Abstract
While mycosis fungoides (MF) is typically an indolent malignancy, it may infrequently undertake an aggressive course. We used proteomic analyses to identify a biomarker of the aggressive course of MF. Results of this investigation demonstrated that PARP-1, heat shock protein family A (Hsp70) member 1 like (HSAP1L), Hsp70 member 1A (HSPA1A), ATP-depending RNA-helicase (DDX17), and the α isoform of lamina-associated polypeptide 2 (TMPO) had higher expression in aggressive disease versus non-aggressive. Moreover, PARP-1 was overexpressed in patients with early stage of MF who developed later an aggressive disease. PARP-1 was evaluated as a new target for therapy, demonstrating the selective dose-dependent cytotoxic effect of PARP inhibitors on Sézary cells in comparison with non-malignant lymphocytes. In conclusion, we believe that PARP-1 may serve not only as a biomarker at initial biopsies for a disease that may become aggressive but also as a new therapeutic target of advanced MF and Sézary syndrome.
Keywords: PARP-1, cutaneous lymphoma, mycosis fungoides, Sezary syndrome, biomarkers
Background
Cutaneous T cell lymphoma (CTCL) is a clonal malignancy that is derived from cutaneous mature T lymphocytes. Mycosis fungoides (MF) and Sezary syndrome (SzS, a leukemic variant of CTCL) are the most common types of CTCL with no cure. The median survival of patients with stage IV disease is 18 months, even in the context of multiple lines of therapy (1). With no method currently available to predict aggressive behavior, treating MF that runs the aggressive course involves frequent clinical monitoring and expensive work up which could be reduced by early screening approaches.
The utilization of targeted proteomics in MF was due to the prior difficulty in working with formalin-fixed-paraffin-embedded (FFPE) samples and recovering the proteins from them. Innovative work by Hood et al. (2) and others have shown the ability to utilize FFPE tissue samples in proteomics-based analyses with the nearly equivalent recovery of peptides from FFPE tissue and fresh-frozen tissue (2, 3). We have performed global proteomic studies of patient samples from non-aggressive and aggressive MF to identify biomarkers of aggressive MF in patients from early stage biopsy tissues.
Questions addressed
Is Poly [ADP-ribose] polymerase 1 (PARP-1) a biomarker of advanced MF? May PARP-1 serve as a target for therapy of CTCL?
Experimental Design
Patients from the Cutaneous Lymphoma Clinic of the University of Pittsburgh Medical Center (Pittsburgh, PA, USA) were selected. Global proteomic analysis(2, 3) of non-aggressive and aggressive MF patient samples were performed to determine the biomarkers of aggressive MF in patients with early-stage biopsy tissues. FFPE specimens from patients of each group underwent laser microdissection to isolate malignant T cells for proteomic analysis. For Luminex analysis, the peripheral blood was collected and data analysis was performed using four-parametric-curve fitting5. Cleaved PARP-1 (Asp214) was analyzed by xMap™ technology6. A minimum of six doses of AZD2461 was used to establish the IC50 for incubated Sezary cells.
Results
A retrospective analysis was used to identify patients whose MF had an aggressive or non-aggressive course. The aggressive disease was defined as a patient's overall survival at least twice less than expected based on published data by Kim Y et al. (1). Comparision through proteomic analysis of proteins present in non-aggressive and aggressive disease identified several differences in protein expression (Fig. 1A). The following proteins including PARP-1, heat shock protein family A (Hsp70) member 1 like (HSAP1L), Hsp70 member 1A (HSPA1A), ATP-depending RNA-helicase (DDX17), and the α isoform of lamina-associated polypeptide 2 (TMPO) were increased in aggressive disease samples vs. non-aggressive (Fig 1B). PARP-1 is a nuclear protein involved in regulating DNA damage and has been observed to have multiple roles in several cancers (4-6). PARP-1 has also been found to be an important protein in the BRCA mutations of ovarian and breast cancers (7). Using immunohistochemistry, we compared the