Abstract
In the pharmaceutical industry, the analysis of atropisomers is of considerable interest from a scientific and regulatory perspective. The compound of interest contains two stereogenic axes due to the hindered rotation around the single bonds connecting the aryl groups, which results in four potential configurational isomers (atropisomers). The separation of the four atropisomers is achieved on a derivatized β-cyclodextrin bonded stationary phase. Further investigation shows that low temperature conditions, including sample preparation (−70 °C), sample storage (−70 °C), and chromatographic separation (6 °C), were critical to preventing interconversion. LC-UV-Laser Polarimetric analysis identified peak 1/2 as a pair of enantiomers and peak 3/4 as another. Thermodynamic analysis of the retention data indicated that the separation of the pairs of enantiomers is primarily enthalpy controlled as indicated by the positive slope of the van’t Huff plot. The difference in absolute Δ (Δ H), ranged from 2.20 kJ/mol to 2.42 kJ/mol.
Keywords: Atropisomer separation, Chiral HPLC, Thermodynamic parameters, β-cyclodextrin stationary phase, Chiral separation mechanism
1. Introduction
The identification of the enantiomer of interest is of great interest to the pharmaceutical community due to the fact that one isomer may have beneficial health properties and the unwanted isomer may have little pharmacological effect, or even show toxicity [1]. Among all the separation techniques used for chiral separation, such as supercritical fluid chromatography (SFC) [2], [3], high performance liquid chromatography (HPLC) [4], gas chromatography (GC) [5], capillary electrophoresis (CE) [6], etc. HPLC is the most commonly used technique due to its maturity and wide applications [7]. In-depth reviews of the analytical methods and pharmacology of drug stereochemistry [8], chiral separation techniques with an emphasis on practical approaches, and pharmaceutical applications have been published [9], [10]. The chiral recognition mechanism for different stationary phases such as macrocyclic antibiotics [11], polysaccharides [12], [13] and cyclodextrins [14] using HPLC has been investigated. Additionally, several literature references have discussed the thermodynamic aspects of chiral compound retention mechanism [15], [16], [17], [18].
Atropisomerism is a unique type of isomerism due to axial chirality, in which a single bond rotation is highly restricted unlike a carbon centered chiral compound [19]. The separation and identification of atropisomers is challenging due to the dynamic nature of the molecular rotation around the hindered axial bond [20]. This dynamic molecular rotation results in a low energy barrier between atropisomers such that on-column interconversion can occur at normal chromatographic operating conditions. However, the analysis of atropisomers is important from a scientific and regulatory perspective, as the bioavailability and physicochemical properties of atropisomers can be substantially different from the target drug [21]. Therefore, having a reliable method for analysis is critical to ensure patient safety. Two recent studies of atropisomers highlight the limited knowledge on the separation mechanism and the interconversion process of these isomers. The separation of two atropisomers of a substituted biphenyl compound was achieved on Chiralcel OD-H column, and the interconversion rates between the two atropisomers were studied [22]. Chromatographic separation of two triphenyl atropisomers with very low energy barrier was accomplished using Obelisc R column. The retention mechanism and interconversion process were studied using molecular modeling and dynamic nuclear magnetic resonance (NMR). The chromatographic kinetic data were complemented with those obtained from NMR studies [23].
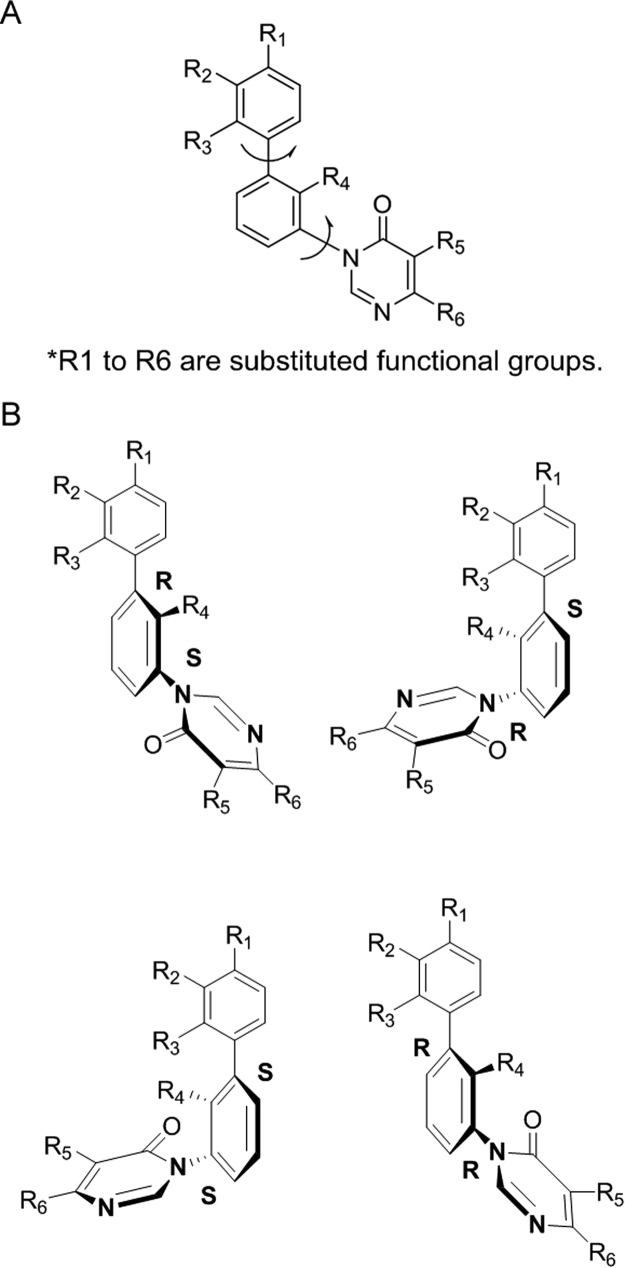
In this study, compound I (Fig. 1), containing three consecutive aryl groups with six differing substitution groups (R1-R6) was investigated. Due to the hindered rotation around the single bonds connecting the aryl groups, there are four potential atropisomers for this compound. Presently, there is no literature discussing the separation of a compound with four atropisomers.
