ABSTRACT
Preclinical evidence suggests that high-dose hypofractionated ionizing radiation (IR) can enhance anti-tumor immunity and result in significant tumor control when combined with immune checkpoint blockade (ICB). However, low-dose daily fractioned IR used for many tumor types including head and neck squamous cell carcinoma results in lymphopenia and may be immunosuppressive. We compared immune correlates, primary tumor and abscopal tumor control rates following the addition of PD-1 mAb to either high-dose hypofractioned (8Gyx2) or low-dose daily fractionated (2Gyx10) IR in syngeneic models of cancer. When compared to 2Gyx10 IR, 8Gyx2 IR preserved peripheral and tumor-infiltrating CD8+ T-lymphocyte accumulation and activation and reduced peripheral and tumor gMDSC accumulation. Regulatory T-lymphocytes were largely unaltered. Type I and I IFN levels and expression of IFN-responsive MHC class I and PD-L1 was enhanced in tumors treated with 8Gyx2 compared to 2Gyx10 IR. Functionally, tumor-specific CD8+ T-lymphocyte IFN responses within tumor draining lymph nodes were enhanced following 8Gyx2 IR but suppressed following 2Gyx10 IR. When combined with PD-1 mAb, reversal of adaptive immune resistance and subsequent enhancement of CD8+ cell dependent primary and abscopal tumor control was observed following 8Gyx2 but not 2Gyx10 IR. These data strongly support that compared to daily fractionated low-dose IR, high-dose hypofractionated IR preserves or enhances anti-tumor immunity and, when combined with PD-1 mAb to reverse adaptive immune resistance, promotes anti-tumor immunity to control primary and distant tumors. These data critically inform the rational design of trials combining IR and ICB.
KEYWORDS: Radiation, resistance, checkpoint, immunity, abscopal
Introduction
Immune checkpoint blockade (ICB) has emerged as a promising treatment option for many cancers. However, only a small subset of patients with recurrent/metastatic head and neck squamous cell carcinoma (HNSCC) demonstrate durable responses to ICB.1,2 Given our current understanding that programmed death (PD)-based ICB primarily reverses adaptive immune resistance only,3 combining ICB with other anti-cancer therapies that have the potential to activate an anti-tumor immune response could increase response rates.
Ionizing radiation (IR) is a mainstay of treatment for many cancer types, including HNSCC.4 Through many mechanisms, IR can both enhance the diversity of tumor antigens visible to the adaptive immune system5–7 and induce serum transducer of interferon genes (STING)-dependent production of type I interferon (IFN) signals needed for the initiation of innate, and subsequently adaptive, anti-tumor immune responses.8,9 Given its widespread availability and use, IR may represent an ideal adjuvant to immunotherapy and given its ability to induce anti-tumor immunity, be additive or synergistic with ICB through reversal of adaptive immune resistance. However, radiation administered in many daily, low-dose fractions for many cancer types can result in significant lymphopenia and immune suppression.10,11
Several preclinical studies have demonstrated additive or synergistic responses with combination IR and ICB,5,9,12–15 but often use single high-dose or hypofractionated IR regimens that are not translatable to cancer types such as HNSCC that are treated with low-dose, daily fractionated IR. Here we report head-to-head results directly comparing immune correlatives and tumor control of daily, low-dose fractionated IR (2Gyx10) and high-dose hypofractionated (8Gyx2) IR in syngeneic models of oral cavity carcinoma and, to establish generalizability of our findings, colon adenocarcinoma. We demonstrated that 8Gyx2 IR results in preservation of peripheral and tumor-infiltrating effector immune subsets, reduction of immunosuppressive cell subsets, increased expression of PD-1/L1 within the tumor microenvironment (TME) and enhancement of tumor tumor-specific immune responses. Treatment of these tumors with 2Gyx10 suppressed anti-tumor immunity. Accordingly, the addition of PD-1 mAb to 8Gyx2 but not 2Gyx10 reversed adaptive immune resistance and resulted in CD8+ cell-dependent control of both primary and abscopal tumors. These results provide necessary pre-clinical data to help guide the evidence-based design of clinical trials combining IR and ICB.
Results
Daily, low-dose fractionated and high-dose hypofractionated IR alone result in similar control of primary tumor growth
To compare primary tumor control following daily, fractionated IR or high-dose, hypofractionated IR, mice bearing established syngeneic MOC1 oral carcinomas or MC38-CEA colon adenocarcinomas were treated with either 2Gyx10 or 8Gyx2 IR. In both models, these IR regimens resulted in similar degrees of modest tumor growth control (Fig. 1A). Given clinical evidence that patients receiving daily fractionated IR develop lymphopenia10,11 and pre-clinical evidence that different IR regimens variably alter the tumor microenvironment,5,9,15 we hypothesized that these two IR regimens would variably alter immune infiltration and activation within the TME despite little difference in primary tumor growth control. To examine this, we performed flow cytometric analysis on tumor and splenic tissues over time at specific intervals following the start of either IR regimen (Fig. 1B).
Figure 1.
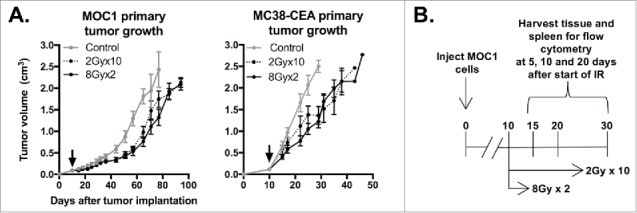
8Gyx2 and 2Gyx10 IR alone results in similar degrees of primary tumor control. A, 5 × 106 MOC1 or 1 × 105 MC38-CEA cells were injected subq into the legs of WT B6 mice (n = 10 mice/group), allowed to engraft, and treated with IR starting day 10 (black arrow). Primary tumor growth was measured 2–3/week. Shown are results from one of three independent experiments, each with similar results. B, schema for acquisition of primary tumor and spleens for immune correlative analysis.
