Abstract
Despite the increasing reports on the incidence of fresh vegetables and fruits as a possible vehicle for human pathogens, there is currently limited knowledge on the growth potential of Escherichia coli O157:H7 on different plant substrates. This study analyzed the selective adhesion and growth of E. coli O157:H7 on chili habanero (Capsicum chinense L.), cucumber (Cucumis sativus), radish (Raphanus sativus), tomato (Lycopersicon esculentum), beet (Beta vulgaris subsp. vulgaris), and onion (Allium cepa L.) under laboratory conditions. The Gompertz parameters were used to determine the growth kinetics. Scanning electron microscopy was used to visualize the adhesion of E. coli O157:H7 on the epicarp of the samples. Predictive models were constructed to compare the growth of E. coli O157:H7 on the samples with different intrinsic factors and to demonstrate the low selectivity of the pathogen. No significant difference was observed in the lag-phase duration (LPD), generation time (GT), and exponential growth rate (EGR) of the pathogen adhered to the samples. The interaction between the microorganism and the substrate was less supportive to the growth of E. coli O157:H7 for onion, whereas for tomato and cucumber, the time for the microorganism to attain the maximum growth rate (M) was significantly longer than that recorded for other samples.
Keywords: E. coli O157:H7, Adhesion, Predictive growth, Low selectivity, Fruit and vegetables
Introduction
Escherichia coli O157:H7 is a foodborne zoonotic pathogen that serving as the causative agent of hemorrhagic colitis and hemolytic uremic syndrome (HUS) in humans. This microorganism possesses a remarkable capability to get transmitted from animal reservoirs (cattle, goats, and sheep) to humans through a variety of food vehicles that plays a critical role in its pathogenicity. E. coli O157:H7 employs multiple mechanisms for colonizing vegetables through adhesion to cell surfaces. The microorganism adheres strongly to tomato skin, spinach leaves and alfalfa sprout roots by curli,1, 2 which is mediated by the filamentous type III secretion system composed of EspA filaments.3 Flagella also play a key role in the leaf attachment process in E. coli O157:H7.4 The internalization of E. coli O157:H7 has been demonstrated under controlled environmental conditions in the seedlings of temperate vegetable crops such as lettuce, tomato, cress, radish, alfalfa, and mung bean,5, 6, 7, 8 and the adhesion and internalization of E. coli O157:H7 in mature leafy vegetables have been demonstrated both experimentally and in field conditions.9, 10, 11, 12, 13
Despite the increasing incidence of fresh produce as a vehicle for human pathogens, there is a dearth of information on the growth potential of E. coli O157:H7 on different plant substrates. Although most of the outbreaks involving the occurrence of E. coli O157:H7 on leafy vegetables have been associated with cross-contamination14, 15, 16, 17 there are very few studies that have investigated the adhesion and growth of E. coli O157:H7 in other mature vegetables.18, 19, 20, 21, 22 Interestingly, many of these vegetables possess a variety of intrinsic factors that can considerably reduce the growth of foodborne microorganisms; however, E. coli O157:H7 manages to successfully survive under these varying conditions.
Assuming that the degree of mutual interaction between bacterial strain and the specific type of plant affects their association, this study aimed to investigate the capacity of E. coli O157:H7 to colonize freshly consumed vegetables and fruits under experimental conditions. The growth of the pathogen on different substrates was compared using the Gompertz equation to obtain bacterial growth curves and linear regression-derived parameters such as the lag-phase duration, maximum microbial population, and specific microbial growth.
Materials and methods
Microorganism and preparation of inoculums
Escherichia coli O157:H7 strain (SS11) used in this study was isolated from the feces of pigs from Santa Elena in Yucatán, Mexico.23 The strain was isolated after pre-enrichment with EC broth (Difco, Detroit, MI, USA) using the immunomagnetic separation procedure (IMS) employing anti-E. coli O157 Dynabeads (Dynal Biotech, Norway) as described by the manufacturer. The IMS-enriched bacterial culture (50 μL) was streaked onto Chromocult coliform agar (Merck, Darmstadt, Germany) and Sorbitol MacConkey agar (CM813; Oxoid Ltd, USA) supplemented with cefixime-potassium tellurite (SR172; Oxoid Ltd.). The phenotype of the microorganism was characterized with the API 20E system (BioMérieux, France). Strain serotype O157:H7 was identified with the immunocapture system and latex agglutination (Tecra Diagnostics, NSW, Australia). Polymerase chain reaction (PCR) was used to confirm the presence of rfbO157 and stx1 genes in the strain SS11 and to confirm the absence of stx2 verotoxin gene. For this purpose, three pairs of oligonucleotide primers were synthesized for the amplification of rfbO157 (420 bp), stx2 (584 bp) and stx1 (306 bp).24, 25, 26 The PCR products were detected by 2% agarose gel-electrophoresis at 100 V and staining with 10 μg/mL ethidium bromide.
The suspensions of E. coli (104 CFU/mL) were prepared for the inoculation of the vegetables and fruits. A single colony of E. coli O157:H7 from a streak plate was suspended in 20 mL of EC selective enrichment broth (Difco) and incubated overnight at 37 °C (16–18 h) in a shaking incubator at 150 rpm (Thermo Scientific, USA). The overnight culture (1 mL) was inoculated into 99 mL of EC broth and incubated at 37 °C till it attained the exponential phase (105 CFU/mL). The bacterial cell concentrations were estimated by comparing the transmittance of the cell suspension with a previously determined standard curve at 540 nm using a UV–visible spectrophotometer. The final concentration of 104 CFU/mL was obtained by diluting the bacterial suspension with 400 mL of EC broth, and the concentration of the inoculum was further confirmed by plating 0.1 mL of an appropriately diluted culture on TSA.
