Abstract
Poly-ADP-ribosylation (PARylation) is a protein posttranslational modification (PTM) that is critically involved in many biological processes that are linked to cell stress responses. It is catalyzed by a class of enzymes known as poly-ADP-ribose polymerases (PARPs). In particular, PARP1 is a nuclear protein that is activated upon sensing nicked DNA. Once activated, PARP1 is responsible for the synthesis of a large number of PARylated proteins and initiation of the DNA damage response (DDR) mechanisms. This observation provided the rationale for developing PARP1 inhibitors for the treatment of human malignancies. Indeed, three PARP1 inhibitors (Olaparib, Rucaparib and Niraparib) have recently received FDA approval for treating ovarian cancer. Moreover, earlier in 2017, Niraparib has also been approved for the treatment of fallopian tube cancer and primary peritoneal cancer. Despite these very exciting progresses in the clinic, the basic signaling mechanism that connects PARP1 to a diverse array of biological processes is still poorly understood. This is, in large part, due to the inherent technical difficulty associated with the analysis of protein PARylation, which is a low-abundance, labile and heterogeneous PTM. The study of PARylation has been greatly facilitated by the recent advances of mass spectrometry-based proteomic technologies tailored to the analysis of this modification. In this Perspective, we discuss these breakthroughs, including their technical development, and applications that provide a global view of the many biological processes regulated by this important protein modification.
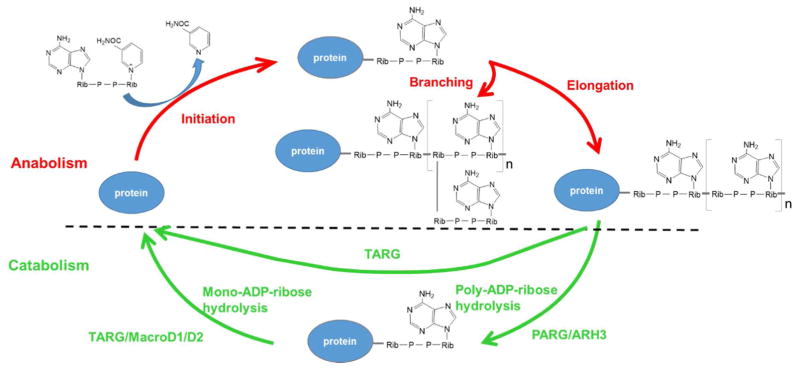
The Life Cycle of Poly-ADP-ribose
ADP-ribosylation is a reversible protein posttranslational modification (PTM) where either one (mono-ADP-ribosylation) or multiple (poly-ADP-ribosylation) units of ADP-ribose are attached to a target protein(1–3). ADP-ribosylation can be catalyzed by different enzymes, including poly-ADP-ribose polymerases (PARPs), bacterial toxins and several NAD+-dependent Sirtuins(1, 2, 4, 5). These enzymes commonly catalyze the reaction in which the ADP-ribose group is transferred from the donor cofactor, NAD+, to the acceptor protein(3). In the case of protein poly-ADP-ribosylation (PARylation) (catalyzed by a number of PARP family members), once initiated, the chain can be further elongated in both a linear and branched fashion, generating PAR polymers whose lengths can often reach 200 units (Figure 1)(1). Compared to other common PTMs, a rather unique feature about ADP-ribosylation is that this modification can occur on amino acids with diverse side chain chemistry, including Asp, Glu, Lys, Arg, Cys and Ser(6).
Figure 1.
Metabolism of Poly-ADP-ribose. A number of enzymes are involved in degrading PAR, with two representative ones (PARG and TARG) shown in the Figure.
The first PARP family member, PARP1, was cloned in 1987(7–9). Numerous efforts have now led to the identification of 16 additional members within the PARP family(10–17). Recent studies have shown that only a subset of the PARPs (i.e. PARP1, PARP2 and Tankyrases) catalyze protein PARylation. The remaining PARPs either catalyze MARylation, or are enzymatically inactive(18). Toward this, a unified nomenclature has been proposed to designate these enzymes as ADP-ribosyltransferases (ARTs) and they are classified into two sub-families, ARTCs (C2/C3 toxins-like) and ARTDs (bacterial diphtheria toxin-like)(19). This Perspective article will focus on PARP1 (ARTD1) and PARP1-dependent downstream signaling events. PARP1 is a bona fide PARylating enzyme, and is arguably the best studied PARP family member. It is a nuclear protein that is critically involved in a wide array of biological processes, including DNA damage repair, epigenetic regulation, transcription, apoptosis, and mRNA metabolism. In particular, PARP1 and PARP1-medaited PARylation events are intimately linked to cellular DNA damage response (DDR)(6, 20). The basal level protein PARylation in a cell is very low, where most PARP1 stays quiescent. However, during genotoxic stress, PARP1 is rapidly recruited to DNA lesions with a time for half-accumulation (t1/2) of about 1.6 sec(20). Upon binding to nicked DNA, its catalytic activity can increase up to 500-fold, resulting the synthesis of a large number of PARylated proteins (including PARP1 itself)(21–23). PARP1 is also one of the most abundant polypeptides in the nuclei (e.g. ~2×106 molecules per cell)(24, 25). On average, one PARP1 molecule is responsible for patrolling approximately 1000 bp of DNA(26). With these unique features, PARP1 efficiently scans the genome to detect DNA strand breaks, and then relay these signals downstream to activate a variety of important biological processes related to DDR (e.g. recruitment of DNA repair machinery and cell cycle arrest, etc).
PARP1 contains several domains, including three N-terminal Zinc Finger motifs, a BRCT domain, a WGR domain and a C-terminal catalytic domain. Our understanding of the function of each of these domains has been greatly facilitated by recent structural and biochemical studies of PARP1(27, 28). Specifically, the first two Zinc Finger motifs of PARP1 are critical for the recognition of various DNA structures, including single- and double-strand breaks, nucleosome linker DNA, etc(29, 30). Furthermore, it has been shown that binding of PARP1 to a DNA double-strand break (DSB) induces a profound conformational change, resulting in the remodeling of an inhibitory motif near the catalytic domain, and hence stimulation of its enzymatic activity(27). Binding of PARP1 to DNA single strand breaks (SSBs) induces a somewhat different intramolecular conformational change, which nevertheless is also able to potently activate PARP1(28).
It has been estimated that PARP1 could account for more than 90% of the PAR synthesized under genotoxic conditions(31). Intriguingly, the half-life of these protein-linked PAR chains is less than 1 min, suggesting the presence, in intact cells, of an efficient mechanism for PAR-catabolism(22). Among the various PAR-degrading enzymes, the best studied is Poly-ADP-ribose glycohydrolase (PARG). PARG possesses both endo- and exo-glycohydrolysis activity that catalyzes the hydrolysis of the ester bond between ADP-ribose units in a PAR chain to generate free ADP-ribose(32). PARG is a highly efficient enzyme that plays a dominant role in regulating PAR-catabolism. Although PARP1 is much more abundant compared to PARG (~50-fold more), the catalytic activity of PARG is 20- to 100-fold higher, resulting a rapid turnover the PAR polymers(26). This temporal control of PAR metabolism provides a mechanism for tight regulation of PAR-dependent downstream signaling events (many of these are of a transient nature), including protein-protein interaction, translocation and sub-organellar structure formation (e.g., stress granules, cajal bodies)(33–35). As expected, depletion of PARG stabilizes PAR polymers, and greatly prolongs their half-life(36). Furthermore, deletion of PARG in mice is early embryonic lethal during the gastrulation stage, a phenotype reminiscent of PARP1/PARP2 double-knock animals(37, 38). These results also provided additional evidence supporting the critical role of PARG in regulating PAR homeostasis.
Despite being a highly active enzyme, PARG, however, is unable to remove the terminal ADP-ribose unit linked to the side chain of an acceptor amino acid (e.g. Asp and Glu)(39). This function instead is fulfilled by another PAR-degrading enzyme called TARG1/C6orf130(40). It has been shown that, in the context of PAR-catabolism, TARG1 is able to complement PARG, by (1) hydrolyzing the glutamate-ADP-ribose ester bond, and thus removing the entire PAR chain from a PARylated protein, (2) reversing protein MARylation, and (3) completing the removal of the terminal ADP-ribose following the PARG reaction(40). Although the exact mechanism remains unclear, patients with TARG1/C6ORF130 mutations develop severe neurodegeneration. These results suggest that there is a potential link between defects in the recycling of ADP-ribosylated peptides and cell death(40). Another type of well-studied enzymes to reverse the mono-ADP-ribosylation is macrodomain proteins such as MacroD1/2. Besides the catalytic activity targeting acidic residues, MacroD2 could also bind to mono-ADP-ribose, but not poly-ADP-ribose(41, 42).
In addition to aforementioned catalytic enzymes, a number of studies have identified several additional enzymes that are also involved in PAR-catabolism. One such enzyme is ARH (ADP-ribosylhydrolase), which specifically hydrolyzes the chemical bond between the target amino acid arginine and ADP-ribose(43). ARH3, on the other side, functions similar to PARG, which digests PAR polymers to release free ADP-ribose(44). Two recent studies also demonstrated that ARH3 is able to directly remove ADP-ribose that is linked to Ser residues(45, 46). Several recently identified enzymes process ADP-ribosylation by a different mechanism, i.e., cleaving the pyrophosphate bonds within an ADP-ribose and generating peptidyl-ribose-5′-phosphate. These enzymes include proteins with the NUDIX (nucleoside diphosphate linked to another moiety X) domain, human NUDT16, bacterial RppH(47, 48), and ENPP1 (ectonucleotide pyrophosphatase/phosphodiesterase 1)(49). Collectively, these enzymes function to ensure a fine control of cellular ADP-ribosylation homeostasis.
Biological Function of Protein ADP-ribosylation
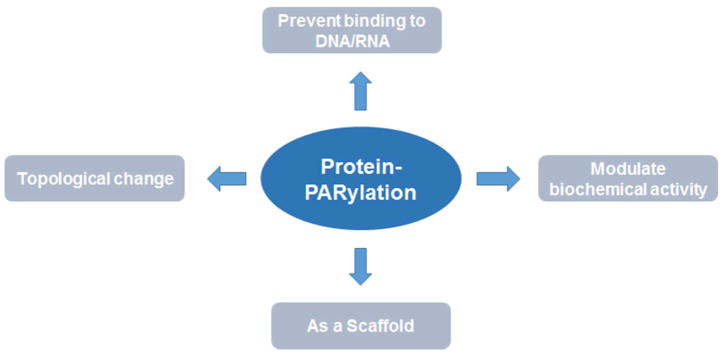
ADP-ribosylation can dramatically alter the function of an acceptor protein, an effect often ascribed to the unique biochemical characteristics of this modification (Figure 2). First, from a structural point of view, PAR resembles DNA and RNA (Figure 1), all of which are bulky, charged and flexible. Because each ADP-ribose unit contains two phosphates, PARylation can lead to a drastic change in the electrostatic property of a protein. As a result, it may prevent a DNA- or RNA-binding protein from interacting with its nucleic acid targets(50). Indeed, a significant fraction of DNA damage-induced PAR polymers are linked to PARP1 itself. Automodification of PARP1 results in its dissociation from DNA due to charge repulsion, which serves an important mechanism to inactivate this enzyme(51). Second, PARylation often introduces a change in the topological feature of an acceptor protein, which could disrupt important protein-protein interactions. For example, studies have pointed p53 as a protein that is PARylated by PARP1 during genotoxic stress. Using site-directed mutagenesis, it was shown that PARP1 modifies p53 on a number of evolutionarily conserved Asp/Glu residues (Glu255, Asp256 and Glu268). PARylation of p53 prevents it from binding to the nuclear export receptor Crm1, promoting the accumulation of p53 in the nucleus and the activation of p53-mediated check point response(52). Third, ADP-ribosylation could function as a mechanism to modulate the biochemical activity of an acceptor protein. This is best illustrated by several bacterial toxins, including diphtheria, cholera, and pertussis toxins, all of which possess MARylating activities. Specifically, once inside the cell, the diphtheria toxin functions by modifying the diphthamide residue of elongation factor 2 with a single mono-ADP-ribose unit. This event prevents its association with the mammalian translation machinery, and thus potently blocks protein synthesis(53).