8Gyx2 alters the accumulation of peripheral and TME immune cell subsets to a greater degree than 2Gyx10
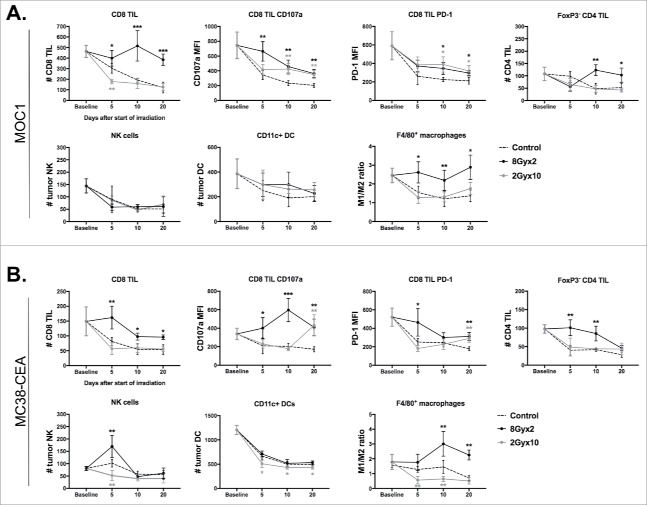
Mice bearing established MOC1 or MC38-CEA tumors were treated with 8Gyx2 or 2Gyx10 IR, and tumor and spleens were harvested at 5, 10 and 20 days after the start of IR for immune correlative analysis. We first evaluated the accumulation of effector immune cells. 8Gyx2 IR resulted in preservation of CD8 and CD4 TIL infiltration that decreased with either tumor progression (control) or 2Gyx10 IR, and generally resulted in higher expression of CD107 a and PD-1 on CD8 TIL in both models (Fig. 2A&B). Similarly, peripheral CD8 T-lymphocyte counts were preserved following 8 Gyx2 but consistently decreased following 2Gyx10 IR in MOC1 tumor-bearing mice (Supplemental Fig. 1). While NK or DC infiltration was minimally altered between the two IR regimens in both models, mature macrophages were consistently polarized more toward an M1 phenotype following 8Gyx2 IR compared to 2Gyx10.
Figure 2.
8Gyx2 and 2Gyx10 IR variably alters effector immune infiltration and activation within the TME. Primary tumor tissues (n = 5/time point) were harvested 5, 10 and 20 days after the start of either 8Gyx2 IR (black lines), 2Gyx10 IR (grey lines) or no IR (dashed lines) and prepared single cell suspensions were analyzed via flow cytometric analysis. A, Effector immune cell infiltration into MOC1 primary tumors. B, Effector immune cell infiltration into MC38-CEA primary tumors. Quantification of tumor infiltration of immune cells presented as absolute number of cells per 1 × 104 live cells analyzed. Quantification of expression of specific markers presented as MFI. Cell subset definitions included CD8 TIL (CD3+CD8+), CD4 TIL (CD3+CD4+FoxP3−), NK (CD3−NK1.1+), DC (CD11 c+, CD11b+/−, PDCA+/−) and macrophages (CD11b+F4/80+CD11 c−). All cells were CD45.2+ and 7AAD−. *, p < 0.05; **, p < 0.01; ***, p < 0.001 (t test) compared to control tumors for each time point.
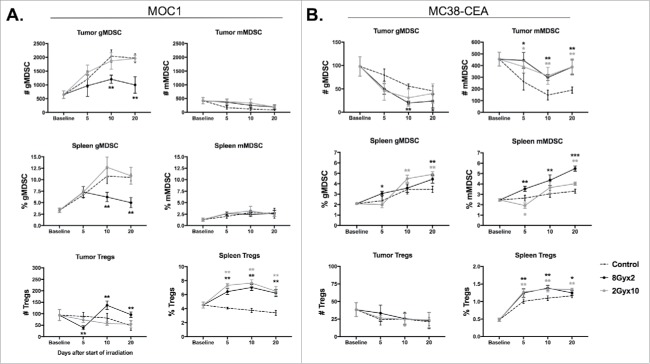
We next assessed accumulation of immunosuppressive cells in the periphery and TME. MOC1 tumors have local immunosuppression driven primarily by gMDSC.16 Notably, 8Gyx2 but not 2Gyx10 IR resulted in a significant reduction in both tumor and peripheral gMDSC (Fig. 3A). gMDSC were also reduced in MC38-CEA tumor-bearing mice, but this is of unclear significance as mMDSC appear to be the dominant MDSC phenotype in these mice, and mMDSC accumulation was consistently increased following IR in both the tumor and periphery (Fig. 3B). Accumulation of Tregs was consistently increased in the periphery after 2Gyx10 IR in both models, but was unaltered in the MC38-CEA TME and only modestly increased in the MOC1 TME after 8Gyx2 IR. Thus, cumulatively, IR with 8Gyx2 appeared to favor greater preservation of effector immune cells with increased expression of activation markers and checkpoints, as well as reduction in gMDSCs, compared to IR with 2Gyx10.
Figure 3.
8Gyx2 and 2Gyx10 IR variably alters tumor and peripheral accumulation of immunosuppressive cell subsets. Primary tumor and splenic tissues (n = 5/time point) were harvested and analyzed as in Fig. 2. A, Immunosuppressive immune cell accumulation in MOC1 primary tumors and spleen. B, Immunosuppressive immune cell accumulation in MC38-CEA primary tumors and spleen. Quantification of tumor accumulation of cells presented as absolute number of cells per 1 × 104 live cells analyzed. Quantification of splenic accumulation of cells presented as percentage of live, CD45.2+ cells analyzed. Cell subset definitions included gMDSC (CD11b+F4/80−Ly6GhiLy6Cint), mMDSC (CD11b+ F4/80−Ly6GlowLy6Chi) and Tregs (CD3+CD4+FoxP3+CD25hi). *, p < 0.05; **, p < 0.01; ***, p < 0.001 (t test) compared to control tumors for each time point.