Selection and preparation of vegetables and fruits
The prepared E. coli O157:H7 suspension was inoculated in triplicate on three vegetables viz., radish (Raphanus sativus), beet (Beta vulgaris subsp. vulgaris), and onion (Allium cepa L.), and on three fruits viz. ripe red tomato (Lycopersicon esculentum L.), cucumber (Cucumis sativus), and green habanero chili (Capsicum chinense L.). Fresh and healthy-looking fruit and vegetable samples were purchased no more than one day prior to the inoculation from a local supermarket and stored at 10 °C till use. The samples with visible defects on the surface such as bruises, cuts, or abrasions were excluded from the experiment. The vegetables were decontaminated by immersing in 200 mg/L of chlorine solution (6.5 mg/L of free chlorine) for 2 min before inoculation. The decontamination procedures were previously standardized based on the absence of growth of Enterobacteriaceae on MacConkey agar. The samples were then rinsed with sterile deionized water for 1 min and air dried with paper towels in a biosafety cabinet (Esco, Infinity® Class II, Australia).
Growth of E. coli O157:H7 on the epicarp layer of fruits and vegetables
The samples were treated for five different periods (0, 3, 5, 7 and 9 h) of incubation to determine the adhesion capacity of the E. coli O157:H7. The samples were completely submerged into 400 mL of E. coli O157:H7 broth (104 CFU/mL) for 30 min and gently shaken at 100 rpm at 37 °C. Then the samples were kept individually in sterile air bubble polyethylene bags (Stomacher® Bag, Seward, UK) for the defined period of time for each treatment. After incubation, the samples were washed thrice with sterile deionized water and air dried in a biosafety cabinet. In addition, two control samples were considered. The first one was an internal control of inoculated samples that were not subjected to the final washes, and second one was a negative control of inoculated samples that were decontaminated with 200 mg/L of chlorine solution in the final wash.
The adhesion of E. coli O157:H7 to the epicarp of vegetables and fruits was determined by spreading the samples on MacConkey agar. Three slices of the vegetable shell (10 g) were cut off aseptically and soaked in 90 mL of phosphate buffered saline (PBS, pH 7.1). After a five-fold serial dilution, 0.1 mL of the sample solution was inoculated on MacConkey agar in duplicate and incubated for 24 h at 35 ± 1 °C. The number of colonies observed in the plates was used to calculate the CFU/g of tissue.
Proximate analysis
AOAC (Association of Analytical Communities) official methods27 were used for analysis of moisture (Method 925.09), total protein (Method 950.48), fat (Method 983.23), and ash contents of the samples (Method 930.05). pH values were measured by placing the flat electrode (Sensorex, Stanton, USA) directly against the surface of the exposed tissue at three different points. Proximal analysis of the epicarp was performed using the skin of the fruits and vegetables.
Scanning electron microscopy (SEM)
The adhesion of E. coli O157:H7 to the exocarp of the samples was visualized using a scanning electron microscope (Philips model XL 30 SEM microscope) operating at an accelerating voltage of 15 kV. A final pressure of 4 Torr (533.3 Pa) was gradually generated in the chamber in order to prevent the samples from drying, prior to performing the analysis.28
Modeling of microbial growth
All the experiments were performed in triplicate. The microbial counts per gram of the sample were converted to log10 values before the calculations. The mathematical modeling of the microbial growth (predicted data) was performed by employing the modified Gompertz equation:
where L(t) is the log of the number of bacteria at time ‘t’ (in hour). A is the asymptotic log of number of bacteria as ‘t’ decreases indefinitely; C is the asymptotic amount of growth (log number) that occurs as ‘t’ increases indefinitely; M is the time (in hour) at which the absolute growth rate is at maximum; and B is the relative growth at time ‘M’.29, 30
The equation was fitted using a nonlinear regression routine of the SYSTAT statistical computer system package (SYSTAT Inc., Evanston, IL, USA). The algorithm was selected from the set of parameters with the lowest residual sum of squares with a 95% confidence interval. The Gompertz parameters (A, B, C, and M) were subsequently used to calculate the growth kinetics, including the exponential growth rate, EGR (log CFU/g/h) = BC/e; the lag-phase duration, LPD (h) = M − 1/B; the generation time, GT (h) = log(2)e/BC; and the maximum population density, MPD (log CFU/g) = A + C.
Statistical analysis
An analysis of variance (ANOVA) was performed to compare the growth parameters of E. coli O157:H7 obtained from the growth curves. The ANOVA and least significance difference (LSD) between the means were calculated at 95% confidence level using the SYSTAT software. The means that differed from the calculated LSD were considered statistically significant (α = 0.05).
