Abstract
[11C]-PK11195 (PK11195) has been widely used with positron emission tomography (PET) to assess levels of the translocator protein 18 kDa (TSPO) as a marker of neuroinflammation. Recent ligands, such as [11C]-PBR28 and [11C]-DPA713, have improved signal-to-noise ratio and specificity for TSPO over PK11195. However, these second generation radiotracers exhibit binding differences due to a single polymorphism (rs6971) that leads to three genotypes: C/C, C/T and T/T associated with high, mixed and low binding affinities, respectively. Here we report that [3H]-DPA-713 in the presence of cholesterol or PK11195 has an accelerated dissociation rate from TSPO in platelets isolated from individuals with the T/T genotype. This allosteric interaction was not observed in platelets isolated from individuals with the C/C or C/T genotype. The results provide a molecular rationale for low binding affinity of T/T TSPO and further support the exclusion of these subjects from PET imaging studies using second generation TSPO ligands.
Translocator protein 18 kDa (TSPO), previously known as the peripheral benzodiazepine receptor, is an outer mitochondrial membrane protein that is thought to be involved in the transport of cholesterol into mitochondria, a required step in steroid biosynthesis. As a component of the outer mitochondrial membrane, TSPO has also been associated with mitochondrial functions like mitochondrial permeability transition pore opening, apoptosis and cell proliferation (Rupprecht et al. 2010). More recent evidence using ligands that target TSPO raise questions about its role in steroidogenesis or cell viability (Selvaraj and Stocco 2015). While the physiological role and therapeutic potential of TSPO are a matter of debate, it is clear that increases in TSPO expression have been documented in various diseases characterized by glial cell activation and neuroinflammation, including Alzheimer's disease, amyotrophic lateral sclerosis, Parkinson's disease and multiple sclerosis (MS) (Rupprecht et al. 2010). As a result, methods to image neuroinflammation by monitoring TSPO levels in disease are of major interest (Turkheimer et al. 2015).
Positron emission tomography (PET) with [11C]-PK 11195, a first generation TSPO ligand, has been used to demonstrate microglial activation in various neurodegenerative, psychiatric, and neurooncologic diseases (Turkheimer et al. 2015). Even though [11C]-PK11195 is a potent (Ki = 9.3 ± 0.5 nM) TSPO radiotracer (James et al. 2008), it exhibits low brain permeability and high non-specific plasma protein binding resulting in PET images with low signal-to-noise ratio and low specificity (Vivash and O'Brien 2016). In an effort to overcome these limitations, second generation TSPO ligands such as [11C]-DPA-713 (Chauveau et al. 2008) and [11C]-PBR28 (Owen et al. 2012) were developed. Early binding studies using [11C]-PK11195 did not differentiate binding among healthy volunteers. In contrast, binding studies with [11C]-PBR28 unveiled three patient populations: one subgroup of high affinity binders (HABs), a subgroup with two distinguishable PBR28 binding sites that was labeled mixed-affinity binders (MABs) and a third subgroup labeled low-affinity binders (LABs); the results suggested that differences in binding affinities among subjects was not simply a reduction in TSPO density and raised the question of potential functional significance for the different binding affinities (Owen et al. 2010). Subsequent studies with several second generation TSPO ligands demonstrated the importance of considering TSPO binding patterns from individual subjects; the three binding affinities were confirmed in brains from healthy subjects and were shown to be in similar proportions to those found in MS brains (Owen et al. 2011). Additional studies showed that a single nucleotide polymorphism (rs6971) was the underlying cause for differences in binding affinities (Kreisl et al. 2013; Owen et al. 2012). The rs6971 polymorphism results in three genotypes and a corresponding amino acid substitution from alanine to threonine at position 147 of TSPO: C/C (Ala/Ala), C/T (Ala/Thr) and T/T (Thr/Thr). Each of these genotypes was found to be associated with the specific binding phenotypes: C/C, high binding affinity; C/T, mixed binding affinity and T/T, low binding affinity. In the clinic, T/T binding has been difficult to distinguish from nonspecific binding so the clinical usefulness of TSPO binding in subjects with this genotype is limited (Turkheimer et al. 2015; Vivash and O'Brien 2016).