8Gyx2 but not 2Gyx10 IR induces expression of IFN and IFN-responsive genes on tumor cells within the TME
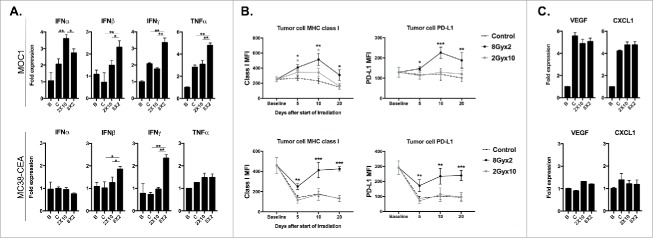
Interferon (IFN)-responsive genes MHC class I and PD-L1 may be altered within the TME following IR.17,18 We next hypothesized that different regimens of IR, through modulation of immune cell subsets within the TME, would alter IFN levels and expression of these genes. Analysis of whole tumor lysates demonstrated enhanced production of type I (IFNβ) and type II IFN (IFNγ) within the TME following 8Gyx2 compared to 2Gyx10 IR (Fig. 4A). This correlated with significantly increased expression of tumor cell MHC class I (H2-Kb/Db) and PD-L1 in both models (Fig. 4B). The effector cytokine TNFα was also expressed to a significantly greater extent in tumors irradiated with 8Gyx2.
Figure 4.
8Gyx2 and 2Gyx10 IR variably alters IFN levels and expression of IFN-responsive MHC class I and PD-L1 within the TME. A, MOC1 (top panels) or MC38-CEA (bottom panels) primary tumor tissues (n = 5/time point) were harvested as in Fig. 2 and analyzed via qRT-PCR for expression of type I IFN (IFNα/β) or effector (IFNγ/TNFα) cytokines. For all PCR data, B refers to baseline (day 10, before the start of IR) tumors. C (control), 2 × 10 Gy and 8Gyx2 tumors were collected and analyzed 10 days after the start of IR (tumor day 20). B, flow cytometric analysis of expression (MFI) of MHC class I (H2-Kb/Db) or PD-L1 on CD45.2−CD31−PDGFR− tumor cells within the TME. C, qRT-PCR analysis for expression of myeloid chemokines (VEGF/CXCL1). *, p < 0.05; **, p < 0.01; ***, p < 0.001 (t test) compared to control tumors for each time point.
Immune correlative analysis of human tissues indicates that expression of PD-L1 on infiltrating immune cells within the TME may also predict responses to PD-based ICB.19 Within the TME of both models, 8Gyx2 but not 2Gyx10 consistently enhanced PD-L1 expression on both total CD45.2+ immune cells and more specifically on CD11b+ myeloid cells (Supplemental Fig. 2). Taken together, these data indicate that 8Gyx2 IR more efficiently induces production of type I IFNs, effector cytokines and IFN-responsive genes MHC class I (H2-Kb/Db) and PD-L1 within the TME compared to 2Gyx10 IR.
Given the significant reduction in gMDSC infiltration into MOC1 tumors, we measured expression of myeloid chemokines within the TME of irradiated tumors. While 8Gyx2 IR reduced gMDSC infiltration, neither IR regimen appeared to significantly do so by altering expression of VEGF or CXCL1 within the TME of either model (Fig. 4C).
2Gyx10 but not 8Gyx2 IR suppresses tumor-specific T-lymphocyte responses in TDLNs
While the above immune correlative data suggest that 8Gyx2 enhances local immunity to a greater degree than 2Gy10 IR, we hypothesized that different IR regimens could directly alter tumor-specific T-lymphocyte responses. To assess this, we sorted T-lymphocytes from the TDLN of tumor-bearing mice following IR and assessed for cellular tumor-specific IFNγ production. Of note, based on our mouse radiation jig design, TDLNs are included in the irradiation fields. Fig. 5 demonstrates IFNγ production over time following the start of IR. In both models, tumor-specific responses decreased with tumor progression (controls), and these baseline responses were significantly suppressed with 2Gyx10 IR. However, these T-lymphocyte responses were either preserved or enhanced at each time point for both models following 8Gyx2 IR. These functional data verify the immune correlative data presented above and strongly suggest that 8Gyx2 IR enhances while 2Gyx10 suppresses tumor-specific T-lymphocyte responses.
Figure 5.
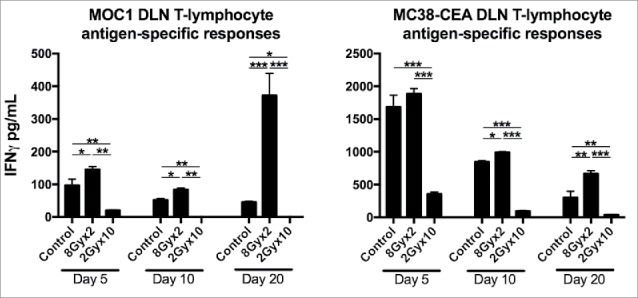
8Gyx2 IR enhances while 2Gyx10 IR suppresses draining lymph node tumor-specific T-lymphocyte responses. T-lymphocytes were sorted from tumor draining lymph nodes 5, 10 and 20 days after the start of either 8Gyx2 IR, 2Gyx10 IR or no IR (n = 5/time point) and analyzed for tumor-specific immune responses. *, p < 0.05; **, p < 0.01; ***, p < 0.001 (t test).
