Abstract
Background
Facial port-wine stains (PWS) are considered by some an aesthetic skin problem, yet impact on quality of life (QoL) has not been objectively documented.
Objective
We sought to (1) characterize the effect of PWS on QoL in adults, (2) to identify the clinical and demographic factors that affect QoL, and (3) to compare our results with QoL studies in other skin conditions.
Methods
In total, 244 adults with facial PWS completed an online QoL survey, which included the Skindex-29 instrument.
Results
QoL in adults with facial PWS was diminished, especially from an emotional perspective. Variables associated with reduced QoL in all Skindex-29 subdomains included comorbid depression, limited facial mobility, and presence of other skin conditions. Persons with hypertrophy had more emotional and symptomatic impairment. The composite dermatologic-specific QoL scores were similar to those of cutaneous T-cell lymphoma, rosacea, alopecia, and vitiligo.
Limitations
Selection bias was a potential limitation, as participants were primarily recruited from patient support groups.
Conclusion
Our analysis demonstrates that the presence of a facial PWS has a significant negative impact on QoL. Dermatologists caring for patients with PWS should inquire about QoL, provide appropriate support and resources, and consider QoL when discussing treatment options and obtaining authorization for these procedures.
Keywords: port-wine stain, quality of life, Skindex-29, Sturge-Weber Syndrome, Klippel-Trenaunay Syndrome
Port-wine stains (PWS) are congenital birthmarks that reflect embryonic vascular development abnormalities.1 The estimated incidence of PWS is 0.3% in newborns.2 At birth, PWS typically presents as pink-to-red macules or patches, though occasionally they appear hypertrophic. The lesions grow proportionally with age and often progressively darken to deep red or purple. By 46 years of age, two-thirds of affected individuals develop soft tissue overgrowth and nodules, causing disfigurement, asymmetry, and spontaneous bleeding.3–5 These changes are attributed to progressive dilation of the malformed vasculature. Although PWS can be sporadic and isolated, it might also be associated with other vascular anomalies and genetic syndromes including Sturge-Weber syndrome and Klippel-Trenaunay syndrome.
A growing body of literature illustrates that skin disease can profoundly influence quality of life (QoL).6–8 Although isolated PWS are generally thought to be asymptomatic (i.e., without pruritus, pain, major functional impairment), previous studies have demonstrated adversely impaired QoL in similarly asymptomatic skin conditions, such as vitiligo and alopecia.9–11 The distinct appearance of PWS and potential complications might significantly impact a person’s psychosocial development and well-being.12,13 Furthermore, the face is intimately associated with personal identity and social interactions. Several publications have shown a negative impact on health-related QoL and psychological adjustment in individuals with facial PWS or other facial differences including cleft lip, skeletal deformities, and scars.14,15 However, these studies did not use validated dermatologic-specific QoL instruments, making it difficult to ascertain the influence of other comorbidities on an individual’s QoL and to compare these results with QoL studies in other skin conditions.
We evaluated the impact of facial PWS on QoL in affected adults using the standardized QoL tool, Skindex-29.16 Furthermore, we examined the independent demographic and clinical factors that influenced QoL and compared our findings to published QoL studies on other dermatologic conditions.
We hypothesized that QoL would be significantly impacted by the presence of a facial PWS, and would be similar to that of other highly visible skin conditions, such as alopecia and vitiligo. We predicted that diminished QoL would correlate with a higher percentage of affected total body surface area (TBSA) and diagnosis of an underlying syndrome. Finally, we expected to find improved QoL with persons who had received laser treatment for their PWS, especially if initiated during infancy or early childhood.
MATERIALS AND METHODS
Survey methods and target population
A 62-question anonymous survey was administered via REDCap, a secure web interface for data collection and management.17 The survey was developed by the authors and piloted among a small cohort of adults with facial PWS. Participants were recruited through the Vascular Birthmarks Foundation, Sturge-Weber Foundation, and Klippel-Trenaunay Foundation via email groups, Facebook groups, and newsletters. Approval was obtained from these organizations prior to distribution of the survey. Recruitment fliers were also displayed in the Beckman Laser Institute Dermatology Clinic at the University of California Irvine. Eligibility criteria included being an adult 18 years or older with a facial PWS and the ability to read and respond to questions independently. This study was granted Institutional Review Board approval by the University of Minnesota (No. 1501P60561, September 15, 2015) and University of California, Irvine (No. 2015–2350, November 4, 2015).
Survey content
The main outcome measure of the survey was the Skindex-29, a widely used and validated dermatology-specific QoL questionnaire that addresses 3 independent domains: emotions, symptoms, and functioning. Item scores were transformed into a scale from 1 to 100 with higher scores indicating higher impact of skin disease.16 We used the cut off values proposed by Nijsten et al to categorize QoL with the Skindex-29 (e.g., very little, mild, moderate, severe, and extremely severe).18
Independent measures addressed 5 additional areas: (1) demographics including education level, (2) socialization with others, (3) medical and dermatologic comorbidities, (4) assessment of PWS clinical severity, and (5) treatment of PWS. Queried comorbid dermatologic conditions included acne, eczema, psoriasis, skin cancer, and other with the option to fill in text. Disease severity was determined by patient estimation of affected TBSA using the approximation that palm surface area roughly equals 1% TBSA.19 We used a figure to ascertain the facial distribution and bilaterality of participants’ PWS (Fig 1); they were able to select one or more facial regions to indicate the area(s) affected.
