ABSTRACT
Although the anti-CCR4 antibody mogamulizumab (moga) shows striking antitumor activity against adult T cell leukemia (ATL), it can also cause fatal immunological pathology such as severe skin rash and graft-versus-host disease, which might be attributed to depletion of CCR4+ regulatory T cells. We previously showed that next generation sequencing enables precise analysis of the T cell receptor (TCR) repertoire, and we here used the technique to reveal the immunological dynamics in moga-treated ATL patients. Treatment with moga resulted in remarkable reduction or elimination of clonal cells, and enhanced reconstitution of non-tumor polyclonal CD4+ T cells and oligoclonal CD8+ T cells. Interestingly, cutaneous T cells infiltrating moga-related skin rashes did not share the same major clones in peripheral blood, which minimizes the possibility of cross-reaction. Thus, deep sequencing of the TCR can reveal the immune reconstitution of moga-treated ATL and provides powerful insights into its mode of action.
KEYWORDS: adult T cell leukemia, mogamulizumab, T cell receptor, immune reconstitution, next generation sequencing
Introduction
Adult T cell leukemia (ATL) is a mature T cell malignancy that occurs in human T-lymphotropic virus type-1 (HTLV-1) carriers,1 and typical ATL cells present as “flower-cells” with polylobulated nuclei.2 Whereas not all the ATL cells exhibit the same morphology, ATL cells can be identified by CADM1 expression.3,4 ATL is one of the most therapy-resistant hematologic malignancies, and even the most intensive chemotherapy, modified LSG15 (mLSG15), can provide short survival of about one year.5 Although allogeneic hematopoietic stem cell transplantation (allo-HSCT) can achieve long-term remission,6 it also raises the risks of therapy-related mortality such as graft-versus-host disease (GVHD), which limits its indication and success.
The recently developed anti-CCR4 antibody, mogamulizumab (moga), has shown substantial anti-ATL activity, even in relapsing or chemotherapy-resistant disease.7,8 Moga crosslinks CCR4 on ATL cells and Fc receptors on NK cells, inducing antibody-dependent cellular cytotoxicity against ATL cells. In addition, it also enhances antitumor immunity by depleting regulatory T cells.9,10 However, there are several obstacles to the use of moga. The treatment can cause fatal adverse events, including Stevens-Johnson syndrome,11 autoimmune encephalitis,4 and GVHD after allo-HSCT,12 which may be attributable to depletion of regulatory T cells. In addition, the best combination of moga and cytotoxic chemotherapy regimen remains unclear, because sequential administration of mLSG15 and moga did not show superior outcome.13 Its efficacy against extramedullary lesions is unsatisfactory.7 To achieve the best therapeutic effects without fatal adverse events, close monitoring and understanding of the immunological dynamics in moga-treated patients is warranted.
We have demonstrated that deep sequencing of the T cell receptor-alpha (TCR-α) by next generation sequencing enables minute TCR repertoire analysis in ATL.4 This technique can elucidate the clonality of ATL cells and non-tumor T cells at single-cell level. Here, we sequentially performed this analysis in ATL patients who received conventional chemotherapy and moga, which clearly visualized polyclonal reconstitution of non-tumor T cell clones by moga.
