ABSTRACT
Tumors are associated with expansion of immunosuppressive cells such as tumor associated macrophages (TAMs), regulatory T cells (Tregs) and myeloid derived suppressor cells (MDSCs). These cells promote tumor growth, angiogenesis, metastasis and immune escape. Cancer patients frequently present symptoms such as anemia, leukocytosis and/or cytopenia; associated with poor prognosis. To uncover tumor-mediated hematopoietic abnormalities and identify novel targets that can be harnessed to improve tumor-specific immune responses, we investigated the hematopoietic stem and progenitor cell compartment in melanoma bearing mice. We show that melanoma growth results in expansion of myeloid lineages such as MDSCs, macrophages and DCs along with a reduction in mature RBCs and platelets. Mature B lymphocytes in the blood and BM of melanoma mice were also reduced. Mice bearing melanoma showed extramedullary hematopoiesis in the spleen. Increased expansion of myeloid lineages occurred directly at the level of stem and progenitor cells. The reduction in mature B lymphocytes resulted from a block at the Pro-B cell stage in the bone marrow. Addition of recombinant IL-3 to bone marrow cells resulted in the expansion of committed myeloid progenitors including common myeloid precursors, granulocyte-monocyte precursors and megakaryocyte-erythrocyte precursors. In vivo, IL-3 receptor stimulation in melanoma bearing mice using an IL-3 antibody also resulted in a robust expansion of committed myeloid progenitors and hematopoietic stem cells. Collectively our findings demonstrate that tumor growth plays a pivotal role in reprogramming the host immune system by impacting hematopoiesis directly at the level of stem cell compartment.
KEYWORDS: immunosuppressive, myeloid derived suppressor cells, tumor microenvironment, tumor associated macrophages
Introduction
Leukemoid reactions defined as leukocyte counts greater than 30–50 × 109/µl of blood in cancer patients have been reported for a variety of cancers.1–5 In most cases the leukocytosis results from elevated neutrophil counts and is accompanied by neutrophil activation, absence of basophilia and dysplastic changes.6 There are indications that leukemoid reactions are associated with a poor prognosis.2,7 While neutrophils and other myeloid cells such as macrophages are vital to innate immunity, their pro-tumorgenic roles are also well documented.8,9 Dysfunctional differentiation of myeloid cells also leads to the generation of myeloid derived suppressor cells (MDSCs) that potently inhibit anti-tumor immunity.10-12 Both acute and chronic inflammation redirect hematopoiesis by favoring granulopoiesis over lymphopoiesis.13 The diversion of lymphoid progenitors towards myeloid lineages may further reduce the pool of lymphocytes required to mount effective anti-tumor immunity. It is therefore crucial to decipher the mechanism regulating cancer associated hematopoietic perturbations, which will greatly help in predicting patient outcomes to therapeutic approaches.
Leukocytes originate from self-renewing long term hematopoietic stem cells (HSCs) through a series of highly regulated steps. Recent studies have shown that inflammation can affect HSCs directly through PAMPs that are recognized by the TLRs expressed on HSCs or indirectly through inflammatory cytokines, such as TNF, IL-1, IL-6 and IL-8, thus regulating the immune response from the progenitor stage.14,15 Tumor cells also upregulate a variety of cytokines and metabolites that shape the tumor microenvironment and the anti-tumor immune response.16-18 It is therefore possible that the leukemoid reaction and myeloproliferation result from the various cytokines released by the tumor cells or by the host in response to the tumor cells. Cytokines such as G-CSF, GM-CSF and IL-6 have been shown to promote proliferation of granulocytic progenitors in response to infection and chronic inflammation.19,20 Rantes, an inflammatory cytokine has been shown to have a direct effect on the fate of stem and progenitor cells and causes myeloid skewing.21 Interestingly, evidence also suggests that depending upon whether hematopoiesis is occurring under physiological conditions or in response to inflammation or injury, distinct developmental paths may be followed up by the HSCs. 22-24 For example c/EBPα is critical for steady state granulopoiesis, but not for inflammation induced HSC skewing.19
In this study we document that B16-F10 melanoma induces a paraneoplastic syndrome characterized by hematopoietic alterations, splenomegaly, granulocytosis in the blood, spleen and bone marrow (BM), significant leukopenia and defective erythropoiesis. The changes in the mature immune cell content occurred at the level of HSCs and extramedullary hematopoiesis was observed in the spleen of these mice. Increased percentage and absolute numbers of common myeloid progenitors (CMPs), granulocyte-monocyte progenitors (GMPs) and megakaryocyte-erythrocyte progenitors (MEPs) were seen in the spleen of melanoma mice. Leukopenia resulted from the block of B cell maturation at the level of pro-B cells to mature B cells. Immature erythropoietic progenitors were also increased in the spleen and BM.
Paraneoplastic syndromes are mediated by tumor derived factors such as cytokines and in order to discover the underlying mechanism, we established an in vitro assay and showed that IL-3 regulates these profound changes in hematopoiesis. IL-3 expression was upregulated in B16-F10 culture supernatants. Addition of recombinant IL-3 to primary BM cells recapitulated the in vivo phenotype with an increase in the frequency of lin- cells, HSCs and myeloid progenitors (MPs). In vivo, administration of the IL-3 antibody, which stabilizes circulating IL-3, to B16-F10 melanoma bearing mice also significantly enhanced myelopoiesis. Overall our data shows that melanoma growth results in aberrant hematopoiesis mediated partly by IL-3.
Materials and methods
Mice
6–8 week old wild type C57 BL/6 mice were purchased from Taconic. Mice were housed under standard specific pathogen free conditions. All animal procedures were carried out in accordance with the University of Michigan's Institutional Animal Care and Use Committee.
Cell line and melanoma model
Mouse melanoma cells, B16-F10 were obtained from American Type Culture Collection (ATCC; Manassas, VA; CRL-6475™). Cell culture reagents were purchased from ThermoFisher Scientific. Cells were cultured in Dulbecco's Modified Eagle's medium (DMEM) with nonessential amino acids, 1% penicillin-streptomycin (pen/strep), 1% L-glutamine and 10% fetal bovine serum. This media is referred to as complete media. Tumors were generated by injecting 2 × 105 (in 200 μl volume) B16-F10 cells subcutaneously into the flanks of 6–8 week old C57BL/6 mice. Non-tumor mice were injected with media alone. Manual calipers were used to monitor tumor size and volume was calculated using the formula as follows: tumor volume = 0.5(L × W2), where L is the smaller of two measurements. The tumors were non ulcerated and the tumor bearing animals did not exhibit any signs of moribund behavior. The mice were bright, alert and responsive. All mice were analyzed at 21 days post tumor implantation when the mean tumor volume was 1384.5 mm3 (supplementary Fig. 1). Some animals were also analyzed at 14 days post implantation when the mean tumor volume was197.1 mm3. For IL-3 studies in vivo, wild type mice were injected with B16-F10 cells as above. 7 days post injection, mice were injected with 100 µg of IL-3 antibody (clone MP2-8F8, BioXcell) or isotype control i.p. every alternate day until day 21. Mice were humanly euthanized at day 21 and tissues were analyzed by flow staining as below.
