Abstract
Background
Filaggrin loss-of-function (FLG-LOF) mutations are an established genetic cause of eczema. These mutations have subsequently been reported to increase the risk of aeroallergen sensitization and allergic airway disease. However, it is unclear whether FLG variants require both eczema and aeroallergen sensitization to influence airway disease development long-term outcomes.
Objective
To examine the effects of FLG-LOF mutations on allergic airway disease outcomes, with eczema and aeroallergen sensitization as intermediate variables, using the Isle of Wight birth cohort.
Methods
Study participants were evaluated at ages 1, 2, 4, 10 and 18 years to ascertain the development of allergic diseases (eczema, asthma and allergic rhinitis) and aeroallergen sensitization (determined by skin prick tests). FLG-LOF mutations were genotyped in 1150 subjects. To understand the complex associations between FLG mutations, intermediate variables (eczema and aeroallergen sensitization) and airway disease, path analysis was performed.
Results
There were significant total effects of FLG-LOF mutations on both asthma and allergic rhinitis at all ages as well as on aeroallergen sensitization up till 10 years old. In the filaggrin-asthma analysis, a direct effect of FLG-LOF mutations was observed on early childhood eczema (age 1 and 2 years) (relative risk (RR) 2.01, 95% CI: 1.74 - 2.31, p < 0.001), and all significant indirect pathways on asthma outcomes passed through eczema at these ages. In contrast, for the filaggrin-rhinitis model, FLG-LOF mutations exerted significant direct effects on early eczema as well as rhinitis at 10 years (RR 1.99; 95% CI: 1.72 - 2.29, p = 0.002).
Conclusion
FLG-LOF mutations are a significant risk factor for later childhood asthma and rhinitis. However, the pathway to asthma is only through early childhood eczema while a direct effect was observed for childhood rhinitis.
Introduction
The overall prevalence and burden of allergic diseases (eczema, rhinitis and asthma) in childhood and adolescence have increased in recent years. (1) These conditions appear to be associated with one another, as illustrated by the “allergic march” concept whereby eczema is often the first manifestation of allergic disease in infancy and early childhood, followed by rhinitis and asthma. (2) However, this concept has recently been challenged in favor of allergic comorbidity where these diseases occur together although overt manifestation may be sequential. (3, 4) Allergic sensitization is a likely common denominator as evidence suggests a close relationship between atopic dermatitis, aeroallergen sensitization (AAS) and asthma/rhinitis. (5–8)
Genetic factors have garnered widespread examination as an explanation for this observed association, of which the role of filaggrin (FLG) mutations has received particular attention as a risk factor for allergic disease development (33). FLG encodes for a skin epidermal protein that contributes to the natural skin barrier protecting against transcutaneous water loss as well as the unwanted entry of environmental allergens. (9, 10) FLG loss-of-function (FLG-LOF) mutations have been established to cause epidermal barrier dysfunction, thereby increasing the risk of eczema. (11) Beyond eczema, there is evidence that FLG mutations are also associated with allergic sensitization and airway allergy in later childhood. (12–16)
The exact mechanism by which FLG-LOF mutations increase allergic airway disease remains to be determined. One proposed pathway is that the presence of prior eczema and skin inflammation are necessary to allow for the mechanical entry of aeroallergens, thus promoting the sequential development of aeroallergen sensitization (AAS) and airway disease in subjects with FLG-LOF mutations. (17–19) An alternative pathway is that a defective skin barrier allows allergens to induce systemic Th2-driven sensitization, independent of eczema. (13, 20)
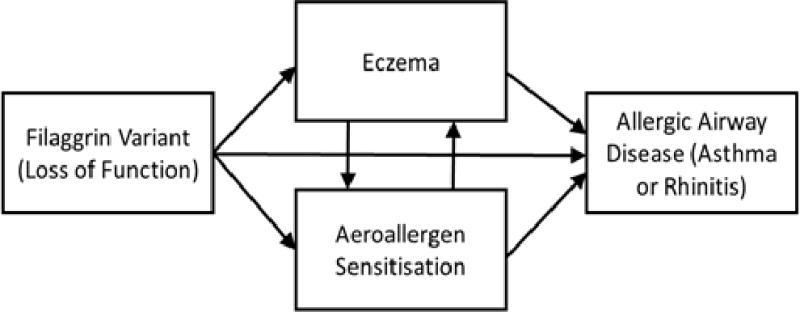
Ziyab et al studied the interactions between FLG-LOF mutations and allergic sensitization on the outcomes of eczema and allergic airway disease in the Isle of Wight cohort. (21) With the same cohort, we further analysed the impact of FLG-LOF mutations on long-term allergic airway disease outcomes up to 18 years old, with eczema and aeroallergen sensitization as intermediate analysis variables. Using path analysis, the relative contributions of eczema and AAS towards the pathogenesis of asthma and rhinitis in FLG variants were evaluated. (Figure 1)
Figure 1.
Methodology
Study Population
The IOW birth cohort consists of all subjects consecutively born on the Isle of Wight between 1 January 1989 and 28 February 1990 (see Supplementary Figure 1). They were recruited at birth and followed up at regular intervals (1, 2, 4, 10 and 18 years of age) in order to study the impact of genetic and environmental factors on the natural development and progression of allergic diseases. (21–23) (Supplementary Figure 2) Exclusion criteria were children who were adopted, perinatal deaths and parental refusal for follow-up. The study was approved by the Isle of Wight Local Research Ethics Committee (06/Q1701/34) and received further approval in 2006 for genetic studies.
Diagnostic Criteria for Allergic Disease
The detailed assessment of the IOW birth cohort has been published previously (24–27). At each follow-up, subjects and/or parents were required to complete study-specific and disease validated questionnaires including International Study of Asthma and Allergy in Childhood (ISAAC) (25). The majority of participants underwent skin prick testing (SPT) at 4, 10 and 18 years to 14 common food and aeroallergens (ALK-Abello, Horsholm, Denmark). (23) Subjects who could not physically attend follow-ups received either a postal- or telephone-based questionnaire to maintain data collection.
A diagnosis of eczema in the past 12 months was made based on the diagnostic criteria by Hanifin and Rajka, that is, a pruritic dermatitis of a chronic and relapsing duration (more than 6 weeks) with a characteristic morphology and distribution. (28) Asthma was defined as having both a history of asthma and either the presence of wheezing or requirement for asthma medications in the prior 12 months. (29) Rhinitis was defined by sneezing, running and / or congested nose present on most days in the past 12 months, in the absence of a cold or flu. (30). Allergic disease diagnoses were confirmed by both the patients’ primary physicians as well as clinicians involved in the study.
