Abstract
In this work a new method is presented for simultaneous colorimetric determination of morphine(MOR) and ibuprofen(IBU) based on the aggregation of citrate-capped gold nanoparticles (AuNPs). Citrate-capped gold nanoparticles were aggregated in the presence of morphine and ibuprofen. The difference in kinetics of AuNPs aggregation in the presence of morphine / ibuprofen was used for simultaneous analysis of morphine and ibuprofen. The formation and size of synthesized Au NPs and the aggregated forms were monitored by infra-Red (IR) spectroscopy and transmission electron microscopy (TEM) respectively.. By adding morphine or ibuprofen the absorbance was decreased at 520 nm and increased at 620 nm. The difference in kinetic profiles of aggregation was applied for simultaneous analysis of MOR and IBU using partial least square regression as an efficient multivariate calibration method. The number of PLS latent variables was optimized by leave-one-out cross-validation method using predicted residual error sum of square. The proposed model exhibited a high capability in simultaneous prediction of MOR and IBU concentrations in real samples. Our results showed linear ranges of 1.33–33.29 µg/mL (R2=0.9904) and 0.28–6.9 µg/mL (R2=0.9902) for MOR and IBU respectively with low detection limits of 0.15 and 0.03 µg/mL(S/N=5).
Keywords: Morphine, Ibuprofen, Simultaneous determination, AuNps, Partial least squares
1. Introduction
Analytical methods based on the spectrophotometric measurements, such as UV–Vis, have attracted increasing interest due to their availability, simplicity and ease of operation [1]. Multivariate spectral calibration is becoming a general method which can leads to quantitative spectral analysis, allowing simultaneous determination of several analytes that coexist in samples with minimum number of preparation steps [2].
Among multivariate calibration methods, partial least squares (PLS) regression as a powerful method has been applied because it can model the effect of the interfering components without primary separation [2].
Gold nanoparticles (AuNPs) are particularly attractive because they have surface modifying capability such as bio-labelling and also very useful for biological applications. Some unique characteristics of AuNPs are particularly large surface area, good bio-compatibility, high conductivity and electro catalytic activity [3], [4], [5]. AuNPs are also useful colorimetric probes because of their distance-dependent optical properties and extremely high extinction coefficients in visible region that make it more sensitive than AgNPs [6], [7]. AuNPs simplicities rely on their unique surface plasmon resonance from red to blue, corresponding to their dispersion and aggregation states [8], respectively. Based on this principle, several colorimetric methods have been developed for the detection of biomolecules [9], 2,4,6-trinitrotoluene [10], drugs [9] and melamine [11], [12].
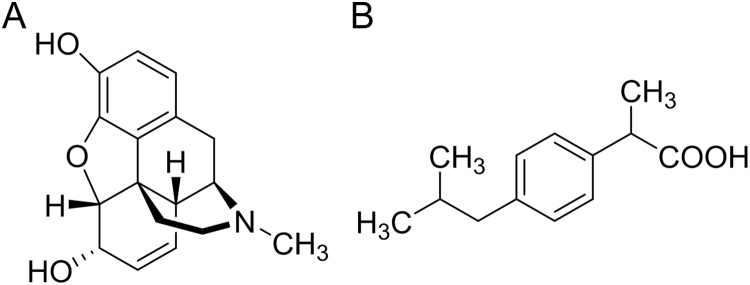
Morphine (MOR), a non-steroidal anti-inflammatory drug (Scheme 1A), is used primarily to treat both acute and chronic severe pain. It is also used for pain due to myocardial infarction and for labor pains [13] and its duration of analgesia is about three to seven hours [13].
Scheme 1.
Structure of (A) morphine and (B) ibuprofen.
Ibuprofen (IBU), is commonly used as an antipyretic drug especially for podiatry (Scheme 1B) [14]. Many procedures have been applied for the quantitative determination of IBU. These procedures include non-aqueous titration [15], polarography [16], colorimetric titration [17], first and second derivative spectrophotometry [18], Infra-red (IR) spectrophotometry [19] and H-Nuclear magnetic resonance (H_NMR) spectroscopy [20]. In addition, several chromatographic procedures are also employed using thin-layer chromatography [21] and gas chromatography [22]. The chromatographic methods are most common direct methods [23] but they are classified as time consuming methods. Also HPLC methods need organic and hazardous solvents.
Various analytical methods have been developed for the determination of morphine. The same as IBU determination, chromatographic method [24], [25] are most commonly methods for MOR detection. Fluorescence [26], enzyme-linked immunosorbent assay [27], immunoassays, such as surface plasmon resonance (SPR) based immunosensors [28] and radioimmunoassays (RIA) [29], molecular imprinting technique [30] amperometric methods [31], chemiluminescence [32] and electrochemical methods [33] are also reported for detection of morphine [34], [35], [36], [37].
