Abstract
Background
Obesity may negatively affect survival in breast cancer (BC), but studies are conflicting, and associations may vary by tumor subtypes and race/ethnicity groups.
Methods
In a retrospective review, we identified 273 women with invasive BC administered Adriamycin/Taxane-based neoadjuvant chemotherapy from 2004–2016 with body mass index (BMI) data at diagnosis. Obesity was defined as BMI ≥30. Associations between obesity and event-free survival (EFS), using STEEP events, and overall survival (OS), using all-cause mortality, were assessed overall and stratified by tumor subtype [[Hormone Receptor Positive (HR+)/HER2−, HER2+, and Triple Negative Breast Cancer (TNBC])] in our diverse population.
Results
Median follow-up was 32.6 months (range 5.7–137.8 months). Overall, obesity was associated with worse EFS (HR 1.71, 95% CI 1.03–2.84, p=0.04) and a trend towards worse OS (p=0.13). In HR+/HER2− disease (n=135), there was an interaction between obesity and hormonal therapy with respect to OS but not EFS. In those receiving tamoxifen (n=33), obesity was associated with worse OS (HR 9.27, 95% CI 0.96–89.3, p=0.05). In those receiving an aromatase inhibitor (n=89), there was no association between obesity and OS. In TNBC (n=44), obesity was associated with worse EFS (HR 2.62, 95% CI 1.03–6.66, p=0.04) and a trend towards worse OS (p=0.06). In HER2+ disease (n=94), obesity was associated with a trend towards worse EFS (HR 3.37, 95% CI 0.97–11.72, p=0.06) but not OS. Race/ethnicity was not associated with survival in any subtype, and there were no interactions with obesity on survival.
Conclusions
Obesity may negatively impact survival, with differences among tumor subtypes.
Keywords: breast cancer, obesity, survival, tumor subtype, race/ethnicity, neoadjuvant chemotherapy
Introduction
Obesity is associated with various poor health outcomes and is implicated in 5–10% of US health care spending.1,2 Epidemiologic studies have suggested that obesity confers increased risk of developing breast cancer (BC)3,4 and is independently associated with poor clinical outcomes in BC.4–7 Proposed mechanisms range from difficulties with screening and chemotherapy dosing to underlying genetic/molecular differences.8
The effect of obesity on BC outcomes, however, is less clear as the impact of obesity may vary by tumor subtype, menopausal status and hormone therapy class.9,10 Some studies have shown the association to be strongest in those with hormone receptor positive (HR+) disease,11,12 while others have suggested an association in triple negative breast cancer (TNBC) and HER2 positive (HER2+) tumors as well.13–16
Rates of obesity also vary among different race/ethnicity groups, with Blacks and Hispanics more likely to be obese as compared to Non-Hispanic Whites.17 Although some have hypothesized that obesity may influence survival differently in various racial/ethnic groups, studies are small and conflicting,18 and health disparities exist among minorities with respect to cancer outcomes which may confound the impact of obesity.19 In Asian women, obesity may predict worse survival,14,18 but in studies of Black and Hispanic women, no differences have been reported in the association between obesity and clinical outcomes as compared to Non-Hispanic White women.20,21 Differences may exist in certain subtypes, such as HR+ disease,18,20,21 or using different markers of obesity, such as waist circumference and waist-to-hip ratio.22
Additionally, few have investigated the impact of obesity in the neoadjuvant setting on event-free (EFS) and overall survival (OS). Most studies have focused on pathologic complete response (pCR), a surrogate marker for survival in the neoadjuvant setting,23 and some report that obese patients have lower rates of pCR with a suggestion of worse surival.24 However, others have shown no differences in pCR or survival between different body mass index (BMI) groups,21,25 and many of the studies are limited to homogenous, clinical trial populations. The impact of obesity on BC survival in the neoadjuvant setting, and in particular the role of tumor subtype and race/ethnicity, is still unknown.
Our study seeks to examine the role of baseline obesity, as measured by BMI, on EFS and OS in women with invasive BC receiving neoadjuvant chemotherapy (NAC) and to explore if tumor subtype influences these associations in our ethnically diverse population.
Methods
Patient Population
We performed a retrospective review of women with invasive breast cancer seeking care at Columbia University Medical Center (CUMC) between January 2004 and February 2016 administered Adriamycin(A)/Taxane(T)-based NAC. Clinicopathologic data were abstracted from medical records by four independent researchers. All data were double-verified, and discrepancies were resolved by oncologists EC and KK. Of the 342 unique women identified, 317 women had undergone surgery at the time of this analysis. We excluded 10 women who were metastatic at diagnosis, 5 women who received non-A/T based NAC (all received CMF), 1 who was pregnant and ultimately did not receive NAC, and 3 women with unknown NAC regimens, resulting in a final cohort of 298 women. Of these, 273 women had complete data on BMI at diagnosis and were included in this analysis. All research was conducted in accordance with CUMC IRB approved protocol (IRB # AAAJ8512).
Clinical and Pathologic Variables
BMI was calculated from height and weight documented at the clinic visit closest to pathologic diagnosis. BMI was defined as weight in kilograms divided by height in meters2. BMI was also collected at the most recent clinic visit to assess change over time. Obesity was classified according to World Health Organization guidelines, and obesity was defined as BMI≥30.26 BSA (body surface area) in meters squared (m2) was calculated using the DuBois formula (BSA = 0.00718 × Height(cm)0.725 × Weight(kg)0.425), and a BSA ≥2.2 was defined as high.27 A diagnosis of diabetes was inferred from physician notes at breast cancer diagnosis.
