCs |
PD-L1 positive TILs |
CD274/PD-L1 Copy Number |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Amplification | Polysomy | Disomy | Deletion | ||||||||
| n | n (%) | Mean (%); Median (%) | Range (%) | n (%) | Mean (%); Median (%) | Range (%) | n (%) | n (%) | n (%) | n (%) | |
| UPS | 57 | 23 (40.4) | 24.9; 10.0 | 0.0–90.0 | 10 (17.5) | 23.1; 15.0 | 0.0–50.0 | 8 (14.0) | 26 (45.6) | 22 (38.6) | 1 (1.8) |
| ASPS | 5 | 1 (20.0) | 70.0; 70.0 | 0.0–70.0 | 1 (20.0) | 0.5; 0.5 | 0.0–0.5 | 0 (0.0) | 1 (3.3) | 29 (96.7) | 0 (0.0) |
| ES | 5 | 1 (20.0) | 5.0; 5.0 | 0.0–5.0 | 1 (20.0) | 5.0; 5.0 | 0.0–5.0 | 1 (4.3) | 3 (13.0) | 19 (82.6) | 0 (0.0) |
| AS | 23 | 8 (34.8) | 25.4; 7.5 | 0.0–80.0 | 2 (8.7) | 5.5; 5.5 | 0.0–10.0 | 0 (0.0) | 5 (62.5) | 2 (25.0) | 1 (12.5) |
| LMS | 8 | 1 (11.1) | 3.0; 3.0 | 0.0–3–0 | 0 (0.0) | 0 (0.0) | 1 (20.0) | 4 (80.0) | 0 (0.0) | ||
| SS | 30 | 2 (6.7) | 10.5; 10.5 | 0.0–20.0 | 1 (3.3) | 10.0; 10.0 | 0.0–10.0 | 0 (0.0) | 0 (0.0) | 5 (100.0) | 0 (0.0) |
| p-value | 0.012 | 0.238 | <0.001 | ||||||||
Cohort description of primary, therapy-naïve UPS
As the prevalence of positive PD-L1 expression was highest in UPS, we decided to more comprehensively characterize this specific subtype of hSTS with regard to PD-L1 expression and the immunologic TME, in particular TILs. Detailed clinicopathological parameters were available for all patients and follow-up data was available for 52/57 patients. Data is described in detail in the Material and Methods section and in Table 2.
Table 2.
Clinicopathological characteristics of patients with therapy-naïve undifferentiated pleomorphic sarcoma (UPS) of soft tissue (n=57).
| Patient characteristics (n=57) | n (%) |
|---|---|
| Mean age (range) | 67 (40–88) |
| Gender | |
| Male | 25 (43.9) |
| Female | 32 (56.1) |
| Localization | |
| Lower extremity | 30 (52.6) |
| Upper extremity | 9 (15.8) |
| Hip / Pelvis | 8 (14.0) |
| Trunk | 7 (12.3) |
| Head and neck region | 2 (3.5) |
| Not available | 1 (1.8) |
| Grading (WHO) | |
| G2 | 14 (24.6) |
| G3 | 43 (75.4) |
| Grading (FNCLCC) | |
| 4 | 3 (5.3) |
| 5 | 11 (19.3) |
| 6 | 20 (35.1) |
| 7 | 18 (31.1) |
| 8 | 5 (8.8) |
| Resection margins | |
| R0 | 32 (56.1) |
| R1 | 15 (26.3) |
| R2 | 6 (10.5) |
| RX | 2 (3.5) |
| Not available | 2 (3.5) |
| TNM stage | |
| pT1a | 5 (8.8) |
| pT1b | 11 (19.3) |
| pT2a | 2 (3.5) |
| pT2b | 37 (64.9) |
| Not available | 2 (3.5) |
| UICC stage | |
| IIA / IIB | 23 (40.4) |
| III | 28 (49.1) |
| IV | 4 (7.0) |
| Not available | 2 (3.5) |
PD-1 expression and subpopulation analysis of TILs in primary UPS
PD-1 was not expressed in TCs. Scattered TILs showed PD-1 staining in 5/57 (8.8%) cases. Quantification of CD3+ and CD8+ TILs resulted in the following values: CD8: mean 12.5%, median 2%, range 0%-60%; CD3: mean 14.2%, median 3%, range 0%-70%. In 6/57 (10.5%) cases, no CD3+ and/or CD8+ TILs were found. Infiltration density of CD3+ and CD8+ TILs was closely correlated. Correlation of absolute percentages of infiltrating CD3+ and CD8+ cells revealed a correlation coefficient of r = 0.968 (p<0.001). Dichotomizing absolute numbers of TIL subsets into subgroups according to infiltration density, we found CD8+ TILs in low density in 39/57 (68.4%) and in high density in 18/57 (31.6%) cases. Respective values for CD3 were: CD3low 38/57 (66.7%) and CD3high 19/57 (33.3%) cases (Table 3). Staining results regarding CD3 and CD8 TILs were concordant in tissue cores and corresponding whole tissue sections in all selected cases tested (n = 9), thereby excluding any possible bias due to tissue heterogeneity.
Table 3.
Distribution of immunological parameters among primary primary, therapy-naïve undifferentiated pleomorphic sarcomas (UPS) of soft tissue. TC Tumor cell; TIL Tumor infiltrating lymphocyte.
| CD3 mean percentage (range) | 14.2 (0.0 – 70.0) |
|---|---|
| High density | 19 (33.3) |
| Low density | 38 (66.7) |
| CD8 mean percentage (range) | 12.5 (0.0 – 60.0) |
| High density | 18 (31.6) |
| Low density | 39 (68.4) |
| PD-L1 TCs mean percentage (range) | 10.1 (0.0–90) |
| Positive | 23 (40.4) |
| Negative | 34 (59.6) |
| PD-L1 TILs mean percentage (range) | 4.1 (0.0–50.0) |
| Positive | 10 (17.5) |
| Negative | 47 (82.5) |
| PD-1 (absolute number of cells) | 0.8 (0.0–25.0) |
| Positive | 5 (8.8) |
| Negative | 52 (91.2) |
Correlation among variables in primary UPS
High density of both CD3+ TILs and CD8+ TILs was significantly correlated with the presence of PD-L1 expression in both, TCs and TILs. In UPS with high density lymphocytic infiltration as seen for CD8+ TILs in 18 cases, positive PD-L1 expression was observed in 14/18 (77.8%) cases. In contrast, only 6/39 (15.4%) cases with low density CD8+ TIL infiltration were PD-L1 positive (p<0.001). Similar results were seen for CD3+ TILs: 15/19 (78.9%) cases with CD3high expressed PD-L1, whereas only 5/38 (13.2%) CD3low cases showed PD-L1 expression (p<0.001). PD-L1 positive TILs were observed in 6 cases, all of which (6/6; 100.0%) displayed a high density CD3+/CD8+ TIL infiltrate (CD3: p = 0.001; CD8: p = 0.001; Supplementary Table 4). PD-1 positive TILs were significantly more frequent in cases with PD-L1 positive TILs (3/5; 60.0% compared to 3/52; 5.8%; p = 0.006)
No significant correlation between density of CD3+ and/or CD8+ lymphocytic infiltration and/or PD-L1 expression on TCs with PD-1 expression was detected. Furthermore, no correlation between PD-L1 expression on TCs and TILs was observed. Intensity of PD-L1 staining on TCs was neither correlated with percentage of PD-L1 expression on TCs, nor was it correlated with density of lymphocytic infiltrate, PD-L1 expression on TILs or PD-1 expression. Furthermore, no statistically significant correlation of CD274/PD-L1 copy number status (amplification, polysomy or deletion) with PD-L1 TC expression and/or staining intensity, PD-1 expression or density of CD3/CD8 TIL infiltration, was found (Table 4).