In this work, a new method based on aggregation of citrate-capped gold nanoparticles (AuNPs) is presented for simultaneous determination of MOR and IBU. The method is based on the difference in the aggregation rate of citrate-capped gold nanoparticles in the presence of MOR and IBU. In the presence of MOR or IBU the absorbance of the solution containing AuNPs, is decreased in 520 nm and increased in 620 nm which shows the aggregation of AuNPs. Difference in the aggregation kinetics (e.g. absorbance increase versus time at 620 nm) was applied for simultaneous analysis of MOR and IBU using partial least squares (PLS) regression as an efficient multivariate calibration method. This method is selective without any initial sample preparation. The proposed method is a simple, fast, and low cost procedure, in contrast to chromatographic methods. This method does not need any expensive apparatus. Also because of applying the chemometrics methods for analyzing, the proposed method needs no primary time-consuming separation process.
2. Experimental
2.1. Chemicals and materials
All used materials and reagents were of analytical grade, solvents were of spectroscopic grade and double distilled water (DDW) was used throughout the experiments. MOR and IBU pure drugs were obtained from the Department of Food and Drug Administration, Urmia, Iran. All chemicals used in the experiments were of analytical grade and were used without further purification. Tri sodium citrate dehydrate, HCl and NaOH were purchased from Merck (Darmstadt, Germany).
2.2. Apparatus
Absorption spectra were recorded with an Agilent 8453 UV–Visible spectrophotometer with a 1 cm quartz cells. The size, morphology and structure of the synthesized AuNPs were characterized by transmission electron microscopy (TEM, Philips-CMC-300 kV). A Metrohm pH meter model 713 pH-meter was used for pH measurements. A 40 kHz universal ultrasonic cleaner water bath (Elmasonic E60H, German) was used. The calculations were performed in MATLAB (Hyper-cube Inc. Version10) software using PLS mfiles.
2.3. Synthesis of gold nanoparticles
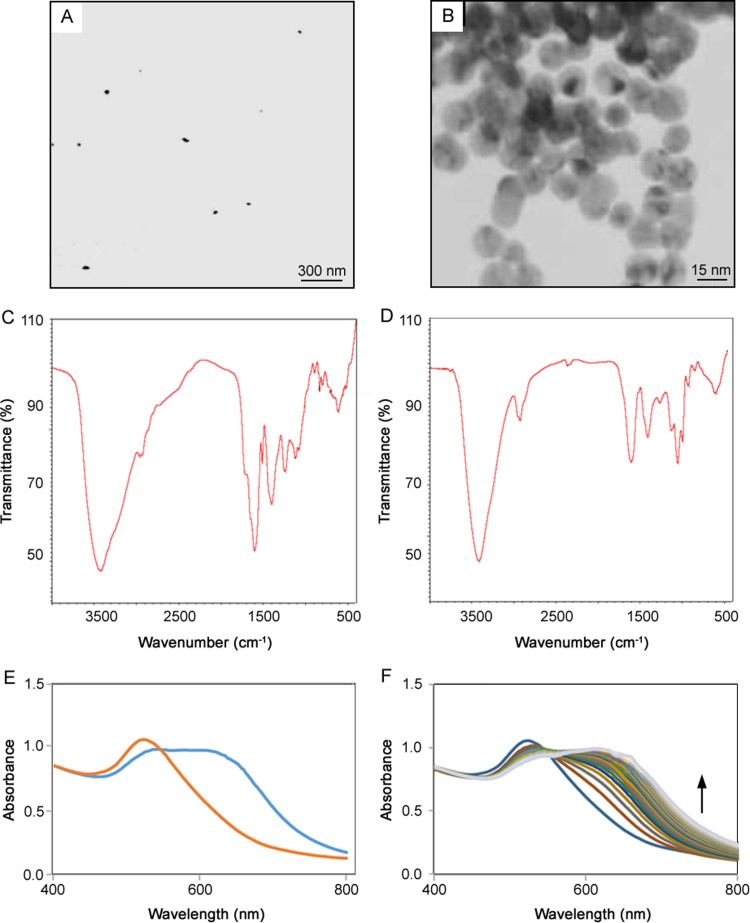
The Au seeds were synthesized according to Ferns method. Briefly, 100 mL of 1 mM aqueous solution of HAuCl4 was heated to boil with stirring; then 10 mL 1% (w/v) aqueous sodium citrate was added. The color of the mixed solution changed from yellow to wine red in a few minutes, indicating the formation of AuNPs. The boiling and stirring were continued for 15 min [3]. The seed solution was cooled to room temperature and was stored in a dark bottle at 4 C. The solution of the prepared citrate-capped AuNPs was wine red, and had a characteristic localized surface plasmon resonance (LSPR) absorption band of AuNPs at 520 nm (λmax) with narrow peak. In the solution, monodisperse AuNPs were red and exhibited a relatively narrow surface plasmon absorption band centered at around 520 nm in the UV–Vis spectrum. In contrast, a solution containing aggregated AuNPs was blue corresponding to a characteristic red shift in the surface plasmon resonance to higher wavelength of 620 nm [3]. The results were presented in Fig. 1 (A–B). Also, Fig. 1 (C, D) show the IR spectra of the synthesized Au Nanoparticles, before and after aggregation. The concentration of the synthesized nano particles were estimated as 10−8 mol L−1 based on the extinction coefficient for 13 nm gold nanoparticles reported in the litratures as 2.8×108. In IR spectra it can be demonstrated the functional groups of PhOH (3000–3600 cm−1), RCOOH (2800–3200 cm−1), OR2 (1000–1300 cm−1), Allyl-OH (3000–3600 cm−1). In the spectrum of aggregated AuNPs using the studied drugs the NR3 bands (1000–1350 1/ cm) obviously exist that prove drugs had interaction whit AuNPs. Intensity of IR spectrum of AuNps when drugs coated them or have interaction with them, are decreased and also the functional group of drug are increase in intensity. The absorbance spectra of synthesized AuNPs before and after aggregation were shon in Fig. 1E. Also Fig. 1F represents the changes of absorbance during time for morphine.