PD-1 mAb plus 8Gyx2 but not 2Gyx10 significantly enhances primary tumor control and survival
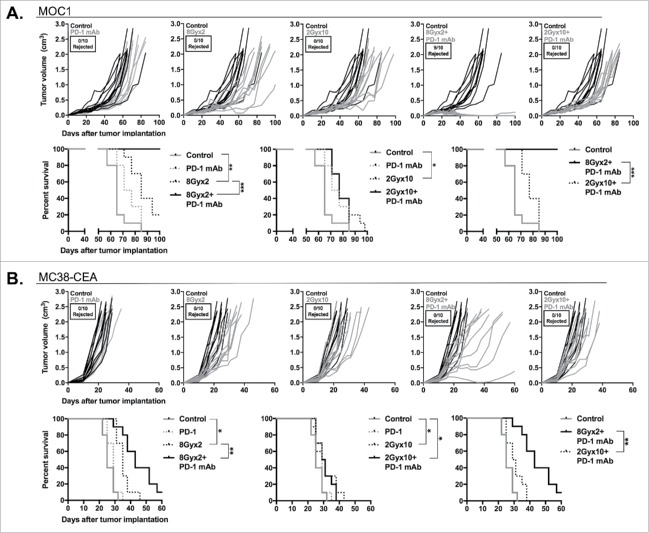
Given the above data, we hypothesized that these different IR regimens would variably alter primary tumor growth when combined with PD-1 mAb. Mice bearing established tumors were treated with either 8Gyx2 or 2Gyx10 with and without PD-1 mAb concurrently. Either IR regimen modestly delayed MOC1 primary tumor growth and enhanced survival, while PD-1 mAb had little effect (Fig. 6A). Combination 2Gyx10 IR plus PD-1 mAb did not enhance primary tumor control or survival. Conversely, the addition of PD-1 mAb to 8Gyx2 IR resulted in the rejection of 90% of MOC1 tumors and significantly delayed the growth of tumors that did not reject, leading to 100% survival. Moreover, all mice that rejected MOC1 tumors resisted challenge with MOC1 in the opposite flank, demonstrating the presence of an anti-tumor immune memory response (Supplemental Fig. 3). Results in mice bearing MC38-CEA tumors were similar (Fig. 6B). While no tumors rejected, the addition of PD-1 mAb to 8Gyx2 but not 2Gyx10 IR resulted in significantly enhanced primary tumor growth control and survival. These data strongly suggest that enhanced immune activation and PD-1/PD-L1 expression within the TME following 8Gyx2 IR are functionally relevant, and that adaptive immune resistance following 8Gyx2 IR can be reversed with the addition of PD-1 mAb to induce significant primary tumor control.
Figure 6.
8Gyx2 plus PD-1 mAb but not 2Gyx10 IR plus PD-1 mAb controls primary tumor growth and enhances survival. MOC1 (A) or MC38-CEA (B) tumors (n = 10 mice/group) were engrafted and treated with either 8Gyx2 or 2Gyx10 IR with or without PD-1 mAb (200 μg/injection, twice weekly × 3 weeks). IR and PD-1 mAb treatments were started concurrently on day 10. Individual tumor growth curves (top panels) and survival (bottom panels) are shown. Results shown are from one of two independent experiments with similar results. *, p < 0.05; **, p < 0.01; ***, p < 0.001 (log-rank test).
PD-1 mAb plus 8Gyx2 but not 2Gyx10 induces control of distant tumors through systemic anti-tumor immunity
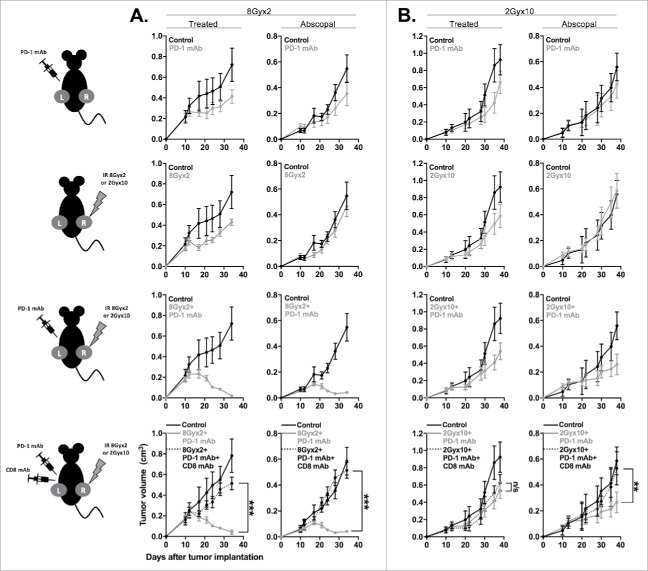
We next aimed to determine if IR plus PD-1 mAb treatment can induce control of distant, untreated (abscopal) tumors in highly responsive MOC1 tumor-bearing mice. Mice were engrafted with bilateral MOC1 tumors, but only the right tumor was treated with IR. Fig. 7A demonstrates that while systemic PD-1 or local 8Gyx2 IR alone induces modest primary and abscopal tumor control, combination 8Gyx2 IR plus PD-1 mAb induces significant control of both primary (treated) and abscopal (untreated) tumors. This primary and abscopal tumor control was completely abrogated following CD8+ cell depletion, supporting the critical role of adaptive immunity in tumor regression.
Figure 7.
8Gyx2 IR plus PD-1 mAb results in CD8+ cell-dependent control of primary and abscopal tumors. MOC1 tumors were simultaneously engrafted in the left leg (primary tumor) and right flank (abscopal tumor) of WT B6 mice (n = 5 mice/group). Mice were treated with 8Gyx2 (A) or 2Gyx10 IR (B) to the left leg tumor only, PD-1 mAb (200 μg/injection, twice weekly × 3 weeks), or the combination, and primary and abscopal tumor growth was measured. Results shown are from one of two independent experiments with similar results. In separate experiments, mice bearing primary and abscopal tumors were treated with combination IR (8Gyx2 or 2Gyx10) plus PD-1 mAb with or without CD8+ cellular depletion (clone YTS169.4, 200 μg/injection twice weekly for 2 weeks beginning the day before the start of treatment) and primary and abscopal tumor growth was measured (bottom panels). **, p < 0.01; ***, p < 0.001 (t test of day 35 tumor measurements).
We further investigated whether similar abscopal effects could be achieved with combination 2Gyx10 IR plus PD-1 mAb, hypothesizing that local immunosuppression from fractionated IR would impact immune responses in a distant tumor to a lesser degree. Fig. 7B demonstrates that while 2Gyx10 IR plus PD-1 mAb induces no control of primary tumor growth beyond that achieved with 2Gyx10 IR alone, a modest delay in abscopal tumor growth is observed that is completely abrogated following CD8+ cell depletion. These data strongly validate that combination 8Gyx2 IR plus PD-1 mAb results in systemic CD8+ cell-dependent anti-tumor immunity that is capable of controlling the growth of distant tumors. These data also suggest that some degree of CD8+ cell-dependent systemic anti-tumor immunity does form following 2Gyx10 IR plus PD-1 mAb, but that this local IR treatment suppresses primary tumor growth control, presumably through IR-induced suppression of local immune cell function.