Age was defined in years at diagnosis and was stratified into <50 years of age and ≥50 years of age. Race/ethnicity was categorized as Non-Hispanic White, Non-Hispanic Black, Hispanic, and Asian/Other based on self-report or physician notes. Tumor size was defined as the largest dimension on any imaging modality prior to any treatment and was stratified at 0–2cm, >2–5cm, and >5cm. Grade was defined as the highest grade seen on any biopsy and was defined as low/intermediate grade (grade 1 and 2) and high grade (grade 3). Estrogen receptor (ER) and progesterone receptor (PR) positivity were defined as 1% or greater expression on any biopsy in accordance with the American Society of Clinical Oncology/College of American Pathologist (ASCO/CAP) guidelines from 2010.28 Tumors were considered HER2-positive if they were 3+ by immunohistochemistry (IHC), demonstrated gene amplification with a ratio of Her-2/CEP17 ≥2.2 by in situ hybridization or had HER2 average copies/cell ≥6 on either the core biopsy or surgical pathology specimen.29 Based on prior studies, subtype groups were defined as a) HR+ (ER and/or PR positive) and HER2 negative, b) HER2 positive regardless of hormonal status, and c) TNBC (ER, PR, and HER2 negative).30 Clinical and pathologic staging were determined based on the American Joint Committee on Cancer (AJCC) TNM Staging Manual, 7th edition. Pathologic complete response (pCR) was defined as no residual invasive disease in the breast or lymph nodes on surgical pathology specimens (ypT0/Tis ypN0).
All women received A-based, T-based, or A/T-based NAC and were dosed based on BSA without adjustments made based on obesity status. Women were considered to have received radiation therapy (XRT) if they received any type of whole breast/chest wall radiation with or without nodal radiation. Hormonal therapy was defined as treatment with tamoxifen only or any aromatase inhibitor (AI) and was categorized as tamoxifen only or any AI use, as some women received tamoxifen and then an AI (classified as AI use). Of the 15 women who were HR+ but did not receive hormonal therapy, 4 deferred due to disease progression, 1 deferred due to pregnancy, 4 refused, 1 was lost to follow-up before initiation, 5 had missing data, and 1 woman received fulvestrant. Surgery type was stratified into lumpectomy or mastectomy with or without lymph node dissection.
Statistical Analysis
Chi-square and t-tests were used to compare relevant clinical and pathologic variables according to obesity status at diagnosis. EFS was based on the STEEP criteria,31 and events were defined as any local/regional or distant metastasis, contralateral invasive breast cancer (excluding in-situ disease), any secondary, non-breast, invasive cancer, and/or death by any cause. EFS and OS were calculated in months from date of diagnosis to date of first event or death (for OS) or last follow-up in those without events. Kaplan Meier survival analysis and the log-rank statistic were used to estimate survival differences between groups based on clinically relevant variables. Cox proportional hazard models were used to estimate the hazard ratio for the association between obesity and EFS and OS, and to evaluate the potential interaction between obesity and other relevant covariates. Given literature suggesting differences in the association between obesity and survival among various tumor subtypes and race/ethnicity groups, stratified analyses were also performed using the a priori determined variable of subtype (HR+/HER2−, HER2+ and TNBC) and race/ethnicity (Non-Hispanic White, Non-Hispanic Black, and Hispanic). All analyses were performed using SAS 9.4 and STATA 12.0 with significance defined as a two-sided p-value of less than or equal to 0.05.
Results
Demographics
BMI at diagnosis ranged from 17.6 to 50.9, with a mean of 28.8 [standard deviation (SD) 6.1]. Of the 273 women, 102 (37%) were classified as obese (BMI ≥30). BMI was measured on average 2.7 months after diagnosis (SD 10.7 months) with a median time of 21 days after diagnosis (range 0–120 months), with 8 women having BMI values from over a year after diagnosis. On average, BMI changed a mean of −0.11 units (SD 3.3 units) with a median change of 0.05 units (range −10.6 to 9.3 units) over an average time period of 30.2 months (SD 22.1 months). Rates of obesity varied significantly among the different race/ethnicity groups (p<0.01): Non-Hispanic White (n=91) 29% obese, Non-Hispanic Black (n=66) 47% obese, Hispanic (n=95) 44% obese, and Asian/Other (n=16) 13% obese. There was a higher prevalence of diabetes (30% vs. 13%) in obese compared with non-obese women, p<0.01. All women received A-based, T-based, or A/T-based NAC, and dosages were not reduced for the 10 women with BSA ≥2.2 or the 14 women with BMI≥ 40, and there were no differences in type of surgery (lumpectomy vs. mastectomy) or XRT as compared to women with normal weight (BMI 18.5 to <25) and BSA <2.2, p>0.05. Otherwise, there were no differences in age, clinical size, clinical stage, grade, subtype (HR+/HER2−, HER2 positive regardless of hormone receptor status, and TNBC), surgery type (lumpectomy vs. mastectomy), hormonal therapies, trastuzumab use, or radiation therapy in obese as compared with non-obese women, p>0.05 (Table 1).
Table 1.
Baseline Characteristics and Clinicopathologic Variables by Obesity
| Variable | None (BMI <30) | Obese (BMI ≥30) | p-value |
|---|---|---|---|
| N=171 | N=102 | ||
| Age (n=273) | |||
| <50 | 75 (44%) | 44 (43%) | 0.91 |
| 50+ | 96 (56%) | 58 (57%) | |
| Race/Ethnicity (n=268) | |||
| Non-Hispanic White | 65 (39%) | 26 (26%) | <0.01 |
| Non-Hispanic Black | 35 (21%) | 31 (31%) | |
| Hispanic | 53 (32%) | 42 (41%) | |
| Asian/Other | 14 (8%) | 2 (2%) | |
| Clinical Stage (n=270) | |||
| I/II | 111 (66%) | 60 (59%) | 0.30 |
| III | 58 (34%) | 41 (41%) | |
| Clinical Size (n=273) | |||
| 0–2cm | 29 (17%) | 10 (10%) | 0.16 |
| >2–5cm | 107 (63%) | 64 (63%) | |
| >5cm | 35 (20%) | 28 (27%) | |
| Grade(n=270) | |||
| Low (I or II) | 60 (36%) | 32 (31%) | 0.47 |
| High (III) | 108 (64%) | 70 (69%) | |
| Subtype (n=273) | |||
| Hormone Receptor +/HER2− | 86 (50%) | 49 (48%) | 0.89 |
| HER2+ | 57 (33%) | 37 (36%) | |
| Triple Negative Breast Cancer | 28 (17%) | 16 (16%) | |
| Surgery Type (n=272) | |||
| Lumpectomy | 56 (33%) | 35 (35%) | 0.75 |
| Mastectomy | 115 (67%) | 66 (65%) | |
| RT (n=267) | |||
| Yes | 136 (82%) | 86 (85%) | 0.50 |
| No | 30 (18%) | 15 (15%) | |
| Hormonal Therapy (n=258) | |||
| Yes | 116 (72%) | 77 (79%) | 0.19 |
| No | 45 (28%) | 20 (21%) | |
| Trastuzumab (n=258) | |||
| Yes | 55 (34%) | 37 (39%) | 0.46 |
| No | 107 (66%) | 59 (61%) | |
| Diabetes (n=272) | |||
| Yes | 22 (13%) | 30 (30%) | <0.01 |
| No | 149 (87%) | 71 (70%) |
RT – Radiation Therapy
Overall Survival Analysis
Median follow-up was 32.6 months (range 5.7–137.8 months). There were a total of 60 events with 57 being recurrence or progression of invasive breast cancer (6 local only, 9 local then distant, and 42 distant) and 3 being death from any cause without evidence of recurrence/progression. There were 29 (28%) events among obese women as compared to 31 (18%) in non-obese women. There were a total of 33 deaths from all causes, with 3 deaths occurring without recurrence/progression. There were 16 (16%) deaths among obese women as compared with 17 (10%) among non-obese women.
