ABSTRACT
Allogeneic hematopoietic cell transplantation (allo-HCT) is a potentially curative therapy for hematologic malignancies. Donor T cells are able to eliminate residual tumor cells after allo-HCT, producing the beneficial graft-versus-tumor (GVT) effect, but can also cause graft-versus-host disease (GVHD) when attacking host normal tissues. We previously reported that granzyme B (GzmB) is involved in activation-induced cell death (AICD) of donor T cells and exerts differential impacts on GVHD and GVT effect. Serine protease inhibitor 6 (Spi6) is the sole endogenous inhibitor of GzmB that can protect immune and tissue cells against GzmB-mediated damage. This study is aimed to delineate the mechanism by which the GzmB-Spi6 axis regulates allogeneic T cell response. Using multiple clinically relevant murine allo-HCT models, we have found that Spi6 is concentrated in mitochondria during allogeneic T cell activation, while Spi6−/− T cells exhibit abnormal mitochondrial membrane potential, mass, reactive oxygen species (ROS) production and increased GzmB-dependent AICD mainly in the form of fratricide. Compared with WT T cells, Spi6−/− T cells exhibit decreased expansion in the host and cause significantly reduced GVHD. Notably, however, Spi6−/− T cells demonstrate the same level of GVT activity as WT T cells, which were confirmed by two independent tumor models. In summary, our findings demonstrate that Spi6 plays a novel and critical role in maintaining the integrity of T cell mitochondrial function during allogeneic response, and suggest that disabling Spi6 in donor T cells may represent a novel strategy that can alleviate GVHD without sacrificing the beneficial GVT effect.
KEYWORDS: Allogeneic hematopoietic cell transplantation (allo-HCT), Graft-versus-host disease (GVHD), Graft-versus-tumor (GVT) effect, Serine protease inhibitor 6 (Spi6), Granzyme B (GzmB)
Introduction
Allogeneic hematopoietic cell transplantation (allo-HCT) is a potentially curative treatment for hematologic malignancies.1,2 Donor T cells are able to eliminate residual tumor cells in the host, producing the beneficial graft-versus-tumor (GVT) effect.3,4 However, cancer relapse resulting from insufficient GVT and graft-versus-host disease (GVHD) remain as two major obstacles that lead to mortality and morbidity after allo-HCT.5 Many laboratory and clinical investigations, including human leukocyte antigen (HLA)-based donor and recipient selection,6,7 pretreatment and selective modulation of donor cells,8,9 optimizing pre-conditioning regimens of hosts,10,11 infection treatment and GVHD prophylaxis, have contributed to the improvement of outcome of allo-HCT.12,13 Most of the therapeutic approaches for GVHD are targeting T cells because of their central role in directly causing GVHD.12 However, It is difficult to suppress GVHD without affecting GVT effect theoretically and clinically because of their shared pathophysiology.12 Therefore, there still is a great need for new therapeutic interventions that can control GVHD without sacrificing the beneficial GVT effect.
We previously reported that GzmB, the key effector molecule in the perforin/granzyme cytotoxic pathway, is not only used by T cells to kill target cells, but also triggers activation-induced cell death (AICD) in donor T cells after allo-HCT, which significantly affects GVHD and GVT effect.14,15 While T cells use GzmB to either kill their target cells or damage themselves, the endogenous GzmB inhibitor is also upregulated.16 Serine protease inhibitor 6 (Spi6) is the sole endogenous GzmB inhibitor, which belongs to OVA family and functions as a suicide substrate when bind to GzmB.17,18 Spi6, as well as its human homologue protease inhibitor 9 (PI9),18 has been reported to play an essential role in protecting immune cells against GzmB-mediated cytotoxicity, subsequently affecting memory T cell development and homeostasis, dendritic cell antigen presentation and regulatory T cell suppressive function.16,19–21 However, it is previously not known whether Spi6 function in conventional T cells has any impact on the outcome of allo-HCT. Therefore, this study aims to determine the contribution of Spi6 in GVHD and GVT effect. To this end, we have utilized both MHC-mismatched and clinically more relevant MHC-matched and haploidentical allo-HCT models for GVHD study. In addition, we have used both the A20 lymphoma and WEHI-3 leukemia models for GVT study. We have systematically analyzed Spi6 expression and Spi6-mediated protecting mechanism and impact on allo-HCT. Our results demonstrate that Spi6 plays a novel role in maintaining donor T cell mitochondrial function, as Spi6 deficiency in donor T cells causes mitochondrial dysfunction and increased fratricidal AICD. Perhaps more interestingly, loss of Spi6 function in donor T cells leads to decreased GVHD without reducing the GVT effect. These differential roles suggest that disabling Spi6 in donor T cells may represent a novel strategy that can alleviate GVHD without sacrificing GVT effect.
Results
Spi6 is upregulated in activated T cells following allogeneic stimulation
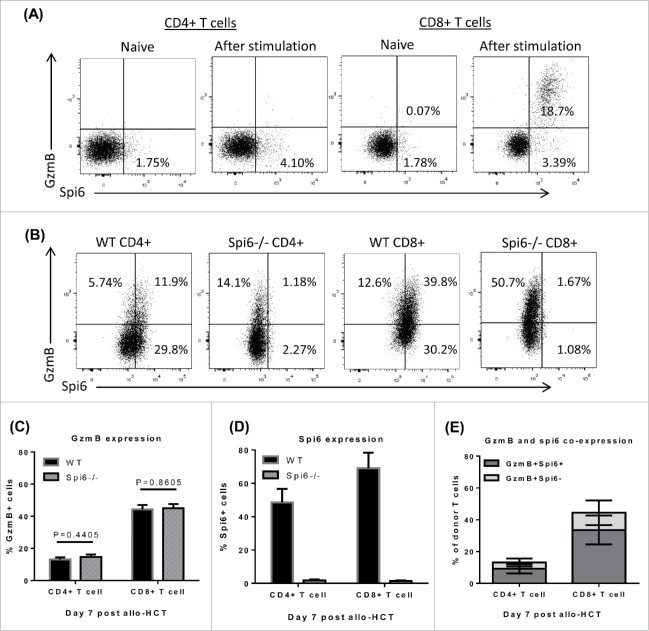
As the endogenous inhibitor of GzmB, Spi6 is expressed in many cell types to defend against GzmB-mediated cytotoxicity.19,22-24 However, whether and how Spi6 affects conventional T cells in allogeneic immune response have not been described. Using flow cytometry analysis, we have found that there is barely any GzmB or spi6 expression in naïve T cells (Fig. 1A). We next examined if Spi6 was upregulated in T cells after allogeneic activation in vitro. We performed MLR with WT or Spi6−/− T cells from C57 BL/6 mice as responders and BMDCs from BALB/c mice as stimulators. After 3 to 4 days in MLR, Spi6 expression is increased in both CD4+ and CD8+ T cells. Particularly for CD8+ T cells, most of cells expressing GzmB also co-express Spi6 (Fig. 1A). Further we examined if spi6 is also upregulated in donor T cells after allo-HCT. After lethal irradiation, BALB/c hosts were injected with TCD-BM combined with WT or spi6−/− pan T cells isolated from C57 BL/6 donors. 7 days later, donor-derived T cells were harvested from host spleens and analyzed for spi6 and GzmB expression (Fig. 1B). GzmB expression appears to be equivalent between WT and Spi6−/− T cells (Fig. 1C). Approximately 50% of CD4+ and 70% of CD8+ WT T cells are Spi6 positive, compared to Spi6−/− T cells showing merely background signal (Fig. 1D). For both CD4+ and CD8+ T cells, the majority of population that are positive for GzmB are also positive for Spi6 (Fig. 1E). Our data indicate that Spi6 is upregulated in donor T cells and co-expressed with GzmB after allogeneic activation.
