Abstract
A selective, sensitive and precise assay based on solid phase extraction and liquid chromatography–tandem mass spectrometry (LC–MS/MS) was developed for the simultaneous determination of amiloride (AMI) and hydrochlorothiazide (HCTZ) in human plasma. Sample clean-up with 250 µL of plasma was done on Phenomenex Strata™-X extraction cartridges using their labeled internal standards (AMI-15N3 and HCTZ-13C,d2). Chromatography was performed on Hypersil Gold C18 (50 mm×3.0 mm, 5 µm) column using acetonitrile with 4.0 mM ammonium formate (pH 4.0, adjusted with 0.1% formic acid) (80:20, v/v) as the mobile phase. Detection was carried out on a triple quadrupole API 5500 mass spectrometer utilizing an electrospray ionization interface and operating in the positive ionization mode for AMI and negative ionization mode for HCTZ. Multiple reaction monitoring was used following the transitions at m/z 230.6/116.0, m/z 233.6/116.0, m/z 296.0/204.9 and m/z 299.0/205.9 for AMI, AMI-15N3, HCTZ and HCTZ-13C,d2, respectively. Calibration curves were linear (r2≥0.9997) over the concentration range of 0.050–50.0 and 0.50–500 ng/mL for AMI and HCTZ, respectively, with acceptable accuracy and precision. The signal-to-noise ratio at the limit of quantitation was ≥14 for both the analytes. The mean recovery of AMI and HCTZ from plasma was 89.0% and 98.7%, respectively. The IS-normalized matrix factors determined for matrix effect ranged from 0.971 to 1.024 for both the analytes. The validated LC–MS/MS method was successfully applied to a bioequivalence study using 5 mg AMI and 50 mg HCTZ fixed dose tablet formulation in 18 healthy Indian volunteers with good reproducibility.
Keywords: Amiloride, Hydrochlorothiazide, LC–MS/MS, Solid phase extraction, Human plasma
1. Introduction
Combination therapy has now become a standard line of treatment for hypertension to reduce the burden of cardiovascular morbidity and mortality [1]. Diuretics, which are used to regulate the excretion of water and salts, are an integral part of fixed-dose combination (FDC) therapy. FDC therapy offers several advantages over monotherapy such as superior antihypertensive efficacy, lower dose strength and fewer side effects. Drugs in FDC generally include a diuretic along with an angiotensin converting enzyme inhibitor, β-receptor or an angiotensin receptor blocker. Additionally, fixed combination of two diuretics is also recognized as an effective option [2], [3]. Amiloride (AMI) and hydrochlorothiazide (HCTZ) are one such combination of diuretic drugs that reduces the amount of water in the body by increasing the flow of urine and thus helps lower blood pressure. AMI is a potassium-conserving drug that possesses weak diuretic and antihypertensive activity compared to thiazide diuretics. It promotes excretion of sodium and chloride, but reduces the excretion of potassium. HCTZ acts by inhibiting the renal tubular reabsorption of sodium and chloride and is principally responsible for the antihypertensive activity of this drug combination. Both the drugs begin to act in about 2 h and their peak plasma concentration is reached in about 3–4 h. The plasma half life of AMI and HCTZ varies from 3 to 9 h and 5.6–14.8 h, respectively [4].
Several methods have been reported to determine AMI as a single analyte in biological samples; however, most of them are very old (more than two decades) and were developed mainly using high performance liquid chromatography (HPLC). While very few recent methods are available for determination of AMI in human plasma [5], [6], detection or screening of AMI along with other diuretics in human urine especially for doping control has been discussed in several reports [7], [8], [9]. On the other hand, a number of methods have been described for the estimation of HCTZ either alone [10], [11], [12] or with other antihypertensive drugs in biological fluids [13], [14], [15], [16], [17], [18], [19], [20]. The simultaneous determination of AMI and HCTZ in human plasma is also a subject of some reports [21], [22], [23]. The first method on their simultaneous analysis was reported by Van der Meer and Brown [21], using a reversed-phase HPLC method. The other two methods employed liquid chromatography–tandem mass spectrometry (LC–MS/MS) using positive/negative ion-switching electrospray ionization [22], [23]. The chromatographic analysis time in both the methods was ≥3.5 min, and the lower limit of quantitation (LLOQ) achieved for AMI was identical (0.1 ng/mL) using both the methods, while the sensitivity of HCTZ was 1.0 ng/mL [22] and 5.0 ng/mL [23], respectively. A comparative summary of different features of these methods is summarized in Table 1.
Table 1.
Comparative summary of chromatographic methods developed for simultaneous determination of amiloride and hydrochlorothiazide.
| Sr. No. | Detection technique; Linear range (ng/mL) | Extraction; Internal standard; Plasma volume | Column; Elution mode and mobile phase; Retention time; Run time | Application | Ref. |
|---|---|---|---|---|---|
| 1 | RP–HPLC; | LLE with ethyl acetate; hydroflumethiazide; 1000 µL | Spherisorb ODS II (125 mm×4.6 mm, 5 µm); Isocratic, acetonitrile: methanol: tetra ethyl ammonium phosphate buffer (pH 2.8) (10:9:100,v/v); AMI-3.67 min and HCTZ- 4.80 min; 10 min | Pharmacokinetic study with 10 healthy volunteers after oral administration of 10/100 mg of AMI/HCTZ tablets | [21] |
| 0–20 for AMI,0–500 for HCTZ | |||||
| 2 | LC–MS/MS; | PP with acetonitrile; rizatriptan; 250 µL | Curosil-PFP (250 mm×4.6 mm, 5 µm); Gradient, 0.15% formic acid in water containing 0.23% ammonium acetate and methanol; AMI-4.63 min and HCTZ- 4.36 min; 6.50 min | Pharmacokinetic study with 18 healthy Chinese subjects after oral administration of 5/50 mg of AMI/HCTZ tablets | [22] |
| 0.1–80 for AMI, 1.0–800 for HCTZ | |||||
| 3 | LC-MS/MS; | SPE on Lichrosep DVB-HL; triamterine and hydrochlorothiazide | Hypurity Advance (100 mm×4.6 mm, 5 µm); Isocratic, 2.0 mM ammonium acetate, pH 3.0 and acetonitrile (30:70, v/v); AMI-1.49 min, HCTZ-2.68 min; 3.50 min | – | [23] |
| 0.1–10 for AMI | |||||
| 5.0–500 for HCTZ | |||||
| 13C, d2; 300 µL | |||||
| 4 | LC-MS/MS; | SPE on Phenomenex Strata™-X; AMI−15N3 and HCTZ-13C, d2; | Hypersil Gold C18 (50 mm×3.0 mm, 5 µm); Isocratic, acetonitrile and 4.0 mM ammonium formate, pH 4.0 (80:20, v/v); AMI-1.28 and HCTZ- 1.72 min; 2.50 min | Bioequivalence study with 18 healthy Indian subjects after oral administration of 5/50 mg of AMI/HCTZ tablets | Present method |
| 0.05–50 for AMI 0.5–500 for HCTZ | |||||
| 250 µL | |||||
AMI: amiloride; HCTZ: hydrochlorothiazide; LLE: liquid-liquid extraction; PP: Protein precipitation; SPE: solid phase extraction
However, due to the presence of very low levels of AMI in human plasma after oral administration of therapeutic doses (5–10 mg), it is essential to develop a rapid and sensitive, yet reliable and rugged method for their simultaneous determination in subject samples. Moreover, to the best of our knowledge, there are no reports on the pharmacokinetics of this drug combination in Indian subjects. Thus, the aim of the present work was to develop and validate a sensitive, selective and rapid LC–MS/MS method for the simultaneous analysis of AMI and HCTZ in human plasma. The salient points of the developed method include shorter chromatographic analysis time, low sample volume, higher sensitivity and use of labeled internal standards (ISs). The method was successfully applied to a bioequivalence study of 5/50 mg AMI/HCTZ FDC formulation in healthy Indian subjects.