Results and discussion
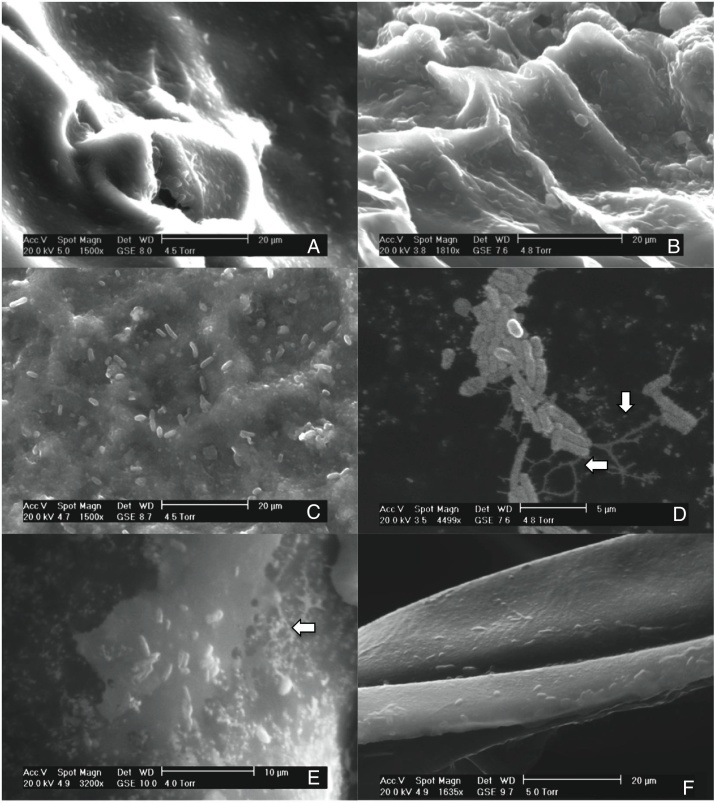
This study investigated the quantification of the growth of E. coli O157:H7 on vegetables and fruits. Although the major disease outbreaks attributed to this pathogen have been associated with leafy vegetables, the results of this study showed that E. coli O157:H7 is also capable of adhering to vegetables with different physicochemical characteristics (Fig. 1). The methodology used in this study for the adhesion of E. coli O157:H7 was validated through internal controls. The bacterial cell that adhered to the surface of the samples formed a matrix with the epidermal layer (biofilm), which provided them adequate support to counter the effects of washing. E. coli O157:H7 strain possesses several fimbrial and nonfimbrial adhesins which usually do not play any role in its pathogenesis, but are involved with its adhesion to surfaces.31 For example, curli-expressing thin-aggregative fimbriae are responsible for binding to eukaryotic extracellular matrix proteins and promoting the formation of E. coli O157:H7 biofilms on inert surfaces.32, 33 According to Torres et al.,31 the formation of biofilms increases the probability of survival of E. coli O157:H7, and hence, facilitates its protection against adverse environmental conditions. The samples from the vegetables and fruits that were not subjected to the final wash prior to plating (internal control) recorded higher bacterial concentration than those that were washed, suggesting the presence of both adhered and free microorganism on the surface of the samples. The SEM images of the samples obtained after the final washing clearly showed a layer of capsule bound to the cell wall of the samples that could possibly be associated with the exopolysaccharides secreted by the microorganism to form the matrix structure (Fig. 1D and E).
Fig. 1.
Scanning electron micrographs demonstrating the adhesion of Escherichia coli O157:H7 on the epicarp of vegetables and fruits, (A) beet, Beta vulgaris subsp. vulgaris (1550×); (B) habanero Chili, Capsicum chinense L., (1810×); (C) tomato, Lycopersicon esculentum (1500×); (D) radish, Raphanus sativus (4499×); (E) cucumber, Cucumis sativus (3200×); (F) onion, Allium cepa L. (1635×).
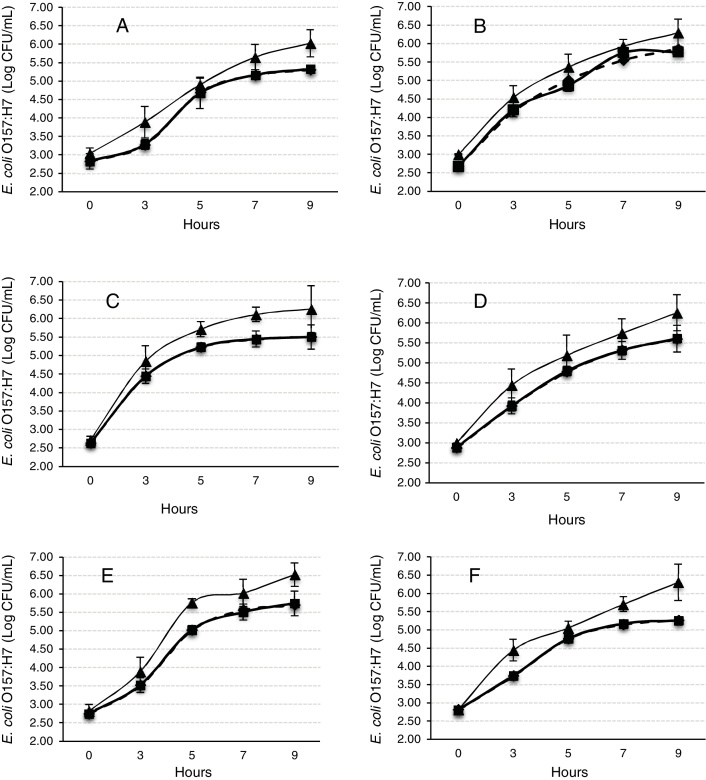
The Gompertz function fits well for the observed data with the sum of squares being less than 1 in all the cases (Fig. 2). Despite of the different intrinsic factors of the vegetables and fruits used in this study that could influence the growth of human pathogenic microorganisms, no significant difference was found in the lag-phase duration (LPD = 2.17 ± 1.03 h; mean ± S.E.), generation time (GT = 0.47 ± 0.07 h), or exponential growth rate (EGR = 0.72 ± 0.12 log CFU mL/h) of the E. coli O157:H7 adhered to the samples (Table 1). Additional information on the proximate composition of the vegetables and fruits under investigation are shown in Table 2. These values are comparable to those reported by Kim et al.,34 for perilla leaves inoculated with E. coli O157:H7 at 36 °C (LPD = 2.40 ± 0.32 h; EGR = 0.55 ± 0.11 CFU/mL/h). The growth of the E. coli O157:H7 strain on the epicarp of the vegetables and fruits was slower than that observed under controlled laboratory conditions. The growth model of Buchanan and Klawitter,35 which considered the same temperature and pH conditions employed in this study (35 ± 1 °C; pH 6.0) displayed faster kinetic values than those observed in