expression of PARP-1 in patients with early MF who developed aggressive disease with those who met expected DSS. Non-aggressive disease samples demonstrated lower IHC scores for PARP-1 staining, 22.5 ± 6.1, where the early MF samples which later developed aggressive disease had higher IHC scores for PARP-1 staining, 73.1 ± 52.9 (Fig.1C and D). Although MF, in general, demonstrated increased expression of PARP-1 compared to normal skin throughout this study, there was a large difference in expression seen on IHC when comparing non-aggressive and aggressive MF. This is not uncommon to PARP-1, as there is evidence in other studies that increased expression in malignant cells of other cancers is related to a worse prognosis (4). We found that patients with tumor stage MF (stage IIB) had a higher level of cleaved PARP than patients with early stage of MF (stage IA) or healthy controls (113.1 ± 88.9 MFI in tumor MF vs. 3.9 ± 3.2 MFI in early MF vs. 14.1 ± 21.4 MFI in control, p < 0.05) (Fig. 2A). The results of that study were confirmed by IHC when PARP-1 was found to be significantly higher in tumor stage samples compared to early stage (Fig. 2B). Comparison of intracellular expressions of PARP-1 in Sézary cells (clonal Vβ positive cells) vs. non-malignant lymphocytes from the same patient (non-clonal Vβ negative) (Fig. 2C) was performed and showed the significantly higher level of intracellular PARP-1 in Sézary cells (65.8 ± 30.8 MFI in Sézary cells vs. 36.2 ± 10.2 MFI in non-malignant lymphocytes, p < 0.05) (Fig. 2D). Several PARP inhibitors (PARPi) have been developed and are currently in use as treatment options for breast and ovarian cancers (4-6). Their effectiveness for these cancers is primarily due to their ability to affect the repair of DNA strand breaks (4, 6). There is also evidence of PARPi's having effectiveness in other hematologic malignancies such as leukemia (8). PARPi's have not been specifically tested for benefit in MF. We tested the use of PARPi's in Sézary syndrome by co-incubating Sézary cells with AZD2461 (Fig. 2E). The IC50 for AZD2461 was 7.2 nM (95% confidence interval = 3.8 to 9.7) for Sézary cells vs. 16.7 nM (95% confidence interval = 10.4 to 21.5) for control lymphocytes (p < 0.05).
Figure 1. PAPR-1 is overexpressed in patients with aggressive MF.
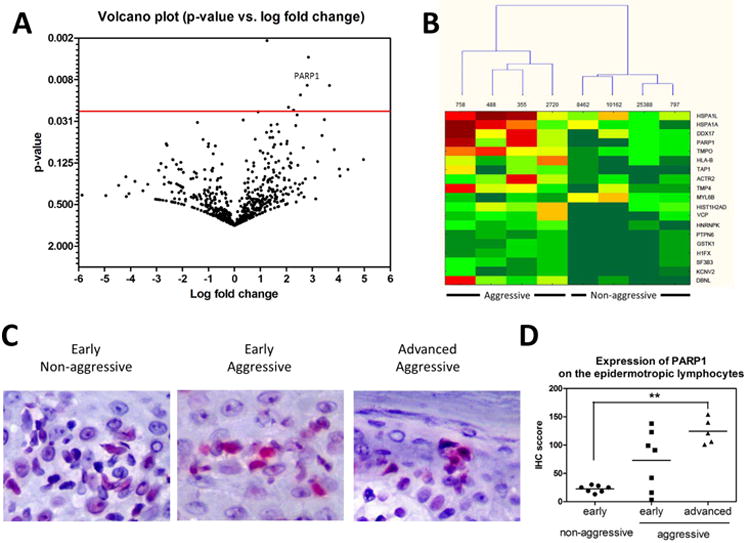
A Volcano plot protein expressed in aggressive (n=4) vs. non-aggressive (n=4) MF. Fold-changes and p-value were plotted against each other. B. Cluster analysis of statistically significant proteins in patients with aggressive (n=4) and non-aggressive (n=4) MF. C. A representative sample of PARP-1 expression in the epidermotropic lymphocytes of patients with the early MF of the non-aggressive course, the the early MF of the aggressive course, and the advanced MF with the aggressive course. IHC, × 400. D. The mean IHC score of expression of PARP-1 on the epidermotropic lymphocytes in patients with the early MF of non-aggressive course (n=7), the early MF of the aggressive course (n=7), and the advanced MF with the aggressive course (n=5). **, p<0.01.
Figure 2. PARP-1 is overexpressed in patients with advanced MF and Sezary syndrome.
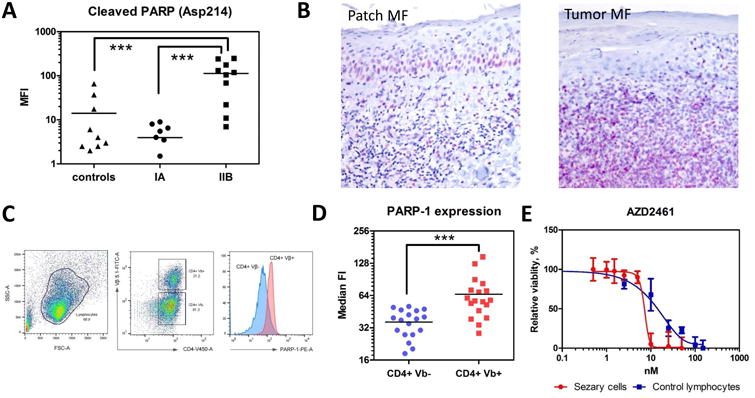
(A) Cleaved PARP (Asp214) by Luminex in the peripheral blood of patients with MF: stage IA (n=7), stage IIB (tumors) (n=10) and healthy volunteers (controls) (n=9). ***, p < 0.001. (B) A representative sample of PARP-1 expression in a patient with patch stage MF (patch MF) vs. a patient with tumor stage MF (tumor MF). IHC, × 200. (C) The gating strategy to determine the PARP-1 expression on CD4+Vβ+ (malignant) and CD4+Vβ- (non-malignant) lymphocytes. (D) The median fluorescent intensity (FI) of intracellular PARP-1 in CD4+Vβ+ (malignant) and CD4+Vβ- (non-malignant) lymphocytes in patients with Sézary syndrome (n=18). ***, p < 0.001. (E) Effect of AZD2461 on the viability of Sezary cells. Cell viability by XTT colorimetric assay 24 hr after co-incubation of lymphocytes with different PARPi. Sézary cells (red) vs. control lymphocytes (blue). AZD2461 doses are shown in the X-axis, whereas the Y-axis shows the relative cell viability.