Figure 1.
Spi6 expression is upregulated after allogeneic T cell activation. (A) MLR was performed using C57 BL/6 WT or Spi6−/− pan T cells as responders and BALB/c BMDCs as stimulators with a ratio of 5:1. After co-culture for 3 days, cells were stained with anti-mouse GzmB and Spi6 antibodies and analyzed by flow cytometry. Representative dot plots are gated on total live T cells (Live/Dead− TCRβ+CD4+ or CD8+). (B-E) BALB/c mice were lethally irradiated on day -1. On day 0, hosts were injected with 3 × 106 TCD-BM cells alone or combined with 1 × 105 WT or Spi6−/− pan T cells isolated from C57 BL/6 donor mice. On day 7, total splenocytes were harvested from host mice and analyzed by flow cytometry. Representative dot plots (B) are gated on total live donor T cells (Live/Dead− H-2Kb+ TCRβ+CD4+ or CD8+) and summary data of GzmB (C), Spi6 (D) expression and co-expression (E) from 3 independent experiments are shown as the percentages of total donor CD4+ or CD8+ cells. Unpaired student T tests were performed to determine statistical significance.
Spi6 protects T cells against mitochondrial damage during allogeneic response
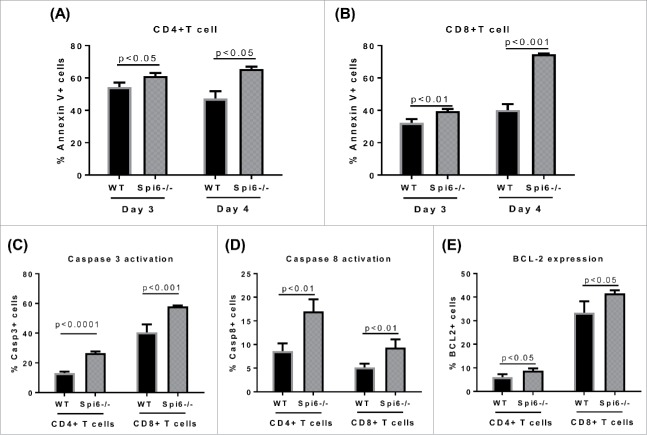
To test if Spi6 can protect T cells against GzmB-dependent cell death, we first performed MLR to mimic allogeneic response in vivo. We isolated pan T cells from C57 BL/6 WT or Spi6−/− mice, co-cultured with BMDCs from BALB/c mice for 3 to 4 days. We observed substantial AICD in both CD4+ and CD8+ T cells by Annexin V staining. Compared with WT T cells, Spi6−/− T cells exhibited significantly increased cell death (Fig. 2A-2B). Also we found that the activity of caspase 3 and caspase 8 was significantly higher in Spi6−/− T cells than in WT T cells, which implicates a protective function of Spi6 in GzmB-induced, caspase-dependent apoptosis (Fig. 2C-2D). On the other hand, it has been reported that caspase-3 mediated cleavage of BCL-2 is involved in promoting cell death, since BCL-2 plays an important role in maintaining cell survival by inhibiting caspase activation.25,26 Therefore, we also compared BCL-2 expression between WT and Spi6−/− T cells. We found that BCL-2 expression was significantly higher in Spi6−/− T cells in than WT T cells (Fig. 2E), which may be due to a protective reaction to the apoptotic process prompted by Spi6 deficiency. Interestingly, using several mito-trackers that allow us to evaluate mitochondrial status and function by flow cytometry, we have found that Spi6−/− T cells exhibit more mitochondrial dysfunction compared with WT T cells. These Spi6−/− T cells showed significantly increased mitochondrial mass, membrane potential and ROS production (Fig. 3A-3B-3C). Using confocal microscopy to visualize Spi6 and mitochondria subcellular localization, we found that Spi6 was co-localized with mitochondria in the cytoplasm after T cell activation (Fig. 3D). Quantification analyses further confirmed the co-localization between Spi6 and mitochondria (Fig. 3E). Together, these data indicate that Spi6 protects T cells against mitochondrial damage as well as caspase activation after allogeneic activation.
Figure 2.
Spi6 protects T cells from apoptotic death after allogeneic activation. MLR was performed using C57 BL/6 WT or Spi6−/− pan T cells as responders and BALB/c BMDCs as stimulators with a ratio of 5:1. After co-culture for 3 to 4 days, Annexin V staining was performed to analyze cell death with flow cytometry. Shown are the percentages of Annexin V+ cells in H-2Kb+TCR+CD4+ (A) or CD8+ (B) T cells. Caspase activity and BCL-2 expression were assessed by flow cytometry 3 days after MLR. Data shown are percentages of cells with active Caspase 3+ (C), Caspase-8 (D) and positive BCL-2 expression (E) in H-2Kb+TCR+CD4+ or CD8+ T cells.
Figure 3.
Spi6 protects T cells from and mitochondrial dysfunction after allogeneic activation. MLR was performed using C57 BL/6 WT or Spi6−/− pan T cells as responders and BALB/c BMDCs as stimulators with a ratio of 5:1. After co-culture for 4 days, Mitochondrial damage was analyzed by flow cytometry to test ROS (A), Mass (B), and potential loss (C). MFIs of CD4+ and CD8+ T cell populations are presented as mean and SEM, and unpaired student t test were performed to determine statistical significance. (D-E) Pan T cells were isolated from WT C57 BL/6 mice, stimulated with anti-CD3 and anti-CD28 conjugated microbeads (visualized as red solid beads) in complete medium with IL-2 100 IU/ml. At day 2, intracellular co-staining of spi6 (green), mitochondria (red) and nucleus (blue) was analyzed by confocal microscopy. (E) White arrows show positive staining of mitochondria and Spi6 co-localization. Co-localization of Spi6 and mitochondria was quantified using Pearson correlation coefficient method using by ImageJ software.