this study (LPD = 1.54 h; GT = 0.33 h; and EGR = 0.92 log CFU/mL/h). This could be an outcome of the variation in the interactions that occur between the substrate and pathogen which might not necessarily occur in a growth medium. Similarly, the duration of the lag phase is dependent on a wide variety of factors, including the initial concentration of the inoculum, the time required to recover from the physical damage or shock due to the transfer, and the time required for the synthesis of essential coenzymes or division factors and other enzymes involved in the metabolism of the substrates present in the medium.36 In general when pathogens colonize plant surfaces, they are required to evade various defense mechanisms of the host plants. Plants possess a variety of complex immune responses, which enable them to detect and initiate counter measures to control undesired proliferation of foreign substances.37 In addition, ethylene, a plant hormone, plays a vital role in the defense against plant pathogens.38 Many microorganisms considerably increase the expression of ethylene; however the transcription level of ethylene induced by E. coli O157 were ten-fold lower, suggesting that either the plants do not sense the presence of the microorganism or that the microorganism evade the defense mechanisms of the plants consequently inhibiting the activation of ethylene.39
Fig. 2.
Observed and predicted data for Escherichia coli O157:H7 adhered to the epicarp of vegetables and fruits, (A) tomato; (B) radish; (C) cucumber; (D) beet; (E) habanero chili; (F) onion (▴: internal control; ♦: predicted data; ■: observed data).
Table 1.
Mathematical modeling of the growth of E. coli O157:H7 adhered to the epicarp of vegetables and fruits. The Gompertz (A, B, C, M) and derived parameters (GT, generation time; EGR, exponential growth rate; LPD, lag-phase duration; MPD, maximum population density) are shown. Data are presented as mean ± standard error (n = 3). The letters between the columns indicate significant differences (p < 0.05).
|
A (log CFU/mL) |
Bgrowth (h) |
C (log CFU/mL) |
M (h) |
GT (h) |
EGR (log CFU/mL/h) |
LPD (h) |
MPD (log CFU/mL) |
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SE | Mean | SE | Mean | SE | Mean | SE | Mean | SE | Mean | SE | Mean | S.E | Mean | S.E | |||||||||
| Tomato | 2.819 | 0.12 | a | 1.498 | 0.60 | a | 2.060 | 0.59 | a | 3.631 | 0.03 | a | 0.398 | 0.11 | a | 0.881 | 0.23 | a | 2.895 | 1.53 | a | 4.279 | 0.54 | a |
| Beet | 2.139 | 0.12 | b | 1.684 | 1.30 | a | 2.729 | 1.20 | a | 2.009 | 0.38 | b | 0.562 | 0.03 | a | 0.540 | 0.03 | a | 2.230 | 1.50 | a | 4.868 | 0.27 | b |
| Radish | 2.507 | 0.15 | a | 1.431 | 0.63 | a | 2.537 | 0.80 | a | 2.032 | 0.13 | b | 0.336 | 0.07 | a | 0.968 | 0.18 | a | 1.026 | 0.37 | a | 5.044 | 0.69 | b |
| Chili | 2.573 | 0.15 | a | 1.344 | 0.92 | a | 2.351 | 1.00 | a | 2.066 | 0.55 | b | 0.632 | 0.07 | a | 0.491 | 0.06 | a | 2.709 | 1.30 | a | 4.924 | 1.05 | b |
| Cucumber | 2.742 | 0.05 | a | 1.790 | 0.84 | a | 1.971 | 0.79 | a | 3.351 | 0.17 | a | 0.413 | 0.10 | a | 0.806 | 0.16 | a | 1.966 | 0.69 | a | 4.314 | 0.85 | a |
| Onion | 2.804 | 0.03 | a | 1.286 | 0.59 | a | 1.921 | 0.61 | a | 2.961 | 0.06 | a | 0.480 | 0.06 | a | 0.647 | 0.08 | a | 2.193 | 0.74 | a | 4.125 | 0.63 | a |
| F-value | 5.193 | 0.053 | 0.147 | 3.766 | 1.953 | 1.847 | 0.349 | 4.052 | ||||||||||||||||
| p-value | 0.009 | 0.998 | 0.977 | 0.045 | 0.159 | 0.178 | 0.873 | 0.048 | ||||||||||||||||
Table 2.