Tissue preparation
Tumor, blood, spleen and bone marrow were obtained from tumor bearing mice at day 21 post tumor implantation (D21) and control mice with no tumors (NT). Blood was collected by submandibular bleeding in EDTA coated tubes (Sarstedt). Complete blood counts and reticulocyte counts were obtained using the Hemavet 950 (Drew Scientific). Tissues were processed into single cell suspensions as detailed in supplementary methods.
Flow cytometry
All antibodies unless indicated were purchased from Biolegend (supplementary table 1). All flow staining was carried out in PBS containing 2% FCS (flow buffer). Prior to flow staining cell suspensions were stained with viability dye (eBioscience) or 7-AAD (Biolegend) to exclude dead cells as per the manufacturer's protocol. To analyze immune cells CD45, CD3, CD4, CD8, CD19, F4/80, CD11c, CD11b and Gr-1 antibodies were used. Gr-1high and Gr-1low populations were analyzed by using CD11b, Ly6G and Ly6C antibodies. Tregs were identified as CD4+, Foxp3+ cells using the Foxp3 staining kit from BD Biosciences. Plasma cells were identified as CD19+, CD138+ cells within the live spleen cells. For hematopoietic stem cell and progenitor analysis, cell suspensions were first stained with CD16/CD32 (FcγRII/III) for 20 mins on ice to prevent non-specific binding of antibodies to the Fc receptors. Cells were then centrifuged at 1500 rpm at 4° C for 5 mins, followed by staining with Lineage cocktail, c-kit, Sca-1, CD34 and IL7Rα antibodies. 5–6 × 106 cells were used for staining from each sample to be able to acquire sufficient number of stem and progenitor populations. LT-HSCs and LRPs were identified using CD150 and CD48 antibodies in combination with Lineage cocktail, sca-1 and c-kit. B cell progenitors were identified using the B220, IgM, CD24 and BP-1 (Ly51; BD Bioscience) antibodies within the live BM cells from NT or tumor bearing mice. RBC lineages were analyzed using Ter119 and CD71 antibodies on spleen and BM samples that did not undergo RBC lysis. Cells were stained with unconjugated CD16/CD32 antibody first to block Fc receptors followed by FITC-CD71 and PB-Ter119 antibodies. Antibody dilutions were used based on the manufacturer's recommendations. All staining was carried out at 4°C for 30 mins. Post staining, cells were washed twice with flow staining buffer and run on the FACSAria flow cytometer (BD Biosciences). Data was analyzed using Flow Jo version 10 (Tree Star, Inc.).
Cytokine array
Proteome Profiler cytokine array from R and D systems was used as described in supplementary methods.
BM culture assays
Bone marrow cells were obtained from the femur and tibia of wild type mice as described in supplementary methods. Cells were subjected to RBC lysis and plated as 5 × 106 cells per well of a 12 well plate in 1 ml media (RPMI + 10% FCS + Pen/Strep + β-mercaptoethanol). For cultures containing B16-F10 CM, cells were grown in media and CM (70%: 30% ratio). For cultures with cytokine additions, cells were grown in media supplemented with IL-3 (20 ng/ml; Peprotech) or C5 a (50 ng/ml; R and D systems) or IL-1Rα (20 ng/ml; Peprotech) or a combination of all 3 cytokines. Cultures were maintained for 3 days after which they were analyzed by flow staining as described above.
Haematoxylin and Eosin staining (HE staining)
Tissues from no tumor and melanoma bearing mice were processed at day 21 for H and E staining as indicated in supplementary methods.
Statistical analysis
Data was analyzed using GraphPad Prism version 7. Animal studies were carried out with at least 5 animals in each group. The statistical test used is indicated in each figure. p < 0.05 were considered significant.
Results
Melanoma growth results in marked alterations in peripheral blood components
Cancer associated myeloproliferation leads to the inhibition of anti-tumor immunity, promotion of tumor angiogenesis and metastasis and is therefore a candidate therapeutic target.7,9-11 In order to understand the tumor-mediated immune changes, we investigated the immune compartment in melanoma bearing mice. Analysis was carried out 21 days post implantation (D21), at which time the average tumor volume was 1384.5 mm3 (supplementary Figure 1A). The mice were bright, alert and responsive and did not exhibit moribund behavior as expected since the median survival of B16-F10 melanoma bearing mice was observed to be 28 days (supplementary Figure 1B). The CD45+ hematopoietic cells from B16-F10 tumors contained approximately 30% myeloid derived suppressor cells (MDSCs). Mice bearing B16-F10 tumors also showed a 5.2 fold increase (40.48 ± 2.21 in tumor mice vs. 7.71 ± 0.80 in control mice) in the level of circulating MDSCs (supplementary Figure 2). The expansion of MDSCs was accompanied by widespread changes in blood cells' content (Fig. 1). Percentage and absolute numbers of white blood cells (WBCs) such as granulocytes and agranulocytes were analyzed. Among the granulocytes, neutrophil percentage was increased in melanoma bearing mice by 2.7 fold (36.4 ± 3.88 vs. 13.18 ± 2.20; Fig. 1B). Percentage and absolute numbers of basophils did not change while a 2.6 fold decrease (0.58 ± 0.13 vs. 1.35 ± 0.26) in eosinophil percentage was observed (Fig. 1B). Percentage of lymphocytes and monocytes were also reduced in tumor bearing mice by 1.3 fold (60.04 ± 3.85 vs. 82.44 ± 3.02) and 3.4 fold (1.75 ± 0.44 vs. 6.04 ± 0.28) respectively as were their absolute numbers (Fig. 1C). Flow cytometric analysis revealed that the reduction in lymphocytes included B cells, CD4 and CD8 T cells (Fig. 1C).
Figure 1.

Melanoma growth induces immune cell dysregulation, anemia and thrombocytopenia: (A) C57 BL/6 mice were subcutaneously injected with 2 × 105 B16-F10 melanoma cells. Mice were euthanized at 21 days post tumor implantation (D21) and blood, spleen and BM was analyzed. No tumor (NT) mice were used as controls. Blood cells analyzed are shown. (B, C and D) White blood cell (WBC) counts, Hemoglobin (HB), hematocrit (HCT), RBC and platelet counts in peripheral blood are shown. Last graph in (C) shows percentage of B cells, CD4 and CD8 T lymphocytes in the blood as analyzed by flow. Data was analyzed by Student's t-test. Mean ± SD are shown. *p < 0.05, ***p < 0.005, ****p < 0.001.