Fig. 1.
Structure of Compound I and its four configurational isomers.
Commercially available software packages, such as DryLab®, can be used for optimizing the chromatographic conditions during the HPLC method development activities [24], [25]. Even though, computer-aided tools have been used to optimize chiral separations [26], to the best of our knowledge, there is no literature reporting the use of computer aided tools in the separation of atropisomers. In this paper, DryLab® software was used to optimize the mobile phase composition for the atropisomer separation. In addition, the current study details the chromatographic separation of the four atropisomers and investigates the thermodynamic properties of the separation process.
2. Materials and methods
2.1. Reagents and materials
HPLC grade acetonitrile and methanol were purchased from EMD Science (Gibbstown, NJ, USA). Ammonium acetate was purchased from Sigma-Aldrich (St. Louis, MO, USA). Ultra pure water from a Millipore Milli-Q system (Milford, MA, USA) was used for the preparation of the mobile phase. Compound I was provided by Bristol-Myers Squibb (New Brunswick, NJ, USA) and exists in three physical forms: Form A, Form B, and racemic mixture.
2.2. HPLC instrumentation and conditions
HPLC measurements utilized an Agilent 1100 HPLC system (Santa Clara, CA, USA), equipped with a quaternary pump, autosampler, column thermostat and variable wavelength detector set to a wavelength of 278 nm. Astec CYCLOBOND I 2000 HP-RSP (250 mm×4.6 mm, 5 µm), purchased from Sigma-Aldrich (St. Louis, MO, USA) was employed for analytical separation. In addition, the following columns were evaluated: Phenomenex Lux Cellulose-1 (150 mm×4.6 mm, 5 µm), Astec CHIROBIOTIC V (250 mm×4.6 mm, 5 µm), Astec CYCLOBOND I 2000 (250 mm×4.6 mm, 5 µm) and Astec CYCLOBOND I 2000 RSP (250 mm×4.6 mm, 5 µm). Initial column screening work was performed using the following conditions: mobile phase was methanol: water (50:50) (v/v) with a column temperature of 6 °C, and flow rate is 1 mL/min. The column temperature for all studies was controlled to ±1 °C. The sample was dissolved in methanol at a concentration of 0.5 mg/mL and the injection volume was 2 µL. Temperature and solution stability investigation were performed using a mobile phase of methanol: water (60:40) (v/v) and the flow rate was 0.7 mL/min. Two injections were made at each testing condition to ensure reproducibility.
LC-UV-Laser Polarimetric analysis was performed using a Waters Alliance 2690 system (Millford, MA, USA), equipped with a model 996 photodiode array detector and a model ALP-2000 Advanced Laser Polarimeter (PDR, Lake Park, FL, USA). A more concentrated sample (2 mg/mL in methanol) was injected (10 µL injection volume) to improve the sensitivity for polarimeter detection. The flow rate was increased to 0.9 mL/min to minimize band broadening, which will also increase sensitivity. A column temperature of 20 °C was used as that was the lowest control limit for the instrument used. The LC-UV-Laser Polarimetric analysis is used for peak identification/correlation only, and the difference in chromatographic condition between this and the sample analysis has no effect on the conclusions made.
All the chromatographic data was collected with Empower software (Waters, Milford, MA, USA).
3. Results and discussion
As discussed earlier, Compound I can have four potential atropisomers. During development, chromatographic separation and quantitation of the four atropisomers was needed to control the atropisomer ratios in the active pharmaceutical ingredient. In order to ensure a robust analytical method, it is important to understand the dynamic nature of atropisomerism and carefully consider the sample preparation procedure, sample solution stability and separation conditions.
3.1. Column selection
Initial chiral HPLC method development efforts focused on identifying a suitable column that could provide the necessary chiral recognition to separate the four isomers. At the early stages of development, while the dynamic nature of compound I was anticipated based on the existing literature of atropisomers [27], [28], the exact thermodynamics of atropisomer interconversion and its impact on the atropisomer ratios were not fully understood. Therefore column screening was initiated with a racemic sample of compound I and the sample preparation consisted of dissolving compound I in methanol at a concentration of 0.5 mg/mL at room temperature. The initial screening consisted of evaluating five different chiral columns using the conditions described earlier: Phenomenex Lux Cellulose-1, Astec CHIROBIOTIC V, Astec CYCLOBOND I 2000, Astec CYCLOBOND I 2000 RSP and Astec CYCLOBOND I 2000 HP-RSP. These columns are based on cellulose, macrocyclic glycopeptides and β-cyclodextrin (β-CD) chiral stationary phases, respectively. The Phenomenex Lux Cellulose-1 and the Astec CHIROBIOTIC V columns did not afford any significant separation of the four atropisomers. As a result, there was no further development using these two columns.
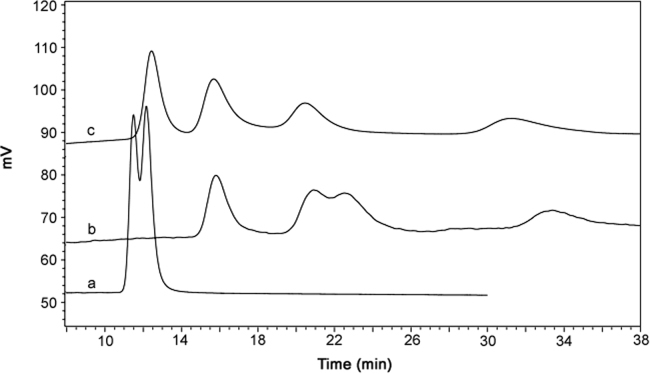
A promising separation was achieved using the β-cyclodextrin (β-CD) stationary phases from Supelco. The column screening results from the Astec CYCLOBOND I 2000 series are shown in Fig. 2. The unmodified β-CD column, CYCLOBOND I 2000, separated the racemic compound I into 2 peaks, with each peak presumably containing a pair of isomers. However, the 2, 3-position hydroxylpropyl ether derivatized β- CD columns, CYCLOBOND I 2000 RSP and CYCLOBOND I 2000 HP-RSP, achieved separation of all four atropisomers.