Aeroallergen Sensitization
Aeroallergen sensitization (AAS) status was determined using skin prick tests (SPTs) performed at the follow-ups. Aeroallergens tested included house dust mite (Dermatophagoides pteronyssinus), cat, dog, Alternaria alternata, Cladosporium herbarium, grass pollen mix, and tree pollen mix (ALK-Albello, Horsholm, Denmark). Positive (histamine) and negative (saline) controls were performed for measurements. At ages 1 and 2 years, SPTs were performed only if subjects demonstrated symptoms of eczema, asthma, or rhinitis. For the purpose of analysis, SPT results for these 2 follow-ups were combined as they occurred within a short time period. At 4, 10 and 18 years, SPTs were routinely performed on children attending the follow-ups to the standard battery of aeroallergens regardless of symptoms. Positive sensitization was defined as having a SPT reaction to at least one aeroallergen with a mean wheal diameter of 3 mm greater than the negative control.
FLG Genotyping
Five variants in the FLG gene (R501X, 2282del4, S3247X, 3702delG, and R2447X) that account for 96% of all FLG mutations in European ancestry were selected for genotyping. (11) Extracted DNA samples were run using GoldenGate Genotyping Assays on the BeadXpressVeracode platform (Illumina, Inc, SanDiego, CA) and analyzed using the genotyping module of the GenomeStudio Software package (Illumina, Inc, SanDiego, CA). Children were determined to have FLG loss-of-function defect if they carry the minor allele for at least one of the following FLG null variants: R501X, 2282del, or S3247X.
Statistical Analysis
Data analysis was first performed with SPSS software (Version 22; IBM, USA). Categorical variables that were assessed included gender, the proportion of patients with aeroallergen sensitization (based on positive or negative SPT), eczema, rhinitis and asthma at each follow-up time point. Statistical significance of the difference in proportions between the two groups were assessed by the chi-squared or Fisher exact test. To explore the mechanisms leading to longitudinal airway disease manifestation in FLG-LOF mutation carriers, path analysis was performed with Mplus software (Version 7, USA). Paths were first mapped out for direct and indirect effects. Risk ratios for each path, defined as the ratio of two probabilities, were derived. Using FLG-LOF as an example, one probability would be having the disease (outcome) if a subject possessed the FLG-LOF mutation, whilst the other probability was having the disease without the mutation. Probabilities were assessed based on probit regressions. A direct effect is defined by a unidirectional path where a determining variable is directly responsible for an outcome, with all other variables remaining constant. (31) An example would be FLG-LOF (A) → Asthma (C). An indirect effect is defined as the effect of a variable on an outcome through another intermediate intervening variable, such as FLG-LOF (A) → Eczema (B) → Asthma (C). The total effect is a combination of direct and indirect effect on the specified outcome, which reflects the overall effect of latent continuous variables leading to a binary outcome. (31) On the basis of time sequence, the path model also includes paths from one variable measured at an earlier age to variables and outcomes measured at later ages. For instance, the initial model included paths from eczema at ages 1 or 2 years to eczema at age 4 and to asthma at age 10. These paths enabled us to evaluate whether eczema at an earlier age influences eczema and/or asthma at a later age. For this study, the total effects of FLG-LOF on allergic airway disease outcomes would include all possible interactions with eczema and aeroallergen sensitization at various time points. Statistically significant paths were determined via stepwise variable selection based on p-values. Separate path models to assess the direct and indirect effects of risk factors of asthma and rhinitis were constructed. Path coefficients and risk ratios were estimated for constructed paths. 95% confidence intervals (CIs) were used and a P value of < 0.05 was regarded as statistically significant.
Results
Study Population Description
1456 children were available for analysis. Subjects were predominantly (99%) of Caucasian ethnicity. The IOW cohort was dynamic in that some subjects were unable to attend one follow up but returned to participate in future assessments. Nonetheless, we had high retention at each assessment from 1 to 18 years (see Supplementary Figure 2). During routine investigation for sensitization, skin prick test results availability at each assessment ranged between 58.4 to 71.1%.
The prevalence of eczema was highest at 2 years of age (21.1%) (Table 1). Thereafter, eczema prevalence decreased at 4 years but remained constant until 18 years. Consistent with the atopic march, allergic airway disease tended to manifest to a greater extent in later childhood and adolescence. Similarly, the prevalence of positive AAS sensitization increased progressively from 17.3% at 4 years to 40.3% at 18 years. There was a significant risk of asthma at 4 years in children with eczema at ages 1 (risk ratio (RR) 2.29; 95% CI: 1.70 - 3.09; p < 0.001) or 2 (RR 1.74; 95% CI: 1.29 - 2.36; p < 0.001) years (see Supplementary Table). Eczema at 4 years was associated with increased asthma presentation at 4 (RR 2.19; 95% CI: 1.63 - 2.95; p < 0.001) and 10 (RR 1.56; 95% CI: 1.07 - 2.27; p = 0.02) years (Supplementary Table). Early life eczema at 1, 2 and 4 years was consistently associated with rhinitis from 4 years onwards (Supplementary Table).
Table 1.
Prevalence of allergic disease and aeroallergen sensitization outcomes in the IOW cohort and FLG-genotyped subpopulation
| Total IOW cohort n/N (%) |
FLG-genotyped subpopulation n/N (%) |
p value | |
|---|---|---|---|
| Gender | |||
| Male | 786/1536 (51.2) | 569/1150 (49.5) | 0.38 |
| Female | 750/1536 (48.8) | 581/1150 (50.5) | 0.38 |
| Eczema | |||
| 1 year | 182/1364 (13.3) | 145/1065 (13.6) | 0.85 |
| 2 years | 259/1227 (21.1) | 209/978 (21.2) | 0.88 |
| 4 years | 147/1214 (12.1) | 121/1008 (12.0) | 0.94 |
| 10 years | 186/1358 (13.7) | 164/1117 (14.6) | 0.48 |
| 18 years | 161/1307 (12.3) | 132/1086 (14.2) | 0.90 |
| Asthma | |||
| 4 years | 182/1214 (15.0) | 151/1006 (15.0) | 1.0 |
| 10 years | 201/1368 (14.7) | 136/1016 (13.4) | 0.37 |
| 18 years | 231/1305 (17.7)# | 162/973 (16.6) | 0.49 |
| Rhinitis | |||
| 4 years | 66/1214 (5.4) | 51/1007 (5.1) | 0.75 |
| 10 years | 205/1362 (15.1) | 184/1121 (16.4) | 0.38 |
| 18 years | 468/1309 (35.8)^ | 405/1087 (37.3) | 0.45 |
| Positive Aeroallergen sensitization | |||
| 1 or 2 years | 79/563 (14.0) | 71/473 (15.0) | 0.65 |
| 4 years | 170/980 (17.3) | 119/725 (16.4) | 0.62 |
| 10 years | 276/1035 (26.7) | 197/765 (25.8) | 0.67 |
| 18 years | 343/851 (40.3)¥ | 248/628 (39.5) | 0.76 |
n: number of patients with specified characteristic or disease; N: total number of patients with available data for analysis at a specified time-point; %: prevalence
There was a significant increase in prevalence of asthma from 10 to 18 years (p = 0.005).