The changes in the blood immune compartment of melanoma mice prompted us to examine the spleen and BM. While the primary lymphoid organs like BM and thymus are sites of generation and maturation of immune cells, secondary lymphoid organs such as the spleen and lymph nodes are sites of antigen and immune cells' interactions. Flow cytometric analysis of the BM CD45+ cells showed a striking reduction in the percentage of CD19+ B cells (9.96 ± 1.99 vs. 26.22 ± 1.37) indicating defective B cell development (supplementary Figure 3Ai). The percentage of CD4+ T cells was marginally increased by 1.2 fold (0.92 ± 0.07 vs. 0.72 ± 0.04) while the percentage of CD8+ T cells was decreased by 2.3 fold (0.28 ± 0.02 vs. 0.67 ± 0.04), likely leading to similar percentages of total CD3+ T cells (CD4 T cells + CD8 T cells) in naïve and melanoma bearing mice (supplementary Figure 3Aii). Melanoma bearing mice showed increased percentage of MDSCs (55.62 ± 2.15 vs. 47.68 ± 2.54) and macrophages (52.84 ± 1.27 vs. 36.06 ± 1.13) while the percentage of DCs remained the same (supplementary Figure 3Aiii-v) in the BM. Percentage of B cells within the spleen of melanoma bearing mice remained normal while a reduction in the CD3 (24.70 ± 0.40 vs. 28.44 ± 0.76) and CD4 T cell (11.10 ± 0.51 vs. 12.98 ± 0.47) content was observed (supplementary Fig. 3Bi-ii). Elevated percentages of MDSCs (3.57 ± 0.35 vs. 1.41 ± 0.13), macrophages (18.22 ± 0.96 vs. 11.35 ± 0.63) and DCs (11.15 ± 0.15 vs. 8.95 ± 0.25) were also observed (supplementary Figure 3Biii-v). The perturbations in hematopoiesis were evident as early as 14 days post tumor implantation, suggesting an immediate impact of tumor growth on the hematopoietic system (supplementary Figure 4). Thus it appears that melanoma growth affects the hematopoietic system in the BM and spleen leading to an increase in myeloid cells and a decrease in cells of the lymphoid lineage.
Mice were also observed to be highly anemic as indicated by a 2.2 fold decrease (15.91 ± 0.23 vs. 7.27 ± 0.95) in hemoglobin content and hematocrit in tumor bearing mice along with a 2 fold reduction (4.80 ± 0.55 vs.10.38 ± 0.20) in the red blood cell (RBC) number (Fig. 1D). In addition, mice showed thrombocytopenia as evident by the 2.9 fold (239.7 ± 37.46 vs. 703.2 ± 53.17) reduction in blood platelet count (Fig. 1D). Overall a marked alteration in RBC and WBC lineages in blood, BM and spleen was seen in tumor bearing mice.
Melanoma growth results in defective erythropoiesis
Melanoma bearing mice were observed to be severely anemic. Therefore, we next analyzed the erythroid precursors in these mice. Interestingly, a 4.8 fold increase (18.94 ± 1.41 vs. 3.98 ± 0.83) in the percentage of reticulocytes and a 5.2 fold increase (1793 ± 335.2 vs. 344.5 ± 15.99) in the absolute number of reticulocytes was observed in the peripheral blood of melanoma mice indicating an increase in erythroid precursor cells which was in contrast with the decreased number of mature RBCs (Fig. 2Ai-ii). To further investigate the apparent discrepancy between the number of mature RBCs and reticulocytes we made use of CD71 and Ter119 expression that have been used to delineate the proerythroblast, early erythroblast, late erythroblast and reticulocyte stages of RBC generation (Fig. 2B).25 In mice the BM and the spleen are sites of erythropoiesis especially under stress 25,26; detailed analysis of melanoma spleens and BM showed an increase in the percentage of CD71+, Ter119- cells (proerythroblasts), increase in CD71+, Ter119+ cells (early erythroblasts) and a decrease in the CD71-, Ter119+ cells (late erythroblasts/reticulocytes) compared to NT mice in the spleen and BM (Fig. 2C-D). Overall, we observed that while the pro and early erythroblasts were increased in the spleen and BM indicating increased erythropoiesis, melanoma bearing mice were anemic likely due to the decreased proportion of late erythroblasts and elevated reticulocytes (Fig. 2).
Figure 2.
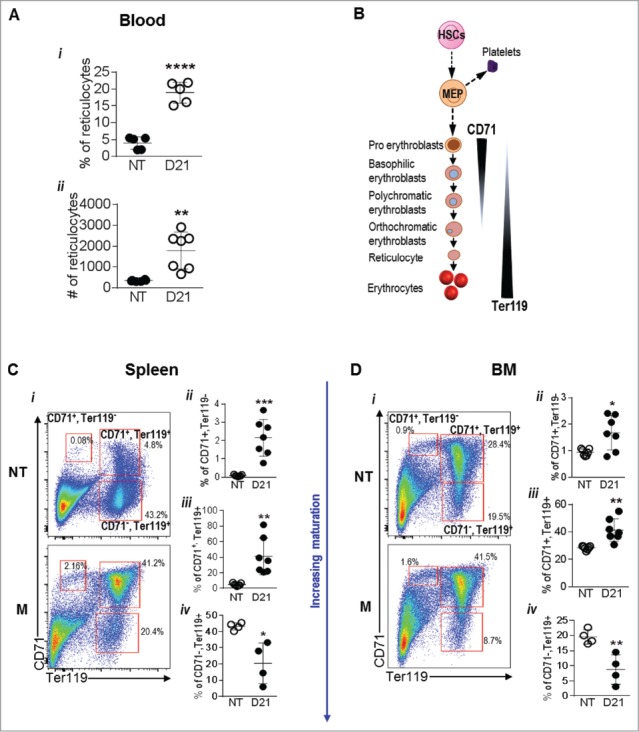
Melanoma growth alters RBC generation in the spleen and bone marrow: (A) Schematic showing erythropoiesis. (B) % and absolute number of reticulocytes in the blood of no tumor (NT) and B16-F10 tumor bearing mice at day 21 (D21). (C and D) RBC lineages in the spleens (C) and bone marrow (D) of NT and D21 mice was analyzed by flow cytometry using the markers CD71 and Ter119. Data was analyzed by Student's t-test. Mean ± SD are shown. *p < 0.05, **p < 0.01, ***p < 0.005.
Spleens from melanoma bearing mice show disruption of normal morphology
Spleen being a reservoir of RBCs and lymphocytes was further examined for alterations in hematopoiesis. The changes in the mature immune cells content were accompanied by gross morphological changes. Splenomegaly was observed in melanoma bearing mice as evidenced by increase in spleen weight (0.22 g ± 0.03 vs. 0.09 ± 0.00) and 4.5 fold increase in total cellularity (Fig. 3Ai-iii). NT mice showed well demarcated separation between the red pulp which contains the sinusoids and the white pulp which contains the lymphoid follicles surrounding the small arteries (Fig 3B). In contrast, total obliteration of normal architecture, such that, there were no distinct red and white pulp was observed in the spleens from melanoma bearing mice (Fig. 3B). A decrease in the size of lymphoid follicles (Fig 3B) was also seen in the spleens of tumor bearing mice along with a prominent increase of multinucleated giant cells, possibly megakaryocytes indicating extra medullary hematopoiesis. In addition, the percentage of CD45+ cells in the spleens of tumor bearing mice was reduced compared to NT animals (48.16 ± 6.02 vs. 94.45 ± 0.90; Fig. 3Ci). To check if the altered histology and reduced % of CD45+ cells observed in the tumor spleen were a result of a germinal center B cell reaction to tumor antigens, we analyzed the plasma cell content in the spleen. No difference in the plasma cell frequency or number was seen between NT and tumor bearing mice (Fig. 3Cii). Interestingly bone marrow cellularity did not change in the melanoma bearing mice and only a marginal reduction (79.98 ± 0.63 vs. 87.9 ± 0.65) in the percentage of CD45+ cells was observed (Fig. 3D i-ii). Bone marrow histology was normal with a meshwork of hematopoietic cells, adipocytes and megakaryocytes (Fig. 3E). Overall our data indicated that melanoma growth resulted in a striking shift in the cells of the immune system possibly as a consequence of extramedullary hematopoiesis. To investigate this possibility we next examined the hematopoietic and progenitor cells in NT and melanoma bearing mice.