In this work, we used kinetic dissociation studies to directly compare the binding of [3H]-DPA-713 in platelet lysates from individuals of each TSPO genotype. We found that in contrast to results from individuals with C/C or C/T genotype, platelets from individuals with the T/T genotype exhibit a unique allosteric binding site that decreases the residence time of [3H]-DPA-713 in the presence of excess cholesterol or PK11195. The results suggest a molecular mechanism for the observed low binding in subjects with the T/T genotype.
Methods
Human Subjects
A Johns Hopkins Institutional Review Board approved this study and all participants provided written informed consent. Peripheral whole blood was collected from healthy adult participants (over age 18 years) who were recruited for a [11C]DPA-713 PET imaging study (Coughlin et al. 2014) through local advertising. Each subject completed a careful clinical interview to confirm no history of medical disease or surgery in the past year and a physical assessment with screening blood work, electrocardiogram and urine toxicology. Eligibility criteria for the healthy controls were previously reported (Coughlin et al. 2014). Each participant underwent TSPO (rs6971) genotype analysis using the rs6971 TaqMan assay (Applied Biosytems®, Life Technologies, Grand Island, NY) on isolated DNA (PureGene® Blood Core Kit C, Qiagen, Valencia, CA) as previously described (Coughlin et al. 2014). One platelet sample (described below) from each of three male individuals (ages 23, 46, and 54 years) was selected to represent data from an individual of each TSPO genotype (C/C, C/T, T/T). Two of the subjects were African American and one was Caucasian. None of the three healthy individuals included in the current study were taking prescribed or over-the-counter medications (Coughlin et al. 2014).
Platelet preparation and [3H]-DPA-713 dissociation measurements
A peripheral whole blood sample from each participant was collected into a glass vacutainer tube (BD vacutainer® catalog No. 364606) containing acid citrate dextrose (22.0 g/L trisodium citrate, 8.0 g/L citric acid, 24.5 g/L dextrose). Platelet-rich plasma (PRP) was isolated by centrifugation at 190 × g for 15 min at 4 °C. The PRP was subsequently centrifuged at 2500 × g for 5 min at 4 °C and then the platelet pellet was resuspended in PBS and stored at -80 °C. Protein concentrations were determined using a colorimetric assay where absorbance was read at 750 nm (Dc Protein Assay Kit; Bio-Rad, Hercules, CA). Dissociation rates were determined as previously described (Limbird 2005). Briefly, the association phase was conducted by incubating lysates of human platelets (0.25 mg protein/mL) in assay buffer (50 mM Tris, 140 mM NaCl, 1.5 mM MgCl2, 5 mM KCl, 1.5 mM CaCl2, pH 7.4 at 37 °C) exhibiting the TSPO genotypes (C/C, T/T and CT) with 100 nM [3H]-DPA-713 (83 Ci/mmol; Quotient Bioresearch) for 10 min. The concentration of radioligand was slightly above the binding affinity (66.4 nM when using T/T genotype) (Owen et al. 2011) to ensure measurable, steady state binding. The dissociation phase was then initiated by addition of excess unlabeled DPA (12 μM), PK11195 (5 μM) or cholesterol (1.2 μM). The ligand being evaluated for allosteric modification was added during the dissociation phase. The amount of [3H]-DPA-713 still bound to the receptor was measured at various times during the first 30 min after addition of unlabeled ligands. Incubations were terminated by vacuum filtration through Whatman GF/B filters pretreated with 0.3% polyethyleneimine. Filters were washed three times with ice-cold 0.1 M NaCl, dried and the radioactivity retained on the filters measured using a scintillation counter. Rate of [3H]-DPA-713 dissociation was first determined in the presence of excess DPA-713 (12 μM) as control. The change in rate of dissociation was then determined in the presence of DPA-713 plus potential allosteric modifiers PK11195 or cholesterol at 5 and 1.2 μM respectively.
Data analysis
GraphPad Prism® (version 5.0; GraphPad Software Inc., La Jolla, CA) was used to perform nonlinear regression analysis; a first order exponential decay equation was used for curve fitting, and determination of dissociation rate constants. Half-lives (t1/2) were obtained from the first-order dissociation constants and correspond to the molecular t1/2 of the TSPO-ligand complex.
