Abstract
While trimethoprim-sulfamethoxazole is considered first-line therapy for Pneumocystis pneumonia prevention in renal transplant recipients, reported adverse drug reactions may limit use and increase reliance on costly and less effective alternatives, often aerosolized pentamidine. We report our experience implementing a protocolized approach to trimethoprim-sulfamethoxazole adverse drug reaction assessment and rechallenge to optimize prophylaxis in this patient cohort. We retrospectively reviewed 119 patients receiving Pneumocystis pneumonia prophylaxis prior to and after protocol implementation. Forty-two patients (35%) had 48 trimethoprim-sulfamethoxazole adverse drug reactions documented either at baseline or during the prophylaxis period, of which 83% were non-immune-mediated and 17% were immune-mediated. Significantly more patients underwent trimethoprim-sulfamethoxazole rechallenge after protocol implementation (4/22 vs. 23/27; P=0.0001), with no recurrence of adverse drug reactions in 74%. In those who experienced a new or recurrent reaction (26%), all were mild and self-limiting with only 1 recurrence of an immune-mediated reaction. After protocol implementation, aerosolized pentamidine-associated costs were reduced. The introduction of a standard approach to trimethoprim-sulfamethoxazole rechallenge in the context of both prior immune and non-immune-mediated reactions was safe and successful in improving the uptake of first-line Pneumocystis pneumonia prophylaxis in renal transplant recipients.
Introduction
While trimethoprim-sulfamethoxazole (TMP-SMX) is considered first-line therapy for Pneumocystis jirovecii pneumonia (PJP) prevention in renal transplant recipients (RTRs), aerosolized pentamidine (AP) is frequently employed as an alternative due to patient-reported TMP-SMX adverse drug reactions (ADRs). In this context, dapsone is also often avoided due to perceived antibiotic sulfonamide cross-reactivity (1). Although atovaquone, too, is recommended as a second- (2) or third-line alternative (3), it is often not employed due to cost (4). The additional costs and adverse effects of AP (5, 6), as well as the superior efficacy of TMP-SMX (7), highlight the need to improve the uptake of selective TMP-SMX rechallenge. We report our experience implementing a protocolized approach to TMP-SMX ADR management to optimize PJP prophylaxis in RTRs.
Materials and Methods
This study was conducted at Austin Health, a tertiary referral renal transplant center in Melbourne, Australia. A TMP-SMX ADR assessment and management protocol for RTRs was developed by the Infectious Diseases and Nephrology units, in consultation with an external expert reviewer who leads an international antibiotic allergy service (Figure 1), and implemented in late July 2015. Common TMP-SMX ADRs were categorized as either Type-A (non-immune-mediated) or Type-B (immune-mediated) according to previously published definitions (8). Following accurate ADR phenotyping, a management plan was defined for each patient involving either (i) TMP-SMX rechallenge (ii) dapsone 100mg orally daily or (iii) AP 300mg every 4 weeks. All oral rechallenges were performed in a supervised outpatient setting. A TMP-SMX oral rechallenge was via a single strength tablet (80mg-400mg), followed by a 2-hour observation period. TMP-SMX prophylactic maintenance dosing (either 160mg-800mg daily or 3-times weekly [clinician preference]) was continued post-challenge if no ADR was observed. Second-line prophylaxis dosing and agent choice was in accordance with the Australian Therapeutic Guidelines: Antibiotic which recommends 1) dapsone 100mg orally daily, followed by 2) AP 300mg every 4 weeks, and 3) atovaquone 1500mg orally daily (3). RTRs at Austin Health routinely receive PJP prophylaxis for 12 months post-transplantation and during and after treatment of rejection episodes. RTR urinary tract infection (UTI) prophylaxis is not routine practice.
Figure 1. TMP-SMX ADR assessment and management protocol.