Fig. 1.
(A)TEM of synthesized AuNPs, (B) TEM of aggregated AuNPs, (C) IR of aggregated AuNPs, (D) IR of AuNPs, (E) spectrum of synthesized AuNPs and aggregated AuNPs spectrum in optimum condition and (F) aggregation spectra of AuNPs up to 10 min in the presence of morphine 10 µg/mL. Conditions: temperature 25 °C, ionic strength 1 mmol/L and pH 6.
3. Results and discussion
3.1. Optimization of NaCl concentration
Ionic strength has a crucial role in the aggregation of nano particles. It can be attributed to the ability of strong electrolytes to constrict the aroused electrical double-layer from the capping agent around the nano particles. Although the addition of an alectrolyte is necessary for starting the aggregation, it was also found that by increasing the ion strength above a certain limit, the aggregation of nanoparticles occurred even in the absence of analytes [1]. Therefore the effect of ionic strength (electrolyte concentration) should be studied. The results these study were presented in Figs. S1 and 1 mmol L−1 of NaCl was used as optimized value.
3.2. Optimization of pH
Because of the presence of hydroxyl, carboxyl and amine groups in drugs, pH is another critical parameter that should be taken into consideration. Also electrostatic interactions are dominantly responsible for aggregation of AuNPs in the presence of drugs [6]. The results of interaction of studied drugs with AuNPs were presented in Fig. S2. in order to increase the possibility of electrostatic interactions, the best condition is achievable in which the drug molecules are available in the nanoparticle surroundings [38]. Our studied showed that the synthesized AuNps are stable at pH 6 and also the drugs have the most favorable structure for interaction with AuNPs in the pH range of 6–7 (see Fig. S2). Therefore pH 6 was chosen for further studies.
3.3. Optimization of incubation time
Different incubation times were examined to find the optimum value. The results indicated that AuNPs started to aggregate right after mixing with MOR and IBU in optimum conditions and was suppressed and spectral change was detectable in 10 min which considered to be the optimum incubation time. So we selected this range of time as the end time for simultaneous kinetic study (Fig. S3).
3.4. The effect of temperature
The results (Fig. S4) showed that by increasing the temperature the aggregation was also increased. Because the aim of our study was simultaneous kinetic analysis of MOR and IBU using PLS, a temperature should be selected so that a good difference between the kinetic profiles of the analytes is attainable. Based on the results 25 °C was selected for further experiments.
3.5. PLS model development
PLS calibrations for both drugs were constructed by using non-linear iterative partial least squares (NIPALS) algorithm. A training set of 27 standard samples (20 samples as calibration set and 7 samples as prediction set) were taken from different mixtures of MOR and IBU. The correlation between different calibration samples has to be avoided because collinear component in the training set data will tend to cause under-fitting in the PLS models [39].