Fig 1.
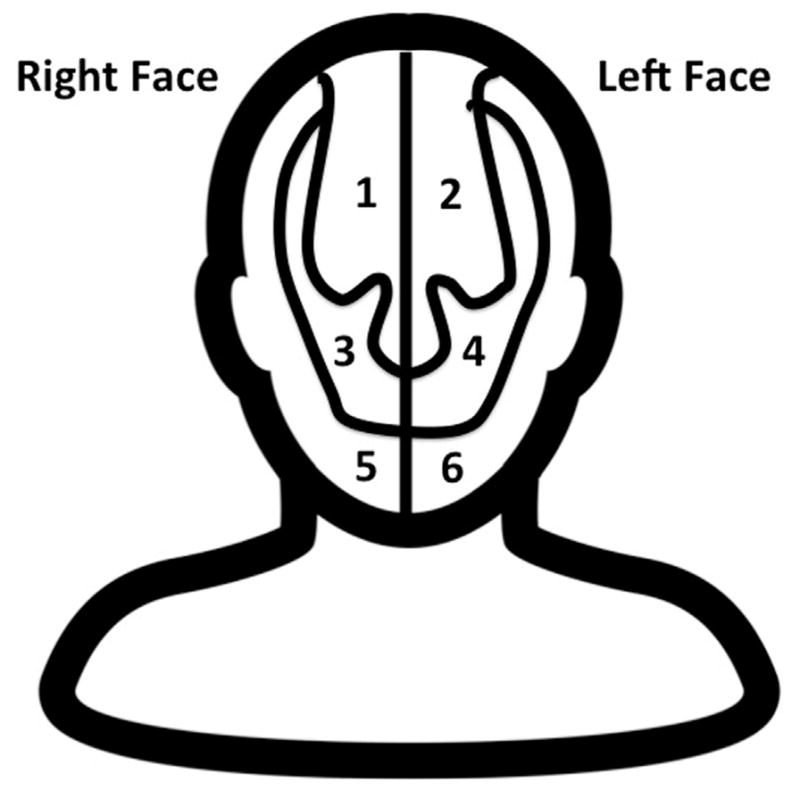
Figure shown to participants to help them indicate the distribution of their facial PWS.
Analysis
Descriptive statistics were used to analyze the survey sample. Domain scores and a composite score for the Skindex-29 were calculated according to the scoring procedures.16 These scores were the outcome variables. Demographic and clinical characteristics were used as independent variables. Simple linear regression and analysis of variance models were used to assess univariate associations between each outcome and the set of independent variables. Multiple linear regression models were used to determine which independent variables remained significant while adjusting for other variables. The independent variables used in each model were determined using a stepwise selection procedure. P values less than .05 were considered statistically significant. Data were analyzed using SAS V9.3 (SAS Institute Inc, Cary, NC).
RESULTS
Patient characteristics
In total, 265 adults with facial PWS attempted the survey between November 7, 2015, and March 1, 2016. Of these, 244 (90.0%) completed the survey and were included in the final analyses. A majority were Caucasian (86.1%) and female (74.6%) (Table I). Participant age ranged from 18 to 75 years (mean, 38.9 years; standard deviation [SD] = 13.2). Disease severity was estimated using percent affected TBSA (mean 8.0; SD = 12.9). Seventy-six participants (31.2%) reported involvement of body region(s) beyond the face (Table II). A majority (77.1%) reported undergoing ≥1 laser treatment(s) of their facial PWS (Table III). Of those who received laser treatment, the average age at first treatment was 15.5 years (SD = 14.3).
Table I.