Table 4.
Correlation of variables among each other. TC Tumor cell; TIL Tumor infiltrating lymphocyte.
| CD3 | CD8 | PD-L1 | ||||
|---|---|---|---|---|---|---|
| density | density | TCs | PD-L1 TILs | PD-1 TILs | CD274/PD-L1 copy number | |
| CD3 density | x | r=0.961 | r=0.650 | r=0.485 | r=0.175 | r=0.071 |
| p<0.001 | p<0.001 | p=0.001 | p=0.128 | p=0.590 | ||
| CD8 density | x | r=0.608 | r=0.505 | r=0.190 | r=0.057 | |
| p<0.001 | p<0.001 | p=0.124 | p=0.666 | |||
| PD-L1 TCs | x | r=0.227 | r=0.162 | r=-0.126 | ||
| p=0.087 | p=0.390 | p=0.340 | ||||
| PD-L1 TILs | x | r=0.500 | r=0.139 | |||
| p=0.001 | p=0.295 | |||||
| PD-1 TILs | x | r=-0.053 | ||||
| p=0.836 | ||||||
| CD274/PD-L1 | x | |||||
| copy number |
Correlation of variables with clinicopathological data of primary UPS
Cases with a high density CD3+ lymphocytic infiltrate were more frequently detected in lower pT-stages at diagnosis (p = 0.024). Among CD3high hSTS, 8/18 (44.4%) cases were diagnosed as pT1 a/b and 10/18 (55.6%) as pT2 a/b. CD3low cases were staged as pT1 a/b in 8/37 (21.6%) and pT2 a/b in 29/37 (78.4%). No further correlation of any of the parameters with UICC stage, pN- or M category, WHO grade and/or FNCLCC score was observed, nor was there a correlation with patients´ age or gender.
Survival analysis
In univariate survival analysis, both, high UICC stage and a positive resection margin were a prognostic factor for poor overall survival (OS) (p = 0.001 and p<0.001, respectively), disease-specific survival (DSS) (p = 0.002 and p<0.001, respectively) and disease-free survival (DFS) (p = 0.001 and p = 0.002, respectively). WHO grade 3 was prognostic for unfavourable OS (p = 0.008) and DSS (p = 0.013) compared to WHO grade 2. In addition, older age was a prognostic factor for poor OS (p = 0.038), but not for DSS and DFS. In contrast, pT-category and gender were not recognized as prognostic factors.
Patients with PD-L1 positive UPS showed a significantly better mean OS, DSS and DFS compared to patients with PD-L1 negative UPS (p = 0.039; p = 0.023; p = 0.051; Fig. 2a). Mean OS (DSS; DFS) of patients with PD-L1 positive TCs was 146.3 months (115.9; 111.5), whereas patients with PD-L1 negative TCs showed a mean OS (DSS; DFS) of only 64.7 months (46.7; 54.3). PD-L1 expression on TILs showed no association with survival, neither did CD274/PD-L1 copy number status. Patients´ outcome was influenced by the density of the T-lymphocytic inflammatory infiltrate. Favourable OS (p = 0.009), DSS (p = 0.003) and DFS (p = 0.047) was observed in patients with high density CD8+ TIL infiltrate with a mean survival time for OS 132.5 months (DSS: 191.7 months; DFS: 117.7 months) compared to poor OS (DSS; DFS) in patients with low density CD8+ TIL infiltrate with mean OS 45.8 months (DSS: 58.7 months; DFS: 54.2 months) (Fig. 2b). These survival differences were mirrored by comparable differences when analyzing the CD3+ lymphocytic infiltrate. Patients with high density CD3+ lymphocytic infiltrate showed significantly better OS (p = 0.014) and DSS (p = 0.006) and a trend for better DFS (p = 0.071) compared to patients with low density CD3+ infiltrate. Mean OS (DSS; DFS) of patients with UPS containing CD3high was 122.4 months (176.3 months, 110.2 months), while respective values for patients with CD3low were 46.5 months (60.0 months; 55.6 months) (Supplementary Fig. 1). PD-1 expression was of no significant prognostic value.
Figure 2.
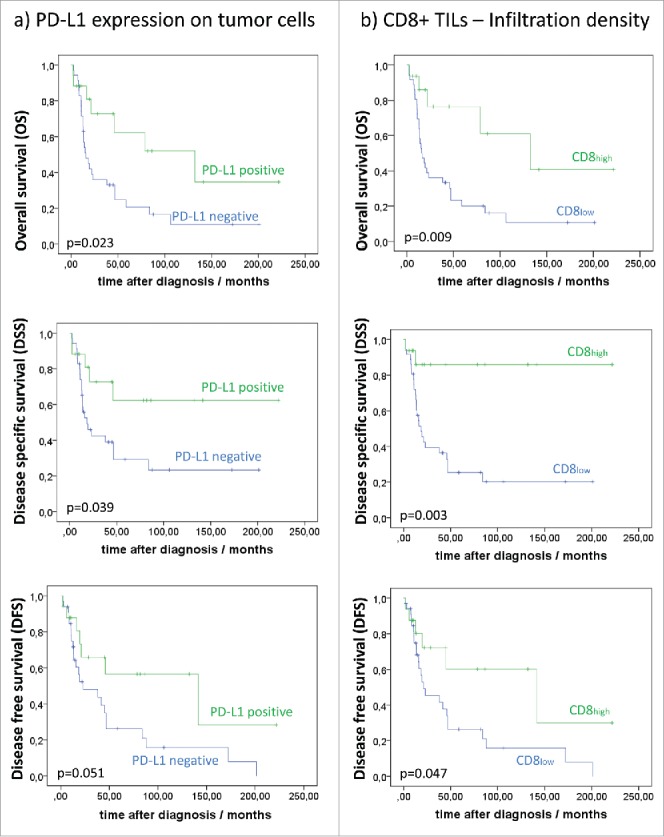
Association of infiltration density of (a) PD-L1 expression on tumor cells (TCs) and (b) CD8+ TILs with overall survival (OS), disease-specific survival (DSS) and disease-free survival (DFS).
Multivariate analysis including status of resection margin, UICC stage, WHO grade, age and gender revealed, that PD-L1 expression on TCs was an independent prognostic factor for OS (p = 0.047) and showed statistical trends for DSS (p = 0.096) and DFS (p = 0.087). Density of CD8+ lymphocytic infiltrate was an independent prognostic factor for OS, DSS and DFS (OS p = 0.001; DSS p = 0.003; DFS p = 0.020). Similarly, prognostic impact of CD3+ TILs density with respect to OS and DSS was independent of UICC stage, WHO grade, age and gender and showed a statistical trend for DFS (OS p = 0.002; DSS p = 0.004; DFS p = 0.036; Table 5; Table 6).