Discussion
PD-based ICB induces objective anti-tumor responses in 15–20% of patients with recurrent/metastatic cancer.1,2 Based upon our current understanding of how PD-based ICB reverses adaptive immune resistance,3 there is great interest in combining PD-based ICB with other anti-cancer therapies that have the capacity to induce anti-tumor immunity to enhance these response rates. IR can have stimulatory effects on a variety of cell types within the TME aside from induction of DNA damage and cell death, and specifically has been shown to induce IFN-dependent expression of MHC class I and PD-L1,17,18,20,21 enhance expression of innate immune ligands,22 expand diversity of T-cell receptor repertoires within tumors,5 and promote cGAS-dependent sensing of cytoplasmic DNA and subsequent STNG-dependent production of type I IFN.8,9
Herein we demonstrate significant, CD8+ cell dependent primary and abscopal tumor control with 8Gyx2 but not 2Gyx10 when combined with PD-1 mAb. Previous reports have demonstrated additive or synergistic tumor control of IR with PD- or CTLA-4-based ICB, but most have used high single or hypofractionated doses of IR ranging from 6–20 Gy. Deng at al. demonstrated CD8+ cell-dependent enhanced tumor control of TUBO and MC38 tumors with 12Gyx1 plus PD-L1 mAb.13 Demaria et al. and Ruocco et al. demonstrated similar enhanced control of primary tumors and formation of metastases in 4T1 tumor-bearing mice with either one or two fractions of 12 Gy plus CTLA-4 mAb.12,14 Of reports that investigate different radiation regimens, Dewan et al. reported superior control of primary and abscopal tumor growth CTLA-4 mAb with 8Gyx3 IR compared to 20Gyx1 or 6Gyx5.15 This same group more recently demonstrated that higher (>12–18 Gy) doses of IR induce the expression of an endonuclease (Trex1) that degrades cytoplasmic DNA induced by irradiation, blunting cGAS- and STING-dependent type I IFN responses.9 Our results demonstrating enhanced CD8+ cell-dependent primary and abscopal tumor control with the addition of PD-1 mAb to 8Gyx2 IR across two independent cancer models are consistent with these studies. The addition of PD-based ICB reverses adaptive immune resistance induced by IR-dependent upregulation of CD8 TIL PD-1 and PD-L1 within the TME on both tumor and other infiltrating immune cells.
By direct comparison, our results are among the first to definitively demonstrate local immunosuppression, measured by reduction in effector immune infiltration in the TME and TDLN tumor-specific T-lymphocyte responses, following daily fractionated IR (2Gyx10). The demonstration of modest but statistically significant CD8+ cell-dependent abscopal but not primary tumor control following 2Gyx10 IR plus PD-1 mAb further supports local immunosuppression mediated by this IR regimen. Dovedi et al. demonstrated enhanced tumor control following the addition of PD-1 or PD-L1 mAb to CT26 colon carcinoma, 4T1 breast carcinoma and 4434 melanoma tumor-bearing mice treated with 2Gyx5 IR along with IFN-dependent induction of tumor cell PD-L1 expression.21 While one explanation for why 5 consecutive days of low-dose IR appears to positively enhance anti-tumor immunity in this report could be variability between models, another possibility could be length of treatment. Our results demonstrate more profound loss of tumor-specific T-lymphocyte responses at 10 days after the start if IR (corresponding to the last day of 2Gyx10 IR) compared to 5 days after the start of IR. It is possible that stopping daily IR after 5 days would have resulted in less immunosuppression and more robust tumor control in MOC1 and MC38-CEA tumor-bearing mice. We also cannot rule out that daily handling of the mice receiving daily radiotherapy contributed in some way to the observed immunosuppression, but feel this is unlikely given that all mice were handled often and similarly for tumor measurements. Mechanistically, immunosuppression following daily fractionated IR is likely due to circulation of inherently IR sensitive T-lymphocytes through the radiation field over many consecutive days.23,24 Alternatively, Kuo et al. have demonstrated that tumor secreted galectin-1, which can induce T-lymphocyte apoptosis, is increased following irradiation.11 While not measured here, possibly variable galectin-1 release following different IR regimens in vivo could be interesting to explore.
Our findings of decreased peripheral and TME accumulation of gMDSCs following 8Gyx2 but not 2Gyx10 IR have significant implications for the MOC1 model of HNSCC, where local immunosuppression within the TME is primarily driven by gMDSCs.16,25 Reduction of MDSCs within the TME following IR has been attributed to the myeloid polarizing activity and direct cytotoxicity of TIL derived IFNγ and TNFα, respectively.13,26 Based upon expression of the myeloid chemokines VEGF and CXCL1 within the TME,27,28 reduced chemotaxis does not appear to be the dominant explanation for reduced gMDSC within the MOC1 TME. We demonstrated enhanced local IFN levels within the TME and enhanced IFNγ production capacity in TDLN T-lymphocytes of mice treated with 8Gyx2 IR in both models, but enhanced TNFα in the TME of treated MOC1 tumors only. This correlation suggests that the ability of IR to reduce MDSC levels within the TME may be linked to TNF production. Our analysis did not differentiate between tumor, immune or stromal cell production of TNFα13,29. Our finding of reduced gMDSC in the periphery (spleen) following local IR is less well described, but could have significant implications for immunosuppression within distant or abscopal tumors and deserves further study. Induction of Tregs following IR is often discussed and originates from a report by Schaue et al. demonstrating increased accumulation of peripheral Tregs following increasing doses of IR.30 Our findings of no to minimal changes in accumulation of Tregs within the TME of either model with either IR regimen are consistent with the findings of others13,31 and suggests that the number of infiltrating Tregs does not likely serve as a major resistance mechanism to immune activation following IR treatment in these models.