2. Experimental
2.1. Chemicals and materials
Reference standards of amiloride hydrochloride (AMI, 99.47%), hydrochlorothiazide (HCTZ, 99.88%) and labeled internal standards (ISs), amiloride-15N3 hydrochloride (AMI-15N3 98.54%) and hydrochlorothiazide-13C,d2 (HCTZ-13C,d2, 99.29%) were obtained from Clearsynth Labs Pvt. Ltd. (Mumbai, India). HPLC grade acetonitrile was procured from Mallinckrodt Baker, S.A.de C.V. (Estado de Mexico, Mexico). Ammonium formate and formic acid were purchased from Sigma-Aldrich (St. Louis, MO, USA). Solid phase extraction (SPE) cartridges, Phenomenex Strata™-X (30 mg, 1 mL), were obtained from Phenomenex India (Hyderabad, India). Water used in the study was prepared from Milli-Q water purification system from Millipore (Bangalore, India). Blank human plasma in K3EDTA was obtained from Supratech Micropath (Ahmedabad, India) and was stored at −20 °C until use.
2.2. Liquid chromatography and mass spectrometry conditions
Chromatographic analysis was carried out on a Shimadzu HPLC system (Kyoto, Japan) equipped with a binary LC-20 CE Prominence pump, autosampler (SIL-HTc), a solvent degasser (DGU-20A3 prominence) and a temperature-controlled compartment for column (CTO-10ASVP). AMI and HCTZ were separated on Hypersil Gold C18 (50 mm×3.0 mm, 5 µm) column from Thermo Scientific (Cheshire, UK), using acetonitrile and 4.0 mM ammonium formate (pH 4.0 adjusted with 0.1% formic acid) (80:20, v/v) as the mobile phase. The mobile phase flow rate, autosampler temperature and typical pressure of the system were maintained at 0.700 mL/min, 5 °C and 1400 psi, respectively. A triple quadrupole mass spectrometer MDS SCIEX API-5500 (Toronto, Canada) equipped with electro-spray ionization (ESI) and operated in the positive ionization mode for AMI and negative ionization mode for HCTZ was used for quantitative studies. The source parameters like ion spray voltage, curtain gas, Gas1, Gas2, turbo heater temperature and collision activation dissociation were optimized at 2500/−4500 V, 25/25 psi, 35/35 psi, 70/75 psi, 500/500 °C, and 6/6 for AMI/HCTZ, respectively. The optimized compound dependent mass parameters and multiple reaction monitoring (MRM) transitions for the analytes and ISs are summarized in Table 2. Quadrupole 1 and 2 were set at unit mass resolution. Analyst classic software version 1.5.2 was used to control all parameters of LC and MS/MS.
Table 2.
Optimized compound dependent mass parameters for amiloride, hydrochlorothiazide and their labeled internal standards.
| Analytes and their labeled internal standards | Q1 mass (amu) | Q3 mass (amu) | Dwell time (ms) | Declustering potential (V) | Entrance potential (V) | Collision energy (eV) | Collision cell exit potential (V) |
|---|---|---|---|---|---|---|---|
| Amiloride | 230.6 | 116.0 | 300 | 55.0 | 10.00 | 43.0 | 11.0 |
| Amiloride-15N3 | 233.6 | 116.0 | 300 | 55.0 | 10.00 | 43.0 | 11.0 |
| Hydrochlorothiazide | 296.0 | 204.9 | 300 | −90.0 | −10.00 | −33.0 | −15.0 |
| Hydrochlorothiazide-13C,d2 | 299.0 | 205.9 | 300 | −90.0 | −10.00 | −33.0 | −15.0 |
2.3. Calibrators and quality control samples
The standard stock solutions of AMI and HCTZ (100 µg/mL) were prepared by dissolving requisite amounts of their salts in methanol. Intermediate solutions of AMI and HCTZ (5.0 µg/mL) were prepared in methanol: water (50:50, v/v) respectively. The calibration standards (CSs) for AMI were prepared at 0.05, 0.10, 0.25, 0.50, 1.00, 2.50, 5.00, 10.0, 25.0, and 50.0 ng/mL and at 0.50, 1.00, 2.50, 5.00, 10.0, 25.0, 50.0, 100.0, 250.0 and 500.0 ng/mL for HCTZ. The concentration of quality control samples (QCs) was 40.0/400.0 ng/mL (HQC, high quality control), 20.0/200.0 and 1.50/15.0 ng/mL (MQC-1/2, medium quality control), 0.150/1.50 ng/mL (LQC, low quality control) and 0.05/0.50 ng/mL (LLOQ QC, lower limit of quantification quality control) for AMI/HCTZ, respectively. Separate stock solutions (100 µg/mL) of the internal standards were prepared by dissolving 1.0 mg of reference standards in 10.0 mL of methanol. Their combined working solution (100 ng/mL for AMI-15N3 hydrochloride and HCTZ-13C,d2 respectively) was prepared from their stock solutions in methanol:water (50:50, v/v). The stock solutions were stored at 5 °C, while CSs and QCs were stored at –70 °C until use.