Spi6 predominantly protects T cells from GzmB-mediated fratricide rather than suicide
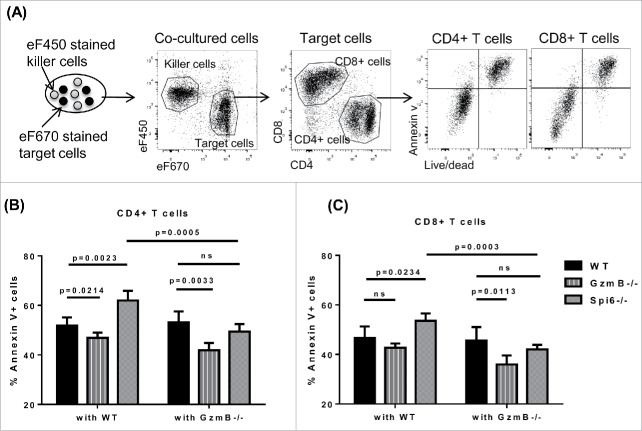
As a key cytotoxic molecule stored in the cytotoxic granules, GzmB has been implicated in T cell suicide and fratricide.24,27 However, whether fratricide or suicide is the dominant mechanism for GzmB-mediated AICD is still unknown. Previous reports including ours show that GzmB-mediated AICD affects various types of T cells.14,15,28 But it is not clear whether such T cells are killed by GzmB leaked from their own granules or delivered from other T cells. Meanwhile, Spi6 has been shown to protect the integrity of cytotoxic granules in T cells and inhibit endogenous GzmB-mediated damage.24 Yet it remains an interesting question whether Spi6 can mediate protection against exogenous GzmB delivered from other T cells. Therefore, we set up a system to determine if Spi6 can protect T cells from suicide only or also from fratricide. Because the complicated environment of in vivo HCT experiments, we used a simplified system, anti-CD3 and anti-CD28 coated plates, to activated T cells, excluding the impact of allo-antigens, APCs, microbiota and exogenous cytokines etc. After being activated in vitro for 48 hours, both CD4+ and CD8+ T cells showed substantial AICD, measured by Annexin V and cell viability dye. Consistent to T cell AICD after allogeneic stimulation, we also observed lower apoptosis in GzmB−/− T cells and higher apoptosis in Spi6−/− T cells (Fig. 4A-4C). Then we performed cell suicide/fratricide assay, using the eF670 cell proliferation dye to stain WT, GzmB−/− or Spi6−/− T cells as target cells, and the eF450 cell proliferation dye to stain WT or GzmB−/− T cells as killer cells (Fig. 4A). After in vitro stimulation and co-culturing of target cells and killer cells with a ratio of 1:1 for 2 days, we analyzed apoptosis by flow cytometry. By gating ef670 or ef450 positive population, we were able to separate target cells from killer cells (Fig. 4A). With this setting, target cells co-cultured with WT killer cells would suffer both suicide and fratricide mediated by GzmB, while co-culturing with GzmB−/− killer cells would exclude GzmB-mediated fratricide. By comparing target cells co-cultured with WT versus GzmB−/− killer cells, we would know if GzmB/Spi6 participate in fratricide or not. By comparing different target cells that all co-cultured with GzmB−/− killer cells, we would know if GzmB/Spi6 participate in suicide or not. Using this system, we found that for both CD4+ and CD8+ T cells, Spi6−/− T cells showed significantly increased apoptosis when co-cultured with WT versus GzmB−/− T cells, clearly indicating the presence of fratricide. We didn't see such a clear difference for either WT or GzmB−/− target cells, probably because both have normal Spi6 to inhibit exogenous GzmB. When all target cells were co-cultured with GzmB−/− killer cells that cannot cause GzmB-induced fratricide, we found that GzmB−/− T cells showed significantly decreased apoptosis compared with WT and Spi6−/− T cells, which indicates that GzmB definitely participated in suicide. Surprisingly, Spi6−/− T cells showed no more apoptosis than WT T cells when both were co-cultured with GzmB−/− killer cells, which strongly suggests that Spi6 predominantly protect T cells from GzmB-mediated fratricide rather than suicide (Fig. 4B-4C). In an attempt to decrease GzmB-independent background cell death, we repeated this experiment using GzmB−/− × Prf1−/− (double KO) T cells (Supplementary Figure 1). However, the double KO T cells exhibited cell death levels similar to GzmB−/− T cells, suggesting that GzmB-dependent cell death is largely perforin-dependent in this system.
Figure 4.
Spi6 predominantly protects T cells from GzmB-mediated fratricide rather than suicide. Pan T cells were isolated from C57 BL/6 WT, GzmB−/− and Spi6−/− mice. Target cells (WT, GzmB−/− and Spi6−/−) were stained with the cell proliferation dye eF670 10 uM and killer cells (WT and GzmB−/−) were stained with the cell proliferation dye eF450 5 uM. Target cells and killer cells were mixed with a ratio of 1:1 and stimulated with plate-bound anti-CD3 + anti-CD28 in complete medium. After co-cultured for 2 days, cells were harvested and analyzed by flow cytometry. (A) Experiment design and flow cytometry gating strategies. (B) Percentage of Annexin V+ CD4+ target T cells after co-culturing with WT or GzmB−/− killer T cells. (C) Percentage of Annexin V+ CD8+ target T cells after co-culturing with WT or GzmB−/− killer T cells. Unpaired student t tests were performed to determine statistical significance.
Spi6−/− T cells exhibit less proliferation and more AICD in vivo after allo-HCT
To test whether Spi6 in donor T cells can affect their function after allo-HCT, we first examined T cell expansion in host mice. Spi6−/− T cells exhibit significantly decreased expansion compared with WT T cells (Fig. 5A-5C). To determine whether the difference in T cell expansion is due to proliferation, we performed in vivo proliferation assay by staining donor T cells with the eF670 cell proliferation dye. When eF670 dilution was examined at day 3 after allo-HCT, Spi6−/− T cells showed significantly less proliferation compared with WT T cells (Fig. 5D). Furthermore, to determine if Spi6 can protect T cell from AICD in vivo, we analyzed donor T cell survival at 5 days after allo-HCT. By using Annexin V combined with the live/dead dye, we found that compared with WT T cells, Spi6−/− T cells exhibited significantly increased apoptosis (Fig. 5E). Moreover, CD8+ T cells showed higher levels of AICD than CD4+ T cells, which is correlated with their different GzmB/Spi6 expression levels after allo-activation (Fig. 1 C). Put these data together, we reason that Spi6−/− T cells showed less proliferation because Spi6−/− dead cells are cleared rapidly in vivo while WT T cells have Spi6-mediated protection and therefore their improved survival led to more accumulated eF670 dilution, which is the readout for higher proliferation. In addition, using CD45.1+ WT and CD45.2+ Spi6−/− T cells, mixed at 1:1 ratio and injected to host mice, we were able to detect donor T cell proliferation in different organs. We confirmed that the proliferation of WT T cells (CD45.1) is significantly higher than Spi6−/− (CD45.2) T cells in the spleen and liver at both day 5 and day 7 after HCT (Fig. 5 F). It has been reported that Spi6 can affect allo-reactive CD8+ T cell memory and effector survival after skin and heart transplantation.21 Therefore, we analyzed the effector/memory phenotypes of donor T cells at day 21 after allo-HCT, but did not observe any difference between WT and Spi6−/− T cells (data not shown). However, higher levels of Spi6 were found in effector T cells than memory T cells in both CD4+ and CD8+ T cells, suggesting that Spi6 may be more important for the function of effector T cells than memory T cells (Fig. 5G).
Figure 5.