Epicarp proximal composition (%) of vegetables and fruits (n = 5).
| Moisture |
Ash |
Crude fiber |
pH |
|||||
|---|---|---|---|---|---|---|---|---|
| Mean | SE | Mean | SE | Mean | SE | Mean | SE | |
| Tomato | 95.4 | 0.23 | 0.74 | 0.04 | 0.57 | 0.22 | 4.77 | 0.08 |
| Beet | 81.6 | 0.25 | 1.69 | 0.13 | 1.11 | 0.24 | 6.44 | 0.07 |
| Radish | 90.0 | 0.76 | 1.34 | 0.37 | 0.65 | 0.08 | 7.03 | 0.04 |
| Chili | 92.3 | 0.35 | 0.76 | 0.01 | 2.98 | 0.19 | 6.30 | 0.40 |
| Cucumber | 94.9 | 0.17 | 0.64 | 0.03 | 1.25 | 0.07 | 7.08 | 0.11 |
| Onion | 95.1 | 0.27 | 0.27 | 0.03 | 0.47 | 0.04 | 5.40 | 0.60 |
E. coli O157:H7 growth was lower in onion than other samples suggesting an interaction that is not completely favorable between the microorganism and the epicarp of the vegetable. In the case of tomato and cucumber, the time for the microorganism to reach the maximum growth rate (M) was significantly longer than that observed for other samples; the maximum population densities (MPD) for these vegetables were also the lowest (Table 1). The variation in the size of the bacterial populations among the different species has been attributed to a range of plant characteristics, including the leaf water content, pH, and the presence of bacterial inhibitory compounds. In addition, it occurs only when the water pressure on the surface of the product overcomes the internal gas pressure, and also it depends on the hydrophobic nature of the surface.40, 41, 42 E. coli grows in a broad pH range of 4.4–10.0 with an optimum pH of 6–7.43 Specifically, E. coli O157:H7 can tolerate harsh acidic conditions, and few other strains have been reported to be able to survive even at pH 2.5–3.0 for over 4 h.44 Arnold and Kaspar,45 found that E. coli O157:H7 becomes more tolerant to acid when it is in the stationary growth phase or is starved during the log-phase of growth. The systemic pH of the vegetables and fruits used in this study is in a suitable range suitable for the growth of the pathogenic microorganism (Table 2), tomato and onion possess the lowest pH (4.77 and 5.40, respectively). This suggests that the pH in tomatoes might have influenced the growth rate of E. coli O157:H7 during the log-phase, as the pathogen took longer to reach the maximum growth rate (M) in tomatoes (Fig. 2, Table 1). This finding is in agreement with a study by Beuchat46 with ripe tomatoes, which reported that the pH range of 3.9–4.4 present in the ripe tomatoes prevents or retards bacterial growth. Del Rosario and Beuchat47 also suggested that vegetables and fruit juices with a pH value less than 4.0 are, in general, not good substrates to support the growth of E. coli O157:H7.
Other researchers have shown that MPD decreases significantly when the concentration of preservatives increases or when natural microbial compounds are present.48 As in the case of cucumber the volatile oils and the methanolic and dichloromethane extracted from the peel of C. sativus exhibited antimicrobial activity.49 Cho et al.,50 reported that the high content of the volatiles such as 2,6-nonadienal and 2-nonenal show strong activity against E. coli O157:H7. In contrast to this, there are no reports on the presence of antimicrobial compounds against human pathogens in the skin of tomato and onion. Although not specifically against E. coli O157:H7, but still some studies have reported antimicrobial activity of onion aqueous extracts against gram-negative microorganisms.51 However, Rounds et al.,52 showed that powdered onion induced the growth of E. coli by 1.2 log CFU/g, indicating that onion, like other vegetables, provides nutrients that enhance the growth of bacteria. Nevertheless, there is no conclusive explanation on the delay of the growth rate of E. coli O157:H7 on those vegetables.
In conclusion, the predictive models presented in this study offer a good comparative account of the growth of E. coli O157:H7 on samples with different intrinsic factors demonstrating low selectivity of the pathogen. The data suggest that selectivity of the bacterium, E. coli O157:H7 strain SS11 slightly depends on the intrinsic factors of the vegetables and fruits under study, with the clear exception of tomato, cucumber and onion, wherein the delay in the growth of the pathogen was significant. However, it is important to note that the growth models constructed under controlled environmental conditions offer limited insights and may vary widely from the observed profile of vegetables and fruits in the field, since this study was designed to inoculate the samples with E. coli O157:H7 without the presence of competitive microflora on the surface. A lower concentration of the microorganism could also be expected in the field than that was employed in the study in the laboratory. The results reported by Brackett,52 suggested that reducing the native microbial populations by washing and sanitizing or by controlled atmosphere storage, enables proliferation of human pathogens on the surface of vegetables. The reduction in the surface populations reduces competition for space and nutrients thereby providing growth potential for pathogenic contaminants. In theory, this scenario can result in an unspoiled product that is unsafe for consumption. Future research in this field should aim to verify the potential for adhesion and internalization of E. coli O157:H7 in the tissues of vegetables under realistic field conditions.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgement
The authors are grateful to Ana Ruth Christopher Ramos from CINVESTAV-Mérida (México) for technical assistance with the scanning electron microscopy. We also thank Daniela A. Acevedo Ojeda from UMM for assistance with PCR analysis. This project was supported by FOMIX-CONACYT – Gobierno del Estado de Yucatán (México), grant no. 108103.