Fig. 2.
Overlay chromatograms of compound I using three CYCLOBOND columns: (a) CYCLOBOND I 2000 column, (b) CYCLOBOND I 2000 RSP column, (c) CYCLOBOND I 2000 HP-RSP column, Screening condition: mobile phase: MeOH: water (50:50, v/v); column temperature: 6 °C; flow rate: 1 mL/min; detection wavelength: 278 nm.
The three CYCLOBOND chiral stationary phases (CSP) used in this study have a similar basis for chiral separations, especially in the reversed-phase mode, which is an inclusion complexing interaction [29]. Β-CD based CSPs consists of seven glucose units with each glucose unit bonded to another through a α - (1, 4) glycosidic linkage. This forms the toroid “bucket”, narrower at one end than the other, in which the chiral separation can occur on the inside cavity surface or on the outside surface. By derivatizing the 2, 3- position of β-CD a potentially new interaction is available to compound I as the inward cavity facing 2, 3-position hydroxyls could create steric interactions which further enhances the inclusion capability and provides an additional flexible hydrogen bonding group with the substituted hydroxypropyl ether, extending the interactive potential for the sterically hindered center of compound I. This appears to be the primary driving force for the separation of the four atropisomers. According to current vendor information and literature [30], compared with CYCLOBOND I 2000 RSP stationary phase, a different linkage is used in the bonding chemistry for CYCLOBOND I 2000 HP-RSP and the substitution level on the β-CD secondary hydroxyl groups is also different, therefore, CYCLOBOND I 2000 HP-RSP provides extra hydrogen bonding capability. This would further enhance the resolution of the four atropisomers. Based on these results, the CYCLOBOND I 2000 HP-RSP column was selected for further studies and no additional efforts were devoted to enhancing the separation on the other CYCLOBOND columns.
3.2. Mobile phase optimization using DryLab®
Acetonitrile as an organic modifier was originally evaluated on CYCLOBOND I 2000 HP-RSP column, and no acceptable separation was achieved. It was found that the addition of methanol to acetonitrile: water mobile phase significantly improved the separation. When ethanol was added as a secondary organic modifier in the acetonitrile: water mixture, only three peaks were observed at a column temperature of 6 °C. While the separation improves as the column temperature increase, it is still not as good as methanol as organic modifier. With longer run time and high column temperature, the potential on column interconversion between the atropisomers is a significant concern in this case. Therefore, methanol is selected as the organic modifier for further evaluation.
In order to further develop the separation conditions, DryLab® was used to optimize the mobile phase composition. DryLab® can be employed to model either one or two variables. In this study, the LC-Isocratic B% mode was selected to predict the optimized mobile phase composition of methanol. Experimental chromatographic separations were performed using the following two mobile phase compositions: methanol: water (55:45, v/v) and methanol: water (70:30, v/v). The two runs were selected based on the following considerations: 1) keep the run time within 30 min; 2) maintain a low separation temperature to avoid on-column interconversion, and 3) take DryLab® recommended 15% difference in organic modifier concentration between the two runs for optimization. This analysis predicts an optimum separation using 60% methanol in the mobile phase. The Drylab prediction and the actual chromatogram achieved using the predicted optimum conditions are shown in Fig. 3. The resolution between the critical pair (peak 1 and 2) obtained from the experiment (1.89) matches that predicted by DryLab® (2.14). Clearly, the experimental chromatogram is consistent with the predicted chromatogram. The utilization of DryLab® in this step is not only beneficial in achieving a robust method, but also reduces the time spent on method development.
Fig. 3.
Comparison of (A) predicted and (B) experimental chromatogram. Mobile phase: MeOH: water (60:40, v/v); column temperature: 6 °C; flow rate: 0.7 mL/min; detection wavelength: 278 nm.
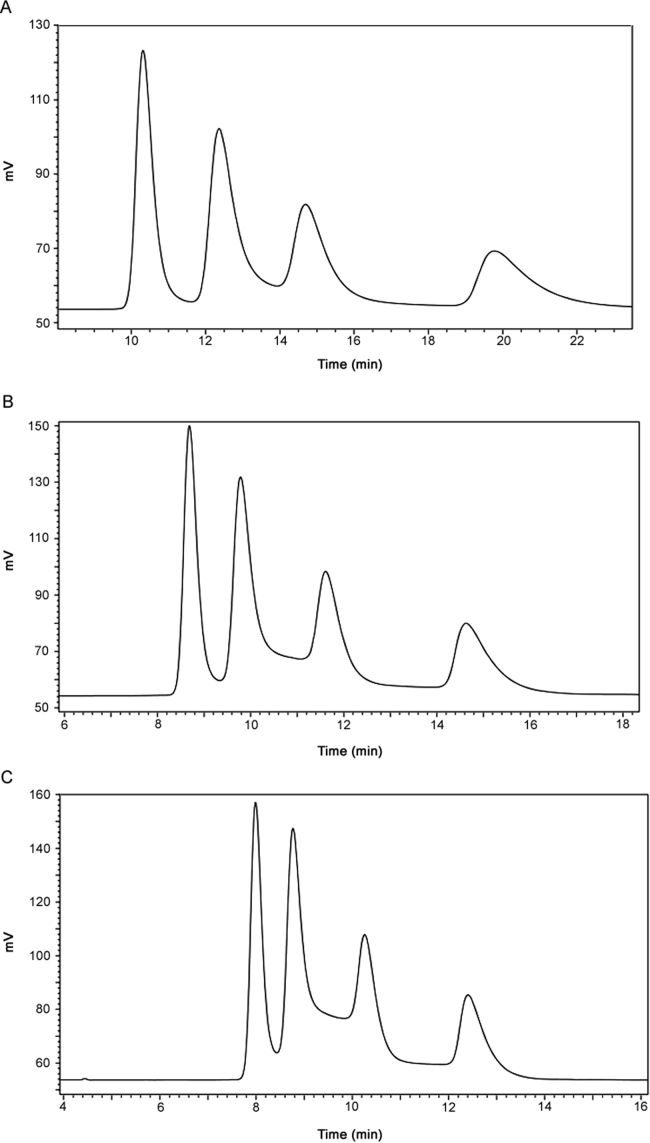
3.3. Effect of column temperature on atropisomer ratio determination
Due to the low energy of activation between each atropisomer, on-column interconversion during sample analysis presents a challenge for reliable quantitation. Therefore, a temperature study was designed to understand the impact of the separation temperature on the atropisomer ratios. Fig. 4 shows the chromatographic separation of Compound I at column temperatures of 6 °C, 20 °C, and 30 °C. At 6 °C, all four atropisomers can be separated. As the column temperature is increased, the retention times of all four atropisomers decrease and the rising baseline between peaks is indicative of faster atropisomer interconversion. This peak-plateau-peak phenomenon is the result of simultaneous interconversion and separation on the column and is consistent with the results described in literature [23]. Therefore, a column temperature to 6 °C is needed to prevent on column atropisomer interconversion and maintain adequate resolution.