Spi6−/− T cells exhibit decreased proliferation and increased AICD after allo-HCT.BALB/c mice were lethally irradiated on day -1. On day 0, hosts were injected with 3 × 106 TCD-BM cells alone or combined with 2 × 106 (A, B, D, E, F and G) or 1 × 105 (C) WT or Spi6−/− Pan T cells isolated from C57 BL/6 donor mice. (A-C) On day 3, 5 and 7 after allo-HCT, splenocytes were harvested from the host mice and analyzed by flow cytometry. Shown are summary of absolute numbers of live donor CD4+ and CD8+ T cells acquired by multiplying the percentages of H-2Kb+Live/Dead−TCRβ+CD4+ and CD8+ cells to total cell numbers harvested from the host spleens. (D) Pan T cells were stained with the cell proliferation dye ef670 before injecting to host mice. On day 3 after allo-HCT, splenocytes were harvested from the host mice and analyzed by flow cytometry. Representative flow plots showing eF670 dilution are gated on H-2Kb+Live/Dead−TCRβ+ CD4+ or CD8+ cells. Summary data show the percentage of eF670 diluted population. (E) On day 5 after allo-HCT, total cells harvested from the host spleens were assessed by Annexin V and Live/Dead dye staining to measure cell death. Representative dot plots are gated on donor T cells (H-2Kb+TCRβ+CD4+ or CD8+) with naïve donor cells serving as negative controls to show background cell death. The percentages of donor T cells that are Annexin V+Live/dead− are shown as summary data. (F) Lethally irradiated BALB/c mice were injected with C57 BL/6 TCD-BM cells combined with WT (CD45.1) and Spi6−/− (CD45.2) Pan T cells mixed at 1:1 ratio. Represented flow plots of WT and Spi6−/− T cell proliferation in liver and spleen were shown at day 5 after allo-HCT. Also shown are summary data of T cell proliferation at day 7 in liver and spleen. (G) Day 21 after allo-HCT, summary data of Spi6 expression on memory (CD44+CD62 L+) or effector (CD44+ CD62 L−) T cell subsets. Unpaired student t tests were performed to determine statistical significance.
Spi6 deficiency in donor T cells diminishes GVHD
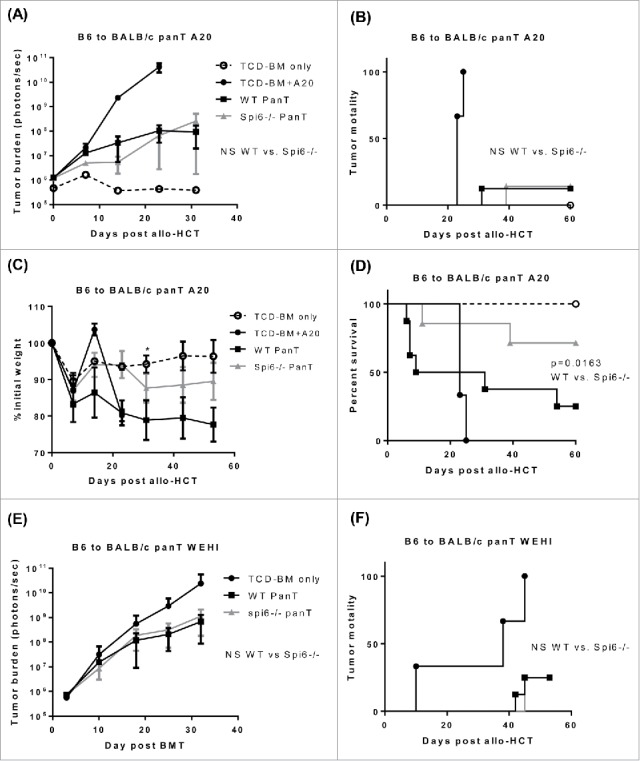
Based on Spi6-mediated protection of T cells from AICD, we hypothesized that Spi6 preserves donor-derived allo-reactive T cells after allo-HCT. To test this hypothesis, we used three murine models. For MHC-mismatched (C57 BL/6 → BALB/c) HCT, we injected TCD-BM combined with WT or Spi6−/− T cells into lethally irradiated hosts. Host mice receiving WT T cells died within 2 months, while only 50% of mice receiving Spi6−/− T cells developed lethal GVHD (Fig. 6A-6B). For haploidentical (C57 BL/6 → B6D2F1) HCT, we injected TCD-BM combined with WT or Spi6−/− T cells. Again, Spi6−/− T cells induced significantly decreased severity and lethality of GVHD (Fig. 6C-6D). Furthermore, we performed MHC-matched yet minor histocompatibility antigen-mismatched (C57 BL/6 → 129/SvJ) HCT and also observed a similar phenotype (Fig. 6E-F). It was recently reported that Spi6−/− Tregs were less potent in suppressing allo-reactive T cells.23 To exclude the influence of Spi6-dependent Treg function, we used Treg-depleted CD25− T cells to perform allo-HCT. We used RAG1−/− BM cells instead of WT BM to exclude the effect of de novo developed donor T cells. Without Tregs, Spi6−/− T cells still caused significantly decreased acute GVHD (Fig. 6G-H). These data indicate that Spi6 preserves donor allo-reactive T cell function in mediating acute GVHD, independent of Treg function.
Figure 6.

Spi6−/− T cells cause less GVHD than WT T cells. (A-B) BALB/c mice were lethally irradiated with 900 rad at day -1. At Day 0, host mice were injected with 3 × 106 TCD-BM cells alone or combined with 2 × 105 WT or Spi6−/− pan T cells isolated from C57 BL/6 donor mice. Host survival (A) and clinical score (B) were monitored after allo-HCT. (C-D) B6D2F1 mice were lethally irradiated with 1000 rad at day -1. At Day 0, host mice were injected with 5 × 106 TCD-BM cells alone or combined with 4 × 106 WT or Spi6−/− pan T cells isolated from C57 BL/6 donor mice. Host survival (C) and clinical score (D) were monitored after allo-HCT. (E-F) 129/SvJ mice were lethally irradiated with 1000 rad at day -1. At Day 0, host mice were injected with 5 × 106 TCD-BM cells alone or combined with 1.5 × 106 WT or Spi6−/− pan T cells isolated from C57 BL/6 donor mice. Host survival (E) and clinical score (F) were monitored after allo-HCT. (G-H) Lethally irradiated BALB/c mice were injected with 3 × 106 RAG1−/−BM cells alone or combined with 4 × 105 WT or Spi6−/− CD25− pan T cells isolated from C57 BL/6 donor mice. Host Survival (G) and clinical GVHD score (H) were monitored after allo-HCT. All of the survival experiments were combined from 2–3 independent experiments. Statistical significances for Kaplan-Meier survival curves are evaluated by Log-rank (Mantel-Cox) tests and clinical GVHD scores are evaluated by two-way ANOVA.
Spi6 deficiency in donor T cells does not affect the GVT effect.
