Abstract
Pain remains highly prevalent in HIV-seropositive (HIV+) patients despite their well-suppressed viremia with combined antiretroviral therapy. Investigating brain abnormalities within the pain matrix, and in relation to pain symptoms, in HIV+ participants may provide objective biomarkers and insights regarding their pain symptoms. We used Patient-Reported Outcome Measurement Information System (PROMIS®) pain questionnaire to evaluate pain symptoms (pain intensity, pain interference and pain behavior), and structural MRI to assess brain morphometry using FreeSurfer (cortical area, cortical thickness and subcortical volumes were evaluated in 12 regions within the pain matrix). Compared to seronegative (SN) controls, HIV+ participants had smaller surface areas in prefrontal pars triangularis (right: p=0.04, left: p=0.007) and right anterior cingulate cortex (p=0.03) and smaller subcortical regions (thalamus: p<0.003 bilaterally; right putamen: p=0.01), as well as higher pain scores (pain intensity-p=0.005; pain interference-p=0.008; p-behavior-p=0.04). Furthermore, higher pain scores were associated with larger cortical areas, thinner cortices and larger subcortical volumes in HIV+ participants; but smaller cortical areas, thicker cortices and smaller subcortical volumes in SN controls (interaction-p=0.009 to p=0.04). These group differences in the pain-associated brain abnormalities suggest that HIV+ individuals have abnormal pain responses. Since these abnormal pain-associated brain regions belong to the affective component of the pain matrix, affective symptoms may influence pain perception in HIV+ patients and should be treated along with their physical pain symptoms. Lastly, associations of lower pain scores with better physical or mental health scores, regardless of HIV-serostatus (p<0.001), suggest adequate pain treatment would lead to better quality of life in all participants.
Keywords: HIV, affective pain, global health, brain morphometry, neuroinflammation
Introduction
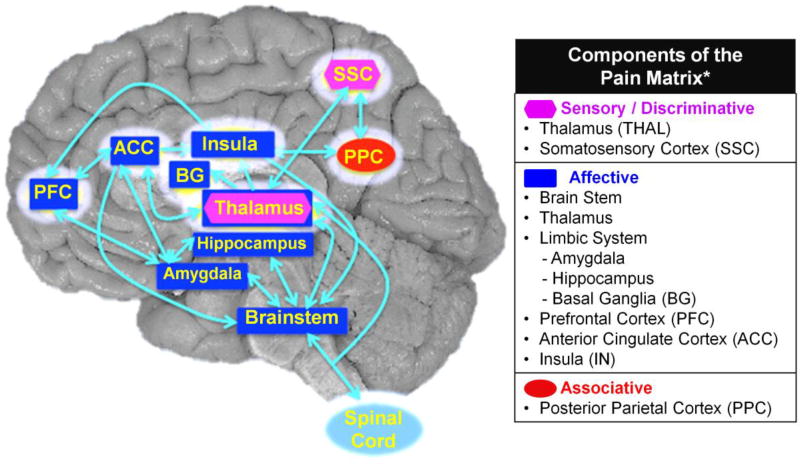
Pain is one of the most common self-reported symptoms in HIV-seropositive (HIV+) individuals (Lawson et al., 2014; Uebelacker et al., 2015). Assessing pain symptoms in HIV+ individuals can be challenging since pain is highly subjective, which may lead to under-treatment of their pain symptoms. Additionally, opioids are often prescribed for pain management in HIV patients, which is risky for those who are co-prescribed sedative medications (Merlin, 2015). Furthermore, opioids could potentiate HIV disease progression (Zou et al., 2011; Liu et al., 2016), and have high propensity for drug dependence (Krashin et al., 2012). Understanding whether brain abnormalities in HIV patients might be related to their subjective pain symptoms may provide insights for their pain management. Recent studies showed brain morphologic abnormalities in patients with chronic pain disorders (Baliki et al., 2011), such as fibromyalgia, chronic back pain, irritable bowel syndrome or neuropathic pain (Schmidt-Wilcke, 2015). Brain regions shown to be abnormal in patients with chronic pain were grouped as the “pain matrix” (Figure 1), which involves more than direct physical pain, but primarily for nociceptive processing and pain modulation (Bushnell et al., 1999; Melzack, 2005; Iannetti et al., 2013). However, whether alterations in brain structures within the pain matrix is related to subjective pain symptoms in HIV patients is unknown, and were investigated in the present study.
Figure 1. Regions of Interest Selected from the Pain Matrix.
The pain matrix (modified from Melzack, 2001) and the highlighted brain regions are those shown to be abnormal in patients with pain (Apkarian et al., 2005; Melzack, 2005; Tracey and Mantyh, 2007; Schweinhardt and Bushnell, 2010; Oertel et al., 2012; Hashmi et al., 2013; Atlas et al., 2014). The pain matrix comprises regions involved in pain processing and pain modulation. These brain regions are grouped into 3 different pain components: sensory or discriminative, affective and associative, which are all interconnected to each other. The sensory or discriminative component comprised mainly of the thalamus (Thal) and the somatosensory cortex (SSC) The affective component includes the brainstem, Thal, the limbic system [amygdala, hippocampus, basal ganglia (BG)], prefrontal cortex (PFC), anterior cingulate cortex (ACC) and, insula (IN). The associative component includes the posterior parietal cortex (PPC). The regions surrounded with white halo are the regions of interest selected for the current study.
We explored the neuroimaging signature for pain in HIV+ individuals by evaluating the relationships between morphometric abnormalities within the pain matrix on brain MRI, and self-reported pain symptoms assessed with National Institute of Health (NIH) Toolbox Patient Reported Outcome Measurement Information System (PROMIS®) pain questionnaire (Amtmann et al., 2010; Cella et al., 2010). Specifically, we aimed to: (1) assess HIV+ individuals on self-reported pain intensity, pain interference and pain behavior; (2) identify brain regions within the pain matrix that show brain abnormalities in HIV+ individuals compared to HIV-seronegative (SN) controls; (3) compare the relationships between these abnormal cortical and subcortical morphometric measures and pain severity in ambulatory HIV+ individuals and in SN controls; and (4) determine the relationships between the pain severity and mental or physical health in HIV+ and SN individuals.
Based on prior studies, we propose the following hypotheses: (1) Since weakened immune system caused by HIV infection may lead to higher risk for painful co-morbidities (Lawson et al., 2014), HIV+ individuals will have higher pain intensity, pain interference and pain behavior scores than SN controls. (2) Brain atrophy may result from chronic neuroinflammation in both HIV-associated brain injury (Gray et al., 2001; Chang et al., 2011) and chronic pain (Apkarian and Scholz, 2006; Moayedi et al., 2012). Therefore, compared to SN controls, HIV+ individuals, especially those with pain symptoms, are expected to show smaller cortical areas, thinner cortices and smaller subcortical brain volumes within the pain matrix. (3) Since greater pain symptoms were associated with more gray matter atrophy both in HIV+ individuals (Keltner et al., 2014) and within the pain matrix in patients with chronic back pain (Fritz et al., 2016), we hypothesize that all participants with greater pain symptoms will have smaller volumes or thinner cortices within the pain matrix. However, this inverse relationship may be more pronounced in HIV+ individuals than SN controls due to the greater neuroinflammation (Gray et al., 2001; Chang et al., 2014). (4) Individuals with chronic pain and HIV+ patients with pain symptoms both had poorer mental and physical health compared to individuals without pain (Breitbart et al., 1996; Merlin et al., 2013; Uebelacker et al., 2015; Mann et al., 2016); therefore, greater pain severity will be associated with poorer mental health and physical health scores in both HIV+ and SN individuals.
Materials and Methods
Participants
A total 106 participants were screened and were randomly but consecutively chosen from 206 participants who were being enrolled in a larger neuroimaging study of brain changes after cognitive training (Chang et al., 2016; Chang et al., 2017). However, 66 (31 HIV+ and 35 SN) of these participants agreed to be co-enrolled in the current study to evaluate the neuroimaging signature of pain (between June 20th 2013 to December 15th, 2015.) The study design, experiments and consent forms were approved by the Cooperative Institutional Review Board of the University of Hawaíi at Mānoa, CHS#20523.
The participants were initially recruited through flyers, referrals from the local community and clinics, or by word-of-mouth. They were screened first by telephone or in person. Potentially qualified participants provided informed consent and signed the approved consent form before under-going further detailed evaluations, including review of the medical records by a study physician to ensure they fulfilled all study criteria. The inclusion criteria for all participants were: (1) men or women of any ethnicity, ages≥18 years; (2) able to provide informed consent, with a minimum of an 8th grade reading level (verified by the Wechsler Test of Adult Reading); (3) HIV-seropositive (with documentation from medical records) or seronegative for HIV (confirmed by an HIV ELISA blood test if screened positive with ClearView® COMPLETE HIV-1/2 Test); (4) HIV+ participants had to be stable on an antiretroviral regimen for 6-months or would remain without antiretroviral treatment during the study. The exclusions criteria for all participants were: (1) history of any co-morbid and confounding psychiatric illness(es) unrelated to HIV-infection; (2) any confounding neurological disorder (e.g., multiple sclerosis, Parkinson’s disease, non-HIV brain infections, neoplasms, cerebral palsy, or significant head trauma with loss of consciousness >30 minutes); (3) significantly abnormal screening laboratory tests (>2 SD) indicating a chronic medical condition that might affect brain function; (4) pregnancy (verified by urine pregnancy test in women of child bearing age); (5) other contraindications for MR studies, such as metallic or electronic implants in the body (e.g. pacemaker, surgical clips, pumps, etc.), or severe claustrophobia.