Fig. 4.
Column temperature effect on the separation of atropisomers. (A) 6 °C, (B) 20 °C, and (C) 30 °C. Mobile phase: MeOH: water (60:40, v/v); flow rate: 0.7 mL/min; detection wavelength: 278 nm.
3.4. Peak correlation of the atropisomers
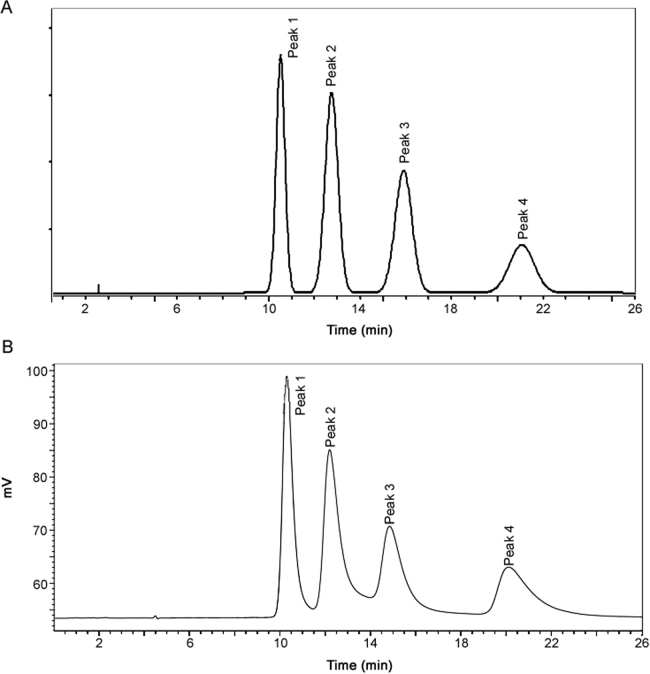
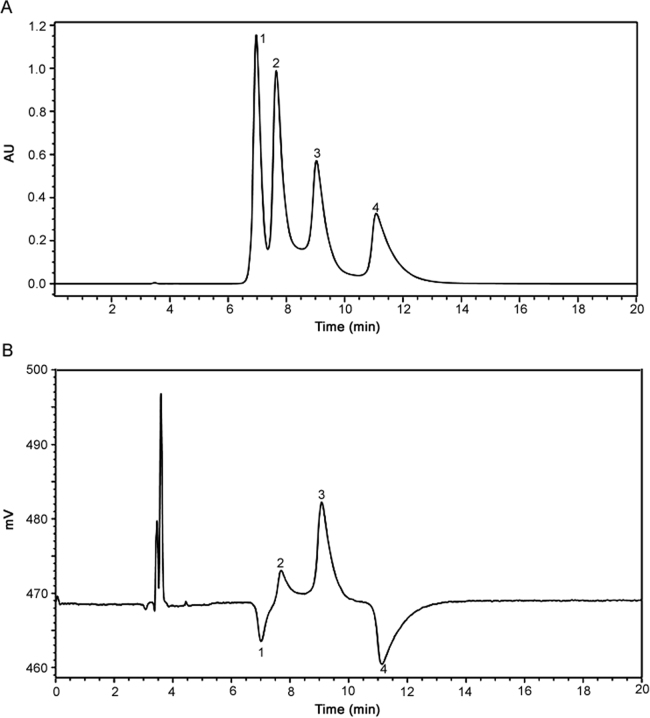
Tandem Laser Polarimetric analysis was utilized to correlate the individual atropisomer peaks observed in the separation with the proposed Compound I atropisomer structures. The results of this analysis in Fig. 5, show that peak 1 and peak 2 have the same peak area in the UV chromatogram but opposite rotation of the plane-polarized light (negative peak reflection for peak 1 and positive peak deflection for peak 2). This indicates that peak 1 and peak 2 are a pair of enantiomers. Peak 3 and peak 4 have the same peak area in the UV chromatogram but opposite rotation of the plane-polarized light (positive peak reflection for peak 3 and negative peak deflection for peak 4) which indicates that peak 3 and peak 4 are another pair of enantiomers. This provides evidence that the peaks are atropisomers and not irrelevant species or impurities.
Fig. 5.
Chromatogram of the atropisomers of compound I (racemic mixture) by LC-UV-Laser Polarimetric detection: (A) UV and (B) polarimetric detector. Mobile phase: MeOH: Water (60:40, v/v), sample concentration: 2 mg/mL of compound I in methanol, flow rate: 0.9 mL/min, column temperature: 20 °C.