Our head-to-head comparison of hypofractionated high dose and daily fractionated low dose IR has significant implications for the rational design of clinical trials investigating combination IR and immunotherapy. The two IR regimens utilized in this study were not biologically equivalent doses (assuming an α/β of 10), and they were not intended to be. Our aim was to compare standard-of-care daily fractionated IR with hypofractionated, higher dose IR, similar to that used for stereotactic body radiation therapy (SBRT). The well-established standard of care approach to treatment of head and neck cancer with radiotherapy consists of 35 daily fractions of low dose (1.8–2 Gy) IR, and HNSCC patients commonly demonstrate lymphopenia following definitive IR treatment.10 There are currently at least 7 clinical trials underway investigating the combination of daily, low-dose fractionated IR and ICB (either PD-1 or CTLA-4 mAb) in patients with previously untreated, locoregionally advanced HNSCC (clinicaltrials.gov). While these studies will likely yield useful clinical and immune correlative information, data such as ours may provide one explanation if these combinations fail to demonstrate enhanced locoregional control or disease-specific survival or evidence of significant anti-tumor immune activity in immune correlative analyses.
Cumulatively, these data add to mounting evidence that different IR regimens can induce profoundly different effects on anti-tumor immunity, and that in the context of using IR in combination with immunotherapy such as ICB, single or hypofractionated high dose IR better induces anti-tumor immunity. Such evidence should be considered in the design of clinical trials combining IR and ICB moving forward.
Materials and methods
Tumor cell culture and in vivo tumor growth
Mouse oral cancer (MOC1) cells were obtained from R. Uppaluri (Washington University in St. Louis) in 2014. MOC1 and MC38-CEA cells were developed and cultured as described,32–35 mycoplasma free and used for all experiments at early passage number. Cells were harvested with TrypLE Select (Thermo) to minimize epitope loss. All experiments were approved by the NIDCD Animal Care and Use Committee. Tumors were established by injecting cells subcutaneously into wild-type C57 BL/6 mice (WT B6; Taconic) and tumor volume was calculated as: (length2 × width)/2. For all irradiation experiments, mice were engrafted with tumor in the right leg. For abscopal experiments, mice were simultaneously engrafted with tumors in the right leg and left flank. In some experiments, mice were treated with PD-1 or CD8 mAb (BioXCell) via intraperitoneal injection.
Irradiation
Non-anaesthetized mice bearing tumors were secured into custom lead-shielded jigs that expose the leg alone to radiation, and irradiated with 2Gyx10 (2 Gy 5 days on, 2 off, 5 on) or 8Gyx2 (8 Gy twice 2 days apart) using a Pentak XRAD320 X-ray irradiator (Precision X-ray, Inc.) at a dose-rate of 2.8 Gy/min. Tumors were allowed to engraft to 0.1 cm3 (5–7 mm) before the start of treatment.
Flow cytometric analysis
To process tissues for flow, spleens were crushed between frosted slides and filtered (40 μM). Tumors were minced, digested using a mouse tumor dissociation kit (Miltenyi) per manufacturer protocol and filtered (70 μM). Following CD16/32 block (Biolegend), single cell suspensions were stained with primary antibodies. Fluorophore-conjugated primary antibodies included anti-mouse CD45.2 clone 104, CD11b clone M1/70, Ly-6 C clone HK1.4, Ly-6G clone 1 A, CD8 clone 53–6.7, NK1.1 clone PK136, CD4 clone GK1.5, FoxP3 clone FJK-16 s, CD11 c clone N418, F4/80 clone BM8, CD107 a clone 1D4B, CD25 clone PC61.5.3, PD-1 clone 29 F.1A12, CD3 clone 145-2C11, PD-L1 clone 10 F.9 G2, PDCA clone 129c1, and H2-KbDb clone 28-8-6. Cells were stained with antibodies for one hour, washed, and analyzed by flow cytometry on a BD Canto using BD FACS Diva software. All cells stained for cell surface marker analysis were stained with 7AAD to determine viability, and isotype controls and a “fluorescence minus one” method were used to determine staining specificity. FoxP3+ regulatory CD4+ T-lymphocytes (Tregs) were stained using the Mouse Regulatory T Cell Staining Kit #1 (eBioscience) per manufacturer protocol. Post-acquisition analysis was performed with FlowJo vX10.0.7r2.
qRT-PCR
Whole tumor lysates were generated using the Tissue Lyser II and RNA was purified using the RNEasy Mini Kit (Qiagen) per the manufacturer's protocol. cDNA was synthesized utilizing a high capacity cDNA reverse transcription kit with RNase inhibitor (Applied Biosystems). A Taqman Universal PCR master mix was used to assess the relative expression of target genes compared to GAPDH on a Viia7 qPCR analyzer (Applied Biosystems). All primers were purchased from Thermo Scientific.
Tumor-specific T-lymphocyte IFN production
T-lymphocytes were sorted from tumor draining lymph nodes (TDLN) using negative magnetic separation (Pan T Cell Isolation Kit II, Miltenyi). T-lymphocytes were consistently enriched from lymph node to ≥90% by flow cytometry. Sorted T-lymphocytes were added to IFNγ pretreated (24 hours, 20 ng/mL) and irradiated (50 Gy) parental tumor cells at a 10:1 E:T ratio and 48 hour supernatants were assessed for IFNγ concentration by ELISA (eBioscience) per manufacturer protocol.
Supplementary Material
Disclosure/conflict of interest
The authors declare no conflict of interest.
Acknowledgments
We thank Drs. Ravindra Uppaluri and Nicole Schmitt for their critical review of this manuscript.
Financial support
This work was supported by the Intramural Research Program of the NIH, NIDCD, project number ZIA-DC000087. MM was supported by through the National Institutes of Health Medical Research Scholars Program, a public-private partnership supported jointly by the NIH and generous contributions to the Foundation for the NIH from Pfizer Inc., The Doris Duke Charitable Foundation, The Newport Foundation, The American Association for Dental Research, The Howard Hughes Medical Institute, and the Colgate-Palmolive Company, as well as other private donors.