Among obese women, 29% achieved pCR as compared with 27% in non-obese women (X2, p=0.75), and there were no differences in rates of pCR between obese and non-obese women when stratified by tumor subtype, p>0.05. On univariate analysis, obesity (BMI≥30 compared with BMI<30) was associated with significantly worse EFS (HR 1.71, 95% CI 1.03–2.84, p=0.04). There was also a trend towards worse OS (HR 1.69, 95% CI 0.85–3.36, p=0.13), Figure 1. Tumor subtype was also significantly associated with worse EFS (logrank test p<0.01) and OS (logrank test p=0.05) with women with TNBC having worse and HER2+ breast cancer having better survival outcomes than those with HR+/HER2− breast cancers (eFigure1).
Figure 1. Kaplan Meier Curves of Obesity with Event-free and Overall Survival.
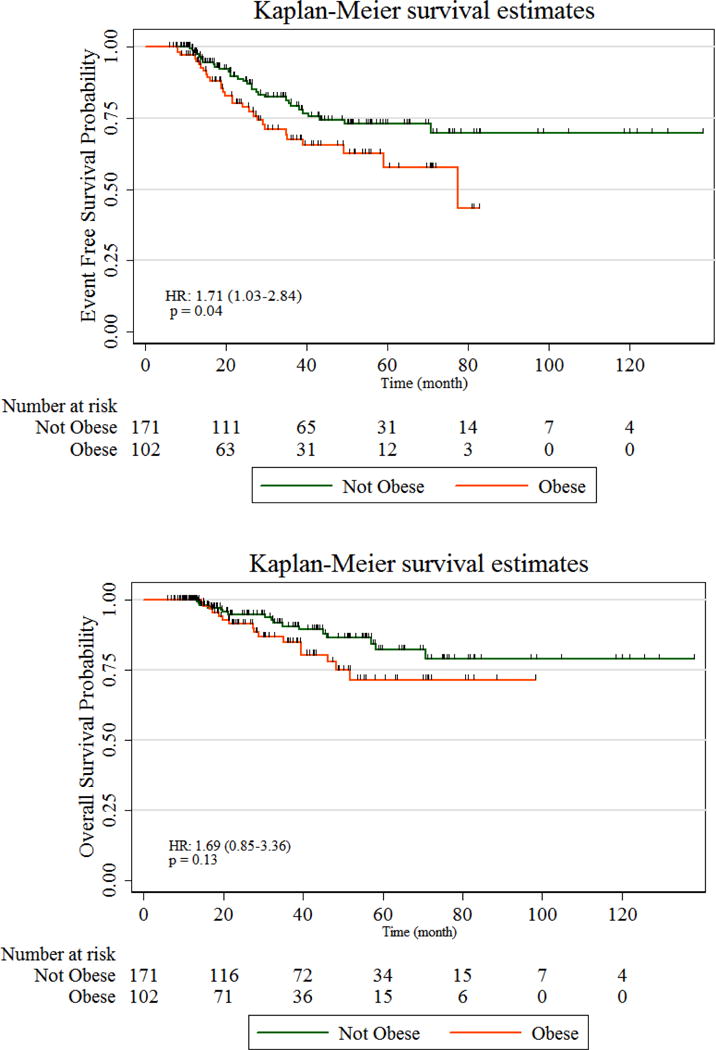
Overall, obesity is associated with worse event-free and a trend towards worse overall survival.
Stratified Analysis by Subtype
HR+/HER2−: Interactions with Hormonal Therapy Class
In women with HR+/HER2− tumors (n= 135), there was an interaction between obesity and hormonal therapy type (AI vs. tamoxifen only) with OS (interaction p=0.03). In those receiving tamoxifen only (n=33), obesity was associated with worse OS (HR 9.27, 95% CI 0.96–89.31, p=0.05). In those receiving AI’s (n=89), there was no association between obesity and OS (p=0.34) Table 2/Figure 2. There was no significant interaction with EFS, although the effects are in the same direction as OS. Although not statistically significant (p=0.06), a higher proportion of women receiving AI’s were obese as compared to those receiving tamoxifen (43% vs. 24%). Of the 7 within this subtype who received no hormonal therapies, 3 were obese, of which 2 had disease progression and died; whereas, 4 were not obese, of which 1 had local recurrence only. Hormonal therapy type was also significantly correlated with age (p<0.01), and older women (≥50 years) were more likely to be receiving an AI than younger women (<50 years). Analysis conducted with age (<50 years vs. ≥50 years) instead of AI vs. tamoxifen only in HR+/HER2− women also showed significant interaction with OS (interaction p=0.03) and trend with EFS (interaction p=0.06), and obesity adversely influenced survival in younger but not older women (data not shown).
Table 2.