3.6. Linear range of calibration curves
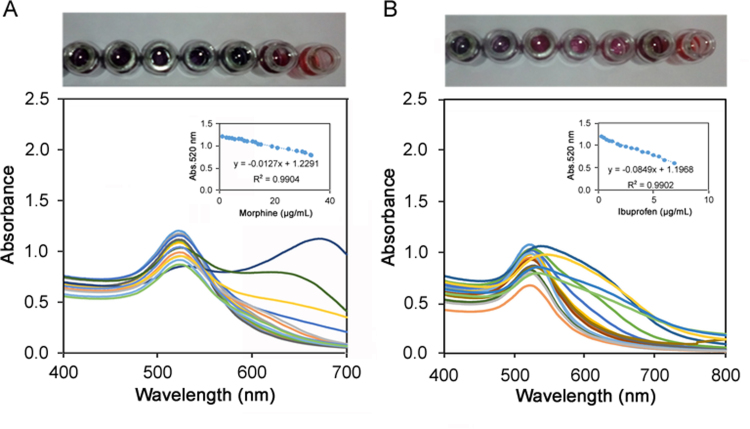
For the mentioned purposes, under optimum experimental conditions, a typical calibration curve was obtained for the determination of MOR and IBU separately. The calibration curves have a linear range of 1.33–33.29 and 0.28–6.9 µg/mL with y=−0.0127x+1.2291, R2=0.9904 and y=−0.0849x+1.1965, R2=0.9902 (Fig. 2) with the detection limit of 0.15 µg/mL (n=5) and 0.03 µg/mL for MOR and IBU respectively. Table 1 shows a comparison between the results obtained by the present method and those obtained by other methods reported for the determination of these drugs. As can be seen in Table 1, present method had a good detection limit and linear range compared to those obtained from reverse phase-high performance liquid chromatography (RP-HPLC), electrochemical method, liquid chromatography- mass spectrometry (LC-MS-MS), gas chromatography- mass spectrometry (GC-MS) and direct aqueous derivatization methods [40], [41], [42], [43], [44], [45], [46], [47]. It should be highlighted that the major advantages of this method were use of very simple method for simultaneous determination of these drugs.
Fig. 2.
Calibration curve (a) morphine 1.33–33.29 µg/mL and (b) ibuprofen 0.28–6.9 µg/mL. Conditions: temperature 25 °C, ionic strength 1 mmol/L, reaction time 10 min, pH 6: AuNPs, 10 n mol / L.
Table 1.
Linear range and limit of detection of the proposed method in comparison with some previous methods for analysis of morphine and ibuprofen.
| Compound | Method | Linear range (μg/mL) | LOD (μg/mL) | Reference |
|---|---|---|---|---|
| Morphine | RP-HPLC | 0.15–2 | 0.05 | [41] |
| LC-MS-MS | 0.002–2 | 0.001 | [42] | |
| Direct aqueous derivatization | 0.008–5.0 | 0.002 | [43] | |
| GC-MS | 0.02–20 | 0.003 | [44] | |
| Spectrophotometry | 0.025–2 | 0.002 | [45] | |
| Ibuprofen | Derivatives of the ratio spectra method | 2–32 | 0.53 | [46] |
| HPLC | 6.1–200 | 1.7 | [47] | |
| Spectrophotometry | 0.28–6.9(IBU) | 0.03(IBU)- | This work | |
| 1.33–33.29 (MOR) | 0.15(MOR) | |||
RP-HPLC: Reverse phase-high performance liquid chromatography; LC-MS-MS: Liquid chromatography-tandem mass spectrometry; GC-MS: Gas chromatography-mass spectrometry; HPLC: High performance liquid chromatography
3.7. Simultaneous determination of MOR and IBU
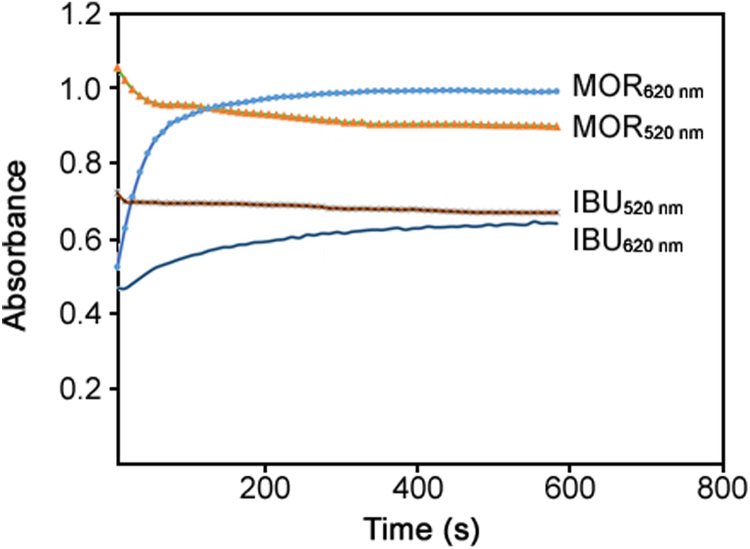
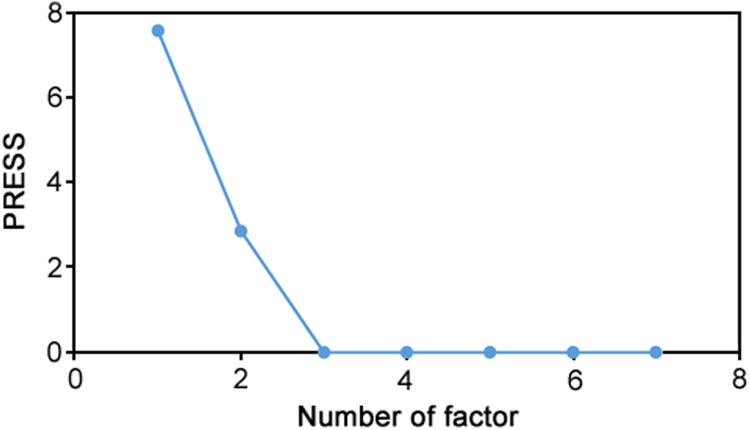
