Abstract
A sensitive and selective method was developed for the separation and characterization of related substances (RSs) in EVT-401 by hyphenated LC–MS techniques. Complete separation of the RSs was achieved with an Inertsil ODS-SP column (250 mm×4.6 mm, 5 µm) by linear gradient elution using a mobile phase consisting of 0.2% formic acid solution, methanol and acetonitrile. EVT-401 was found to be susceptible to acid, alkaline and oxidative stresses, while relatively stable under photolytic and thermal dry stress conditions. Fourteen RSs including six process-related substances and eight degradation products were detected and identified in EVT-401 with positive ESI high-resolution TOF-MS analysis of their parent ions and the corresponding product mass spectra elucidation, and some of them were further verified by chemical synthesis and NMR spectroscopy. The specific LC–MS method developed for separation, identification and characterization of RSs is valuable for EVT-401 manufacturing process optimization and quality control.
Keywords: EVT-401, Related substances, LC–MS, Degradation products
1. Introduction
P2X receptors, which allow non-specific passage of cations (Na+, Ca2+, K+), are a kind of ligand-gated ion channels. Until now, seven kinds of receptors have been found and described [1]. P2X7 receptor, expressed on hematopoietic lineage cells including mast cells, lymphocytes, erythrocytes, fibroblasts, and peripheral macrophages, is a distinct member of the P2X subclass, which has a key role in inflammation and immunity. By inhibiting P2X7 receptor, the treatment of multiple sclerosis, inflammatory bowel disease and rheumatoid arthritis has been achieved [2], [3], [4], [5], [6]. Pfizer's CE-224535 and AstraZeneca's AZD -9056 [7] as oral small molecule P2X7 receptor antagonists have entered clinical trials for rheumatoid arthritis.
EVT-401, 2-(3-fluoro-4-(trifluoromethyl)phenyl)-N-(2-(1-hydroxypropan-2-yl)-6-methyl-1-oxo-1,2-dihydroisoquinolin-5-yl)acetamide, is a P2X7 receptor antagonist developed for the treatment of rheumatoid arthritis by preventing disease progression and symptom exacerbation [8]. As an attractive new P2X7 receptor antagonist, EVT-401 has shown efficacy of combating neuroinflammation. And the Phase I clinical trial of EVT-401 on rheumatoid arthritis has recently been completed [9]. The control of impurities by adopting quality by design (QbD) approach is very important as mentioned in the ICH guidelines for pharmaceutical development [10]. Until now, none of the methods described the identification and characterization of EVT-401 and the RSs. The identification of both process-related substances and degradation products is needed to ensure the good manufacturing of high quality EVT-401.
Hence, the purpose of present work was to determine and characterize the RSs of EVT-401 by LC–MS techniques and some of them were further synthesized and verified by NMR spectroscopy. The results obtained from the study would be of significance for quality control and the stability-indicating study of EVT-401.
2. Experimental
2.1. Chemicals and reagents
EVT-401 (Batch No. 140610-1, purity>99.71%) was obtained from Zhejiang Conba Pharmaceutical Co., Ltd. (Hangzhou, China). The reference related substances (RSs A–F, Table 1) were synthesized in China Pharmaceutical University (Nanjing, China). HPLC-grade methanol, acetonitrile and formic acid were purchased from Tedia (Ohio, USA). Hydrochloric acid, sodium hydroxide and 30% hydrogen peroxide were all of analytical grades and purchased from Nanjing Chemical Reagent Co., Ltd. (Nanjing, China). Water was purified with a Millipore Milli Q-Plus system (Millipore, MA, USA).
Table 1.
EVT-401 and its RSs identified by LC-TOF and LC–MS/MS.
| Impurity code | tR (min) | [M+H]+(m/z) | Ion formula | Score/dif (ppm) | Product ions (m/z) | Abbreviated names | Originsa |
|---|---|---|---|---|---|---|---|
| 1(A) | 5.95 | 233.1285 | C13H17N2O2+ | 98.64/0.00 | 215,175,157 | de-(2-(3-fluoro-4-(trifluoromethyl)phenyl)-acetyl)-EVT-401 | Pr & Dr |
| 2 | 7.91 | 413.1508 | C22H22FN2O5+ | 97.73/−0.08 | 395,377, 349 | 4'''-carboxyl-EVT-401 | Dr |
| 337,215 | |||||||
| 3(B) | 9.21 | 275.1394 | C15H19N2O3+ | 95.19/1.54 | 233,215,175 | 2-(5-amino-6-methyl-1-oxo-1,2- | Pr |
| 157 | dihydroisoquinolin-yl)propyl acetate | ||||||
| 4 | 15.02 | 427.1666 | C23H24FN2O5+ | 97.96/−0.50 | 409,395,337 | 4'''-methoxylformyl-EVT−401 | Dr |
| 5(C) | 17.81 | 263.1026 | C13H15N2O4+ | 98.36/−0.10 | 245,228, 205 | 2-(1-hydroxypropan-2-yl)-6-methyl-5- | Pr |
| 199,188,159 | nitroisoquinolin-1,2-dihydroisoquinolin-one | ||||||