Rhinitis increased in prevalence from 4 to 18 years (p < 0.001).
Aeroallergen sensitization increased in prevalence from 4 to 18 years (p < 0.001).
FLG status
FLG status was tested in 1150 participants, in which 119 had FLG-LOF mutations. No significant differences in eczema, airway disease and AAS were found between the whole study population and the genotyped population (Table 1). The frequency of minor allele occurrences in the cohort had previously been described by Ziyab et al. (32)
Path Analysis
Total Effects
The peak association between FLG-LOF mutations and eczema was noted at 4 years (RR 2.89; 95% CI: 2.57 - 3.24; p < 0.001). There was a significant effect of FLG-LOF mutations on asthma at all ages studied, with a greater association seen at 10 years (RR 1.96; 95% CI: 1.70 - 2.26; p = 0.003) and 18 years of age (RR 2.50; 95% CI: 2.20 - 2.83; p = 0.004) (Table 2). Additionally, FLG-LOF mutations also demonstrated a significant association with rhinitis at all ages, with the strongest association observed at 10 years (RR 2.66; 95% CI: 2.35 - 3.00; p < 0.001). FLG-LOF demonstrated an impact on AAS up to 10 years, but this association was not evident at 18 years.
Table 2.
Total path analysis effects of all FLG-LOF mutations on allergic disease and aeroallergen sensitization outcomes
| Subjects with available disease and FLG-status data at specified time-point |
1 & 2 Years (n / N) |
4 Years (n / N) |
10 Years (n / N) |
#18 Years (n / N) |
Risk Ratio (95% CI) |
p-value |
|---|---|---|---|---|---|---|
| Eczema | ||||||
| 1026 | 26 / 242 | 2.01 (1.74 - 2.31) |
< 0.001 | |||
| 895 | 13 / 110 | 2.89 (2.57 - 3.24) |
< 0.001 | |||
| 1010 | 13 / 131 | 2.26 (1.98 - 2.58) |
< 0.001 | |||
| 975 | 9 / 116 | 1.71 (1.46 - 1.99) |
< 0.001 | |||
| AAS | ||||||
| 143 | 5 / 54 | 1.43 (1.21 - 1.69) |
0.002 | |||
| 841 | 22 / 152 | 1.52 (1.29 - 1.78) |
< 0.001 | |||
| 953 | 38 / 263 | 1.65 (1.41 - 1.92) |
< 0.001 | |||
| Asthma | ||||||
| 1006 | 22 / 151 | 1.49 (1.26 - 1.75) |
0.001 | |||
| 1016 | 15 / 136 | 1.96 (1.70 - 2.26) |
0.003 | |||
| 973 | 18 / 162 | 2.50 (2.20 - 2.83) |
0.004 | |||
| Rhinitis | ||||||
| 1007 | 7 / 51 | 1.76 (1.51 - 2.04) |
0.006 | |||
| 1121 | 33 / 184 | 2.66 (2.35 - 3.00) |
< 0.001 | |||
| 1087 | 57 / 405 | 1.32 (1.10 - 1.57) |
< 0.001 |
n: number of patients with FLG-LOF mutations and specified condition
N: overall number of patients with specified condition at specified time point
AAS: aeroallergen sensitization
No significant total effect on AAS at 18 years was detected.
FLG-LOF mutations were detected in 119 study participants.
Direct Effects
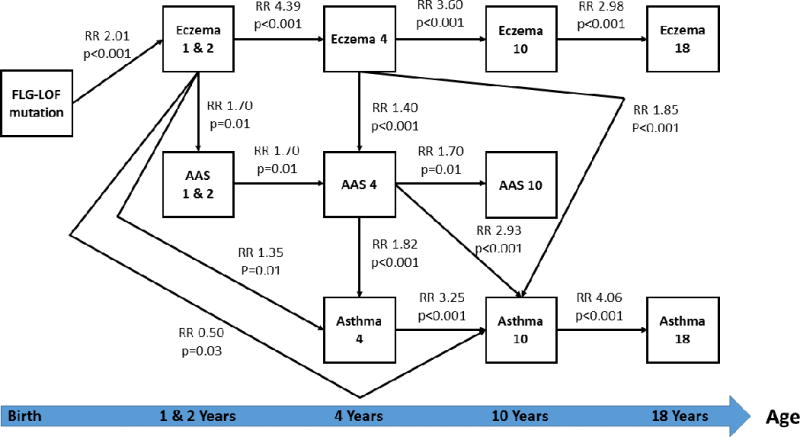
In the FLG-asthma path analyses (Figure 2), no direct effect of FLG-LOF mutations on asthma or aeroallergen sensitization (AAS) was found across all ages. A direct effect of FLG-LOF on eczema at 1 to 2 years of age was seen (RR 2.01, 95% CI: 1.74 - 2.31, p < 0.001). Eczema at 1 to 2 years of age in FLG-LOF variants had multiple direct effects on eczema at 4 years (RR 4.39, 95% CI: 3.99 - 4.82, p < 0.001), early aeroallergen sensitization up to age 4 years as well as on asthma at 4 years of age. Surprisingly, eczema at 1 to 2 years appeared to reduce the risk of asthma at 10 years old (RR 0.50; 95% CI: 0.37 - 0.66; p = 0.03). Eczema at 1 to 4 years had no direct effect on late asthma at 18 years. Eczema at 4 years directly increased the probability of developing aeroallergen sensitization at 4 years (RR 1.40, 95% CI: 1.18 - 1.65, p < 0.001) and asthma only at 10 years (RR 1.85, 95% CI: 1.59 - 2.14, p < 0.001). Later eczema (10 and 18 years) had no direct effects on late asthma and AAS. Both AAS (RR 1.82, 95% CI: 1.57 - 2.11, p < 0.001) and asthma (RR 3.25, 95% CI: 2.91 - 3.62, p < 0.001) at 4 years had direct effects on later asthma at 10 years. Only asthma at 10 years had a direct effect on having asthma at 18 years.