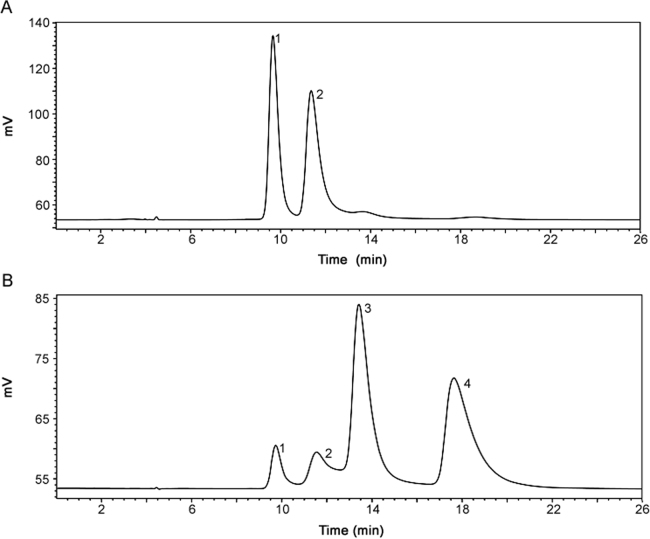
Further identification studies were conducted using two forms (Form A and B) that were isolated and analyzed by single crystal X-ray diffraction (XRD). XRD confirmed that Form A was a racemic mixture of RS/SR configurations, and Form B is a racemic mixture of RR/SS configurations. The UV chromatograms of the two forms are shown in Fig. 6. From the chromatograms, it can be concluded that peak 1 and peak 2 are the RS/SR isomers as those are the only two peaks observed in Form A, Peak 3 and peak 4 are the RR/SS enantiomers as they are the predominate peaks observed in the Form B chromatogram. Furthermore, it is noted that Form B contains small amounts of Form A, indicated by the presence of peak 1 and 2 in the chromatogram. This suggests that interconversion might occur during the sample preparation procedure. Finally, absolute peak assignment of each atropisomer is not currently feasible due to the lack of authentic material of each individual isomer, as they have proven difficult to isolate.
Fig. 6.
Chromatograms of compound I in (A) form A and (B) form B. Mobile phase: MeOH: water (60:40, v/v); column temperature: 6 °C; flow rate: 0.7 mL/min; detection wavelength: 278 nm.
3.5. Sample preparation and solution stability
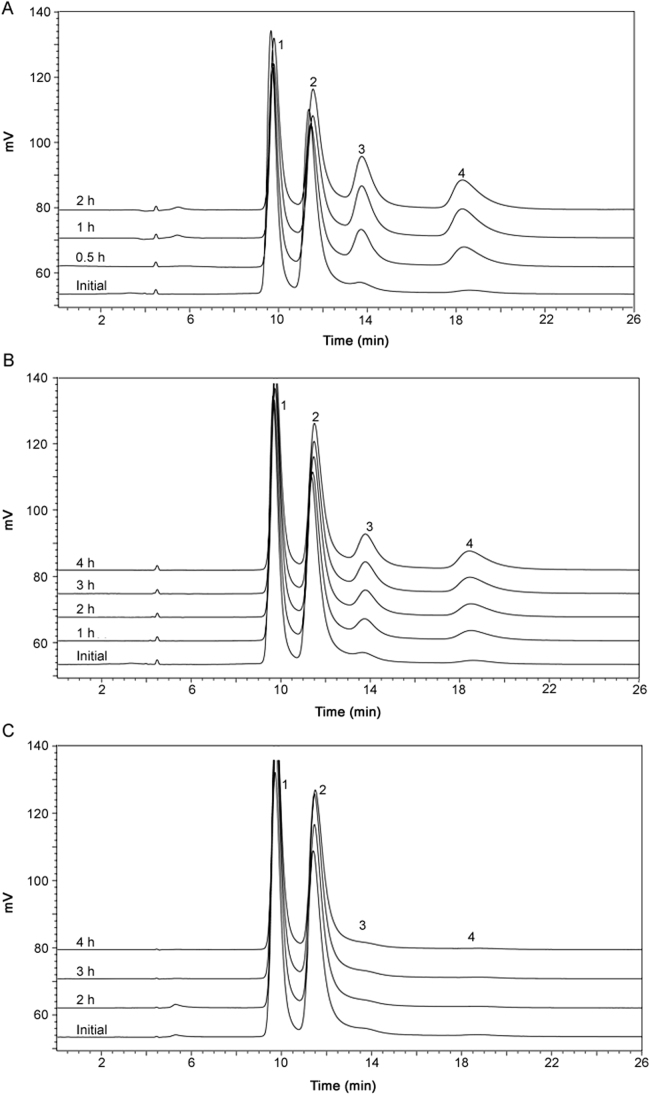
Since the energy barrier between the atropisomers is low and the interconversion of atropisomers could occur during sample preparation, the sample preparation procedure was studied to understand the impact of the sample preparation temperature on atropisomer amounts. Different sample preparations were made using Form A (RS/SR) material and methanol as the diluent. Methanol was allowed to equilibrate at three different temperatures: room temperature (20 ±2 °C), 0 °C, and cooled with dry ice (−70±3 °C) prior to starting sample preparations. For each sample preparation, the time spent on preparation and handling was kept to a minimum and the sample solution was injected immediately after preparation. As shown in Fig. 7, the atropisomer ratio is significantly affected by increasing solvent temperature: the peak areas of peak 3 and peak 4 increased while that of peak 1 and peak 2 decreased. This suggests that as more energy is applied to the system, atropisomer interconversion is enhanced, in this case, RS/SR is converting to SS/RR. These results demonstrate that it is critical to use low temperature solvents during the sample preparation procedure. Therefore, dry ice cooled methanol (−70 °C) was selected in order to prevent interconversion of the atropisomers.
Fig. 7.