| 6(D) | 21.49 | 291.0975 | C14H14N2O5+ | 99.00/0.06 | 231 | 2-(5-nitro−1-oxoisoquinolin−2(1 H)-yl) | Pr |
| propyl acetate | |||||||
| 7 | 22.74 | 453.1435 | C22H21F4N2O4+ | 98.60/−0.74 | 435,395, 249 | 7-hydroxyl-EVT-401 | Dr |
| 231,217, 191 | |||||||
| 8 | 23.74 | 453.1433 | C22H21F4N2O4+ | 98.45/−0.12 | 435,395, 249 | 6-hydromethyl-EVT-401 | Dr |
| 231,217, 191 | |||||||
| 9 | 26.23 | 453.1435 | C22H21F4N2O4+ | 98.42/−0.70 | 435,395, 249 | 3-hydroxyl-EVT-401 | Dr |
| 231,217, 191 | |||||||
| 10 | 27.29 | 453.1433 | C22H21F4N2O4+ | 98.78/−0.12 | 435,395, 249 | 8-hydroxyl-EVT-401 | Dr |
| 231,217, 191 | |||||||
| 11 | 29.85 | 453.1433 | C22H21F4N2O4+ | 99.34/−0.34 | 435,395, 249 | 4-hydroxyl-EVT-401 | Dr |
| 231,217, 191 | |||||||
| 12(E) | 29.85 | 305.1132 | C15H17N2O5+ | 99.09/0.63 | 245 | 2-(6-methyl-5-nitro-1-oxo-1,2- | Pr |
| dihydroisoquinolin-yl)propyl acetate | |||||||
| 13 | 30.89 | 449.1682 | C23H23F3N2O4+ | 97.98/0.1 | 431,409,389 | 3'''-methoxy-EVT-401 | Dr |
| 371 | |||||||
| EVT-401 | 31.98 | 437.1483 | C22H21F4N2O3+ | 94.00/−0.05 | 419,379,359 | EVT-401 | API |
| 215,201,175 | |||||||
| 159 | |||||||
| 14(F) | 42.57 | 479.1588 | C24H23F4N2O4+ | 98.41/0.68 | 439,419 | 1'-acetyl-EVT-401 | Pr |
Pr=Process related substance, Dr=Degradation product
2.2. Test solutions
Stock solutions were prepared accurately by dissolving EVT-401 or the synthetic reference RSs A–F in the diluent consisting of methanol-acetonitrile-water (24:14:62, v/v/v) to a final concentration of 0.5 mg/mL, respectively. 0.1% reference solution was prepared by diluting EVT-401 stock solution (0.5 mg/mL) to a final concentration of 0.5 μg/mL. A mixed reference solution of EVT-401 and the reference RSs was prepared similarly by spiking the EVT-401 solution with a suitable volume of each of the RSs stock solution to produce a final concentration of 0.5 mg/mL for EVT-401 and 10 μg/mL for each of the RSs.
2.3. Instruments
An Agilent 1260 infinity series LC equipped with a DAD coupled to an Agilent TOF mass spectrometer (CA, USA) was used for sample analysis. Samples were separated on an Inertsil ODS-SP column (250 mm×4.6 mm, 5 µm) by using a mobile phase consisting of methanol-acetonitrile-water-formic acid (24:14:62:0.2, v/v/v/v) as solvent A and methanol-acetonitrile-water-formic acid (30:20:50:0.2, v/v/v/v) as solvent B with linear gradient elution. The program in terms of the volume ratio of solvents (A:B) was as follows: 0 min (100:0)→35 min (0:100)→50 min (0:100)→50.1 min (100:0)→60 min (100:0). The flow rate was 1.0 mL/min and the column temperature was set at 35 ℃. Analytes were monitored at 237 nm. The injection volume was 20 μL. TOF-MS was operated in high resolution mode with a dual ESI source in positive mode and protonated purine of m/z 121.0508 (C5H5N4+) and protonated fluoro-phosphazine of m/z 922.0098 (HP-921, C18H19O6N3P3F24+) were used as the internal calibration reference ions. Ionization was performed at 3.5 kV, assisted by nitrogen drying gas flow of 10 L/min at 350 °C with a nebulizer pressure of 275 kPa. The m/z scanning range was from 105 to 1000 at 1 spectra/s. All the operations, acquisition and analysis were controlled by Mass Hunter Workstation.
The liquid chromatography tandem MS (LC–MS/MS) analyses were performed with a Finnigan TSQ mass spectrometer (Thermo electron corporation, San Jose, CA, USA) in positive ESI mode. The product MS spectra were collected with the following conditions: spray voltage set at 5.0 kV, heated capillary temperature at 350 °C, and the nitrogen sheath gas and auxiliary gas at 350 and 50 kPa, respectively. The 0.2 Pa argon gas collision induced fragmentation was performed with the energies optimized for each impurity from 10 to 20 eV.
The NMR spectra were recorded on a Bruker AVANCE 500 MHz NMR system (Fallanden, Switzerland) with all samples dissolved in DMSO-d6 using tetramethylsilane as the chemical shift reference standard.
2.4. Forced degradation
According to the ICH guidelines, the selected stress conditions were as follows: acidic hydrolysis (1 M HCl 1 mL, 90 °C, 12 h), alkaline hydrolysis (1 M NaOH 1 mL, 90 °C, 8 h), oxidation (10% H2O2 1 mL, 80 °C, 4 h), thermal hydrolysis (solid, 150 °C, 5 d) and photolytic degradation (methanol-acetonitrile-water (24:14:62, v/v/v) 1 mL, 4500±500 lx, 3 days). The degradation samples of acidic and alkaline hydrolysis were neutralized with NaOH or HCl solution before dilution, respectively. All degradation samples were diluted with a small amount of methanol before degradation and prepared to a final nominal concentration about 0.5 mg/mL of EVT-401 by the same diluent before analysis.