All 66 participants completed the detailed clinical and neuropsychological evaluations, the Patient Reported Outcome Measurement Information System (PROMIS) online pain questionnaire (Apkarian et al., 2005), and had good quality brain MRI scans.
Image Acquisition for Structural MRI
All MRI studies were performed on a research-dedicated 3 Tesla Siemens TIM Trio scanner (VB17, Siemens Medical Solutions, Erlangen, Germany) using a 12-channel phase-array head coil. Following a localizer scan, a 3D magnetization-prepared rapid gradient echo (MP-RAGE) scan was performed (sagittal, TE/TR/TI=4.47ms/2.2s/1s, 12° flip angle, FOV=320mm, 1.0×1.0×1.0mm resolution, 192 slices, ipat mode GRAPPA, acceleration factor=2, slice thickness=1mm). Next, a 3D-T2-weighted sequence (FLAIR) was acquired (transversal, TE/TR/TI=84/9100/2500ms, slice thickness 3.0mm). Although all participants had repeat structural scans after the cognitive training for the parent study, only the baseline scans prior to any intervention were included in the current study. All scans were reviewed by the study physicians to screen for possible brain lesions or structural abnormalities. All subjects had good quality scans that passed the quality control criteria.
Image Processing and Analysis
All MR scans were processed using the surface-based method in FreeSurfer 5.3 (Dale et al., 1999). The program automatically reconstructed and labeled 40 subcortical and 70 cortical regions in each hemisphere. Brain structures were automatically-registered using an existing brain template [Montreal Neurological Institute (MNI)] prior to segmentation and after extraction from the skull. All regions were visually inspected to ensure accurate segmentation. Manual editing of the volumetric segmentation was performed only if gross misalignment was detected on visual inspection. Editing was performed blinded to the subjects’ HIV-serostatus or pain scale severity in order to minimize bias. Regional cortical area, cortical thickness, and subcortical volume for the anatomic regions of interest (ROIs) were automatically determined in each hemisphere.
Seven cortical and five subcortical ROIs from each hemisphere were selected for this study based on previous brain imaging studies on pain (Apkarian et al., 2005; Melzack, 2005; Tracey and Mantyh, 2007; Schweinhardt and Bushnell, 2010; Oertel et al., 2012; Hashmi et al., 2013). These ROIs are part of the pain matrix, which includes the prefrontal cortex (PFC), cingulate cortex (CC), somatosensory cortex (SSC), posterior parietal cortex (PPC), insula, basal ganglia, thalamus, hippocampus, amygdala, and brainstem (Fig. 1). The cortical ROIs selected for the current study included anterior cingulate cortex (ACC), postcentral and supramarginal regions of the SSC, pars triangularis of the inferior frontal gyrus and orbitofrontal cortex (OFC) of the PFC, insula, and precuneus of the PPC. The subcortical ROIs included subcomponents of the basal ganglia (caudate, putamen, pallidum and accumbens) and thalamus.
Pain Measures
All participants completed the Patient Reported Outcomes Measurement Information System (PROMIS®) pain questionnaire (Apkarian et al., 2005) at the time of enrollment. The pain questionnaire comprises 10 questions on global health status (PROMIS SF v1.1 Global Health), 3 questions on pain intensity (PROMIS Scale v1.0 Pain Intensity 3a), 4 to 12 computer adaptive test (CAT) questions on pain interference (PROMIS Bank v1.1 Pain Interference), and 4 to 12 CAT questions on pain behavior (PROMIS Bank v1.0 Pain Behavior). Pain intensity questions determined “how much it hurts”, pain interference defined “how the pain affected the participant’s daily life”, and pain behavior evaluated “how the participant manifested his/her pain”. All scores for each of the 3 pain domains and for the global health status assessment were normalized using the transmutation table provided by PROMIS which accounted for age, sex, race, and education level (Cella et al., 2010). The normalized data were expressed as T-scores. Individuals with T-scores ≥60 for pain intensity, pain interference and pain behavior were considered above population average for pain severity, and those with T-scores ≤40, were below population average. The global health status consisted of 2 domains, physical health and mental health. Individuals who had T-scores of ≥60 were one standard deviation healthier than the general population, and those that had T-scores ≤40 1 standard deviation less healthy than the general population.
Statistical Analysis
Statistical analyses were performed with R studio-Version 0.99.335; (RStudioTeam, 2015) or the SAS Enterprise Guide 7.1 software. Group differences in age, sex distribution, race distribution and other participant characteristics were analyzed using Chi-square tests for categorical variables, and two-sample T-tests for continuous variables. All brain morphometric analyses of volume and surface area used estimated total intracranial volume (eTIV) as a covariate to correct for the total volume of the cranium (Buckner et al., 2004; Malone et al., 2015; Sargolzaei et al., 2015). Since cortical thickness does not scale with head size (Barnes et al., 2010), only age and sex were used as covariates for cortical thickness analysis.
One-way analysis of covariance [ANCOVA] was used to determine the HIV+ status effect on pain measures (Aim 1) and brain morphometry (Aim 2). Two-way ANCOVAs further evaluated 1) the main and interactive effects of pain measures and HIV status on brain morphometric measures (cortical area, cortical thickness, and subcortical volume) (Aim 3), and 2) the main and additive effects of pain scores and HIV status on global mental health outcomes (Aim 4). Post hoc analyses using linear regressions were performed across both subject groups for the measures that did not show interactive effects. Normality was checked using QQ-plots. All results with P-values<0.05 were considered significant and P-values between 0.05–0.09 were considered trends. Bonferroni correction was not applied because the measures selected were based on a priori hypotheses derived from prior publications.
Results
Participant Characteristics (Table 1)
The two subject groups (35 HIV and 31 SN controls) had similar mean age, sex proportion, race distribution, mean years of education, Hollingshead Index of Social Position (ISP), and Center for Epidemiologic Studies-Depression Scale (CES-D) scores. Only a few HIV (n=3, 8.8%) and SN participants (n=5, 16.1%) had frequent headaches. However, similar to previous studies (Schütz and Robinson-Papp, 2013; Ekenze et al., 2014), many more HIV+ individuals had distal neuropathy (56%) than SN controls (26%). Of the 11 participants taking pain medications during the study, 7 used opioids (oxycodone, hydrocodone), 7 also used nonsteriodal anti-inflammatory drugs (NSAIDS) (aspirin, ibuprofen, naproxen), 2 used tramadol, and 1 used a muscle relaxant (carisoprodol).
Pain Measures
HIV+ participants scored higher than SN controls on all three pain measures (pain intensity, pain interference, and pain behavior, Table 2). Correlation analysis between these pain measures and HIV+ participants’ characteristics (age, HIV disease duration, CD4 count, and viral load) showed significance only between duration of HIV+ diagnosis and two pain measures: pain intensity (r=+0.37, p=0.03) and pain behavior (r=+0.39, p=0.02).
Brain Morphometric Measures
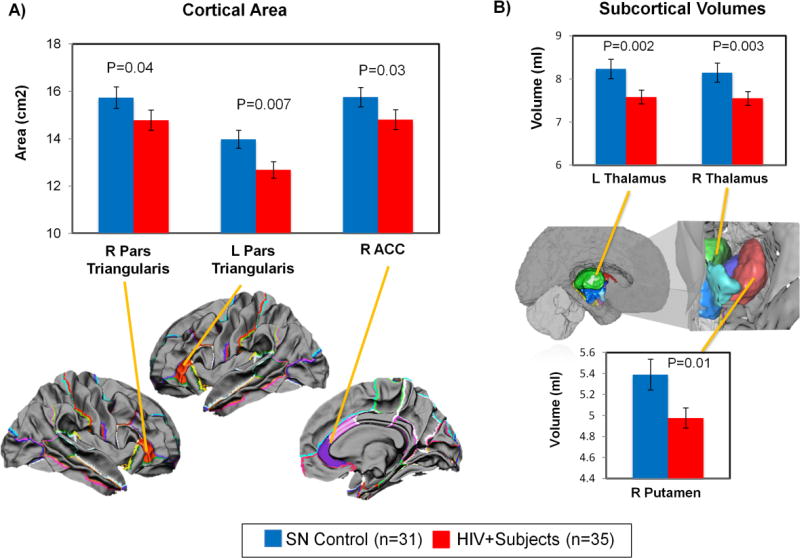
Cortical and subcortical morphometry measures were compared between HIV+ and SN subjects, using a one-way ANCOVA. Independent of pain status, HIV+ individuals had smaller cortical areas in bilateral pars triangularis (left: −9.2%. p=0.007, right: −6.1%. p=0.04) and right ACC (−6.0%, p=0.03) (Figure 2A), and smaller volumes in bilateral thalamus (left: −7.9%, p=0.002; right: −7.3%, p=0.003) and right putamen (−7.3%, p=0.01) (Figure 2B).