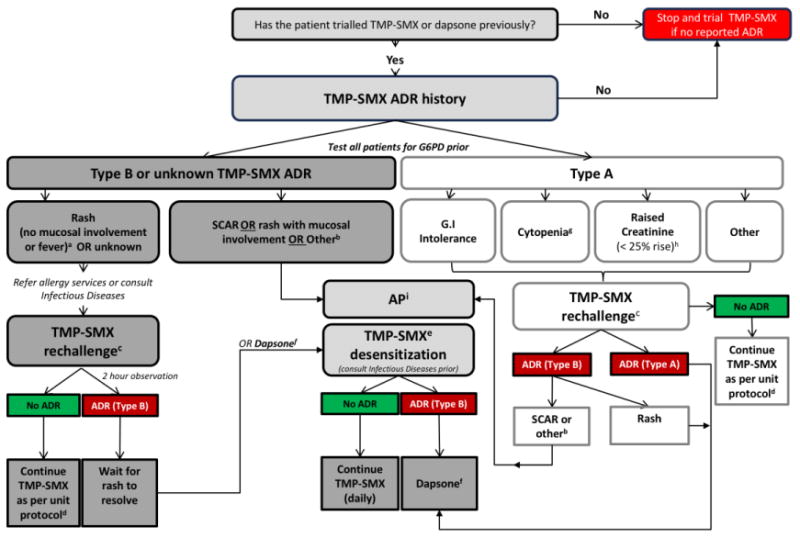
PJP, Pneumocystis jirovecii pneumonia; TMP-SMX, trimethoprim-sulfamethoxazole; ADR, adverse drug reaction; G6PD, glucose-6-phosphate dehydrogenase; GI, gastrointestinal intolerance; SCAR, severe cutaneous adverse reaction; AP, aerosolized pentamidine
a If TMP-SMX-associated rash within last two years, can consider dapsone rather than rechallenge
b Drug fever, acute interstitial nephritis, immune complex deposition
c Oral single dose challenge and observe for two hours (TMP-SMX 80mg-400mg). Patients reviewed 96 hours post-oral challenge to ensure no serious adverse event. Repeat follow-up as per treating physician.
d Preferred prophylaxis strategy generally TMP-SMX 160mg-800mg daily or 3 times weekly.
e For all patients proceed with TMP-SMX desensitization or alternatively, dapsone therapy may be employed.
f Prescribe dapsone 100mg orally daily. Ensure G6PD deficiency screen negative prior to use. Dapsone monitoring includes full blood examination to check for anemia and/or hemolysis. Methemoglobulinemia can occur (normally symptomatic at >10%). Consider blood gas if symptomatic (e.g. central cyanosis, short of breath). May need to reduce dose to 50mg daily if toxicities occur. If ADR follows dapsone therapy then pentamidine usage should be considered.
g For discussion on a case-by-case basis with treating unit. Avoid if TMP-SMX is the only implicated drug in cytopenia.
h Avoid rechallenge if creatinine clearance < 40ml/min in a patient with previous renal impairment attributed to TMP-SMX.
i Requires preauthorization from Infectious Diseases
A retrospective audit of PJP prophylaxis in RTRs (Ethics Approval: LNR/17/Austin/5) was conducted over two 12-month periods before and after protocol implementation (August 2014-July 2015 and August 2015-July 2016). RTRs receiving PJP prophylaxis were identified from pharmacy dispensing records. Subjects were excluded if RTRs were followed outside of Austin Health. A PJP prophylaxis course was defined as continuous therapy until cessation documented in the medical record. Baseline demographics and PJP prophylaxis courses were recorded from medical and pharmacy records. Hospital day admission costs for AP administration were ascertained from health information costings.
Results
A total of 166 RTRs were prescribed PJP prophylaxis during the study period, of which 119 (208 courses) were followed-up at Austin Health and included in the study. The majority of patients were male (66%), having undergone their first transplant (90.8%), with a median age-adjusted Charlson comorbidity score of 4 (IQR: 3,6) (Table 1).
Table 1. Patient clinical characteristics.
| Patient clinical characteristics | N = 119 n (%) |
|---|---|
|
| |
| Age (years) at PJP prophylaxis commencement | |
| Median (IQR) | 56 (45,64) |
|
| |
| Charlson Age-Comorbidity Index | |
| Median (IQR) | 4 (3,6) |
|
| |
| Sex | |
| Male | 79 (66.4) |
|
| |
| Underlying renal disease | |
| Diabetes mellitus | 23 (19) |
| IgA nephropathy | 23 (19) |
| Polycystic kidney disease | 13 (11) |
| Reflux nephropathy | 11 (9) |
| Focal segmental glomerulosclerosis | 10 (9) |
| Glomerulonephritis (unspecified) | 8 (7) |
| Unknown cause | 4 (3) |
| Renovascular | 4 (3) |
| ANCA vasculitis | 4 (3) |
| Hypertension | 2 (2) |
| Systemic lupus erythematosus | 2 (2) |
| Renal calculi | 2 (2) |
| Anti-glomerular basement membrane disease | 2 (2) |
| Other | 11 (9) |
|
| |
| Indication for PJP prophylactic course | |
| Prophylaxis, initial | 72 (60) |
| Prophylaxis, prolonged | 27 (23) |
| Prophylaxis, rejection | 14 (12) |
| Prophylaxis, secondary | 6 (5) |
|
| |
| No. renal transplants at PJP prophylaxis commencement | |
| Median (range) | 1 (1-3) |
|
| |
| Documented antibiotic ADR | |
| - Any, prior to PJP prophylaxis commencement | 32 (27) |
| - TMP-SMX, prior to PJP prophylaxis commencement | 10 (8) |
| - TMP-SMX, totala – n ADRs reported | 42 (35) |
| ○ Type Ab | 40 (83) |
| ○ Type B (immediate)c | 0 (0) |
| ○ Type B (delayed)c | 8 (17) |
ADR, adverse drug reaction; PJP, Pneumocystis jirovecii pneumonia; TMP-SMX, trimethoprim-sulfamethoxazole
Patients may have ≥1 ADR documented either prior to or during the PJP prophylaxis period. Total 48 ADRs reported.