Figure 2.
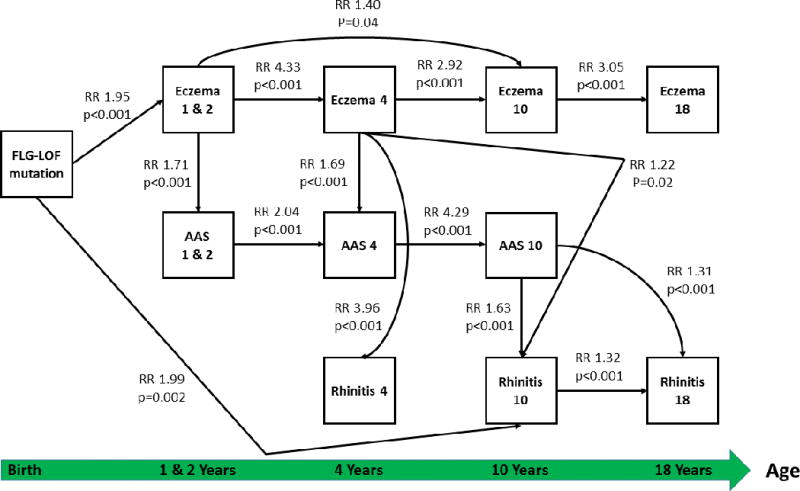
Direct effects on rhinitis at 4 years arose from eczema at 4 years only (RR 3.96, 95% CI: 3.58 - 4.37, p < 0.001) (Figure 3). For rhinitis at 10 years, a direct effect of FLG-LOF mutations (RR 1.99; 95% CI: 1.72 - 2.29, p = 0.002), earlier eczema at 4 years (RR 1.22; 95% CI: 1.01 - 1.46, p = 0.02) and aeroallergen sensitization at 10 years (RR 1.63; 95% CI: 1.39 - 1.90; p < 0.001) was observed. Late AAS at 10 years also had a direct effect on rhinitis at 18 years (RR 1.32, 95% CI: 1.10 - 1.57, p < 0.001).
Figure 3.
Indirect Effects
The most statistically significant indirect effects of FLG-LOF mutations on asthma at all ages occurred through early eczema (1, 2 and 4 years) and early aeroallergen sensitization (AAS) at 4 years (Table 3). FLG-LOF had an indirect effect on asthma at age 4 through eczema at 1-2 years but the rest of the indirect effects of FLG-LOF on asthma involved both, eczema and AAS as intermediaries. Likewise, for rhinitis at 4 years, FLG-LOF mutations had an indirect effect that required early eczema (1, 2 and 4 years) but not AAS. For rhinitis at 10 and 18 years, FLG-LOF variants exerted their effects via early eczema as well as through AAS at 4 and 10 years. There were no significant paths whereby FLG-mutations influenced airway disease outcomes through AAS without the presence of eczema.
Table 3.
Indirect pathways depicting the most significant associations linking FLG mutations and airway disease outcomes
| FLG status | 1 and 2 years | 4 years | 10 years | 18 years | Path co-efficient | P value |
|---|---|---|---|---|---|---|
|
| ||||||
| FLG-LOF → | Eczema→ | Eczema→AAS→Asthma | 3.17 | 0.002 | ||
| FLG-LOF → | Eczema→ | Eczema→AAS→ | Asthma | 3.14 | 0.002 | |
| FLG-LOF → | Eczema→ | Eczema→AAS→ | Asthma→ | Asthma | 3.08 | 0.002 |
| FLG-LOF → | Eczema→ | Eczema→ Rhinitis | 2.65 | 0.008 | ||
| FLG-LOF → | Eczema→ | Eczema→AAS→ | AAS→Rhinitis | 3.27 | 0.001 | |
| FLG-LOF → | Eczema→ | Eczema→AAS→ | AAS→ | Rhinitis | 3.33 | 0.001 |
AAS: aeroallergen sensitization
Discussion
We sought to study the time-dependent interplay between FLG-LOF mutations and sequential development of aeroallergen sensitization and allergic airway disease. Our study has found that the association of FLG deficiency with allergic disease outcomes extends up to 18 years of age, as compared to data from cohort studies which demonstrated that FLG-LOF mutations have an impact on allergic disease up till 11 years of age. (17, 33) FLG-LOF mutations exerted total effects not only on eczema and allergic airway disease at all ages studied but also on aeroallergen sensitization up to 10 years of age. Except for one direct path linking FLG-LOF mutation and rhinitis at 10 years old, all other paths passed through eczema at 1 and 2 years to give rise to airway disease, thus emphasising the role of eczema during early childhood in increasing the probability of persistent airway disease expression in FLG variants.
Two biologic pathways have been proposed to increase the risk of FLG-LOF: one suggests that a barrier permeability impairment attributable to FLG-LOF mutations without eczema is the main factor, and a second assumes that eczematous skin inflammation contributes to both the barrier defect and mediation of systemic Th2 inflammation. Recent studies appear to favor the latter mechanism in asthma. Skin barrier defects in eczema patients have been reported to be associated with higher aeroallergen sensitization. (5) In a study by Thyssen et al, FLG mutations were not associated with food and aeroallergen sensitization without concomitant atopic dermatitis. (19) Palmer et al found that FLG mutations were more strongly associated with a combined eczema-asthma phenotype than with asthma alone. (11) Marenholz et al performed case-control association analysis and similarly found the strongest association with the “eczema plus asthma” phenotype in FLG variants. (18) Palmer et al however, did not evaluate atopic sensitization, whilst Marenholz et al’s study evaluated atopic sensitization as an outcome of FLG-LOF effects, but not as a risk factor for disease development. We defined eczema and aeroallergen sensitization as intermediate variables as this would be more intuitive in a pathophysiologic disease model. We then attempted to dissect and quantify the contributions of these factors in a longitudinal manner on allergic airway disease outcomes. An important result was derived from indirect path analysis, where it was repeatedly demonstrated that FLG-LOF mutations required early eczema followed by aeroallergen sensitization as pre-requisites to influence asthma presentations at all ages. Similar to a previous analysis conducted by Ziyab et al, our analysis detected a limit of effect of FLG-LOF mutations upon aeroallergen sensitization up to 10 years of age. (32) This corresponded to a decrease in eczema prevalence in late childhood and adolescence in our cohort, suggesting that resolution of eczema and maturation of the skin barrier with age allow for reduced sensitization in the later years.