To determine the contribution of Spi6 in the GVT effect, we performed C57 BL/6 → BALB/c allo-HCT, with A20 lymphoma cells inoculating into the hosts immediately before BM and T cell transplantation. Using bioluminescence imaging to monitor tumor burden, we found that 1 × 105 WT and Spi6−/− T cells control tumor growth equally efficiently, in comparison to significantly higher tumor burden in mice receiving TCD-BM only (Fig. 7A-B). No tumor-associated death occurred for recipients of WT or Spi6−/− T cells, suggesting that Spi6−/− T cells had intact anti-tumor activity compared with WT T cells (Fig. 7C). However, recipients of Spi6−/− T cells showed weight loss that was significantly lower compared with recipients of WT T cells (Fig. 7D), suggesting that Spi6−/− T cells cause less severe GVHD in these tumor-bearing mice. Moreover, we compared host overall survival including tumor-associated and GVHD-associated mortality. Host mice receiving Spi6−/− T cells exhibit significantly improved overall survival compared with counterparts receiving WT T cells (Fig. 7E).
Figure 7.
Spi6−/− T cells exhibit equivalent GVT activity to that of WT T cells. BALB/c mice were lethally irradiated with 900 rad at day -1. At Day 0, host mice were injected with 1 × 105 luciferase-expressing A20 tumor cells by tail vein injection, and then immediately injected with 3 × 106 TCD-BM cells alone or combined with 1 × 105 WT or Spi6−/− pan T cells isolated from C57 BL/6 donor mice. Tumor burden was measured by bioluminescence imaging. Representative graphs of tumor burden are shown in (A) and quantification is shown in (B). Tumor-induced mortality (C) and body weight loss (D) were monitored after allo-HCT. Tumor-induced mortality (C) and overall survival (E) combined from 2 independent experiments is shown as Kaplan-Meier survival curves with Log-rank (Mantel-Cox) tests. Statistical significance for tumor burden and body weight loss is evaluated by two-way ANOVA.
Since no tumor-associated mortality was observed in hosts receiving 1 × 105 WT or Spi6−/− T cells, we suspected that perhaps the T cell dose is too high relative to the tumor cell dose. Therefore, we decreased T cell dose to 5 × 104, and observed substantial tumor signal for all recipients. However, we observed no difference in tumor burden between mice receiving WT and Spi6−/− T cells (Fig. 8A). 1 out of 8 mice died from tumor growth in both groups receiving Spi6−/− and WT T cells (Fig. 8B). Again, body weight loss and GVHD-associated mortality were observed in the WT group but not in the Spi6−/− group (Fig. 8C-8D), and most of the mice receiving WT T cells died from GVHD before their tumor burden showed substantial increases (Fig. 8A-8D). Next, to rule out a tumor model-specific effect, we used the WEHI-3 myeloid leukemia cells to inoculate the BALB/c mice immediately before allo-HCT. Again, we observed similar results: there was no difference for host tumor burden and tumor-associated mortality between mice that received WT or Spi6−/− T cells (Fig. 8E-8F). Taken together, these data indicate that Spi6 deficiency in donor T cells significantly alleviates GVHD but does not affect the GVT response, which leads to significantly improved overall survival in the tumor-bearing host mice.
Figure 8.
Spi6 deficiency in donor T cells does not affect GVT effect in two tumor models. (A-D) BALB/c mice were lethally irradiated with 900 rad at day -1. At Day 0, host mice were injected with 1 × 105 luciferase-expressing A20 tumor cells by tail vein, and then immediately injected with 3 × 106 TCD-BM cells alone or combined with 5 × 104 WT or Spi6−/− pan T cells isolated from C57 BL/6 donor mice. Tumor burden was measured by bioluminescence imaging. After allo-HCT, tumor burden (A), tumor-associated mortality (B), body weight loss (C) and overall survival (D) were monitored. (E-F) BALB/c mice were lethally irradiated with 900 rad at day -1. At Day 0, host mice were injected with 4 × 104 luciferase-expressing WEHI-3 tumor cells, and then immediately injected with 3 × 106 TCD-BM cells alone or combined with 1 × 105 WT or Spi6−/− pan T cells isolated from C57 BL/6 donor mice. Tumor burden (E) was monitored by bioluminescence imaging. Tumor-associated mortality (F) was also monitored. Log-rank (Mantel-Cox) tests were used to determine statistical significance for tumor-associated mortality and overall survival. Statistical significance for tumor burden and body weight loss is evaluated by two-way ANOVA.
Discussion
GzmB is an effector molecule used by cytotoxic lymphocytes to kill infected or transformed cells. It is also a critical regulator for immune cell homeostasis.29 Our recent work has revealed essential regulatory roles played by GzmB in T cell allogeneic response. In the setting of allo-HCT, our previous studies demonstrate that GzmB can not only be used by donor T cells to kill tumor cells and damage normal tissues, but also regulate donor T cell AICD thereby affecting T cell expansion. In this study, we tested the role of Spi6, the sole endogenous inhibitor of GzmB, in the setting of murine allo-HCT. Unlike GzmB, Spi6 is only expressed in the cytoplasm and is functional inside of the cell.30 Expression of Spi6 can be found in many types of cells to protect against GzmB-dependent damage, including T cells, NKT cells, DCs and mesenchymal stem cells.21,22,24,31 We have found that Spi6 expression is upregulated in donor T cells after allo-HCT and protected T cells from AICD which resulted in increased cell expansion. Mitochondrial related cell death increased in Spi6−/− T cells, which indicates that Spi6 can protect against mitochondrial damage by GzmB during allogeneic T cell activation. Interestingly, we have found that Spi6 predominantly protects T cells from fratricide rather than suicide in the setting of allogeneic response, which has not been reported previously. Importantly, using multiple and clinically relevant allo-HCT models, we have found that disabling Spi6 in donor T cells significantly decreased severity and lethality of GVHD without sacrificing the beneficial GVT effect. Therefore, these findings reveal Spi6 as a novel target that has the potential to improve the effectiveness of allo-HCT.
Although an underlying mechanism involving donor T cell expansion may be shared by host-reactive T cells mediating GVHD and tumor-reactive T cells mediating the GVT effect,12 the different impact on GVHD and GVT may be due to the fundamental difference between how they react with and damage their respective target cells. We postulate the potential reasons why Spi6 affects only GVHD but not GVT may attribute to two aspects. First, TCR avidity to GVHD target cells versus tumor cells is different. T cells recognize target cells via TCR binding to specific antigenic peptide presented by MHC (pepMHC) on the surface of APCs and infected or transformed cells.32 The TCR-pepMHC interaction avidity determines the subsequent TCR signaling strength, and different avidity may trigger various levels of T cell reponse.33 Higher avidity TCR can trigger strong T cell activation34,35 than lower avidity TCR.36,37 For our study, we suspect that high avidity T cells are more likely to express GzmB as well as Spi6, they are host-reactive and cause GVHD, while low or intermediate avidity T cells are more likely to be tumor-reactive mediating the GVT effect, which do not express or express lower levels of GzmB and Spi6 and kill tumor cells by other cytotoxic pathways. Since almost all host tissue cells express MHC I molecules, donor T cells can easily recognize and kill a normal host cell that expresses “foreign” antigens. Although some of these host-reactive T cells that can recognize tumor cells are also important to the GVT effect, tumor cells may have evolved mechanisms to evade T cell recognition and cytotoxicity, including down-regulation of MHC I expression and upregulation of anti-apoptotic molecules.38 On the other side, there are unique features that make malignant cells distinct from normal cells, such as expression of tumor associated or specific antigens including mutated self-antigens, which could also serve as low avidity targets for donor T cells to recognize.39 Secondly, Spi6 function in different T cell phenotypes may also account for its differential effects on GVHD and GVT. We have found that Spi6 expression is higher in effector T cells than memory T cells, which is consistent with our previous reports showing that GzmB mainly causes AICD in CD8+ and CD4+ effector T cells.14,15,40 These findings suggest that the GzmB-Spi6 axis may predominantly function during donor-derived effector T cell clonal expansion. In support of this notion, a recent study showed that Spi6 deficiency in CD8+ T cells causes decreased clonal expansion of short-lived effector T cells but do not change long-term memory T cell pool. Those memory precursors have minimal or low GzmB expression and do not rely on Spi6 for survival.21 Notably, while donor-derived effector T cells are mainly responsible for GVHD, memory T cells are more important to GVT effect rather than GVHD.41 Therefore, Spi6 function in different T cell phenotypes may also explain why only GVHD but not GVT effect is affected.