References
- 1.Seiwert TY, Burtness B, Mehra R, Weiss J, Berger R, Eder JP, Heath K, McClanahan T, Lunceford J, Gause C, et al.. Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): an open-label, multicentre, phase 1b trial. Lancet Oncol. 2016;17:956–65. doi: 10.1016/S1470-2045(16)30066-3. PMID:27247226 [DOI] [PubMed] [Google Scholar]
- 2.Ferris RL, Blumenschein G Jr, Fayette J, Guigay J, Colevas AD, Licitra L, Harrington K, Kasper S, Vokes EE, Even C, et al.. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N Engl J Med. 2016;375:1856–67. doi: 10.1056/NEJMoa1602252. PMID:27718784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taube JM, Klein A, Brahmer JR, Xu H, Pan X, Kim JH, Chen L, Pardoll DM, Topalian SL, Anders RA. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin Cancer Res. 2014;20:5064–74. doi: 10.1158/1078-0432.CCR-13-3271. PMID:24714771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bourhis J, Overgaard J, Audry H, Ang KK, Saunders M, Bernier J, Horiot JC, Le Maître A, Pajak TF, Poulsen MG, et al.. Hyperfractionated or accelerated radiotherapy in head and neck cancer: a meta-analysis. Lancet. 2006;368:843–54. doi: 10.1016/S0140-6736(06)69121-6. PMID:16950362 [DOI] [PubMed] [Google Scholar]
- 5.Twyman-Saint/sVictor C, Rech AJ, Maity A, Rengan R, Pauken KE, Stelekati E, Benci JL, Xu B, Dada H, Odorizzi PM, et al.. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature. 2015;520:373–7. doi: 10.1038/nature14292. PMID:25754329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hodge JW, Sharp HJ, Gameiro SR. Abscopal regression of antigen disparate tumors by antigen cascade after systemic tumor vaccination in combination with local tumor radiation. Cancer Biother Radiopharm. 2012;27:12–22. doi: 10.1089/cbr.2012.1202. PMID:22283603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chakraborty M, Abrams SI, Coleman CN, Camphausen K, Schlom J, Hodge JW. External beam radiation of tumors alters phenotype of tumor cells to render them susceptible to vaccine-mediated T-cell killing. Cancer Res. 2004;64:4328–37. doi: 10.1158/0008-5472.CAN-04-0073. PMID:15205348 [DOI] [PubMed] [Google Scholar]
- 8.Deng L, Liang H, Xu M, Yang X, Burnette B, Arina A, Li XD, Mauceri H, Beckett M, Darga T, et al.. STING-Dependent Cytosolic DNA Sensing Promotes Radiation-Induced Type I Interferon-Dependent Antitumor Immunity in Immunogenic Tumors. Immunity. 2014;41:843–52. doi: 10.1016/j.immuni.2014.10.019. PMID:25517616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vanpouille-Box C, Alard A, Aryankalayil MJ, Sarfraz Y, Diamond JM, Schneider RJ, Inghirami G, Coleman CN, Formenti SC, Demaria S. DNA exonuclease Trex1 regulates radiotherapy-induced tumour immunogenicity. Nat Commun. 2017;8:15618. doi: 10.1038/ncomms15618. PMID:28598415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Campian JL, Sarai G, Ye X, Marur S, Grossman SA. Association between severe treatment-related lymphopenia and progression-free survival in patients with newly diagnosed squamous cell head and neck cancer. Head Neck. 2014;36:1747–53. doi: 10.1002/hed.23535. PMID:24174270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuo P, Bratman SV, Shultz DB, von Eyben R, Chan C, Wang Z, Say C, Gupta A, Loo BW Jr, Giaccia AJ, et al.. Galectin-1 mediates radiation-related lymphopenia and attenuates NSCLC radiation response. Clin Cancer Res. 2014;20:5558–69. doi: 10.1158/1078-0432.CCR-14-1138. PMID:25189484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Demaria S, Kawashima N, Yang AM, Devitt ML, Babb JS, Allison JP, Formenti SC. Immune-mediated inhibition of metastases after treatment with local radiation and CTLA-4 blockade in a mouse model of breast cancer. Clin Cancer Res. 2005;11:728–34. PMID:15701862 [PubMed] [Google Scholar]
- 13.Deng L, Liang H, Burnette B, Beckett M, Darga T, Weichselbaum RR, Fu YX. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest. 2014;124:687–95. doi: 10.1172/JCI67313. PMID:24382348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ruocco MG, Pilones KA, Kawashima N, Cammer M, Huang J, Babb JS, Liu M, Formenti SC, Dustin ML, Demaria S. Suppressing T cell motility induced by anti-CTLA-4 monotherapy improves antitumor effects. J Clin Invest. 2012;122:3718–30. doi: 10.1172/JCI61931. PMID:22945631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dewan MZ, Galloway AE, Kawashima N, Dewyngaert JK, Babb JS, Formenti SC, Demaria S. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin Cancer Res. 2009;15:5379–88. doi: 10.1158/1078-0432.CCR-09-0265. PMID:19706802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davis RJ, Moore EC, Clavijo PE, Friedman J, Cash HA, Chen Z, Silvin C, Van Waes C, Allen C. Anti-PD-L1 efficacy can be enhanced by inhibition of myeloid derived suppressor cells with a selective inhibitor of PI3Kdelta/gamma. Cancer Res. 2017;77(10):2607–19. doi: 10.1158/0008-5472.CAN-16-2534. PMID:28364000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lugade AA, Sorensen EW, Gerber SA, Moran JP, Frelinger JG, Lord EM. Radiation-induced IFN-gamma production within the tumor microenvironment influences antitumor immunity. J Immunol. 2008;180:3132–9. doi: 10.4049/jimmunol.180.5.3132. PMID:18292536 [DOI] [PubMed] [Google Scholar]
- 18.Garnett CT, Palena C, Chakraborty M, Tsang KY, Schlom J, Hodge JW. Sublethal irradiation of human tumor cells modulates phenotype resulting in enhanced killing by cytotoxic T lymphocytes. Cancer Res. 2004;64:7985–94. doi: 10.1158/0008-5472.CAN-04-1525. PMID:15520206 [DOI] [PubMed] [Google Scholar]