Chromatograms of sample solution prepared using MeOH at (a) −70 °C, (b) 0 °C, and (c) 20 °C. Mobile phase: MeOH: water (60:40, v/v); column temperature: 6 °C; flow rate: 0.7 mL/min; detection wavelength: 278 nm.
Further experiments were conducted to evaluate the stability of the prepared sample solutions over time at three different sample solution storage temperatures: room temperature (20 °C), 0 °C, and −70 °C. As shown in Fig. 8, significant interconversion is observed when the solution was kept at room temperature for only 30 min. The peak areas of peak 1 and 2 decreased approximately by 31% while the areas of peak 3 and 4 increased by the same amount. When the solution was kept at 0 °C, the interconversion rate is much lower. After 1 h, the peak areas of 1 and 2 decreased approximately ~7.5%, while the peak areas of peak 3 and 4 increased proportionately. The interconversion between the atropisomers could be controlled for up to 4 h by reducing the storage temperature to −70 °C. Therefore, once prepared, all sample solutions were stored at −70 °C to enable accurate determination of the true atropisomer composition of a sample.
Fig. 8.
Chromatograms of sample solutions prepared in cooled MeOH (−70 °C), stored at different temperatures: (A) 20 °C, (B) 0 °C, and (C) −70 °C. Mobile phase: MeOH: water (60:40, v/v); column temperature: 6 °C; flow rate: 0.7 mL/min; detection wavelength: 278 nm.
3.6. Thermodynamic analysis
Solute retention is usually expressed in terms of the retention factor, k′, by the van’t Hoff equation, (Eq. (1)), where Δ H and Δ S are the enthalpy change and entropy change, respectively, when the analyte transfers from the mobile phase to the stationary phase. T is the temperature, in absolute value, R is the universal gas constant and φ is the phase ratio of the column. If the enthalpy and entropy changes are constant with temperature change, a linear van’t Hoff plot (ln k′ vs. 1/T) can be obtained. The slope and intercept are −ΔH/R and ΔS/R+lnφ, respectively.
| (1) |
For chiral separations, where enantioselectivity, α is expressed as k2/k1, where subscripts 2 and 1 represent the more and less retained enantiomers, the van’t Hoff relation can be also described as Eq. (2):
| (2) |
where Δ (ΔH) is the diffference in enthalpy changes between the two enantiomers (ΔH2−ΔH1), Δ (ΔS) is the diffference in entropy changes between the two enatiomers (ΔS2−ΔS1). For a linear plot of lnα versus 1/T, the slope and intercept are Δ (ΔH)/R and Δ (ΔS)/R, respectively.
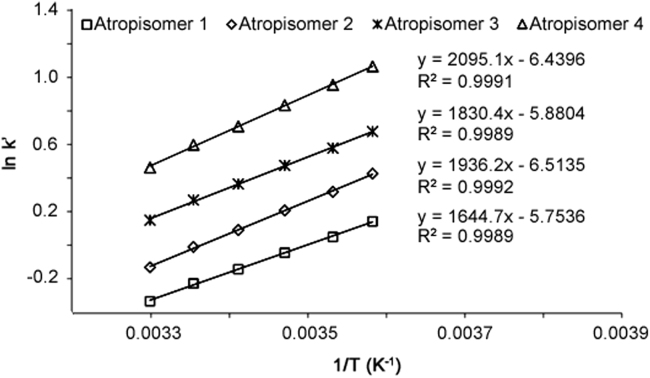
The retention behavior of compound I was determined over the temperature range 6–30 °C at approximately 5 °C intervals. The retention time of each atropisomer was obtained in duplicate. Plots of ln k′ versus 1/T were obtained for all the four atropisomers with linear correlation coefficients (R2) of 0.9989 and above as shown in Fig. 9. As noted previously, the retention times decreased with increasing column temperature. The Δ H value for each atropisomer obtained from the slope of van’t Hoff plot is listed in Table 1. The absolute Δ H values were in the range of 13.7–17.4 kJ/mol and the absolute Δ (ΔH) range from 2.20 kJ/mol to 2.42 kJ/mol. These values are consistent with the values reported by R.M. Woods et. al. [15] which describes the enantiomeric separation of a variety of biaryl atropisomers. The analyte/CSP interactions are common for all four atropisomers, while the difference in the interaction reflected by Δ (ΔH) is sufficient to provide reasonable separation of the atropisomers.
Fig. 9.
van’t Hoff plot for the four atropisomers of compound I.
Table 1.
Thermodynamic parameters for atropisomers.
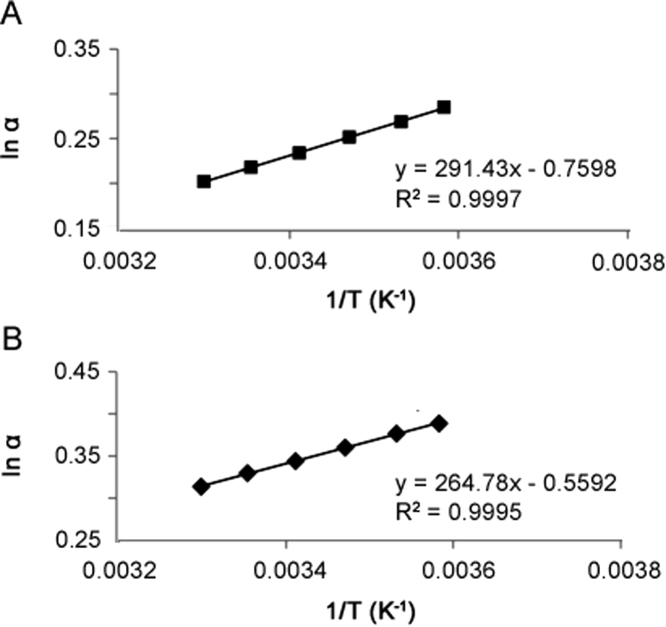
As stated above, Form A consists of atropisomers 1 and 2 as a pair of enantiomers (RS/SR) and Form B consists of atropisomers 3 and 4 as a pair of enantiomers (SS/RR). Using the retention factors data, the apparent enantioselectivity (α) was determined for the two pair of enantiomers (Table 2), respectively. The ln α versus 1/T plots were obtained for the two pair of enantiomers as shown in Fig. 10. Both pairs of enatiomers show a linear relationship with a correlation coefficient R2 higher than 0.999. This indicates that the separation mechanism remains unchanged for the four atropisomers during the temperature range studied. The negative enthalpic terms obtained for all four atropisomers indicate that the association process between each atropisomer and the stationary phase is an enthalpically favored process. Additionally, the changes in enthalpies provide excellent selectivity of the enantiomer separation throughout the temperature range studied as noted by the resolution, Rs ranging from 1.43 to 2.60. In addition, as illustrated in Table 2, the resolutions and enantioselectivities between each pair of enantiomers increases with decreasing temperature which is typical for an enthalpy driven separation. This indicates that the more retained enantiomer experiences a stronger enantioselective interaction with the CSP. He et al. [31] reported similar improvement of chiral separations by decreasing the column temperature for enantiomers, oxzepam and lorazepam on Astec Cyclobond I 2000 RSP column.