Model of Obesity and Survival (Event-Free and Overall) by subtype, adjusted for hormonal therapy in Hormone Receptor Positive (HR+)/HER2− disease
| Model of Obesity and Survival: Stratified by Subtype and Hormonal Therapy | |||||
|---|---|---|---|---|---|
| Obesity and EFS | |||||
| N | Events | HR (95% CI) | p-value | ||
| HR+/HER2− | |||||
| AI | Obese (BMI 30+) | 38 | 6 | 0.92 (0.32, 2.60) | 0.87 |
| Not Obese (BMI<30) | 51 | 9 | 1 | ||
| Tamoxifen | Obese (BMI 30+) | 8 | 4 | 2.47 (0.72, 8.49) | 0.15 |
| Not Obese (BMI<30) | 25 | 7 | 1 | ||
| HER2+ | Obese (BMI 30+) | 37 | 7 | 3.37 (0.97–11.72) | 0.06 |
| Not Obese (BMI<30) | 57 | 4 | 1 | ||
| TNBC | Obese (BMI 30+) | 16 | 10 | 2.62 (1.03–6.66) | 0.04 |
| Not Obese (BMI<30) | 28 | 8 | 1 | ||
| Obesity and OS | |||||
|---|---|---|---|---|---|
| N | Deaths | HR (95% CI) | p-value | ||
| HR+/HER2−* | |||||
| AI | Obese (BMI 30+) | 38 | 2 | 0.45 (0.09–2.26) | 0.34 |
| Not Obese (BMI<30) | 51 | 6 | 1 | ||
| Tamoxifen | Obese (BMI 30+) | 8 | 3 | 9.27 (0.96–89.31) | 0.05 |
| Not Obese (BMI<30) | 25 | 1 | 1 | ||
| HER2+ | Obese (BMI 30+) | 37 | 2 | 1.35(0.22–8.19) | 0.75 |
| Not Obese (BMI<30) | 57 | 3 | 1 | ||
| TNBC | Obese (BMI 30+) | 16 | 7 | 3.00 (0.95–9.51) | 0.06 |
| Not Obese (BMI<30) | 28 | 5 | 1 | ||
P-value for the interaction between obesity and hormonal therapy for HR+/HER2− is 0.23
P-value for the interaction between obesity and hormonal therapy for HR+/HER2− is 0.03
Abbreviations: AI – Aromatase Inhibitor, EFS – Event-Free survival, OS – Overall Survival, HR – Hazard Ratio, BMI – Body Mass Index, TNBC – Triple Negative Breast Cancer, HR – Hormone Receptor, CI – Confidence Interval
Figure 2. Kaplan-Meier Curves: Hormone Receptor Positive (HR+)/Her2− (Event-free and Overall survival): Stratified by Tamoxifen vs. Aromatase Inhibitor (AI) use.
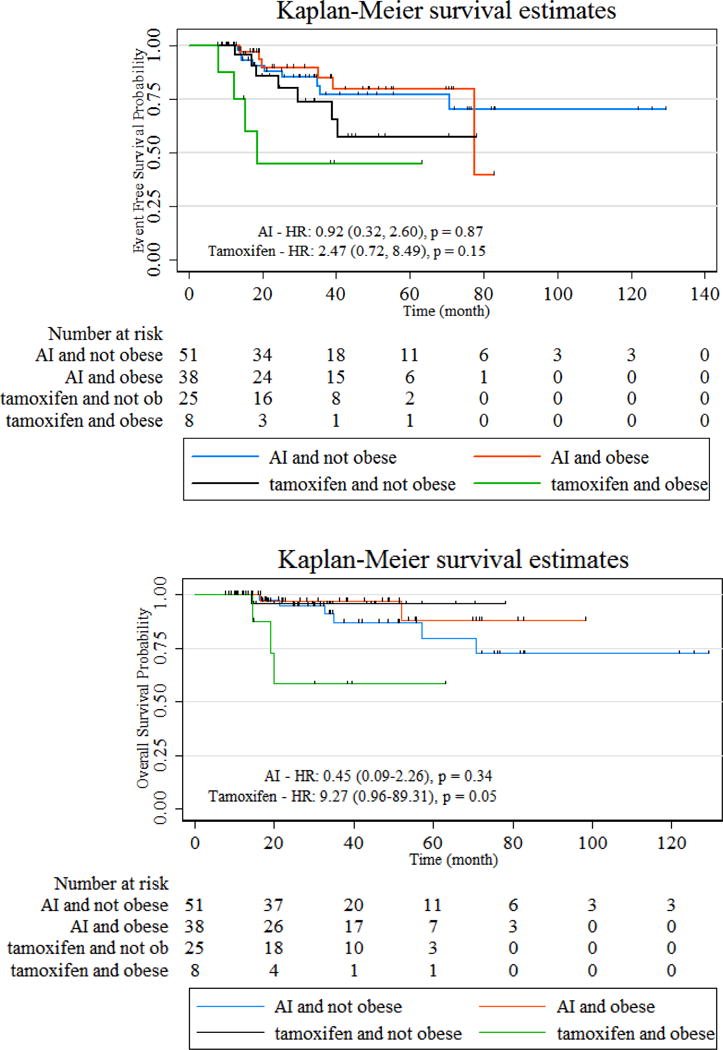
In HR+ women, there is a significant interaction between hormonal therapy and obesity with overall survival (OS), and in women receiving tamoxifen only, but not AIs, obesity is associated with significantly worse OS. There is no significant interaction with event-free survival, although effects are in the same direction.
HER2 Positive
In women with HER2+ tumors regardless of hormone receptor status (n=94), obesity was associated with a trend to worse EFS (HR 3.37, 95% CI 0.97–11.72, p=0.06) but not OS (HR 1.35, 95% CI 0.22–8.19, p=0.75), Table 2/eFigure2. In those with HER2+ tumors, survival (EFS and OS) did not differ between those with HR+ and HR− disease, p>0.05 (data not shown).
TNBC
In women with TNBC (n=44), obesity was associated with worse EFS (HR 2.62, 95% CI 1.03–6.66, p=0.04) and a trend towards worse OS (HR 3.00, 95% CI 0.95–9.51, p=0.06), Table 2/Figure 3.
Figure 3. Kaplan-Meier Curves (Event-free and Overall Survival) Triple Negative Breast Cancer (TNBC).