Several other findings were derived from FLG-asthma path analyses. First, although eczema at 1 and 2 years was associated with a reduced direct effect on asthma at 10 years, the total effect of FLG-LOF mutation on asthma at this age remained high, which was through eczema at 1 and 2 years and allergen sensitization at 4 years. This means that eczema at 1 and 2 years does have an overall increased risk of asthma at age 10 but this is mediated by allergen sensitization at 4 years, where the barrier defect plus skin inflammation promote allergen entry as well as stimulates atopic responses. The German Multicentre Asthma Study reported similar findings where children with eczema in early life only developed asthma if they were atopic. In non-atopic children, there was no increase in asthma risk. (34) It is unlikely asthma at age 10 is significantly contributed by early life viral infection induced wheezing, as most viral wheezes would have improved by that age. (35) It is possible that a longer period of eczema up to 4 years is required to alter the immunologic development of asthma for this to have a direct effect on asthma. Second, there was a progressively stronger total effect of FLG-LOF on persistent asthma at 10 and 18 years. We postulate that distinct asthma phenotypes stratified by age could account for this observation. For instance, in this cohort, 40% asthma was non-atopic at age 10, which may not have been predicted by eczema at age 1 and 2, as early life eczema is largely an atopic phenotype. (36) In a late/persistent asthma phenotype, the direct effect of aeroallergen sensitization at 4 years and the most significant indirect paths having to pass through aeroallergen sensitization at this age highlight the importance of combined interactions between early eczema and aeroallergen sensitization. An abnormal increase in skin permeability and inflammation facilitate epicutaneous sensitization. (5, 19) This may promote a prolonged Th2 type inflammatory response that accounts for the later presentation of asthma. The documentation of ongoing systemic eosinophilic inflammation in adolescent asthma was observed in a previous report in this cohort. (37) Our study supports this observation whereby aeroallergen sensitization at 4 years leads to asthma at 10 years which in turn strongly predicts asthma persistence into adolescence via a significant direct effect. In contrast, the pathogenesis of early childhood asthma appears to be heterogenous. On the background of the highest prevalence of eczema occurring at age 2 years in our cohort, the presence of a direct effect of eczema at 1 and 2 years increased the probability of an early asthma phenotype at 4 years. These findings are consistent with results from the Copenhagen Prospective Study on Asthma in Childhood, where early onset of asthma was followed by later development of sensitization. (38) This suggests that factors other than aeroallergen sensitization could contribute to early asthma. One possible contribution could arise from Staphylococcus aureus skin infections in FLG-LOF variants. FLG breakdown products urocanic acid (UCA) and pyrrolidone carboxylic acid (PCA) are able to attenuate S. aureus survival by lowering the pH of the stratum corneum (39). A deficiency of these products is associated with increased S. aureus skin colonization in FLG-LOF variants. (40) Moreover, interactions between Staphylococcal enterotoxins, toll-like receptors and specific IgE have been reported to upregulate bronchial inflammation. (41, 42) Whether S. aureus can directly affect asthma outcomes in these individuals requires further analysis of the mechanistic pathways.
Asthma and allergic rhinitis are thought to share similar pathogenetic mechanisms as described in the “one airway one disease” concept, due to similarities in their respective mucosae. (43) The discordance in the increasing prevalence of these two conditions in some epidemiologic studies, as well as a difference in response to certain therapeutics, however, point to inherent differences in these disorders. (44–46) Our study found a direct effect of FLG-LOF mutation on rhinitis at 10 years, whereas no direct effects of FLG mutations on asthma were found. This is similar to the findings by Weidinger et al, in which an association between FLG mutations and allergic rhinitis was observed independent of eczema. (47) In the same study, nasal biopsies were performed on healthy controls which noted strong FLG expression in the stratified epithelium of the nasal vestibule lining. A deficiency of FLG in the nasal region and resultant local inflammation in FLG variants could thus predispose to rhinitis without requiring eczema. Furthermore, late aeroallergen sensitization at 10 years had direct effects on persistent rhinitis at 10 and 18 years whereas this was not observed for asthma. Nonetheless, we found that eczema in FLG variants remained an important intermediate in exerting indirect effects on rhinitis pathogenesis. The combined presence of epicutaneous sensitization and local nasal allergic sensitization could explain why rhinitis prevalence in our cohort increased by almost 7-fold from 4 to 18 years old.
A strength of our study was the availability of a continuous time-frame of data for longitudinal analysis. (17, 48, 49) Information for FLG genotype status was available for a high proportion (79.0%) of study participants, and baseline characteristics between the sampled population and the whole cohort were similar. (50) As the IOW cohort consisted of consecutive births, selection bias was minimized. The participation rate remained high throughout the study (84 - 94.3%), thus minimizing loss-to-follow-up bias. The asthma definition from the ISAAC study was first published in 1993, and this was adopted for our study participants from age 10 years onwards. (51) At age 4 years, it is recognized that not all wheezing equates to asthma. Nonetheless, we found that asthma diagnoses determined by our physicians at this age were accurate, and a previous report on the IOW cohort showed that applying the ISAAC definition retrospectively to this age group did not alter the prevalence of asthma diagnosis. (52)
There were several study limitations. Firstly, data on aeroallergen sensitization at 1 and 2 years was limited to symptomatic children, thereby potentially introducing selection bias. However, previous IOW studies did not find significantly higher rates of sensitization when comparing this age group with subjects at 4 years. (21, 32) Secondly, path analysis poses some limitations. It describes associations but does not establish cause-and-effect relationships between variables. Paths with feedback loops (an example being mutual continued interactions between eczema and aeroallergen sensitization) cannot be assessed in this analysis, although both factors were included in the model for their effects on asthma at later ages. It is recognized that rhinitis itself can be an independent risk factor for asthma development and control. (53) In our analysis, we evaluated separate path models for asthma and rhinitis with the intention of evaluating them as overall outcome variables, thus we were unable to study the interaction between these airway diseases. Links between these two conditions could be further explored in another path study model.