2.4. Sample preparation
Prior to extraction, all frozen subject samples, CSs and QCs were thawed and allowed to equilibrate at room temperature. To an aliquot of 250 µL spiked plasma/subject sample, 25 µL of mixed IS solution was added and vortexed for 30 s. Further, 100 µL of 10 mM ammonium formate in water was added and vortex mixed for another 30 s. The samples were then centrifuged at 14,000g for 5 min at 10 °C. Prior to loading, SPE cartridges were conditioned by passing 1.0 mL of methanol followed by 1.0 mL of 10 mM ammonium formate solution. After draining the plasma matrix by applying positive nitrogen pressure, the cartridges were washed with 1.0 mL of 10 mM ammonium formate in water, followed by 1.0 mL of 10% (v/v) methanol in water. The cartridges were dried for 1.0 min and the samples were eluted with 500 µL of acetonitrile and 4.0 mM ammonium formate, pH 4.0 (80:20, v/v) mixture into pre-labeled vials. After brief vortexing, 5.0 µL of the eluant was used for injection in the chromatographic system.
2.5. Method validation procedures
The protocols followed for method validation were as per the USFDA guidance [24] and are similar to those of our previous work [25]. System suitability experiment was performed by injecting six consecutive injections using aqueous standard mixture of the analytes and ISs. The precision (% CV) of system suitability test was observed in the range of 0.49%–1.12% for the retention time and 0.29%–1.92% for the area response for both the analytes and ISs. The signal-to-noise ratio for system performance was ≥14 for AMI and HCTZ. Carryover assessment was carried out through the following sequence of injections, extracted blank plasma→ULOQ sample→two extracted blank plasma samples→LLOQ sample→extracted blank plasma.
Selectivity of the method was assessed for potential matrix interferences in eight different sources (5 K3EDTA, 1 heparinized, 1 haemolysed and 1 lipemic) of blank human plasma by extraction and inspection of the resulting chromatograms for interfering peaks. This was also checked for commonly used medications like paracetamol, ranitidine, diclofenac, caffeine, acetylsalicylic acid and ibuprofen by human volunteers.
Five calibration lines containing ten non-zero concentrations were used to determine linearity. A linear, 1/x2, least-squares regression algorithm was used to plot the peak area ratio (analyte/IS) from multiple reaction monitoring versus concentration. The linear equations were then used to calculate the predicted concentrations in all samples within the analytical runs. Re-injection reproducibility for extracted samples was also checked by reinjection of an entire analytical run after storage at 5 °C.
Intra-day accuracy and precision were evaluated by replicate analysis of plasma samples on the same day. The analytical run consisted of a calibration curve and six replicates of LLOQ QC, LQC, MQC-2, MQC-1 and HQC samples. The inter-day accuracy and precision were assessed by analysis of five precision and accuracy batches on three different days.
Extraction recovery of the analytes and ISs from human plasma was evaluated in six replicates by comparing the mean peak area responses of pre-extraction fortified samples to those of post-extraction fortified samples. Absolute matrix effect (expressed as matrix factors) was assessed by comparing the mean area responses of post-extraction fortified samples to those of neat samples prepared in elution solution. The relative matrix effect was determined by assessment of precision (% CV) values for slopes of calibration lines from eight different plasma lots. Ion suppression/enhancement effects on the MRM LC–MS/MS sensitivity were evaluated by post column analyte infusion experiment. Briefly, a standard solution containing AMI, AMI-15N3, HCTZ and HCTZ-13C,d2 (at ULOQ level) was infused post column into the mobile phase at 5 µL/min employing infusion pump. Aliquots of 5 µL of extracted control blank plasma sample were then injected into the column and chromatograms were acquired for the analytes and ISs.
Stock solutions of the analytes and ISs were checked for short-term stability at room temperature (25 °C) and long-term stability at 5 °C. Stability results were evaluated by measuring the area response (analyte/IS) of stability samples against freshly prepared comparison samples with identical concentration. Bench-top stability at room temperature, freeze-thaw stability at –20 °C and –70 °C, auto-sampler stability (wet extract) at 5 °C, processed sample stability at 25 °C and long-term stability at –20 °C and –70 °C were performed at LLOQ QC and HQC concentration level for both the analytes using six replicates. The area ratio response (analyte/IS) of stability samples was compared against freshly prepared comparison standards with identical concentration.
To prove method ruggedness for determination of the analytes, two batches were studied for accuracy (%) and precision (% CV). The first batch was evaluated on two Hypersil Gold C18 (50 mm×3.0 mm, 5 µm) columns with different batch numbers, while the second batch was analyzed by two different analysts. The dilution integrity experiment was performed at twice the ULOQ concentration (100 and 1000 ng/mL for AMI and HCTZ). Six replicates samples of 1/5th and 1/10th concentration were prepared and their concentrations were calculated by applying the dilution factor of 5 and 10 respectively against the freshly prepared calibration curve standards.
2.6. Application of the method for bioequivalence study and incurred sample reanalysis (ISR)
The design for bioequivalence study was an open label, balanced, randomized, two-treatment, two-period, two-sequence, single dose, crossover study in 18 healthy adult Indian subjects under fasting condition. All the subjects were informed of the aim and risks involved in the study and written consent was obtained. An independent ethics committee approved the study protocol. Health check-up for all subjects was done with general physical examination, ECG and laboratory tests like hematology, biochemistry and urine examination. The study was conducted in accordance with International Conference on Harmonization, E6 Good Clinical Practice guidelines [26]. The subjects were orally administered a single dose of test/reference formulation with 240 mL of water after a wash-out period of 7 days. Blood samples were collected in vacutainers containing K3EDTA at 0.00 (pre-dose), 0.25, 0.50, 0.75, 1.00, 1.25, 1.50, 1.75, 2.00, 2.50, 3.00, 3.50, 4.00, 4.50, 5.00, 5.50, 6.00, 8.00, 10.0, 12.0, 16.0, 24.0, 36.0, 48.0, 72.0 and 96.0 h of administration of drug. Blood samples were centrifuged at 1811g at 4 °C for 15 min; plasma was separated and stored at −70 °C until use. The pharmacokinetic parameters of AMI and HCTZ were estimated by non-compartmental model using WinNonlin software version 5.2.1 (Certara, Princeton, NJ 08540, USA).