3. Results and discussion
3.1. Method development and optimization of chromatographic conditions
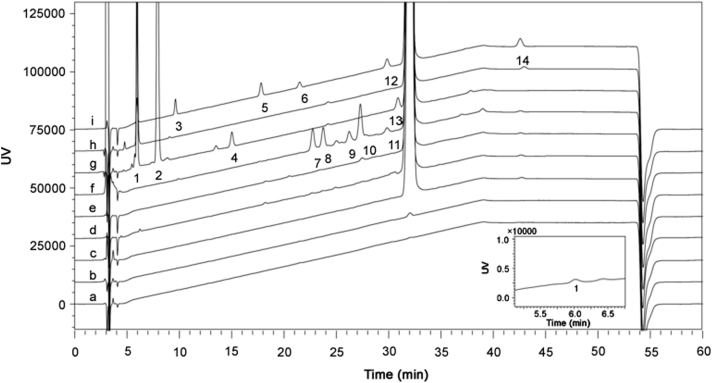
To achieve good separation for active pharmaceutical ingredient (API) and potential impurities, and compatibility with LC–MS detection, a selective method should be developed. Different components of the mobile phases, proportions, buffer concentrations and column brands were tested to obtain a better separation. Finally, the mobile phases consisting of methanol-acetonitrile-water-formic acid were chosen to achieve a better separation and LC–MS compatibility with the UV detection was set at 237 nm because of a compromise between the sensitivity and the base line drifting. Typical HPLC results are shown in Fig. 1, and 14 RSs were detected in all test samples.
Fig. 1.
HPLC–UV chromatograms of EVT-401 test and stressed solutions: blank (a); 0.1% reference solution (b); EVT-401 (c); photolysis (d); thermal (e); oxidation (f); alkaline (g); acid (h); mixture of reference RSs and EVT-401 (i). Inset: Partial magnification of EVT-401.
3.2. Chromatographic characteristics of related substances
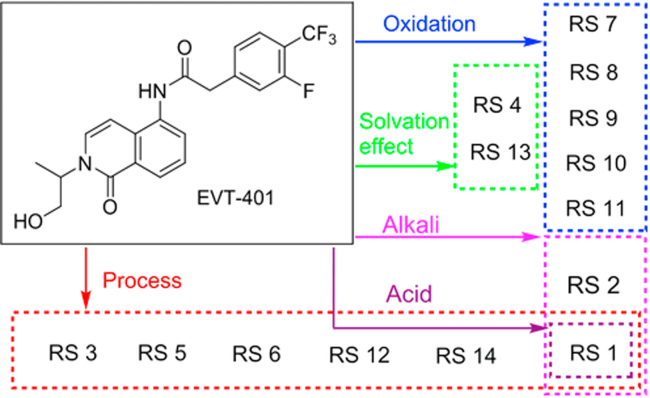
Among the 14 RSs (Fig. 1), six process-related substances and eight degradation products were detected in all test samples. Among them, two process-related substances (RSs 1 and 14) were found in the API with the contents lower than 0.1% based on the external 0.1% self-reference method. The content of RS 1 had a significant increase in the stress conditions of acidic and alkaline hydrolysis. The content of RS 14 was not found in alkaline hydrolysis, and reduced in the other four stress conditions. Significant degradation of EVT-401 was observed under acidic (RS 1), alkaline (RSs 1 and 2) and oxidative (RSs 7–11) stress conditions, but no obvious degradation was observed in photolysis and thermal dry stress conditions. RSs 4 and 13 were produced under methanol solvation, which were not found during the storage of EVT-401. All the RSs 1–14 were well separated from EVT-401.
3.3. MS characteristics of EVT-401 and the RSs
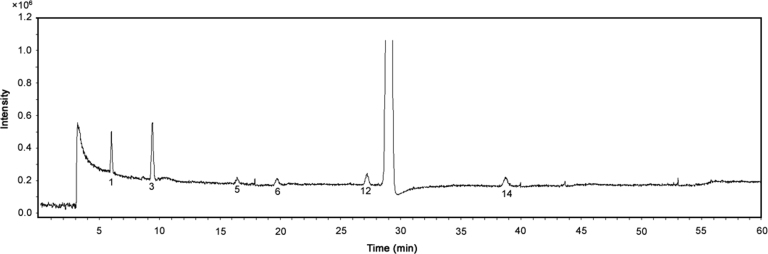
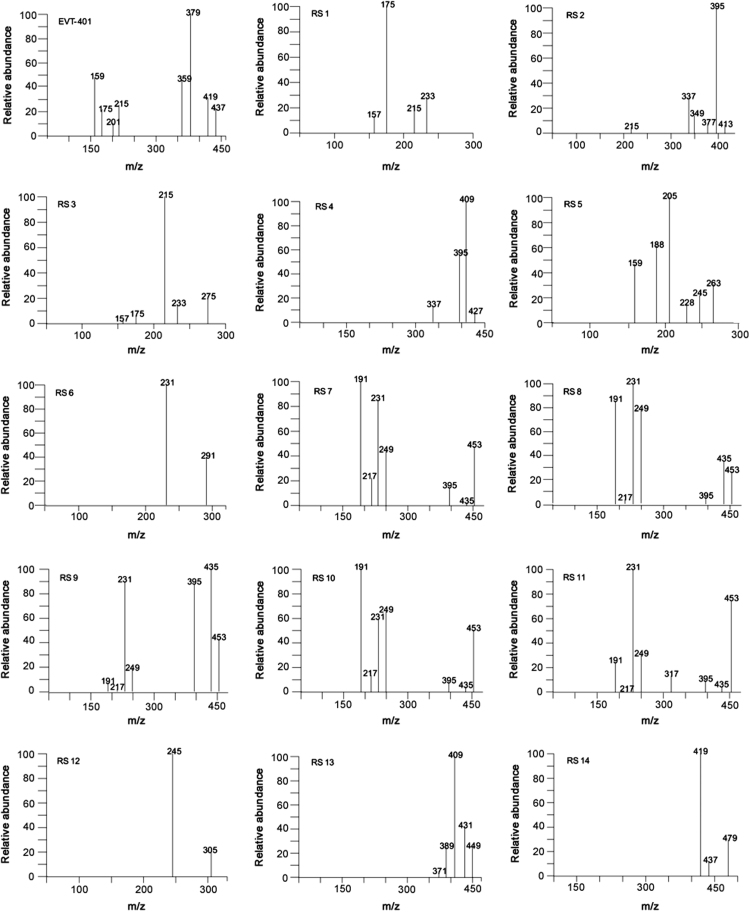
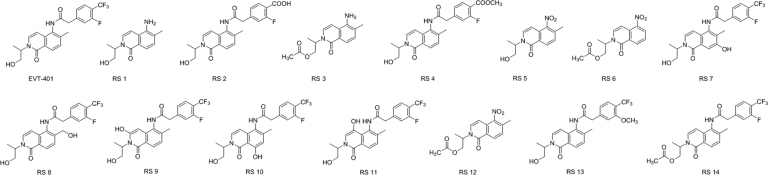
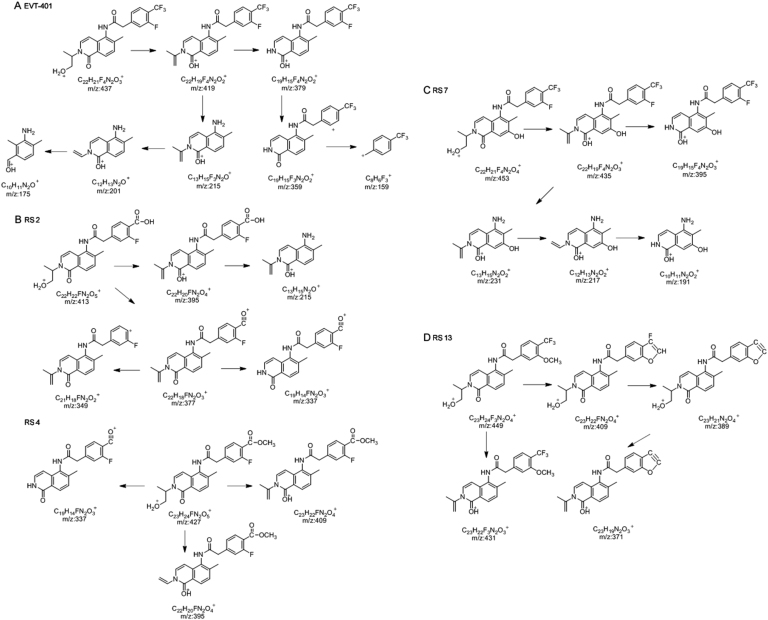