While the observations in our study should be validated with other birth or longitudinal birth cohorts, our study has several clinical implications. With insights gained into the effects of FLG-LOF mutations, it is important to determine airway disease severity in adolescence and progression into adulthood in affected individuals. Translational studies have largely focused on characterizing the skin barrier defect and local inflammatory cytokines in the context of eczema. Our findings highlight how these mechanisms affect asthma and rhinitis presentations to different extents. To understand susceptibility to each condition, further studies are required to understand the pathways in which cytokines, local allergens and possibly the microbiome correlate with airway inflammation. Third, therapeutic strategies targeted at ameliorating eczema and preventing skin infections in FLG variant carriers are being studied. (54) It is possible that such strategies may also be able to influence allergic airway disease outcomes. However, this requires long term follow-up.
In conclusion, distinct time-ordered paths are involved in asthma and rhinitis development. Early eczema is an important risk factor in FLG variants for asthma manifestation up to 18 years old. The pathogenetic mechanisms for rhinitis are different since eczema and local nasal FLG defects (direct effect) can contribute to disease. This study has increased our understanding of associations between FLG mutations and airway disease, and can provide a platform for future studies of allergic disease pathogenesis.
Supplementary Material
Acknowledgments
We would like to acknowledge the help of all the staff at The David Hide Asthma and Allergy Research Centre in undertaking the 18-year and previous assessments of the 1989 Isle of Wight birth cohort. The 18-year assessment and other analyses were funded by grants from the National Institute of Health, USA (R01 HL082925 and R01 AI061471).
Footnotes
DR ADRIAN KWOK WAI CHAN (Orcid ID : 0000-0002-6892-984X)
DR HONGMEI ZHANG (Orcid ID : 0000-0003-3557-0364)
PROFESSOR GRAHAM ROBERTS (Orcid ID : 0000-0003-2252-1248)
References
- 1.Asher MI, Montefort S, Bjorksten B, Lai CK, Strachan DP, Weiland SK, et al. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC Phases One and Three repeat multicountry cross-sectional surveys. Lancet. 2006;368(9537):733–43. doi: 10.1016/S0140-6736(06)69283-0. [DOI] [PubMed] [Google Scholar]
- 2.Spergel JM, Paller AS. Atopic dermatitis and the atopic march. Journal of Allergy & Clinical Immunology. 2003;112(6 Suppl):S118–27. doi: 10.1016/j.jaci.2003.09.033. [DOI] [PubMed] [Google Scholar]
- 3.Belgrave DC, Granell R, Simpson A, Guiver J, Bishop C, Buchan I, et al. Developmental profiles of eczema, wheeze, and rhinitis: two population-based birth cohort studies. PLoS Medicine / Public Library of Science. 2014;11(10):e1001748. doi: 10.1371/journal.pmed.1001748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bousquet J, Anto JM, Wickman M, Keil T, Valenta R, Haahtela T, et al. Are allergic multimorbidities and IgE polysensitization associated with the persistence or re-occurrence of foetal type 2 signalling? The MeDALL hypothesis. Allergy. 2015;70(9):1062–78. doi: 10.1111/all.12637. [DOI] [PubMed] [Google Scholar]
- 5.De Marchi F, Piacentini GL, Piazza M, Sandri M, Boner AL, Peroni DG. Correlation of skin barrier impairment in atopic dermatitis with aeroallergen sensitization. Allergy Asthma Proc. 2015;36(6):127–33. doi: 10.2500/aap.2015.36.3872. [DOI] [PubMed] [Google Scholar]
- 6.Celakovska J, Ettlerova K, Ettler K, Vaneckova J, Bukac J. Sensitization to aeroallergens in atopic dermatitis patients: association with concomitant allergic diseases. J Eur Acad Dermatol Venereol. 2015;29(8):1500–5. doi: 10.1111/jdv.12891. [DOI] [PubMed] [Google Scholar]
- 7.Bantz SK, Zhu Z, Zheng T. The Atopic March: Progression from Atopic Dermatitis to Allergic Rhinitis and Asthma. J Clin Cell Immunol. 2014;5(2) doi: 10.4172/2155-9899.1000202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lazic N, Roberts G, Custovic A, Belgrave D, Bishop CM, Winn J, et al. Multiple atopy phenotypes and their associations with asthma: similar findings from two birth cohorts. Allergy. 2013;68(6):764–70. doi: 10.1111/all.12134. [DOI] [PubMed] [Google Scholar]
- 9.Mildner M, Jin J, Eckhart L, Kezic S, Gruber F, Barresi C, et al. Knockdown of filaggrin impairs diffusion barrier function and increases UV sensitivity in a human skin model. Journal of Investigative Dermatology. 2010;130(9):2286–94. doi: 10.1038/jid.2010.115. [DOI] [PubMed] [Google Scholar]
- 10.Sandilands A, Sutherland C, Irvine AD, McLean WH. Filaggrin in the frontline: role in skin barrier function and disease. Journal of Cell Science. 2009;122(Pt 9):1285–94. doi: 10.1242/jcs.033969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Palmer CN, Irvine AD, Terron-Kwiatkowski A, Zhao Y, Liao H, Lee SP, et al. Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nature Genetics. 2006;38(4):441–6. doi: 10.1038/ng1767. [DOI] [PubMed] [Google Scholar]
- 12.Marenholz I, Nickel R, Ruschendorf F, Schulz F, Esparza-Gordillo J, Kerscher T, et al. Filaggrin loss-of-function mutations predispose to phenotypes involved in the atopic march. Journal of Allergy & Clinical Immunology. 2006;118(4):866–71. doi: 10.1016/j.jaci.2006.07.026. [DOI] [PubMed] [Google Scholar]