Results and Discussion
Change in the dissociation rate of a radioligand is a well-established method to identify allosteric modulation of receptor radioligand interactions and has been used to demonstrate allosteric modulation in several experimental systems (for review see (Limbird 2005)). Likewise, residence time of a receptor-ligand complex can significantly influence its biological function; in fact, TSPO ligand residence times were recently used to predict their neurostereoidogenic efficacy (Costa et al. 2016). In this study we followed the dissociation rate of [3H]-DPA-713 from TSPO in platelet lysates from individuals of each TSPO genotype in the presence and absence of competing ligands (PK11195 and cholesterol). We report the presence of allosteric modulation when using PK11195 and cholesterol in individuals with the T/T genotype that results in acceleration of DPA-713 rate of dissociation and a corresponding decrease in residence time.
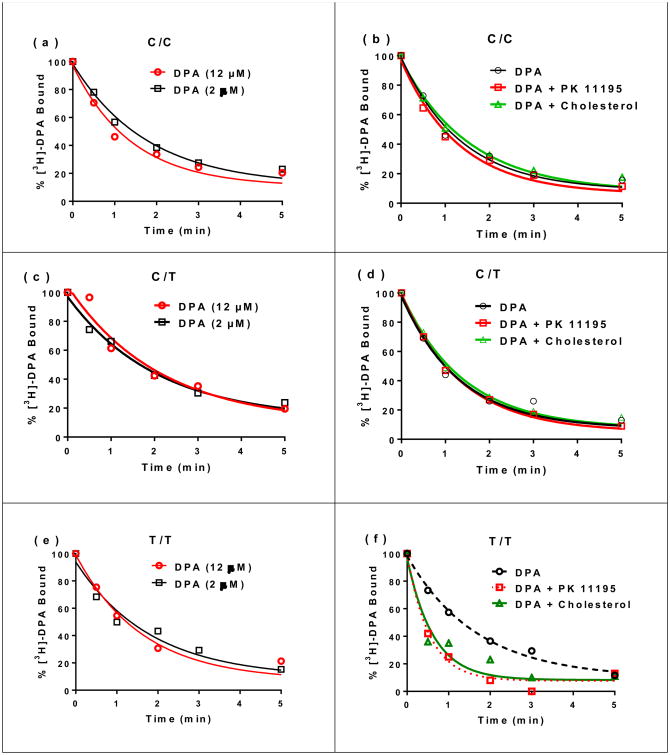
Before performing dissociation measurements of [3H]-DPA-713 in the presence of excess DPA-713, we first determined the concentration of DPA-713 that was sufficient to displace [3H]-DPA-713 while preventing the radiolabeled ligand from binding to TSPO again. Association phase was initiated by incubating platelets isolated from each subject with high (C/C)-, mixed (C/T)- or low (T/T)-affinity phenotype with [3H]-DPA-713. After maximal association was observed (approximately 10 min), [3H]-DPA-713 dissociation was initiated by the addition of 2 or 12 μM DPA-713. Dissociation rates were the same when using 2 and 12 μM for TSPO from all three genotypes (Fig 1a, 1c and 1e and Table 1). Lack of acceleration of dissociation rates when using 2 vs. 12 μM DPA-713 confirmed that 2 μM DPA-713 was sufficient to displace and subsequently prevent further association of the radiolabeled ligand.
Fig 1.
Rate of [3H]-DPA-713 dissociation from TSPO when using platelets isolated from human subjects with C/C, C/T, and T/T rs6971 genotypes. Left Panels: dissociation of [3H]-DPA-713 in the presence of 2 and 12 μM DPA-713 when using (a) C/C, (c) C/T and (e) T/T genotypes. Right Panels: rate of [3H]-DPA-713 dissociation in the presence of excess unlabeled DPA-713 (12 μM) and effect on this rate by PK 11195 (5 μM) and cholesterol (1.2 μM) when using (b) C/C, (d) C/T and (f) T/T genotypes.
Table 1.