Figure 2. Effect of HIV on Brain Morphologic Measures (Cortical Area and Subcortical Volume).
Effect of HIV on Brain Morphologic Measures (cortical area, cortical thickness and subcortical volume). (A) In the cortical brain regions, HIV+ subjects had smaller cortical areas than SN controls in the right anterior cingulate cortex (ACC) and bilateral pars triangularis of the inferior frontal region. (B) In the subcortical regions, HIV+ subject had smaller volumes than SN controls in the right putamen and bilateral thalamus. P-values for group differences are from one-way ANCOVA (co-varied for age, sex and eTIV for cortical area and subcortical volumes; co-varied for age and sex for cortical thickness).
Brain regions that showed significant morphometric abnormalities in HIV+ individuals belonged to the affective-motivational component of the pain matrix (Price, 1992; Treede et al., 1999; Price, 2000; Johansen et al., 2001; Apkarian et al., 2005; Oertel et al., 2012; Hashmi et al., 2013).
Relationships between Brain Morphometry and Pain Measures
We also explored possible group differences in the relationship between brain morphometry and pain measures. Only three brain regions (left supramarginal area, left putamen volume and right pallidum volume) showed significant pain effects on the two-way ANCOVAs. However, the pain effects were different between HIV+ individuals and SN controls, showing higher pain scores with larger cortical area and subcortical volumes in HIV+ individuals, but smaller brain measures in SN controls. Hence, these interactive effects are described and illustrated below.
Interactions between Pain Assessments and HIV Status on Brain Morphometry
Pain Intensity
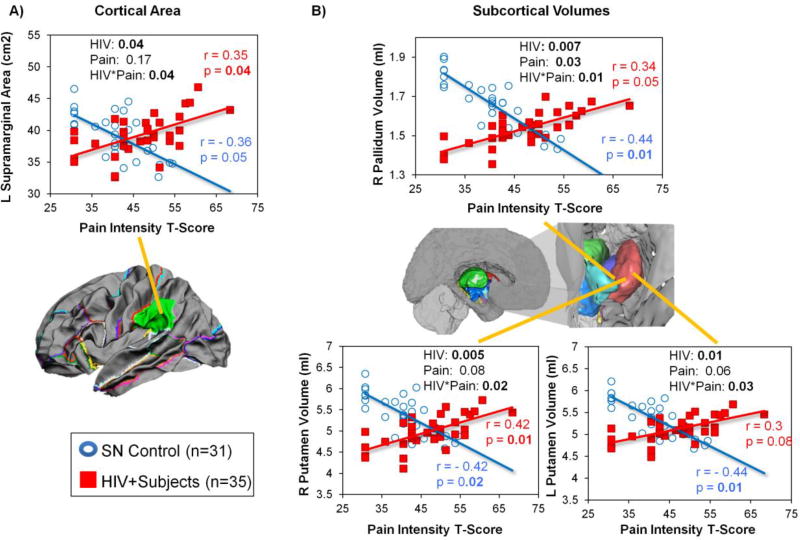
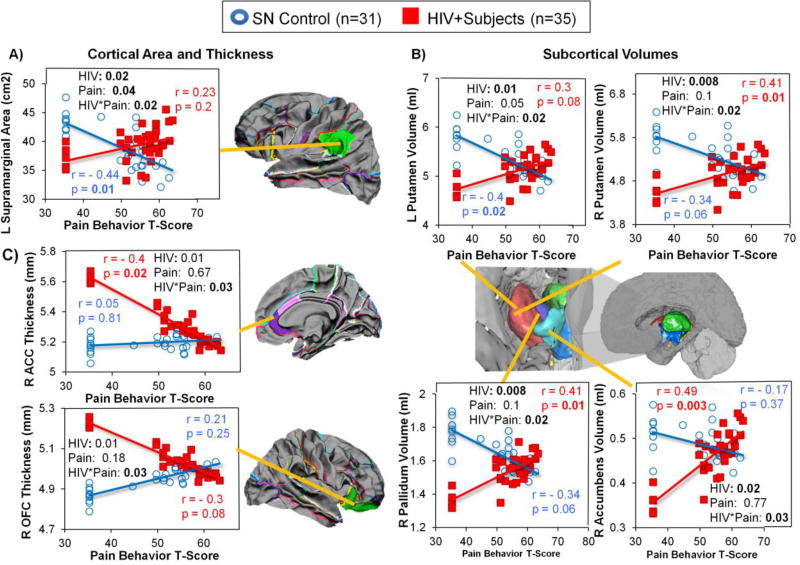
Pain intensity-by-HIV status interactions were found in the left supramarginal area (Figure 3A), right pallidum volume (Figure 3B), and bilateral putamen volumes (Figure 3B). For all of these regions, HIV individuals with higher pain intensity had larger cortical areas and subcortical volumes, whereas SN controls with higher pain intensity had smaller cortical areas and subcortical volumes.
Figure 3. Group Differences in the Relationships between Pain Intensity and Brain Morphologic Measures in HIV+ Participants and SN Controls.
HIV+ individuals and SN controls showed opposite relationships between pain intensity and brain morphometry in the L supramarginal area, bilateral putamen volumes, and R pallidum volume. These graphs show that HIV individuals with higher pain intensity tended to have bigger cortical area in the L supramarginal region and larger subcortical volume in the R pallidum and bilateral putamen, while SN controls with higher pain intensity scores had smaller left supramarginal area, and smaller volumes in the right pallidum and bilateral putamen.
Pain Interference
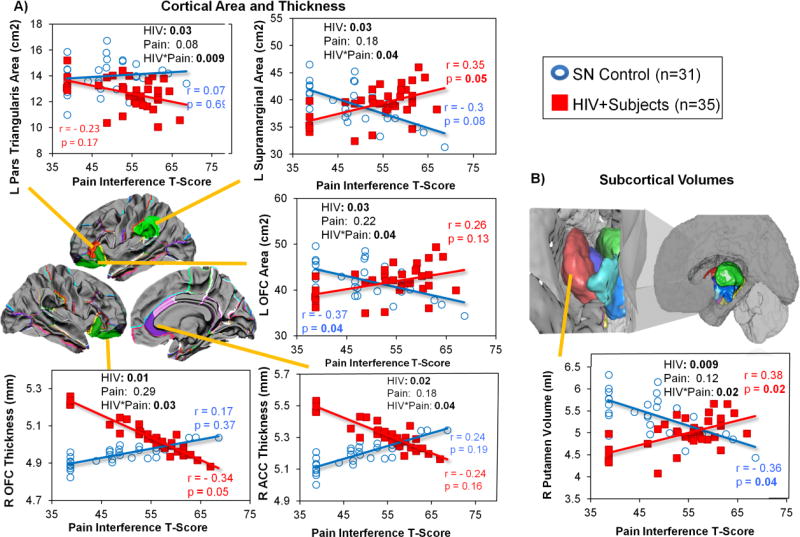
Pain interference-by-HIV serostatus interactions on brain morphometry were found in several cortical and subcortical regions (Figure 4). HIV+ individuals with higher pain interference scores had smaller cortical areas in left pars triangularis, thinner right OFC, and thinner right ACC, while SN controls with higher pain interference scores had larger areas and thicker cortices in these same brain regions (Figure 4A). In contrast, higher pain interference scores were associated with larger cortical areas in the left supramarginal and left OFC region in HIV+ individuals but with smaller areas in the SN controls (Figure 4A).
Figure 4. Group Differences in the Relationships between Pain Interference and Brain Morphologic Measures in HIV+ Participants and SN Controls.
Group differences in the relationship between Pain Interference and Brain Morphologic Measures. HIV-by-Pain interaction is observed in the L pars triangularis area, L supramarginal area, L orbitofrontal cortex (OFC) area, R orbitofrontal cortex (OFC) thickness, R anterior cingulate cortex (ACC) thickness, and R putamen volume. Graphs showed that HIV individuals with higher pain interference score had: (1) larger cortical area in the L supramarginal and L orbitofrontal regions but smaller cortical area in the L pars triangularis; (2) thinner cortices in the R OFC and R ACC; and (3) larger subcortical volume in the R putamen, while SN controls with higher pain scores typically showed the opposite relationships on these brain measures.
Amongst the subcortical ROIs, a pain interference-by-HIV serostatus interaction was found only in the right putamen. Higher pain interference scores were associated with larger right putamen volumes in HIV subjects but smaller right putamen volumes in SN controls (Figure 4B).