In summary, our data indicate that Spi6 can protect donor T cells from GzmB-induced AICD after allo-HCT, especially by protecting mitochondrial function. This protection is critical for the expansion of donor T cells that causes GVHD but does not affect the beneficial GVT effect. Therefore, this study suggests that disabling Spi6 in donor T cells may represent a novel strategy that can alleviate GVHD without sacrificing the GVT effect.
Materials and methods
Animals and tumor cells
C57 BL/6 J (H-2b) WT, C57 BL/6 J CD45.1 and 129/SvJ (H-2b) mice were obtained from the Jackson Laboratory. BALB/c (H-2d) and B6D2F1/Cr mice were purchased from Charles River–Frederick. Spi6−/− mice in the C57 BL/6 J strain were originally developed by Dr. Ashton-Rickardt's laboratory at the University of Chicago and obtained from Dr. Abdi's laboratory in Harvard University.23,24 GzmB−/− mice in C57 BL/6 J strain were developed as described.42 Luciferase-expressing A20 and WEHI-3 cells were developed from their respective parental lines as described beore.14,42 All mice were maintained in SPF housing, and all experiments were conducted in accordance with the animal care guidelines at Roswell Park Cancer Institute, using protocols approved by the animal studies committee.
Reagents and antibodies
Antibodies including anti-mouse TCRβ, CD4, CD8, CD44, CD62 L, H-2Kb, H-2Kd, CaspGLOW fluorescein active caspase-3 staining kits and Annexin V apoptosis detection kits were purchased from eBioscience. Rabbit anti-mouse Spi6 antibody was purchased from Hycult Biotech. FITC-Dnk anti-rabbit IgG was purchased from Jackson Immuno Research Lab. MitoSOX red mitochondrial superoxide indicator, MitoTracker red, MitoTracker orange CMTMRos and MitoTracker green FM were purchased from Life technologies. CD90.2 microbeads and Pan T isolation kit II were purchased from Miltenyi Biotec.
Donor cell preparation
Donor bone marrow (BM) cells were isolated from WT C57 BL/6 mice. T cell depletion (TCD) was performed by using anti-CD90.2 microbeads (purity >95%). Donor pan T cells were purified from the spleens of C57 BL/6 WT or Spi6−/− mice by using Pan T isolation kit II (purity >94%). Donor CD25− T cells were purified with the same kit combined with biotin-conjugated anti-CD25 antibody (purity >94%).
Bone marrow transplantation for GVHD and GVT
For MHC-mismatched HCT, BALB/c hosts (H-2d) were irradiated with 900 rad from a Cs-137 source. One day later, the hosts were injected intravenously with 3 × 106 TCD-BM cells only or combined with indicated types and doses of T cells isolated from C57 BL/6 (H-2b) WT or Spi6−/− mice. For MHC-matched, minor antigen-mismatched HCT, 129/SvJ hosts (H-2b) were irradiated with 1000 rad. One day later, the hosts were injected intravenously with 5 × 106 BM cells only or combined with 4 × 106 T cells isolated from C57 BL/6 WT or Spi6−/− mice. For the parent to F1 HCT, B6D2 F/Cr mice were irradiated with 1000 rad. One day later, the hosts were injected intravenously with 5 × 106 TCD-BM cells only or combined with 4 × 106 T cells isolated from C57 BL/6 WT or Spi6−/− mice. For GVHD study, the host mice were weighed once to twice every week and monitored for clinical GVHD score and survival. For GVT study, host mice were injected intravenously with syngeneic tumor cells right before BM and T cell injection. Tumor burdens were measured by bioluminescence imaging every week and tumor mortality and overall survival were monitored.
Clinical GVHD scoring criteria
The clinical GVHD manifestations are weight loss; change in posture, activity, fur texture, hair loss and in some cases diarrhea. Clinical GVHD is evaluated comprehensively with a scoring system as follows: a) Posture: normal-0, hunching at rest-1, severe hunching impairs movement-2; b) Activity: normal-0, mild decrease-1, stationary unless stimulated-2; c) Fur texture: normal-0, moderate ruffling-1, severe ruffling and poor grooming-2; d) Skin: normal-0, scaling, mild hair loss-1, areas of denuded skin-2; e) Diarrhea: no-0, mild-1, severe-2; f) Body weight loss: no body weight loss or gain weight-0, ≤ 5%-1, 5–10%-2, 10–15%-3, >15%-4; g) Vitality: Live-0, Dead-10.
Mixed lymphocyte reaction (MLR)
BM derived dendritic cells (BMDCs) as stimulators were isolated from BALB/c mice and cultured in 5% RPMI with 5% GM-CSF for 7 days and LPS (100 ng/ml) treated at day 6. Pan T cells as responders were isolated from splenocytes of C57 BL/6 WT or Spi6−/− mice. 0.25 × 106 responders and 0.05 × 106 stimulators were co-cultured in 200 ul 10% RPMI/well in 96-well plate for 3 to 4 days. Cells were harvested and washed once with 1 ml DPBS before staining for flow cytometry.
Mitochondrial damage measurement by flow cytometry
After MLR, cells were washed with 1 ml DPBS once for flow staining. After routine surface marker staining, cells were washed twice with FACS buffer before staining for mitochondrial trackers (all purchased from Thermo Fisher Scientific). For mitochondrial reactive oxygen species (Mito-ROS) staining, cells were stained with 5 uM MitoSOX red reagents in 0.5 ml RPMI with 10% FBS for 30 min at 37 °C; For mitochondrial mass staining, cells were stained with 200 nM MitoTracker Green reagents in 0.5 ml RPMI for 30 min at 37 °C; For mitochondrial potential staining, cells were stained with 500 nM MitoTracker Orange reagents in 0.5 ml RPMI for 30 min at 37 °C. Stained cells were then washed twice with FACS buffer before flow cytometry analyses.