Table 5.
Survival associations for immunologic variables in undifferentiated pleomorphic sarcomas (UPS).
| Number | OS | DSS | DFS | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n=52 | (Events) | Mean OS (SD) | p-value | (Events) | Mean DSS (SD) | p-value | (Events) | Mean DFS (SD) | p-value | |
| CD3 density | ||||||||||
| High | 17 | 6 | 122.4 (27.9) | 0.002* | 3 | 176.3 (23.6) | 0.004* | 7 | 110.2 (27.6) | 0.036* |
| Low | 35 | 29 | 46.5 (11.0) | 25 | 60.0 (13.9) | 24 | 55.6 (13.0) | |||
| CD8 density | ||||||||||
| High | 16 | 5 | 132.5 (28.6) | 0.001* | 2 | 191.7 (19.8) | 0.002* | 6 | 117.7 (28.8) | 0.020* |
| Low | 36 | 30 | 45.8 (10.7) | 26 | 58.8 (13.5) | 25 | 54.2 (12.6) | |||
| PD-L1 tumor cells | ||||||||||
| Positive | 17 | 7 | 115.9 (26.7) | 0.046* | 5 | 146.3 (27.6) | 0.039 | 7 | 111.5 (27.2) | 0.051 |
| Negative | 35 | 28 | 46.7 (11.3) | 23 | 64.7 (15.1) | 24 | 54.3 (12.9) | |||
| PD-L1 TILs | ||||||||||
| TILs positive | 5 | 1 | 64.9 (17.5) | 0.126 | 0 | All cases censored | 0.055 | 1 | 69.7 (14.4) | 0.193 |
| TILs negative | 47 | 34 | 62.1 (12.3) | 28 | 30 | 67.1 (12.7) | ||||
| PD-1 TILs | ||||||||||
| Positive | 4 | 1 | 154.1 (40.8) | 0.166 | 1 | 154.1 (40.8) | 0.297 | 2 | 140.6 (70.0) | 0.359 |
| Negative | 48 | 34 | 61.2 (12.2) | 27 | 86.8 (15.7) | 29 | 66.6 (12.7) | |||
| CD274/PD-L1 Copy number | ||||||||||
| Amplification | 7 | 4 | 87.8 (34.9) | 0.867 | 3 | 108.8 (38.7) | 0.877 | 4 | 108.8 (44.7) | 0.394 |
| Polysomy | 25 | 17 | 42.8 (10.1) | 14 | 46.8 (11.4) | 16 | 40.0 (8.8) | |||
| Disomy | 19 | 13 | 81.2 (22.0) | 6 | 107.8 (24.6) | 10 | 95.3 (21.8) | |||
| Deletion | 1 | 1 | 18.4 (0.0) | 1 | 18.4 (0.0) | 1 | 18.4 (0.0) |
multivariate statistical analysis
Table 6.
Multivariate analysis including (a) PD-L1 expression on tumor cells, (b) CD8+ lymphocytic infiltrate, (c) CD3+ lymphocytic infiltrate. HR Hazard Ratio; CI Confidence Interval ; OS Overall Survival ; DSS Disease-specific Survival ; DFS Disease-free Survival.
| OS |
DSS |
DFS |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 95% CI | 95% CI | 95% CI | |||||||||||
| HR | Min. | Max. | p-value | HR | Min. | Max. | p-value | HR | Min. | Max. | p-value | ||
| (a) PD-L1 Tumor cells | |||||||||||||
| Age | 1,040 | 1,005 | 1,075 | 0.025 | 1,025 | 0.989 | 1,063 | 0.181 | 1,032 | 0.995 | 1,070 | 0.092 | |
| Gender | male | 1,000 | 0.999 | 1,000 | 0.716 | 1,000 | 0.638 | ||||||
| female | 1,000 | 0.429 | 2,335 | 1,194 | 0.459 | 3,107 | 0.812 | 0.341 | 1,935 | ||||
| WHO Grade | 2 | 0.214 | 0.164 | 0.398 | |||||||||
| 3 | 2,348 | 0.611 | 9,017 | 3,135 | 0.628 | 15,642 | 1,769 | 0.471 | 6,638 | ||||
| UICC stage | IIa/b | 1,000 | 0.080 | 1,000 | 0.159 | 1,000 | 0.089 | ||||||
| III | 2,300 | 0.919 | 5,758 | 1,910 | 0.728 | 5,011 | 1,752 | 0.639 | 4,805 | ||||
| IV | 4,571 | 1,028 | 20,313 | 4,089 | 0.894 | 18,705 | 5,651 | 1,199 | 26,620 | ||||
| Resection margin | tumor free | 1,000 | 0.128 | 1,000 | 0.041 | 1,000 | 0.483 | ||||||
| tumor infiltrated | 2,269 | 1,028 | 5,009 | 3,145 | 1,296 | 7,636 | 1,702 | 0.717 | 4,041 | ||||
| unclear | 0.000 | 0.000 | 0.000 | 0.000 | . | 0.000 | 0.000 | . | |||||
| PD-L1 TCs | low | 1,000 | 0.047 | 1,000 | 0.096 | 1,000 | 0.087 | ||||||
| high | 2,408 | 1,011 | 5,735 | 2,351 | 0.860 | 6,428 | 2,184 | 0.893 | 5,340 | ||||
| (b) CD8+ lymphocytic infiltrate | |||||||||||||
| Age | 1,055 | 1,017 | 1,094 | 0.004 | 1,038 | 0.998 | 1,080 | 0.063 | 1,041 | 1,002 | 1,081 | 0.041 | |
| Gender | male | 1,000 | 0.998 | 1,000 | 0.759 | 1,000 | 0.813 | ||||||
| female | 1,001 | 0.443 | 2,262 | 1,153 | 0.463 | 2,871 | 0.903 | 0.387 | 2,108 | ||||
| WHO Grade | 2 | 1,000 | 0.709 | 1,000 | 0.631 | 1,000 | 0.731 | ||||||
| 3 | 1,323 | 0.303 | 5,777 | 1,536 | 0.267 | 8,836 | 1,280 | 0.314 | 5,216 | ||||
| UICC stage | IIa/b | 1,000 | 0.009 | 1,000 | 0.022 | 1,000 | 0.033 | ||||||
| III | 5,055 | 1,605 | 15,917 | 4,668 | 1,333 | 16,350 | 2,819 | 0.873 | 9,100 | ||||
| IV | 5,251 | 1,202 | 22,943 | 4,846 | 1,076 | 21,816 | 6,691 | 1,459 | 30,682 | ||||
| Resection margin | tumor free | 1,000 | 0.030 | 1,000 | 0.017 | 1,000 | 0.323 | ||||||
| tumor infiltrated | 3,617 | 1,398 | 9,356 | 4,920 | 1,644 | 14,724 | 2,096 | 0.799 | 5,503 | ||||
| unclear | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | |||||||
| CD8+ TILs | low | 1,000 | 0.001 | 1,000 | 0.003 | 1,000 | 0.020 | ||||||
| high | 6,105 | 2,041 | 18,258 | 10,536 | 2,186 | 50,774 | 3,317 | 1,209 | 9,106 | ||||
| (c) CD3+ lymphocytic infiltrate | |||||||||||||
| Age | 1,052 | 1,015 | 1,091 | 0.006 | 1,035 | 0.996 | 1,076 | 0.079 | 1,039 | 1,001 | 1,079 | 0.047 | |
| Gender | male | 1,000 | 0.874 | 1,000 | 0.582 | 1,000 | 0.856 | ||||||
| female | 1,069 | 0.470 | 2,433 | 1,298 | 0.513 | 3,280 | 0.924 | 0.393 | 2,172 | ||||
| WHO Grade | 2 | 1,000 | 0.555 | 1,000 | 0.437 | 1,000 | 0.629 | ||||||
| 3 | 1,540 | 0.367 | 6,463 | 1,967 | 0.357 | 10,854 | 1,400 | 0.358 | 5,482 | ||||
| UICC stage | IIa/b | 1,000 | 0.016 | 1,000 | 0.046 | 1,000 | 0.044 | ||||||
| III | 4,178 | 1,391 | 12,551 | 3,547 | 1,089 | 11,553 | 2,460 | 0.794 | 7,621 | ||||
| IV | 4,888 | 1,122 | 21,287 | 4,378 | 0.980 | 19,551 | 6,357 | 1,387 | 29,143 | ||||
| Resection margin | tumor free | 1,000 | 0.031 | 1,000 | 0.015 | 1,000 | 0.350 | ||||||
| tumor infiltrated | 3,410 | 1,367 | 8,503 | 4,607 | 1,637 | 12,959 | 2,003 | 0.783 | 5,127 | ||||
| unclear | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | |||||||
| CD3+ TILs | low | 1,000 | 0.002 | 1,000 | 0.004 | 1,000 | 0.036 | ||||||
| high | 4,775 | 1,753 | 13,006 | 6,694 | 1,820 | 24,619 | 2,749 | 1,067 | 7,083 | ||||
Discussion