EVT-401 and RSs 1–14 were characterized through hyphenated LC-ESI-MS techniques (Fig. 2, Fig. 3). The chromatographic and mass spectrometric data are summarized in Table 1; the structures and the plausible fragmentation mechanisms are proposed in Fig. 4, Fig. 5.
Fig. 2.
Typical LC-TOF chromatogram of reference RSs and EVT-401.
Fig. 3.
Product mass spectra of [M+H]+ ions of EVT-401 and its RSs. EVT-401 (m/z 437), RS 1 (m/z 233), RS 2 (m/z 413), RS 3 (m/z 275), RS 4 (m/z 427), RS 5 (m/z 263), RS 6 (m/z 291), RS 7 (m/z 453), RS 8 (m/z 453), RS 9 (m/z 453), RS 10 (m/z 453), RS 11 (m/z 453), RS 12 (m/z 305), RS 13 (m/z 449) and RS 14 (m/z 479).
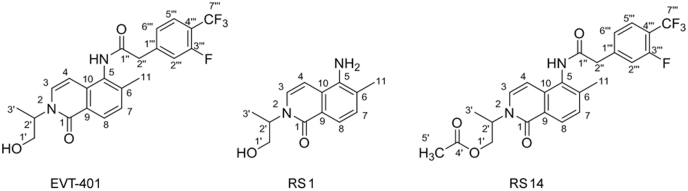
Fig. 4.
Structures of EVT-401 and its RSs.
Fig. 5.
Fragmentation characteristics of the product mass spectra of the [M+H]+ ions of EVT-401 and its main RSs. (A) EVT-401, (B) RSs 2 and 4, (C) RS 7, and (D) RS 13.
3.3.1. MS/MS fragmentation patterns of EVT-401
Because of the structural similarities between EVT-401 and its RSs, the mass spectra of them have some intrinsically identical characteristics. The analysis of the mass spectra of EVT-401 was essential for the determination of its RSs.
The positive ESI-TOF-MS full scan accurate mass determination found two adduct ions of EVT-401 at m/z 437.1483 and 459.1296, respectively. They were consistent with the [M+H]+ and [M+Na]+ ions of EVT-401([C22H21F4N2O3]+ and [C22H20F4N2O3Na]+), respectively. The MS/MS spectrum of EVT-401 [M+H]+ ion displayed characteristic product ions at m/z 419, 379, 359, 215, 201, 175 and 159 (Fig. 3). The formation of product ion with m/z 419 was attributed to the neutral loss of H2O from the parent [M+H]+ ion and a neutral loss of CH3-CH=CH-OH to produce the product ion with m/z 379. The three major product ions at m/z 215, 201 and 175 were generated via the C-N bond cleavage at the amide bond site, forming three cations corresponding to the 5-amino-6-methyl-2-(prop-1-en-2-yl)isoquinolin-1(2H)-one moiety, 5-amino-6-methyl-2-vinylisoquinolin-1(2H)-one moiety and 5-amino-6-methylisoquinolin-1(2H)-one moiety, respectively (Fig. 4, Fig. 5).
3.3.2. Characterization of RSs 1, 3, 5, 6, 12 and 14
Based on the consistent chromatographic retentions of reference RSs as shown in Fig. 1, Fig. 2, protonated molecules [M+H]+ and their MS/MS spectra (Fig. 3), RSs 1, 3, 5, 6, 12 and 14 were identified as RSs A, B, C, D, E and F, respectively (Table 1 and Fig. 4).