Patient social and demographic characteristics
| Study population N = 244 |
% | |
|---|---|---|
| Sex | ||
| Male | 62 | 25.4 |
| Female | 182 | 74.6 |
| Age, years | ||
| Mean | 38.9 | - |
| Ethnicity | ||
| Caucasian | 210 | 86.1 |
| African American | 6 | 2.5 |
| Asian | 11 | 4.5 |
| Hispanic/Latino | 13 | 5.3 |
| Other | 12 | 4.9 |
| Highest level of education | ||
| <12th grade | 13 | 5.3 |
| High school or GED | 34 | 13.9 |
| Some college | 65 | 26.6 |
| College graduate | 63 | 25.8 |
| Graduate or professional degree | 69 | 28.3 |
| Special education services | ||
| Yes | 28 | 11.5 |
| Medical comorbidities | ||
| Anxiety | 82 | 33.6 |
| Depression | 64 | 26.2 |
| Headaches | 53 | 21.7 |
| Migraines | 41 | 16.8 |
| Seizures | 28 | 11.5 |
| Learning disability | 21 | 8.6 |
| Autism | 3 | 1.2 |
| Relationship status | ||
| Single | 73 | 29.9 |
| Dating | 24 | 9.8 |
| Married/engaged | 125 | 51.2 |
| Divorced | 18 | 7.4 |
| Widowed | 4 | 1.6 |
| Number of close friends | ||
| 0 | 7 | 2.9 |
| 1–3 | 91 | 37.3 |
| 4–6 | 77 | 31.6 |
| 7–9 | 31 | 12.7 |
| 10≤ | 38 | 15.6 |
| Frequency of socializing | ||
| <1 x/week | 54 | 22.2 |
| 1 x/week | 38 | 15.6 |
| 2–3 x/week | 70 | 28.8 |
| 4–5 x/week | 29 | 11.9 |
| 6 ≤ x/week | 52 | 21.4 |
| Do you associate a negative connotation with the word “stain”? | ||
| Yes | 102 | 41.8 |
| Preferred name for PWS | ||
| Port-wine stain | 90 | 36.9 |
| Port-wine birthmark | 88 | 36.1 |
| Vascular birthmark | 33 | 13.5 |
| Vascular malformation | 15 | 6.2 |
| Other | 18 | 7.4 |
GED, General Education Diploma; PWS, port-wine stain.
Table II.
Port-wine stain disease characteristics
| Study population N = 244 |
% | |
|---|---|---|
| Association with a syndrome | ||
| No | 141 | 57.8 |
| Yes, Sturge Weber Syndrome | 80 | 32.8 |
| Yes, Klippel-Trenaunay Syndrome | 16 | 6.6 |
| Yes, other | 7 | 2.9 |
| Facial location | ||
| Unilateral | 200 | 82.0 |
| Bilateral | 44 | 18.0 |
| Total number of areas involved* | ||
| 1 | 90 | 36.9 |
| 2 | 71 | 29.1 |
| 3 | 48 | 19.7 |
| 4 | 15 | 6.2 |
| 5 | 3 | 1.2 |
| 6 | 17 | 7.0 |
| Other areas of involvement (n = 76) | ||
| Neck | 68 | 27.9 |
| Arm or hand | 52 | 21.3 |
| Leg or foot | 47 | 19.3 |
| Back/trunk | 25 | 10.3 |
| Genitals | 16 | 6.6 |
| Hypertrophy of PWS | ||
| Yes | 88 | 36.1 |
| Texture of PWS | ||
| Normal skin | 170 | 69.7 |
| Papules | 61 | 25.0 |
| Nodules | 13 | 5.3 |
| Limitation of facial mobility | ||
| Yes | 54 | 22.1 |
| Color of PWS | ||
| Light pink | 10 | 4.1 |
| Pink | 78 | 32.0 |
| Red | 52 | 21.3 |
| Light purple | 69 | 28.3 |
| Deep purple | 35 | 14.3 |
| Body surface area of PWS† | ||
| <1% | 31 | 12.7 |
| 1%–5% | 158 | 64.8 |
| 6%–10% | 16 | 6.6 |
| 11%–15% | 9 | 3.7 |
| 16%–25% | 6 | 2.5 |
| 26%–50% | 10 | 4.1 |
| >50% | 14 | 5.7 |
Table III.
Treatment of facial port-wine stains
| Underwent laser treatment n = 188 | % (Total = 77%) | |
|---|---|---|
| Most recent treatment | ||
| ≤6 months | 33 | 17.7 |
| 7–12 months | 16 | 8.6 |
| 2–5 years | 30 | 16.0 |
| >5 years | 108 | 57.8 |
| Number of treatments | ||
| 1 | 9 | 4.8 |
| 2–10 | 65 | 34.6 |
| 11–20 | 40 | 21.3 |
| 21–30 | 25 | 13.3 |
| 31–50 | 28 | 14.9 |
| 51–100 | 9 | 4.8 |
| >100 | 12 | 6.4 |
| Age at first treatment | ||
| 0–3 months | 14 | 7.5 |
| 4–6 months | 8 | 4.3 |
| 7–11 months | 5 | 2.7 |
| 1–5 years | 30 | 16.0 |
| 6–10 years | 25 | 13.3 |
| 11–17 years | 45 | 23.9 |
| 18–25 years | 24 | 12.8 |
| 26–40 years | 23 | 12.2 |
| 41–60 years | 13 | 6.9 |
| >60 years | 1 | 0.5 |
| Positive impact | ||
| Yes | 114 | 60.6 |
| Perceived impact on PWS | ||
| Improved | 127 | 67.6 |
| Stayed same | 58 | 30.9 |
| Worsened | 3 | 1.6 |
PWS, Port-wine stain.
Patients’ preferred terminology
Most respondents preferred that health care providers use the terms “port-wine stain” (36.9%) or “port-wine birthmark” (36.1%) when discussing their skin condition. Less frequently, respondents favored “vascular birthmark” (13.5%) or “vascular malformation” (6.2%). One hundred and two (41.8%) respondents reported a perceived negative connotation with the word “stain” in reference to their skin condition.