In the present study, PD-L1 protein expression on TCs was seen in 6.7%-40.4% of cases, with highest prevalence in UPS, and lowest prevalence in LMS and SS. PD-L1 expression on TILs occurred in 0.0–20.0% of cases, with highest frequency seen in ASPS and AS. Frequency of PD-L1 expression in our cohort was low in comparison to studies by Movva et al, who detected PD-L1 on 50% of sarcoma samples and Kim et al, who found PD-L1 on TCs in 65% and on TILs in 58% of cases. In contrast, Paydas et al reported PD-L1 positive TCs in 30% of cases and D´Angelo et al in 12% of cases (respective value for TILs were 29% and 30%).11,13,14,33 These striking differences in frequency of PD-L1 expression in sarcomas may be explained by several factors, three of which constitute a general problem in PD-L1 testing7: (1) Use of different cut-off values for definition of PD-L1 positivity; (2) Use of different immunohistochemical assays and antibodies for assessment of PD-L1 expression and (3) dynamic, time-dependent PD-L1 expression which might be influenced by preoperative treatment protocols. Moreover, most previous studies on PD-L1 in sarcoma examined cohorts comprising a multitude of (low and high-grade) sarcoma subtypes with low absolute numbers of distinct subentities and unknown neoadjuvant treatments. In the present study, we report absolute percentages of PD-L1 positivity without applying pre-set cut-offs as suggested by several large studies.34–38 In addition, we used the PD-L1 antibody clone SP263 for immunohistochemistry, which has been recommended as companion diagnostic in previous studies targeting PD-1/PD-L1 axis.7,39,40 To avoid possible confounding effects of preoperative treatment on tumor immunology and TME, we included exclusively primary resected, therapy-naïve hSTS in our study.
Copy number gains on chromosome 9p24 containing the genes CD274/PDL1 and PD-L2/PDCD1LG2 were observed in 14% of UPS and 4.3% of angiosarcomas in our cohort. Amplification of chromosome 9p24 represents a well-described mechanism of immune evasion in several lymphoma and epithelial cancer subtypes such as triple-negative breast and head and neck cancers.26–28 Immune evasion is mediated in part by increased tumoral expression of PD-L1 and consequent activation of the PD-1 regulatory pathway on TILs. The identification of 9p24 copy-number gain as a potential mechanism for PD-L1 overexpression in the present study may provide a rationale for treatment of sarcoma patients, especially in a subgroup of UPS with PD-L1 amplification. We found a significant correlation between CD274/PD-L1 gene amplification and the PD-L1 immunohistochemical status, however, three amplified cases were PD-L1 immunonegative. The reason for this discrepancy is currently not clear, but may involve posttranscriptional or posttranslational modifications.
Both, PD-L1 and PD-1 expression in TCs and TILs and TIL density have been studied as potential prognostic biomarkers in various tumor types, including sarcomas. In our analysis, PD-L1 was a positive prognostic biomarker for OS, DSS and DFS in univariate analysis which was confirmed in multivariate analysis. In the current literature, impact of PD-L1 expression on prognosis in hSTS is controversial. Two recent studies concluded that PD-L1 positivity on TCs and PD-1 positivity in TILs are negative prognostic factors,10,14 whereas others did not find any prognostic impact of PD-L1 expression in sarcomas.11 In general, the role of PD-L1 expression as a prognostic factor is unclear. Depending on disease entity and preset cut-off values, positive expression is either associated with improved clinical outcome (HNSCC), negative clinical outcome (gastric cancer, pancreatic cancer), positive or negative outcome (MM, NSCLC, colorectal cancer) or it doesn't show any association with outcome (cervical cancer, squamous cell carcinoma of the lung) (reviewed in31). PD-L1 is a dynamic marker whose expression is dependent on the local micromilieu. To characterize the TME in UPS in more detail, we investigated – in addition to PD-L1 expression – the expression of PD-1, and TIL T-cell subpopulations with antibodies directed against CD3, a general T-cell marker, and CD8, a marker of cytotoxic T-cells. Ligation of PD-1 and PD-L1 induces T-cell anergy and therefore contributes to immune escape.6–8 CD8+ cytotoxic TILs are capable of direct tumor cell killing and other subpopulations of CD3+ T-cells (e.g. CD4+ T-cells) orchestrate the interplay of different immune cell types for example by cytokine expression including INFγ8,18,19,41 These tumor-specific cell populations, involved in adaptive immunity, are essential for inhibition of tumor growth. Our analysis revealed a close interdependence of PD-L1/PD-1 expression and T-cell populations. PD-L1 expression in TCs was significantly more frequent in UPS with PD-L1 positive TILs and both, PD-L1 expression in TCs and TILs were correlated with high density CD3+ and CD8+ lymphocytic infiltrate.
Overall, studies investigating TILs in sarcomas are rare, often lumping together low and high grade sarcomas representing a multitude of genetically, morphologically and clinically distinct and different sub entities.11,15,42,43 In our UPS cohort, high density of both CD3+ and CD8+ T-cell infiltrate was observed in approximately one third of cases. This frequency is similar to results by Sorbye et al, who found a high density of CD3+ (CD8+) intratumoral T-cells in 25% (18%) of cases and by D´Angelo et al who reported high density CD3+ (CD8+) T-cells in 43% (19%) of cases. Confirming and extending these previous studies, the presence of T-cells within tumor tissue suggests that the adaptive immunity plays an active role in anti-tumor response in hSTS, particularly in UPS.