Type-A ADR are typically a non-immune-mediated dose-dependent effects (e.g. gastrointestinal upset, serum creatinine rise).
Type-B ADRs are typically immune-mediated unpredictable reactions that can be both immediate (IgE mediated) or delayed (T-cell mediated). These delayed T-cell mediated reactions can range from mild maculopapular exanthems (MPE) to severe cutaneous adverse drug reactions (SCAR).
Forty-two patients (35%) had 48 TMP-SMX ADRs documented during the audit period. These included 10 patients with ADRs noted prior to their commencement of PJP prophylaxis. Forty (83%) were Type-A ADRs including cytopenia (32/48, 67%), all in the context of concurrent mycophenolate mofetil (MMF) and/or valganciclovir. Other Type-A ADRs included elevated serum creatinine (SeCr) (5/48, 10%), nausea (2/48, 4%), and elevated liver function tests (1/48, 2%). Eight (17%) were mild maculopapular exanthem (MPE) Type-B ADRs. Thirty-two patients (27%) in this cohort reported ADRs to any antibiotic prior to commencing PJP prophylaxis. There was a higher number of patients with a documented TMP-SMX ADR that underwent TMP-SMX challenge after protocol implementation compared to before (4/22 vs. 23/27; P=0.0001). Of the 27 patients that underwent rechallenge, there was no recurrence of TMP-SMX ADR in 74%, including with 3 immune-mediated ADRs. In the 26% that experienced a new or recurrent ADR, all were mild and self-limiting with only 1 recurrent immune-mediated reaction (MPE). Dapsone was employed in 4 patients, 75% (3/4) after protocol implementation with none experiencing Type-B immune-mediated sequelae.
There was a non-significant reduction in RTR AP doses, from 91 (21 RTRs; median: 3 doses per patient [IQR 1-5]), to 68 (19 RTRs; median: 2 doses per patient [IQR 1-5]) after protocol implementation. As a result, estimated AP administration costs reduced by 25% (AUD$68,768 vs. AUD$51,387).
Discussion
Although limited by a short study period and cohort size, our findings suggest that selective TMP-SMX rechallenge is a safe and effective method of optimizing PJP prophylaxis in RTRs, similar to the HIV setting (9). As often seen in clinical practice, Type-A ADRs contributed to a significant number of antibiotic allergy labels (10). Mitsides et al. (11) reported similar rates of TMP-SMX ADR and discontinuation (38%) in 290 RTRs receiving PJP prophylaxis, predominantly due to SeCr rise. This rise is likely due to inhibition of tubular Cr secretion by trimethoprim which can result in reversible increases in SeCr higher than 35% in patients with pre-existing renal impairment (11) and, therefore, likely to recur on TMP-SMX rechallenge. We did not observe similar rates of SeCr rise in our cohort, possibly due to the intermittent dose routinely used as it is likely that this ADR is dose-related (12). Although reversible, increases in SeCr in RTRs may elicit concerns of acute rejection and therefore, treating physicians should be aware of this phenomenon. We observed high TMP-SMX discontinuation due to cytopenias. In many cases of presumed TMP-SMX-induced cytopenia, the contribution of other immunosuppression agents (e.g. MMF) and antivirals (e.g. valganciclovir), is underappreciated. Avoidance of TMP-SMX or dapsone in the setting of these Type-A ADR histories is potentially unnecessary.