Pain Behavior
The surface area of the left supramarginal region (Figure 5A) and volumes of the subcortical regions (bilateral putamen, right pallidum, and left accumbens, Figure 5B) showed pain behavior-by-HIV serostatus interactions. For all of these interactions, higher pain behavior scores were associated with larger brain areas in the cortical regions and volumes in the subcortical regions in HIV+ participants, but smaller brain measures in SN controls. In contrast, the opposite relationships were observed in the right OFC and right ACC thickness (Figure 5C), in which pain behavior scores correlated negatively with thickness in HIV+ participants but positively in SN controls.
Figure 5. Group Differences in the Relationships between Pain Behavior and Brain Morphologic Measures in HIV+ Participants and SN Controls.
Group Differences in the relationships between Pain Behavior and Brain Morphologic Measures. HIV-by-Pain interaction is observed in the L supramarginal area, R anterior cingulate cortex (ACC) thickness, R lateral OFC thickness, R pallidum volume, L accumbens volume and, bilateral putamen volumes. Graphs showed that HIV individuals with higher pain behavior score tend to have: (1) bigger cortical area in the L supramarginal region and L medial OFC; (2) thinner cortex in the R lateral OFC; and (3) larger subcortical volumes in the R pallidum, L accumbens and, bilateral putamen, but SN controls showed the opposite relationship in these brain measures.
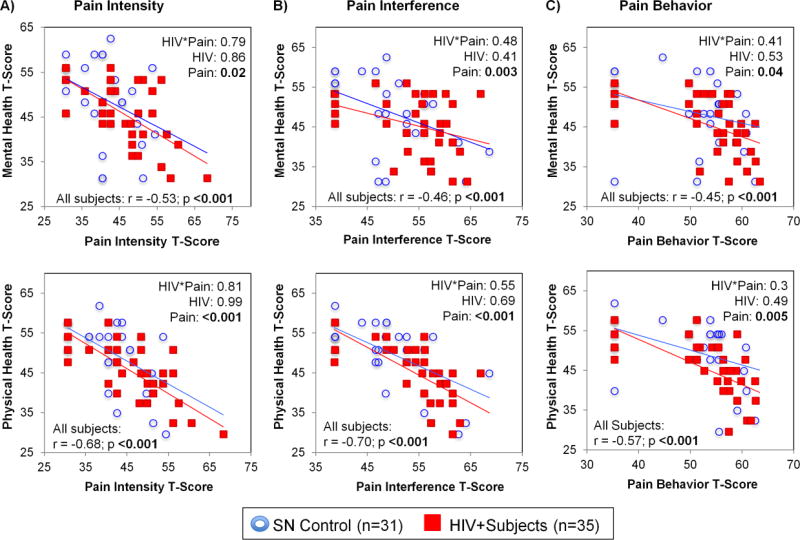
Pain Measures and Global Health Status (Figure 6)
Figure 6. Relationships Between Pain Measures (Intensity, Interference and Behavior) and Mental or Physical Health (2-way ANCOVA).
Relationships Between Pain Measures [Pain Intensity (A), Pain Interference (B), and Pain Behavior (C)] and Mental or Physical Health Scores in HIV+ individuals and SN controls. Individuals with higher pain intensity, pain interference, and pain behavior scores consistently had poorer mental and physical health, regardless of HIV status. No pain-by-HIV serostatus interaction or HIV-serostatus effects on these global health measures were observed. For Global Health (Physical and Mental), T-score ≥ 60 means score is 1 SD better than US general population while T-score ≤ 40 is 1 SD worst than US general population. T-score generated based on US population July 2006-March 2007 and Sept 2007-Mar 2008 (n = 21,133 + 967; mean age 48.2; 52% female; 82% white, 9% black, 8% multiracial, 1% other; 3% < HS, 16% HS, 39% some college, 24% college, 19% post-baccalaureate)
On two-way ANCOVA, main effects of all pain measures (T-scores of pain intensity, pain interference and pain behavior) were found on mental and physical health scores regardless of HIV serostatus. Individuals with higher pain intensity, pain interference, and pain behavior scores consistently had poorer mental and physical health, regardless of HIV serostatus. The strongest associations were found between physical health score and pain Interference (r=−0.7, p<0.001) or pain intensity (r=−0.68, p<0.001). No pain-by-HIV serostatus interaction or HIV-serostatus effects on these global health measures were observed.
Discussion
This is the first study to use PROMIS® pain questionnaires to evaluate pain symptoms in HIV+ individuals, and determine whether their pain symptoms are related to morphometric brain abnormalities within the pain matrix. There were four major findings. 1) HIV+ individuals had higher pain scores than SN controls which might have resulted from direct effects of the virus or reactive inflammation causing painful nerve damage (Kemp et al., 2008; Grovit-Ferbas and Harris-White, 2010; Hao, 2013), and residual painful side-effects from past antiretroviral medications [i.e. didanosine or stavudine] (Moore et al., 2000; Schütz and Robinson-Papp, 2013) in some participants. 2) Compared to SN controls, HIV+ patients had smaller cortical areas and subcortical volumes in brain regions that comprise the affective component of the pain matrix (bilateral pars triangularis, right ACC and right putamen). These alterations might have resulted from neuronal degeneration mediated by chronic HIV-associated neuroinflammation (Adle-Biassette et al., 1999; Kaul et al., 2001; Corasaniti et al., 2003; Hong and Banks, 2015). 3) In most brain regions, larger volumes and thinner cortices were associated with higher pain measures in HIV+ participants, but lower pain measures in SN controls. These opposite associations suggest both acute and chronic inflammation in the HIV+ participants, with brain swelling and neurodegeneration, but primarily neuronal loss from chronic neuroinflammation in SN controls. 4) Regardless of HIV-serostatus, participants with lower pain scores had better global health status (physical and mental), suggesting that pain symptoms had greater impact on an individual’s quality of life, with or without a concomitant chronic disease (Breitbart et al., 1996; Merlin et al., 2013; Uebelacker et al., 2015; Mann et al., 2016).
Pain in HIV+ Individuals
The prevalence of distal neuropathy in our HIV+ participants (56%) was consistent with other studies in adult HIV+ individuals (42% – 57%) (Ellis et al., 2010; Ekenze et al., 2014; Phillips et al., 2014). The higher pain severity on PROMIS® in our HIV+ compared to SN individuals was consistent with prior findings using other instruments (i.e. Brief Pain Inventory (Breitbart et al., 1996; Merlin et al., 2012; Phillips et al., 2014; Uebelacker et al., 2015), Wisconsin Brief Pain Questionnaire (Mphahlele et al., 2012), Numerical Rating Scale (Huang et al., 2013), or McGill Pain Questionnaire (Serchuck et al., 2010; Parker et al., 2014)). Although our HIV+ participants were stable clinically, more prevalent and pronounced pain symptoms were still present, some from painful neuropathy caused by direct effects of the virus, or persistent side effects of past medications (i.e. didanosine or stavudine) (Kemp et al., 2008; Grovit-Ferbas and Harris-White, 2010; Hao, 2013; Uebelacker et al., 2015). The multicenter CNS Antiretroviral Therapy Effects Research (CHARTER) cohort demonstrated that past antiretroviral medications can influence pain symptoms in HIV patients (Malvar et al., 2015). Moreover, our HIV+ participants as a group had been diagnosed for ~16 years, and those with longer HIV disease duration also had more pain symptoms, consistent with reports indicating prolonged HIV-infection may increase risk of acquiring painful co-morbidities (i.e. neuropathy) (Stewart et al., 1996; Glare, 2001; Lawson et al., 2014).
Pain experienced by HIV+ individuals is typically chronic pain from either peripheral or central sensitization (Carr, 2004; Nair et al., 2009). Ongoing HIV-infection may trigger local tissue inflammation leading to hypersensitization of peripheral nervous system receptors, while chronic infection (e.g., HIV or other co-occurring viruses) may further lead to central sensitization. With central sensitization, prolonged exposure of brain cells to inflammatory neurotransmitters (e.g., glutamate), neuromodulators (e.g., BDNF), and immune mediators (e.g., cytokines or chemokines), may trigger hyperactivity of postsynaptic nociceptive neurons (Merlin, 2015).
Brain morphometry in HIV+ individuals
The brain atrophy in our HIV+ patients, regardless of pain symptoms, is consistent with previous findings (Becker et al., 2011; Sarma et al., 2014). Brain volume loss was thought to result from the direct neurotoxic effects of HIV viral proteins, or indirect effects of HIV-mediated glial inflammatory responses; both processes may lead to neurodegeneration (Wiley et al., 1991; Masliah et al., 1992; Adle-Biassette et al., 1995).