Figure 3.
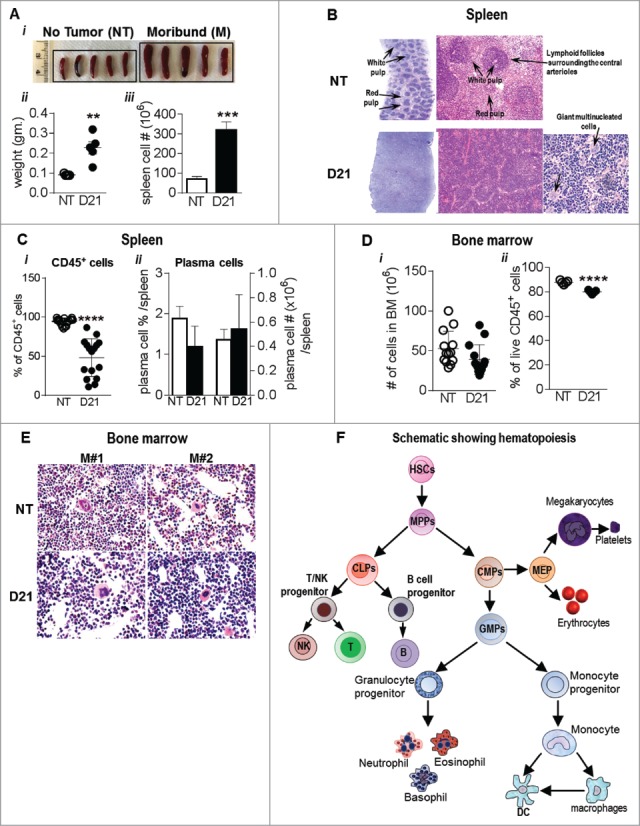
Spleens from melanoma bearing mice show features of extramedullary hematopoiesis: (Ai) Image showing splenomegaly in B16-F10 melanoma bearing mice compared to NT mice. (ii) Spleen weights and (iii) total cell # post RBC lysis are also indicated. (B) Representative images showing HE stains of paraffin embedded spleen sections from NT and melanoma mice. (C) Percentage of (i) CD45+ hematopoietic cells and (ii) plasma cells (CD138+, CD19+) in NT and B16-F10 melanoma spleens were analyzed by flow cytometry. (Di) Total number of bone marrow cells from NT and B16-F10 melanoma mice (2 femur and 2 tibia) and (ii) % of CD45+ hematopoietic cells in the bone marrow post RBC lysis as analyzed by flow cytometry. (E) Representative images showing HE stains of paraffin embedded bone sections from WT and melanoma bearing mice. (F) Schematic showing hematopoiesis and generation of mature cells. Data was analyzed by Student's t-test. Mean ± SD are shown. **p < 0.01, ***p < 0.005, ****p < 0.001.
Melanoma bearing mice show extramedullary myelopoieis
Development of mature immune cells and RBCs occurs through a sequential differentiation of progenitor cells that are themselves derived from the HSCs (Fig. 3F). While hematopoiesis in adults is restricted to the bone marrow, our data indicated that melanoma bearing mice exhibited extramedullary hematopoiesis. Flow cytometric analysis was used to identify the hematopoietic and progenitor cells in the spleen. Lineage negative cells (Lin-) were identified as cells that lack the expression of markers expressed by mature cells using a cocktail of antibodies against T lymphocytes (CD3), B lymphocytes (B220), monocytes/macrophages (CD11b, Gr-1, B220), granulocytes (Gr-1) and erythrocytes (Ter 119). The frequency (1.8 fold, 5.11 ± 0.63 vs. 2.81 ± 0.14) and number of lin- cells was increased (1.7 fold) in melanoma mice spleens indicating an increase in the content of progenitor and immature cells (Fig. 4Bii-iii). Within the lin- compartment, LSKs identified as c-kit+, sca-1+ cells also showed an increase of 7.6 fold in LSK frequency (0.014 ± 0.00 vs. 0.002 ± 0.00) and a 4.9 fold increase in absolute numbers (Fig. 4Civ-v).
Figure 4.

Extramedullary hematopoiesis in spleen results in increased stem and progenitor cells: (A) spleens of NT and melanoma mice 21 days post tumor implantation (D21) were analyzed by flow cytometry. (i) Dot plots show gating strategy. (ii-iii) Lin- cells were identified using the lineage antibody cocktail. (iv-v) Within the Lin- cells, the LSK and progenitor cell population was gated as c-kit+, sca-1+ cells. (vi-vii) Myeloid progenitors (MPs) were gated as c-kit+, sca-1− cells. (Bi) Dot plot showing gating of oligopotent myeloid progenitors. (ii-iii) Common myeloid progenitors (CMPs), iv-v) granulocyte-monocyte precursors (GMPs) and (vi-vii) megakaryocyte-erythroid progenitors (MEPs) were gated as CD34+, FcgRII/IIIlow cells, CD34+, FcgRII/IIIhigh and CD34−, FcgRII/IIIlow cells respectively within the MPs. (Ci-iii) Common lymphoid progenitors (CLPs) were identified as IL-7Rα+ Sca-1+ cells within the Lin−, c-kitlow population. Absolute cell numbers in all panels were obtained by multiplying the frequency of each population by the number of live cells in the spleen. Data was analyzed by Student's t-test. Mean ± SD are shown. *p < 0.05, **p < 0.01, ***p < 0.005, ns is not significant.
The HSC population contains a mixture of cells with long term (LT) hematopoietic reconstituting activity and those that have no renewal capacity and give rise to downstream lineages (lineage restricted progenitors; LRPs) characterized by CD150 and CD48 markers.27 Melanoma bearing mice showed a 2.3 fold decrease in LT-HSCs numbers and an increase in LRPs (7.1 fold) further indicating enhanced generation of committed progenitors (supplementary Figure 5). MP frequency was increased by 17 fold (1.42 ± 0.30 vs. 0.08 ± 0.01) with a corresponding increase of 27 fold in the absolute number of MPs (Fig. 4Dvi-vii). Further analysis revealed an increase in the frequency and number of CMPs, GMPs and MEPs, indicating that myelopoiesis was enhanced in melanoma bearing mice (Fig. 4Bi-vii). Evidence of extramedullary hematopoiesis was seen in the spleens from B16-F10 melanoma mice, 14 days post tumor implantation with an increase in the frequency of MPs, CMPs and GMPs (supplementary Figure 6). No differences in the LSK frequencies or absolute numbers were seen in the bone marrow of melanoma bearing mice compared to NT mice (supplementary Fig. 7-8). Given that melanoma bearing mice showed decreased lymphocyte percentages, we also analyzed the CLP population in these mice (Fig. 4C). Surprisingly, no difference in CLP frequency and absolute numbers were observed between NT and melanoma bearing mice (Fig. 4Ci-iii) suggesting that the decrease in B cell generation may occur after CLP differentiation. The earliest identifiable B cell precursor post-CLPs is the Pre pro B cell (Fig. 5A). Flow staining was used to track B cell development from the Pre pro B cell stage to immature B cell stage in the bone marrow of melanoma bearing mice (Fig. 5Bi). Results showed a 3 fold increase in the percentage of Pre pro B cells (47.65 ± 5.74 vs. 18.23 ± 1.56) in melanoma bearing mice compared to NT mice (Fig. 5Bii). While the proportion of pro B cells did not change, the percentage of pre B cells (2.8 fold, 15.37 ± 3.21 vs. 43.51 ± 1.20) and immature B cells (6.6 fold, 2.58 ± 1.13 vs. 16.6 ± 1.84) was drastically reduced in melanoma bearing mice, indicating a block at the level of pro B cells to pre B cell stage (Fig. 5Biii-v).