Even more pronounced, when analyzing the prognostic impact of the various immunologic variables in UPS, we identified a high density lymphocytic infiltrate, especially of CD8+ TILs, as an independent positive prognostic marker with respect to OS, DSS and DFS. While a positive prognostic impact of TILs is widely accepted in epithelial cancers (colorectal and ovarian cancer) and malignant melanoma18,19,32 to the best of our knowledge, our study is the first to demonstrate a prognostic role of high density CD3+ and CD8+ T-cell infiltrate in sarcomas, specifically in the UPS subgroup. Previous studies on sarcomas, often hampered by the limitations described above either showed no impact of CD3+ and CD8+ TILs on prognosis or – in univariate analysis11 – revealed an association of low CD3+ infiltration with better survival.
Our study clearly has limitations, as case numbers of rare sarcoma entities included in the study are low. In addition, our study is performed on tissue microarrays containing only small tumor areas. To circumvent bias due to tissue heterogeneity, Botti et al44 suggested to include multiple tumor cores representative for the whole tumor area for evaluation of PD-L1 IHC using TMAs. Along these lines, we included a high number of tissue cores / tumor (3–6) in our TMAs. In addition, previous studies were able to confirm staining results based on TMA on whole slides.45 In our study, scoring based on TMAs was concordant with scoring of whole slide sections in selected cases, making any bias unlikely.
In conclusion, we provide a comprehensive analysis of PD-L1 expression on TCs and TILs in primary resected, therapy-naïve hSTS, using a well established immunohistochemical assay demonstrating tumoral PD-L1 positivity in 26% and amplification of the corresponding CD274/PD-L1 gene locus in 7.1% of cases, with highest rate of PD-L1 expression (40%) and PD-L1 amplification (14%) in UPS. 66.7% of CD274/PD-L1 amplified cases show concordant PD-L1 protein expression. PD-L2 protein expression was not detected. Furthermore, we provide evidence that both CD3+ and CD8+ TIL density within the TME and PD-L1 expression on TILs are potential prognostic factors in UPS. These findings represent novel insights into the immunologic landscape of sarcomas and provide a foundation for further therapeutic intervention into this tumor type.
Material and methods
Tissue samples
One hundred and twenty eight formalin-fixed paraffin-embedded (FFPE) tumor samples of patients with hSTS, undergoing surgery at the Klinikum Rechts der Isar of the Technical University of Munich, Germany, between 1995 and 2015 were included. Cases comprised 57 UPS, 30 SS, 23 AS (including 1 radiation associated AS), 8 LMS, 5 ASPS and 5 ES. All tumors were primary resected/biopsied without neoadjuvant treatment prior to surgery. Hematoxylin & eosin stained sections were reviewed by two pathologists (MB, KSp). Diagnosis was based on morphology, and ancillary immunohistochemical and molecular analysis in selected cases (e.g. MDM2 fluorescence in situ hybridization in n = 57 UPS revealed no amplification of the respective gene locus (0/57)). Grading was performed according to the current World Health Organization (WHO) Classification of Tumors of the Soft Tissue and Bone1 and the Classification of the French Fédération National des Centre de Lutte contre le Cancer (FNCLCC).46 Staging at the time of diagnosis was performed according to the UICC (Union international contre le cancer) tumor, node, metastasis (TNM) classification (7th edition).47 All procedures of the study were in full accordance with the Helsinki Declaration of 1975 and approval for the study was obtained from the Ethics Review Committee of the Technical University of Munich (147/17 S).
For the subgroup of UPS, detailed clinical information was available for 52/57 patients: At the time of diagnosis, synchronous metastases were present in 4 patients. 36 patients died during follow-up, 28 deaths were disease-related. Mean follow-up was 19 months (2 – 84 months) for deceased patients, whose deaths were disease-specific, 60 months (60 – 132 months) for deceased patients, whose deaths were not disease-related and 78 months (6 – 222 months) for patients, who were alive at the last follow-up (Table 2).
Tissue microarray construction
FFPE tumor samples were assembled into a tissue microarray (TMA) using a Tissue Microarrayer (Beecher Instruments) with a core size of 0.6 mm. A minimum of 3, where feasible up to 6 tumor cores were taken from areas with vital tumor tissue previously marked by two pathologists (MB, KSp).
Immunohistochemistry
Immunohistochemistry (IHC) was performed on 2 µm sections from each TMA using primary antibodies against PD-L1 (SP263; Ventana Medical Systems, Roche; ready to use), PD-L2 (D7U8 C; Cell Signaling; 1:50), PD-1 (11RQ-22; 1:50; Cell Marque), CD3 (MRQ-39; 1:500; Cell Marque) and CD8 (C8/144B; Dako; 1:50). Stainings were run on an automated immunostainer with an optiVIEW DAB detection kit (Ventana Medical Systems,). Evaluation of immunohistochemical stainings was performed simultaneously by two pathologists (MB, KSp) who were blinded to clinicopathological data and follow-up.
Membranous expression of PD-L1 was assessed in TCs and TILs, which were identified based on morphologic evaluation. Absolute percentage of positive TCs was counted. Staining intensity was assessed in a 3-tiered grading system including “weak staining” (1+), “intermediate staining” (2+), “strong staining” (3+). PD-L1 staining in TILs was assessed documenting the percentage of PD-L1 positive TILs across tumor area. Staining intensity of PD-L1 in TILs was not taken into account since no obvious differences in staining intensity were noted. The corresponding tumor cores on the TMA of each case were analyzed and the mean value of positivity across the cores was calculated and documented as result. To exclude misinterpretation of tumor-associated macrophages (TAMs) as TCs or TILs, immunohistochemical double staining of PD-L1 (28–8, 1:500, Abcam) / and the macrophage marker CD163 (EdHu1, 1:800, AbD Serotec) on a BondRxm system with a ChromoPlex DualDetection Kit) was performed in selected cases (n = 15). In analogy, expression of PD-1 in TILs was assessed by counting the absolute number of PD-1 positive TILs across all corresponding tumor cores.
Density of CD3+ and CD8+ TILs was evaluated by counting the percentage of positive TILs across tumor area, taking into account sarcoma cells and tumor stroma whereas tumor necrosis and peritumoral tissue were excluded from the analysis. Again, corresponding tumor cores of each case were analyzed and the density across all cores was calculated and documented as result (Fig. 3).
Figure 3.