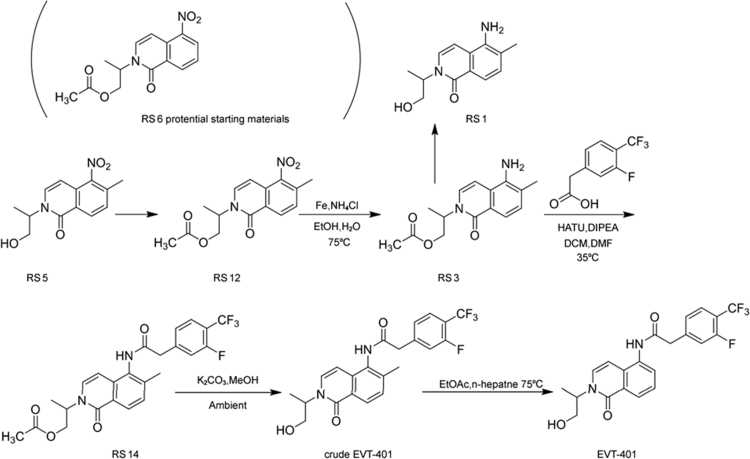
Six process-related substances (RSs 1, 3, 5, 6, 12 and 14) were found during the optimization research for process parameters and characterized through LC–MS techniques. According to the manufacturing processes of EVT-401 (Fig. 6), the origins of these process-related substances were located, and the effective approaches were designed and conducted to reduce and even eliminate them as much as possible in the API. The LC–MS/MS determination and elucidation of their fragment ions figured out all their possible structures. And then they were further verified through synthesis and spectroscopic determination.
Fig. 6.
The synthetic route for EVT-401.
RS 1(A) was the major process-related substance formed by the hydrolysis of the ester bond of the residual intermediate compound RS 3(B), as well as the hydrolysis degradation of EVT-401 under acidic and basic stress conditions. By adjusting the reaction temperature and time, the content of RS 1(A) could be significantly reduced. RS 3(B) was the intermediate compound, which was attributed to the reduction of nitro of RS 12(E). And RS 14(F) was formed by the reaction of RS 3 with 2-(3-fluoro-4-(trifluoromethyl) phenyl) acetic acid.
Most of the above discussed process-related substances can be eliminated by the adjustment of the mole ratios of the reaction materials, in addition to the commonly employed purity control methods of the starting materials or re-crystallization in the final steps. The modified synthesis processes were successfully applied for high-quality EVT-401 manufacturing, as most of the impurities were eliminated completely in EVT-401, while only RSs 1 and 14 with contents in the range from 0.01% to 0.1% were found in the finished products.
3.3.3. Characterization of the degradation products RS 2, RSs 7–11
Significant degradation of EVT-401 was observed under acidic (RS 1), alkaline (RSs 1 and 2) and oxidative (RSs 7–11) stress conditions, but no obvious degradation was observed in photolysis stress and thermal dry stress (Fig. 1). The identification and characterization of these degradants were important for the quality control of EVT-401.
RS 2 was the major degradation product under alkaline stress with much decreased chromatographic retention, suggesting that the polarity of RS 2 was significantly increased compared with that of EVT-401. The accurate mass of the [M+H]+ ion of RS 2 determined by ESI-TOF-MS was m/z 413.1508, corresponding to ion formula of [C22H22FN2O5]+, which was 24 amu smaller than EVT-401 [C22H21F4N2O3]+, corresponding to the conversion of the –CF3 into –COOH. The product mass spectrum of RS 2 (Fig. 3) [M+H]+ contained major product ions at m/z 215, the same as the product ion found in API, confirming the presence of the structure of isoquinoline. The product mass spectrum contained ions at m/z 395, 377, 349 and 337 due to the neutral losses of H2O, 2H2O, H2O + HCOOH and H2O + CH3-CH=CH-OH from the [M+H]+ ion, respectively, indicating the presence of carboxyl group. Therefore, RS 2 was confirmed to be 4'''-carboxyl-EVT-401 (Fig. 4, Fig. 5).
RSs 7–11 were all the oxidative degradation products, which were found to have similar ESI-TOF-MS characteristics. The ESI-TOF-MS characteristics of the corresponding protonated molecules formula for RS 7 (m/z 453.1435), RS 8 (m/z 453.1433), RS 9 (m/z 453.1435), RS 10 (m/z 453.1433), and 11 (m/z 453.1436) were all found to be [C22H21F4N2O4]+, indicating that they were isomers with 16 amu higher than that of EVT-401 ([C22H21F4N2O3]+) , resulting from the oxidation with one O atom or a hydroxyl substitution to EVT-401. The reversed phase HPLC retention of RSs 7–11 was all decreased compared with that of EVT-401, indicating the addition of a polar hydroxyl group substitution in the structure of EVT-401.
The product mass spectra of RSs 7–11 contained the same major product ions at m/z 435 and 395, which were 16 amu higher than those of EVT-401 (m/z 419, 379), giving further evidence of a polar hydroxyl group substitution in the structure of EVT-401. Moreover, the characteristic product ions of RSs 7–11 (Fig. 3) at m/z 231, 217 and 191 were 16 amu higher than those of EVT-401 (m/z 215, 201 and 175), confirming the presence of hydroxyl substitution in the structure of isoquinoline.
Considering the formation of intra-molecular hydrogen bond should result in the decreased polarity and the readily oxidation characteristic of the aniline ring under oxidation stress, RSs 7–11 were deduced to be 7-hydroxy derivative, 11-hydroxy derivative, 3-hydroxy derivative, 8-hydroxy derivative and 5-hydroxy derivative of isoquinoline, respectively (Fig. 4, Fig. 5).
3.3.4. Characterization of RSs 4 and 13
RSs 4 and 13 were produced by the action of the methanol solvation effect, and were not found during the storage of EVT-401.
Both RSs 4 and 2 were products under the alkaline stress, which were found to have similar structures.