Figure 2.
Potential biological function of protein Poly-ADP-ribosylation.
PARylation may also be gain-of-function to serve as a scaffold for recruiting other proteins. Indeed, a number of PAR-binding motifs (PBMs) have been identified in recent years, including WWE, PBZ, BRCT, FHA, OB-fold, RRM and macro domain(2). These PBMs are present in a wide range of proteins involved in DNA damage response, chromatin remodeling and RNA processing. For example, upon sensing DNA strand breaks, PARP1 modifies itself and those proteins in proximity. These protein-linked PAR polymers recruit a protein called XRCC1 via binding to its BRCT domain. This then triggers the formation of a large protein complex involved in the repair of DNA single strand breaks (SSBs)(54). As another example, it was recently demonstrated that the oligonucleotide/oligosaccharide-binding (OB)-fold is another PAR binding motif(55). It is present in a number of proteins involved in DDR, including hSSB1, hSSB2, CTC1 and MEIOB. OB-fold binds tightly (Kd = 150 nM) to the iso-ADP-ribose unit within a PAR polymer, and mediates the early recruitment of these DDR-related proteins to the sites of DNA damage. In the case of hSSB1, it was shown that PAR-dependent recruitment is critical for hSSB1-regulated DSB repair, which likely proceeds via the nonhomologous end joining (NHEJ) pathway(55).
Besides regulating these molecular events, activation of PARP1 is also associated with important physiological outcomes. Over-stimulation of PARP1 has a profound impact on NAD+ metabolism, which serves as the major pathway for NAD+ catabolism. Specifically, cellular NAD+ concentrations drop to 20% of their normal levels following a mere 15 min of DNA damage-induced PARP1 activation(56, 57). Depletion of the cellular NAD+ pool leads to forced NAD+ synthesis through the salvage pathway, which dramatically lowers the cellular ATP level and eventually causes cell death (e.g., necrosis)(1). During apoptosis, PARP1 is cleaved by activated caspases, generating a 24 kDa DNA-binding domain, and an 89 kDa C-terminal fragment that contains the catalytic domain(58). Because the PARP1 C-terminal domain is unable to bind to nicked DNA, PARP1 is inactivated, and ATP is thus spared for the execution of the apoptotic program. Finally, PARP1 is also known to regulate another form of programmed cell death called parthanatos(59). During parthanatos, the accumulation of PAR causes the translocation of apoptosis-inducing factor (AIF) from mitochondria to the nucleus(60, 61). Recent studies have shown that AIF is able to bind to a nuclease called macrophage migration inhibitory factor (MIF). In so doing, AIF recruits MIF to the nucleus, which cleaves genomic DNA, and eventually leads to parthanatos(62).
Clinical Development of PARP1 Inhibitors
The observation that PARP1 is involved in mediating cell stress responses raises the intriguing possibility that PARP1 could be explored as a potential therapeutic target for treating human diseases, such as cancer(63). In particular, BRCA1/2 are tumor suppressor proteins that play a critical role in mediating DNA double-strand break (DSB) repair(64–66). Mutations of BRCA1/2 lead to genome instability, which underlies the pathogenesis of a significant fraction of breast cancer and ovarian cancer (and to a lesser extent, prostate cancer and pancreatic cancer)(67–69). BRCA1/2-deficient tumors have an aggressive phenotype, and these patients often have early onset diseases, and poor prognosis(70–72). In two landmark studies, it was shown that BRCA1/2-mutated cancer cells are homologous recombination (HR)-deficient. They rely on PARP1 for genome integrity, and as a result, can be selectively killed by PARP1 inhibitors via a “synthetic lethality” mechanism(73, 74). The validity of this synthetic lethality concept is further corroborated by studies which showed that PARP1 inhibitor (PARPi) resistance can stem from the development of secondary “revertant” mutations in BRCA1/2 that restore the expression of the wild-type BRCA1/2 protein, and then the HR function(75, 76).
In addition, a number of recent studies suggest that PARPi may also kill tumor cells (BRCA1/2-mutant and beyond) via a “trapping” mechanism(77). Specifically, the binding of PARP1 to nicked DNA triggers its auto-PARylation. Auto-modified PARP1 is then released from the site of damaged DNA due to the steric hindrance and charge repulsion of the PAR polymers. Upon the treatment of PARPi, PARP1 is unable to modify itself. As a result, the PARP1-inhibitor complex is stabilized and is trapped at the DNA lesion(78). This complex is highly toxic, which could block the progression of the replication fork, and interfere with subsequent DNA replication. It has been suggested that the cytotoxicity of at least some of the PARPi can be ascribed to these trapped PARP1 complexes(78). Consistent with this notion, PARP1-deleted cells are highly resistant to PARPi, suggesting that the functional consequence of PARP1 deletion is not equivalent to PARP1 inhibition(77, 78).
These abovementioned studies provided a solid rationale to develop PARPi to treatment cancer. However, initial efforts to target PARP1 clinically suffered a major setback, due to the disappointing results of a phase III study of Iniparib in metastatic, triple-negative breast cancer (TNBC)(79). Iniparib was originally designed to act as a covalent inhibitor of PARP1. However, subsequent studies demonstrated that this compound is not a genuine PARP1 inhibitor, and it has no activity against PARP1 in vitro, or in intact cells(36, 80, 81). Other drug discovery programs have focused on developing NAD+-competitive inhibitors of PARP1 (PARPi)(82–86) (Table 1). Results from these initiatives have turned out to be very encouraging, with a number of agents gaining FDA-approval, including Olaparib, Rucaparib and Niraparib (treating ovarian cancer and beyond)(87–89). It is important to note that even though many PARPi have been optimized to potently inhibit the catalytic activity of PARP1, they are not equivalent with respect to inducing PARP1 trapping, a scenario that may explain the differential cytotoxic effects of these compounds(77). Indeed, new generation of PARPi (e.g. Talazoparib/BMN673) with more potent PARP1-trapping activity has been developed(85).
Table 1.
Characteristics of PARP 1 inhibitors in clinical development.
| PARP1 inhibitor | Company | IC50 (nm) | Route | Clinical trial (phase) |
|---|---|---|---|---|
| Olaparib (AZD-2281) | AstraZeneca | PARP1=5.0 PARP2=1.0 |
Oral |
|
| Rucaparib | Clovis Oncology | PARP1=1.4 | Oral |
|
| Niraparib | Tesaro | PARP1=3.8 PARP2=2.1 |
Oral |
|
| Talazoparib | Pfizer | PARP1=0.57 | Oral |
|
| Veliparib (ABT-888) | Abbvie | PARP1=5.2 PARP2=2.9 |
Oral |
|
Although the primary target of these abovementioned compounds is PARP1, additional PARP family members and other NAD+-utilizing enzymes may share a similar NAD+-binding pocket, and therefore could also be blocked by these compounds(90, 91). This may explain some of the off-targets effects observed for these compounds.
Challenges for the Study of PARP downstream signaling
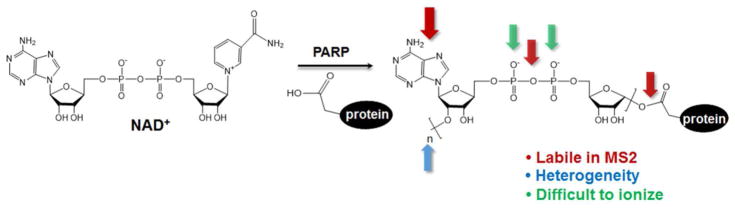
Contrary to the fruitful efforts of characterizing the upstream regulators of PARP1, its genuine downstream targets remain poorly defined. The best described PARP1 substrates are confined in the DNA repair pathways, yet recent studies have suggested the involvement of PARP1 in many other biological processes(92, 93). Mass spectrometry-based proteomic approaches are powerful tools to study protein posttranslational modifications (PTMs)(94). However, the application of these systematic approaches to studying PARP1 downstream signaling has been hampered by many of the intrinsic difficulties associated with the analysis of this modification(36) (Figure 3): (1) PARylated proteins are of low abundances, and are rapidly turned over in vivo. (2) During cell lysis, PARP1 could be activated by sheared DNA, a scenario that introduces artificial PARylation. Similarly, if not properly inactivated, PAR-catabolizing enzymes (e.g., PARG) could degrade PARylated proteins in the cell lysis step. (3) Mono- and Poly-ADP-ribose moieties are linked to amino acids with diverse side chain chemistries. (4) Unlike many other protein modifications (e.g., phosphorylation, acetylation and methylation), PARylation is a heterogeneous PTM that does not possess a defined mass shift. (5) The topology of the PAR polymers is highly complex. They range from 2 to 200 units, and can be linear and/or branched(1). (6) The adenosine moiety, pyrophosphate bond and the amino acid linkage are chemically labile. ADP-ribosylation is also a highly unstable PTM which readily decomposes in conventional tandem MS conditions, yielding neutral-loss fragments instead of sequence-specific ions(95, 96). (7) A PAR chain can extend to hundreds of ADP-ribose units, with each ADP-ribose containing two phosphate groups. As a result, PARylated peptides are acidic, and are difficult to ionize (peptides are typically analyzed by MS using positive ion mode).
Figure 3.
Challenges for mass spectrometric analysis of protein Poly-ADP-ribosylation. A representative side chain linkage (Asp-/Glu-ADP-Ribosylation) is shown.
In the following section, we will discuss recent progresses on MS-based technologies that overcome some of these abovementioned challenges. We will also explore how these approaches help address several important questions in the field of PARP/ADP-ribosylation biology, including (1) what is the identity of many ADP-ribosylated proteins? (2) If a protein is ADP-ribosylated, at which residue it is modified? (3) What are the substrates of different PARPs? (4) How ADP-ribosylation affects these proteins functionally? (5) How these ADP-ribosylated proteins mediate the signaling output of PARPs?