As mentioned above, in order to to select the number of factors in PLS algorithm a cross-validation method leaving out one sample at a time, was employed. Increase in the intensity of the absorbance at 620 nm was directly related to the level of MOR and/or IBU in the sample (Fig. 3a–b). The rate of aggregation of the synthesized AuNPs by citrate were correlated to MOR and IBU level by different rate constants. The calibration and prediction sets were collected by monitoring the absorbance increase at 620 nm and were used in PLS process. For the mentioned set of 20 sample kinetic profiles, PLS1 and PLS2 algorithms were applied and by these calibrations the amount of the samples left out during the calibration process was calculated. Changes in predicted residual error sum of squares (PRESS) in PLS2 calibration as a function of the number of PLS latent variables are given in Fig. 4. As can be seen 3 components were enough to construct the PLS2 model. Nonlinearity in the absorbance–concentration relationship and interaction between the factors could be considered as the other sources of chemical factors.
Fig. 3.
Change in absorbance of AuNPs at 520 and 620 nm versus time (kinetic profiles) by injection of morphine and ibuprofen. Conditions: morphine/ ibuprofen concentration: 5.0 µg/mL, temperature 25 °C, ionic strength 1 mmol/L, pH of 6: AuNPs, 10 n mol / L.
Fig. 4.
Plot of PRESS against the number of factors for simultaneous determination of morphine and ibuprofen.
The predicted values of MOR and IBU levels in the calibration and prediction samples and their corresponding relative prediction errors are listed in Table 2., Table 3., Table 4.. It was observed that the predicted values were very close to the actual amounts and the relative prediction errors were lower than 5.0%. This confirmed the success of PLS regression for accurate prediction of MOR and IBU amounts in samples. It would be beneficial to evaluate whether the results of PLS1 and PLS2 multivariate calibration resulted in a more appropriate model. For this comparison and the validation of the models, some statistical parameters including root mean square error of prediction (RMSEP), root mean square error of cross validation (RMSECV) and root mean square error of calibration (RMSEC) were calculated. The calculated statistical parameters are given in Table 4. One can see from Table 2., Table 3. that the calibration and prediction results of PLS are more consistent than those of univariate calibration.
Table 2.
The level of reference value of IBU and MOR in the prediction set by PLS modeling of kinetic profiles.
| Reference value (µg L−1) |
Predicted value (µg L−1) |
|||||
|---|---|---|---|---|---|---|
| No. | IBU | MOR | PLS1 |
PLS2 |
||
| IBU | MOR | IBU | MOR | |||
| 1 | 460 | 230 | 418.9 | 221.5 | 418.98 | 221.14 |
| 2 | 530 | 280 | 555.06 | 285.5 | 555.07 | 285.49 |
| 3 | 300 | 160 | 289.43 | 156.9 | 289.46 | 156.8 |
| 4 | 660 | 360 | 652.4 | 359 | 652.4 | 359.01 |
| 5 | 660 | 370 | 663.26 | 367.7 | 663.25 | 367.83 |
| 6 | 580 | 330 | 594.99 | 332.4 | 594.96 | 332.57 |
| 7 | 600 | 330 | 600.3 | 333.4 | 600.35 | 333.54 |
| R.S.E. (%) | 3.55 | 1.45 | 3.56 | 1.49 | ||
| R.S.E.t (%) | 3.19 | |||||
Table 3.