The accurate mass of the [M+H]+ ion of RS 4 determined by ESI-TOF-MS was m/z 427.1666, corresponding to ion formula of [C23H24FN2O5]+, which was 14 amu bigger than RS 2 [C22H22FN2O5]+, corresponding to the conversion of –COOH into –COOCH3. The MS/MS spectrum of RS 4 (Fig. 3) displayed similar characteristic fragment ions as RS 2 at m/z 395 and 337, corresponding to neutral losses of CH3OH and CH3OH + CH3-CH=CH-OH, respectively. And the characteristic fragment at m/z 409 was due to the neutral losses of H2O. Therefore, RS 4 was confirmed to be 4'''-methoxylformyl-EVT-401 because of the esterification reaction between RS 2 and methanol (Fig. 4, Fig. 5).
The accurate mass of the [M+H]+ ion of RS 13 determined by ESI-TOF-MS was m/z 449.1682, corresponding to ion formula of [C23H23F3N2O4]+, which was 12 amu bigger than EVT-401 [C22H21F4N2O3]+, corresponding to the conversion of –F into –OCH3. The characteristic fragments [M+H-2HF]+ at m/z 409 and [M+H-3HF]+ at m/z 389 were consistent with the ortho effect of –OCH3, while the product mass spectrum contained ions at m/z 431 and 371 due to the neutral losses of H2O and 3HF+H2O from the parent [M+H]+ ion, respectively. Based on the above discussed fragmentation pattern, RS 13 was assigned as 3'''-methoxy-EVT-401 (Fig. 4, Fig. 5).
3.4. Characterization of the process-related substances RSs 1 and 14 by NMR
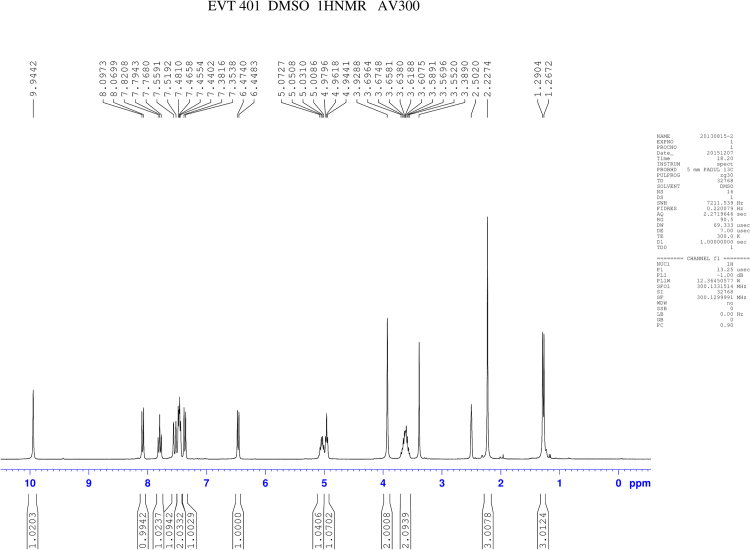
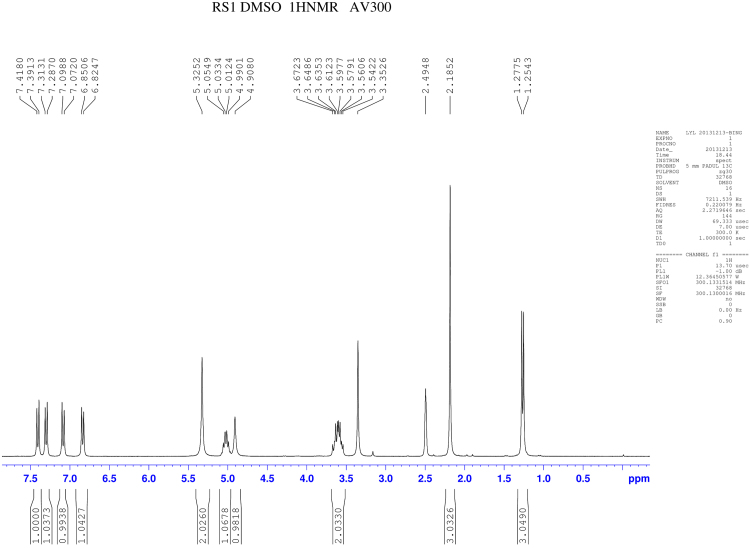
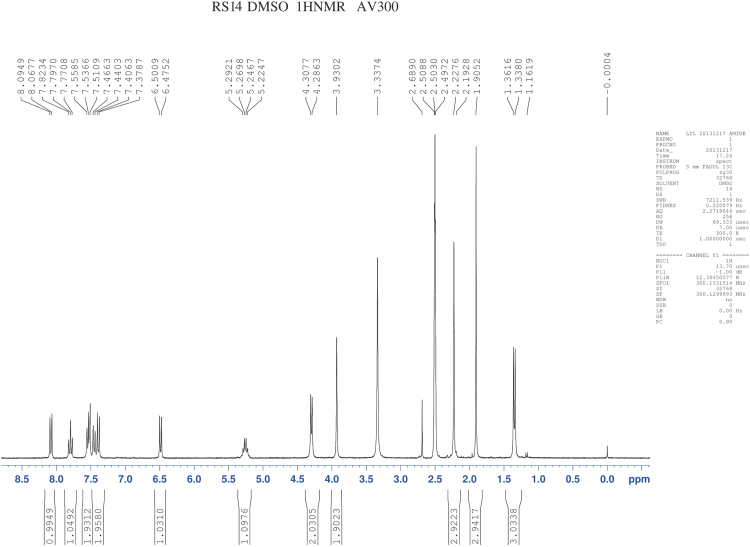
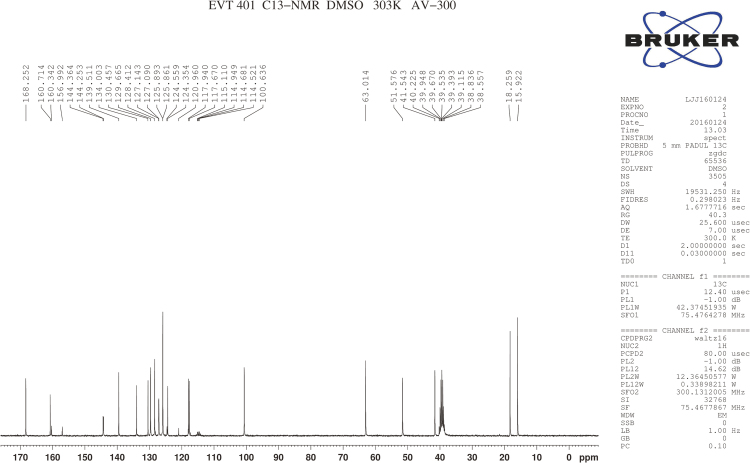
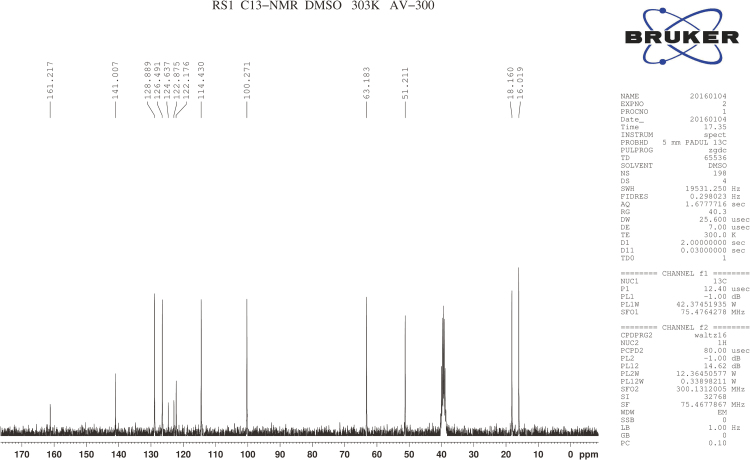
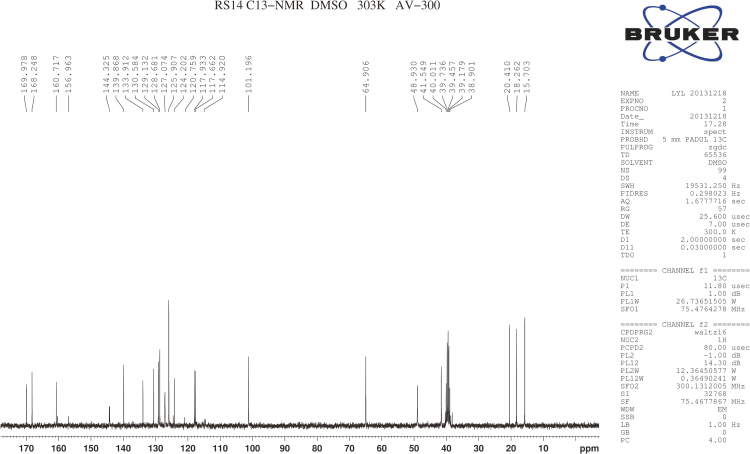
RSs 1 and 14 were found in EVT-401 and the contents of RS 1 and RS 14 in the stress conditions have a certain degree of increase or decrease. The RSs were further verified through synthesis and NMR determination (Figs. S1 and S2). The 1H and 13C NMR spectral data of EVT-401 and process-related RS 1 and 14 are summarized in Table 2 and Fig. 7.
Table 2.
The NMR assignments for EVT-401, RSs 1 and 14.
| Position | EVT-401 |
RS 1 |