The reproducibility of the method was assessed by ISR of 94 samples near the Cmax and the elimination phase in the pharmacokinetic profile of the drugs. The results obtained were compared with the data obtained earlier for the same sample using the same procedure. As per the acceptance criterion at least two-thirds of the original results and repeat results should be within 20% of each other [27].
3. Results and discussion
3.1. Method development
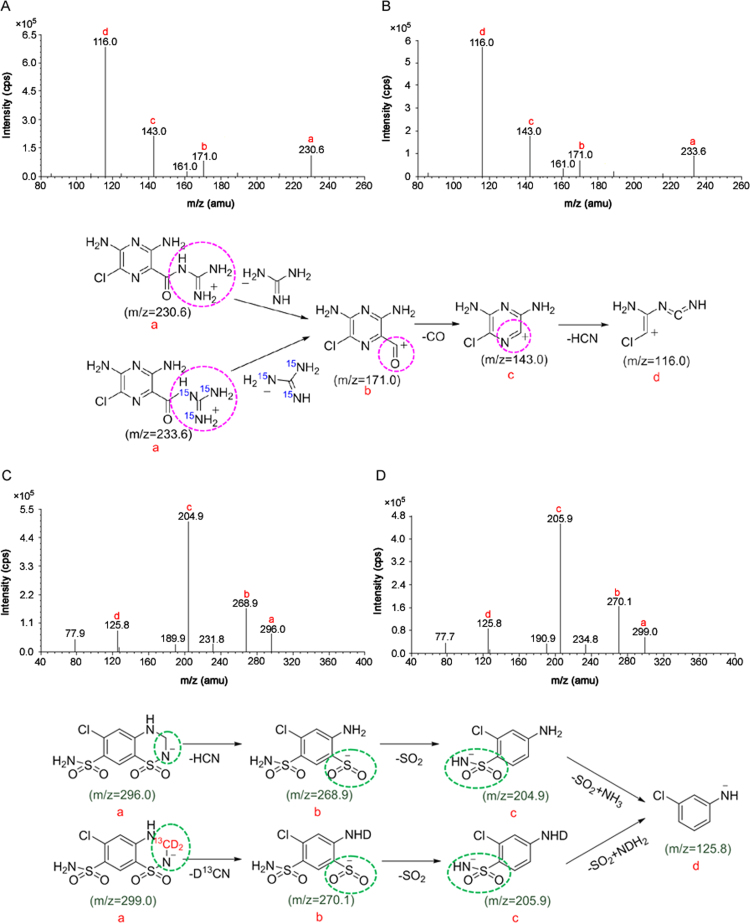
Based on our previous work related to determination of lisinopril and HCTZ in human plasma [20], full scan mass spectra were recorded in the negative as well as positive electrospray ionization mode to maximize response for both the drugs. It was found that the signal intensity for AMI was much higher in the positive mode, while HCTZ gave superior response in the negative mode. The same observation has been reported earlier by Song et al. [22]. The Q1 MS spectra in the positive mode showed predominant protonated molecular ions [M+H]+ at m/z 230.6 and 233.6 for AMI and AMI-15N3, respectively, and abundant deprotonated molecular ions [M-H]- at m/z 296.0 and 299.0 for HCTZ and HCTZ-13C,d2, respectively, in the negative ionization mode. Several fragments were generated by applying collision energy of 43 eV and −33 eV for AMI and HCTZ, respectively, as shown in Fig. 1. The most stable and consistent product ions observed in Q3 MS for AMI, AMI-15N3, HCTZ and HCTZ-13C,d2 were at m/z 116.0 (elimination of guanidine moiety, CO and HCN), 116.0, 204.9 (elimination of HCN and NH3), and 205.9, respectively. For switching of positive and negative scan mode, the inter-channel delay was set at 50 ms, with a dwell time of 300 ms and no cross talk was observed between the MRMs of AMI and AMI-15N3 which have identical product ions. For correct identification of AMI and HCTZ, qualifier transitions of m/z 143.0 and 268.9, respectively were also monitored.
Fig. 1.
Product ion mass spectra of (A) amiloride (m/z 230.6→116.0), (B) amiloride-15N3, IS (m/z 233.6→116.0) in positive ionization mode, (C) hydrochlorothiazide (m/z 296.0→204.9) and (D) hydrochlorothiazide-13C, d2, IS (m/z 299.0 → 205.9) in the negative ionization mode and their proposed fragmentation pathways.
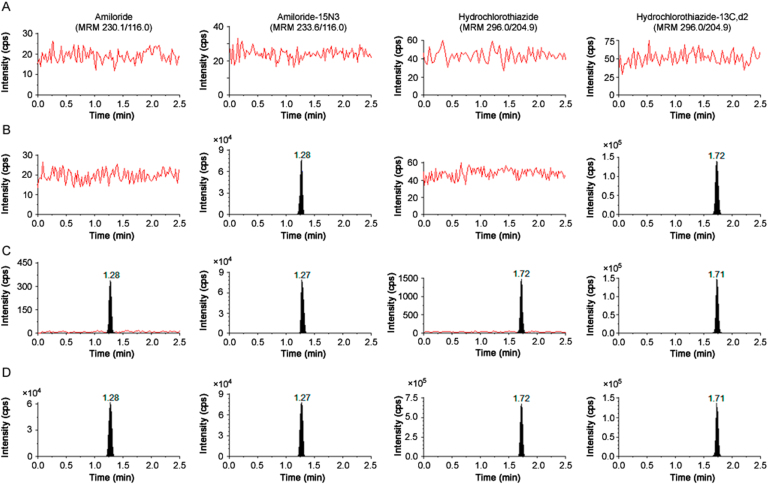
Chromatographic analysis was tested on three different reversed phase columns namely, ACE C18 (50 mm×4.6 mm, 5.0 µm), Gemini C18 (50 mm×4.6 mm, 5.0 µm) and Hypersil Gold C18 (50 mm×3.0 mm, 5 µm). One previous method based on LC–MS/MS used a mixture of formic acid, ammonium acetate and methanol under linear gradient conditions, but this mobile phase could not provide chromatographic separation between the analytes using a Curosil-PFP column. For mobile phase selection, different combinations of acetonitrile/methanol and acidic buffers (ammonium formate/ammonium acetate) in the pH range of 3.0–5.5 were tested to have adequate retention, sufficient response and sharp peak shapes. As observed in our previous work with lisinopril and HCTZ [20], acetonitrile as organic modifier provided higher sensitivity than methanol. Similarly, the signal obtained employing ammonium formate buffer was higher than ammonium acetate buffer for both the drugs. Although baseline separation (Rs≥1.32) was achieved on all three columns, Hypersil Gold C18 provided the desired sensitivity, adequate retention and good peak shape for both the analytes using acetonitrile and 4.0 mM ammonium formate (pH 4.0, adjusted with 0.1% formic acid) (80:20, v/v) within 2.5 min, which was shorter than reported methods (3.5–10 min) [21], [22], [23]. Fig. 2 shows representative chromatograms for double blank plasma, blank plasma spiked with ISs, at LLOQ concentration and subject samples for both the drugs. The retention time for AMI and HCTZ was 1.28 and 1.72, respectively, with a resolution factor of 2.32. The labeled ISs used for both the drugs had similar chromatographic behavior, extraction efficiency and mass spectrometric response. Furthermore, post column analyte infusion chromatograms for qualitative assessment of matrix effect showed no suppression or enhancement in the signal at the retention time of AMI, HCTZ or their ISs (Fig. S1).