Associate Editor: Beatriz Ernestina Cabilio Guth
References
- 1.Jeter C., Matthysse A.G. Characterization of the binding of diarrhea-genic strains of E. coli to plant surfaces and the role of curli in the interaction of the bacteria with alfalfa sprouts. Mol Plant Microbe Intract. 2005;18:1235–1242. doi: 10.1094/MPMI-18-1235. [DOI] [PubMed] [Google Scholar]
- 2.Macarisi D., Patel J., Bauchan G., Giron J.A., Sharma V.K. Role of curli and cellulose expression in adherence of Escherichia coli O157:H7 to spinach leaves. Foodborne Pathog Dis. 2012;9:160–167. doi: 10.1089/fpd.2011.1020. [DOI] [PubMed] [Google Scholar]
- 3.Knutton S. Electron microscopical methods in adhesion. Meth Enzymol. 1995;253:145–158. doi: 10.1016/s0076-6879(95)53015-0. [DOI] [PubMed] [Google Scholar]
- 4.Xicohtencatl-Cortes J., Sánchez-Chacón E., Saldana Z., Freer E., Giron J.A. Interaction of Escherichia coli O157:H7 with leafy green produce. J Food Protect. 2009;72:1531–1537. doi: 10.4315/0362-028x-72.7.1531. [DOI] [PubMed] [Google Scholar]
- 5.Johannessen G.S., Bengtsson G.B., Heier B.T., Bredholt S., Wasteson Y., Rorvik L.M. Potential uptake of Escherichia coli O157:H7 from organic manure into crisphead lettuce. Appl Environ Microbiol. 2005;71:2221–2225. doi: 10.1128/AEM.71.5.2221-2225.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Franz E., Visser A.A., van Diepeningen A.D., Klerks M.M., Termorshuizen A.J., van Bruggen A.H. Quantification of contamination of lettuce by GFP-expressing Escherichia coli O157:H7 and Salmonella enterica serovar Typhimurium. Food Microbiol. 2007;24:106–112. doi: 10.1016/j.fm.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 7.Grant J., Wendelboe A.M., Wendel A. Spinach-associated Escherichia coli O157:H7 outbreak, Utah and New México. Emerg Infect Dis. 2008;14:1633–1636. doi: 10.3201/eid1410.071341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harapas D., Premier R., Tomkins B., Franz P., Ajilouni S. Persistence of Escherichia coli on injured vegetable plants. Int J Food Microbiol. 2009;138:232–237. doi: 10.1016/j.ijfoodmicro.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 9.Vijayakumar C., Wolf-Hall C.E. Evaluation of household sanitizers for reducing levels of Escherichia coli on iceberg lettuce. J Food Protect. 2002;65:1646–1650. doi: 10.4315/0362-028x-65.10.1646. [DOI] [PubMed] [Google Scholar]
- 10.Singh P.K., Parsek M.R., Greenberg E.P., Welsh M.J. A component of innate immunity prevents bacterial biofilm development. Nature. 2002;417:552–555. doi: 10.1038/417552a. [DOI] [PubMed] [Google Scholar]
- 11.Boyer R.R., Sumner S.S., Williams R.C., Pierson M.D., Popham D.L., Kniel K.E. Influence of curli expression by Escherichia coli O157:H7 on the cell's overall hydrophobicity, charge, and ability to attach to lettuce. J Food Protect. 2007;70:1339–1345. doi: 10.4315/0362-028x-70.6.1339. [DOI] [PubMed] [Google Scholar]
- 12.Chang J.M., Fang T.J. Survival of Escherichia coli O157:H7 and Salmonella enterica serovars Typhimurium in iceberg lettuce and the antimicrobial effect of rice vinegar against E. coli O157:H7. Food Microbiol. 2007;24:745–751. doi: 10.1016/j.fm.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 13.McEvoy J.L., Luo Y., Conway W., Zhou B., Feng H. Potential of Escherichia coli O157:H7 to grow on field-cored lettuce as impacted by postharvest storage time and temperature. Int J Food Microbiol. 2009;128:506–509. doi: 10.1016/j.ijfoodmicro.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 14.Seltzer J., Rush J., Kinsey J. The Food Industry Center, University of Minnesota; 2015. Natural Selection: 2006 E. coli Recall of Fresh Spinach. A Case Study by the Food Industry Center. Available from: http://ageconsearch.umn.edu/bitstream/54784/2/Natural%20Selection.pdf. Accessed 30.08.09. [Google Scholar]
- 15.Wendel A.M., Johnson D.H., Sharapov U. Multistate outbreak of Escherichia coli O157:H7 infection associated with consumption of packaged spinach, August–September 2006: the Wisconsin investigation. Clin Infect Dis. 2009;48:1079–1086. doi: 10.1086/597399. [DOI] [PubMed] [Google Scholar]
- 16.Ethelberg S., Lisby M., Bottiger B. Outbreaks of gastroenteritis linked to lettuce, Denmark, January 2010. Euro Surveill. 2010;15:6. [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention (CDC) 2015. Multistate Outbreak of Shiga Toxin-producing E. coli O157:H7 Infections Linked to Ready-to-Eat Salads. Available from: http://www.cdc.gov/ecoli/2013/O157H7-11-13/index.html. Accessed 30.08.13. [Google Scholar]
- 18.Han Y., Sherman D.M., Linton R.H., Nielsen S.S., Nelson P.E. The effects of washing and chlorine dioxide gas on survival and attachment of Escherichia coli O157: H7 to green pepper surfaces. Food Microbiol. 2000;17:521–533. [Google Scholar]