Results and Discussion
First, we sequentially analyzed peripheral blood samples from five ATL patients treated with conventional chemotherapy (mLSG15) and moga (Fig. 1A-B). At diagnosis, flow cytometry revealed that the majority of T cells exhibited a CD3dimCD4+CADM1+CD7− phenotype, and TCR repertoire analysis showed that the T cells comprised one or double clonal populations, namely, ATL cells (Fig. 1A-1/1B-1 and Table S1 A-1/1B-1). Although primary chemotherapy with mLSG15 reduced the frequencies of ATL clones, they remained the major clones, and reconstitution of non-tumor polyclonal T cell clones was incomplete (Fig. 1A-2/1B-2 and Table S1 A-2/1B-2). By contrast, moga treatment resulted in a dramatic reduction or elimination of ATL clones and substantial reconstitution of non-tumor polyclonal T cell clones (Fig. 1A-3 and Table S1 A-3). Interestingly, while CD4+ T cells showed remarkable polyclonal reconstitution, CD8+ T cells showed “skewed” oligoclonal reconstitution (Fig. 1B-3 (CD4 and CD8) and Table S1B-3 (CD4 and CD8)), indicating that a number of major CD8+ T cell clones emerged and expanded after moga treatment. However, when CADM1+CD7− ATL re-appeared 7 months later, the same ATL clone increased, and polyclonal T cell clones were suppressed again (Fig. 1A-4 and Table S1 A-4). The results of flow cytometry and TCR repertoire analysis were always consistent with one another, supporting that the CD3dimCD4+CADM1+CD7− population was exclusively composed of ATL cells.3,4 Thus, while moga reduced or eliminated ATL clones, it also resulted in striking reconstitution of polyclonal CD4+ T cells and oligoclonal CD8+ T cells. By contrast, conventional chemotherapy mLSG15░did not produce such effects in our cases. These observations may indicate that moga can achieve deeper remission, though their effects vary case to case. The analyses of these two patients and three more patients are summarized in Tables S3 and S4 (cases #1, 2, 3, 5, and 6). Interestingly, case #6 had two abnormal clones before moga treatment (TRAV9-2/TRAJ31 and TRAV13-1/TRAJ4). While moga selectively reduced the former one, the latter one was resistant to moga (Tables S3 and S4). It is known that some ATL cases are resistant to moga,7,8 but the responses to moga might depend on each ATL clone even in a patient. In addition, the frequencies of abnormal lymphocytes in peripheral blood morphologically-defined, CADM1+ CD4 T cells, and % clonal cells are summarized in Figure S1 as for the cases #1 to #6. As they decreased similarly, they might be useful to monitor therapeutic effects of anti-ATL therapy.
Figure 1.
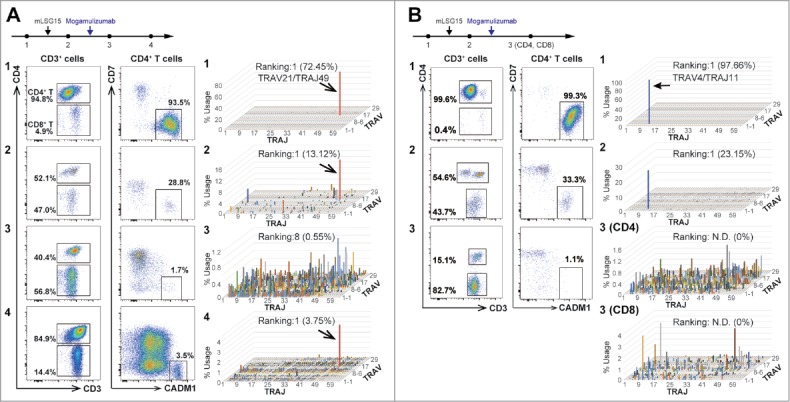
Sequential analysis of peripheral blood T cells by high-resolution flow cytometry and TCRα, V, and J assignment (A) Results from an acute type ATL patient who received both conventional chemotherapy mLSG15 (A-1 to A-2) and moga (A-2 to A-3). Flow cytometric scattergrams illustrating percentages of CD4+/8+ and CADM1+CD7− (ATL) cells. For TCR repertoire analysis, 3D graphs show the frequencies of TRAV/J clones. ATL cells with a CADM1+CD7− phenotype decreased remarkably and reconstitution of polyclonal T cells was observed after moga treatment (A-3); however, 7 months later, a CADM1+CD7− cell population and the TRAV21/J49 clone reappeared and polyclonal T cells were suppressed (A-4). (B) Results from another acute type ATL patient who received mLSG15 (B-1 to B-2) and moga (B-2 to B-3). After moga treatment, PBMCs were sorted into CD4+ and CD8+ T cells, and subjected to TCR repertoire analysis (B-3 (CD4) and B-3 (CD8)). Data are representative of five independent patients. N.D.: not detected.