RS 14 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| δc/ppm | δH/ppm | Mult.a | Integ. H | δc/ppm | δH/ppm | Mult.a | Integ. H | δc/ppm | δH/ppm | Mult.a | Integ. H | |
| 1 | 156.99 | – | – | – | 161.22 | – | – | – | 156.96 | – | – | – |
| 2 | – | – | – | – | – | – | – | – | – | – | – | – |
| 3 | 120.96 | 7.376 | d | 1 | 122.88 | 7.405 | d | 1 | 124.20 | 7.548 | d | 1 |
| 4 | 100.64 | 6.461 | d | 1 | 100.27 | 7.085 | d | 1 | 101.20 | 6.488 | d | 1 |
| 5 | 139.51 | – | – | – | 141.01 | – | – | – | 139.87 | 7.797 | d | 1 |
| 6 | 134.00 | – | – | – | 128.89 | – | – | – | 133.91 | – | – | – |
| 7 | 130.45 | 7.481 | d | 1 | 126.49 | 6.838 | d | 1 | 130.58 | 7.511 | d | 1 |
| 8 | 117.94 | 8.084 | d | 1 | 114.43 | 7.300 | d | 1 | 117.93 | 8.081 | d | 1 |
| 9 | 124.55 | – | – | – | 124.64 | – | – | – | 125.91 | – | – | – |
| 10 | 125.86 | – | – | – | 122.18 | – | – | – | 127.08 | – | – | – |
| 11 | 18.26 | 2.227 | s | 3 | 18.16 | 2.185 | s | 3 | 18.262 | 2.193 | s | 3 |
| 1' | 63.01 | 3.624 | m | 2 | 63.18 | 3.607 | m | 2 | 64.91 | 4.297 | d | 2 |
| 2' | 51.58 | 5.041 | d | 1 | 51.21 | 5.023 | m | 1 | 48.93 | 5.258 | dd | 1 |
| 3' | 15.92 | 1.279 | d | 3 | 16.02 | 1.266 | d | 3 | 15.70 | 1.350 | d | 3 |
| 4' | – | – | – | – | – | – | – | – | 169.68 | – | – | – |
| 5' | – | – | – | – | – | – | – | – | 20.41 | 1.905 | s | 3 |
| 1'' | 168.25 | – | – | – | – | – | – | – | 168.25 | – | – | – |
| 2'' | 41.54 | 3.929 | s | 2 | – | – | – | – | 41.55 | 3.930 | s | 2 |
| 1''' | 144.31 | – | – | – | – | 144.33 | – | – | – | |||
| 2''' | 114.60 | 7.368 | d | 1 | – | – | – | – | 114.92 | 7.393 | d | 1 |
| 3''' | 160.53 | – | – | – | – | – | – | – | 160.71 | – | – | – |
| 4''' | 115.03 | – | – | – | – | – | – | – | 117.66 | – | – | – |
| 5''' | 127.12 | 7.794 | t | 1 | – | – | – | – | 129.13 | – | – | – |
| 6''' | 125.89 | 7.460 | d | 1 | – | – | – | – | 128.68 | 7.453 | d | 1 |
| 7''' | 124.46 | – | – | – | – | – | – | – | 120.76 | – | – | – |
| –OH | – | 4.962 | t | 1 | – | 4.908 | s | 1 | - | – | – | – |
| –NH– | – | 9.944 | s | 1 | – | 5.325 | s | 2 | - | 9.928 | s | 1 |
s: singlet; d: doublet; t: triplet; m: multiplet; dd: double-doublet; and br: broad.