Smaller areas in the pars triangularis and ACC in HIV+ patients compared to SN controls are consistent with prior findings of smaller than normal gray matter volumes in the frontal and cingulate cortices (Fischer et al., 1999; Küper et al., 2011; Wang et al., 2016). Similarly, subcortical atrophy in the thalamus and putamen of our HIV+ participants is also expected (Becker et al., 2011; Chang et al., 2011; Wade et al., 2015), and likely reflects neurodegeneration from HIV-induced neurotoxicity, since subcortical regions typically showed the highest viral burden (Ranki et al., 1995; Gray et al., 2001).
Furthermore, compared to SN controls, HIV+ participants had more brain atrophy and higher scores on the affective component of pain measures. These findings suggest that abnormalities in brain morphometry within the pain matrix may be useful biomarkers to assess pain in ambulatory HIV+ individuals.
Pain and brain morphology
The few brain regions that showed associations with pain effects showed opposite effects between HIV+ participants and SN controls, suggesting that pain related-brain alterations might have resulted from different or mixed types of pain processes, such as acute and chronic pain (Borsook et al., 2013). Patients with acute pain showed larger brain volumes (Cleary and Heinricher, 2013), likely due to cellular swelling and edema from ongoing acute inflammation (May, 2008; Freund et al., 2011; Ceko et al., 2013; Mole et al., 2014). Similarly, healthy individuals who underwent repeated painful stimulation showed enlarged brain volumes (Teutsch et al., 2008). In contrast, chronic pain patients tended to have smaller brain volumes (Robinson et al., 2011), likely as a consequence of neuronal loss, maladaptive plasticity, or retrograde neuronal degeneration in response to tissue injury or prolonged inflammation (May, 2008; Freund et al., 2011; Ceko et al., 2013; Mole et al., 2014).
Brain morphologic abnormalities in HIV+ individuals with pain
Our HIV+ individuals showed evidence of both acute and chronic pain since those with higher pain scores had larger cortical areas and subcortical volumes, as well as thinner cortices. The acute inflammation may be due to the various neurotoxic processes that led to brain swelling, while chronic inflammation in response to ongoing HIV infection may lead to neuronal loss and neurodegeneration (Rodriguez-Raecke et al., 2009; Baliki et al., 2011).
In healthy individuals, pain sensitivity positively correlated with thicker cortical gray matter in brain regions that are implicated in sensory, affective and cognitive aspects of pain (Erpelding et al., 2012). In contrast, our HIV+ individuals with higher pain scores showed thinner cortices in right OFC and right ACC (affective components of pain matrix), which suggests that acute pain may also lead to brain injury involved in “psychological pain” in these individuals. Direct neuronal damage from HIV may lead to functional loss and tissue shrinkage (Gustin et al., 2011), while chronic ongoing inflammation may lead to indirect central and peripheral sensitization from repeated neuronal stimulation or prolonged exposure of neuronal cells to neurotoxic inflammatory mediators (Ji et al., 2014).
Cortical thickness may provide a good surrogate measure for overall pain dysfunction in those with chronic pain (Erpelding et al., 2012; Frøkjær et al., 2012; Desouza et al., 2013). The thinner cortices in regions involved in the affective pain component, such as OFC and ACC, in HIV+ individuals with higher pain interference and pain behavior scores also may lead to an impaired pain response (Lloyd et al., 2016). Similar correlations between cortical thickness and pain interference and behavior scores were found in chronic pain patients with interruptions in the pain modulation network, which led to increased pain perception and impaired pain aversion response (Moont et al., 2011; Desouza et al., 2013). Other studies, using different instruments to measure pain, also found more affective symptoms and distress in HIV+ individuals (Price, 1992, 2000; Evans et al., 2003). Additionally, using a conditioned pain paradigm and chemically-induced lesions in the ACC of rodents, atrophy from neuronal damage in the ACC was associated with higher pain interference and pain behavior scores, but reduced pain avoidance and pain aversive behavior (Johansen et al., 2001; Qu et al., 2011; Yan et al., 2012; Barthas et al., 2015).
Physical and Mental Health of HIV+ Individuals with Pain
We hypothesized that the dual insults from pain and HIV infection on the brain would lead to lower global health status (mental and physical health scores) in HIV+ participants relative to the SN individuals. However, in this clinically-stabled group of HIV+ participants, we did not find significant associations between HIV disease and global health status, or significant pain-by-HIV disease interactions on global health status. Across both groups, individuals with higher pain scores consistently had lower physical and mental health scores compared to those with low pain scores, suggesting that only pain, but not HIV-seropositivity, contributed to the poorer quality of life. This finding validates prior reports that HIV+ patients with well-controlled pain had better quality of life (Merlin et al., 2013; Uebelacker et al., 2015). Therefore, better and more aggressive pain management strategies are important for the well-being of HIV+ individuals with pain.
This study has several limitations. Since this is a cross-sectional study, the associations between pain, HIV disease and brain abnormalities do not imply causality. Longitudinal evaluations or treatment studies are needed to further delineate these relationships. Although the pain assessments from PROMIS® showed robust group effects, the sample size is moderate for a morphometry study. Future studies with larger sample sizes are needed to validate brain morphometry as biomarkers for pain measures in HIV-infected individuals. Since the PROMIS® pain questionnaire did not assess specific pain characteristics, such as the type, location, or duration of pain symptoms, further research correlating these characteristics with abnormal brain measures is needed.
In summary, many of our HIV+ individuals manifest symptoms of pain despite well-controlled viremia. In the HIV+ participants, the association between higher pain scores and larger cortical areas and subcortical volumes suggest ongoing acute inflammation, while the pain-severity-associated thinner cortices suggest concomitant chronic inflammation as a consequence of ongoing HIV disease. In SN controls, the pain-severity-associated smaller brain volumes also suggest neurodegeneration from chronic inflammation.
The opposite relationships between morphometry and pain scores in HIV+ individuals and SN controls suggest that the pain response in HIV+ subjects is altered. These abnormal pain-associated brain regions in HIV+ participants involved the affective component of the pain matrix, which further suggests a psychological influence on their pain response. Therefore, pain management for HIV+ patients should include treatment of their affective symptoms. Lastly, the association of low pain scores in participants with better global health further demonstrates the importance of adequate pain treatment for better quality of life in all participants.
Supplementary Material
Table 1.
Participant Characteristics (Mean±S.E.M)
| SN Controls (n=31) |
HIV+ Participants (n=35) |
P-value from T-test or χ2 |
|
|---|---|---|---|
| Age (years) | 50.2 ± 2 | 54.1 ± 1.6 | 0.12 |
| Number of Men (%) | 29 (93.6) | 32 (91.4) | 0.75 |
| Race (% Asian/White/ American Indian/ Pacific Islander/ Black or African American/ More than one race) | 22.6/ 48.4/ 0/ 22.6/ 0/ 6.5 | 17.1/ 37.1/ 5.7/ 14.3/ 8.6/ 17.1 | 0.2 |
| Education (years) | 15.2 ± 0.5 | 14.3 ± 0.4 | 0.16 |
| Index of Social Position (ISP) | 38.3 ± 2.9 | 38 ± 2.8 | 0.94 |
| # using pain medication* (%) | 5 (16) | 7 (20) | 0.17 |
| CES-D score | 9.7 ± 2.1 | 11.1 ± 1.8 | 0.68 |
| # with Distal Neuropathy** (%) | 8 (25.8) | 19 (55.9) | 0.01 |
| # with headaches** (%) | 5 (16.1) | 3 (8.8) | 0.4 |
| CD4 Count (#/mm3) | 584.4 ± 52.8 | ||
| Viral Load (copies/mL) | 83 ± 39.7 | ||
| log Viral Load (log copies/mL) | 1.46 ± 0.08 | ||
| Duration of HIV Diagnosis (months) | 193.3± 17.3 | ||
| # on Antiretroviral Treatment (%) | 34 (97.1%) |
Pain medications reported include opioids (oxycodone, hydrocodone, 7/12,,58%), nonsteriodal anti-inflammatory drugs (NSAIDS, aspirin, ibuprofen, naproxen, 7/12, 58%), Tramadol (2/12, 17%), and muscle relaxant (carisoprodol, 1/12, 8%)
N=65. One HIV subject had missing data on headache and distal neuropathy.
Table 2.
Difference Between Pain Measures* in HIV+ Participants and SN controls (mean ± S.E.)
| Average T-scores | SN Control (n=31) | HIV+ Participants (n=35) |
P-value from Unpaired T-test |
|---|---|---|---|
| Pain Intensity | 40.8 ± 1.3 | 46.7 ± 1.5 | 0.005 |
| Pain Interference | 48.6 ± 1.5 | 54.2 ± 1.3 | 0.008 |
| Pain Behavior | 49.0 ± 1.7 | 53.6 ± 1.4 | 0.04 |
Pain Measures: T-score ≥ 60 is 1 SD worst than US general population; T-score ≤ 40 is 1 SD better than US general population. T-score generated is based on US population July 2006–March 2007; Sept 2007–Mar 2008 (n = 21,133 + 967; mean age 48.2; 52% female; 82% white, 9% black, 8% multiracial, 1% other; 3% < HS, 16% HS, 39% some college, 24% college, 19% post-baccalaureate)
Acknowledgments
This study was supported by grants from the National Institute on Drug Abuse (R01-DA-035659, K24-DA16170) and the National Institute on Minority Health and Health Disparities (G12MD-007601). We thank all the research participants for their participation in the study, the community providers who referred the participants, and all the clinical and technical research staff (especially Drs. Tamara Andres, Ahnate Lim and Vanessa Douet) at the University of Hawaii Neuroscience and MR Research Program at the Queen's Medical Center, who assisted in data collection or provided advice on this project. We also thank the Masters in Clinical Research (MSCR) Program of the University of Hawaii – Manoa, led by Dr. Rosanne Harrigan, for the opportunity to conduct this thesis work. We thank Dr. Jim Davis, for his guidance in statistical analyses.