Skindex-29 in patients with PWS
The mean Skindex-29 composite score in patients with facial PWS was 24.6 (SD = 19.1), indicating that overall the presence of a facial PWS had a moderate negative influence on QoL. The mean Skindex-29 subscores were 34.4 ± 25.8 (emotions), 14.9 ± 18.4 (symptoms), and 24.3 ± 22.3 (functioning). These scores show that the most significant adverse impact was on the emotional realm, followed by functioning and symptoms.
Univariate associations with facial port-wine stain
Univariate associations of demographic and clinical measures are presented in Table IV. Women had more emotional (P = .0295) and symptomatic (P = .0134) impairment in relation to their facial PWS than men. Number of close friends was inversely related to the emotions score (P = .0333). Participants who reported fewer social engagements had higher subscale and composite scores (composite, P = .0004). Participants with comorbid skin conditions were more adversely affected in all 3 subdomains (emotions, P = .0155; symptoms, P <.0001; functioning P = .0382) than those without other skin diseases. Anxiety and depression were the most commonly reported co-morbidities overall and were associated with higher subscale and composite scores (composite score for anxiety and depression, P <.0001).
Table IV.
Univariate associations* of demographic and clinical measures with Skindex-29 outcomes
| Emotions | Symptoms | Functioning | Composite score | |||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||
| Variable | Beta (SE) | P value | Beta (SE) | P value | Beta (SE) | P value | Beta (SE) | P value |
| Age, years | −0.0 (0.1) | .9778 | 0.3 (0.1) | .0008 | 0.2 (0.1) | .0772 | 0.2 (0.1) | .0815 |
| Sex | .0295 | .0134 | .8648 | .0661 | ||||
| Female | 8.2 (3.8) | 6.7 (2.7) | 0.6 (3.3) | 5.2 (2.8) | ||||
| Male | ref | ref | ref | ref | ||||
| White | −7.7 (4.8) | .1074 | −1.8 (3.4) | .5940 | −9.8 (4.1) | .0165 | −6.4 (3.5) | .0674 |
| Education | .2084 | .0199 | .2932 | .0789 | ||||
| <High school | 1.7 (7.8) | 3.0 (5.5) | 9.5 (6.7) | 4.7 (5.7) | ||||
| High school/GED | 12.8 (5.4) | 12.2 (3.8) | 9.2 (4.7) | 11.4 (4.0) | ||||
| Some college | 4.1 (4.4) | 7.4 (3.1) | 3.2 (3.8) | 4.9 (3.3) | ||||
| College | 2.5 (4.5) | 5.6 (3.1) | 2.9 (3.9) | 3.6 (3.3) | ||||
| Graduate | ref | ref | ref | ref | ||||
| Comorbid skin condition | 8.2 (3.4) | .0155 | 11.4 (2.3) | <.0001 | 6.1 (2.9) | .0382 | 8.6 (2.5) | .0006 |
| Anxiety | 14.1 (3.4) | <.0001 | 7.0 (2.5) | .0049 | 11.3 (2.9) | .0002 | 10.8 (2.5) | <.0001 |
| Depression | 21.1 (3.5) | <.0001 | 7.4 (2.6) | .0056 | 17.9 (3.0) | <.0001 | 15.4 (2.6) | <.0001 |
| Special education | −11.7 (5.1) | .0232 | 1.0 (3.7) | .7866 | −3.8 (4.5) | .4022 | −4.8 (3.8) | .2077 |
| Close friends | .0333 | .0571 | .0001 | .0030 | ||||
| 0–3 | 14.4 (4.9) | 5.6 (3.5) | 17.7 (4.1) | 12.5 (3.6) | ||||
| 4–6 | 10.3 (5.1) | 6.1 (3.6) | 9.0 (4.3) | 8.5 (3.7) | ||||
| 7–9 | 8.2 (6.2) | −2.6 (4.4) | 6.0 (5.2) | 3.9 (4.5) | ||||
| 10≤ | ref | ref | ref | ref | ||||
| Socializations | .0640 | .0008 | <.0001 | .0004 | ||||
| None | 12.7 (5.0) | 10.0 (3.5) | 17.9 (4.1) | 13.5 (3.6) | ||||
| 1/week | 8.2 (5.5) | 15.1 (3.8) | 12.4 (4.6) | 11.9 (3.9) | ||||
| 2–3/week | 4.3 (4.7) | 5.3 (3.3) | 6.3 (3.9) | 5.3 (3.4) | ||||
| 4–5/week | −0.9 (5.9) | 2.7 (4.1) | −1.5 (4.9) | 0.1 (4.3) | ||||
| 6≤/week | ref | ref | ref | ref | ||||
| Negative connation of “stain” | 16.2 (3.2) | <.0001 | 7.7 (2.3) | .0011 | 14.5 (2.7) | <.0001 | 12.8 (2.3) | <.0001 |
| Associated syndrome | −2.0 (3.4) | .5567 | 6.9 (2.3) | .0034 | 0.2 (2.9) | .9404 | 1.7 (2.5) | .4869 |
| PWS severity, 1–6 | 2.1 (1.2) | .0767 | 3.4 (0.8) | <.0001 | 2.0 (1.0) | .0521 | 2.5 (0.9) | .0042 |
| Bilateral PWS | 5.7 (4.3) | .1851 | 10.9 (3.0) | .0003 | 4.7 (3.7) | .2101 | 7.1 (3.2) | .0254 |
| Size of PWS, palms | 0 (0.1) | .8318 | 0.4 (0.1) | <.0001 | 0.1 (0.1) | .6372 | 0.2 (0.1) | .1038 |