Identification of the Constituents of the PARylated Proteome and PAR-interactome
As an important approach to understanding how PARP1 regulates its downstream signaling events, affinity purification approaches have been employed to capture PARylated proteins for their subsequent mass spectrometric (MS) identification. Towards this, a number of studies have been carried out to characterize the PARP-dependent PARylated proteome (ensemble of the PARylated proteins) and PAR-interactome (ensemble of the proteins that interact with PAR)(97–100). These studies utilized different enrichment reagents to isolate PARylated proteins, including the Af1521 macrodomain, 10H antibody and catalytically inactive PARG. In order to preserve the interaction between PAR and these abovementioned affinity enrichment agents, the experiments were commonly performed under non-denaturing conditions. When interpreting the results from these specific experiments, it is important to consider the following: First, PARP1 could be activated by sheared DNA during the cell lysis step, resulting the formation of non-physiological PARylated proteins. This could be prevented by the addition of PARP1 inhibitors in cell lysis buffer(97). Second, the hits identified from these studies likely include not only the PARylated protein (PARylated proteome), but also additional proteins that interact with these hits in a PAR dependent- or independent-manner (PAR-interactome), under these native conditions. Third, these studies identified both overlapping and distinct hits, owing to the unequal binding affinities and specificities of these abovementioned PAR-enrichment agents(101).
Dani et al., employed the Af1521 macrodomain combined with shotgun proteomics experiments to characterize the cellular mono-ADP-ribosylated proteins(98) (Table 2). In these experiments, they demonstrated that the Af1521 macrodomain (mAf1521) binds tightly to not only free ADP-ribose (Kd = 0.13 μM), but also ADP-ribose attached to an acceptor protein. Another unique feature about mAf1521 is that this protein domain is able to bind to ADP-ribose conjugated to different amino acids, including Arg, Cys and diphthamide, indicating the recognition of the ADP-ribose is unaffected by the amino acid side chain chemistry. Dani et al., was able to increase the specificity of mAf1521 by employing a “two-step” enrichment strategy. Postnuclear and membrane fractions were first subjected to pull-down using mAf1521/G42E (an mAf1521 mutation that abolishes ADP-ribose binding), which eliminates the non-specifically bound proteins. The flow-through fraction was then incubated, in the second step, with wide-type mAf1521 to specifically isolate the ADP-ribosylated proteins. Combined with shotgun proteomic analysis, this strategy allows the identification of a number of ADP-ribosylated proteins that are involved in regulating cytoskeleton, cell metabolism and mRNA splicing. Because these experiments were performed under basal conditions (without the treatment of genotoxic agents), most of the identified hits were mono-ADP-ribosylated (MARylated) proteins(98).
Table 2.
Summary of the different MS methodologies for the analysis of the ADP-ribosylated proteome. (site) indicates the studies where ADP-ribosylation site information is obtained. CID, collision-induced dissociation; HCD, higher-energy collisional dissociation; ETD, electron transfer dissociation; ETcaD, electron-transfer dissociation with supplemental collisional activation; EThcD, electron-transfer/higher-energy collision dissociation.
| Affinity purification | Binding Specificity | Fragmentation MS2 | Reference |
|---|---|---|---|
| Af1521 macrodomain | Bind to both mono- and poly-ADP-ribose | CID | Dani et al., 2009 |
| Af1521 macrodomain | Bind to both mono- and poly-ADP-ribose | HCD | Jungmichel et al., 2009 |
| 10H antibody | Bind to poly-ADP-ribose (only units >10) | CID | Gagné et al., 2008 |
| PARG-DEAD (catalytically inactive PARG) | Bind to poly-ADP-ribose | CID | Gagné et al., 2012 |
| IMAC | For phosphopeptide enrichment | CID (site) | Chapman et al., 2013 |
| IMAC | For phosphopeptide enrichment | CID/HCD (site) | Daniels et al., 2014 |
| Boronate affinity chromatography | Boron forms ester bonds with the 1,2-cis-diol moiety in ADP-ribose; Asp-/Glu-ADP-ribose converted to hydroxamic acid | CID (site) | Zhang et al., 2013 |
| IMAC | For phosphopeptide enrichment | ETD (site) | Leidecker et al., 2016 |
| Af1521 macrodomain | Bind to both mono- and poly-ADP-ribose | HCD/ETD (site) | Martello et al., 2016 |
| Af1521 macrodomain | Bind to both mono- and poly-ADP-ribose | HCD/ETcaD/EThcD (site) | Bilan et al., 2017 |
mAf1521 is known to bind to not only mono-ADP-ribose, but also poly-ADP-ribose. This property has been exploited for the enrichment of ADP-ribosylated proteins from cells that have been exposed to genotoxic agents. Indeed, Jungmichel et al., used various DNA damaging agents, including H2O2, MMS, UV radiation and ionizing radiation to activate PARP1, and then used mAf1521 to isolate ADP-ribosylated proteins for their subsequent mass spectrometry identification (both PARylated proteome as well as PAR-interactome)(97). Of note, both PARP1 inhibitors and PARG inhibitors were included the lysis buffer to prevent the non-physiological introduction and removal of PAR chains during the cell lysis step. Jungmichel et al., were able to identify a total of 235 proteins that belong to the PARylated proteome and/or PAR interactome. More than 70% of the identified hits were nuclear proteins, a finding consistent with the notion that the majority of the PARylated proteins generated under genotoxic stress are mediated by PARP1, which itself is a nuclear protein. In a separate study, Gagné et al., used mAf1521 together with two other PAR-binding agents (i.e., 10H antibody and catalytically inactive PARG) to isolate the PARylated proteome and PAR interactome from cells that have been treated with a DNA-alkylating agents, MNNG(99). Intriguingly, it was found in these experiments that a significant fraction of the PARylated proteins were commonly identified in both the mAf1521 and PARG catalytically dead mutant pulldown samples (PARG also uses a macrodomain to bind to PARylated proteins). In contrast, the hits identified from the 10H enriched fraction contained more distinct proteins (e.g., mitochondrial proteins), a finding that can, at least in part, be ascribed to the superior affinity of the 10H antibody to longer PAR polymers (i.e., more than 20 ADP-ribose units)(99). Of note, a recent study showed that mAf1521 possesses intrinsic catalytic activity that cleaves protein-ADP-ribose linkages(41). This issue can be overcome, however, by performing the enrichment step at low temperature conditions (4 °C) (102).
One common theme that emerged from these proteome-wide studies is that PARylated proteins and PAR-binding proteins are involved in not only the canonical DNA damage response, but also many other important biological processes, including transcription control, RNA metabolism and epigenetic regulation. Further hypothesis-driven research is needed to elucidate the detailed mechanism by which PARP1 regulates these signaling events. In addition, it is currently unknown whether some of these proteins bind to PAR with specific topology. We expect that a more complete understanding of the PAR-interactome will be obtained by using structurally defined PAR chains. Finally, besides nicked DNA, PARP1 could also be activated by other stimuli. For example, PARP1 can be activated by binding to phosphorylated ERK2, an event that further promotes ERK2-catlayzed phosphorylation of the transcription factor Elk1(103). It is therefore important to also characterize the PARylated proteome in unstressed cells, and study how PARP1 is linked to additional biological processes through modulating the PAR-interaction network.
Site-specific Characterization of the ADP-ribosylated Proteome
After a PARylated protein is identified, the exact attachment site becomes the next question. This turns out to be a major challenge, again because of the heterogeneous, low-abundance and labile nature of this modification. Furthermore, many amino acids have been shown to be ADP-ribosylated, including Asp/Glu, Ser/Thr, Lys, Arg, Asn, Cys and diphthamide(36, 96, 102, 104–107). These side chain chemistries can be broadly divided into three groups, i.e. N-glycosides (i.e., Lys, Arg, diphthamide and Asn), O-glycosides (i.e., Asp/Glu and Ser/Thr) and others (i.e., Cys). Because of their distinct characteristics (e.g., chemical stability to NH2OH and alkali)(107), it has become clear that different methodologies are required for their optimal analysis. For example, the ester bond between Asp/Glu and ADP-ribose is highly sensitive to NH2OH. In contrast, ADP-ribose that is conjugated to Ser/Thr/Tyr is resistant to NH2OH treatment(108). Also ADP-ribosylation events in the N-glycoside group are in general resistant to this compound(107), although it has been suggested that ADP-ribose-conjugated to Arg can be slowly released upon NH2OH treatment(108). Several recent studies suggest that Asp/Glu-ADP-ribosylation represents a major form of cellular ADP-ribosylation, at least under H2O2 treatment conditions. For example, using the NH2OH derivatization method (see discussion below), Zhang et al., previously showed that GAR1, a protein involved in ribosome biogenesis and telomere maintenance, is PARylated at several Asp/Glu residues. Mutation of these residues completely abolishes its PARylation in an in vitro PARylation assay, suggesting that these sites are the major PAR-acceptor residues for this protein(36). As one of the most abundant ADP-ribosylated proteins in the cell, Karch et al found that a major fraction of histone proteins are modified by PARP1 on Asp/Glu, because NH2OH treatment nearly abolishes the PAR signal(109). In another study, Kraus lab developed various immunological reagents that recognize different forms of ADP-ribosylation (i.e., mono-, oligo- and poly-). They demonstrated that NH2OH could eliminate all forms of ADP-ribosylation signals in H2O2-treated HeLa cells(110). Finally, Zhen et al recently showed that the proteomic profile of D/E-ADP-ribosylation among various H2O2-treated breast (cancer) cell lines is very similar to the total PAR pattern detected by an immunoblotting assay, suggesting that D/E-ADP-ribosylation contributes to a major fraction of cellular PARylation under these conditions(111).
Biochemical studies have led to the identification of PARylation sites in several proteins, including histone H1, histone H2B, seminal ribonuclease and p53, with 1–4 modification sites pinpointed in each case(52, 112–114). However, these efforts were generally limited to traditional methods of amino acid sequencing coupled to in vitro PARylation reaction. Using ADP-ribosylated peptides and auto-modified PARP1 as model systems, mass spectrometry-based studies have tackled the labile and heterogeneous nature of PARylation. For example, in order to address the structural heterogeneity of PAR, Tao et al., used a PARP1 mutant (E988Q) that catalyzes only MARylation, but not PARylation. By using LC-MS/MS experiments, they pinpointed three PARP1 ADP-ribosylation sites, namely D387, E488 and E491 that cluster in/near its BRCT domain(115). In another study, Chapman et al., reduced the complexity of PAR by treating automodified PARP1 with phosphodiesterase (PDE). PDE cleaves the pyrophosphate bond within ADP-ribose, thereby converting a PARylated peptide into a peptide modified with a terminal ribose-5′-phosphate. Subsequent tandem MS experiments using CID (collision-induced fragmentation) produced sufficient fragment ions that led to the identification of eight novel automodification sites on PARP1(116).
In more recent years, several streamlined approaches have been developed for the site-specific analysis of the mammalian ADP-ribosylated proteome. The design of these strategies contains a number of common modules: (1) lysis procedures that preserve native PARylation. (2) affinity enrichment methods that allow the isolation of PARylated peptides/proteins. (3) chemical or enzymatic methods that convert a PAR polymer into a small mass tag that is more suitable for subsequent MS analysis. In so doing, the treatment also effectively reduces the negative charges on the modified peptide, and facilitates its ionization. Furthermore, in certain cases, this mass tag itself might also be used as an affinity handle for the isolation and enrichment of the modified peptides. (4) Optimized MS2 and bioinformatic procedures for sequencing the peptides, and identifying the ADP-ribosylation sites.