Footnotes
Conflict of Interest: None of the authors have any conflicts of interest regarding the data presented in this manuscript.
References
- Adle-Biassette H, Levy Y, Colombel M, Poron F, Natchev S, Keohane C, Gray F. Neuronal apoptosis in HIV infection in adults. Neuropathol Appl Neurobiol. 1995;21:218–227. doi: 10.1111/j.1365-2990.1995.tb01053.x. [DOI] [PubMed] [Google Scholar]
- Adle-Biassette H, Chrétien F, Wingertsmann L, Héry C, Ereau T, Scaravilli F, Tardieu M, Gray F. Neuronal apoptosis does not correlate with dementia in HIV infection but is related to microglial activation and axonal damage. Neuropathol Appl Neurobiol. 1999;25:123–133. doi: 10.1046/j.1365-2990.1999.00167.x. [DOI] [PubMed] [Google Scholar]
- Amtmann D, Cook KF, Jensen MP, Chen WH, Choi S, Revicki D, Cella D, Rothrock N, Keefe F, Callahan L, Lai JS. Development of a PROMIS item bank to measure pain interference. Pain. 2010;150:173–182. doi: 10.1016/j.pain.2010.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apkarian AV, Bushnell MC, Treede RD, Zubieta JK. Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain. 2005;9:463–484. doi: 10.1016/j.ejpain.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Apkarian V, Scholz J. Shared mechanisms between chronic pain and neurodegenerative disease. 2006;3:319–326. [Google Scholar]
- Atlas LY, Lindquist MA, Bolger N, Wager TD. Brain mediators of the effects of noxious heat on pain. Pain. 2014;155:1632–1648. doi: 10.1016/j.pain.2014.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baliki MN, Schnitzer TJ, Bauer WR, Apkarian AV. Brain morphological signatures for chronic pain. PLoS One. 2011;6:e26010. doi: 10.1371/journal.pone.0026010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes J, Ridgway GR, Bartlett J, Henley SM, Lehmann M, Hobbs N, Clarkson MJ, MacManus DG, Ourselin S, Fox NC. Head size, age and gender adjustment in MRI studies: a necessary nuisance? Neuroimage. 2010;53:1244–1255. doi: 10.1016/j.neuroimage.2010.06.025. [DOI] [PubMed] [Google Scholar]
- Barthas F, Sellmeijer J, Hugel S, Waltisperger E, Barrot M, Yalcin I. The anterior cingulate cortex is a critical hub for pain-induced depression. Biological psychiatry. 2015;77:236–245. doi: 10.1016/j.biopsych.2014.08.004. [DOI] [PubMed] [Google Scholar]
- Becker JT, Sanders J, Madsen SK, Ragin A, Kingsley L, Maruca V, Cohen B, Goodkin K, Martin E, Miller EN, Sacktor N, Alger JR, Barker PB, Saharan P, Carmichael OT, Thompson PM Multicenter AIDS Cohort Study. Subcortical brain atrophy persists even in HAART-regulated HIV disease. Brain imaging and behavior. 2011;5:77–85. doi: 10.1007/s11682-011-9113-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsook D, Erpelding N, Becerra L. Losses and gains: chronic pain and altered brain morphology. Expert Rev Neurother. 2013;13:1221–1234. doi: 10.1586/14737175.2013.846218. [DOI] [PubMed] [Google Scholar]
- Breitbart W, McDonald MV, Rosenfeld B, Passik SD, Hewitt D, Thaler H, Portenoy RK. Pain in ambulatory AIDS patients. I: Pain characteristics and medical correlates. Pain. 1996;68:315–321. doi: 10.1016/s0304-3959(96)03215-0. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Head D, Parker J, Fotenos AF, Marcus D, Morris JC, Snyder AZ. A unified approach for morphometric and functional data analysis in young old demented adults using automated atlas-based head size normalization: reliability and validation against manual measurement of total intracranial volume. Neuroimage. 2004;23:724–738. doi: 10.1016/j.neuroimage.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Bushnell MC, Duncan GH, Hofbauer RK, Ha B, Chen JI, Carrier B. Pain perception: is there a role for primary somatosensory cortex? Proc Natl Acad Sci U S A. 1999;96:7705–7709. doi: 10.1073/pnas.96.14.7705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr D. Pain in HIV/AIDS: A Major Health Problem. In: Carr D, editor. International Association for the Study of Pain. 2004. [Google Scholar]
- Ceko M, Bushnell MC, Fitzcharles MA, Schweinhardt P. Fibromyalgia interacts with age to change the brain. NeuroImage Clinical. 2013;3:249–260. doi: 10.1016/j.nicl.2013.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cella D, et al. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005-2008. J Clin Epidemiol. 2010;63:1179–1194. doi: 10.1016/j.jclinepi.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Andres M, Sadino J, Jiang CS, Nakama H, Miller E, Ernst T. Impact of apolipoprotein E epsilon4 and HIV on cognition and brain atrophy: antagonistic pleiotropy and premature brain aging. Neuroimage. 2011;58:1017–1027. doi: 10.1016/j.neuroimage.2011.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Jiang C, Cunningham E, Buchthal S, Douet V, Andres M, Ernst T. Effects of APOE ε4, age, and HIV on glial metabolites and cognitive deficits. Neurology. 2014;82:2213–2222. doi: 10.1212/WNL.0000000000000526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Lohaugen GC, Douet V, Miller EN, Skranes J, Ernst T. Neural correlates of working memory training in HIV patients: study protocol for a randomized controlled trial. Trials. 2016;17(1):62. doi: 10.1186/s13063-016-1160-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Løhaugen GC, Andres T, Jiang CS, Douet V, Tanizaki N, Walker C, Castillo D, Lim A, Skranes J, Otoshi C, Miller EN, Ernst TM. Adaptive working memory training improved brain function in human immunodeficiency virus-seropositive patients. Ann Neurol. 2017;81(1):17–34. doi: 10.1002/ana.24805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleary DR, Heinricher MM. Adaptations in responsiveness of brainstem pain-modulating neurons in acute compared with chronic inflammation. Pain. 2013;154:845–855. doi: 10.1016/j.pain.2013.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corasaniti MT, Rotiroti D, Nappi G, Bagetta G. Neurobiological mediators of neuronal apoptosis in experimental neuroAIDS. Toxicology letters. 2003;139:199–206. doi: 10.1016/s0378-4274(02)00434-4. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical Surface-Based Analysis I: Segmentation and Surface Reconstruction. NeuroImage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Desouza DD, Moayedi M, Chen DQ, Davis KD, Hodaie M. Sensorimotor and Pain Modulation Brain Abnormalities in Trigeminal Neuralgia: A Paroxysmal, Sensory-Triggered Neuropathic Pain. PLoS One. 2013;8:e66340. doi: 10.1371/journal.pone.0066340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekenze OS, Nwosu CM, Ogunniyi A. Frequency and risk factors for distal sensory polyneuropathy in HIV infection in a developing country. International journal of STD & AIDS. 2014;25:178–183. doi: 10.1177/0956462413498226. [DOI] [PubMed] [Google Scholar]
- Ellis RJ, Rosario D, Clifford DB, McArthur JC, Simpson D, Alexander T, Gelman BB, Vaida F, Collier A, Marra CM, Ances B, Atkinson JH, Dworkin RH, Morgello S, Grant I. Continued high prevalence and adverse clinical impact of human immunodeficiency virus-associated sensory neuropathy in the era of combination antiretroviral therapy: the CHARTER Study. Arch Neurol. 2010;67:552–558. doi: 10.1001/archneurol.2010.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erpelding N, Moayedi M, Davis KD. Cortical thickness correlates of pain and temperature sensitivity. Pain. 2012;153:1602–1609. doi: 10.1016/j.pain.2012.03.012. [DOI] [PubMed] [Google Scholar]
- Evans S, Weinberg BA, Spielman L, Fishman B. Assessing negative thoughts in response to pain among people with HIV. Pain. 2003;105:239–245. doi: 10.1016/s0304-3959(03)00220-3. [DOI] [PubMed] [Google Scholar]
- Fischer CP, Jorgen G, Gundersen H, Pakkenberg B. Preferential loss of large neocortical neurons during HIV infection: a study of the size distribution of neocortical neurons in the human brain. Brain Res. 1999;828:119–126. doi: 10.1016/s0006-8993(99)01344-x. [DOI] [PubMed] [Google Scholar]