Rate constants of dissociation (koff in min-1), residence times (RT = 1/koff in min) and half-lives (t½ in min) corresponding to kinetic traces shown in Fig 1.
| Displacer of [3H]-DPA-713 | Dissociation Parameters | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| C/C | C/T | T/T | |||||||
| koff | RT | t1/2 | koff | RT | t1/2 | koff | RT | t1/2 | |
| DPA-713 (12 μM) | 0.74 | 1.35 | 0.94 | 0.76 | 1.28 | 0.92 | 0.56a | 1.78 | 1.24 |
| DPA-713 (12 μM) + PK11195 (5 μM) | 0.76 | 1.31 | 0.91 | 0.75 | 1.33 | 0.93 | 1.90a | 0.52 | 0.36 |
| DPA-713 (12 μM) + Cholesterol (1.2 μM) | 0.65 | 1.53 | 1.07 | 0.72 | 1.38 | 0.96 | 1.58a | 0.63 | 0.43 |
One phase exponential decay fits for T/T dissociation traces (Fig 1f) exhibit 95% confidence interval that koff for [3H]-DPA-713 is faster when PK 11195 or cholesterol are present than when [3H]-DPA-713 dissociates in the presence of DPA-713.
Even though preliminary experiments showed 2 μM DPA-713 was already in excess to prevent detection of rebinding of [3H]-DPA-713 at 100 nM after it dissociated, we used 12 μM DPA-713 in subsequent experiments to insure a large excess of unlabeled ligand. When using the C/C or the C/T genotype, the rate of dissociation of [3H]-DPA-713 in the presence of excess DPA-713 was the same as when using excess PK11195 or cholesterol to trigger DPA-713 dissociation (Fig 1b and 1d and Table 1). In contrast, when using platelets from the individual with T/T genotype, both PK11195 and cholesterol accelerated the rate of dissociation of [3H]-DPA-713 (Fig 1f and Table 1). The rate constant of dissociation was approximately 2.8 to 3.4-fold higher when using DPA-713 plus PK11195 (1.90 min-1) or cholesterol (1.58 min-1) vs. DPA-713 alone (0.56 min-1) (Table 1). The residence time and the molecular t1/2 of the TSPO-ligand complex obtained from the corresponding dissociation rate constants were correspondingly lower when using platelets from the subject with T/T versus platelets from subjects with the C/C or C/T genotype (Table 1). The results indicate that TSPO from individuals with the T/T genotype has an allosteric site that upon binding to cholesterol or PK11195 accelerates dissociation of DPA-713 from TSPO thus reducing its residence time.
This is the first report on a TSPO allosteric site in individuals with the T/T genotype. Although some crystal work using wild type and mutant TSPOs was recently reported and rationalizations were made to explain binding differences (Guo et al. 2015; Li et al. 2015), no allosteric site was predicted or described.
In summary, using dissociation kinetics of the second generation TSPO ligand [3H]-DPA-713 we identify, for the first time, unique allosteric interactions for TSPO from individuals with the T/T rs6971 polymorphism. We report that PK11195 and cholesterol bind to this allosteric site which in turn makes [3H]-DPA-713 dissociate faster than when using excess DPA-713 alone (Fig 1f). In contrast, these allosteric interactions were not observed in TSPO from individuals with the C/C or C/T genotype (Fig 1b and 1d). The results provide a rationale at the protein level for why individuals undergoing PET imaging with a second generation radiotracer who have the T/T genotype exhibit lower binding affinities than individuals with similar TSPO and cholesterol levels who have C/C or C/T genotype. Subjects with the T/T genotype exhibit an allosteric interaction between the DPA-713 binding site and another site where an endogenous ligand like cholesterol or the synthetic ligand PK11195 bind and bring about an acceleration of the dissociation rate with a corresponding reduction in residence time. In contrast, PK11195 and cholesterol appear to bind to the same site as DPA-713 in subjects with C/C or C/T genotype. The results support the exclusion of individuals with the T/T genotype from in vivo TSPO PET studies using second generation TSPO ligands. Third generation radiotracers that do not show genotype binding effects in humans may necessitate the identification of a new binding site; these are being pursued but have not yet been identified. TSPO PET imaging remains a challenging field where there are issues of specificity (TSPO binds to microglia and astrocytes) and sensitivity where only severe neurological disease states exhibit robust TSPO PET imaging without resolution of microglia subtypes.