- 19.Vilain RE, Menzies AM, Wilmott JS, Kakavand H, Madore J, Guminski A, Liniker E, Kong BY, Cooper AJ, Howle JR, et al.. Dynamic changes in PD-L1 expression and immune infiltrates early during treatment predict response to PD-1 blockade in melanoma. Clin Cancer Res. 2017;23(17):5024–33. doi: 10.1158/1078-0432.CCR-16-0698. PMID:28512174 [DOI] [PubMed] [Google Scholar]
- 20.Reits EA, Hodge JW, Herberts CA, Groothuis TA, Chakraborty M, Wansley EK, Camphausen K, Luiten RM, de Ru AH, Neijssen J, et al.. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J Exp Med. 2006;203:1259–71. doi: 10.1084/jem.20052494. PMID:16636135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dovedi SJ, Adlard AL, Lipowska-Bhalla G, McKenna C, Jones S, Cheadle EJ, Stratford IJ, Poon E, Morrow M, Stewart R, et al.. Acquired resistance to fractionated radiotherapy can be overcome by concurrent PD-L1 blockade. Cancer Res. 2014;74:5458–68. doi: 10.1158/0008-5472.CAN-14-1258. PMID:25274032 [DOI] [PubMed] [Google Scholar]
- 22.Gameiro SR, Jammeh ML, Wattenberg MM, Tsang KY, Ferrone S, Hodge JW. Radiation-induced immunogenic modulation of tumor enhances antigen processing and calreticulin exposure, resulting in enhanced T-cell killing. Oncotarget. 2014;5:403–16. doi: 10.18632/oncotarget.1719. PMID:24480782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heylmann D, Rodel F, Kindler T, Kaina B. Radiation sensitivity of human and murine peripheral blood lymphocytes, stem and progenitor cells. Biochim Biophys Acta. 2014;1846:121–9. PMID:24797212 [DOI] [PubMed] [Google Scholar]
- 24.Crocenzi T, Cottam B, Newell P, Wolf RF, Hansen PD, Hammill C, Solhjem MC, To YY, Greathouse A, Tormoen G, et al.. A hypofractionated radiation regimen avoids the lymphopenia associated with neoadjuvant chemoradiation therapy of borderline resectable and locally advanced pancreatic adenocarcinoma. J Immunother Cancer. 2016;4:45. doi: 10.1186/s40425-016-0149-6. PMID:27532020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clavijo PE, Moore EC, Chen J, Davis RJ, Friedman J, Kim Y, Van Waes C, Chen Z, Allen CT. Resistance to CTLA-4 checkpoint inhibition reversed through selective elimination of granulocytic myeloid cells. Oncotarget. 2017;8(34):55804–20. PMID:28915554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Filatenkov A, Baker J, Mueller AM, Kenkel J, Ahn GO, Dutt S, Zhang N, Kohrt H, Jensen K, Dejbakhsh-Jones S, et al.. Ablative tumor radiation can change the tumor immune cell microenvironment to induce durable complete remissions. Clin Cancer Res. 2015;21:3727–39. doi: 10.1158/1078-0432.CCR-14-2824. PMID:25869387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Highfill SL, Cui Y, Giles AJ, Smith JP, Zhang H, Morse E, Kaplan RN, Mackall CL. Disruption of CXCR2-mediated MDSC tumor trafficking enhances anti-PD1 efficacy. Sci Transl Med. 2014;6:237ra67. doi: 10.1126/scitranslmed.3007974. PMID:24848257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Davis RJ, Van Waes C, Allen CT. Overcoming barriers to effective immunotherapy: MDSCs, TAMs, and Tregs as mediators of the immunosuppressive microenvironment in head and neck cancer. Oral Oncol. 2016;58:59–70. doi: 10.1016/j.oraloncology.2016.05.002. PMID:27215705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang B, Karrison T, Rowley DA, Schreiber H. IFN-gamma- and TNF-dependent bystander eradication of antigen-loss variants in established mouse cancers. J Clin Invest. 2008;118:1398–404. doi: 10.1172/JCI33522. PMID:18317595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schaue D, Ratikan JA, Iwamoto KS, McBride WH. Maximizing tumor immunity with fractionated radiation. Int J Radiat Oncol Biol Phys. 2012;83:1306–10. doi: 10.1016/j.ijrobp.2011.09.049. PMID:22208977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rodriguez-Ruiz ME, Rodriguez I, Garasa S, Barbes B, Solorzano JL, Perez-Gracia JL, Labiano S, Sanmamed MF, Azpilikueta A, Bolaños E, et al.. Abscopal effects of radiotherapy are enhanced by combined immunostimulatory mAbs and are dependent on CD8 T cells and crosspriming. Cancer Res. 2016;76:5994–6005. doi: 10.1158/0008-5472.CAN-16-0549. PMID:27550452 [DOI] [PubMed] [Google Scholar]
- 32.Cash H, Shah S, Moore E, Caruso A, Uppaluri R, Van Waes C, Allen C. mTOR and MEK1/2 inhibition differentially modulate tumor growth and the immune microenvironment in syngeneic models of oral cavity cancer. Oncotarget. 2015;6:36400–17. PMID:26506415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Farsaci B, Higgins JP, Hodge JW. Consequence of dose scheduling of sunitinib on host immune response elements and vaccine combination therapy. Int J Cancer. 2012;130:1948–59. doi: 10.1002/ijc.26219. PMID:21633954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Judd NP, Winkler AE, Murillo-Sauca O, Brotman JJ, Law JH, Lewis JS Jr, Dunn GP, Bui JD, Sunwoo JB, Uppaluri R. ERK1/2 regulation of CD44 modulates oral cancer aggressiveness. Cancer Res. 2012;72:365–74. doi: 10.1158/0008-5472.CAN-11-1831. PMID:22086849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Robbins PF, Kantor JA, Salgaller M, Hand PH, Fernsten PD, Schlom J. Transduction and expression of the human carcinoembryonic antigen gene in a murine colon carcinoma cell line. Cancer Res. 1991;51:3657–62. PMID:1712245 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.