Figure 5.
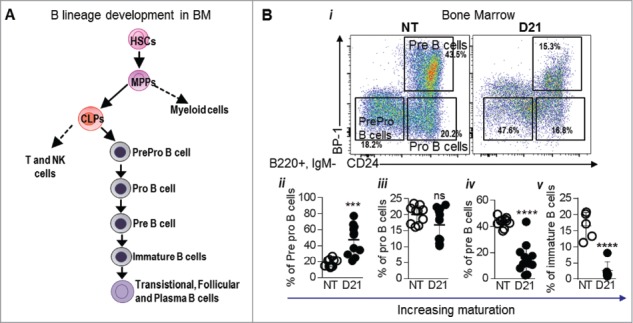
Melanoma growth blocks B cell development at the Pro B cell stage: (A) Schematic showing B cell development from the level of CLPs. (Bi) Various stages of B cell generation in the BM were identified using CD24 and BP-1 (Ly51) marker within the B220+, IgM- cells in the BM. Cells were gated as PrePro B cells (CD24-, BP-1+), Pro B cells (CD24+, BP-1-) and Pre B cells (CD24+, BP-1+). Immature B cells were gated as B220+, IgM+ and IgD- cells. (ii-v) Proportions of PrePro B cells, Pro B cells, Pre B cells and immature B cells in the BM of NT and B16-F10 melanoma bearing mice 21 days post tumor implantation (D21) are shown. Data was analyzed by Student's t-test. Mean ± SD are shown. ****p < 0.001.
Melanoma induces myelopoiesis in an IL-3 dependent manner
Melanoma bearing mice demonstrated increased myelopoiesis and reduced B cell generation. To investigate if secreted factors by melanoma cells could induce the expansion of immature myeloid cells and impact hematopoiesis, we performed BM culture assays. Flow cytometric analysis of BM cells exposed to B16-F10 conditioned media (CM) revealed a higher percentage of F4/80+ macrophages compared to cells grown in regular RPMI (20.14 ± 3.35 vs. 7.33 ± 0.50; Fig. 6Aii). Interestingly we also saw an increase (21.70 ± 0.86 vs. 4.26 ± 0.24) in the percentage of CD45+, Gr-1+, CD11b- cells indicating an increase in the early myeloid lineage progenitors (Fig. 6Aiii). Furthermore we also saw increased frequency of lin- cells (6.84 ± 0.76 vs. 3.71 ± 0.51) and LSKs (0.96 ± 0.13 vs. 0.33 ± 0.06) in BM cells cultured in the presence of B16-F10 CM (Fig. 6Bi-ii). Overall it appeared that culturing BM cells in B16-F10 CM could partially recapitulate the phenotype observed in vivo. We therefore performed cytokine array analysis of CM from B16-F10 cells in order to identify the factors regulating the observed hematopoietic alterations. IL-3, C5a and IL-1Rα were found to be highly expressed by B16-F10 cells. To test the role of each of these cytokines, BM cells were grown in RPMI media supplemented with recombinant IL-3 or C5a or IL-1Rα or all 3 together. Flow cytometric analysis showed that addition of IL-3 to RPMI media resulted in an increase in the frequency of lin- cells (1.9 fold, 9.58 ± 0.78 vs. 4.85 ± 0.8) and LSKs (1.9 fold, 0.78 ± 0.09 vs. 0.42 ± 0.07; Fig. 6Di-ii). A marked increase of 4.6 fold was also seen in MP frequency in the B16-F10 CM group (1.54 ± 0.23 vs. 0.32 ± 0.04; Fig. 6Diii). Not only that, but within the MPs we also saw an increase in the frequency of CMPs (3.7 fold, 0.18 ± 0.01 vs. 0.05 ± 0.00) and GMPs (6.8 fold, 67.83 ± 1.96 vs. 47.9 ± 1.89), the precursors that would eventually give rise to granulocytes, DCs and macrophages (Fig. 6Div-vi). Supplementing RPMI with C5a or IL-1Rα did not have any effect on the content of hematopoietic lineages. Our data thus indicated that IL-3 skews hematopoiesis towards myeloid lineages.
Figure 6.

IL-3 skews hematopoiesis towards myeloid lineage: (A) Bone marrow cells from wild type mice were cultured with B16-F10 cells' culture supernatant (CM) for 3 days after which the cells were analyzed by flow cytometry for (i) MDSCs, (ii) F4/80+ and (iii) Gr-1+ cells. (B) Cultures were also analyzed for (i) Lin- and (ii) LSK cells. (C) Cytokine array analysis was performed on culture supernatant from B16-F10 CM or plain media (Ctrl). Bar graphs show the pixel intensity of each spot. Red boxes indicate the cytokines that were highly expressed in B16-F10-CM. (Di-vi) BM cells were cultured with RPMI media supplemented with recombinant C5 a (20 ng/ml), IL-3 (50 ng/ml), IL-1Rα (20 ng/ml) or a combination of all 3 cytokines for 3 days. Cultures were then analyzed by flow cytometry for stem and progenitor cells. Data was analyzed by Student's t-test. Mean ± SD are shown. *p < 0.05, **p < 0.01, ***p < 0.005, ****p < 0.001.
IL-3 antibody administration enhances melanoma induced extramedullary myelopoiesis
In order to investigate the role of IL-3 in impacting myelopoiesis in melanoma bearing mice, we administered anti-IL-3 antibody or an isotype control antibody to melanoma bearing mice as indicated in the figure and analyzed the hematopoietic compartment in the spleens (Fig. 7A). IL-3 antibody administration resulted in increased frequency and absolute counts of lin- cells (1.2 fold and 1.6 fold), LSKs (2 fold and 2.4 fold) and MPs (1.8 fold and 2.3 fold) as compared to isotype administered group (Fig. 7Bi-vi). No effect on the frequency or absolute CLP counts was seen, indicating that IL-3 only regulates myelopoiesis in melanoma bearing mice (Fig. 7Bvii-viii). While the frequency of CMP, GMP and MEPs did not show a significant increase in the presence of IL-3 antibody, absolute numbers of CMP (3.7 fold) and GMP (2.2 fold) were increased in mice treated with the IL-3 antibody (Fig. 7Bix-xiv). IL-3 antibody administration also did not have an effect on the B cell development as indicated by the similar proportions of committed B cell lineages in isotype control and IL-3 Ab group (Fig. 7Ci-ii).