As an example, Zhang et al., developed an integrated approach towards the site-specific analysis of the cellular Asp-/Glu-ADP-ribosylated proteome(36). In this method, cells are lysed using an SDS buffer to inactivate PAR-metabolizing enzymes. PARylated peptides are enriched by boronate affinity chromatography (boron forms ester bonds with the 1,2-cis-diol moiety in ADP-ribose). Because boronate beads are chemically stable, harsh washing conditions (e.g., SDS buffer) can be applied to remove the non-specifically bound peptides. The Asp-/Glu-ADP-ribosylated peptides are eluted using NH2OH treatment, during which an ADP-ribosylated Asp/Glu residue is converted into a hydroxamic acid derivative. Compared to PARylation, the hydroxamic acid is a mass tag that is amenable to subsequent MS experiments: (1) it has a defined mass shift (+15.0109 Da). (2) it is very stable, and hence can be analyzed on any mass spectrometers with conventional tandem MS capabilities (e.g. collision-induced dissociation). (3) the hydroxamic acid is a small moiety that does not suppress the ionization of modified peptides. Zhang et al., were able to identify a total of 1,048 unique, unambiguously assigned Asp/Glu-ADP-ribosylation sites on 340 proteins. Based on the identified sites, they were able to extract a number of PARylation motifs, including PXE*, E*P, PXXE* and E*XXG. Even though PARylation can occur on a variety of amino acids (see more discussion below), they do not seem to be randomly modified, because some of these modified residues are highly conserved from an evolution point of view. In keeping with PARP1 being a nuclear polypeptide, these modified proteins are involved in a wide array of nuclear biological processes, including DNA damage repair, transcription regulation, epigenetic control and mRNA metabolism.
In the second method, Daniels et al., treated PARylated peptides with phosphodiesterase (PDE) or Nudix hydrolases to convert a PARylated peptide into a peptide modified with a terminal ribose-5′-phosphate(47, 117). The ribose-5′-phosphate moiety is a dual-function tag. First, because it contains a phosphate group, it can be used as an affinity handle to isolate the modified peptides using established strategies for phosphopeptide enrichment (e.g., IMAC and TiO2 beads)(118). Second, it has a defined mass shift (212 Da) that is more amenable to the subsequent MS analysis.
Using a method involving Nudix hydrolase digestion, IMAC enrichment and ETD (electron transfer dissociation) fragmentation (119), Leidecker was able to identify 12 unique ADP-ribosylation sites in human cancer cells, and report Ser as a new acceptor amino acid of ADP-ribosylation. Furthermore, these modified Ser residues often have a preceding Lys, serving as a potential motif for Ser-ADP-ribosylation (120). Alternatively, PARylated peptides can also be treated with PARG, which converts PAR polymers into a MAR unit that remains conjugated to the peptide. These MARylated peptides can be isolated by the Af1521 protein domain. Using LC-MS/MS experiments, more than 900 modification sites were identified in mammalian cells and liver samples. Intriguingly, it was found that Lys residues were the dominant ADP-ribosylation site during oxidative stress, whereas Arg represents the major acceptor residue in the liver(102). A detailed comparison of these methodologies is summarized in Table 2.
One important but poorly addressed question is whether there are mechanisms, under specific cellular conditions, that regulate different patterns of ADP-ribosylation, including amino acid acceptor, mono vs. poly, etc. Various lines of evidence support Asp/Glu as the major acceptor of ADP-ribosylation under H2O2-treated condition, whereas it has been shown that a large fraction of ADP-ribose is linked to arginine in normal liver samples(102). For histone proteins, Ahel and Matic groups found that Ser represents another ADP-ribosylation residue. Importantly, these modification events are dramatically increased when cells are exposed to H2O2, indicating that histone Ser ADP-ribosylation responds to DNA damage(120). More recently, they showed that Ser-ADP-ribosylation is regulated by a protein called HPF1 (histone PARylation factor 1). Specifically, the addition of HPF-1 to PARP1/2-mediated in vitro PARylation reaction is able to induce Ser-ADP-ribosylation of histones and PARP1(121). These results point to the intriguing question of whether there are additional regulatory factors that determine the amino acid specificity of ADP-ribosylation.
As an extremely unstable PTM, it is obvious that an optimized MS2 sequencing strategy is the key to the successful analysis of ADP-ribosylation sites. Early efforts of using the canonical collision-induced dissociation (CID) methods to sequence an ADP-ribosylated generally failed(95). In CID, peptides collide with noble gas, during which their kinetic energy is converted to internal energy. Because it is a slow fragmentation technique, the internal energy is distributed and channeled towards the weakest linkage in the molecule, leading to its breakage(122). The pyrophosphate bond, adenine moiety and amino acid side-chain linkage within ADP-ribose are much more labile than the peptide backbone. As a result, CID analysis of ADP-ribosylated peptides typically leads to the degradation of the ADP-ribose unit without releasing useful sequence specific ions. For HCD (higher energy collisional dissociation), even though the decomposition of ADP-ribose could yield diagnostic ions indicating the presence of an ADP-ribosylated peptide, the breakage of the amino acid-ADP-ribose linkage still occurs(106). More recently, several fragmentation techniques, including electron-transfer dissociation (ETD), have been developed that specifically target the peptide backbone(123). These gentler tandem MS approaches have been successfully used to sequence certain ADP-ribosylated peptides. However, compared to CID, ETD has several notable drawbacks, including low sensitivity and charge-state dependence(120). Furthermore, it is important to recognize that the stability of the linkage between ADP-ribose and various amino acids differs dramatically. For example, while the N-glycosidic bond between Arg and ADP-ribose is remarkably stable during tandem MS analyses, the linkage between Glu and ADP-ribose is much more labile(96). Whether all types of ADP-ribosylated amino acids can equally survive in ETD (or other tandem MS conditions) is unknown. Because the identification of an ADP-ribosylation site is dependent on detecting an intact ADP-ribose moiety on that residue, this stability issue could introduce potential bias, and cause an unequal representation of the modified residues(96). A systematic comparison of the stability of the various ADP-ribose-amino acid linkages (e.g., Asp, Glu, Arg, Lys and Ser, etc.) under different tandem MS conditions (i.e., CID, HCD and ETD) using molecularly-defined standards will be highly informative to understand the bias and limitation of each MS2 method. In this regard, Asp/Glu-ADP-ribosylated peptides can be treated with NH2OH to generate the hydroxamic acid derivative, which is stable, and is compatible with conventional CID(36, 111). However, this method is limited to Asp/Glu-ADP-ribosylation. Finally, many potential ADP-ribosylation sites tend to cluster in the primary sequence, which lead to the formation of sequence-specific ions with only subtle differences. It is imperative to generate high quality MS2 spectra, and use algorithms optimized for PTM site-assignment to confidently and unambiguously pinpoint the ADP-ribosylation sites. With the advent of improved MS and bioinformatic strategies, we have the reason to believe that additional unknown target sites will be discovered.
The abovementioned chemical and enzymatic treatments, however, will invariably lead to the loss of the topological information encoded in the PAR polymers, an issue reminiscent of the analysis of other polymeric and heterogeneous protein modifications (e.g. glycosylation and ubiquitination). Improved methods that allow the comprehensive analysis of both the modification site on proteins, as well as the structure features of PAR itself will provide additional insights that are critical towards understanding the potential functional role of this modification.
Functional Interrogation of the Downstream Signaling Network of PARPs
With many of ADP-ribosylation sites identified, researchers are now able to use classical biochemical and cell biological methods (e.g. site-directed mutagenesis) to address the functional role of this modification in a site-specific manner. For example, Li et al., showed that ADP-ribosylation of PTEN is mediated by a PARP called tankyrase, and the function of ADP-ribosylation is to regulate the stability of PTEN(124). Furthermore, as an important step towards the functional study of the PARP downstream signaling network, mass spectrometry technologies can be applied, in a quantitative fashion, to characterize the remodeling of the ADP-ribosylated proteome upon specific perturbation (e.g., DNA damage or PARP1 inhibitor treatment)(36, 102, 125).
Recent progresses in the development of new methods, including PAR-detection reagents, chemical genetic approaches, and ADP-ribosylation-specific genomic assays, have greatly facilitated the functional interpretation of the downstream signaling network of PARP. Because all the PARPs use NAD+ as the cofactor to catalyze ADP-ribosylation, one important question relates to what is the substrate specificity of these various PARP family members. To address this, a bump-and-hole approach could be used where an orthogonal NAD+ variant can specifically bind to engineered PARPs but not wild-type counterparts, which allows the affinity purification of the modified proteins(126, 127). Gibson et al., developed an NAD+ analog-sensitive approach that allows the identification of the proteome and genome targets of specific PARP family members(127). They were able to pinpoint hundreds of ADP-ribosylation sites on substrate proteins of PARP1, PARP2 and PARP3. Furthermore, they followed the site-specific function of ADP-ribosylation on NELF (negative elongation factor), which is a protein complex that plays a critical role in the negative regulation of transcription elongation via pausing RNA polymerase II (Pol II). Intriguingly, it was found ADP-ribosylation of NELF-E hampers its binding to RNA, and then inhibits the pausing ability.
It has been well recognized that DNA strand breaks are potent activators of PARP1. However, the mechanisms of how PARP1 activity is regulated in vivo and how this affects the context-dependent PARP1 signaling are poorly understood. Using the hydroxylamine chemistry, Zhen et al., recently performed global characterization of the Asp/Glu-ADP-ribosylated proteome in a panel of cell lines, including benign breast epithelial cells, as well as cells derived from common subtypes of breast cancer(111). Despite similar expression levels, PARP1 is differentially activated in these breast (cancer) cells in response to H2O2 treatment. Furthermore, many proteins are modified by PARP1 in a cell-specific manner, including those involved in transcription, mRNA splicing and protein synthesis. In particular, PARP1 targets in MDA-MB-468 cells are enriched with ribosomal proteins. A number of these PARylation sites are located at the interface between the 40S and 60S ribosomal subunits, pointing to the intriguing possibility that PARylation could interfere with the assembly of ribosomal particles, and thereby regulate protein translation during genotoxic stress.
Finally, characterization of ADP-ribosylation at the site level opens up new lines of understanding of its crosstalk with other PTMs. One notable example is the histone proteins, which can be modified by a variety of PTMs, including phosphorylation, ubiquitination, NEDDylation, SUMOylation, methylation, acetylation and ADP-ribosylation(128). Indeed, it has been shown that among the 12 Ser-ADP-ribosylation sites on histones, six of them can also undergo phosphorylation(120). It is conceivable that as one or more of these Ser residues become ADP-ribosylated, that can prevent them from being phosphorylated, a scenario reminiscent of the antagonism between Ser phosphorylation and O-GlcNAc modification(129). Similarly, ADP-ribosylation of Lys could block other PTMs on Lys, including ubiquitination, acetylation and methylation. The functional consequence of these potential crosstalk mechanisms obviously warrants future investigation.