Avoidance of antibiotic sulfonamides in patients with a history of non-antibiotic sulfonamide allergy is largely unnecessary. Large cohort studies have demonstrated the absence of cross-reactivity between antibiotic (TMP-SMX, dapsone, sulfadiazine) and non-antibiotic sulfonamides (e.g. furosemide, hydrochlorothiazide, acetazolamide, oral hypoglycemics) (13), and a low rate of cross-reactivity between antibiotic sulfonamides (1, 14, 15). Despite this, clinician avoidance remains high (13, 16). The non-antibiotic sulfonamides do not contain the structural region implicated in the allergic response (i.e. N1 heterocyclic ring; an N-containing ring attached to the N1 nitrogen of the sulfonamide group and arylamine group at the N4 position). A greater understanding of the true rates of cross-reactivity is likely to increase clinician confidence in rechallenge amongst RTRs.
In both our RTR cohort and other patient groups (17, 18), rechallenge with TMP-SMX in patients with a history of benign MPE has proven successful. Whilst desensitization is an appropriate alternative approach in delayed hypersensitivity (18), the immunological mechanism for tolerance remains unknown (19), and it carries the logistical concerns of acute care admission and additional cost. The benefits of TMP-SMX over alternative agents include the additional coverage of toxoplasmosis (20, 21), possibly nocardiosis (22), and potential risk reduction of sepsis and UTIs in RTRs (23). The introduction of a standard approach to TMP-SMX ADR assessment and rechallenge resulted in an increase in TMP-SMX utilization and reduction in AP, including associated hospital costs, and should be considered to optimize RTR PJP prophylaxis in transplant units with appropriate expertise.
Acknowledgments
K.F.U and J.A.T are supported by NHMRC postgraduate research scholarships
Abbreviations
- ADRs
Adverse drug reactions
- AP
aerosolized pentamidine
- MPE
maculopapular exanthem
- PJP
Pneumocystis jirovecii pneumonia
- RTRs
renal transplant recipients
- SeCr
serum creatinine
- TMP-SMX
trimethoprim-sulfamethoxazole
Footnotes
Disclosure: The authors of this manuscript have conflicts of interest to disclose as described by the American Journal of Transplantation. K.F.U has sat on an advisory board for MSD.
References
- 1.Beumont MG, Graziani A, Ubel PA, MacGregor RR. Safety of dapsone as Pneumocystis carinii pneumonia prophylaxis in human immunodeficiency virus-infected patients with allergy to trimethoprim/sulfamethoxazole. Am J Med. 1996;100(6):611–616. doi: 10.1016/s0002-9343(96)00008-3. [DOI] [PubMed] [Google Scholar]
- 2.Martin SI, Fishman JA, the A. S. T. Infectious Diseases Community of Practice Pneumocystis Pneumonia in Solid Organ Transplantation. Am J Transplant. 2013;13(s4):272–279. doi: 10.1111/ajt.12119. [DOI] [PubMed] [Google Scholar]
- 3.Antibiotic Expert Group. Therapeutic guidelines: antibiotic. Version 15. Melbourne: Therapeutic Guidelines Limited; 2014. [Google Scholar]
- 4.Panel on Opportunistic Infections in HIV-Infected Adults and Adolescents. [Accessed: 2/9/17];Guidelines for the prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from the Centers for Disease Control and Prevention, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. Available from: http://aidsinfo.nih.gov/contentfiles/lvguidelines/adult_oi.pdf.
- 5.Macesic N, Urbancic K, Ierino F, Grayson ML. Is aerosolized pentamidine for Pneumocystis pneumonia prophylaxis in renal transplant recipients not as safe as one might think? Antimicrob Agents Chemother. 2016;60(4):2502–2504. doi: 10.1128/AAC.02290-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ward C, O'Donovan D, Lever AM. Usage and cost of a nebulised pentamidine clinic in a large teaching hospital. J Infect. 2009;58(3):248–250. doi: 10.1016/j.jinf.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 7.Stern A, Green H, Paul M, Vidal L, Leibovici L. Prophylaxis for Pneumocystis pneumonia (PCP) in non-HIV immunocompromised patients. Cochrane Database Syst Rev. 2014;(10) doi: 10.1002/14651858.CD005590.pub3. CD005590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rawlins MD, Thompson TJ. Textbook of adverse drug reactions. Oxford; Oxford University Press; 1977. [Google Scholar]