- 13.Palmer CN, Ismail T, Lee SP, Terron-Kwiatkowski A, Zhao Y, Liao H, et al. Filaggrin null mutations are associated with increased asthma severity in children and young adults. Journal of Allergy & Clinical Immunology. 2007;120(1):64–8. doi: 10.1016/j.jaci.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 14.McLean WH, Palmer CN, Henderson J, Kabesch M, Weidinger S, Irvine AD. Filaggrin variants confer susceptibility to asthma. Journal of Allergy & Clinical Immunology. 2008;121(5):1294–5. doi: 10.1016/j.jaci.2008.02.039. author reply 5–6. [DOI] [PubMed] [Google Scholar]
- 15.Basu K, Palmer CN, Lipworth BJ, McLean WH, Terron-Kwiatkowski A, Zhao Y, et al. Filaggrin null mutations are associated with increased asthma exacerbations in children and young adults. Allergy. 2008;63(9):1211–7. doi: 10.1111/j.1398-9995.2008.01660.x. [DOI] [PubMed] [Google Scholar]
- 16.Weidinger S, O’Sullivan M, Illig T, Baurecht H, Depner M, Rodriguez E, et al. Filaggrin mutations, atopic eczema, hay fever, and asthma in children. Journal of Allergy & Clinical Immunology. 2008;121(5):1203–9.e1. doi: 10.1016/j.jaci.2008.02.014. [DOI] [PubMed] [Google Scholar]
- 17.Henderson J, Northstone K, Lee SP, Liao H, Zhao Y, Pembrey M, et al. The burden of disease associated with filaggrin mutations: a population-based, longitudinal birth cohort study. Journal of Allergy & Clinical Immunology. 2008;121(4):872–7.e9. doi: 10.1016/j.jaci.2008.01.026. [DOI] [PubMed] [Google Scholar]
- 18.Marenholz I, Nickel R, Ruschendorf F, Schulz F, Esparza-Gordillo J, Kerscher T, et al. Filaggrin loss-of-function mutations predispose to phenotypes involved in the atopic march. Journal of Allergy & Clinical Immunology. 2006;118(4):866–71. doi: 10.1016/j.jaci.2006.07.026. [DOI] [PubMed] [Google Scholar]
- 19.Thyssen JP, Tang L, Husemoen LL, Stender S, Szecsi PB, Menne T, et al. Filaggrin gene mutations are not associated with food and aeroallergen sensitization without concomitant atopic dermatitis in adults. J Allergy Clin Immunol. 2015;135(5):1375–8.e1. doi: 10.1016/j.jaci.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 20.Bonnelykke K, Pipper CB, Tavendale R, Palmer CN, Bisgaard H. Filaggrin gene variants and atopic diseases in early childhood assessed longitudinally from birth. Pediatric Allergy & Immunology. 2010;21(6):954–61. doi: 10.1111/j.1399-3038.2010.01073.x. [DOI] [PubMed] [Google Scholar]
- 21.Ziyab AH, Karmaus W, Zhang H, Holloway JW, Steck SE, Ewart S, et al. Allergic sensitization and filaggrin variants predispose to the comorbidity of eczema, asthma, and rhinitis: results from the Isle of Wight birth cohort. Clin Exp Allergy. 2014;44(9):1170–8. doi: 10.1111/cea.12321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arshad SH, Tariq SM, Matthews S, Hakim E. Sensitization to common allergens and its association with allergic disorders at age 4 years: a whole population birth cohort study. Pediatrics. 2001;108(2) doi: 10.1542/peds.108.2.e33. [DOI] [PubMed] [Google Scholar]
- 23.Roberts G, Zhang H, Karmaus W, Raza A, Scott M, Matthews S, et al. Trends in cutaneous sensitization in the first 18 years of life: results from the 1989 Isle of Wight birth cohort study. Clinical & Experimental Allergy. 2012;42(10):1501–9. doi: 10.1111/j.1365-2222.2012.04074.x. [DOI] [PubMed] [Google Scholar]
- 24.Arshad SH, Hide DW. Effect of environmental factors on the development of allergic disorders in infancy. J Allergy Clin Immunol. 1992;90(2):235–41. doi: 10.1016/0091-6749(92)90077-f. [DOI] [PubMed] [Google Scholar]
- 25.Tariq SM, Matthews SM, Hakim EA, Stevens M, Arshad SH, Hide DW. The prevalence of and risk factors for atopy in early childhood: a whole population birth cohort study. J Allergy Clin Immunol. 1998;101(5):587–93. doi: 10.1016/S0091-6749(98)70164-2. [DOI] [PubMed] [Google Scholar]
- 26.Kurukulaaratchy RJ, Fenn M, Twiselton R, Matthews S, Arshad SH. The prevalence of asthma and wheezing illnesses amongst 10-year-old schoolchildren. Respir Med. 2002;96(3):163–9. doi: 10.1053/rmed.2001.1236. [DOI] [PubMed] [Google Scholar]
- 27.Scott M, Raza A, Karmaus W, Mitchell F, Grundy J, Kurukulaaratchy RJ, et al. Influence of atopy and asthma on exhaled nitric oxide in an unselected birth cohort study. Thorax. 2010;65(3):258–62. doi: 10.1136/thx.2009.125443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hanifin JM, Rajka G. Diagnostic features of atopic dermatitis. Acta Derm Venereol Suppl (Stockh) 1980;92:44–47. [Google Scholar]
- 29.Asher MI, Keil U, Anderson HR, Beasley R, Crane J, Martinez F, et al. International Study of Asthma and Allergies in Childhood (ISAAC): rationale and methods. European Respiratory Journal. 1995;8(3):483–91. doi: 10.1183/09031936.95.08030483. [DOI] [PubMed] [Google Scholar]
- 30.Kurukulaaratchy RJ, Karmaus W, Raza A, Matthews S, Roberts G, Arshad SH. The influence of gender and atopy on the natural history of rhinitis in the first 18 years of life. Clinical & Experimental Allergy. 2011;41(6):851–9. doi: 10.1111/j.1365-2222.2011.03765.x. [DOI] [PubMed] [Google Scholar]
- 31.Land KC. Principles of path analysis. Sociological methodology. 1969;1:3–37. [Google Scholar]
- 32.Ziyab AH, Karmaus W, Yousefi M, Ewart S, Schauberger E, Holloway JW, et al. Interplay of filaggrin loss-of-function variants, allergic sensitization, and eczema in a longitudinal study covering infancy to 18 years of age. PLoS ONE [Electronic Resource] 2012;7(3):e32721. doi: 10.1371/journal.pone.0032721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brough HA, Simpson A, Makinson K, Hankinson J, Brown S, Douiri A, et al. Peanut allergy: effect of environmental peanut exposure in children with filaggrin loss-of-function mutations. J Allergy Clin Immunol. 2014;134(4):867–75.e1. doi: 10.1016/j.jaci.2014.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Illi S, von Mutius E, Lau S, Niggemann B, Gruber C, Wahn U. Perennial allergen sensitization early in life and chronic asthma in children: a birth cohort study. Lancet (London, England) 2006;368(9537):763–70. doi: 10.1016/S0140-6736(06)69286-6. [DOI] [PubMed] [Google Scholar]