Conclusions and perspectives
The fundamental understanding of PARP biology has been facilitated by technological advances that allow the characterization of the ADP-ribosylated proteome in a global, quantitative and site-specific manner. These studies have provided promising candidate PARP targets that connect PARP to the many biological processes regulated by this important family of proteins. Further technological progress will clearly be required in order to answer additional questions, including differentiating PARylated vs. MARylated proteins, dissecting the topology of protein-linked PAR chains, and elucidating the spatial-temporal regulation of ADP-ribosylation sites, etc. It is expected that these data sets will serve as an invaluable resource, which will seed future hypothesis-driven research that helps delineate the signaling and functional properties of this therapeutically important protein modification.
Acknowledgments
This work was supported in part by grants from the Welch Foundation (I-1800 to Y.Y.) and NIH (R01 GM122932 to Y.Y.).
References
- 1.D’Amours D, Desnoyers S, D’Silva I, Poirier GG. Poly(ADP-ribosyl)ation reactions in the regulation of nuclear functions. Biochem J. 1999;342:249–268. [PMC free article] [PubMed] [Google Scholar]
- 2.Gibson BA, Kraus WL. New insights into the molecular and cellular functions of poly(ADP-ribose) and PARPs. Nat Rev Mol Cell Biol. 2012;13:411–424. doi: 10.1038/nrm3376. [DOI] [PubMed] [Google Scholar]
- 3.Hottiger MO. Nuclear ADP-Ribosylation and Its Role in Chromatin Plasticity, Cell Differentiation, and Epigenetics. Annu Rev Biochem. 2015;84:227–263. doi: 10.1146/annurev-biochem-060614-034506. [DOI] [PubMed] [Google Scholar]
- 4.Krueger KM, Barbieri JT. The family of bacterial ADP-ribosylating exotoxins. Clin Microbiol Rev. 1995;8:34–47. doi: 10.1128/cmr.8.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hawse WF, Wolberger C. Structure-based mechanism of ADP-ribosylation by sirtuins. J Biol Chem. 2009;284:33654–33661. doi: 10.1074/jbc.M109.024521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu C, Vyas A, Kassab MA, Singh AK, Yu X. The role of poly ADP-ribosylation in the first wave of DNA damage response. Nucleic Acids Res. 2017;45:8129–8141. doi: 10.1093/nar/gkx565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alkhatib HM, Chen DF, Cherney B, Bhatia K, Notario V, Giri C, Stein G, Slattery E, Roeder RG, Smulson ME. Cloning and expression of cDNA for human poly(ADP-ribose) polymerase. Proc Natl Acad Sci U S A. 1987;84:1224–1228. doi: 10.1073/pnas.84.5.1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suzuki H, Uchida K, Shima H, Sato T, Okamoto T, Kimura T, Miwa M. Molecular cloning of cDNA for human poly(ADP-ribose) polymerase and expression of its gene during HL-60 cell differentiation. Biochem Biophys Res Commun. 1987;146:403–409. doi: 10.1016/0006-291x(87)90543-2. [DOI] [PubMed] [Google Scholar]
- 9.Uchida K, Morita T, Sato T, Ogura T, Yamashita R, Noguchi S, Suzuki H, Nyunoya H, Miwa M, Sugimura T. Nucleotide sequence of a full-length cDNA for human fibroblast poly(ADP-ribose) polymerase. Biochem Biophys Res Commun. 1987;148:617–622. doi: 10.1016/0006-291x(87)90921-1. [DOI] [PubMed] [Google Scholar]
- 10.Ame JC, Rolli V, Schreiber V, Niedergang C, Apiou F, Decker P, Muller S, Hoger T, Menissier-de Murcia J, de Murcia G. PARP-2, A novel mammalian DNA damage-dependent poly(ADP-ribose) polymerase. The Journal of biological chemistry. 1999;274:17860–17868. doi: 10.1074/jbc.274.25.17860. [DOI] [PubMed] [Google Scholar]
- 11.Ame JC, Spenlehauer C, de Murcia G. The PARP superfamily. BioEssays: news and reviews in molecular, cellular and developmental biology. 2004;26:882–893. doi: 10.1002/bies.20085. [DOI] [PubMed] [Google Scholar]
- 12.Boehler C, Gauthier LR, Mortusewicz O, Biard DS, Saliou JM, Bresson A, Sanglier-Cianferani S, Smith S, Schreiber V, Boussin F, Dantzer F. Poly(ADP-ribose) polymerase 3 (PARP3), a newcomer in cellular response to DNA damage and mitotic progression. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:2783–2788. doi: 10.1073/pnas.1016574108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaminker PG, Kim SH, Taylor RD, Zebarjadian Y, Funk WD, Morin GB, Yaswen P, Campisi J. TANK2, a new TRF1-associated poly(ADP-ribose) polymerase, causes rapid induction of cell death upon overexpression. The Journal of biological chemistry. 2001;276:35891–35899. doi: 10.1074/jbc.M105968200. [DOI] [PubMed] [Google Scholar]
- 14.Kickhoefer VA, Siva AC, Kedersha NL, Inman EM, Ruland C, Streuli M, Rome LH. The 193-kD vault protein, VPARP, is a novel poly(ADP-ribose) polymerase. The Journal of cell biology. 1999;146:917–928. doi: 10.1083/jcb.146.5.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith S, Giriat I, Schmitt A, de Lange T. Tankyrase, a poly(ADP-ribose) polymerase at human telomeres. Science (New York, NY) 1998;282:1484–1487. doi: 10.1126/science.282.5393.1484. [DOI] [PubMed] [Google Scholar]
- 16.Otto H, Reche PA, Bazan F, Dittmar K, Haag F, Koch-Nolte F. In silico characterization of the family of PARP-like poly(ADP-ribosyl)transferases (pARTs) BMC genomics. 2005;6:139. doi: 10.1186/1471-2164-6-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ma Q, Baldwin KT, Renzelli AJ, McDaniel A, Dong L. TCDD-inducible poly(ADP-ribose) polymerase: a novel response to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Biochemical and biophysical research communications. 2001;289:499–506. doi: 10.1006/bbrc.2001.5987. [DOI] [PubMed] [Google Scholar]
- 18.Vyas S, Matic I, Uchima L, Rood J, Zaja R, Hay RT, Ahel I, Chang P. Family-wide analysis of poly(ADP-ribose) polymerase activity. Nat Commun. 2014;5:4426. doi: 10.1038/ncomms5426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hottiger MO, Hassa PO, Luscher B, Schuler H, Koch-Nolte F. Toward a unified nomenclature for mammalian ADP-ribosyltransferases. Trends in biochemical sciences. 2010;35:208–219. doi: 10.1016/j.tibs.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 20.Haince JF, McDonald D, Rodrigue A, Dery U, Masson JY, Hendzel MJ, Poirier GG. PARP1-dependent kinetics of recruitment of MRE11 and NBS1 proteins to multiple DNA damage sites. J Biol Chem. 2008;283:1197–1208. doi: 10.1074/jbc.M706734200. [DOI] [PubMed] [Google Scholar]
- 21.Wielckens K, George E, Pless T, Hilz H. Stimulation of poly(ADP-ribosyl)ation during Ehrlich ascites tumor cell “starvation” and suppression of concomitant DNA fragmentation by benzamide. J Biol Chem. 1983;258:4098–4104. [PubMed] [Google Scholar]
- 22.Alvarez-Gonzalez R, Althaus FR. Poly(ADP-ribose) catabolism in mammalian cells exposed to DNA-damaging agents. Mutat Res. 1989;218:67–74. doi: 10.1016/0921-8777(89)90012-8. [DOI] [PubMed] [Google Scholar]
- 23.Simonin F, Poch O, Delarue M, de Murcia G. Identification of potential active-site residues in the human poly(ADP-ribose) polymerase. J Biol Chem. 1993;268:8529–8535. [PubMed] [Google Scholar]
- 24.Ludwig A, Behnke B, Holtlund J, Hilz H. Immunoquantitation and size determination of intrinsic poly(ADP-ribose) polymerase from acid precipitates. An analysis of the in vivo status in mammalian species and in lower eukaryotes. J Biol Chem. 1988;263:6993–6999. [PubMed] [Google Scholar]
- 25.Yamanaka H, Penning CA, Willis EH, Wasson DB, Carson DA. Characterization of human poly(ADP-ribose) polymerase with autoantibodies. J Biol Chem. 1988;263:3879–3883. [PubMed] [Google Scholar]
- 26.Lautier D, Lagueux J, Thibodeau J, Menard L, Poirier GG. Molecular and biochemical features of poly (ADP-ribose) metabolism. Mol Cell Biochem. 1993;122:171–193. doi: 10.1007/BF01076101. [DOI] [PubMed] [Google Scholar]
- 27.Langelier MF, Planck JL, Roy S, Pascal JM. Structural basis for DNA damage-dependent poly(ADP-ribosyl)ation by human PARP-1. Science. 2012;336:728–732. doi: 10.1126/science.1216338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ali AAE, Timinszky G, Arribas-Bosacoma R, Kozlowski M, Hassa PO, Hassler M, Ladurner AG, Pearl LH, Oliver AW. The zinc-finger domains of PARP1 cooperate to recognize DNA strand breaks. Nat Struct Mol Biol. 2012;19:685–692. doi: 10.1038/nsmb.2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Langelier MF, Planck JL, Roy S, Pascal JM. Crystal structures of poly(ADPribose) polymerase-1 (PARP-1) zinc fingers bound to DNA: structural and functional insights into DNA-dependent PARP-1 activity. J Biol Chem. 2011;286:10690–10701. doi: 10.1074/jbc.M110.202507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Caldecott KW, Aoufouchi S, Johnson P, Shall S. XRCC1 polypeptide interacts with DNA polymerase beta and possibly poly (ADP-ribose) polymerase, and DNA ligase III is a novel molecular ‘nick-sensor’ in vitro. Nucleic Acids Res. 1996;24:4387–4394. doi: 10.1093/nar/24.22.4387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shieh WM, Ame JC, Wilson MV, Wang ZQ, Koh DW, Jacobson MK, Jacobson EL. Poly(ADP-ribose) polymerase null mouse cells synthesize ADP-ribose polymers. J Biol Chem. 1998;273:30069–30072. doi: 10.1074/jbc.273.46.30069. [DOI] [PubMed] [Google Scholar]
- 32.Min W, Wang ZQ. Poly (ADP-ribose) glycohydrolase (PARG) and its therapeutic potential. Front Biosci (Landmark Ed) 2009;14:1619–1626. doi: 10.2741/3329. [DOI] [PubMed] [Google Scholar]