| Hypertrophy | 11.6 (3.4) | .0007 | 14.2 (2.3) | <.0001 | 10.9 (2.9) | .0002 | 12.2 (2.4) | <.0001 |
| PWS color | .0312 | <.0001 | .0031 | .0008 | ||||
| Light pink | −26.2 (9.1) | −8.8 (6.2) | −15.8 (7.8) | −16.9 (6.6) | ||||
| Pink | −3.3 (5.2) | −8.6 (3.5) | −4.7 (4.4) | −5.5 (3.8) | ||||
| Red | 0 (5.6) | −3.0 (3.8) | 1.9 (4.8) | −0.4 (4.0) | ||||
| Purple | 1.1 (5.3) | 7.2 (3.6) | 7.0 (4.5) | 5.1 (3.8) | ||||
| Dark purple | ref | ref | ref | ref | ||||
| Limited facial mobility | 15.6 (3.9) | <.0001 | 12.1 (2.7) | <.0001 | 15.1 (3.3) | <.0001 | 14.2 (2.8) | <.0001 |
| No laser treatment | −8.8 (3.9) | .0248 | −4.1 (2.8) | .1384 | −3.9 (3.4) | .2473 | −5.6 (2.9) | .0525 |
| Age of 1st laser treatment, years | 0.2 (0.1) | .1162 | 0.3 (0.1) | .0011 | 0.4 (0.1) | .0019 | 0.3 (0.1) | .0027 |
GED, General Education Diploma; PWS, port-wine stain; ref, reference standard for statistical tests; SE, standard error.
Simple linear regression/ANOVA models.
The presence of tissue hypertrophy (P <.0001) and size of PWS (P <.0001) were associated with higher symptom scores. Bilateral facial PWS correlated with higher symptom (P = .0003) and composite (P = .0254) scores.
Patients who had never received laser treatment for their facial PWS had significantly lower scores on the emotion scale (P = .0248). The untreated group was older (mean age, 44; SD = 14) than those who had received treatment (mean age, 37; SD = 12; t-test, P = .0005). The untreated subset had less severe PWS ([mean PWS severity, 1.8; SD = 0.9] vs [mean PWS severity, 2.4; SD = 1.5]; Wilcoxon’s rank sum test, P = .0149). This group also reported fewer bilateral lesions (5.4% vs 21.8%; Fisher’s exact test, P = .0048) and less skin comorbidity (25.0% vs 41.0%; Fisher’s exact test, P = .0401).
Multivariate associations with facial port-wine stain
Several independent variables were identified as having associations with reduced QoL across all 3 subdomains, including comorbid depression (emotions, P < .0001; symptoms, P = .0175; functioning, P < .0001), limited facial mobility (emotions, P = .0006; symptoms, P = .0066; functioning, P = .0002), and presence of other skin conditions (emotions, P = .0044; symptoms, P < .0001; functioning P = .0308). Older patients (beta = −0.3; P = .0020) and those who received special education services (P = .0009) had less emotional impairment. Those with hypertrophy had more emotional (P = .0212) and symptomatic (P <.0001) impairment. Adults who reported more close friends (P = .0227) and frequent social engagements (P = .0427) had significantly less functional impairment due to their PWS.
Skin-specific QOL in facial PWS compared with other skin conditions
The Skindex-29 composite and domain scores were used to compare QoL in persons with facial PWS to 13 other dermatologic diseases and persons without skin disease (Table V).6,8,9,11,20–26 Across all Skindex-29 subscales, QoL for adults with facial PWS (composite score, 24.6) was lower than QoL for persons without skin disease (composite score, 9). Facial PWS was the third-lowest mean Skindex-29 score (composite score and subscore), with the emotion subscore being the most affected (mean, 34.4; SD = 25.8). Notably, the emotion subscore for adults with facial PWS was more adversely altered than that in those with nonmelanoma skin cancer/actinic keratosis (NMSC/AK), alopecia, rosacea, and cutaneous T-cell lymphoma (CTCL). The emotional burden for facial PWS was similar to that of rosacea, vitiligo, and epidermolysis bullosa. Patients with facial PWS were also more severely impacted in the functioning domain (mean, 24.3; SD = 22.3) than patients with NMSC/AK, vitiligo, alopecia, rosacea, acne vulgaris, CTCL, or psoriasis. Facial PWS symptom scores (mean, 14.9; SD = 18.4) were lower than all other skin conditions, except vitiligo. The composite dermatologic-specific QoL scores were similar to those of CTCL, rosacea, alopecia, and vitiligo.