- 19.Ongeng D., Muyanja C., Geeraerd A.H., Springael D., Ryckeboer J. Survival of Escherichia coli O157:H7 and Salmonella enterica serov Typhimurium in manure and manure-amended soil under tropical climatic conditions in Sub-Saharan Africa. J Appl Microbiol. 2011;110:1007–1022. doi: 10.1111/j.1365-2672.2011.04956.x. [DOI] [PubMed] [Google Scholar]
- 20.Abadias M., Alegre I., Oliveira M., Altisent R., Viñas I. Growth potential of Escherichia coli O157:H7 on fresh-cut fruits (melon and pineapple) and vegetables (carrot and escarole) stored under different conditions. Food Control. 2012;27:37–44. [Google Scholar]
- 21.Forslund A., Ensink J.H.J., Markussen B. Escherichia coli contamination and health aspects of soil and tomatoes (Solanum lycopersicum L.) subsurface drip irrigated with on-site treated domestic wastewater. Water Res. 2012;46:5917–5934. doi: 10.1016/j.watres.2012.08.011. [DOI] [PubMed] [Google Scholar]
- 22.Chang W.S., Afsah-Hejri L., Rukayadi Y. Quantification of Escherichia coli O157:H7 in organic vegetables and chickens. Int Food Res J. 2013;20:1023–1029. [Google Scholar]
- 23.Gullian M., Duran-Casanova G., Isla-Esquivel M.L., Suárez-Wegan E., Alarcón-Sánchez A. Estudio de factores predisponentes de enfermedad diarreica aguda en la comunidad de San Simón, Yucatán en base a un análisis de vulnerabilidad nutricional y ambiental. Población y Salud en Mesoamérica. 2011;9(5) Available from: http://revistas.ucr.ac.cr/index.php/psm/article/view/733. [Google Scholar]
- 24.Cebula T.A., Payne W.L., Feng P. Simultaneous identification of strains of Escherichia coli serotype O157: H7 and their Shiga-like toxin type by mismatch amplification mutation assay-multiplex PCR. J Clin Microbiol. 1995;33:248–250. doi: 10.1128/jcm.33.1.248-250.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maurer J.J., Schmidt D., Petrosko P., Sanchez S., Bolton L., Lee M.D. Development of primers to O-antigen biosynthesis genes for specific detection of Escherichia coli O157 by PCR. Appl Environ Microbiol. 1999;65:2954–2960. doi: 10.1128/aem.65.7.2954-2960.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Son I., Binet R., Maounounen-Laasri A., Lin A., Hammack T.S., Kase J.A. Detection of five Shiga toxin-producing Escherichia coli genes with multiplex PCR. Food Microbiol. 2014;40:31–40. doi: 10.1016/j.fm.2013.11.016. [DOI] [PubMed] [Google Scholar]
- 27.AOAC . 18th ed. AOAC International; Gaithersburg, Maryland 20877-2417, USA: 2005. Official Methods of Analysis of AOAC International. [Google Scholar]
- 28.El Abed S., Koraichi-Ibnsouda S., Latrache H., Hamadi F. Scanning electron microscopy (SEM) and environmental SEM: suitable tools for study of adhesion stage and biofilm formation. In: Kazmiruk V., editor. Scanning Electron Microscopy. 2012. [chapter 35]. Available from: http://www.intechopen.com/books/scanning-electron-microscopy/scanning-electron-microscopy-sem-and-environnmental-sem-suitable-tools-for-study-of-adhesion-stage-a. Accessed 30.08.15. [Google Scholar]
- 29.Gibson A.M., Bratchell N., Roberts T.A. Predicting microbial growth: growth response of salmonellae in a laboratory medium as affected by pH, sodium chloride and storage temperature. Int Appl Microbiol. 1988;6:155–178. doi: 10.1016/0168-1605(88)90051-7. [DOI] [PubMed] [Google Scholar]
- 30.Zwietering M.H., Jongenburger I., Rombouts F.M., van’t Rict K. Modelling of the bacterial growth curve. Appl Environ Microbiol. 1990;56:1875–1881. doi: 10.1128/aem.56.6.1875-1881.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Torres A.G., Jeter C., Langley W., Matthysse A.G. Differential binding of Escherichia coli O157:H7 to alfalfa, human epithelial cells, and plastic is mediated by a variety of surface structures. Appl Environ Microbiol. 2005;71:8008–8015. doi: 10.1128/AEM.71.12.8008-8015.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cookson A.L., Cooley W.A., Woodward M.J. The role of type 1 and curli fimbriae of Shiga toxin-producing Escherichia coli in adherence to abiotic surfaces. Int J Med Microbiol. 2002;292:195–205. doi: 10.1078/1438-4221-00203. [DOI] [PubMed] [Google Scholar]
- 33.Ryu J.H., Kim H., Frank J.F., Beuchat L.R. Attachment and biofilm formation on stainless steel by Escherichia coli O157:H7 as affected by curli production. Lett Appl Microbiol. 2004;39:359–362. doi: 10.1111/j.1472-765X.2004.01591.x. [DOI] [PubMed] [Google Scholar]
- 34.Kim J., Ro E., Yoon K. Comparison of growth kinetics of various pathogenic E. coli on fresh perilla leaf. Foods. 2013;2:364–373. doi: 10.3390/foods2030364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Buchanan R.L., Klawitter L.A. The effect of incubation temperature, initial pH, and sodium chloride on the growth kinetics of Escherichia coli O157:H7. Food Microbiol. 1992;9:185–196. [Google Scholar]