- 33.Catara G, Grimaldi G, Schembri L, Spano D, Turacchio G, Lo Monte M, Beccari AR, Valente C, Corda D. PARP1-produced poly-ADP-ribose causes the PARP12 translocation to stress granules and impairment of Golgi complex functions. Sci Rep. 2017;7:14035. doi: 10.1038/s41598-017-14156-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kotova E, Jarnik M, Tulin AV. Poly (ADP-ribose) polymerase 1 is required for protein localization to Cajal body. PLoS Genet. 2009;5:e1000387. doi: 10.1371/journal.pgen.1000387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leung AK, Vyas S, Rood JE, Bhutkar A, Sharp PA, Chang P. Poly(ADP-ribose) regulates stress responses and microRNA activity in the cytoplasm. Mol Cell. 2011;42:489–499. doi: 10.1016/j.molcel.2011.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang Y, Wang J, Ding M, Yu Y. Site-specific characterization of the Asp- and Glu- ADP-ribosylated proteome. Nat Methods. 2013;10:981–984. doi: 10.1038/nmeth.2603. [DOI] [PubMed] [Google Scholar]
- 37.Koh DW, Lawler AM, Poitras MF, Sasaki M, Wattler S, Nehls MC, Stoger T, Poirier GG, Dawson VL, Dawson TM. Failure to degrade poly(ADP-ribose) causes increased sensitivity to cytotoxicity and early embryonic lethality. Proc Natl Acad Sci U S A. 2004;101:17699–17704. doi: 10.1073/pnas.0406182101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Menissier de Murcia J, Ricoul M, Tartier L, Niedergang C, Huber A, Dantzer F, Schreiber V, Ame JC, Dierich A, LeMeur M, Sabatier L, Chambon P, de Murcia G. Functional interaction between PARP-1 and PARP-2 in chromosome stability and embryonic development in mouse. EMBO J. 2003;22:2255–2263. doi: 10.1093/emboj/cdg206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Slade D, Dunstan MS, Barkauskaite E, Weston R, Lafite P, Dixon N, Ahel M, Leys D, Ahel I. The structure and catalytic mechanism of a poly(ADP-ribose) glycohydrolase. Nature. 2011;477:616–620. doi: 10.1038/nature10404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sharifi R, Morra R, Appel CD, Tallis M, Chioza B, Jankevicius G, Simpson MA, Matic I, Ozkan E, Golia B, Schellenberg MJ, Weston R, Williams JG, Rossi MN, Galehdari H, Krahn J, Wan A, Trembath RC, Crosby AH, Ahel D, Hay R, Ladurner AG, Timinszky G, Williams RS, Ahel I. Deficiency of terminal ADP-ribose protein glycohydrolase TARG1/C6orf130 in neurodegenerative disease. EMBO J. 2013;32:1225–1237. doi: 10.1038/emboj.2013.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jankevicius G, Hassler M, Golia B, Rybin V, Zacharias M, Timinszky G, Ladurner AG. A family of macrodomain proteins reverses cellular mono-ADP-ribosylation. Nat Struct Mol Biol. 2013;20:508–514. doi: 10.1038/nsmb.2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rosenthal F, Feijs KL, Frugier E, Bonalli M, Forst AH, Imhof R, Winkler HC, Fischer D, Caflisch A, Hassa PO, Luscher B, Hottiger MO. Macrodomain-containing proteins are new mono-ADP-ribosylhydrolases. Nature structural & molecular biology. 2013;20:502–507. doi: 10.1038/nsmb.2521. [DOI] [PubMed] [Google Scholar]
- 43.Takada T, Okazaki IJ, Moss J. ADP-ribosylarginine hydrolases. Molecular and cellular biochemistry. 1994;138:119–122. doi: 10.1007/BF00928452. [DOI] [PubMed] [Google Scholar]
- 44.Niere M, Mashimo M, Agledal L, Dolle C, Kasamatsu A, Kato J, Moss J, Ziegler M. ADP-ribosylhydrolase 3 (ARH3), not poly(ADP-ribose) glycohydrolase (PARG) isoforms, is responsible for degradation of mitochondrial matrix-associated poly(ADP-ribose) The Journal of biological chemistry. 2012;287:16088–16102. doi: 10.1074/jbc.M112.349183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fontana P, Bonfiglio JJ, Palazzo L, Bartlett E, Matic I, Ahel I. Serine ADP-ribosylation reversal by the hydrolase ARH3. Elife. 2017;6 doi: 10.7554/eLife.28533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Abplanalp J, Leutert M, Frugier E, Nowak K, Feurer R, Kato J, Kistemaker HVA, Filippov DV, Moss J, Caflisch A, Hottiger MO. Proteomic analyses identify ARH3 as a serine mono-ADP-ribosylhydrolase. Nat Commun. 2017;8:2055. doi: 10.1038/s41467-017-02253-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Daniels CM, Thirawatananond P, Ong SE, Gabelli SB, Leung AK. Nudix hydrolases degrade protein-conjugated ADP-ribose. Sci Rep. 2015;5:18271. doi: 10.1038/srep18271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Palazzo L, Thomas B, Jemth AS, Colby T, Leidecker O, Feijs KL, Zaja R, Loseva O, Puigvert JC, Matic I, Helleday T, Ahel I. Processing of protein ADP-ribosylation by Nudix hydrolases. The Biochemical journal. 2015;468:293–301. doi: 10.1042/BJ20141554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Palazzo L, Daniels CM, Nettleship JE, Rahman N, McPherson RL, Ong SE, Kato K, Nureki O, Leung AK, Ahel I. ENPP1 processes protein ADP-ribosylation in vitro. The FEBS journal. 2016;283:3371–3388. doi: 10.1111/febs.13811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ferro AM, Olivera BM. Poly(ADP-ribosylation) in vitro. Reaction parameters and enzyme mechanism. J Biol Chem. 1982;257:7808–7813. [PubMed] [Google Scholar]
- 51.Mendoza-Alvarez H, Alvarez-Gonzalez R. Poly(ADP-ribose) polymerase is a catalytic dimer and the automodification reaction is intermolecular. J Biol Chem. 1993;268:22575–22580. [PubMed] [Google Scholar]
- 52.Kanai M, Hanashiro K, Kim SH, Hanai S, Boulares AH, Miwa M, Fukasawa K. Inhibition of Crm1-p53 interaction and nuclear export of p53 by poly(ADP-ribosyl)ation. Nat Cell Biol. 2007;9:1175–1183. doi: 10.1038/ncb1638. [DOI] [PubMed] [Google Scholar]
- 53.Collier RJ. Understanding the mode of action of diphtheria toxin: a perspective on progress during the 20th century. Toxicon. 2001;39:1793–1803. doi: 10.1016/s0041-0101(01)00165-9. [DOI] [PubMed] [Google Scholar]
- 54.Masson M, Niedergang C, Schreiber V, Muller S, Menissier-de Murcia J, de Murcia G. XRCC1 is specifically associated with poly(ADP-ribose) polymerase and negatively regulates its activity following DNA damage. Mol Cell Biol. 1998;18:3563–3571. doi: 10.1128/mcb.18.6.3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang F, Chen Y, Li M, Yu X. The oligonucleotide/oligosaccharide-binding fold motif is a poly(ADP-ribose)-binding domain that mediates DNA damage response. Proc Natl Acad Sci U S A. 2014;111:7278–7283. doi: 10.1073/pnas.1318367111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Goodwin PM, Lewis PJ, Davies MI, Skidmore CJ, Shall S. The effect of gamma radiation and neocarzinostatin on NAD and ATP levels in mouse leukaemia cells. Biochim Biophys Acta. 1978;543:576–582. doi: 10.1016/0304-4165(78)90312-4. [DOI] [PubMed] [Google Scholar]
- 57.Skidmore CJ, Davies MI, Goodwin PM, Halldorsson H, Lewis PJ, Shall S, Zia’ee AA. The involvement of poly(ADP-ribose) polymerase in the degradation of NAD caused by gamma-radiation and N-methyl-N-nitrosourea. Eur J Biochem. 1979;101:135–142. doi: 10.1111/j.1432-1033.1979.tb04225.x. [DOI] [PubMed] [Google Scholar]
- 58.Boulares AH, Yakovlev AG, Ivanova V, Stoica BA, Wang G, Iyer S, Smulson M. Role of poly(ADP-ribose) polymerase (PARP) cleavage in apoptosis. Caspase 3-resistant PARP mutant increases rates of apoptosis in transfected cells. J Biol Chem. 1999;274:22932–22940. doi: 10.1074/jbc.274.33.22932. [DOI] [PubMed] [Google Scholar]
- 59.Dawson TM, Dawson VL. Mitochondrial Mechanisms of Neuronal Cell Death: Potential Therapeutics. Annu Rev Pharmacol Toxicol. 2017;57:437–454. doi: 10.1146/annurev-pharmtox-010716-105001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yu SW, Wang H, Poitras MF, Coombs C, Bowers WJ, Federoff HJ, Poirier GG, Dawson TM, Dawson VL. Mediation of poly(ADP-ribose) polymerase-1- dependent cell death by apoptosis-inducing factor. Science. 2002;297:259–263. doi: 10.1126/science.1072221. [DOI] [PubMed] [Google Scholar]
- 61.Wang Y, Dawson VL, Dawson TM. Poly(ADP-ribose) signals to mitochondrial AIF: a key event in parthanatos. Exp Neurol. 2009;218:193–202. doi: 10.1016/j.expneurol.2009.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang Y, An R, Umanah GK, Park H, Nambiar K, Eacker SM, Kim B, Bao L, Harraz MM, Chang C, Chen R, Wang JE, Kam TI, Jeong JS, Xie Z, Neifert S, Qian J, Andrabi SA, Blackshaw S, Zhu H, Song H, Ming GL, Dawson VL, Dawson TM. A nuclease that mediates cell death induced by DNA damage and poly(ADP-ribose) polymerase-1. Science. 2016;354 doi: 10.1126/science.aad6872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Virag L, Szabo C. The therapeutic potential of poly(ADP-ribose) polymerase inhibitors. Pharmacol Rev. 2002;54:375–429. doi: 10.1124/pr.54.3.375. [DOI] [PubMed] [Google Scholar]
- 64.Yoshida K, Miki Y. Role of BRCA1 and BRCA2 as regulators of DNA repair, transcription, and cell cycle in response to DNA damage. Cancer Sci. 2004;95:866–871. doi: 10.1111/j.1349-7006.2004.tb02195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wu J, Lu LY, Yu X. The role of BRCA1 in DNA damage response. Protein Cell. 2010;1:117–123. doi: 10.1007/s13238-010-0010-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Venkitaraman AR. Cancer susceptibility and the functions of BRCA1 and BRCA2. Cell. 2002;108:171–182. doi: 10.1016/s0092-8674(02)00615-3. [DOI] [PubMed] [Google Scholar]