Fig. 2.
MRM ion-chromatograms of amiloride, amiloride-15N3, hydrochlorothiazide and hydrochlorothiazide-13C, d2 in (A) double blank plasma, (B) blank plasma spiked with IS, (C) at LLOQ concentration of analyte with IS, and (D) real subject sample at Cmax after oral administration of 5 mg amiloride +50 mg hydrochlorothiazide tablet formulation.
Both AMI (pKa 8.7) [22] and HCTZ (pKa 7.9 and 9.2) [18] are basic drugs and their extraction from human plasma has been reported using all three conventional extraction techniques, namely, protein precipitation (PP) [22], liquid-liquid extraction (LLE) [21] and SPE [23]. As reported by Jangid et al. [23], the mean extraction recovery for AMI and HCTZ obtained using SPE was in the range of 39.8%–42.3% and 77.1%–86.4%, respectively. However, Song et al. [22] reported quantitative recoveries in the range of 82.2%–98.9% for both the drugs using PP with acetonitrile. Nevertheless, based on our previous work with antihypertensive drug combinations [18], [20], SPE was used in the present work to obtain clear extracts with less interference and thus obtain higher sensitivity (AMI: 0.05 ng/mL and HCTZ: 0.50 ng/mL) than the existing methods [21], [22], [23]. Correct choice of solvent for sample washing was essential for removing all endogenous matrix components and was successfully achieved using 10 mM ammonium formate and 10% (v/v) methanol. Highly precise and quantitative recovery was obtained for AMI (85.7%–91.2%) and HCTZ (97.6%–99.4%) under the established extraction conditions using 250 µL plasma sample as shown in Table 3. There was minimal interference from the matrix effect as evident from the IS-normalized matrix factors, which ranged from 0.971 to 1.024 for both the drugs (Table 3).
Table 3.
Extraction recovery and matrix factor for amiloride and hydrochlorothiazide.
| Quality control level (ng/mL) | Mean area response (n=6) |
Recovery (%) |
Matrix factor |
|||||
|---|---|---|---|---|---|---|---|---|
| A (post-extraction spiking) | B (pre-extraction spiking) | C (neat samples in mobile phase) | Analyte (B/A) | IS | Analyte (A/C) | IS | IS-normalized (Analyte/IS) | |
| Amiloride | ||||||||
| 40.0 | 1862411 | 2,102,044 | 2,091,586 | 88.6 | 85.9 | 1.005 | 0.993 | 1.012 |
| 20.0 | 934855 | 1,025,060 | 1,040,670 | 91.2 | 92.5 | 0.985 | 0.977 | 1.008 |
| 1.50 | 66251 | 77,306 | 79,126 | 85.7 | 87.8 | 0.977 | 0.986 | 0.991 |
| 0.15 | 6714 | 7435 | 7518 | 90.3 | 88.1 | 0.989 | 1.019 | 0.971 |
| Hydrochlorothiazide | ||||||||
| 400.0 | 7834266 | 7921401 | 7,929,330 | 98.9 | 99.1 | 0.999 | 0.976 | 1.024 |
| 200.0 | 4010865 | 4088547 | 4,138,206 | 98.1 | 97.9 | 0.988 | 1.015 | 0.973 |
| 15.0 | 300581 | 298775 | 303,005 | 99.4 | 99.5 | 0.992 | 0.995 | 0.997 |
| 1.50 | 29466 | 30192 | 31,155 | 97.6 | 98.5 | 0.969 | 0.986 | 0.983 |
IS: internal standard
3.2. Method validation results
The quality of data can be directly affected if material from prior injection contaminates subsequent analyses. Thus, carryover (column and autosampler) experiment was performed by injecting extracted double blank plasma sample following the highest CSs (AMI: 50 ng/mL and HCTZ: 500 ng/mL). The results showed minimal carryover (≤0.25% of LLOQ concentration) for both the drugs.
The calibration curves were created over the validated concentration range of 0.05–50.0 ng/mL and 0.50–500 ng/mL for AMI and HCTZ, respectively by plotting the peak area ratios of the analyte to IS versus the nominal concentration of the analyte. All five curves gave good linearity with the correlation coefficient value, r2≥0.9997 for both the analytes. The typical regression equations calculated for AMI and HCTZ were y=(1.0097±0. 0057) x − (0.03752±0.00267) and y=(1.0041±0.0171) x – (0.59254±0.00028), respectively. The LLOQ values obtained were 0.05 ng/mL for AMI and 0.50 ng/mL for HCTZ at a signal-to-noise ratio of 14 and 24, respectively. The intra-batch and inter-batch precision (% CV) and accuracy for AMI and HCTZ at different QCs are summarized in Table 4. The results showed acceptable accuracy and precision for both the drugs in human plasma.
Table 4.