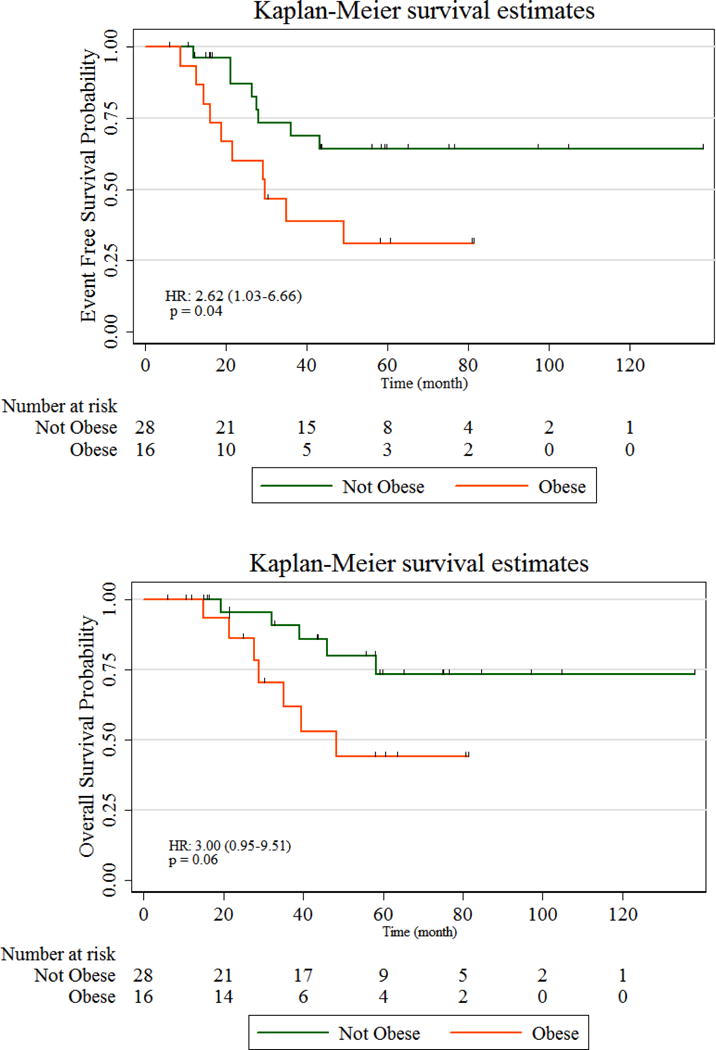
In TNBC, obesity is associated with worse event-free survival and a trend towards worse overall survival.
Role of Race/Ethnicity
The race/ethnicity analyses were limited to Non-Hispanic White, Non-Hispanic Black and Hispanic given the small number of Asian/Others. There were no significant differences in survival (EFS and OS) between Non-Hispanic Whites, Non-Hispanic Blacks and Hispanics either overall or stratified by the three tumor subtype groups, p>0.05 (eFigure3). There were no interactions between race/ethnicity and obesity with either EFS (interaction p=0.24) or OS (interaction p=0.45), after adjusting for tumor subtype.
Discussion
Overall, obesity was associated with worse survival, and the association was strongest in HR+/HER2− and TNBC with a trend in HER2+ BC. In women with HR+/HER2− disease, there was a significant interaction between obesity and hormonal therapy class with survival. However, there were no differences in survival by race/ethnicity and no significant interaction between race/ethnicity and obesity on survival.
Our results are consistent with prior studies conducted in the neoadjuvant setting (Table 3). Fontanella et al. found that obese and very obese women receiving NAC had shorter disease-free and overall survival compared with normal weight women, particularly in Luminal A and TNBC.24 Karatas et al. also found that obese women had lower rates of pCR, and shorter survival than normal/underweight women in all subtypes.23 Although adjuvant studies have suggested that obesity may primarily influence survival in HR+/HER2− disease12, we found that obesity conferred worse clinical outcomes in HER2+ and TNBC as well, and recent studies suggest that obesity may influence survival in all subtypes.4 Hao et al. found that being overweight (BMI >24) was associated with worse OS in multivariate models of 1,106 Chinese women with TNBC,14 and Widschwendter et al. found that in German women, severe obesity was associated with worse survival, but only in TNBC.16 Moreover, in two trials before and after the introduction of trastuzumab therapy, obesity was associated with worse survival in women with HER2+ BC.13,15 Interestingly, obesity was associated with worse EFS but not OS in our cohort of HER2+ BC, suggesting that advances in therapy at the time of progression, such as pertuzumab and trastuzumab emtansine, are improving overall survival in this subtype32,33.
Table 3.
Current Neoadjuvant Study examining Obesity and Survival in Breast Cancer in Context of Other Published Neoadjuvant Studies
| Authors (Year) | Population (n) | Obesity and Survival | Subtype | Strengths/Limitations |
|---|---|---|---|---|
| Current Study: Liu et al. (2017) | 273 | Obesity (BMI≥30) was significantly associated with worse EFS and a trend towards worse OS. This was seen in TNBC but not HER2+ BC. In HR+ BC, there was an interaction with hormonal therapy/age, and younger women receiving tamoxifen who were obese had worse OS. | Significant association in HR+ and TNBC with trend in HER2+ breast cancer | - Diverse population (36% Hispanic, 24% Black) - Interaction by hormonal therapy/age in HR+ breast cancer |
| Warner et al. (2016) | 1797 (Pooled analysis of 4 neoadjuvant trials) | No overall association between BMI and pCR but there was a trend associating increasing BMI with lower pCR rates in HR+/HER2+ BC. | Trend for association in HR+/HER2+ breast cancer | - Homogenous population [majority White (83%)] - Limited to pCR, not survival |
| Karatas et al. (2016) | 295 | Obese women (BMI≥30) had lower rates of pCR and worse recurrence- free survival and OS as compared with normal/underweight women (BMI<25). | All subtypes | - Turkish women only - Limited to Stage II and III BC |
| Erbes et al. (2016) | 324 | No association between BMI and pCR, but in the accompanying meta-analysis, overweight/obese women (BMI≥25) had significantly lower rates of pCR than normal weight women (BMI<25). | No association in any subtype | - German women only - Limited to pCR, not survival |
| Fontanella et al. (2015) | 8872 (Pooled analysis of 8 neoadjuvant trials) | Obese (BMI≥30) and very obese (BMI≥40) women had shorter disease- free survival and OS compared with normal weight women (BMI<25). | Association seen in luminal and TNBC | - Large study - Homogenous German population |
| Tichy et al. (2015) | 349 | BMI significantly associated with worse OS on univariate analysis. | All subtypes | - Diverse population (30% African- American and 70% non-African American) - Study mainly looked at association of race and survival in multivariate models |
Abbreviations: EFS: Event-free survival, OS: Overall survival, TNBC: Triple Negative Breast Cancer; BC: Breast Cancer, HR: Hormone Receptor, pCR: pathological complete response
The mechanism of obesity’s influence on survival is hypothesized to involve increased peripheral conversion of androgens to estrogen by aromatase – a process known as aromatization, which suggests that this association may be more pronounced in HR+ tumors.34,35 As the negative influence of obesity may be present in all subtypes, the mechanism may also involve other non-estrogen related pathways.34,35 Some have postulated that obesity and subsequent insulin resistance affect molecular signaling pathways including IGF-1 leading to a pro-carcinogenic state and dysregulation of growth.35,36 Obesity may also lead to a chronic inflammatory state that can affect cytokine signaling and lead to growth/proliferation of cancer.37 Moreover, obesity may impact epigenetic markers and gene methylation,38 and mouse models suggest that chronic obesity can lead to epigenetic reprogramming, up-regulation of inflammatory signaling, and mammary tissue growth.39
As in prior studies,20,21 we found no differences in survival between different race/ethnicity groups and no interactions between race/ethnicity and obesity on survival. Although there were no significant differences in tumor subtypes by race/ethnicity, there were significant differences in hormonal therapy utilization with Non-Hispanic Blacks less likely to have hormone therapies be listed in the medical record or have missing data (p=0.03), suggesting potential differences in medical treatment or preference/adherence between the race/ethnicity groups. We found no differences in type of surgery, XRT or trastuzumab use (p>0.05) between Non-Hispanic Whites, Non-Hispanic Blacks, and Hispanics.