Footnotes
Authors declare that they do not have conflict of interest
References
- Chauveau F, Boutin H, Van Camp N, Dolle F, Tavitian B. Nuclear imaging of neuroinflammation: a comprehensive review of [11C]PK11195 challengers. Eur J Nucl Med Mol Imaging. 2008;35:2304–2319. doi: 10.1007/s00259-008-0908-9. [DOI] [PubMed] [Google Scholar]
- Costa B, Da Pozzo E, Giacomelli C, Barresi E, Taliani S, Da Settimo F, Martini C. TSPO ligand residence time: a new parameter to predict compound neurosteroidogenic efficacy. Sci Rep. 2016;6:18164. doi: 10.1038/srep18164. doi:srep18164 [pii]10.1038/srep18164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coughlin JM, et al. Regional brain distribution of translocator protein using [(11)C]DPA-713 PET in individuals infected with HIV. J Neurovirol. 2014;20:219–232. doi: 10.1007/s13365-014-0239-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, et al. Protein structure. Structure and activity of tryptophan-rich TSPO proteins. Science. 2015;347:551–555. doi: 10.1126/science.aaa1534. doi:347/6221/551[pii]10.1126/science.aaa1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James ML, et al. DPA-714, a new translocator protein-specific ligand: synthesis, radiofluorination, and pharmacologic characterization. J Nucl Med. 2008;49:814–822. doi: 10.2967/jnumed.107.046151. doi:jnumed.107.046151[pii]10.2967/jnumed.107.046151. [DOI] [PubMed] [Google Scholar]
- Kreisl WC, et al. A genetic polymorphism for translocator protein 18 kDa affects both in vitro and in vivo radioligand binding in human brain to this putative biomarker of neuroinflammation. J Cereb Blood Flow Metab. 2013;33:53–58. doi: 10.1038/jcbfm.2012.131. doi:jcbfm2012131 [pii]10.1038/jcbfm.2012.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Liu J, Zheng Y, Garavito RM, Ferguson-Miller S. Protein structure. Crystal structures of translocator protein (TSPO) and mutant mimic of a human polymorphism. Science. 2015;347:555–558. doi: 10.1126/science.1260590. doi:347/6221/555[pii]10.1126/science.1260590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limbird LE. Cell Surface Receptors Third. Third. Springer; New York: 2005. [Google Scholar]
- Owen DR, et al. Mixed-affinity binding in humans with 18-kDa translocator protein ligands. J Nucl Med. 2011;52:24–32. doi: 10.2967/jnumed.110.079459. doi:jnumed.110.079459[pii]10.2967/jnumed.10.079459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen DR, et al. Two binding sites for [3H]PBR28 in human brain: implications for TSPO PET imaging of neuroinflammation. J Cereb Blood Flow Metab. 2010;30:1608–1618. doi: 10.1038/jcbfm.2010.63. doi:jcbfm201063[pii]10/1038/jcbfm.2010.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen DR, et al. An 18-kDa translocator protein (TSPO) polymorphism explains differences in binding affinity of the PET radioligand PBR28. J Cereb Blood Flow Metab. 2012;32:1–5. doi: 10.1038/jcbfm.2011.147. doi:jcbfm2011147[pii]10.1038/jcbfm.2011.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupprecht R, et al. Translocator protein (18 kDa) (TSPO) as a therapeutic target for neurological and psychiatric disorders. Nat Rev Drug Discov. 2010;9:971–988. doi: 10.1038/nrd3295. doi:nrd3295[pii]10.1038/nrd3295. [DOI] [PubMed] [Google Scholar]
- Selvaraj V, Stocco DM. The changing landscape in translocator protein (TSPO) function. Trends Endocrinol Metab. 2015;26:341–348. doi: 10.1016/j.tem.2015.02.007. doi:S1043-2760(15)00033-8[pii]10.1016/j.tem.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkheimer FE, Rizzo G, Bloomfield PS, Howes O, Zanotti-Fregonara P, Bertoldo A, Veronese M. The methodology of TSPO imaging with positron emission tomography. Biochem Soc Trans. 2015;43:586–592. doi: 10.1042/BST20150058. doi:BST20150058[pii]10.1042/BST20150058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivash L, O'Brien TJ. Imaging Microglial Activation with TSPO PET: Lighting Up Neurologic Diseases? Journal of Nuclear Medicine. 2016;57:165–168 d. doi: 10.2967/jnumed.114.141713. oi:10.2967/jnumed.114.141713. [DOI] [PubMed] [Google Scholar]