Table V.
Skindex-29 scores of adults with facial port-wine stain and other dermatologic conditions
| Diagnosis | Sample size | Symptoms, mean (SD) | Emotions, mean (SD) | Functioning, mean (SD) | Composite |
|---|---|---|---|---|---|
| Vulvodynia20 | 280 | 50.0 (17.0) | 50.0 (20.0) | 44.0 (22.0) | 48.0 |
| DM26 | 41 | 44.9 (24.3) | 50.4 (26.1) | 28.2 (26.6) | 41.2 |
| CLE26 | 178 | 41.3 (23.8) | 49.1 (27.8) | 28.4 (25.6) | 39.6 |
| Epidermolysis Bullosa22 | 75 | 49.0 (25.0) | 35.0 (26.0) | 31.0 (24.0) | 38.3 |
| Eczema21 | 102 | 48.0 (23.0) | 41.0 (27.0) | 26.0 (26.0) | 38.3 |
| Pemphigus8 | 126 | 37.0 (22.0) | 37.0 (22.0) | 33.0 (23.0) | 35.7 |
| Psoriasis21 | 44 | 42.0 (21.0) | 39.0 (27.0) | 23.0 (27.0) | 34.7 |
| Acne vulgaris21 | 63 | 30.0 (19.0) | 41.0 (25.0) | 16.0 (16.0) | 29.0 |
| CTCL25 | 95 | 32.0 (23.0) | 29.0 (18.0) | 22.0 (22.0) | 27.7 |
| Rosacea26 | 29 | 33.0 (20.0) | 33.0 (20.0) | 16.0 (18.0) | 27.3 |
| Facial PWS | 244 | 14.9 (18.4) | 34.4 (25.8) | 24.3 (22.3) | 24.6 |
| Alopecia26 | 7 | 31.0 (24.0) | 27.0 (33.0) | 14.0 (23.0) | 24.0 |
| Vitiligo26 | 245 | 13.9 (14.6) | 35.9 (23.6) | 16.7 (19.5) | 22.2 |
| NMSC/AK26 | 136 | 29.0 (20.0) | 20.0 (19.0) | 9.0 (14.0) | 19.3 |
| No skin disease26 | 107 | 14.0 (12.0) | 9.0 (13.0) | 4.0 (8.0) | 9.0 |
Diagnoses are listed in order of highest to lowest composite Skindex-29 scores.
Bold underscores the results of this study as compared to previously published studies.
AK, Actinic keratosis; CLE, cutaneous lupus erythematosus; CTCL, cutaneous T-cell lymphoma; DM, dermatomyositis; NMSC, non-melanoma skin cancer; PWS, port-wine stain; SD, standard deviation.
DISCUSSION
We hypothesized that the presence of facial PWS would significantly affect QoL, and the effect would be similar to that of other highly visible skin conditions such as alopecia and vitiligo. We predicted that diminished QoL would correlate with greater percent affected TBSA and diagnosis of an underlying syndrome. Finally, we expected to find improved QoL in individuals who received laser treatment for their PWS, especially if initiated during infancy or early childhood.
As predicted, individuals with facial PWS had similarly adversely affected QoL to patients with other dermatologic conditions such as alopecia areata and vitiligo, supporting the conclusion that these diseases are not simply cosmetic but can profoundly influence a person’s emotional and physical well-being.
Our results indicate that facial PWS, regardless of the presence of an associated genetic syndrome, impacted QoL in all domains (emotions, symptoms, and functioning), but it most significantly influenced the emotion domain.
In our analysis, young age was associated with lower QoL, specifically with respect to emotions. This result is similar to a recent study examining QoL in patients with cutaneous lupus erythematosus, in which young age correlated with reduced QoL.21 These findings suggest that older patients may have improved coping mechanisms compared with their younger counterparts.
Improved emotional QoL in patients with PWS was associated with larger social circles and more frequent social engagements. This finding is unsurprising since social isolation is a known risk factor for depression. In our analysis, the presence of comorbid depression was independently associated with lower QoL scores in all 3 domains. This is concerning because depression and anxiety were commonly reported among our study population (26.2% and 33.6%, respectively). Furthermore, previous epidemiologic studies have identified a relatively high prevalence of psychiatric disorders in patients with a variety of dermatologic skin conditions.27 Studies have shown that QoL predicts mental health more accurately than dermatologic disease severity.27,28 This concept was illustrated in our analyses: QoL was associated with depression but not with disease severity (size of PWS).
We were not able to assess whether laser treatment improved QoL because the untreated subset had less severe disease, less bilaterality of their PWS, and fewer skin comorbidities. Furthermore, a majority of study responders initiated treatment at a relatively older age and early treatment is thought to achieve better results.