Figure 7.

IL-3 antibody administration enhances B16-F10 melanoma induced hematopoiesis towards myeloid lineages: (A) Schematic showing experimental design to test the role of IL-3. (Bi-xiv) Effect of isotype control (iso) or IL-3 antibody (IL-3 Ab) administration on hematopoietic stem and progenitor cell populations from B16-F10 melanoma mice are shown. (Ci-ii) Effect on committed B cell progenitors is shown. Data was analyzed by Student's t-test. Mean ± SD are shown. *p < 0.05, **p < 0.01, ns is not significant.
Discussion
Cancer associated hematopoietic dysregulation leads not just to myeloproliferation, but also to the suppression of host immunity and thus represents a novel therapeutic target. Anemia creates another burden for a patient coping with cancer and affects overall prognosis. Anemia in cancer patients has a multifactorial origin and the roles of tumor derived factors in its causation are now being appreciated. Under physiological conditions, hematopoiesis occurs in the fetal liver, spleen and yolk sac but only in the BM in adults. Our results show that melanoma development induces hematopoiesis inappropriately within the spleen. Extra medullary hematopoiesis (EMH) can occur in the spleen under conditions of bone marrow failure. The severe anemia and decreased RBCs in melanoma bearing mice also pointed to failure of BM hematopoiesis. The BM morphology as indicated by the H and E stains and flow cytometric analysis was revealed to be normal. Thus it appears that the EMH observed in melanoma bearing mice is akin to that seen in spleen and liver during an immune response following infection.28 Acute infection and inflammation can also trigger neutrophil mobilization from the BM and blood into the inflammatory sites followed by accelerated or emergency granulopoiesis in the BM 29 and usually represents increased GMP proliferation.19 In contrast, melanoma bearing mice showed an expansion of not only GMPs but also CMPs and MEPs. Additionally an increase in the LRP population and a decrease in LT-HSCs was observed indicating that myelopoiesis was occurring at the cost of HSC renewal. Our results also indicated that melanoma bearing mice were highly anemic despite increased levels of pro- and early erythroblasts and reticulocytes. The condition is reminiscent of conditions of chronic anemia such as hemolytic anemia.30 The lower percentage of late erythroblasts we observed is likely due to the early release of these immature cells into the circulation thereby expanding the pool of circulating reticulocytes. This release of immature RBCs has been linked to erythropoietin.25,30
A central observation of our study is the decreased proportion of mature B cells. A model proposed by Takizawa et al. suggests that myelopoiesis and lymphopoiesis compete for the same developmental resources and under conditions of systemic infection, reduced lymphoid supportive growth and retention signals lead to lymphocyte mobilization thus creating a vacant niche space for enhanced myelopoiesis.15 Furthermore, CLPs have been shown to be reprogrammed to generate DCs and pathogens and TLR ligands are capable of suppressing the differentiation of Pre pro B cells.31 Since CLP frequency was not altered, the decrease in mature B cells in the bone marrow and blood of tumor bearing mice observed in our study could result from enhanced mobilization of immature B cells or increased apoptosis of pro B cells, thereby limiting the pool available for further development. In fact, it has been demonstrated that during alum/infection or PAMPs induced emergency granulopoiesis, cytokines such as IL-1, IL-3, IL-6, G-CSF and GM-CSF cause BM mobilization of lymphocytes to lymphoid organs such as the spleen.15 Our experiments using the IL-3 antibody interestingly show a trend of decreased CD19+ B cells in the BM with no effect on the B cell progenitors, suggesting an increase in the egress of B cells from the bone marrow. Nevertheless, it is evident that melanoma growth resulted in a decrease in the peripheral B cell pool.
While the administration of the IL-3 antibody did not have any impact on B cell progenitors, administration of IL-3 antibody greatly facilitated the expansion of myeloid progenitors, specifically the CMPs, GMPs and MEPs. An increase in the absolute numbers was observed suggesting that IL-3 may serve to enhance the proliferation of these precursor cells. Of note, while the MP2-8F8 antibody has been used to neutralize the IL-3 activity in in vitro assays, in vivo it actually enhances the bioavailability of IL-3 and therefore facilitates its effect on hematopoietic cells.32 This effect has been made use of before to stimulate IL-3 receptor stimulation.33 IL-3 is a pleotropic cytokine that is produced by activated T lymphocytes, mast cells, eosinophils and neutrophils. Bone marrow cells exposed to IL-3 showed expansion of myeloid progenitors and the receptor for IL-3 is expressed on HSCs, CMPs and GMPs. CLPs and MEPs do not express IL-3R and explains why recombinant IL-3 or IL-3 antibody fail to modulate MEPs and CLPs.15 Notably, IL-3 has been demonstrated to be expressed by melanocytic lesions and furthermore its levels were found to correlate with tumor progression.34
Based on our findings we propose a model where melanoma growth either directly through IL-3 or other tumor derived PAMPs and TLR ligands induces hematopoietic skewing to myelopoiesis (Supplementary figure 9). Tumor growth mimics chronic inflammation and triggers BM mobilization and activation of extramedullary hematopoiesis to meet the demands of myeloid cell production. Tumor derived factors or host factors activated in response to tumor growth also impact erythropoiesis and lymphopoiesis thus creating an environment amenable to tumor progression. Leukemoid reaction has been described in a variety of solid tumors such as bladder carcinoma, sarcoma, lung cancer and cervical cancer.2,35-38 Wen-Chao Wu et al. demonstrated a 4–7 fold increase in circulating hematopoietic stem and progenitor cells such as GMPs in the peripheral blood of 90 patients with solid tumors.39 Importantly, they also found that the frequency of these circulating GMPs correlated with tumor progression.39 Paraneoplastic syndrome with granulocytosis was observed in 6 patients with metastatic melanoma.40 Infection, bone marrow metastasis and corticosteroid therapy were ruled out as the underlying mechanisms and high levels of serum GCSF in these patients were found to directly correlate with the elevated WBC counts. The metastatic melanoma was believed to be the source of the ectopic GCSF.40 Two other studies have also identified leukocytosis in melanoma patients with elevated GCSF production by the melanoma tumor and increased serum levels.41,42 Another recent case report also showed leukocytosis in a stage IIIA malignant melanoma patient with a BRAF V600E mutation.43 Interestingly, GCSF in combination with Fms like tyrosine kinase 3 ligand (Flt3L) and GMCSF can lead to the expansion of myeloid progenitors in the context of mammary tumors.44 The observed hematopoietic changes observed in this study with mammary tumors were associated with altered histone methylation in the myeloid progenitors.44 Elevated neutrophil and leukocyte counts in melanoma patients have been found to be associated with poor overall survival and reduced progression free survival by multiple studies.45 Moreover, a study in 1983 also showed that patients with normal lymphocyte counts had a better prognosis than patients with lymphopenia and a positive correlation between lymphocyte counts and treatment response has been also observed.45,46 Thus it is apparent that identification of factors resulting in this paraneoplastic syndrome is crucial for development of anti-melanoma therapies with enhanced efficacy. Understanding the mechanism regulating this tumor-induced paraneoplastic syndrome will also aid in predicting patient responses to anti-tumor therapies and impact survival.