Table 2.
Column temperature effect on selectivity (α) and resolution (Rs) for the two pair of enantiomers of compound I.
| Temperature (ºC) |
Atropisomers 1 and 2 |
Atropisomers 3 and 4 |
||
|---|---|---|---|---|
| α | Rs | α | Rs | |
| 6 | 1.33 | 1.89 | 1.48 | 2.60 |
| 10 | 1.31 | 1.87 | 1.46 | 2.60 |
| 15 | 1.29 | 1.80 | 1.43 | 2.59 |
| 20 | 1.26 | 1.70 | 1.41 | 2.57 |
| 25 | 1.24 | 1.60 | 1.39 | 2.49 |
| 30 | 1.22 | 1.43 | 1.37 | 2.36 |
Fig. 10.
Plot of lnα Vs. 1/T for two pair of enantiomers of compound I. (A): Atropisomer 1 and 2, (B): Atropisomer 3 and 4.
4. Conclusion
The analysis of atropisomers is of considerable interest from both scientific and regulatory perspective. However, achieving reliable quantification of atropisomers using HPLC can be very challenging due to the inter-conversion of the isomers during the analysis process. Four atropisomers of Compound I were successfully separated using HPLC by employing a secondary hydroxyl derivatized β-Cyclodextrin (CD) bonded stationary phase. All aspects of the analysis must be controlled to ensure confidence in the quantitation of the atropisomer ratios due to rapid interconversion between the four atropisomers. Chromatographic peak coalescence or peak-plateau-peak phenomenon has been observed when atropisomer interconversion occurs under non-optimal analysis conditions. Low temperature conditions were used for sample preparation, sample storage, and chromatographic separation to successfully control the interconversion and enable accurate determination of the atropisomer ratios for Compound I.
The thermodynamic analysis of the retention data shows that the enantioseparation of the two pairs of enantiomers is primarily enthalpy controlled. The differences in enthalpies provide excellent selectivity of the enantiomer separation within the temperature range studied.
Acknowledgements
The authors would like to thank Michael Galella of Bristol-Myers Squibb Co. for providing the single crystal x-ray analysis, and Lingfeng He, Yingru Zhang, and Jun Dai of Bristol-Myers Squibb Co. for helpful discussions during the HPLC method development.
Footnotes
Peer review under responsibility of Xi'an Jiaotong University.
References
- 1.Smith S.W. Chiral toxicology: it is the same thing … only different. Toxicol. Sci. 2009;110:4–30. doi: 10.1093/toxsci/kfp097. [DOI] [PubMed] [Google Scholar]
- 2.Yaku K., Aoe K., Nishimura N. Chiral resolution of four optical isomers of diltiazem hydrochloride on Chiralcel columns by packed-column supercritical fluid chromatography. J. Chromatogr. A. 1997;785:185–193. [Google Scholar]
- 3.Patel M.A., Riley F., Ashraf-Khorassani M. Supercritical fluid chromatographic resolution of water soluble isomeric carboxyl/amine terminated peptides facilitated via mobile phase water and ion pair formation. J. Chromatogr. A. 2012;1233:85–90. doi: 10.1016/j.chroma.2012.02.024. [DOI] [PubMed] [Google Scholar]
- 4.Loukotková L., Tesařová E., Bosáková Z. Comparison of HPLC enantioseparation of substituted binaphthyls on CD-, polysaccharide- and synthetic polymer-based chiral stationary phases. J. Sep. Sci. 2010;33:1244–1254. doi: 10.1002/jssc.200900796. [DOI] [PubMed] [Google Scholar]
- 5.Schurig V. Separation of enantiomers by gas chromatography. J. Chromatogr. A. 2001;906:275–299. doi: 10.1016/s0021-9673(00)00505-7. [DOI] [PubMed] [Google Scholar]
- 6.Preinerstorfer B., Laemmerhofer M., Lindner W. Advances in enantioselective separations using electromigration capillary techniques. Electrophoresis. 2009;30:100–132. doi: 10.1002/elps.200800607. [DOI] [PubMed] [Google Scholar]
- 7.Ward T.J., Ward K.D. Chiral separations: a review of current topics and trends. Anal. Chem. 2012;84:626–635. doi: 10.1021/ac202892w. [DOI] [PubMed] [Google Scholar]
- 8.Jozwiak K., Lough W.J., Wainer I.W., editors. Drug Stereochemistry: Analytical Methods And Pharmacology. Third edition. CRC Press; 2012. [Google Scholar]
- 9.Subramanian G., editor. Chiral Separation Techniques: A Practical Approach. Third edition. Wiley-VCH; 2007. [Google Scholar]
- 10.Ahaja S., editor. Chiral Separation Methods For Pharmaceutical And Biotechnological Products. Wiley; 2010. [Google Scholar]
- 11.Ilisz I., Pataja Z., Aranyi A. Macrocyclic antibiotic selectors in direct HPLC enantioseparations. J. Sep. Purif. Rev. 2012;41:207–249. [Google Scholar]
- 12.Chankvetadze B. Recent developments on polysaccharide-based chiral stationary phases for liquid-phase separation of enantiomers. J. Chromatogr. A. 2012;1269:26–51. doi: 10.1016/j.chroma.2012.10.033. [DOI] [PubMed] [Google Scholar]