- 67.Campeau PM, Foulkes WD, Tischkowitz MD. Hereditary breast cancer: new genetic developments, new therapeutic avenues. Hum Genet. 2008;124:31–42. doi: 10.1007/s00439-008-0529-1. [DOI] [PubMed] [Google Scholar]
- 68.Pal T, Permuth-Wey J, Betts JA, Krischer JP, Fiorica J, Arango H, LaPolla J, Hoffman M, Martino MA, Wakeley K, Wilbanks G, Nicosia S, Cantor A, Sutphen R. BRCA1 and BRCA2 mutations account for a large proportion of ovarian carcinoma cases. Cancer. 2005;104:2807–2816. doi: 10.1002/cncr.21536. [DOI] [PubMed] [Google Scholar]
- 69.Li M, Chen Q, Ma T, Yu X. Targeting reactive nitrogen species suppresses hereditary pancreatic cancer. Proc Natl Acad Sci U S A. 2017;114:7106–7111. doi: 10.1073/pnas.1702156114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Castro E, Goh C, Olmos D, Saunders E, Leongamornlert D, Tymrakiewicz M, Mahmud N, Dadaev T, Govindasami K, Guy M, Sawyer E, Wilkinson R, Ardern-Jones A, Ellis S, Frost D, Peock S, Evans DG, Tischkowitz M, Cole T, Davidson R, Eccles D, Brewer C, Douglas F, Porteous ME, Donaldson A, Dorkins H, Izatt L, Cook J, Hodgson S, Kennedy MJ, Side LE, Eason J, Murray A, Antoniou AC, Easton DF, Kote-Jarai Z, Eeles R. Germline BRCA mutations are associated with higher risk of nodal involvement, distant metastasis, and poor survival outcomes in prostate cancer. J Clin Oncol. 2013;31:1748–1757. doi: 10.1200/JCO.2012.43.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Foulkes WD, Wong N, Brunet JS, Begin LR, Zhang JC, Martinez JJ, Rozen F, Tonin PN, Narod SA, Karp SE, Pollak MN. Germ-line BRCA1 mutation is an adverse prognostic factor in Ashkenazi Jewish women with breast cancer. Clin Cancer Res. 1997;3:2465–2469. [PubMed] [Google Scholar]
- 72.Stoppa-Lyonnet D, Ansquer Y, Dreyfus H, Gautier C, Gauthier-Villars M, Bourstyn E, Clough KB, Magdelenat H, Pouillart P, Vincent-Salomon A, Fourquet A, Asselain B. Familial invasive breast cancers: worse outcome related to BRCA1 mutations. J Clin Oncol. 2000;18:4053–4059. doi: 10.1200/JCO.2000.18.24.4053. [DOI] [PubMed] [Google Scholar]
- 73.Bryant HE, Schultz N, Thomas HD, Parker KM, Flower D, Lopez E, Kyle S, Meuth M, Curtin NJ, Helleday T. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature. 2005;434:913–917. doi: 10.1038/nature03443. [DOI] [PubMed] [Google Scholar]
- 74.Farmer H, McCabe N, Lord CJ, Tutt ANJ, Johnson DA, Richardson TB, Santarosa M, Dillon KJ, Hickson I, Knights C, Martin NMB, Jackson SP, Smith GCM, Ashworth A. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434:917–921. doi: 10.1038/nature03445. [DOI] [PubMed] [Google Scholar]
- 75.Edwards SL, Brough R, Lord CJ, Natrajan R, Vatcheva R, Levine DA, Boyd J, Reis-Filho JS, Ashworth A. Resistance to therapy caused by intragenic deletion in BRCA2. Nature. 2008;451:1111–1115. doi: 10.1038/nature06548. [DOI] [PubMed] [Google Scholar]
- 76.Barber LJ, Sandhu S, Chen L, Campbell J, Kozarewa I, Fenwick K, Assiotis I, Rodrigues DN, Reis Filho JS, Moreno V, Mateo J, Molife LR, De Bono J, Kaye S, Lord CJ, Ashworth A. Secondary mutations in BRCA2 associated with clinical resistance to a PARP inhibitor. J Pathol. 2013;229:422–429. doi: 10.1002/path.4140. [DOI] [PubMed] [Google Scholar]
- 77.Murai J, Huang SY, Das BB, Renaud A, Zhang Y, Doroshow JH, Ji J, Takeda S, Pommier Y. Trapping of PARP1 and PARP2 by Clinical PARP Inhibitors. Cancer Res. 2012;72:5588–5599. doi: 10.1158/0008-5472.CAN-12-2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lord CJ, Ashworth A. PARP inhibitors: Synthetic lethality in the clinic. Science. 2017;355:1152–1158. doi: 10.1126/science.aam7344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Guha M. PARP inhibitors stumble in breast cancer. Nat Biotechnol. 2011;29:373–374. doi: 10.1038/nbt0511-373. [DOI] [PubMed] [Google Scholar]
- 80.Liu X, Shi Y, Maag DX, Palma JP, Patterson MJ, Ellis PA, Surber BW, Ready DB, Soni NB, Ladror US, Xu AJ, Iyer R, Harlan JE, Solomon LR, Donawho CK, Penning TD, Johnson EF, Shoemaker AR. Iniparib nonselectively modifies cysteine-containing proteins in tumor cells and is not a bona fide PARP inhibitor. Clin Cancer Res. 2012;18:510–523. doi: 10.1158/1078-0432.CCR-11-1973. [DOI] [PubMed] [Google Scholar]
- 81.Patel AG, De Lorenzo SB, Flatten KS, Poirier GG, Kaufmann SH. Failure of iniparib to inhibit poly(ADP-Ribose) polymerase in vitro. Clin Cancer Res. 2012;18:1655–1662. doi: 10.1158/1078-0432.CCR-11-2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Menear KA, Adcock C, Boulter R, Cockcroft XL, Copsey L, Cranston A, Dillon KJ, Drzewiecki J, Garman S, Gomez S, Javaid H, Kerrigan F, Knights C, Lau A, Loh VM, Jr, Matthews IT, Moore S, O’Connor MJ, Smith GC, Martin NM. 4-[3-(4- cyclopropanecarbonylpiperazine-1-carbonyl)-4-fluorobenzyl]-2H-phthalazin- 1-one: a novel bioavailable inhibitor of poly(ADP-ribose) polymerase-1. J Med Chem. 2008;51:6581–6591. doi: 10.1021/jm8001263. [DOI] [PubMed] [Google Scholar]
- 83.Thomas HD, Calabrese CR, Batey MA, Canan S, Hostomsky Z, Kyle S, Maegley KA, Newell DR, Skalitzky D, Wang LZ, Webber SE, Curtin NJ. Preclinical selection of a novel poly(ADP-ribose) polymerase inhibitor for clinical trial. Mol Cancer Ther. 2007;6:945–956. doi: 10.1158/1535-7163.MCT-06-0552. [DOI] [PubMed] [Google Scholar]
- 84.Jones P, Altamura S, Boueres J, Ferrigno F, Fonsi M, Giomini C, Lamartina S, Monteagudo E, Ontoria JM, Orsale MV, Palumbi MC, Pesci S, Roscilli G, Scarpelli R, Schultz-Fademrecht C, Toniatti C, Rowley M. Discovery of 2-{4-[(3S)-piperidin-3- yl]phenyl}-2H-indazole-7-carboxamide (MK-4827): a novel oral poly(ADP-ribose)polymerase (PARP) inhibitor efficacious in BRCA-1 and -2 mutant tumors. J Med Chem. 2009;52:7170–7185. doi: 10.1021/jm901188v. [DOI] [PubMed] [Google Scholar]
- 85.Shen Y, Rehman FL, Feng Y, Boshuizen J, Bajrami I, Elliott R, Wang B, Lord CJ, Post LE, Ashworth A. BMN 673, a novel and highly potent PARP1/2 inhibitor for the treatment of human cancers with DNA repair deficiency. Clin Cancer Res. 2013;19:5003–5015. doi: 10.1158/1078-0432.CCR-13-1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Donawho CK, Luo Y, Penning TD, Bauch JL, Bouska JJ, Bontcheva-Diaz VD, Cox BF, DeWeese TL, Dillehay LE, Ferguson DC, Ghoreishi-Haack NS, Grimm DR, Guan R, Han EK, Holley-Shanks RR, Hristov B, Idler KB, Jarvis K, Johnson EF, Kleinberg LR, Klinghofer V, Lasko LM, Liu X, Marsh KC, McGonigal TP, Meulbroek JA, Olson AM, Palma JP, Rodriguez LE, Shi Y, Stavropoulos JA, Tsurutani AC, Zhu GD, Rosenberg SH, Giranda VL, Frost DJ. ABT-888, an orally active poly(ADP-ribose) polymerase inhibitor that potentiates DNA-damaging agents in preclinical tumor models. Clin Cancer Res. 2007;13:2728–2737. doi: 10.1158/1078-0432.CCR-06-3039. [DOI] [PubMed] [Google Scholar]
- 87.Ledermann J, Harter P, Gourley C, Friedlander M, Vergote I, Rustin G, Scott C, Meier W, Shapira-Frommer R, Safra T, Matei D, Macpherson E, Watkins C, Carmichael J, Matulonis U. Olaparib maintenance therapy in platinum-sensitive relapsed ovarian cancer. N Engl J Med. 2012;366:1382–1392. doi: 10.1056/NEJMoa1105535. [DOI] [PubMed] [Google Scholar]
- 88.Mirza MR, Monk BJ, Herrstedt J, Oza AM, Mahner S, Redondo A, Fabbro M, Ledermann JA, Lorusso D, Vergote I, Ben-Baruch NE, Marth C, Madry R, Christensen RD, Berek JS, Dorum A, Tinker AV, du Bois A, Gonzalez-Martin A, Follana P, Benigno B, Rosenberg P, Gilbert L, Rimel BJ, Buscema J, Balser JP, Agarwal S, Matulonis UA. Niraparib Maintenance Therapy in Platinum-Sensitive, Recurrent Ovarian Cancer. N Engl J Med. 2016;375:2154–2164. doi: 10.1056/NEJMoa1611310. [DOI] [PubMed] [Google Scholar]
- 89.Swisher EM, Lin KK, Oza AM, Scott CL, Giordano H, Sun J, Konecny GE, Coleman RL, Tinker AV, O’Malley DM, Kristeleit RS, Ma L, Bell-McGuinn KM, Brenton JD, Cragun JM, Oaknin A, Ray-Coquard I, Harrell MI, Mann E, Kaufmann SH, Floquet A, Leary A, Harding TC, Goble S, Maloney L, Isaacson J, Allen AR, Rolfe L, Yelensky R, Raponi M, McNeish IA. Rucaparib in relapsed, platinum-sensitive high-grade ovarian carcinoma (ARIEL2 Part 1): an international, multicentre, open-label, phase 2 trial. Lancet Oncol. 2017;18:75–87. doi: 10.1016/S1470-2045(16)30559-9. [DOI] [PubMed] [Google Scholar]
- 90.Wahlberg E, Karlberg T, Kouznetsova E, Markova N, Macchiarulo A, Thorsell AG, Pol E, Frostell A, Ekblad T, Oncu D, Kull B, Robertson GM, Pellicciari R, Schuler H, Weigelt J. Family-wide chemical profiling and structural analysis of PARP and tankyrase inhibitors. Nat Biotechnol. 2012;30:283–288. doi: 10.1038/nbt.2121. [DOI] [PubMed] [Google Scholar]