Fig. 7.
Structure numbers of EVT-401, RSs 1 and 14.
1H NMR spectra of EVT-401 and RS 1 showed extra signals of hydroxyl resonance peak at about δ 4.9 (s, 1H), and the amino resonance peak of RS 1 at about δ 5.3 (s, 1H). Due to the effect on paramagnetic shielding of carbonyl, the carbonyl carbon resonance peak lies in the bigger chemical shift region at about δ 170–160. In addition, compared with RS 1, the obvious chemical shift increases of EVT-401 and RS 14 were observed for the amino at about δ 9.9 (s, 1H).
The 13C NMR spectra of C-3''' showed the signals for the shielding effect of F, the chemical shift generating a change at about δ160. And the split peaks of benzene indicate the connection with F. Moreover, a characteristic quartet-resonance peak was observed at δ120, confirming the structure of CF3.
4. Conclusions
The impurity profiling of EVT-401 was carried out according to ICH guidelines. EVT-401 was found to be unstable under acidic, alkaline and oxidative stresses. Six process-related substances and eight degradation products in EVT-401 API were characterized by LC–MS and mass spectrometric techniques (Fig. 8). Most of them had much higher polarity than that of EVT-401, and the most susceptible positions for degradation in the structure of EVT-401 were found to be the amide group. The results obtained in the study will play a crucial role in providing scientific reference for the optimization of manufacturing processes and the quality assessment of EVT-401 and its formulations.
Fig. 8.
Correlation of the RSs in EVT-401 and the stress conditions.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Acknowledgments
The authors gratefully acknowledge Zhejiang Conba Pharmaceutical Co., Ltd. (Hangzhou, China) for graciously providing samples of EVT-401.
Footnotes
Peer review under responsibility of Xi'an Jiaotong University.
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.jpha.2017.03.008.
Appendix A. Supplementary material
Fig. S1 (A).
1H NMR spectra of EVT-401.
.
Fig. S1 (B).
1H NMR spectra of RS1.
.
Fig. S1 (C).
1H NMR spectra of RS14.
.
Fig. S2 (A).
13C NMR spectra of EVT-401.
.
Fig. S2 (B).
13C NMR spectra of RS1.
.
Fig. S2 (C).
13C NMR spectra of RS14.
.
References
- 1.Cotrina M.L., Nedergaard M. Physiological and pathological functions of P2X7 receptor in the spinal cord. Purinergic Signal. 2009;5:223–232. doi: 10.1007/s11302-009-9138-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Donnelly-Roberts D.L., Jarvis M.F. Discovery of P2X7 receptor-selective antagonists offers new insights into P2X7 receptor function and indicates a role in chronic pain atates. Br. J. Pharmacol. 2007;151:571–579. doi: 10.1038/sj.bjp.0707265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arulkumaran N., Unwin R.J., Tam F.W. A potential therapeutic role for P2X7 receptor (P2X7R) antagonists in the treatment of inflammatory diseases. Expert. Opin. Investig. Drugs. 2011;20:897–915. doi: 10.1517/13543784.2011.578068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carroll W.A., Donnelly-Roberts D., Jarvis M.F. Selective P2X(7), receptor antagonists for chronic inflammation and pain. Purinergic Signal. 2009;5:63–73. doi: 10.1007/s11302-008-9110-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Le Feuvre R.A., Brough D., Touzani O. Role of P2X7 receptors in ischemic and excitotoxic brain injury in vivo. J. Cereb. Blood Flow Metab. 2003;23:381–384. doi: 10.1097/01.WCB.0000048519.34839.97. [DOI] [PubMed] [Google Scholar]
- 6.Bartlett R., Stokes L., Sluyter R. The P2X7 receptor channel: recent developments and the use of P2X7 antagonists in models of disease. Pharmacol. Rev. 2014;66:638–675. doi: 10.1124/pr.113.008003. [DOI] [PubMed] [Google Scholar]
- 7.The U.S. National Institutes of Health, AZD9056 Relative Bioavailability Study, 2009 〈https://www.clinicaltrials.gov/ct2/show/NCT00908934?Term=AZD+9056&rank=1〉 (Accessed 24 March, 2017).
- 8.M.G. Kelly, J. Kincaid, Bicycloheteroaryl compounds as P2X7 modulators and uses thereof, US 7297700, 2007.
- 9.Evotec, Pipeline-Clinical assets, 〈https://www.evotec.com/articles/en/Products-and-Alliances/Pipeline-Clinical-assets/4/64〉 (Accessed 24 March, 2017).
- 10.International Conference on Harmonisation (ICH) Guideline, Stability testing of new drug substances and products Q1A(R2), February, 2003.