- 13.Shen J., Okamoto Y. Efficient separation of enantiomers using stereoregular chiral polymers. Chem. Rev. 2016;116:1094–1138. doi: 10.1021/acs.chemrev.5b00317. [DOI] [PubMed] [Google Scholar]
- 14.Dai Y., Wang S., Tang W. Cyclodextrin-based chiral stationary phases for high-performance liquid chromatography, ch 3. In: Tang W., Ng S.-C., Sun D., editors. Modified Cyclodextrins For Chiral Separation. Springer-Verlag Berlin Heidelberg; 2013. pp. 67–101. [Google Scholar]
- 15.Woods R.M., Patel D.C., Lim Y. Enantiomeric separation of biaryl atropisomers using cyclofructan based chiral stationary phases. J. Chromatogr. A. 2014;1357:172–181. doi: 10.1016/j.chroma.2014.04.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Darrouzain F., Matoga M., Cavalli E. The hermodynamic approach for studying both the retention and complexation mechanisms with hydroxy-propyl-β-cyclodextrin of a phenoxy-propionic acid herbicide series. Talanta. 2004;64:836–843. doi: 10.1016/j.talanta.2004.02.042. [DOI] [PubMed] [Google Scholar]
- 17.Slama I., Jourdan E., Villet A. Temperature and solute molecular size effects on the retention and enantioselectivity of a series of d, l dansyl amino acids on a vancomycin-based chiral stationary phase. Chromatographia. 2003;58:399–404. [Google Scholar]
- 18.Ilisz I., Grecsó N., Palkó M. Structural and temperature effects on enantiomer separations of bicyclo[2.2.2]octane-based 3-amino-2-carboxylic acids on cinchona alkaloid-based zwitterionic chiral stationary phases. J. Pharm. Biomed. Anal. 2014;98:130–139. doi: 10.1016/j.jpba.2014.05.012. [DOI] [PubMed] [Google Scholar]
- 19.Peluso P., Fabbri D., Dettori M.A. High-performance liquid chromatographic enantioseparation of atropisomeric biphenyls on seven chiral stationary phases. Curr. Org. Chem. 2011;15:1208–1229. [Google Scholar]
- 20.Yan T.Q., Riley F., Philippe L. Chromatographic resolution of atropisomers for toxicity and biotransformation studies in pharmaceutical research. J. Chromatogr. A. 2015;1398:108–120. doi: 10.1016/j.chroma.2015.04.023. [DOI] [PubMed] [Google Scholar]
- 21.LaPlante S.R., Fader L.D., Fandrick K.R. Assessing atropisomer axial chirality in drug discovery and development. J. Med. Chem. 2011;54:7005–7022. doi: 10.1021/jm200584g. [DOI] [PubMed] [Google Scholar]
- 22.Shi Y., Huang M., Macor J.E. Characterization of the in vitro atropisomeric interconversion rates of an endothelin A antagonist by enantioselective liquid chromatography. J. Chromatogr. A. 2005;1078:67–73. doi: 10.1016/j.chroma.2005.04.091. [DOI] [PubMed] [Google Scholar]
- 23.Bu X., Skrdla P.J., Dormer P.G. Separation of triphenyl atropisomers of a pharmaceutical compound on a novel mixed mode stationary phase: a case study involving dynamic chromatography, dynamic NMR and molecular modeling. J. Chromatogr. A. 2010;1217:7255–7264. doi: 10.1016/j.chroma.2010.09.042. [DOI] [PubMed] [Google Scholar]
- 24.Jayaraman K., Alexander A.J., Hu Y. A stepwise strategy employing automated screening and DryLab modeling for the development of robust methods for challenging high performance liquid chromatography separations: a case study. Anal. Chim. Acta. 2011;696:116–124. doi: 10.1016/j.aca.2011.04.010. [DOI] [PubMed] [Google Scholar]
- 25.Hoang T.H., Cuerrier D., McClintock S. Computer-assisted method development and optimization in high-performance liquid chromatography. J. Chromatogr. A. 2003;991:281–287. doi: 10.1016/s0021-9673(03)00206-1. [DOI] [PubMed] [Google Scholar]
- 26.Lämmerhofer M., Di Eugenio P., Molnar I. Computerized optimization of the high-performance liquid chromatographic enantioseparation of a mixture of 4-dinitrophenyl amino acids on a quinine carbamate-type chiral stationary phase using DRYLAB. J. Chromatogr. B. 1997;689:123–135. doi: 10.1016/s0378-4347(96)00366-0. [DOI] [PubMed] [Google Scholar]
- 27.Oki M. Wiley Interscience; 1983. Topics in Stereochemistry Vol. 1 Atropisomerism. [Google Scholar]
- 28.Barrett K.T., Metrano A.J., Rablen P.R. Spontaneous transfer of chirality in an atropisomerically enriched two-axis system. Nature. 2014;509:71–75. doi: 10.1038/nature13189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Del Valle E.M.M. Cyclodextrins and their uses: a review. Process Biochem. 2004;39:1033–1046. [Google Scholar]
- 30.T.E. Beesley, Updates in the technology and application of chiral stationary phases, LCGC LC column technology supplement, 2006, 28-31.
- 31.He H., Liu Y., Sun C. Effect of temperature on enantiomer separation of oxzepam and lorazepam by high-performance liquid chromatography on a beta-cyclodextrin derivatized bonded chiral stationary phase. J. Chromatogr. Sci. 2004;42:62–66. doi: 10.1093/chromsci/42.2.62. [DOI] [PubMed] [Google Scholar]