- Freund P, Weiskopf N, Ward NS, Hutton C, Gall A, Ciccarelli O, Craggs M, Friston K, Thompson AJ. Disability, atrophy and cortical reorganization following spinal cord injury. Brain. 2011;134:1610–1622. doi: 10.1093/brain/awr093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz HC, McAuley JH, Wittfeld K, Hegenscheid K, Schmidt CO, Langner S, Lotze M. Chronic Back Pain Is Associated With Decreased Prefrontal and Anterior Insular Gray Matter: Results From a Population-Based Cohort Study. J Pain. 2016;17:111–118. doi: 10.1016/j.jpain.2015.10.003. [DOI] [PubMed] [Google Scholar]
- Frøkjær JB, Bouwense SA, Olesen SS, Lundager FH, Eskildsen SF, van Goor H, Wilder-Smith OH, Drewes AM. Reduced cortical thickness of brain areas involved in pain processing in patients with chronic pancreatitis. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2012;10:434–438. e431. doi: 10.1016/j.cgh.2011.11.024. [DOI] [PubMed] [Google Scholar]
- Glare PA. Pain in patients with HIV infection: issues for the new millennium. Eur J Pain. 2001;(5 Suppl A):43–48. doi: 10.1053/eujp.2001.0279. [DOI] [PubMed] [Google Scholar]
- Gray F, Adle-Biassette H, Chretien F, Lorin de la Grandmaison G, Force G, Keohane C. Neuropathology and neurodegeneration in human immunodeficiency virus infection. Pathogenesis of HIV-induced lesions of the brain, correlations with HIV-associated disorders and modifications according to treatments. Clin Neuropathol. 2001;20:146–155. [PubMed] [Google Scholar]
- Grovit-Ferbas K, Harris-White ME. Thinking about HIV: the intersection of virus, neuroinflammation and cognitive dysfunction. Immunol Res. 2010;48:40–58. doi: 10.1007/s12026-010-8166-x. [DOI] [PubMed] [Google Scholar]
- Gustin SM, Peck CC, Wilcox SL, Nash PG, Murray GM, Henderson LA. Different pain, different brain: thalamic anatomy in neuropathic and non-neuropathic chronic pain syndromes. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:5956–5964. doi: 10.1523/JNEUROSCI.5980-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao S. The Molecular and Pharmacological Mechanisms of HIV-Related Neuropathic Pain. Curr Neuropharmacol. 2013;11:499–512. doi: 10.2174/1570159X11311050005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashmi JA, Baliki MN, Huang L, Baria AT, Torbey S, Hermann KM, Schnitzer TJ, Apkarian AV. Shape shifting pain: chronification of back pain shifts brain representation from nociceptive to emotional circuits. Brain. 2013;136:2751–2768. doi: 10.1093/brain/awt211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S, Banks WA. Role of the immune system in HIV-associated neuroinflammation and neurocognitive implications. Brain Behav Immun. 2015;45:1–12. doi: 10.1016/j.bbi.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang KT, Owino C, Gramelspacher GP, Monahan PO, Tabbey R, Hagembe M, Strother RM, Njuguna F, Vreeman RC. Prevalence and correlates of pain and pain treatment in a western Kenya referral hospital. J Palliat Med. 2013;16:1260–1267. doi: 10.1089/jpm.2013.0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iannetti GD, Salomons TV, Moayedi M, Mouraux A, Davis KD. Beyond metaphor: contrasting mechanisms of social and physical pain. Trends in cognitive sciences. 2013;17:371–378. doi: 10.1016/j.tics.2013.06.002. [DOI] [PubMed] [Google Scholar]
- Ji RR, Xu ZZ, Gao YJ. Emerging targets in neuroinflammation-driven chronic pain. Nat Rev Drug Discov. 2014;13:533–548. doi: 10.1038/nrd4334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen JP, Fields HL, Manning BH. The affective component of pain in rodents: direct evidence for a contribution of the anterior cingulate cortex. Proc Natl Acad Sci U S A. 2001;98:8077–8082. doi: 10.1073/pnas.141218998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaul M, Garden GA, Lipton SA. Pathways to neuronal injury and apoptosis in HIV-associated dementia. Nature. 2001;410:988–994. doi: 10.1038/35073667. [DOI] [PubMed] [Google Scholar]
- Keltner JR, et al. HIV-associated distal neuropathic pain is associated with smaller total cerebral cortical gray matter. J Neurovirol. 2014;20:209–218. doi: 10.1007/s13365-014-0236-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp WL, Burns DK, Brown TG. Inflammation and Repair. 1. The McGraw-Hill Companies; 2008. Pathology: The Big Picture. (2 C, ed) [Google Scholar]
- Krashin DL, Merrill JO, Trescot AM. Opioids in the management of HIV-related pain. Pain Physician. 2012;15:ES157–168. [PubMed] [Google Scholar]
- Küper M, Rabe K, Esser S, Gizewski ER, Husstedt IW, Maschke M, Obermann M. Structural gray and white matter changes in patients with HIV. Journal of neurology. 2011;258:1066–1075. doi: 10.1007/s00415-010-5883-y. [DOI] [PubMed] [Google Scholar]
- Lawson E, Sabin C, Perry N, Richardson D, Gilleece Y, Churchill D, Dean G, Williams D, Fisher M, Walker-Bone K. Is HIV Painful? An Epidemiologic Study of the Prevalence and Risk Factors for Pain in HIV-infected Patients. The Clinical journal of pain. 2014 doi: 10.1097/AJP.0000000000000162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Liu X, Tang SJ. Interactions of Opioids and HIV Infection in the Pathogenesis of Chronic Pain. Front Microbiol. 2016;7:103. doi: 10.3389/fmicb.2016.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd DM, Helbig T, Findlay G, Roberts N, Nurmikko T. Brain Areas Involved in Anticipation of Clinically Relevant Pain in Low Back Pain Populations With High Levels of Pain Behavior. J Pain. 2016;17:577–587. doi: 10.1016/j.jpain.2016.01.470. [DOI] [PubMed] [Google Scholar]
- Malone IB, Leung KK, Clegg S, Barnes J, Whitwell JL, Ashburner J, Fox NC, Ridgway GR. Accurate automatic estimation of total intracranial volume: a nuisance variable with less nuisance. Neuroimage. 2015;104:366–372. doi: 10.1016/j.neuroimage.2014.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malvar J, Vaida F, Sanders CF, Atkinson JH, Bohannon W, Keltner J, Robinson-Papp J, Simpson DM, Marra CM, Clifford DB, Gelman B, Fan J, Grant I, Ellis RJ, Group C. Predictors of new-onset distal neuropathic pain in HIV-infected individuals in the era of combination antiretroviral therapy. Pain. 2015;156:731–739. doi: 10.1097/01.j.pain.0000461252.75089.bf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann R, Sadosky A, Schaefer C, Baik R, Parsons B, Nieshoff E, Stacey BR, Tuchman M, Nalamachu S. Burden of HIV-Related Neuropathic Pain in the United States. J Int Assoc Provid AIDS Care. 2016;15:114–125. doi: 10.1177/2325957415592474. [DOI] [PubMed] [Google Scholar]
- Masliah E, Achim CL, Ge N, DeTeresa R, Terry RD, Wiley CA. Spectrum of human immunodeficiency virus-associated neocortical damage. Annals of neurology. 1992;32:321–329. doi: 10.1002/ana.410320304. [DOI] [PubMed] [Google Scholar]
- May A. Chronic pain may change the structure of the brain. Pain. 2008;137:7–15. doi: 10.1016/j.pain.2008.02.034. [DOI] [PubMed] [Google Scholar]
- Melzack R. Evolution of the neuromatrix theory of pain. The Prithvi Raj Lecture: presented at the third World Congress of World Institute of Pain, Barcelona 2004. Pain Pract. 2005;5:85–94. doi: 10.1111/j.1533-2500.2005.05203.x. [DOI] [PubMed] [Google Scholar]
- Merlin JS. Chronic Pain in Patients With HIV Infection: What Clinicians Need To Know. Top Antivir Med. 2015;23:120–124. [PMC free article] [PubMed] [Google Scholar]
- Merlin JS, Westfall AO, Chamot E, Overton ET, Willig JH, Ritchie C, Saag MS, Mugavero MJ. Pain is independently associated with impaired physical function in HIV-infected patients. Pain Med. 2013;14:1985–1993. doi: 10.1111/pme.12255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlin JS, Cen L, Praestgaard A, Turner M, Obando A, Alpert C, Woolston S, Casarett D, Kostman J, Gross R, Frank I. Pain and physical and psychological symptoms in ambulatory HIV patients in the current treatment era. Journal of Pain and Symptom Management. 2012;43:638–645. doi: 10.1016/j.jpainsymman.2011.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moayedi M, Weissman-Fogel I, Salomons TV, Crawley AP, Goldberg MB, Freeman BV, Tenenbaum HC, Davis KD. Abnormal gray matter aging in chronic pain patients. Brain Res. 2012;1456:82–93. doi: 10.1016/j.brainres.2012.03.040. [DOI] [PubMed] [Google Scholar]