Supplementary Material
Funding Statement
This work was supported by NIH research grant # R01CA184728 (X.C.) and a generous donation from Brendan and Elise McCarthy to the RPCI Alliance BMT Donation Fund (X.C. and P.L.M.). This work utilized Shared Resources supported by the Roswell Park Cancer Institute's Comprehensive Cancer Center Support Grant CA016056.
Disclosure of conflicts of interest
The authors have no special/competing interests to disclose.
Acknowledgments
We thank Dr. Abdi's for sharing Spi6−/− mice for our research and technical assistance.
References
- 1.Shlomchik WD. Graft-versus-host disease. Nat Rev Immunol. 2007;7(5):340–52. doi: 10.1038/nri2000. PMID:17438575. [DOI] [PubMed] [Google Scholar]
- 2.Copelan EA. Hematopoietic stem-cell transplantation. N Engl J Med. 2006;354(17):1813–26. doi: 10.1056/NEJMra052638. PMID:16641398. [DOI] [PubMed] [Google Scholar]
- 3.Horowitz MM, Gale RP, Sondel PM, Goldman JM, Kersey J, Kolb HJ, Rimm AA, Ringdén O, Rozman C, Speck B, et al.. Graft-versus-leukemia reactions after bone marrow transplantation. Blood. 1990;75(3):555–62. PMID:2297567. [PubMed] [Google Scholar]
- 4.Kolb HJ, Schmid C, Barrett AJ, Schendel DJ. Graft-versus-leukemia reactions in allogeneic chimeras. Blood. 2004;103(3):767–76. doi: 10.1182/blood-2003-02-0342. PMID:12958064. [DOI] [PubMed] [Google Scholar]
- 5.Pasquini MC ZX. Current uses and outcomes of hematopoietic stem cell transplantation: CIBMTR Summary Slides. http://www.cibmtr.org; 2015. [Google Scholar]
- 6.Fleischhauer K, Beelen DW. HLA mismatching as a strategy to reduce relapse after alternative donor transplantation. Semin Hematol. 2016;53(2):57–64. doi: 10.1053/j.seminhematol.2016.01.010. PMID:27000727. [DOI] [PubMed] [Google Scholar]
- 7.Tiercy JM. How to select the best available related or unrelated donor of hematopoietic stem cells? Haematologica. 2016;101(6):680–7. doi: 10.3324/haematol.2015.141119. PMID:27252513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li Pira G, Di Cecca S, Montanari M, Moretta L, Manca F. Specific removal of alloreactive T-cells to prevent GvHD in hemopoietic stem cell transplantation: rationale, strategies and perspectives. Blood Rev. 2016;30(4):297–307. doi: 10.1016/j.blre.2016.03.001. PMID:27066851. [DOI] [PubMed] [Google Scholar]
- 9.Im HJ, Koh KN, Seo JJ. Recent advances in haploidentical hematopoietic stem cell transplantation using ex vivo T cell-depleted graft in children and adolescents. Blood Res. 2016;51(1):8–16. doi: 10.5045/br.2016.51.1.8. PMID:27104186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diaconescu R, Flowers CR, Storer B, Sorror ML, Maris MB, Maloney DG, Sandmaier BM, Storb R. Morbidity and mortality with nonmyeloablative compared with myeloablative conditioning before hematopoietic cell transplantation from HLA-matched related donors. Blood. 2004;104(5):1550–58. doi: 10.1182/blood-2004-03-0804. PMID:15150081.16258490 [DOI] [PubMed] [Google Scholar]
- 11.Baron F, Storb R. Current roles for allogeneic hematopoietic cell transplantation following nonmyeloablative or reduced-intensity conditioning. Clin Adv Hematol Oncol. 2005;3(10):799–819. PMID:16258490. [PubMed] [Google Scholar]
- 12.Fowler DH. Shared biology of GVHD and GVT effects: potential methods of separation. Crit Rev Oncol Hematol. 2006;57(3):225–44. doi: 10.1016/j.critrevonc.2005.07.001. PMID:16207532. [DOI] [PubMed] [Google Scholar]
- 13.Hobbs GS, Perales MA. Effects of T-Cell Depletion on Allogeneic Hematopoietic Stem Cell Transplantation Outcomes in AML Patients. J Clin Med. 2015;4(3):488–503. doi: 10.3390/jcm4030488. PMID:26239251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bian G, Ding X, Leigh ND, Tang Y, Capitano ML, Qiu J, McCarthy PL, Liu H, Cao X. Granzyme B-mediated damage of CD8+ T cells impairs graft-versus-tumor effect. J Immunol. 2013;190(3):1341–50. doi: 10.4049/jimmunol.1201554. PMID:23264653. [DOI] [PubMed] [Google Scholar]
- 15.Du W, Leigh ND, Bian G, O'Neill RE, Mei L, Qiu J, Chen GL, Hahn T, Liu H, McCarthy PL, et al.. Granzyme B-Mediated Activation-Induced Death of CD4+ T Cells Inhibits Murine Acute Graft-versus-Host Disease. J Immunol. 2015;195(9):4514–23. doi: 10.4049/jimmunol.1500668. PMID:26392464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Phillips T, Opferman JT, Shah R, Liu N, Froelich CJ, Ashton-Rickardt PG. A role for the granzyme B inhibitor serine protease inhibitor 6 in CD8+ memory cell homeostasis. J Immunol. 2004;173(6):3801–09. doi: 10.4049/jimmunol.173.6.3801. PMID:15356127. [DOI] [PubMed] [Google Scholar]
- 17.Bird PI. Serpins and regulation of cell death. Results Probl Cell Differ. 1998;24:63–89. doi: 10.1007/978-3-540-69185-3_4. PMID:9949832. [DOI] [PubMed] [Google Scholar]
- 18.Sun J, Ooms L, Bird CH, Sutton VR, Trapani JA, Bird PI. A new family of 10 murine ovalbumin serpins includes two homologs of proteinase inhibitor 8 and two homologs of the granzyme B inhibitor (proteinase inhibitor 9). J Biol Chem. 1997;272(24):15434–15441. doi: 10.1074/jbc.272.24.15434. PMID:9182575. [DOI] [PubMed] [Google Scholar]
- 19.Lovo E, Zhang M, Wang L, Ashton-Rickardt PG. Serine protease inhibitor 6 is required to protect dendritic cells from the kiss of death. J Immunol. 2012;188(3):1057–63. doi: 10.4049/jimmunol.1102667. PMID:22227570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Su Y, Jevnikar AM, Huang X, Lian D, Zhang Z. Spi6 protects alloreactive CD4(+) but not CD8 (+) memory T cell from granzyme B attack by double-negative T regulatory cell. Am J Transplant. 2014;14(3):580–93. doi: 10.1111/ajt.12614. PMID:24730048. [DOI] [PubMed] [Google Scholar]
- 21.Azzi J, Ohori S, Ting C, Uehara M, Abdoli R, Smith BD, Safa K, Solhjou Z, Lukyanchykov P, Patel J, et al.. Serine protease inhibitor-6 differentially affects the survival of effector and memory alloreactive CD8-T cells. Am J Transplant. 2015;15(1):234–41. doi: 10.1111/ajt.13051. PMID:25534448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ansari AW, Temblay JN, Alyahya SH, Ashton-Rickardt PG. Serine protease inhibitor 6 protects iNKT cells from self-inflicted damage. J Immunol. 2010;185(2):877–83. doi: 10.4049/jimmunol.1000651. PMID:20543105. [DOI] [PubMed] [Google Scholar]