Supplementary Material
Funding Statement
This work was supported by National Institutes of Health/National Institute of Neurological Disorders & Stroke (NIH/NINDS) Grants R37-NS094804, R01-NS074387, R21-NS091555 to M.G.C.; NIH/NINDS Grants R01-NS076991, R01-NS082311, and R01-NS096756 to P.R.L.; NIH/NIBIB R01-EB022563 to M.G.C. and P.R.L.; the Center for RNA Biomedicine and the University of Michigan Comprehensive Cancer Center and M-Cube funding through the University of Michigan Medical School to M.G.C.; Leah's Happy Hearts; ChadTough Foundation; the Phase One Foundation; and the Department of Neurosurgery, University of Michigan Medical School.
Conflict-of-interest disclosure
The authors declare no competing financial interests.
Acknowledgment
We are grateful to Karin Muraszko for her outstanding academic leadership and Stephen Napolitan for excellent administrative support.
Author contributions
N.K., Y.L., M.S., M.A., and P.K. performed experiments; N.K., H.D.A. and M.G.C. analyzed results; N.K. made the figures; N.K., P.R.L., and M.G.C. designed the research and wrote the paper.
References
- 1.He H, Zhang Z, Ge J, Zhou W. Leukemoid reaction associated with transitional cell carcinoma: a case report and literature review. Nigerian J Clin Practice. 2014;17:391–4. doi: 10.4103/1119-3077.130256. [DOI] [PubMed] [Google Scholar]
- 2.Kasuga I, Makino S, Kiyokawa H, Katoh H, Ebihara Y, Ohyashiki K. Tumor-related leukocytosis is linked with poor prognosis in patients with lung carcinoma. Cancer. 2001;92:2399–405. doi: 10.1002/1097-0142(20011101)92:9%3c2399::AID-CNCR1588%3e3.0.CO;2-W. PMID:11745296. [DOI] [PubMed] [Google Scholar]
- 3.Matsumoto M, Yazawa Y, Kanzaki M. An autopsy case of liposarcoma with granulocytic leukemoid reaction. Acta Pathologica Japonica. 1976;26:399–408. PMID:961420. [DOI] [PubMed] [Google Scholar]
- 4.Melhem MF, Meisler AI, Saito R, Finley GG, Hockman HR, Koski RA. Cytokines in inflammatory malignant fibrous histiocytoma presenting with leukemoid reaction. Blood. 1993;82:2038–44. PMID:7691245. [PubMed] [Google Scholar]
- 5.Nasser SM, Choudry UH, Nielsen GP, Ott MJ. A leukemoid reaction in a patient with a dedifferentiated liposarcoma. Surgery. 2001;129:765–7. doi: 10.1067/msy.2001.109498. PMID:11391379. [DOI] [PubMed] [Google Scholar]
- 6.Potasman I, Grupper M. Leukemoid reaction: spectrum and prognosis of 173 adult patients. Clin Infect Dis. 2013;57:e177–81. doi: 10.1093/cid/cit562. PMID:23994818. [DOI] [PubMed] [Google Scholar]
- 7.Wilcox RA. Cancer-associated myeloproliferation: old association, new therapeutic target. Mayo Clinic Proc. 2010;85:656–63. doi: 10.4065/mcp.2010.0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Powell DR, Huttenlocher A. Neutrophils in the Tumor Microenvironment. Trends Immunol. 2016;37:41–52. doi: 10.1016/j.it.2015.11.008. PMID:26700397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim J, Bae JS. Tumor-Associated Macrophages and Neutrophils in Tumor Microenvironment. Mediators Inflammat. 2016;2016:6058147. doi: 10.1155/2016/6058147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marvel D, Gabrilovich DI. Myeloid-derived suppressor cells in the tumor microenvironment: expect the unexpected. J Clin Invest. 2015;125:3356–64. doi: 10.1172/JCI80005. PMID:26168215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kumar V, Patel S, Tcyganov E, Gabrilovich DI. The nature of myeloid-derived suppressor cells in the tumor microenvironment. Trends Immunol. 2016;37:208–20. doi: 10.1016/j.it.2016.01.004. PMID:26858199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Talmadge JE, Gabrilovich DI. History of myeloid-derived suppressor cells. Nat Rev Cancer. 2013;13:739–52. doi: 10.1038/nrc3581. PMID:24060865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Glatman Zaretsky A, Engiles JB, Hunter CA. Infection-induced changes in hematopoiesis. J Immunol. 2014;192:27–33. doi: 10.4049/jimmunol.1302061. PMID:24363432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.King KY, Goodell MA. Inflammatory modulation of HSCs: viewing the HSC as a foundation for the immune response. Nat Rev Immunol. 2011;11:685–92. doi: 10.1038/nri3062. PMID:21904387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takizawa H, Boettcher S, Manz MG. Demand-adapted regulation of early hematopoiesis in infection and inflammation. Blood. 2012;119:2991–3002. doi: 10.1182/blood-2011-12-380113. PMID:22246037. [DOI] [PubMed] [Google Scholar]
- 16.Spranger S, Sivan A, Corrales L, Gajewski TF. Tumor and host factors controlling antitumor immunity and efficacy of cancer immunotherapy. Adv Immunol. 2016;130:75–93. doi: 10.1016/bs.ai.2015.12.003. PMID:26923000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kusmartsev S, Gabrilovich DI. Effect of tumor-derived cytokines and growth factors on differentiation and immune suppressive features of myeloid cells in cancer. Cancer Metastasis Rev. 2006;25:323–31. doi: 10.1007/s10555-006-9002-6. PMID:16983515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prensner JR, Chinnaiyan AM. Metabolism unhinged: IDH mutations in cancer. Nat Med. 2011;17:291–3. doi: 10.1038/nm0311-291. PMID:21383741. [DOI] [PubMed] [Google Scholar]
- 19.Hirai H, Zhang P, Dayaram T, Hetherington CJ, Mizuno S, Imanishi J, Akashi K, Tenen DG. C/EBPbeta is required for ‘emergency’ granulopoiesis. Nat Immunol. 2006;7:732–9. doi: 10.1038/ni1354. PMID:16751774. [DOI] [PubMed] [Google Scholar]
- 20.Ueda Y, Cain DW, Kuraoka M, Kondo M, Kelsoe G. IL-1R type I-dependent hemopoietic stem cell proliferation is necessary for inflammatory granulopoiesis and reactive neutrophilia. J Immunol. 2009;182:6477–84. doi: 10.4049/jimmunol.0803961. PMID:19414802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ergen AV, Boles NC, Goodell MA. Rantes/Ccl5 influences hematopoietic stem cell subtypes and causes myeloid skewing. Blood. 2012;119:2500–9. doi: 10.1182/blood-2011-11-391730. PMID:22289892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boiko JR, Borghesi L. Hematopoiesis sculpted by pathogens: Toll-like receptors and inflammatory mediators directly activate stem cells. Cytokine. 2012;57:1–8. doi: 10.1016/j.cyto.2011.10.005. PMID:22079335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheshier SH, Prohaska SS, Weissman IL. The effect of bleeding on hematopoietic stem cell cycling and self-renewal. Stem Cells Dev. 2007;16:707–17. doi: 10.1089/scd.2007.0017. PMID:17999593. [DOI] [PubMed] [Google Scholar]