Moga sometimes causes a severe skin rash,11,14,15 which may be associated with depletion of regulatory T cells. Given that moga treatment resulted in propagation of some oligoclonal CD8+ T cells (Fig. 1) and CD8+ T cells dominantly infiltrate moga-associated skin rashes,4 the T cell clones increased by moga treatment in peripheral blood may infiltrate the skin and induce eruption. If this were the case, the same T cell clones would be expected to be simultaneously detected both in peripheral blood and skin; therefore, peripheral blood and skin tissue samples from four patients with moga-related rash were subjected to TCR repertoire analysis.
ATL clones were depleted and polyclonal reconstitution occurred in peripheral blood after administration of moga, whereas several major clones were detected among CD8+ T cells (Fig. 2A/B and Table S2 A/B). By contrast, the skin tissues showed CD8+-dominant T cell infiltration in the epidermis and dermis with mild liquefaction degeneration. The clonality of T cells differed by case, with both polyclonal (Fig. 2A and Table S2 A) and oligoclonal (Fig. 2B and Table S2B) T cell infiltration observed. Interestingly, most of the major clones were not shared in peripheral blood and the skin tissues (Fig. 2A/B and Table S2 A/B). The analyses of these two patients and two more patients are summarized in Tables S3 and S4, corresponding to cases #3, 4, 5, and 7. Furthermore, we additionally performed TCR-β repertoire analyses as for these four patients (Table S5). Although there were a few overlapped repertoires in peripheral blood and the skin especially in case #7, most of the clones were not shared.
Figure 2.
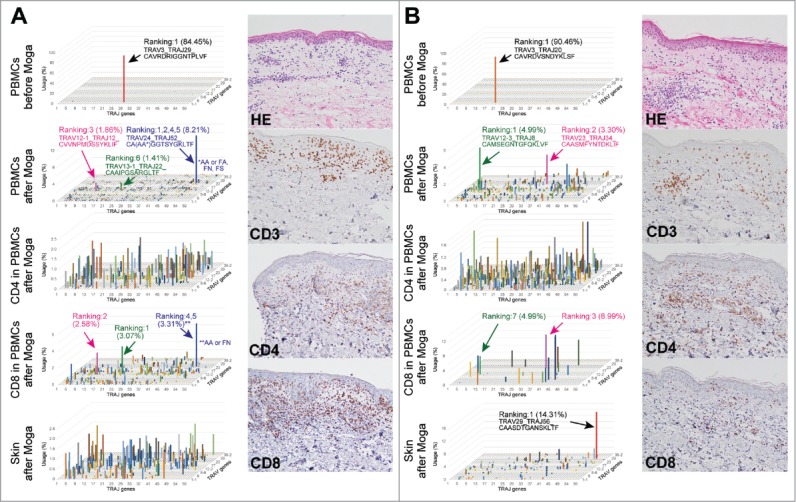
Peripheral blood T cells and cutaneous infiltrating T cells do not share the same major T cell clones after treatment with moga (A, B) PBMCs, sorted CD4+/CD8+ T cells, and skin tissues were subjected to TCR repertoire analysis in two ATL cases with moga-related skin rash. 3D graphs show the frequencies of TRAV/J clones. In panel A, the blue bar consists of three clones with different amino acid sequences in the CDR3 region, but sharing TRAV24/J52. H&E and CD3/4/8 staining of the samples of tissue from the skin rash are also shown. Data are representative of four independent patients.
It is often difficult to differentiate ATL cells from normal T cells only by morphology. Kobayashi et al. and we previously showed that the CD3+CD4+CADM1+CD7− cells are exclusively clonal ATL cells.3,4 Based on our analyses, we propose that we may trace ATL tumor burden quite correctly by detecting CD4+CADM1+CD7− cells by flow cytometry, though it should be thoroughly tested in the future.