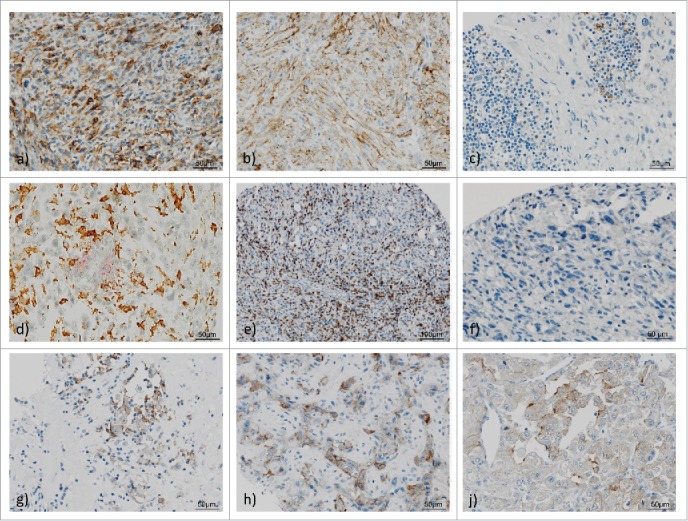
PD-L1, PD-1, CD3 and CD8 expression in high-grade sarcoma of soft tissue and tumor-infiltrating lymphocytes (TILs). 1 a)–1f) Representative examples of undifferentiated pleomorphic sarcoma (UPS) showing membraneous PD-L1 expression in tumor cells with (a) strong staining (3+), (b) intermediate staining (2+); c) UPS with PD-L1 positive TILs; d) UPS with double staining: red: PD-L1 positive lymphocytes, brown: CD163 positive histiocytes, intermingled negative TCs; (e) CD3+ TILs in high infiltration density; (f). UPS with scarce PD-1 positive TILs. 1 g)–1j) PD-L1 staining in representative high-grade sarcomas of soft tissue: (g) Epithelioid sarcoma, (e) Angiosarcoma, (f) Alveolar soft part sarcoma.
PD-L1 Fluorescence in Situ hybridization
Dual-color FISH analysis was performed on 2 µm sections from TMAs as previously described, taking into account at least 20 nuclei per TMA core (CDC274/PD-L1 and PDCD1LG2/PD-L2/CEP9, Zytovision).28 CD274/PD-L1 copy number was evaluated following criteria defined by Schildhaus et al48 with slight modification: PD-L1 high-level amplification was defined as PD-L1/CEP9 ratio ≥2.0 or clusters of >15 PD-L1 signals in >10% of tumor nuclei. Low-level amplification was defined as clusters of >5 PD-L1 signals in >50% of tumor nuclei, polysomy 9 was defined as average PD-L1 copy number >3 signals/cell and PD-L1 deletion was defined as PD-L1/CEP9 ratio <0.8 (Fig. 4).
Figure 4.
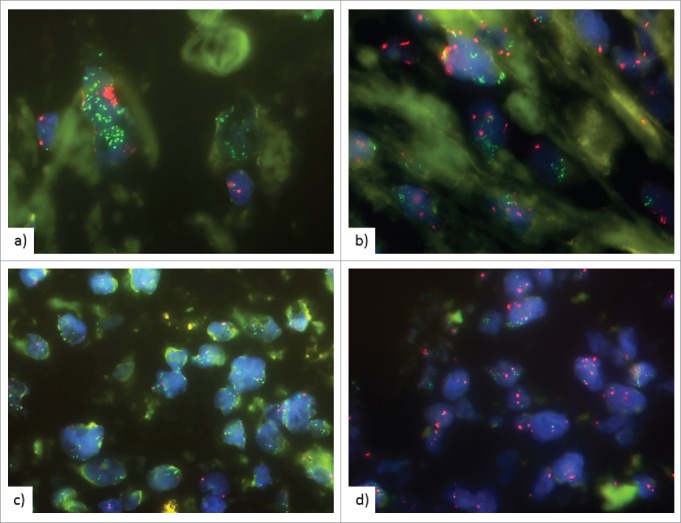
Fluorescence in situ hybridization (FISH) of PD-L1 gene locus. CD274/PD-L1 gene is labelled in green, centromer 9 in red. FISH analysis showing (a), (b) high level amplification with clusters of >15 PD-L1 signals in >10% of tumor nuclei, (c) high level amplification with PD-L1/CEP9 ratio ≥2.0 (d) Low-level amplification with clusters of >5 PD-L1 signals in >50% of tumor nuclei.
Statistics
Descriptive and exploratory statistical analyses were performed using SPSS 23 (SPSS Inc.). The distribution of qualitative data was compared between groups using χ2-test or Fisher's exact test. Likewise, quantitative data was compared between groups using the non-parametric Mann-Whitney U test. To investigate the relationship between continuous variables, the non-parametric Spearman-Rho correlation was applied. Survival probabilities were plotted with the Kaplan-Meier method, a log-rank test was used to probe for the significance of differences in survival probabilities. Multivariate survival analysis was performed with the Cox proportional hazard model.
Using DSS as an endpoint for the determination of the optimal prognostic cut-off values, ROC curve analyses were performed for PD-L1 expression on TCs and TILs, PD-1 expression and CD3+ and CD8+ infiltration density. With the highest Youden's index, the cut-off value for PD-L1 positivity on TCs cells respectively on TILs was set at >1% PD-L1 expression (TCs) respectively >5% PD-L1 expression (TILs). PD-1 positivity on TILs was defined to be ≥ 1 PD-1 positive TIL. High CD3+ and CD8+ infiltration density was defined as >15% TILs covering tumor area whereas low density was stated when CD3+ and CD8+ positive TILs covered ≤15% (Supplementary Fig. 2). All statistical tests were performed on exploratory two-sided 5% significance level.
Supplementary Material
Disclosure/conflict of interest
The authors declare no conflict of interest.
Acknowledgments
The authors thank Klara Fizi, Petra Meyer and Kerstin Schrager for expert technical assistance and Axel Ullrich for generous support.