Conclusion
In this study, we applied novel techniques to perform the first proteomic analysis of biomarkers of aggressive disease in MF. We have demonstrated that PARP-1 has increased expression in an early-stage disease that will become aggressive, compared to an early-stage disease that will not follow an aggressive disease course. PARP-1 could represent an additional stain performed for biopsies once the diagnosis is made, to help determine disease course and proper treatment. The effectiveness of PARP inhibitor on Sézary cells demonstrates that PARP-1 has a role in the pathogenesis of MF and supports PARP-1 as a potential new target in MF treatment.
Acknowledgments
We are grateful to Marie Acquafondata for excellent assistance with PARP-1 immunohistochemistry. We would like to thank Ludmila Velikokhatnaya, Denise Prosser, and Anna Lokshin for the performing Luminex array on cleaved PARP-1. This work was funded by National Cancer Institute grant 5P50CA121973-08 and a research grant from Actelion Pharmaceuticals.
Footnotes
Author contribution: David Lemchak, Swati Banerjee, Shaunak Digambar, Brian Hood performed the experiments. Thomas Conrads, Jaroslav Jedrych, Larisa Geskin, and Oleg Akilov supervised the experiments and analyzed the data. David Lemchak and Oleg Akilov drafted the manuscript. Oleg Akilov designed the study and finalized the manuscript.
Conflicts of Interest: The authors have declared no conflicting interest.
References
- 1.Kim YH, Liu HL, Mraz-Gernhard S, et al. Long-term outcome of 525 patients with mycosis fungoides and Sezary syndrome: clinical prognostic factors and risk for disease progression. Archives of dermatology. 2003;139:857–866. doi: 10.1001/archderm.139.7.857. [DOI] [PubMed] [Google Scholar]
- 2.Hood BL, Darfler MM, Guiel TG, et al. Proteomic analysis of formalin-fixed prostate cancer tissue. Mol Cell Proteomics. 2005;4:1741–1753. doi: 10.1074/mcp.M500102-MCP200. [DOI] [PubMed] [Google Scholar]
- 3.Sprung RW, Jr, Brock JW, Tanksley JP, et al. Equivalence of protein inventories obtained from formalin-fixed paraffin-embedded and frozen tissue in multidimensional liquid chromatography-tandem mass spectrometry shotgun proteomic analysis. Mol Cell Proteomics. 2009;8:1988–1998. doi: 10.1074/mcp.M800518-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kruk A, Ociepa T, Urasinski T, et al. PARP-1 expression in CD34+ leukemic cells in childhood acute lymphoblastic leukemia: relation to response to initial therapy and other prognostic factors. Polish journal of pathology : official journal of the Polish Society of Pathologists. 2015;66:239–245. doi: 10.5114/pjp.2015.54957. [DOI] [PubMed] [Google Scholar]
- 5.Javle M, Curtin NJ. The potential for poly (ADP-ribose) polymerase inhibitors in cancer therapy. Therapeutic advances in medical oncology. 2011;3:257–267. doi: 10.1177/1758834011417039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin KY, Kraus WL. PARP Inhibitors for Cancer Therapy. Cell. 2017;169:183. doi: 10.1016/j.cell.2017.03.034. [DOI] [PubMed] [Google Scholar]
- 7.Wei W, Li Y, Lv S, et al. PARP-1 may be involved in angiogenesis in epithelial ovarian cancer. Oncology letters. 2016;12:4561–4567. doi: 10.3892/ol.2016.5226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bai XT, Moles R, Chaib-Mezrag H, et al. Small PARP inhibitor PJ-34 induces cell cycle arrest and apoptosis of adult T-cell leukemia cells. Journal of hematology & oncology. 2015;8:117. doi: 10.1186/s13045-015-0217-2. [DOI] [PMC free article] [PubMed] [Google Scholar]