- Mole TB, MacIver K, Sluming V, Ridgway GR, Nurmikko TJ. Specific brain morphometric changes in spinal cord injury with and without neuropathic pain. NeuroImage Clinical. 2014;5:28–35. doi: 10.1016/j.nicl.2014.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moont R, Crispel Y, Lev R, Pud D, Yarnitsky D. Temporal changes in cortical activation during conditioned pain modulation (CPM), a LORETA study. Pain. 2011;152:1469–1477. doi: 10.1016/j.pain.2011.01.036. [DOI] [PubMed] [Google Scholar]
- Moore RD, Wong WM, Keruly JC, McArthur JC. Incidence of neuropathy in HIV-infected patients on monotherapy versus those on combination therapy with didanosine, stavudine and hydroxyurea. AIDS. 2000;14:273–278. doi: 10.1097/00002030-200002180-00009. [DOI] [PubMed] [Google Scholar]
- Mphahlele NR, Mitchell D, Kamerman PR. Pain in ambulatory HIV-positive South Africans. Eur J Pain. 2012;16:447–458. doi: 10.1002/j.1532-2149.2011.00031.x. [DOI] [PubMed] [Google Scholar]
- Nair SN, Mary TR, Prarthana S, Harrison P. Prevalence of Pain in Patients with HIV/AIDS: A Cross-sectional Survey in a South Indian State. Indian J Palliat Care. 2009;15:67–70. doi: 10.4103/0973-1075.53550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oertel BG, Preibisch C, Martin T, Walter C, Gamer M, Deichmann R, Lötsch J. Separating brain processing of pain from that of stimulus intensity. Human brain mapping. 2012;33:883–894. doi: 10.1002/hbm.21256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker R, Stein DJ, Jelsma J. Pain in people living with HIV/AIDS: a systematic review. Journal of the International AIDS Society. 2014;17:18719. doi: 10.7448/IAS.17.1.18719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips TJ, Brown M, Ramirez JD, Perkins J, Woldeamanuel YW, Williams AC, Orengo C, Bennett DL, Bodi I, Cox S, Maier C, Krumova EK, Rice AS. Sensory, psychological, and metabolic dysfunction in HIV-associated peripheral neuropathy: A cross-sectional deep profiling study. Pain. 2014;155:1846–1860. doi: 10.1016/j.pain.2014.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price DD. The affective-motivational dimension of pain. A two-stage model. APS Journal. 1992;1:229–239. [Google Scholar]
- Price DD. Psychological and neural mechanisms of the affective dimension of pain. Science. 2000;288:1769–1772. doi: 10.1126/science.288.5472.1769. [DOI] [PubMed] [Google Scholar]
- Qu C, King T, Okun A, Lai J, Fields HL, Porreca F. Lesion of the rostral anterior cingulate cortex eliminates the aversiveness of spontaneous neuropathic pain following partial or complete axotomy. Pain. 2011;152:1641–1648. doi: 10.1016/j.pain.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranki A, Nyberg M, Ovod V, Haltia M, Elovaara I, Raininko R, Haapasalo H, Krohn K. Abundant expression of HIV Nef and Rev proteins in brain astrocytes in vivo is associated with dementia. AIDS. 1995;9:1001–1008. doi: 10.1097/00002030-199509000-00004. [DOI] [PubMed] [Google Scholar]
- Robinson ME, Craggs JG, Price DD, Perlstein WM, Staud R. Gray matter volumes of pain-related brain areas are decreased in fibromyalgia syndrome. J Pain. 2011;12:436–443. doi: 10.1016/j.jpain.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Raecke R, Niemeier A, Ihle K, Ruether W, May A. Brain gray matter decrease in chronic pain is the consequence and not the cause of pain. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:13746–13750. doi: 10.1523/JNEUROSCI.3687-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RStudioTeam. Integrated Development for R Studio. USA: R Studio Inc.; 2015. [Google Scholar]
- Sargolzaei S, Sargolzaei A, Cabrerizo M, Chen G, Goryawala M, Pinzon-Ardila A, Gonzalez-Arias SM, Adjouadi M. Estimating Intracranial Volume in Brain Research: An Evaluation of Methods. Neuroinformatics. 2015;13:427–441. doi: 10.1007/s12021-015-9266-5. [DOI] [PubMed] [Google Scholar]
- Sarma MK, Nagarajan R, Keller MA, Kumar R, Nielsen-Saines K, Michalik DE, Deville J, Church JA, Thomas MA. Regional brain gray and white matter changes in perinatally HIV-infected adolescents. NeuroImage Clinical. 2014;4:29–34. doi: 10.1016/j.nicl.2013.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Wilcke T. Neuroimaging of chronic pain. Best Pract Res Clin Rheumatol. 2015;29:29–41. doi: 10.1016/j.berh.2015.04.030. [DOI] [PubMed] [Google Scholar]
- Schütz SG, Robinson-Papp J. HIV-related neuropathy: current perspectives. HIV AIDS (Auckl) 2013;5:243–251. doi: 10.2147/HIV.S36674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweinhardt P, Bushnell MC. Pain imaging in health and disease--how far have we come? The Journal of clinical investigation. 2010;120:3788–3797. doi: 10.1172/JCI43498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serchuck LK, Williams PL, Nachman S, Gadow KD, Chernoff M, Schwartz L, Team I. Prevalence of pain and association with psychiatric symptom severity in perinatally HIV-infected children as compared to controls living in HIV-affected households. AIDS care. 2010;22:640–648. doi: 10.1080/09540120903280919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart GJ, Irvine SS, Scott M, Kelleher AD, Marriott DJ, McKnight I, Pethebridge AM, Wodak A, Ziegler J. Managing HIV. Part 1: Principles. 1.2 Strategies of care in managing HIV. Med J Aust. 1996;164:99–104. [PubMed] [Google Scholar]
- Teutsch S, Herken W, Bingel U, Schoell E, May A. Changes in brain gray matter due to repetitive painful stimulation. Neuroimage. 2008;42:845–849. doi: 10.1016/j.neuroimage.2008.05.044. [DOI] [PubMed] [Google Scholar]
- Tracey I, Mantyh PW. The cerebral signature for pain perception and its modulation. Neuron. 2007;55:377–391. doi: 10.1016/j.neuron.2007.07.012. [DOI] [PubMed] [Google Scholar]
- Treede RD, Kenshalo DR, Gracely RH, Jones AK. The cortical representation of pain. Pain. 1999;79:105–111. doi: 10.1016/s0304-3959(98)00184-5. [DOI] [PubMed] [Google Scholar]
- Uebelacker LA, Weisberg RB, Herman DS, Bailey GL, Pinkston-Camp MM, Stein MD. Chronic Pain in HIV-Infected Patients: Relationship to Depression, Substance Use Mental Health and Pain Treatment. Pain Med. 2015;16:1870–1881. doi: 10.1111/pme.12799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade BS, Valcour VG, Wendelken-Riegelhaupt L, Esmaeili-Firidouni P, Joshi SH, Gutman BA, Thompson PM. Mapping abnormal subcortical brain morphometry in an elderly HIV+ cohort. NeuroImage Clinical. 2015;9:564–573. doi: 10.1016/j.nicl.2015.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Liu Z, Liu J, Tang Z, Li H, Tian J. Gray and white matter alterations in early HIV-infected patients: Combined voxel-based morphometry and tract-based spatial statistics. Journal of magnetic resonance imaging : JMRI. 2016;43:1474–1483. doi: 10.1002/jmri.25100. [DOI] [PubMed] [Google Scholar]
- Wiley CA, Masliah E, Morey M, Lemere C, DeTeresa R, Grafe M, Hansen L, Terry R. Neocortical damage during HIV infection. Annals of neurology. 1991;29:651–657. doi: 10.1002/ana.410290613. [DOI] [PubMed] [Google Scholar]
- Yan N, Cao B, Xu J, Hao C, Zhang X, Li Y. Glutamatergic activation of anterior cingulate cortex mediates the affective component of visceral pain memory in rats. Neurobiol Learn Mem. 2012;97:156–164. doi: 10.1016/j.nlm.2011.11.003. [DOI] [PubMed] [Google Scholar]
- Zou S, Fitting S, Hahn YK, Welch SP, El-Hage N, Hauser KF, Knapp PE. Morphine potentiates neurodegenerative effects of HIV-1 Tat through actions at μ-opioid receptor-expressing glia. Brain. 2011;134:3616–3631. doi: 10.1093/brain/awr281. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.