Intra-batch and inter-batch precision and accuracy for amiloride and hydrochlorothiazide.
| Nominal concentration (ng/mL) | Intra-batch (n=6) |
Inter-batch (n=30; 6 from each batch) |
||||
|---|---|---|---|---|---|---|
| Mean conc. found (ng/mL) | Precision (% CV) | Accuracy (%) | Mean conc. found (ng/mL) | Precision (% CV) | Accuracy (%) | |
| Amiloride | ||||||
| 40.0 | 38.9 | 3.20 | 97.2 | 40.5 | 2.93 | 101.3 |
| 20.0 | 20.6 | 3.94 | 102.7 | 19.7 | 3.79 | 98.3 |
| 1.50 | 1.49 | 2.27 | 99.3 | 1.46 | 3.03 | 97.3 |
| 0.15 | 0.151 | 4.27 | 100.6 | 0.153 | 2.86 | 102.2 |
| 0.05 | 0.049 | 6.24 | 98.5 | 0.050 | 5.72 | 100.7 |
| Hydrochlorothiazide | ||||||
| 400.0 | 402.9 | 0.66 | 100.6 | 404.3 | 1.15 | 101.0 |
| 200.0 | 199.7 | 1.05 | 99.8 | 204.4 | 1.65 | 102.1 |
| 15.0 | 14.73 | 4.63 | 98.2 | 14.81 | 3.52 | 98.7 |
| 1.50 | 1.521 | 1.40 | 101.3 | 1.504 | 0.97 | 100.2 |
| 0.50 | 0.484 | 4.95 | 96.7 | 0.493 | 3.50 | 98.6 |
CV: Coefficient of variation
The selectivity of the method was evaluated in eight different plasma sources. There was no obvious interference of matrix in any of the samples under the optimized chromatographic conditions. Additionally, the relative matrix effect in lipemic and haemolysed plasma samples in addition to normal K3EDTA plasma was also checked. The precision (% CV) in the measurement of slopes of calibration lines prepared in these plasma sources was 2.56 and 2.95 for AMI and HCTZ, respectively (Table S1).
The stability of both the drugs was investigated in stock solutions and in plasma samples. The working and stock solutions of AMI and HCTZ and their ISs kept for short-term stability remained stable at room temperature up to 18 h, and for a minimum period of 60 days under refrigerated conditions (5 °C) for long-term stability. Bench-top stability at 25 °C, processed sample stability at 25 °C and autosampler stability at 5 °C were established up to 16 h, 24 h and 36 h, respectively, without any change in the concentration of the drugs. Both the drugs were stable up to a minimum of six freeze-thaw cycles and for 120 days in human plasma samples stored at −20 °C and −70 °C for long-term stability. The results for different stability tests in plasma are shown in Table 5.
Table 5.
Stability of amiloride and hydrochlorothiazide in plasma under different conditions (n=6).
| Storage conditions | Amiloride |
Hydrochlorothiazide |
||||
|---|---|---|---|---|---|---|
| Nominal conc. (ng/mL) | Conc. found (ng/mL, mean±SD) | Change (%) | Nominal conc. (ng/mL) | Conc. found (ng/mL, mean±SD) | Change (%) | |
| Bench-top stability (25 °C, 16 h) | 40.0 | 39.8±0.42 | −0.45 | 400.0 | 399.5±6.34 | −0.13 |
| 0.05 | 0.049±0.002 | −1.60 | 0.50 | 0.506±0.012 | 1.28 | |
| Freeze-thaw stability (20 °C) | 40.0 | 40.3±0.69 | 0.85 | 400.0 | 392.2±5.40 | −1.95 |
| 0.05 | 0.047±0.003 | −5.60 | 0.50 | 0.468±0.037 | −6.40 | |
| Freeze-thaw stability (−70 °C) | 40.0 | 39.5 ±0.47 | −1.25 | 400.0 | 400.7±6.58 | 0.17 |
| 0.05 | 0.048±0.004 | −4.40 | 0.50 | 0.496±0.009 | −0.84 | |
| Processed sample stability (25 °C, 24 h) | 40.0 | 40.2±1.07 | 0.45 | 400.0 | 409.6±11.40 | 2.41 |
| 0.05 | 0.053±0.005 | 6.80 | 0.50 | 0.486±0.013 | −2.76 | |
| Autosampler stability (5 °C, 36 h) | 40.0 | 40.5±0.30 | 1.20 | 400.0 | 405.4±6.21 | 1.35 |
| 0.05 | 0.051±0.007 | 1.60 | 0.50 | 0.492±0.059 | −1.68 | |
| Long-term stability (−20 °C, 120 days) | 40.0 | 40.8±0.70 | 2.05 | 400.0 | 394.1±7.85 | −1.47 |
| 0.05 | 0.054±0.004 | 7.20 | 0.50 | 0.507±0.024 | 1.36 | |
| Long-term stability (−70 °C, 120 days) | 40.0 | 40.7±0.77 | 1.63 | 400.0 | 405.1±4.52 | 1.28 |
| 0.05 | 0.055±0.003 | 9.60 | 0.50 | 0.488±0.009 | −2.44 | |
SD: Standard deviation.
Dilution integrity test was performed to verify dilution reliability of samples having concentration above ULOQ level. The precision (% CV) values for 5-fold and 10-fold dilution were in the range of 1.4%–4.8% and the accuracy results were within 95.1%–102.5% for AMI and HCTZ. The precision and accuracy for method ruggedness on two different Hypersil Gold C18 columns and with different analysts were within 1.58%–4.66% and 97.1%–103.6%, respectively, for both the drugs.
3.3. Application of the method for bioequivalence study and ISR
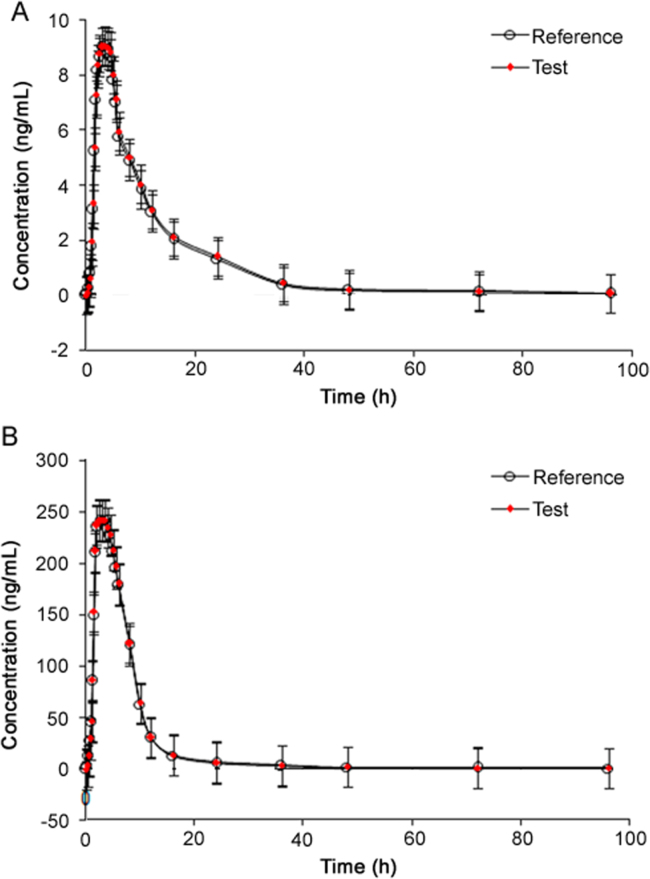
The bioequivalence study was carried out using a single dose of 5 mg AMI and 50 mg HCTZ FDC test formulation (Generic Company, India) and a reference formulation, BIDURET (5 mg AMI +50 mg HCTZ) FDC tablets from GlaxoSmithKline Pharmaceutical Limited, Worli, Mumbai, India. The pharmacokinetic profiles of AMI and HCTZ obtained after oral administration of 5 mg AMI and 50 mg HCTZ FDC tablets to 18 healthy Indian volunteers are shown in Fig. 3. The mean values of pharmacokinetic parameters obtained for test and reference formulations are summarized in Table 6. Comparison of the results obtained with reported methods for this FDC was not possible due to unavailability of pharmacokinetic data in recent reports [22], [23]. Van der Meer and Brown [21] studied the pharmacokinetics of AMI (10 mg) and HCTZ (100 mg) using two oral formulations. The Tmax values obtained in our work were in good agreement with their results, while the Cmax and AUC values were comparable based on dose strength for both the drugs. Nevertheless, the ratios of mean log-transformed parameters and their 90% confidence intervals for Cmax, AUC0–96 h and AUC0-inf were within the acceptance criterion of 80%–125%, which proves that the test and reference formulations were pharmacokinetically equivalent in terms of rate and extent of drug absorption.