- 91.Knezevic CE, Wright G, Rix LLR, Kim W, Kuenzi BM, Luo Y, Watters JM, Koomen JM, Haura EB, Monteiro AN, Radu C, Lawrence HR, Rix U. Proteome-wide Profiling of Clinical PARP Inhibitors Reveals Compound-Specific Secondary Targets. Cell Chem Biol. 2016;23:1490–1503. doi: 10.1016/j.chembiol.2016.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bock FJ, Todorova TT, Chang P. RNA Regulation by Poly(ADP-Ribose) Polymerases. Mol Cell. 2015;58:959–969. doi: 10.1016/j.molcel.2015.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gupte R, Liu Z, Kraus WL. PARPs and ADP-ribosylation: recent advances linking molecular functions to biological outcomes. Genes Dev. 2017;31:101–126. doi: 10.1101/gad.291518.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Olsen JV, Mann M. Status of large-scale analysis of post-translational modifications by mass spectrometry. Mol Cell Proteomics. 2013;12:3444–3452. doi: 10.1074/mcp.O113.034181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hengel SM, Goodlett DR. A Review of Tandem Mass Spectrometry Characterization of Adenosine Diphosphate-Ribosylated Peptides. Int J Mass Spectrom. 2012;312:114–121. doi: 10.1016/j.ijms.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Matic I, Ahel I, Hay RT. Reanalysis of phosphoproteomics data uncovers ADP-ribosylation sites. Nat Methods. 2012;9:771–772. doi: 10.1038/nmeth.2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Jungmichel S, Rosenthal F, Altmeyer M, Lukas J, Hottiger MO, Nielsen ML. Proteome-wide identification of poly(ADP-Ribosyl)ation targets in different genotoxic stress responses. Mol Cell. 2013;52:272–285. doi: 10.1016/j.molcel.2013.08.026. [DOI] [PubMed] [Google Scholar]
- 98.Dani N, Stilla A, Marchegiani A, Tamburro A, Till S, Ladurner AG, Corda D, Di Girolamo M. Combining affinity purification by ADP-ribose-binding macro domains with mass spectrometry to define the mammalian ADP-ribosyl proteome. Proc Natl Acad Sci U S A. 2009;106:4243–4248. doi: 10.1073/pnas.0900066106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gagne JP, Pic E, Isabelle M, Krietsch J, Ethier C, Paquet E, Kelly I, Boutin M, Moon KM, Foster LJ, Poirier GG. Quantitative proteomics profiling of the poly(ADP-ribose)- related response to genotoxic stress. Nucleic Acids Res. 2012;40:7788–7805. doi: 10.1093/nar/gks486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gagne JP, Isabelle M, Lo KS, Bourassa S, Hendzel MJ, Dawson VL, Dawson TM, Poirier GG. Proteome-wide identification of poly(ADP-ribose) binding proteins and poly(ADP-ribose)-associated protein complexes. Nucleic Acids Res. 2008;36:6959–6976. doi: 10.1093/nar/gkn771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Daniels CM, Ong SE, Leung AK. The Promise of Proteomics for the Study of ADP-Ribosylation. Mol Cell. 2015;58:911–924. doi: 10.1016/j.molcel.2015.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Martello R, Leutert M, Jungmichel S, Bilan V, Larsen SC, Young C, Hottiger MO, Nielsen ML. Proteome-wide identification of the endogenous ADP-ribosylome of mammalian cells and tissue. Nat Commun. 2016;7:12917. doi: 10.1038/ncomms12917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cohen-Armon M, Visochek L, Rozensal D, Kalal A, Geistrikh I, Klein R, Bendetz-Nezer S, Yao Z, Seger R. DNA-independent PARP-1 activation by phosphorylated ERK2 increases Elk1 activity: a link to histone acetylation. Mol Cell. 2007;25:297–308. doi: 10.1016/j.molcel.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 104.Gagne JP, Ethier C, Defoy D, Bourassa S, Langelier MF, Riccio AA, Pascal JM, Moon KM, Foster LJ, Ning Z, Figeys D, Droit A, Poirier GG. Quantitative site-specific ADP-ribosylation profiling of DNA-dependent PARPs. DNA Repair (Amst) 2015;30:68–79. doi: 10.1016/j.dnarep.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 105.Messner S, Altmeyer M, Zhao H, Pozivil A, Roschitzki B, Gehrig P, Rutishauser D, Huang D, Caflisch A, Hottiger MO. PARP1 ADP-ribosylates lysine residues of the core histone tails. Nucleic Acids Res. 2010;38:6350–6362. doi: 10.1093/nar/gkq463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bonfiglio JJ, Colby T, Matic I. Mass spectrometry for serine ADP-ribosylation? Think o-glycosylation! Nucleic Acids Res. 2017;45:6259–6264. doi: 10.1093/nar/gkx446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ueda K, Hayaishi O. ADP-ribosylation. Annu Rev Biochem. 1985;54:73–100. doi: 10.1146/annurev.bi.54.070185.000445. [DOI] [PubMed] [Google Scholar]
- 108.Cervantes-Laurean D, Jacobson EL, Jacobson MK. Preparation of low molecular weight model conjugates for ADP-ribose linkages to protein. Methods Enzymol. 1997;280:275–287. doi: 10.1016/s0076-6879(97)80119-x. [DOI] [PubMed] [Google Scholar]
- 109.Karch KR, Langelier MF, Pascal JM, Garcia BA. The nucleosomal surface is the main target of histone ADP-ribosylation in response to DNA damage. Mol Biosyst. 2017;13:2660–2671. doi: 10.1039/c7mb00498b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gibson BA, Conrad LB, Huang D, Kraus WL. Generation and Characterization of Recombinant Antibody-like ADP-Ribose Binding Proteins. Biochemistry (Mosc) 2017;56:6305–6316. doi: 10.1021/acs.biochem.7b00670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhen Y, Zhang Y, Yu Y. A Cell-Line-Specific Atlas of PARP-Mediated Protein Asp/Glu-ADP-Ribosylation in Breast Cancer. Cell Rep. 2017;21:2326–2337. doi: 10.1016/j.celrep.2017.10.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ogata N, Ueda K, Hayaishi O. ADP-ribosylation of histone H2B. Identification of glutamic acid residue 2 as the modification site. J Biol Chem. 1980;255:7610–7615. [PubMed] [Google Scholar]
- 113.Ogata N, Ueda K, Kagamiyama H, Hayaishi O. ADP-ribosylation of histone H1. Identification of glutamic acid residues 2, 14, and the COOH-terminal lysine residue as modification sites. J Biol Chem. 1980;255:7616–7620. [PubMed] [Google Scholar]
- 114.Suzuki H, Quesada P, Farina B, Leone E. In vitro poly(ADP-ribosyl)ation of seminal ribonuclease. J Biol Chem. 1986;261:6048–6055. [PubMed] [Google Scholar]
- 115.Tao Z, Gao P, Liu HW. Identification of the ADP-ribosylation sites in the PARP-1 automodification domain: analysis and implications. J Am Chem Soc. 2009;131:14258–14260. doi: 10.1021/ja906135d. [DOI] [PubMed] [Google Scholar]
- 116.Chapman JD, Gagne JP, Poirier GG, Goodlett DR. Mapping PARP-1 auto- ADP-ribosylation sites by liquid chromatography-tandem mass spectrometry. J Proteome Res. 2013;12:1868–1880. doi: 10.1021/pr301219h. [DOI] [PubMed] [Google Scholar]
- 117.Daniels CM, Ong SE, Leung AK. Phosphoproteomic approach to characterize protein mono- and poly(ADP-ribosyl)ation sites from cells. J Proteome Res. 2014;13:3510–3522. doi: 10.1021/pr401032q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Huttlin EL, Jedrychowski MP, Elias JE, Goswami T, Rad R, Beausoleil SA, Villen J, Haas W, Sowa ME, Gygi SP. A tissue-specific atlas of mouse protein phosphorylation and expression. Cell. 2010;143:1174–1189. doi: 10.1016/j.cell.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Syka JE, Coon JJ, Schroeder MJ, Shabanowitz J, Hunt DF. Peptide and protein sequence analysis by electron transfer dissociation mass spectrometry. Proc Natl Acad Sci U S A. 2004;101:9528–9533. doi: 10.1073/pnas.0402700101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Leidecker O, Bonfiglio JJ, Colby T, Zhang Q, Atanassov I, Zaja R, Palazzo L, Stockum A, Ahel I, Matic I. Serine is a new target residue for endogenous ADP-ribosylation on histones. Nat Chem Biol. 2016;12:998–1000. doi: 10.1038/nchembio.2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bonfiglio JJ, Fontana P, Zhang Q, Colby T, Gibbs-Seymour I, Atanassov I, Bartlett E, Zaja R, Ahel I, Matic I. Serine ADP-Ribosylation Depends on HPF1. Molecular cell. 2017;65:932–940. e936. doi: 10.1016/j.molcel.2017.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mitchell Wells J, McLuckey SA. Collision - Induced Dissociation (CID) of Peptides and Proteins. 2005;402:148–185. doi: 10.1016/S0076-6879(05)02005-7. [DOI] [PubMed] [Google Scholar]
- 123.Chi A, Bai DL, Geer LY, Shabanowitz J, Hunt DF. Analysis of intact proteins on a chromatographic time scale by electron transfer dissociation tandem mass spectrometry. Int J Mass Spectrom. 2007;259:197–203. doi: 10.1016/j.ijms.2006.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Li N, Zhang Y, Han X, Liang K, Wang J, Feng L, Wang W, Songyang Z, Lin C, Yang L, Yu Y, Chen J. Poly-ADP ribosylation of PTEN by tankyrases promotes PTEN degradation and tumor growth. Genes Dev. 2015;29:157–170. doi: 10.1101/gad.251785.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bilan V, Selevsek N, Kistemaker HAV, Abplanalp J, Feurer R, Filippov DV, Hottiger MO. New Quantitative Mass Spectrometry Approaches Reveal Different ADP-ribosylation Phases Dependent On the Levels of Oxidative Stress. Molecular & cellular proteomics: MCP. 2017;16:949–958. doi: 10.1074/mcp.O116.065623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Carter-O’Connell I, Jin H, Morgan RK, David LL, Cohen MS. Engineering the substrate specificity of ADP-ribosyltransferases for identifying direct protein targets. J Am Chem Soc. 2014;136:5201–5204. doi: 10.1021/ja412897a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gibson BA, Zhang Y, Jiang H, Hussey KM, Shrimp JH, Lin H, Schwede F, Yu Y, Kraus WL. Chemical genetic discovery of PARP targets reveals a role for PARP-1 in transcription elongation. Science. 2016;353:45–50. doi: 10.1126/science.aaf7865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Huang H, Sabari BR, Garcia BA, Allis CD, Zhao Y. SnapShot: histone modifications. Cell. 2014;159:458–458. e451. doi: 10.1016/j.cell.2014.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hart GW, Slawson C, Ramirez-Correa G, Lagerlof O. Cross talk between O-GlcNAcylation and phosphorylation: roles in signaling, transcription, and chronic disease. Annu Rev Biochem. 2011;80:825–858. doi: 10.1146/annurev-biochem-060608-102511. [DOI] [PMC free article] [PubMed] [Google Scholar]