- 24.Cain DW, Snowden PB, Sempowski GD, Kelsoe G. Inflammation triggers emergency granulopoiesis through a density-dependent feedback mechanism. PloS One. 2011;6:e19957. doi: 10.1371/journal.pone.0019957. PMID:21655273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dzierzak E, Philipsen S. Erythropoiesis: development and differentiation. Cold Spring Harbor Perspectives Med. 2013;3:a011601. doi: 10.1101/cshperspect.a011601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dumitriu B, Patrick MR, Petschek JP, Cherukuri S, Klingmuller U, Fox PL, Lefebvre V. Sox6 cell-autonomously stimulates erythroid cell survival, proliferation, and terminal maturation and is thereby an important enhancer of definitive erythropoiesis during mouse development. Blood. 2006;108:1198–207. doi: 10.1182/blood-2006-02-004184. PMID:16627753. [DOI] [PubMed] [Google Scholar]
- 27.Kiel MJ, Yilmaz OH, Iwashita T, Yilmaz OH, Terhorst C, Morrison SJ. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121:1109–21. doi: 10.1016/j.cell.2005.05.026. PMID:15989959. [DOI] [PubMed] [Google Scholar]
- 28.O'Malley DP. Benign extramedullary myeloid proliferations. Modern Pathol. 2007;20:405–15. doi: 10.1038/modpathol.3800768. [DOI] [PubMed] [Google Scholar]
- 29.Basu S, Hodgson G, Zhang HH, Katz M, Quilici C, Dunn AR. “Emergency” granulopoiesis in G-CSF-deficient mice in response to Candida albicans infection. Blood. 2000;95:3725–33. PMID:10845903. [PubMed] [Google Scholar]
- 30.Bessman JD. Reticulocytes In: Walker HK, Hall WD, Hurst JW, eds. Clinical methods: The history, physical, and laboratory examinations. Boston, 1990. [PubMed] [Google Scholar]
- 31.Nagai Y, Garrett KP, Ohta S, Bahrun U, Kouro T, Akira S, Takatsu K, Kincade PW. Toll-like receptors on hematopoietic progenitor cells stimulate innate immune system replenishment. Immunity. 2006;24:801–12. doi: 10.1016/j.immuni.2006.04.008. PMID:16782035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jones AT, Ziltener HJ. Enhancement of the biologic effects of interleukin-3 in vivo by anti-interleukin-3 antibodies. Blood. 1993;82:1133–41. PMID:8353280. [PubMed] [Google Scholar]
- 33.Ohmori K, Luo Y, Jia Y, Nishida J, Wang Z, Bunting KD, Wang D, Huang H. IL-3 induces basophil expansion in vivo by directing granulocyte-monocyte progenitors to differentiate into basophil lineage-restricted progenitors in the bone marrow and by increasing the number of basophil/mast cell progenitors in the spleen. J Immunol. 2009;182:2835–41. doi: 10.4049/jimmunol.0802870. PMID:19234178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reed JA, McNutt NS, Bogdany JK, Albino AP. Expression of the mast cell growth factor interleukin-3 in melanocytic lesions correlates with an increased number of mast cells in the perilesional stroma: implications for melanoma progression. J Cutaneous Pathol. 1996;23:495–505. doi: 10.1111/j.1600-0560.1996.tb01441.x. [DOI] [PubMed] [Google Scholar]
- 35.Ito N, Matsuda T, Kakehi Y, Takeuchi E, Takahashi T, Yoshida O. Bladder cancer producing granulocyte colony-stimulating factor. N Eng J Med. 1990;323:1709–10. doi: 10.1056/NEJM199012133232418. [DOI] [PubMed] [Google Scholar]
- 36.Jardin F, Vasse M, Debled M, Dominique S, Courville P, Callonnec F, Buchonnet G, Thiberville L, Tilly H. Intense paraneoplastic neutrophilic leukemoid reaction related to a G-CSF-secreting lung sarcoma. Am J Hematol. 2005;80:243–5. doi: 10.1002/ajh.20454. PMID:16247754. [DOI] [PubMed] [Google Scholar]
- 37.Dukes JW, Tierney LM Jr. Paraneoplastic leukemoid reaction as marker for transitional cell carcinoma recurrence. Urology. 2009;73:928. doi: 10.1016/j.urology.2008.05.023. PMID:18718647. [DOI] [PubMed] [Google Scholar]
- 38.Nimieri HS, Makoni SN, Madziwa FH, Nemiary DS. Leukemoid reaction response to chemotherapy and radiotherapy in a patient with cervical carcinoma. Annals Hematol. 2003;82:316–7. [DOI] [PubMed] [Google Scholar]
- 39.Wu WC, Sun HW, Chen HT, Liang J, Yu XJ, Wu C, Wang Z, Zheng L. Circulating hematopoietic stem and progenitor cells are myeloid-biased in cancer patients. Proc Natl Acad Sci U S A. 2014;111:4221–6. doi: 10.1073/pnas.1320753111. PMID:24591638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Davis JL, Ripley RT, Frankel TL, Maric I, Lozier JN, Rosenberg SA. Paraneoplastic granulocytosis in metastatic melanoma. Melanoma Res. 2010;20:326–9. doi: 10.1097/CMR.0b013e328339da1e. PMID:20440226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schniewind B, Christgen M, Hauschild A, Kurdow R, Kalthoff H, Klomp HJ. Paraneoplastic leukemoid reaction and rapid progression in a patient with malignant melanoma: establishment of KT293, a novel G-CSF-secreting melanoma cell line. Cancer Biol Therapy. 2005;4:23–7. doi: 10.4161/cbt.4.1.1447. [DOI] [PubMed] [Google Scholar]
- 42.Lilly MB, Devlin PE, Devlin JJ, Rado TA. Production of granulocyte colony-stimulating factor by a human melanoma cell line. Exp Hematol. 1987;15:966–71. PMID:3498641. [PubMed] [Google Scholar]
- 43.Gouveia E, Sousa M, Passos MJ, Moreira A. Paraneoplastic leukemoid reaction in a patient with BRAF V600E-mutated metastatic malignant melanoma. BMJ Case Reports. 2015;1–5. doi: 10.1136/bcr-2014-208645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sio A, Chehal MK, Tsai K, Fan X, Roberts ME, Nelson BH, Grembecka J, Cierpicki T, Krebs DL, Harder KW. Dysregulated hematopoiesis caused by mammary cancer is associated with epigenetic changes and hox gene expression in hematopoietic cells. Cancer Res. 2013;73:5892–904. doi: 10.1158/0008-5472.CAN-13-0842. PMID:23913828. [DOI] [PubMed] [Google Scholar]
- 45.Bouwhuis MG, ten Hagen TL, Eggermont AM. Immunologic functions as prognostic indicators in melanoma. Mol Oncol. 2011;5:183–9. doi: 10.1016/j.molonc.2011.01.004. PMID:21367679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bernengo MG, Lisa F, Meregalli M, De Matteis A, Zina G. The prognostic value of T-lymphocyte levels in malignant melanoma. A five-year follow-up. Cancer. 1983;52:1841–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