References
- 1.Christopher D, Fletcher JA, Krishnan U. WHO classification of tumours of soft tissue and bone. International agency for research on cancer 4th edition Lyon; 2013:110–1. [Google Scholar]
- 2.Brennan MF, Antonescu CR, Maki RG. Management of soft tissue sarcoma. New York: Springer; 2013. [Google Scholar]
- 3.Stiller CA, Trama A, Serraino D, Rossi S, Navarro C, Chirlaque MD, Casali PG, RARECARE Working Group . Descriptive epidemiology of sarcomas in Europe: report from the RARECARE project. Eur J Cancer. 2013;49:684–95. doi: 10.1016/j.ejca.2012.09.011. PMID:23079473. [DOI] [PubMed] [Google Scholar]
- 4.Sant M, Allemani C, Santaquilani M, Knijn A, Marchesi F, Capocaccia R, EUROCARE Working Group. EUROCARE-4. Survival of cancer patients diagnosed in 1995–1999. Results and commentary. Eur J Cancer. 2009;45:931–91. doi: 10.1016/j.ejca.2008.11.018. PMID:19171476. [DOI] [PubMed] [Google Scholar]
- 5.Maretty-Nielsen K, Aggerholm-Pedersen N, Safwat A, Jorgensen PH, Hansen BH, Baerentzen S, Pedersen AB, Keller J. Prognostic factors for local recurrence and mortality in adult soft tissue sarcoma of the extremities and trunk wall: a cohort study of 922 consecutive patients. Acta Orthop. 2014;85:323–32. doi: 10.3109/17453674.2014.908341. PMID:24694277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Teng MW, Ngiow SF, Ribas A, Smyth MJ. Classifying cancers based on T-cell infiltration and PD-L1. Cancer Res. 2015;75:2139–45. doi: 10.1158/0008-5472.CAN-15-0255. PMID:25977340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patel SP, Kurzrock R. PD-L1 expression as a predictive biomarker in cancer immunotherapy. Mol Cancer Ther. 2015;14:847–56. doi: 10.1158/1535-7163.MCT-14-0983. PMID:25695955. [DOI] [PubMed] [Google Scholar]
- 8.Spranger S. Mechanisms of tumor escape in the context of the T-cell-inflamed and the non-T-cell-inflamed tumor microenvironment. Int Immunol. 2016;28:383–91. doi: 10.1093/intimm/dxw014. PMID:26989092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen DS, Mellman I. Elements of cancer immunity and the cancer-immune set point. Nature. 2017;541:321–30. doi: 10.1038/nature21349. PMID:28102259. [DOI] [PubMed] [Google Scholar]
- 10.Kim C, Kim EK, Jung H, Chon HJ, Han JW, Shin KH, Hu H, Kim KS, Choi YD, Kim S, et al.. Prognostic implications of PD-L1 expression in patients with soft tissue sarcoma. BMC Cancer. 2016;16:434. doi: 10.1186/s12885-016-2451-6. PMID:27393385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.D'Angelo SP, Shoushtari AN, Agaram NP, Kuk D, Qin LX, Carvajal RD, Dickson MA, Gounder M, Keohan ML, Schwartz GK, et al.. Prevalence of tumor-infiltrating lymphocytes and PD-L1 expression in the soft tissue sarcoma microenvironment. Hum Pathol. 2015;46:357–65. doi: 10.1016/j.humpath.2014.11.001. PMID:25540867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tseng WW, Malu S, Zhang M, Chen J, Sim GC, Wei W, Ingram D, Somaiah N, Lev DC, Pollock RE, et al.. Analysis of the intratumoral adaptive immune response in well differentiated and dedifferentiated retroperitoneal liposarcoma. Sarcoma. 2015;2015:547460. doi: 10.1155/2015/547460. PMID:25705114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paydas S, Bagir EK, Deveci MA, Gonlusen G. Clinical and prognostic significance of PD-1 and PD-L1 expression in sarcomas. Med Oncol. 2016;33:93. doi: 10.1007/s12032-016-0807-z. PMID:27421997. [DOI] [PubMed] [Google Scholar]
- 14.Kim JR, Moon YJ, Kwon KS, Bae JS, Wagle S, Kim KM, Park HS, Lee H, Moon WS, Chung MJ, et al.. Tumor infiltrating PD1-positive lymphocytes and the expression of PD-L1 predict poor prognosis of soft tissue sarcomas. PLoS One. 2013;8:e82870. doi: 10.1371/journal.pone.0082870. PMID:24349382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sorbye SW, Kilvaer T, Valkov A, Donnem T, Smeland E, Al-Shibli K, Bremnes RM, Busund LT. High expression of CD20+ lymphocytes in soft tissue sarcomas is a positive prognostic indicator. Oncoimmunology. 2012;1:75–7. doi: 10.4161/onci.1.1.17825. PMID:22720216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tseng WW, Somaiah N, Engleman EG. Potential for immunotherapy in soft tissue sarcoma. Hum Vaccin Immunother. 2014;10:3117–24. doi: 10.4161/21645515.2014.983003. PMID:25625925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tawbi HA, Burgess M, Bolejack V, Van Tine BA, Schuetze SM, Hu J, D'Angelo S, Attia S, Riedel RF, Priebat DA, et al. Pembrolizumab in advanced soft-tissue sarcoma and bone sarcoma (SARC028): a multicentre, two-cohort, single-arm, open-label, phase 2 trial. The Lancet Oncology. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Galon J, Pages F, Marincola FM, Angell HK, Thurin M, Lugli A, Zlobec I, Berger A, Bifulco C, Botti G, et al.. Cancer classification using the Immunoscore: a worldwide task force. J Transl Med. 2012;10:205. doi: 10.1186/1479-5876-10-205. PMID:23034130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Galon J, Mlecnik B, Bindea G, Angell HK, Berger A, Lagorce C, Lugli A, Zlobec I, Hartmann A, Bifulco C, et al.. Towards the introduction of the ‘Immunoscore’ in the classification of malignant tumours. J Pathol. 2014;232:199–209. doi: 10.1002/path.4287. PMID:24122236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Galon J, Fox BA, Bifulco CB, Masucci G, Rau T, Botti G, Marincola FM, Ciliberto G, Pages F, Ascierto PA, et al.. Immunoscore and Immunoprofiling in cancer: an update from the melanoma and immunotherapy bridge 2015. J Transl Med. 2016;14:273. doi: 10.1186/s12967-016-1029-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39:1–10. doi: 10.1016/j.immuni.2013.07.012. PMID:23890059. [DOI] [PubMed] [Google Scholar]
- 22.Spranger S, Spaapen RM, Zha Y, Williams J, Meng Y, Ha TT, Gajewski TF. Up-regulation of PD-L1, IDO, and T(regs) in the melanoma tumor microenvironment is driven by CD8(+) T cells. Sci Transl Med. 2013;5:200ra116. doi: 10.1126/scitranslmed.3006504. PMID:23986400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taube JM, Anders RA, Young GD, Xu H, Sharma R, McMiller TL, Chen S, Klein AP, Pardoll DM, Topalian SL, et al.. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med. 2012;4:127ra37. doi: 10.1126/scitranslmed.3003689. PMID:22461641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne MC, et al.. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192:1027–34. doi: 10.1084/jem.192.7.1027. PMID:11015443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, Chmielowski B, Spasic M, Henry G, Ciobanu V, et al.. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515:568–71. doi: 10.1038/nature13954. PMID:25428505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Green MR, Monti S, Rodig SJ, Juszczynski P, Currie T, O'Donnell E, Chapuy B, Takeyama K, Neuberg D, Golub TR, et al.. Integrative analysis reveals selective 9p24.1 amplification, increased PD-1 ligand expression, and further induction via JAK2 in nodular sclerosing Hodgkin lymphoma and primary mediastinal large B-cell lymphoma. Blood. 