The level of reference value of IBU and MOR in the calibration set by PLS modeling of kinetic profiles.
| Reference value (µg L−1) |
Predicted value (µg L−1) |
||||||
|---|---|---|---|---|---|---|---|
| No | IBU | MOR | PLS1 |
PLS2 |
|||
| IBU | MOR | IBU | MOR | ||||
| 1 | 700 | 400 | 704.59 | 365.16 | 704.62 | 365 | |
| 2 | 520 | 260 | 524.96 | 262.55 | 524.96 | 262.54 | |
| 3 | 370 | 190 | 364.83 | 190.68 | 364.83 | 190.7 | |
| 4 | 290 | 160 | 293.77 | 165.03 | 293.77 | 165.06 | |
| 5 | 320 | 170 | 320.16 | 171.75 | 320.15 | 171.77 | |
| 6 | 320 | 170 | 315.2 | 173.44 | 315.2 | 173.47 | |
| 7 | 350 | 200 | 346.6 | 193.8 | 346 | 193.82 | |
| 8 | 460 | 230 | 464.14 | 232.29 | 464.14 | 232.28 | |
| 9 | 450 | 270 | 453.87 | 274.37 | 453.87 | 274.38 | |
| 10 | 740 | 290 | 737.16 | 299.62 | 737.16 | 299.52 | |
| 11 | 180 | 80 | 152.7 | 79.4 | 152.64 | 79.94 | |
| 12 | 170 | 100 | 152.46 | 103.04 | 152.49 | 102.89 | |
| 13 | 170 | 100 | 172.58 | 100.65 | 172.57 | 100.67 | |
| 14 | 330 | 220 | 331.5 | 218 | 331.52 | 217.95 | |
| 15 | 350 | 200 | 346.44 | 199.97 | 346.44 | 200 | |
| 16 | 160 | 90 | 162.17 | 91.43 | 162.17 | 91.45 | |
| 17 | 240 | 130 | 241.6 | 133.55 | 241.6 | 133.56 | |
| 18 | 650 | 370 | 655.49 | 376.18 | 655.49 | 376.22 | |
| 19 | 640 | 360 | 639.75 | 367.63 | 639.74 | 367.69 | |
| 20 | 200 | 100 | 194.34 | 110.29 | 194.34 | 110.34 | |
| R.S.E. (%) | 1.91 | 4.03 | 1.92 | 4.04 | |||
| R.S.E. t (%) | 2.55 | ||||||
Table 4.
Statistical parameters of the PLS1 and PLS2 calibration models developed for simultaneously determination of IBU and MOR with kinetic data.
| Model of prediction |
||
|---|---|---|
| Statistical parameters | PLS1 (%) | PLS2 (%) |
| RMSEP | 4.7 | 4.69 |
| RMSECV | 4.1 | 4.2 |
| RMSEC | 5.1 | 5.2 |
The relative standard error and the total relative standard error were calculated using following equations and represented in Table 2., Table 3..
3.8. Interference effect
The influences of foreign coexisting substances such as naproxen, ascorbic acid, tramadol, codeine, acetaminophen, saccharides, amino acids and ions were tested. As listed in Table S1, some of the examined coexisting substances had remarkable interference on the assay. From the results, the interference of naproxen, ascorbic acid, Na2NO2, tryptophan, tyrosine, glucose, sucrose, fructose and lactose were very weak. Among the tested substances K+, Na+, NO3-, I-, Cl-, Mg2+, Fe3+ and Ca2+, codeine and cysteine could be present with relatively higher concentrations without any problems but cefexime, ceftriaxone, NH2OH, Mn2+, Cd2+, SO42-, Ca2+,Zr2+,Co2+, Zn2+, Ni 2+, Al 3+, Fe 2+ and Cu2+ could only be allowed with relatively low concentrations. The allowed concentrations of these interfering substances however, were still rather higher than those of MOR and IBU which indicated that this method had a good selectivity between drugs and other species.
3.9. Real sample analysis
In order to test the applicability of the proposed method, it was applied to determine IBU and MOR in spiked serum and urine samples. The constructed PLS model was applied to estimate the concentration of IBU and MOR in these spiked samples. The obtained results showed good recoveries (99.4–110.7 ℅) (Table S2). The results demonstrated the potential applicability of this method for simultaneous detection of IBU and MOR in real samples.
3.10. Conclusion
The SPR of the AuNPs, synthesized by the reduction of gold ion with citrate, were used as a novel analytical tool for the determination of drugs and species based on the aggregation of these nanoparticles. A direct relationship was found between the aggregation rate recorded in λmax at about 520 and 620 nm. Multivariate calibration modeling of the kinetic absorbance data by PLS regression produced accurate results and the relative prediction errors were almost lower than 5%. In comparison with available analytical methods for simultaneous determination of IBU and MOR, the proposed method had following advantages: (i) it needed lower amounts of reagents; (ii) the proposed method had a simple, fast, and low cost procedure; and (iii)in contrast to chromatographic methods, this method did not need any expensive apparatus.