- 36.Schultz D., Kishony R. Optimization and control in bacterial lag phase. BMC Biol. 2013;11:120. doi: 10.1186/1741-7007-11-120. Available from: http://www.biomedcentral.com/1741-7007/11/120. Accessed 30.08.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Berger C.N., Sodha S.V., Shaw R.K. Fresh fruit and vegetables as vehicles for the transmission of human pathogens. Environ Microbiol. 2010;12:2385–2397. doi: 10.1111/j.1462-2920.2010.02297.x. [DOI] [PubMed] [Google Scholar]
- 38.Broekaert W.F., Delaure S.L., De Bolle M.F., Cammue B.P. The role of ethylene in host–pathogen interactions. Annu Rev Plant Physiol. 2006;44:393–416. doi: 10.1146/annurev.phyto.44.070505.143440. [DOI] [PubMed] [Google Scholar]
- 39.Thilmony R., Underwood W., He S.Y. Genome wide transcriptional analysis of the Arabidopsis thaliana interaction with the plant pathogen Pseudomonas syringae pv. tomato DC3000 and the human pathogen Escherichia coli O157:H7. Plant J. 2006;43:34–53. doi: 10.1111/j.1365-313X.2006.02725.x. [DOI] [PubMed] [Google Scholar]
- 40.Burnett S.L., Chen J., Beuchat L.R. Attachment of Escherichia coli O157:H7 to the surface and internal structures of apples as detected by confocal laser microscopy. Appl Environ Microbiol. 2000;66:4679–4687. doi: 10.1128/aem.66.11.4679-4687.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yadav V., Mandhan R., Dabur R., Chhillar A.K., Gupta J., Sharma G.L. A fraction from Escherichia coli with anti-Aspergillus properties. J Med Microbiol. 2004;54:375–379. doi: 10.1099/jmm.0.45748-0. [DOI] [PubMed] [Google Scholar]
- 42.Zhuang R.Y., Beuchat L.R., Angulo F.J. Fate of Salmonella montevideo on and in raw tomatoes as affected by temperature and treatment with chlorine. Appl Environ Microbiol. 1995;61:2127–2131. doi: 10.1128/aem.61.6.2127-2131.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Desmarchelier P.M., Fegan N. Enteropathogenic Escherichia coli. In: Hocking A.D., editor. Foodborne Microorganisms of Public Health Significance. Australian Institute of Food Sci and Tech (NSW Branch); Sydney: 2003. pp. 267–310. [chapter 9] [Google Scholar]
- 44.Molina P.M., Parma A.E., Sanz M.E. Survival in acidic medium of Shiga toxin-producing Escherichia coli O157:H7 and non-O157:H7 isolated in Argentina. BMC Microbiol. 2003;3:17. doi: 10.1186/1471-2180-3-17. Available from: http://www.biomedcentral.com/1471-2180/3/17. Accessed 30.08.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Arnold K.W., Kaspar C.W. Starvation- and stationary-phase-induced acid tolerance in Escherichia coli O157:H7. Appl Environ Microbiol. 1995;61:2037–2039. doi: 10.1128/aem.61.5.2037-2039.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Beuchat L.R. Ecological factors influencing survival and growth of human pathogens on raw fruits and vegetables. Microb Infect 2002. 2002;4:413–423. doi: 10.1016/s1286-4579(02)01555-1. [DOI] [PubMed] [Google Scholar]
- 47.Del Rosario B.A., Beuchat L.R. Survival and growth of enterohemorrhagic Escherichia coli O157:H7 in cantaloupe and watermelon. J Food Prot. 1995;1:105–107. doi: 10.4315/0362-028X-58.1.105. [DOI] [PubMed] [Google Scholar]
- 48.Andrés S.C., Giannuzzi L., Zaritzky N.E. The effect of temperature on microbial growth in Apple cubes packed in film and preserved by use of orange juice. Int J Food Sci Technol. 2004;39:927–933. [Google Scholar]
- 49.Sotiroudis G., Melliou E., Sotiroudis T.G., Chinou I. Chemical analysis, antioxidant and antimicrobial activity of three Greek cucumber (Cucumis sativus) cultivars. J Food Biochem. 2010;34:61–78. [Google Scholar]
- 50.Cho I.H., Choi E.S., Lim H.G., Lee H.H. Purification and characterization of six fibrinolytic serine-proteases from earthworm Lumbricus rubellus. J Biochem Mol Biol. 2004;37:199–205. doi: 10.5483/bmbrep.2004.37.2.199. [DOI] [PubMed] [Google Scholar]
- 51.Azu N., Onyeagba R. Antimicrobial properties of extracts of Allium cepa (Onions) and Zingiber officinale (Ginger) on Escherichia coli, Salmonella typhi and Bacillus subtilis. J Trop Med. 2006;3(2) Available from: http://ispub.com/IJTM/3/2/11056. Accessed 30.08.15. [Google Scholar]
- 52.Rounds J.M., Rigdon C.E., Muhl L.J. Non-O157 Shiga toxin-producing Escherichia coli associated with venison. Emerg Infect Dis. 2012;18:279–282. doi: 10.3201/eid1802.110855. [DOI] [PMC free article] [PubMed] [Google Scholar]; Brackett R.E. Shelf stability and safety of fresh produce as influenced by sanitation and disinfection. J Food Prot. 1992;55:804–814. doi: 10.4315/0362-028X-55.10.808. [DOI] [PubMed] [Google Scholar]