Factors such as tissue hypertrophy and decreased facial mobility were associated with low QoL. Early laser treatment of PWS might reduce the severity of these factors and the likelihood and severity of other unwanted outcomes, such as development of nodules, and psychosocial morbidity.3,29 Many experts support initiation of laser treatment in infancy for best results.30,31 The average age of first laser treatment in our study population was relatively old (15.5 years), well beyond the recommended age window for initiation of treatment. Future studies will have to evaluate the impact of early treatment on QoL.
Although we are able to draw a number of important conclusions from this study, several limitations should be acknowledged. First, the primary recruitment sources were various patient support and advocacy groups, the persons from which might not represent the facial PWS population as a whole (selection bias). Second, only adults who were able to read and respond to questions independently were included in the study, which again might not represent our study population and contribute to selection bias. Third, the higher frequency of female respondents could make our findings less generalizable to the PWS population as a whole. Finally, clinical severity (affected TBSA, bilaterality of facial lesion, and presence of associated tissue hypertrophy) was determined by patients, rather than by a dermatologist, and might therefore be inaccurate.
In conclusion, our analysis demonstrates that the presence of a facial PWS has a significant negative affect on QoL. Dermatologists caring for patients with PWS should inquire about QoL, provide appropriate support and resources, and consider QoL when discussing treatment options and obtaining authorization for these procedures.
Acknowledgments
Funding source: Research was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health Award Number UL1TR000114.
Thanks to Karen Ball and Anne Howard of the Sturge-Weber Foundation and Dr Linda Rozell-Shannon of the Vascular Birthmark Foundation, the Klippel-Trenaunay Foundation, and University of California Irvine Dermatology Clinic for generously distributing study recruitment materials. Scott Lunos provided statistical support. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations used
- PWS
port-wine stain
- QoL
quality of life
- TBSA
total body surface area
Footnotes
Conflicts of interest: Dr Kristen Kelly received research support from the Sturge-Weber Foundation and the NIH (HD065536); research funding from the Sturge-Weber Foundation; equipment to perform research from Candela-Synderon, Light Sciences Oncology, and Novartis; and consultant fees from MundiPharma. Dr Dorota Korta, Solveig Hagen, and Katherine Grey have nothing to disclose.
References
- 1.Waelchli R, Aylett SE, Robinson K, Chong WK, Martinez AE, Kinsler VA. New vascular classification of port-wine stains: improving prediction of Sturge-Weber risk. Br J Dermatol. 2014;171(4):861–867. doi: 10.1111/bjd.13203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jacobs AH, Walton RG. The incidence of birthmarks in the neonate. Pediatrics. 1976;58(2):218–222. [PubMed] [Google Scholar]
- 3.Geronemus RG, Ashinoff R. The medical necessity of evaluation and treatment of port-wine stains. J Dermatol Surg Oncol. 1991;17(1):76–79. doi: 10.1111/j.1524-4725.1991.tb01597.x. [DOI] [PubMed] [Google Scholar]
- 4.Enjolras O, Riché MC. Port-wine stains. Arch Dermatol. 1985;121(7):834. [PubMed] [Google Scholar]
- 5.Minkis K, Geronemus RG, Hale EK. Port wine stain progression: a potential consequence of delayed and inadequate treatment? Lasers Surg Med. 2009;41(6):423–426. doi: 10.1002/lsm.20788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahmed A, Leon A, Butler DC, Reichenberg J. Quality-of-life effects of common dermatological diseases. Semin Cutan Med Surg. 2013;32(2):101–109. doi: 10.12788/j.sder.0009. [DOI] [PubMed] [Google Scholar]
- 7.Finlay AY. Quality of life in dermatology: after 125 years, time for more rigorous reporting. Br J Dermatol. 2014;170(1):4–6. doi: 10.1111/bjd.12737. [DOI] [PubMed] [Google Scholar]
- 8.Paradisi A, Sampogna F, Di Pietro C, et al. Quality-of-life assessment in patients with pemphigus using a minimum set of evaluation tools. J Am Acad Dermatol. 2009;60(2):261–269. doi: 10.1016/j.jaad.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 9.Amer AAA, Gao X-H. Quality of life in patients with vitiligo: an analysis of the dermatology life quality index outcome over the past two decades. Int J Dermatol. 2016;55(6):608–614. doi: 10.1111/ijd.13198. [DOI] [PubMed] [Google Scholar]
- 10.Ingordo V, Cazzaniga S, Medri M, et al. To what extent is quality of life impaired in vitiligo? A multicenter study on Italian patients using the dermatology life quality index. Dermatology. 2014;229(3):240–247. doi: 10.1159/000363407. [DOI] [PubMed] [Google Scholar]