Footnotes
Peer review under responsibility of Xi'an Jiaotong University.
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.jpha.2017.03.001.
Appendix A. Supplementary material
Supplementary material
.
References
- 1.Gao L., Ren S. prediction of nitrophenol-type compounds using chemometrics and spectrophotometry. Anal. Biochem. 2010;405:184–186. doi: 10.1016/j.ab.2010.06.032. [DOI] [PubMed] [Google Scholar]
- 2.Madrakian T., Afkhami A., Borazjani M. partial least-squares regression for the simultaneous determination of aluminum and beryllium in geochemical samples using xylenol orange Spectrochim . Acta Part A. 2005;61:2988–2990. doi: 10.1016/j.saa.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 3.Hoflich K., Yang R.B., Berger A. the direct writing of plasmonic gold nanostructures by electron-beam-induced deposition, Adv. Mater. 2011;23:2657–2661. doi: 10.1002/adma.201004114. [DOI] [PubMed] [Google Scholar]
- 4.Zeng Ch, Chen Y., Kirschbaum K. Structural patterns at all scales in a nonmetallic chiral Au133 (SR) 52 nanoparticle. Sci. Adv. 2015;1:e1500045. doi: 10.1126/sciadv.1500045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lu W., Wang L., Li J. quantitative investigation of the poly-adenine DNA dissociation from the surface of gold nanoparticles. Sci. Rep. 2015;5:158–160. doi: 10.1038/srep10158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu B., Tan H., Chen Y. Visual detection of silver (I) ions by a chromogenic reaction catalyzed by gold nanoparticles. Microchim Acta. 2013;180:331–335. [Google Scholar]
- 7.Chansuvarn W., Imyim A. Visual and colorimetric detection of mercury (II) ion using gold nanoparticles stabilized with a dithia-diaza ligand. Microchim. Acta. 2012;176:64–66. [Google Scholar]
- 8.Vilela D., Gonzalez M.C., Escarpa A. sensing colorimetric approaches based on gold and silver nanoparticles aggregation: chemical creativity behind the assay. Anal. Chim. Acta. 2012;751:24–26. doi: 10.1016/j.aca.2012.08.043. [DOI] [PubMed] [Google Scholar]
- 9.Xue Y., Zhao H., Wu Z. Colorimetric detection of Cd2+ using gold nanoparticles functionalized with 6-mercaptonicotinic acid and L-Cysteine. Analyst. 2011;136:3725–3730. doi: 10.1039/c1an15238f. [DOI] [PubMed] [Google Scholar]
- 10.Jiang Y., Zhao H., Zhu N. A simple assay for direct colorimetric visualization of trinitrotoluene at picomolar levels using gold nanoparticles. Angew. Chem. Int. Ed. 2008;47:8601–8605. doi: 10.1002/anie.200804066. [DOI] [PubMed] [Google Scholar]
- 11.Kong B., Zhu A., Luo Y. sensitive and selective colorimetric visualization of cerebral dopamine based on double molecular recognition. Angew. Chem. Int. Ed. 2011;50:1837–1839. doi: 10.1002/anie.201007071. [DOI] [PubMed] [Google Scholar]
- 12.Ai K., Liu Y., Lu L. Hydrogen-Bonding Recognition-Induced Color Change of Gold Nanoparticles for Visual Detection of Melamine in Raw Milk and Infant Formula. J. Am. Chem. Soc. 2009;131:9496–9499. doi: 10.1021/ja9037017. [DOI] [PubMed] [Google Scholar]
- 13.M. S. Hynninen, D.C. H. Cheng, I. Hossain, et al. Canad. J Anesthesia, 47 (2000) pp. 1182–1187. [DOI] [PubMed]
- 14.Tucci J., Bandiera E., Darwiche R. Paracetamol and Ibuprofen for paediatric Pain and Fever. J. Pharma. Pract. Res. 2009;39:223–225. [Google Scholar]
- 15.McCloskey K., Cranswick N., Connell T. Making decisions to limit treatment in life-limiting and life-threatening conditions in children: a framework for practice. Arch. Dis. Child. 2015;100:1–23. doi: 10.1136/archdischild-2014-306666. [DOI] [PubMed] [Google Scholar]
- 16.El-Didamony A., Hafeez S. Spectrophotometric determination of thioridazine hydrochloride in tablets and biological fluids by ion-pair and oxidation reactions. Spec. Int. J. 2012;27:129–141. [Google Scholar]
- 17.Swaroopa G., Rani N. Polarographic determination of ibuprofen by calibration method using acetic acid and different maxima suppressors. Int. Res. J. of Sci. Eng. 2015;3:214–223. [Google Scholar]