2010;116:3268–77. doi: 10.1182/blood-2010-05-282780. PMID:20628145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barrett MT, Anderson KS, Lenkiewicz E, Andreozzi M, Cunliffe HE, Klassen CL, Dueck AC, McCullough AE, Reddy SK, Ramanathan RK, et al.. Genomic amplification of 9p24.1 targeting JAK2, PD-L1, and PD-L2 is enriched in high-risk triple negative breast cancer. Oncotarget. 2015;6:26483–93. doi: 10.18632/oncotarget.4494. PMID:26317899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Straub M, Drecoll E, Pfarr N, Weichert W, Langer R, Hapfelmeier A, Götz C, Wolff KD, Kolk A, Specht K. CD274/PD-L1 gene amplification and PD-L1 protein expression are common events in squamous cell carcinoma of the oral cavity. Oncotarget. 2016;7:12024–34. doi: 10.18632/oncotarget.7593. PMID:26918453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–64. doi: 10.1038/nrc3239. PMID:22437870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parsa AT, Waldron JS, Panner A, Crane CA, Parney IF, Barry JJ, Cachola KE, Murray JC, Tihan T, Jensen MC, et al.. Loss of tumor suppressor PTEN function increases B7-H1 expression and immunoresistance in glioma. Nat Med. 2007;13:84–8. doi: 10.1038/nm1517. PMID:17159987. [DOI] [PubMed] [Google Scholar]
- 31.Wang X, Teng F, Kong L, Yu J. PD-L1 expression in human cancers and its association with clinical outcomes. Onco Targets Ther. 2016;9:5023–39. doi: 10.2147/OTT.S105862. PMID:27574444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gooden MJ, de Bock GH, Leffers N, Daemen T, Nijman HW. The prognostic influence of tumour-infiltrating lymphocytes in cancer: a systematic review with meta-analysis. Br J Cancer. 2011;105:93–103. doi: 10.1038/bjc.2011.189. PMID:21629244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Movva S, Wen W, Chen W, Millis SZ, Gatalica Z, Reddy S, von Mehren M, Van Tine BA. Multi-platform profiling of over 2000 sarcomas: identification of biomarkers and novel therapeutic targets. Oncotarget. 2015;6:12234–47. doi: 10.18632/oncotarget.3498. PMID:25906748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gandhi L, Balmanoukian A, Hui R, Hamid O, Rizvi NA, Leighl N, Gubens M, Goldman JW, Lubiniecki GM, Emancipator K, et al.. Abstract CT105: MK-3475 (anti-PD-1 monoclonal antibody) for non-small cell lung cancer (NSCLC): Antitumor activity and association with tumor PD-L1 expression. Cancer Research. 2014;74:CT105–CT. doi: 10.1158/1538-7445.AM2014-CT105. [DOI] [Google Scholar]
- 35.Gettinger SN, Shepherd FA, Antonia SJ, Brahmer JR, Chow LQM, Juergens RA, Borghaei H, Shen Y, Harbison C, Alaparthy S, et al.. First-line nivolumab (anti-PD-1; BMS-936558, ONO-4538) monotherapy in advanced NSCLC: Safety, efficacy, and correlation of outcomes with PD-L1 status. ASCO Annual Meeting Proceedings; 2014:8024. [Google Scholar]
- 36.Grosso J, Horak CE, Inzunza D, Cardona DM, Simon JS, Gupta AK, Sankar V, Park JS, Kollia G, Taube JM, et al.. Association of tumor PD-L1 expression and immune biomarkers with clinical activity in patients (pts) with advanced solid tumors treated with nivolumab (anti-PD-1; BMS-936558; ONO-4538). ASCO Annual Meeting Proceedings; 2013:3016. [Google Scholar]
- 37.Hodi FS, Sznol M, Kluger HM, McDermott DF, Carvajal RD, Lawrence DP, Topalian SL, Atkins MB, Powderly JD, Sharfman WH, et al.. Long-term survival of ipilimumab-naive patients (pts) with advanced melanoma (MEL) treated with nivolumab (anti-PD-1, BMS-936558, ONO-4538) in a phase I trial. ASCO Annual Meeting Proceedings; 2014:9002. [Google Scholar]
- 38.Kefford R, Ribas A, Hamid O, Robert C, Daud A, Wolchok JD, Joshua AM, Hodi FS, Gangadhar TC, Hersey P, et al.. Clinical efficacy and correlation with tumor PD-L1 expression in patients (pts) with melanoma (MEL) treated with the anti-PD-1 monoclonal antibody MK-3475. ASCO Annual Meeting Proceedings; 2014:3005. [Google Scholar]
- 39.Grigg C, Rizvi NA. PD-L1 biomarker testing for non-small cell lung cancer: truth or fiction? J Immunother Cancer. 2016;4:48. doi: 10.1186/s40425-016-0153-x. PMID:27532023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rizvi NA, Brahmer JR, Ou S-HI, Segal NH, Khleif S, Hwu W-J, Gutierrez M, Schoffski P, Hamid O, Weiss J, et al.. Safety and clinical activity of MEDI4736, an anti-programmed cell death-ligand 1 (PD-L1) antibody, in patients with non-small cell lung cancer (NSCLC). American Society of Clinical Oncology; 2015. [Google Scholar]
- 41.de Visser KE, Eichten A, Coussens LM. Paradoxical roles of the immune system during cancer development. Nat Rev Cancer. 2006;6:24–37. doi: 10.1038/nrc1782. PMID:16397525. [DOI] [PubMed] [Google Scholar]
- 42.Sorbye SW, Kilvaer TK, Valkov A, Donnem T, Smeland E, Al-Shibli K, Bremnes RM, Busund LT. Prognostic impact of peritumoral lymphocyte infiltration in soft tissue sarcomas. BMC Clin Pathol. 2012;12:5. doi: 10.1186/1472-6890-12-5. PMID:22375962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fujii H, Arakawa A, Utsumi D, Sumiyoshi S, Yamamoto Y, Kitoh A, Ono M, Matsumura Y, Kato M, Konishi K, et al.. CD8(+) tumor-infiltrating lymphocytes at primary sites as a possible prognostic factor of cutaneous angiosarcoma. Int J Cancer. 2014;134:2393–402. doi: 10.1002/ijc.28581. PMID:24243586. [DOI] [PubMed] [Google Scholar]
- 44.Botti G, Scognamiglio G, Cantile M. PD-L1 immunohistochemical detection in tumor cells and tumor microenvironment: main considerations on the use of tissue micro arrays. Int J Mol Sci. 2016;17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dislich B, Stein A, Seiler CA, Kroll D, Berezowska S, Zlobec I, Galvan J, Slotta-Huspenina J, Walch A, Langer R. Expression patterns of programmed death-ligand 1 in esophageal adenocarcinomas: comparison between primary tumors and metastases. Cancer Immunol Immunother. 2017;66:777–86. doi: 10.1007/s00262-017-1982-2. PMID:28289861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guillou L, Coindre J-M, Bonichon F, Nguyen B, Terrier P, Collin F, Vilain MO, Mandard AM, Le Doussal V, Leroux A, et al.. Comparative study of the national cancer institute and french federation of cancer centers sarcoma group grading systems in a population of 410 adult patients with soft tissue sarcoma. J Clin Oncol. 1997;15:350–62. doi: 10.1200/JCO.1997.15.1.350. PMID:8996162. [DOI] [PubMed] [Google Scholar]
- 47.Wittekind C. [2010 TNM system: on the 7th edition of TNM classification of malignant tumors]. Pathologe. 2010;31:331–2. doi: 10.1007/s00292-010-1349-3. PMID:20703480. [DOI] [PubMed] [Google Scholar]
- 48.Schildhaus HU, Heukamp LC, Merkelbach-Bruse S, Riesner K, Schmitz K, Binot E, Paggen E, Albus K, Schulte W, Ko YD, et al.. Definition of a fluorescence in-situ hybridization score identifies high- and low-level FGFR1 amplification types in squamous cell lung cancer. Mod Pathol. 2012;25:1473–80. doi: 10.1038/modpathol.2012.102. PMID:22684217. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