- 35.Kurukulaaratchy R, Fenn M, Waterhouse L, Matthews S, Holgate S, Arshad S. Characterization of wheezing phenotypes in the first 10 years of life. Clinical & Experimental Allergy. 2003;33(5):573–8. doi: 10.1046/j.1365-2222.2003.01657.x. [DOI] [PubMed] [Google Scholar]
- 36.Kurukulaaratchy R, Fenn M, Matthews S, Arshad S. Characterisation of atopic and non-atopic wheeze in 10 year old children. Thorax. 2004;59(7):563–8. doi: 10.1136/thx.2003.010462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arshad SH, Raza A, Lau L, Bawakid K, Karmaus W, Zhang H, et al. Pathophysiological characterization of asthma transitions across adolescence. Respir Res. 2014;15:153. doi: 10.1186/s12931-014-0153-7. England. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bonnelykke K, Pipper CB, Tavendale R, Palmer CN, Bisgaard H. Filaggrin gene variants and atopic diseases in early childhood assessed longitudinally from birth. Pediatric Allergy & Immunology. 2010;21(6):954–61. doi: 10.1111/j.1399-3038.2010.01073.x. [DOI] [PubMed] [Google Scholar]
- 39.Miajlovic H, Fallon PG, Irvine AD, Foster TJ. Effect of filaggrin breakdown products on growth of and protein expression by Staphylococcus aureus. Journal of Allergy and Clinical Immunology. 2010;126(6):1184–90.e3. doi: 10.1016/j.jaci.2010.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boguniewicz M, Leung DY. Recent insights into atopic dermatitis and implications for management of infectious complications. J Allergy Clin Immunol. 2010;125(1):4–13. doi: 10.1016/j.jaci.2009.11.027. quiz 4–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Novak N, Bieber T. FcepsilonRI-Toll-like receptor interaction in atopic dermatitis. Curr Probl Dermatol. 2011;41:47–53. doi: 10.1159/000323295. [DOI] [PubMed] [Google Scholar]
- 42.Huvenne W, Hellings PW, Bachert C. Role of staphylococcal superantigens in airway disease. Int Arch Allergy Immunol. 2013;161(4):304–14. doi: 10.1159/000350329. [DOI] [PubMed] [Google Scholar]
- 43.Bousquet J, Vignola AM, Demoly P. Links between rhinitis and asthma. Allergy. 2003;58(8):691–706. doi: 10.1034/j.1398-9995.2003.00105.x. [DOI] [PubMed] [Google Scholar]
- 44.Lee SL, Wong W, Lau YL. Increasing prevalence of allergic rhinitis but not asthma among children in Hong Kong from 1995 to 2001 (Phase 3 International Study of Asthma and Allergies in Childhood) Pediatr Allergy Immunol. 2004;15(1):72–8. doi: 10.1046/j.0905-6157.2003.00109.x. [DOI] [PubMed] [Google Scholar]
- 45.Selnes A, Nystad W, Bolle R, Lund E. Diverging prevalence trends of atopic disorders in Norwegian children. Results from three cross-sectional studies. Allergy. 2005;60(7):894–9. doi: 10.1111/j.1398-9995.2005.00797.x. [DOI] [PubMed] [Google Scholar]
- 46.Patil VK, Kurukulaaratchy RJ, Venter C, Grundy J, Roberts G, Dean T, et al. Changing prevalence of wheeze, rhinitis and allergic sensitization in late childhood: findings from 2 Isle of Wight birth cohorts’ 12-years apart. Clin Exp Allergy. 2015 doi: 10.1111/cea.12534. [DOI] [PubMed] [Google Scholar]
- 47.Weidinger S, O’Sullivan M, Illig T, Baurecht H, Depner M, Rodriguez E, et al. Filaggrin mutations, atopic eczema, hay fever, and asthma in children. Journal of Allergy & Clinical Immunology. 2008;121(5):1203–9.e1. doi: 10.1016/j.jaci.2008.02.014. [DOI] [PubMed] [Google Scholar]
- 48.Schuttelaar ML, Kerkhof M, Jonkman MF, Koppelman GH, Brunekreef B, de Jongste JC, et al. Filaggrin mutations in the onset of eczema, sensitization, asthma, hay fever and the interaction with cat exposure. Allergy. 2009;64(12):1758–65. doi: 10.1111/j.1398-9995.2009.02080.x. [DOI] [PubMed] [Google Scholar]
- 49.Bisgaard H, Simpson A, Palmer CN, Bonnelykke K, McLean I, Mukhopadhyay S, et al. Gene-environment interaction in the onset of eczema in infancy: filaggrin loss-of-function mutations enhanced by neonatal cat exposure. PLoS Medicine/Public Library of Science. 2008;5(6):e131. doi: 10.1371/journal.pmed.0050131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Venkataraman D, Soto-Ramirez N, Kurukulaaratchy RJ, Holloway JW, Karmaus W, Ewart SL, et al. Filaggrin loss-of-function mutations are associated with food allergy in childhood and adolescence. Journal of Allergy & Clinical Immunology. 2014;134(4):876–82.e4. doi: 10.1016/j.jaci.2014.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pearce N, Weiland S, Keil U, Langridge P, Anderson HR, Strachan D, et al. Self-reported prevalence of asthma symptoms in children in Australia, England, Germany and New Zealand: an international comparison using the ISAAC protocol. Eur Respir J. 1993;6(10):1455–61. [PubMed] [Google Scholar]
- 52.Soto-Ramirez N, Ziyab AH, Karmaus W, Zhang H, Kurukulaaratchy RJ, Ewart S, et al. Epidemiologic methods of assessing asthma and wheezing episodes in longitudinal studies: measures of change and stability. Journal of Epidemiology. 2013;23(6):399–410. doi: 10.2188/jea.JE20120201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Leynaert B, Neukirch F, Demoly P, Bousquet J. Epidemiologic evidence for asthma and rhinitis comorbidity. Journal of Allergy and Clinical Immunology. 2000;106(5, Supplement):S201–S5. doi: 10.1067/mai.2000.110151. [DOI] [PubMed] [Google Scholar]
- 54.Heimall J, Spergel JM. Filaggrin mutations and atopy: consequences for future therapeutics. Expert Rev Clin Immunol. 2012;8(2):189–97. doi: 10.1586/eci.11.100. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