Fig. 3.
Mean plasma concentration-time profiles of (A) amiloride and (B) hydrochlorothiazide after oral administration of 5 mg amiloride +50 mg hydrochlorothiazide fixed dose of test and reference formulation to 18 healthy Indian subjects.
Table 6.
Mean pharmacokinetic (±SD) parameters following oral administration of 5 mg amiloride +50 mg hydrochlorothiazide combination tablet formulation to18 healthy Indian subjects under fasting condition.
| Parameter | Amiloride |
Hydrochlorothiazide |
||
|---|---|---|---|---|
| Test | Reference | Test | Reference | |
| Cmax (ng/mL) | 9.09±2.15 | 9.06±1.49 | 242.32±52.11 | 240.79±69.32 |
| Tmax (h) | 3.50±0.40 | 3.40±0.50 | 2.85±0.35 | 3.01±0.20 |
| t1/2 (h) | 9.45±0.45 | 9.05±0.31 | 8.65±0.58 | 9.10±0.78 |
| AUC 0-96 (h·ng/mL) | 109.23±42.49 | 103.45±35.28 | 1905.34±120.17 | 1875.16±143.11 |
| AUC 0-inf (h·ng/mL) | 118.53±31.75 | 111.73±25.92 | 2002.61±148.86 | 1968.92±156.79 |
| Kel (1/h) | 0.073±0.008 | 0.077±0.009 | 0.080±0.021 | 0.076±0.016 |
Cmax: maximum plasma concentration; Tmax: time point of maximum plasma concentration;
t1/2: half-life of drug elimination during the terminal phase; AUC0-96: area under the plasma concentration-time
curve from zero hour to 96 h; AUC0-inf: area under the plasma concentration-time curve from 0 h
to infinity; Kel: elimination rate constant; SD: standard deviation.
Further, ISR results showed a % change of ±15% from the initial analysis, which are within the acceptance criteria of ±20% (Fig. S2).
4. Conclusions
The present work describes a rapid, selective and sensitive LC–MS/MS method using ESI source for the simultaneous determination of AMI (positive mode) and HCTZ (negative mode) in human plasma. The major advantages of this method include higher sensitivity and shorter analysis time (2.5 min), which renders it highly suitable for the analysis of large numbers of plasma samples obtained from clinical studies. The lowest concentration quantified was 0.05 ng/mL for AMI and 0.50 ng/mL for HCTZ using 250 µL of plasma samples with suitable accuracy and precision. Moreover, this is the first report on the pharmacokinetics of AMI and HCTZ in healthy Indian subjects using an FDC formulation of these drugs. The method was successfully applied to a bioequivalence study with good reproducibility.
Acknowledgments
One of the authors, Jaivik V. Shah, would like to acknowledge University Grants Commission (UGC), New Delhi, India, for BSR Fellowship F 4-1/2009 (BSR)/7–74/2007 and to the Department of Chemistry, Gujarat University, Ahmedabad, India, for this research work.
Footnotes
Peer review under responsibility of Xi'an Jiaotong University.
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.jpha.2017.03.007.
Appendix A. Supplementary material
Supplementary material
.
References
- 1.Circelli M., Nicolini G., Egan C.G. Efficacy and safety of delapril/indapamide compared to different ACE-inhibitor/hydrochlorothiazide combinations: a meta-analysis. Int. J. Gen. Med. 2012;5:725–734. doi: 10.2147/IJGM.S35220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mancia G., De Backer G., Dominiczak A. 2007 ESH-ESC practice guidelines for the management of arterial hypertension: ESH-ESC task force on the management of arterial hypertension. J. Hypertens. 2007;25:1751–1762. doi: 10.1097/HJH.0b013e3282f0580f. [DOI] [PubMed] [Google Scholar]
- 3.Gradman A.H., Basile B.L., Carter B.I. American society of hypertension writing group, combination therapy in hypertension. J. Clin. Hypertens. (Greenwich) 2011;13:146–154. doi: 10.1111/j.1751-7176.2010.00397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.GlaxoSmithKline Pharmaceuticals Limited, BIDURET (amiloride+hydrochlorothiazide) oral tablets. Prescribing Information, Dr. Annie Besant Road, Worli, Mumbai 400030, India.