Whereas the importance of immune reconstitution of T cells after hematopoietic stem cell transplantation is well acknowledged and associated with long-term prognosis and therapy-related mortality,16,17 immune cell reconstitution has been discussed less in the context of cytotoxic chemotherapy. Given the association of ATL with immunodeficiency18,19 and the strong immunomodulatory effects exerted by moga, understanding of the dynamics of immune cell reconstitution is particularly important to avoid fatal adverse events. Here, deep sequencing of the TCR-alpha repertoires can uncover this phenomenon after conventional chemotherapy and treatment with moga. In our analyses, moga not only depleted or eliminated ATL clones, but also enhanced reconstitution of non-tumor polyclonal T cells, which did not occur in response to conventional chemotherapy. In addition, CD8+ T cells exhibited oligoclonal reconstitution after treatment with moga. It may represent enhanced ATL-specific immunity,10 which cannot be confirmed with our current methods. As described previously, it is questionable whether conventional chemotherapy and moga can synergistically suppress ATL13; this might be because conventional chemotherapy inhibits reconstitution of T cell immunity by moga.
Depletion of regulatory T cells using moga may lead to deterioration of autoimmune symptoms, partly due to cross-reaction by the same ATL-specific clones. If so, the same T cell clones should be detected in both peripheral blood and the affected tissue. However, in this study, peripheral blood and skin tissues from patients suffering from skin eruptions did not share the same major T cell clones. These results suggest that the skin rash may not be a consequence of cross-reaction, but rather reflects alterations of T cell homeostasis or cytokine-release syndrome. Our data suggest that the immunological dynamics and polarization in moga-treated patients varies depending on the tissue site.
In conclusion, TCR repertoire analysis by deep sequencing reveals reconstitution of polyclonal and oligoclonal T cell clones after moga treatment. This phenomenon was observed only after moga treatment and may be essential for its effects. Although further investigations are warranted, our method would help to clarify the mode of action of moga.
Methods
TCR repertoire analysis
PBMCs and sorted CD4+/CD8+ T cells, or the skin tissues were collected from ATL patients with written informed consent, and subjected to TCR repertoire analysis. All the studies were performed in accordance with the guidelines set out in the Declaration of Helsinki and were approved by the Saga University Institutional Review Board. TCR repertoire consists of combination of diverse TRAV and J chain usage and amino acid sequences of CDR3 region. Sequencing of the T cell receptor α (TRA) locus was performed at Repertoire Genesis Incorporation using an unbiased gene amplification method with Miseq or GS Junior, and the repertoire analysis software, Repertoire Genesis, was used as previously described.4 Between 103 and 105 valid reads were generated. Cell numbers and extracted RNA contents of the samples are summarized in Table S6.
Flow cytometry
Peripheral blood mononuclear cells (PBMCs) were collected from nine ATL patients. Cells were stained using a combination of APC-H7-anti-CD3 (SK7), PE-anti-CD7 (M-T701), and PerCP-Cy5.5-anti-CD4 (SK3) (BD Biosciences, Franklin Lakes, NJ), FITC-anti-CCR4 (205410; R&D systems, Minneapolis, MN), and Alexa Flour 647-anti-CADM1 (3E1; MBL, Nagoya, Japan). Multicolor flow cytometric analysis was performed using a FACSCanto II analyzer (BD Biosciences), and data were analyzed using Flowjo software (TreeStar, Ashland, OR).
Supplementary Material
Category
Brief Report
Conflicts of interest
No potential conflicts of interest were disclosed.