- 9.Lin D, Li WK, Rieder MJ. Cotrimoxazole for prophylaxis or treatment of opportunistic infections of HIV/AIDS in patients with previous history of hypersensitivity to cotrimoxazole. Cochrane Database Syst Rev. 2007;(2) doi: 10.1002/14651858.CD005646.pub2. CD005646. [DOI] [PubMed] [Google Scholar]
- 10.Trubiano JA, Cairns KA, Evans JA, Ding A, Nguyen T, Dooley MJ, et al. The prevalence and impact of antimicrobial allergies and adverse drug reactions at an Australian tertiary centre. BMC Infect Dis. 2015;15:572. doi: 10.1186/s12879-015-1303-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mitsides N, Greenan K, Green D, Middleton R, Lamerton E, Allen J, et al. Complications and outcomes of trimethoprim-sulphamethoxazole as chemoprophylaxis for pneumocystis pneumonia in renal transplant recipients. Nephrology (Carlton) 2014;19(3):157–163. doi: 10.1111/nep.12201. [DOI] [PubMed] [Google Scholar]
- 12.Delanaye P, Mariat C, Cavalier E, Maillard N, Krzesinski JM, White CA. Trimethoprim, creatinine and creatinine-based equations. Nephron Clin Pract. 2011;119(3):c187–193. doi: 10.1159/000328911. [DOI] [PubMed] [Google Scholar]
- 13.Strom BL, Schinnar R, Apter AJ, Margolis DJ, Lautenbach E, Hennessy S, et al. Absence of cross-reactivity between sulfonamide antibiotics and sulfonamide nonantibiotics. N Engl J Med. 2003;349(17):1628–1635. doi: 10.1056/NEJMoa022963. [DOI] [PubMed] [Google Scholar]
- 14.Zawodniak A, Lochmatter P, Beeler A, Pichler WJ. Cross-reactivity in drug hypersensitivity reactions to sulfasalazine and sulfamethoxazole. Int Arch Allergy Immunol. 2010;153(2):152–156. doi: 10.1159/000312632. [DOI] [PubMed] [Google Scholar]
- 15.Carr A, Tindall B, Penny R, Cooper DA. Patterns of multiple-drug hypersensitivities in HIV-infected patients. AIDS. 1993;7(11):1532–1533. doi: 10.1097/00002030-199311000-00023. [DOI] [PubMed] [Google Scholar]
- 16.Wall GC, Dewitt JE, Haack S, Fornoff A, Eastman DK, Koenigsfeld CF. Knowledge and attitudes of American pharmacists concerning sulfonamide allergy cross-reactivity. Pharm World Sci. 2010;32(3):343–346. doi: 10.1007/s11096-010-9389-6. [DOI] [PubMed] [Google Scholar]
- 17.Carr A, Penny R, Cooper DA. Efficacy and safety of rechallenge with low-dose trimethoprim-sulphamethoxazole in previously hypersensitive HIV-infected patients. AIDS. 1993;7(1):65–71. doi: 10.1097/00002030-199301000-00010. [DOI] [PubMed] [Google Scholar]
- 18.Pyle RC, Butterfield JH, Volcheck GW, Podjasek JC, Rank MA, Li JT, et al. Successful outpatient graded administration of trimethoprim-sulfamethoxazole in patients without HIV and with a history of sulfonamide adverse drug reaction. J Allergy Clin Immunol Pract. 2014;2(1):52–58. doi: 10.1016/j.jaip.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 19.Legendre DP, Muzny CA, Marshall GD, Swiatlo E. Antibiotic hypersensitivity reactions and approaches to desensitization. Clin Infect Dis. 2014;58(8):1140–1148. doi: 10.1093/cid/cit949. [DOI] [PubMed] [Google Scholar]
- 20.Fernandez-Sabe N, Cervera C, Farinas MC, Bodro M, Munoz P, Gurgui M, et al. Risk factors, clinical features, and outcomes of toxoplasmosis in solid-organ transplant recipients: a matched case-control study. Clin Infect Dis. 2012;54(3):355–361. doi: 10.1093/cid/cir806. [DOI] [PubMed] [Google Scholar]
- 21.Martina MN, Cervera C, Esforzado N, Linares L, Torregrosa V, Sanclemente G, et al. Toxoplasma gondii primary infection in renal transplant recipients. Two case reports and literature review Transpl Int. 2011;24(1):e6–12. doi: 10.1111/j.1432-2277.2010.01173.x. [DOI] [PubMed] [Google Scholar]
- 22.Fishman JA. Infection in Solid-Organ Transplant Recipients. N Engl J Med. 2007;357(25):2601–2614. doi: 10.1056/NEJMra064928. [DOI] [PubMed] [Google Scholar]
- 23.Horwedel TA, Bowman LJ, Saab G, Brennan DC. Benefits of sulfamethoxazole-trimethoprim prophylaxis on rates of sepsis after kidney transplant. Transpl Infect Dis. 2014;16(2):261–269. doi: 10.1111/tid.12196. [DOI] [PubMed] [Google Scholar]