- 5.Huclová J., Satínský D., Pavlícek O. Using on-line solid phase extraction for determination of amiloride in human urine by sequential injection technique. Anal. Chim. Acta. 2006;573–574:376–382. doi: 10.1016/j.aca.2006.01.024. [DOI] [PubMed] [Google Scholar]
- 6.Myung C.S., Bae J.W., Park Y.S. Analytical HPLC method validation of amiloride and its pharmacokinetic study in humans. J. Liq. Chromatogr. Relat. Technol. 2008;31:2455–2466. [Google Scholar]
- 7.Qin Y., Wang X.B., Wang C. Application of high-performance liquid chromatography–mass spectrometry to detection of diuretics in human urine. J. Chromatogr. B. 2003;794:193–203. doi: 10.1016/s1570-0232(03)00422-7. [DOI] [PubMed] [Google Scholar]
- 8.Morra V., Davit P., Capra P. Fast gas chromatographic/mass spectrometric determination of diuretics and masking agents in human urine. Development and validation of a productive screening protocol for antidoping analysis. J. Chromatogr. A. 2006;1135:219–229. doi: 10.1016/j.chroma.2006.09.034. [DOI] [PubMed] [Google Scholar]
- 9.Deventer K., Pozo O.J., Eenoo P. Van. Qualitative detection of diuretics and acidic metabolites of other doping agents in human urine by high-performance liquid chromatography–tandem mass spectrometry Comparison between liquid–liquid extraction and direct injection. J. Chromatogr. A. 2009;1216 doi: 10.1016/j.chroma.2009.06.003. (5819–582) [DOI] [PubMed] [Google Scholar]
- 10.Liu F., Xua Y., Gaob S. Determination of hydrochlorothiazide in human plasma by liquid chromatography/tandem mass spectrometry. J. Pharm. Biomed. Anal. 2007;44:1187–1191. doi: 10.1016/j.jpba.2007.04.020. [DOI] [PubMed] [Google Scholar]
- 11.Rajasekhar D., Kumar I.J., Venkateswarlu P. High performance liquid chromatography/ negative ion electrospray tandem mass spectrometry method for the measurement of hydrochlorothiazide in human plasma: application to a comparative bioavailability study. Eur. J. Mass Spectrom. 2009;15:715–721. doi: 10.1255/ejms.1038. [DOI] [PubMed] [Google Scholar]
- 12.Pei B.F., Guo H., Zheng L.C. Determination of hydrochlorothiazide in human plasma by HPLC: application to a pharmacokinetic study. Lat. Am. J. Pharm. 2014;33:1470–1474. [Google Scholar]
- 13.Singh B., Lokhandae R.S., Dwiwedi A. Improved simultaneous quantitation of candesartan and hydrochlorothiazide in human plasma by UPLC-MS/MS and its application in bioequivalence studies. J. Pharm. Anal. 2014;4:144–152. doi: 10.1016/j.jpha.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qiu X., Wang Z., Wang B. Simultaneous determination of irbesartan and hydrochlorothiazide in human plasma by ultra performance liquid chromatography tandem mass spectrometry and its application to a bioequivalence study. J. Chromatogr. B: Analyt. Technol. Biomed. Life Sci. 2014;957:110–115. doi: 10.1016/j.jchromb.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 15.Koyuturk S., Can N.O., Atkosar Z. A novel dilute and shoot HPLC assay method for quantification of irbesartan and hydrochlorothiazide in combination tablets and urine using second generation C18-bonded monolithic silica column with double gradient elution. J. Pharm. Biomed. Anal. 2014;97:103–110. doi: 10.1016/j.jpba.2014.04.026. [DOI] [PubMed] [Google Scholar]
- 16.Gadepalli S.G., Deme P., Kuncha M. Simultaneous determination of amlodipine, valsartan and hydrochlorothiazide by LC-ESI-MS/MS and its application to pharmacokinetics in rats. J. Pharm. Anal. 2014;4:399–406. doi: 10.1016/j.jpha.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aydoǧmuş Z. Simultaneous determination of aliskiren, amlodipine and hydrochlorothiaizide in spiked human plasma and urine by high performance liquid chromatography. J. Anal. Chem. 2015;70:502–509. [Google Scholar]
- 18.Shah P.A., Sharma P., Shah J.V. Simultaneous analysis of losartan, its active metabolite, and hydrochlorothiazide in human plasma by a UPLC-MS/MS method. Turk. J. Chem. 2015;39:714–733. [Google Scholar]
- 19.Patel J.R., Pethani T.M., Vachhani A.N. Development and validation of bioanalytical method for simultaneous estimation of ramipril and hydrochlorothiazide in human plasma using liquid chromatography-tandem mass spectrometry. J. Chromatogr. B. 2014;970:53–59. doi: 10.1016/j.jchromb.2014.08.023. [DOI] [PubMed] [Google Scholar]
- 20.J.V. Shah , P.A. Shah , P.V. Shah , et al., Fast and sensitive LC–MS/MS method in negative ionization mode for the simultaneous determination of lisinopril and hydrochlorothiazide in human plasma, J. Pharm. Anal. 〈https://doi.org/10.1016/j.jpha.2016.11.004〉. [DOI] [PMC free article] [PubMed]
- 21.Van der Meer M.J., Brown L.W. Simultaneous determination of amiloride and hydrochlorothiazide in plasma by reversed-phase high-performance liquid chromatography. J. Chromatogr. 1987;423:351–357. doi: 10.1016/0378-4347(87)80363-8. [DOI] [PubMed] [Google Scholar]
- 22.Song M., Hang T., Zhao H. Simultaneous determination of amiloride and hydrochlorothiazide in human plasma by liquid chromatography/tandem mass spectrometry with positive/negative ion-switching electrospray ionisation. Rapid Commun. Mass Spectrom. 2007;21:3427–3434. doi: 10.1002/rcm.3235. [DOI] [PubMed] [Google Scholar]
- 23.Jangid A.G., Tale R.H., Vaidya V.V. A single, selective and simple validated method for simultaneous estimation of amiloride and hydrochlorothiazide in human plasma by liquid chromatography–tandem mass spectrometry. Biomed. Chromatogr. 2012;26:95–100. doi: 10.1002/bmc.1632. [DOI] [PubMed] [Google Scholar]
- 24.Guidance for Industry, Bioanalytical Method Validation, US Department of Health and Human Services, Food and Drug Administration Centre for Drug Evaluation and Research (CDER), Centre for Veterinary Medicine (CVM), May 2001.
- 25.Shah J.V., Patel D.P., Shah P.A. Simultaneous quantification of atenolol and chlorthalidone in human plasma by ultra-performance liquid chromatography-tandem mass spectrometry. Biomed. Chromatogr. 2016;30:208–216. doi: 10.1002/bmc.3537. [DOI] [PubMed] [Google Scholar]
- 26.Guidance for Industry: ICH E6 Good Clinical Practice, U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER), Centre for Biologics Evaluation and Research (CBER), April 1996.
- 27.Yadav M., Shrivastav P.S. Incurred sample reanalysis: a decisive tool in bioanalytical research. Bioanalysis. 2011;3:1007–1024. doi: 10.4155/bio.11.76. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material