Our finding of a significant interaction between obesity and hormonal therapy in HR+/HER2− BC is unexpected and hypothesis-generating. Prior studies have investigated the efficacy of AI’s vs. tamoxifen in obese vs. non-obese women in mostly post-menopausal women, and results are mixed.40 In post-menopausal women, some studies have shown that obesity was associated with worse survival in women receiving anastrazole but not tamoxifen10; however, others have shown no difference in benefit of letrozole vs. tamoxifen41 as well as a significant benefit of exemestane over tamoxifen in obese women.40 We found that in those receiving tamoxifen, obesity predicted worse OS, but in those receiving AI’s, obesity did not predict worse survival. Studies show that aromatase activity is elevated in the mammary tissue of obese mice42, and the inhibition of peripheral conversion of fat to estrogen by aromatase may be particularly critical in obese women. In vivo studies have shown that AI levels are not reduced in obese women43 and that, compared to non-obese women, estrogen levels are not elevated in obese women receiving AI’s,44,45 but are elevated in obese women not receiving AI’s.44 AI’s may block a significant pathway linking obesity and poor survival, potentially conferring a protective effect as compared with tamoxifen in obese women.
Interestingly, we found that hormonal therapy was also significantly correlated with age, defined as a categorical variable with a cutoff of age 50, which may be a surrogate for menopausal status, and we saw a similar interaction between obesity and age with survival. This further suggests that menopausal status and the hormonal milieu, as influenced by anti-estrogen therapies, may influence the role of obesity on survival in this estrogen sensitive BC population. A recent meta-analysis found that although obesity is associated with worse survival overall, there may be a suggestion of larger effect size in pre-menopausal versus post-menopausal women.5 The various mechanisms relating obesity to worse survival may vary by menopausal status and hormonal therapies and differentially influence survival in HR+ BC as compared with other subtypes. Unfortunately, in our study, we have not comprehensively and reliably collected data on menopausal status and instead use age (≥50) as a surrogate. However, prior studies have shown that, although definitions for menopause can vary in the literature, this only translates into slight differences in menopausal status-specific breast cancer rates.46
Strengths of our study include modern NAC regimens, rigorous data collection techniques, a diverse population that is a majority non-White, and assessment of interactions with multiple variables including tumor subtype, race/ethnicity and hormonal therapies. We are also the first to identify an interaction between obesity, hormonal therapy class, and tumor subtype on survival. Limitations include a relatively small sample size, incomplete assessment of menopausal status and hormonal therapy utilization and the retrospective nature of our study owing to potential for residual confounding.
Future studies should clarify the interaction between obesity and hormonal therapy classes, particularly different types of AI’s as well as duration of therapy, in HR+ disease and explore the role of menopausal status. Additionally, BMI may not capture central adiposity,35 and future studies should also examine other measures of obesity, as studies suggest that waist circumference and waist-to-hip ratio may be independent predictors of all-cause mortality,47 particularly in Black patients.48
Conclusion
In women receiving NAC for invasive BC, baseline obesity is associated with worse outcomes, particularly in those with HR+/HER2− disease receiving tamoxifen compared with AI’s and in those with TNBC. Clinically, screening for and treating obesity may be important in assessing prognosis and improving survival.
Supplementary Material
Acknowledgments
Funding: This study was funded by the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant Number KL2 TR000081.
Abbreviations
- BMI
Body Mass Index
- NAC
neoadjuvant chemotherapy
- HR+
hormone receptor positive
- TNBC
triple negative breast cancer
- pCR
pathologic complete response
- EFS
event-free survival
- OS
overall survival
Footnotes
Compliance with Ethical Standards
Conflict of Interest: Author EC has received funding from Merck and has served as a consultant for Eisai. Author KK has served as a consultant for Lilly, Biotheranostics, Amgen, Eisai, and Novartis. All other authors have no conflicts of interest to declare.
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Informed consent: Informed consent was obtained from all individual participants included in the study.