References
- 1.Ishitsuka K, Tamura K. Human T-cell leukaemia virus type I and adult T-cell leukaemia-lymphoma. Lancet Oncol. 2014;15:e517−26. doi: 10.1016/S1470-2045(14)70202-5. PMID:25281470. [DOI] [PubMed] [Google Scholar]
- 2.Shimoyama M, Minato K, Tobinai K, Nagai M, Setoya T, Takenaka T, Ishihara K, Watanabe S, Hoshino H, Miwa M, et al.. Atypical adult T-cell leukemia-lymphoma: diverse clinical manifestations of adult T-cell leukemia-lymphoma. Jpn J Clin Oncol 1983;13 Suppl 2:165–87. PMID:6603526. [PubMed] [Google Scholar]
- 3.Kobayashi S, Nakano K, Watanabe E, Ishigaki T, Ohno N, Yuji K, Oyaizu N, Asanuma S, Yamagishi M, Yamochi T, et al.. CADM1 expression and stepwise downregulation of CD7 are closely associated with clonal expansion of HTLV-I-infected cells in adult t-cell leukemia/lymphoma. Clin Cancer Res. 2014;20:2851–61. doi: 10.1158/1078-0432.CCR-13-3169. PMID:24727323. [DOI] [PubMed] [Google Scholar]
- 4.Ureshino H, Shindo T, Nishikawa H, Watanabe N, Watanabe E, Satoh N, Kitaura K, Kitamura H, Doi K, Nagase K, et al.. Effector Regulatory T Cells Reflect the Equilibrium between Antitumor Immunity and Autoimmunity in Adult T-cell Leukemia. Cancer Immunol Res. 2016;4:644–9. doi: 10.1158/2326-6066.CIR-15-0303. PMID:27215229. [DOI] [PubMed] [Google Scholar]
- 5.Tsukasaki K, Utsunomiya A, Fukuda H, Shibata T, Fukushima T, Takatsuka Y, Ikeda S, Masuda M, Nagoshi H, Ueda R, et al.. VCAP-AMP-VECP compared with biweekly CHOP for adult T-cell leukemia-lymphoma: Japan Clinical Oncology Group Study JCOG9801. J Clin Oncol. 2007;25:5458–64. doi: 10.1200/JCO.2007.11.9958. PMID:17968021. [DOI] [PubMed] [Google Scholar]
- 6.Hishizawa M, Kanda J, Utsunomiya A, Taniguchi S, Eto T, Moriuchi Y, Tanosaki R, Kawano F, Miyazaki Y, Masuda M, et al.. Transplantation of allogeneic hematopoietic stem cells for adult T-cell leukemia: a nationwide retrospective study. Blood. 2010;116:1369–76. doi: 10.1182/blood-2009-10-247510. PMID:20479287. [DOI] [PubMed] [Google Scholar]
- 7.Ishida T, Joh T, Uike N, Yamamoto K, Utsunomiya A, Yoshida S, Saburi Y, Miyamoto T, Takemoto S, Suzushima H, et al.. Defucosylated anti-CCR4 monoclonal antibody (KW-0761) for relapsed adult T-cell leukemia-lymphoma: a multicenter phase II study. J Clini Oncol. 2012;30:837–42. doi: 10.1200/JCO.2011.37.3472. PMID:22312108. [DOI] [PubMed] [Google Scholar]
- 8.Yamamoto K, Utsunomiya A, Tobinai K, Tsukasaki K, Uike N, Uozumi K, Yamaguchi K, Yamada Y, Hanada S, Tamura K, et al.. Phase I study of KW-0761, a defucosylated humanized anti-CCR4 antibody, in relapsed patients with adult T-cell leukemia-lymphoma and peripheral T-cell lymphoma. J Clin Oncol. 2010;28:1591–8. doi: 10.1200/JCO.2009.25.3575. PMID:20177026. [DOI] [PubMed] [Google Scholar]
- 9.Sugiyama D, Nishikawa H, Maeda Y, Nishioka M, Tanemura A, Katayama I, Ezoe S, Kanakura Y, Sato E, Fukumori Y, et al.. Anti-CCR4 mAb selectively depletes effector-type FoxP3+CD4+ regulatory T cells, evoking antitumor immune responses in humans. Proc. Natl. Acad. Sci. USA. 2013;110:17945–50. doi: 10.1073/pnas.1316796110. PMID:24127572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sugata K, Yasunaga J, Miura M, Akari H, Utsunomiya A, Nosaka K, Ezoe S, Kanakura Y, Sato E, Fukumori Y, et al.. Enhancement of anti-STLV-1/HTLV-1 immune responses through multimodal effects of anti-CCR4 antibody. Sci Rep. 2016;6:27150. doi: 10.1038/srep27150. PMID:27250643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ishida T, Ito A, Sato F, Kusumoto S, Iida S, Inagaki H, Morita A, Akinaga S, Ueda R. Stevens-Johnson Syndrome associated with mogamulizumab treatment of adult T-cell leukemia / lymphoma. Cancer science. 