- 23.Azzi J, Skartsis N, Mounayar M, Magee CN, Batal I, Ting C, Moore R, Riella LV, Ohori S, Abdoli R, et al.. Serine protease inhibitor 6 plays a critical role in protecting murine granzyme B-producing regulatory T cells. J Immunol. 2013;191(5):2319–2327. doi: 10.4049/jimmunol.1300851. PMID:23913965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang M, Park SM, Wang Y, Shah R, Liu N, Murmann AE, Wang CR, Peter ME, Ashton-Rickardt PG. Serine protease inhibitor 6 protects cytotoxic T cells from self-inflicted injury by ensuring the integrity of cytotoxic granules. Immunity. 2006;24(4):451–61. doi: 10.1016/j.immuni.2006.02.002. PMID:16618603. [DOI] [PubMed] [Google Scholar]
- 25.Kirsch DG, Doseff A, Chau BN, Lim DS, de Souza-Pinto NC, Hansford R, Kastan MB, Lazebnik YA, Hardwick JM. Caspase-3-dependent cleavage of Bcl-2 promotes release of cytochrome c. Journal of Biological Chemistry. 1999;274(30):21155–61. doi: 10.1074/jbc.274.30.21155. PMID:10409669. [DOI] [PubMed] [Google Scholar]
- 26.Swanton E, Savory P, Cosulich S, Clarke P, Woodman P. Bcl-2 regulates a caspase-3/caspase-2 apoptotic cascade in cytosolic extracts. Oncogene. 1999;18(10). doi: 10.1038/sj.onc.1202490. PMID:10086332. [DOI] [PubMed] [Google Scholar]
- 27.Viswanathan K, Bot I, Liu L, Dai E, Turner PC, Togonu-Bickersteth B, Richardson J, Davids JA, Williams JM, Bartee MY, et al.. Viral cross-class serpin inhibits vascular inflammation and T lymphocyte fratricide; a study in rodent models in vivo and human cell lines in vitro. PLoS One. 2012;7(9):e44694. doi: 10.1371/journal.pone.0044694. PMID:23049756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sharma V, Delgado M, Ganea D. Granzyme B, a new player in activation-induced cell death, is down-regulated by vasoactive intestinal peptide in Th2 but not Th1 effectors. J Immunol. 2006;176(1):97–110. PMID:16365400. [DOI] [PubMed] [Google Scholar]
- 29.Voskoboinik I, Whisstock JC, Trapani JA. Perforin and granzymes: function, dysfunction and human pathology. Nat Rev Immunol. 2015;15(6):388–400. PMID:25998963. [DOI] [PubMed] [Google Scholar]
- 30.Ashton-Rickardt PG. An emerging role for Serine Protease Inhibitors in T lymphocyte immunity and beyond. Immunol Lett. 2013;152(1):65–76. PMID:23624075. [DOI] [PubMed] [Google Scholar]
- 31.Rizzitelli A, Meuter S, Vega Ramos J, Bird CH, Mintern JD, Mangan MS, Villadangos J, Bird PI. Serpinb9 (Spi6)-deficient mice are impaired in dendritic cell-mediated antigen cross-presentation. Immunol Cell Biol. 2012;90(9):841–51. PMID:22801574. [DOI] [PubMed] [Google Scholar]
- 32.Rudolph MG, Stanfield RL, Wilson IA. How TCRs bind MHCs, peptides, and coreceptors. Annu Rev Immunol. 2006;24:419–66. PMID:16551255. [DOI] [PubMed] [Google Scholar]
- 33.Stone JD, Chervin AS, Kranz DM. T-cell receptor binding affinities and kinetics: impact on T-cell activity and specificity. Immunology. 2009;126(2):165–76. PMID:19125887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Foley MH, Forcier T, McAndrew E, Gonzalez M, Chen H, Juelg B, Walker BD, Irvine DJ. High avidity CD8+ T cells efficiently eliminate motile HIV-infected targets and execute a locally focused program of anti-viral function. PLoS One. 2014;9(2):e87873. PMID:24551068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Keane NM, Roberts SG, Almeida CA, Krishnan T, Chopra A, Demaine E, Laird R, Tschochner M, Carlson JM, Mallal S, et al.. High-avidity, high-IFNgamma-producing CD8 T-cell responses following immune selection during HIV-1 infection. Immunol Cell Biol. 2012;90(2):224–34. PMID:21577229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Speiser DE, Kyburz D, Stubi U, Hengartner H, Zinkernagel RM. Discrepancy between in vitro measurable and in vivo virus neutralizing cytotoxic T cell reactivities. Low T cell receptor specificity and avidity sufficient for in vitro proliferation or cytotoxicity to peptide-coated target cells but not for in vivo protection. J Immunol. 1992;149(3):972–80. PMID:1634779. [PubMed] [Google Scholar]
- 37.Alexander-Miller MA, Leggatt GR, Berzofsky JA. Selective expansion of high- or low-avidity cytotoxic T lymphocytes and efficacy for adoptive immunotherapy. Proc Natl Acad Sci U S A. 1996;93(9):4102–07. doi: 10.1073/pnas.93.9.4102. PMID:8633023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–74. doi: 10.1016/j.cell.2011.02.013. PMID:21376230. [DOI] [PubMed] [Google Scholar]
- 39.Zilberberg J, Feinman R, Korngold R. Strategies for the identification of T cell-recognized tumor antigens in hematological malignancies for improved graft-versus-tumor responses after allogeneic blood and marrow transplantation. Biol Blood Marrow Transplant. 2015;21(6):1000–7. doi: 10.1016/j.bbmt.2014.11.001. PMID:25459643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Du W, Leigh ND, Bian G, Alqassim E, O'Neill RE, Mei L, Qiu J, Liu H, McCarthy PL, Cao X. Granzyme B Contributes to the Optimal Graft-Versus-Tumor Effect Mediated by Conventional CD4+ T Cells. J Immunol Res Ther. 2016;1(1):22–8. PMID:27774524. [PMC free article] [PubMed] [Google Scholar]
- 41.Li N, Matte-Martone C, Zheng H, Cui W, Venkatesan S, Tan HS, McNiff J, Demetris AJ, Roopenian D, Kaech S, et al.. Memory T cells from minor histocompatibility antigen-vaccinated and virus-immune donors improve GVL and immune reconstitution. Blood. 2011;118(22):5965–76. doi: 10.1182/blood-2011-07-367011. PMID:21917752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cao X, Cai SF, Fehniger TA, Song J, Collins LI, Piwnica-Worms DR, Ley TJ. Granzyme B and perforin are important for regulatory T cell-mediated suppression of tumor clearance. Immunity. 2007;27(4):635–46. doi: 10.1016/j.immuni.2007.08.014. PMID:17919943. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.