References
- 1.Bhaskaran K, Douglas I, Forbes H, dos-Santos-Silva I, Leon DA, Smeeth L. Body-mass index and risk of 22 specific cancers: a population-based cohort study of 5.24 million UK adults. Lancet. 2014;384(9945):755–765. doi: 10.1016/S0140-6736(14)60892-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tsai AG, Williamson DF, Glick HA. Direct medical cost of overweight and obesity in the USA: a quantitative systematic review. Obesity reviews: an official journal of the International Association for the Study of Obesity. 2011;12(1):50–61. doi: 10.1111/j.1467-789X.2009.00708.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gathirua-Mwangi WG, Zollinger TW, Murage MJ, Pradhan KR, Champion VL. Adult BMI change and risk of Breast Cancer: National Health and Nutrition Examination Survey (NHANES) 2005–2010. Breast cancer (Tokyo, Japan) 2015;22(6):648–656. doi: 10.1007/s12282-015-0638-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jiralerspong S, Goodwin PJ. Obesity and Breast Cancer Prognosis: Evidence, Challenges, and Opportunities. J Clin Oncol. 2016;34(35):4203–4216. doi: 10.1200/JCO.2016.68.4480. [DOI] [PubMed] [Google Scholar]
- 5.Chan DS, Vieira AR, Aune D, et al. Body mass index and survival in women with breast cancer-systematic literature review and meta-analysis of 82 follow-up studies. Annals of oncology: official journal of the European Society for Medical Oncology. 2014;25(10):1901–1914. doi: 10.1093/annonc/mdu042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Niraula S, Ocana A, Ennis M, Goodwin PJ. Body size and breast cancer prognosis in relation to hormone receptor and menopausal status: a meta-analysis. Breast cancer research and treatment. 2012;134(2):769–781. doi: 10.1007/s10549-012-2073-x. [DOI] [PubMed] [Google Scholar]
- 7.Protani M, Coory M, Martin JH. Effect of obesity on survival of women with breast cancer: systematic review and meta-analysis. Breast cancer research and treatment. 2010;123(3):627–635. doi: 10.1007/s10549-010-0990-0. [DOI] [PubMed] [Google Scholar]
- 8.Allott EH, Hursting SD. Obesity and cancer: mechanistic insights from transdisciplinary studies. Endocrine-related cancer. 2015;22(6):R365–386. doi: 10.1530/ERC-15-0400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pfeiler G, Konigsberg R, Fesl C, et al. Impact of body mass index on the efficacy of endocrine therapy in premenopausal patients with breast cancer: an analysis of the prospective ABCSG-12 trial. J Clin Oncol. 2011;29(19):2653–2659. doi: 10.1200/JCO.2010.33.2585. [DOI] [PubMed] [Google Scholar]
- 10.Sestak I, Distler W, Forbes JF, Dowsett M, Howell A, Cuzick J. Effect of body mass index on recurrences in tamoxifen and anastrozole treated women: an exploratory analysis from the ATAC trial. J Clin Oncol. 2010;28(21):3411–3415. doi: 10.1200/JCO.2009.27.2021. [DOI] [PubMed] [Google Scholar]
- 11.Dignam JJ, Wieand K, Johnson KA, Fisher B, Xu L, Mamounas EP. Obesity, tamoxifen use, and outcomes in women with estrogen receptor-positive early-stage breast cancer. J Natl Cancer Inst. 2003;95(19):1467–1476. doi: 10.1093/jnci/djg060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sparano JA, Wang M, Zhao F, et al. Obesity at diagnosis is associated with inferior outcomes in hormone receptor-positive operable breast cancer. Cancer. 2012;118(23):5937–5946. doi: 10.1002/cncr.27527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crozier JA, Moreno-Aspitia A, Ballman KV, Dueck AC, Pockaj BA, Perez EA. Effect of body mass index on tumor characteristics and disease-free survival in patients from the HER2-positive adjuvant trastuzumab trial N9831. Cancer. 2013;119(13):2447–2454. doi: 10.1002/cncr.28051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hao S, Liu Y, Yu KD, Chen S, Yang WT, Shao ZM. Overweight as a Prognostic Factor for Triple-Negative Breast Cancers in Chinese Women. PLoS One. 2015;10(6):e0129741. doi: 10.1371/journal.pone.0129741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mazzarella L, Disalvatore D, Bagnardi V, et al. Obesity increases the incidence of distant metastases in oestrogen receptor-negative human epidermal growth factor receptor 2-positive breast cancer patients. Eur J Cancer. 2013;49(17):3588–3597. doi: 10.1016/j.ejca.2013.07.016. [DOI] [PubMed] [Google Scholar]
- 16.Widschwendter P, Friedl TW, Schwentner L, et al. The influence of obesity on survival in early, high-risk breast cancer: results from the randomized SUCCESS A trial. Breast cancer research: BCR. 2015;17:129. doi: 10.1186/s13058-015-0639-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. Jama. 2012;307(5):491–497. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 18.Bandera EV, Maskarinec G, Romieu I, John EM. Racial and ethnic disparities in the impact of obesity on breast cancer risk and survival: a global perspective. Advances in nutrition (Bethesda, Md) 2015;6(6):803–819. doi: 10.3945/an.115.009647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hunt BR, Hurlbert MS. Black: white disparities in breast cancer mortality in the 50 largest cities in the United States, 2005–2014. Cancer epidemiology. 2016 doi: 10.1016/j.canep.2016.07.018. [DOI] [PubMed] [Google Scholar]
- 20.Tichy JR, Deal AM, Anders CK, Reeder-Hayes K, Carey LA. Race, response to chemotherapy, and outcome within clinical breast cancer subtypes. Breast cancer research and treatment. 2015;150(3):667–674. doi: 10.1007/s10549-015-3350-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Warner ET, Ballman KV, Strand C, et al. Impact of race, ethnicity, and BMI on achievement of pathologic complete response following neoadjuvant chemotherapy for breast cancer: a pooled analysis of four prospective Alliance clinical trials (A151426) Breast cancer research and treatment. 2016;159(1):109–118. doi: 10.1007/s10549-016-3918-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hall IJ, Newman B, Millikan RC, Moorman PG. Body size and breast cancer risk in black women and white women: the Carolina Breast Cancer Study. Am J Epidemiol. 2000;151(8):754–764. doi: 10.1093/oxfordjournals.aje.a010275. [DOI] [PubMed] [Google Scholar]
- 23.Karatas F, Erdem GU, Sahin S, et al. Obesity is an independent prognostic factor of decreased pathological complete response to neoadjuvant chemotherapy in breast cancer patients. Breast (Edinburgh, Scotland) 2016 doi: 10.1016/j.breast.2016.05.013. [DOI] [PubMed] [Google Scholar]