2013;104:647–50. doi: 10.1111/cas.12116. PMID:23360455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fuji S, Inoue Y, Utsunomiya A, Moriuchi Y, Uchimaru K, Choi I, Otsuka E, Henzan H, Kato K, Tomoyose T, et al.. Pretransplantation Anti-CCR4 Antibody Mogamulizumab Against Adult T-Cell Leukemia/Lymphoma Is Associated With Significantly Increased Risks of Severe and Corticosteroid-Refractory Graft-Versus-Host Disease, Nonrelapse Mortality, and Overall Mortality. J Clin Oncol. 2016;34:3426–33. doi: 10.1200/JCO.2016.67.8250. PMID:27507878. [DOI] [PubMed] [Google Scholar]
- 13.Ishida T, Jo T, Takemoto S, Suzushima H, Uozumi K, Yamamoto K, Uike N, Saburi Y, Nosaka K, Utsunomiya A, et al.. Dose-intensified chemotherapy alone or in combination with mogamulizumab in newly diagnosed aggressive adult T-cell leukaemia-lymphoma: a randomized phase II study. Br J Haematol. 2015;169:672–82. doi: 10.1111/bjh.13338. PMID:25733162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yonekura K, Kanzaki T, Gunshin K, Kawakami N, Takatsuka Y, Nakano N, Tokunaga M, Kubota A, Takeuchi S, Kanekura T, et al.. Effect of anti-CCR4 monoclonal antibody (mogamulizumab) on adult T-cell leukemia-lymphoma: cutaneous adverse reactions may predict the prognosis. J Dermatol. 2014;41:239–44. doi: 10.1111/1346-8138.12419. PMID:24628073. [DOI] [PubMed] [Google Scholar]
- 15.Yonekura K, Tokunaga M, Kawakami N, Takeda K, Kanzaki T, Nakano N, Kubota A, Takeuchi S, Takatsuka Y, Seto M, et al.. Cutaneous Adverse Reaction to Mogamulizumab May Indicate Favourable Prognosis in Adult T-cell Leukaemia-lymphoma. Acta Derm Venereol. 2016;96:1000–2. doi: 10.2340/00015555-2421. PMID:27025906. [DOI] [PubMed] [Google Scholar]
- 16.Talvensaari K, Clave E, Douay C, Rabian C, Garderet L, Busson M, Garnier F, Douek D, Gluckman E, Charron D, et al.. A broad T-cell repertoire diversity and an efficient thymic function indicate a favorable long-term immune reconstitution after cord blood stem cell transplantation. Blood. 2002;99:1458–64. doi: 10.1182/blood.V99.4.1458. PMID:11830500. [DOI] [PubMed] [Google Scholar]
- 17.Thomson BG, Robertson KA, Gowan D, Heilman D, Broxmeyer HE, Emanuel D, Kotylo P, Brahmi Z, Smith FO. Analysis of engraftment, graft-versus-host disease, and immune recovery following unrelated donor cord blood transplantation. Blood. 2000;96:2703–11. PMID:11023501. [PubMed] [Google Scholar]
- 18.Verdonck K, Gonzalez E, Van Dooren S Vandamme AM, Vanham G, Gotuzzo E. Human T-lymphotropic virus 1: recent knowledge about an ancient infection. Lancet Infect Dis. 2007;7:266–81. doi: 10.1016/S1473-3099(07)70081-6. PMID:17376384. [DOI] [PubMed] [Google Scholar]
- 19.Yasunaga J, Sakai T, Nosaka K, Etoh K, Tamiya S, Koga S, Mita S, Uchino M, Mitsuya H, Matsuoka M. Impaired production of naive T lymphocytes in human T-cell leukemia virus type I-infected individuals: its implications in the immunodeficient state. Blood. 2001;97:3177–83. doi: 10.1182/blood.V97.10.3177. PMID:11342446. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