- 11.Janković S, Perić J, Maksimović N, et al. Quality of life in patients with alopecia areata: a hospital-based cross-sectional study. J Eur Acad Dermatol Venereol. 2016;30(5):840–846. doi: 10.1111/jdv.13520. [DOI] [PubMed] [Google Scholar]
- 12.Lanigan SW, Cotterill JA. Psychological disabilities amongst patients with port wine stains. Br J Dermatol. 1989;121(2):209–215. doi: 10.1111/j.1365-2133.1989.tb01800.x. [DOI] [PubMed] [Google Scholar]
- 13.Malm M, Carlberg M. Port-wine stain–a surgical and psychological problem. Ann Plast Surg. 1988;20(6):512–516. doi: 10.1097/00000637-198806000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Masnari O, Schiestl C, Rossler J, et al. Stigmatization predicts psychological adjustment and quality of life in children and adolescents with a facial difference. J Pediatr Psychol. 2013;38(2):162–172. doi: 10.1093/jpepsy/jss106. [DOI] [PubMed] [Google Scholar]
- 15.van der Horst CM, de Borgie CA, Knopper JL, Bossuyt PM. Psychosocial adjustment of children and adults with port wine stains. Br J Plast Surg. 1997;50(6):463–467. doi: 10.1016/s0007-1226(97)90335-0. [DOI] [PubMed] [Google Scholar]
- 16.Chren M, Lasek R, Flocke S. Improved discriminative and evaluative capability of a refined version of Skindex, a quality-of-life instrument for patients with skin diseases. Arch Dermatol. 1997;133:1433–1440. [PubMed] [Google Scholar]
- 17.Harris P, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap) - A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inf. 2009;42(3):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nijsten T, Sampogna F, Abeni D. Categorization of Skindex-29 scores using mixture analysis. Dermatology. 2008;218(2):151–154. doi: 10.1159/000182253. [DOI] [PubMed] [Google Scholar]
- 19.Rhodes J, Clay C, Phillips M. The surface area of the hand and the palm for estimating percentage of total body surface area: results of a meta-analysis. Br J Dermatol. 2013;169(1):76–84. doi: 10.1111/bjd.12290. [DOI] [PubMed] [Google Scholar]
- 20.Ponte M, Klemperer E, Sahay A, Chren M. Effects of vulvodynia on quality of life. J Am Acad Dermatol. 2009;60(1):70–76. doi: 10.1016/j.jaad.2008.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klein R, Moghadam-Kia S, Taylor L, et al. Quality of life in cutaneous lupus erythematosus. J Am Acad Dermatol. 2011;64(5):849–858. doi: 10.1016/j.jaad.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tabolli S, Sampogna F, Di Pietro C, et al. Quality of life in patients with epidermolysis bullosa. Br J Dermatol. 2009;161(4):869–877. doi: 10.1111/j.1365-2133.2009.09306.x. [DOI] [PubMed] [Google Scholar]
- 23.Sampogna F, Tabolli S, Mastroeni S, et al. Quality of life impairment and psychological distress in elderly patients with psoriasis. Dermatology. 2007;215(4):341–347. doi: 10.1159/000107628. [DOI] [PubMed] [Google Scholar]
- 24.Jones-Caballero M, Chren M, Soler B, Pedrosa E, Peñas P. Quality of life in mild-to-moderate acne: relationship to clinical severity and factors influencing change with treatment. J Eur Acad Dermatology Venereol. 2007;21(2):219–226. doi: 10.1111/j.1468-3083.2006.01907.x. [DOI] [PubMed] [Google Scholar]
- 25.Sampogna F, Frontani M, Baliva G, et al. Quality of life and psychological distress in patients with cutaneous lymphoma. Br J Dermatol. 2009;160(4):815–822. doi: 10.1111/j.1365-2133.2008.08992.x. [DOI] [PubMed] [Google Scholar]
- 26.Goreshi R, Chock M, Foering K, et al. Quality of life in dermatomyositis. J Am Acad Dermatol. 2011;65(6):1107–1116. doi: 10.1016/j.jaad.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Picardi A, Abeni D, Melchi CF, Puddu P, Pasquini P. Psychiatric morbidity in dermatological outpatients: an issue to be recognized. Br J Dermatol. 2000;143(5):983–991. doi: 10.1046/j.1365-2133.2000.03831.x. [DOI] [PubMed] [Google Scholar]
- 28.Sampogna F, Picardi A, Chren M-M, et al. Association between poorer quality of life and psychiatric morbidity in patients with different dermatological conditions. Psychosom Med. 2004;66(4):620–624. doi: 10.1097/01.psy.0000132869.96872.b2. [DOI] [PubMed] [Google Scholar]
- 29.Brightman LA, Geronemus RG, Reddy KK. Laser treatment of port-wine stains. Clin Cosmet Investig Dermatol. 2015;8:27–33. doi: 10.2147/CCID.S53118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ashinoff R, Geronemus RG. Flashlamp-pumped pulsed dye laser for port-wine stains in infancy: earlier versus later treatment. J Am Acad Dermatol. 1991;24(3):467–472. doi: 10.1016/0190-9622(91)70075-d. [DOI] [PubMed] [Google Scholar]
- 31.Astner S, Anderson RR. Treating vascular lesions. Dermatol Ther. 2005;18(3):267–281. doi: 10.1111/j.1529-8019.2005.05025.x. [DOI] [PubMed] [Google Scholar]