- 24.Fontanella C, Lederer B, Gade S, et al. Impact of body mass index on neoadjuvant treatment outcome: a pooled analysis of eight prospective neoadjuvant breast cancer trials. Breast cancer research and treatment. 2015;150(1):127–139. doi: 10.1007/s10549-015-3287-5. [DOI] [PubMed] [Google Scholar]
- 25.Erbes T, Stickeler E, Rucker G, et al. BMI and Pathologic Complete Response to Neoadjuvant Chemotherapy in Breast Cancer: A Study and Meta-Analysis. Clinical breast cancer. 2016;16(4):e119–132. doi: 10.1016/j.clbc.2016.02.018. [DOI] [PubMed] [Google Scholar]
- 26.WHO. Physical status: the use and interpretation of anthropometry. Geneva: WHO; 1995. [Google Scholar]
- 27.Du Bois D, Du Bois EF. A formula to estimate the approximate surface area if height and weight be known. 1916. Nutrition. 1989;5(5):303–311. discussion 312–303. [PubMed] [Google Scholar]
- 28.Hammond ME, Hayes DF, Wolff AC, Mangu PB, Temin S. American society of clinical oncology/college of american pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. Journal of oncology practice/American Society of Clinical Oncology. 2010;6(4):195–197. doi: 10.1200/JOP.777003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tchrakian N, Flanagan L, Harford J, Gannon JM, Quinn CM. New ASCO/CAP guideline recommendations for HER2 testing increase the proportion of reflex in situ hybridization tests and of HER2 positive breast cancers. Virchows Archiv: an international journal of pathology. 2015 doi: 10.1007/s00428-015-1871-z. [DOI] [PubMed] [Google Scholar]
- 30.Zhang C, Wang S, Israel HP, et al. Higher locoregional recurrence rate for triple-negative breast cancer following neoadjuvant chemotherapy, surgery and radiotherapy. SpringerPlus. 2015;4:386. doi: 10.1186/s40064-015-1116-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hudis CA, Barlow WE, Costantino JP, et al. Proposal for standardized definitions for efficacy end points in adjuvant breast cancer trials: the STEEP system. Journal of Clinical Oncology. 2007;25(15):2127–2132. doi: 10.1200/JCO.2006.10.3523. [DOI] [PubMed] [Google Scholar]
- 32.Luen SJ, Salgado R, Fox S, et al. Tumour-infiltrating lymphocytes in advanced HER2-positive breast cancer treated with pertuzumab or placebo in addition to trastuzumab and docetaxel: a retrospective analysis of the CLEOPATRA study. The Lancet Oncology. 2017;18(1):52–62. doi: 10.1016/S1470-2045(16)30631-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Verma S, Miles D, Gianni L, et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med. 2012;367(19):1783–1791. doi: 10.1056/NEJMoa1209124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matthews SB, Thompson HJ. The Obesity-Breast Cancer Conundrum: An Analysis of the Issues. International journal of molecular sciences. 2016;17(6) doi: 10.3390/ijms17060989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Renehan AG, Zwahlen M, Egger M. Adiposity and cancer risk: new mechanistic insights from epidemiology. Nature reviews Cancer. 2015;15(8):484–498. doi: 10.1038/nrc3967. [DOI] [PubMed] [Google Scholar]
- 36.Ford NA, Lashinger LM, Allott EH, Hursting SD. Mechanistic targets and phytochemical strategies for breaking the obesity-cancer link. Frontiers in oncology. 2013;3:209. doi: 10.3389/fonc.2013.00209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lashinger LM, Ford NA, Hursting SD. Interacting inflammatory and growth factor signals underlie the obesity-cancer link. J Nutr. 2014;144(2):109–113. doi: 10.3945/jn.113.178533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hair BY, Troester MA, Edmiston SN, et al. Body mass index is associated with gene methylation in estrogen receptor-positive breast tumors. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2015;24(3):580–586. doi: 10.1158/1055-9965.EPI-14-1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rossi EL, de Angel RE, Bowers LW, et al. Obesity-Associated Alterations in Inflammation, Epigenetics, and Mammary Tumor Growth Persist in Formerly Obese Mice. Cancer prevention research (Philadelphia, Pa) 2016;9(5):339–348. doi: 10.1158/1940-6207.CAPR-15-0348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goodwin PJ. Obesity and endocrine therapy: host factors and breast cancer outcome. Breast (Edinburgh, Scotland) 2013;22(Suppl 2):S44–47. doi: 10.1016/j.breast.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 41.Ewertz M, Gray KP, Regan MM, et al. Obesity and risk of recurrence or death after adjuvant endocrine therapy with letrozole or tamoxifen in the breast international group 1–98 trial. J Clin Oncol. 2012;30(32):3967–3975. doi: 10.1200/JCO.2011.40.8666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Subbaramaiah K, Howe LR, Bhardwaj P, et al. Obesity is associated with inflammation and elevated aromatase expression in the mouse mammary gland. Cancer prevention research (Philadelphia, Pa) 2011;4(3):329–346. doi: 10.1158/1940-6207.CAPR-10-0381. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 43.Hubalek M, Oberguggenberger A, Beer B, et al. Does obesity interfere with anastrozole treatment? Positive association between body mass index and anastrozole plasma levels. Clinical breast cancer. 2014;14(4):291–296. doi: 10.1016/j.clbc.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 44.Diorio C, Lemieux J, Provencher L, Hogue JC, Vachon E. Aromatase inhibitors in obese breast cancer patients are not associated with increased plasma estradiol levels. Breast cancer research and treatment. 2012;136(2):573–579. doi: 10.1007/s10549-012-2278-z. [DOI] [PubMed] [Google Scholar]
- 45.Sini V, Lunardi G, Cirillo M, et al. Body mass index and circulating oestrone sulphate in women treated with adjuvant letrozole. Br J Cancer. 2014;110(5):1133–1138. doi: 10.1038/bjc.2014.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Phipps AI, Ichikawa L, Bowles EJA, et al. Defining Menopausal Status in Epidemiologic Studies: A Comparison of Multiple Approaches and their Effects on Breast Cancer Rates. Maturitas. 2010;67(1):60–66. doi: 10.1016/j.maturitas.2010.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.George SM, Bernstein L, Smith AW, et al. Central adiposity after breast cancer diagnosis is related to mortality in the Health, Eating, Activity, and Lifestyle study. Breast cancer research and treatment. 2014;146(3):647–655. doi: 10.1007/s10549-014-3048-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sun X, Nichols HB, Robinson W, Sherman ME, Olshan AF, Troester MA. Post-diagnosis adiposity and survival among breast cancer patients: influence of breast cancer subtype. Cancer Causes Control. 2015;26(12):1803–1811. doi: 10.1007/s10552-015-0673-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