- 18.Diani Saraan S.M., Sinaga S.M. development method for determination of ternary mixture of paracetamol, ibuprofen and caffeine in tablet dosage form using zero-crossing derivative spectrophotometric. Int. J. Pharm. Tech. Res. 2015;7:349–353. [Google Scholar]
- 19.Hassan W.S. determination of ibuprofen and paracetamol in binary mixture using chemo metric-assisted spectrophotometric methods. Am. J. Appl. Sci. 2008;15:1005–1012. [Google Scholar]
- 20.El-Brashy A., Eid M., Talaat W. Kinetic Spectrophotometric Method for The Determination of ibuprofen in Pharmaceuticals and Biological Fluids. Int. J. Biomed. Sci. 2006;2:406–413. [PMC free article] [PubMed] [Google Scholar]
- 21.Al-Abachi M.Q., Al-Safi S.A. Spectrophotometric Kinetic Methods for the Determination of ibuprofen in Pure Form and Pharmaceutical Preparations. Iraq. J. Sci. 2015;56:2704–2717. [Google Scholar]
- 22.Fokou P. Valere Tsouh, Kwadwo Nyarko A., Appiah-Opong R. Update on Medicinal Plants with Potency on Mycobacterium ulcerans. Bio-Med. Res. Int. 2015;16:16–20. doi: 10.1155/2015/917086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qureshi T., Memon N., Memon S.Q. Determination of Ibuprofen Drug in Aqueous Environmental Samples by Gas Chromatography–mass Spectrometry without Derivatization. Colum. Int. Pub. Am. J. Mod. Chroma. 2014;1:45–54. [Google Scholar]
- 24.Alvi S.N., Yusuf A., Al Gaai E. Rapid determination of ibuprofen concentration in human plasma by high performance liquid chromatography, word J. pharm. pharmacist. J. Pharm. Pharmacist. Sci. 2014;3:1767–1777. [Google Scholar]
- 25.Virupaxappa B.S., Shivaprasad K.H., Kulkarni Raviraj M. Kinetic Estimation of Ibuprofen and Nimesulide in Pharmaceuticals. Asi. J. Res. Chem. 2011;659:665–670. [Google Scholar]
- 26.Szkutnik-Fiedler D., Grzekowiak E. HPLC-UV determination of morphine in human plasma and its application to the clinical study. Act. Pol. Pharm. Drug Res. 2011;68:473–479. [PubMed] [Google Scholar]
- 27.Mabuchi M., Takatsuka S., Matsuoka M. Determination of morphine, morphine-3-glucuronide and morphine-6-glucuronide in monkey and dog plasma by HPLC-electrospray ionization mass spectrometry. J. Pharm. Biomed. Anal. 2004;35:563–574. doi: 10.1016/j.jpba.2004.01.026. [DOI] [PubMed] [Google Scholar]
- 28.Li J.F., Dong C., S-Contin M. (Morphine) clinical pharmacology - prescription drugs and medications at Rx List. Spectrochim. Acta A. 2009;71:1938–1940. [Google Scholar]
- 29.Atta N.F., Gala A. Electrochemical Morphine Sensing Using Gold Nanoparticles Modified Carbon Paste Electrode. Int. J. Electrochem. Sci. 2011;6:5066–5081. [Google Scholar]
- 30.Hao H.X., Zhou H., Chang J. Molecularly imprinted polymers for determination of morphine, plasmon resonance spectroscopy. Chin. Chem. Lett. 2011;22:477–480. [Google Scholar]
- 31.Gu Y., Yan X., Li C. Biomimetic sensor based on molecularly imprinted polymer with nitroreductase-like activity for morphine detection. Biosens. Bioelectron. 2016;15:393–399. doi: 10.1016/j.bios.2015.09.060. [DOI] [PubMed] [Google Scholar]
- 32.Ho K.C., Chen C.Y., Hsu H.C. Amperometric detection of morphine at a Prussian blue-modified indium tin oxide electrode. Biosens. Bioelectron. 2004;20:3–8. doi: 10.1016/j.bios.2003.11.027. [DOI] [PubMed] [Google Scholar]
- 33.Yeh W.M., Ho K.C. Amperometric morphine sensing using a molecularly imprinted polymer-modified electrode. Anal. Chim. Acta. 2005;542:76–79. [Google Scholar]
- 34.Idris A.M., Alnajjar A.O. Application of ion mobility spectrometry for determination of morphine in human urine. Talanta. 2008;77:522–525. [Google Scholar]
- 35.Atta N.F., Gala A., Ahmed R.A. Sensitive electrochemical determination of morphine using gold nanoparticles–ferrocene modified carbon paste electrode. Electro. Anal. 2011;23:737–739. [Google Scholar]
- 36.Verstraete A.G. Detection times of drugs of abuse in blood, urine, and oral fluid. Ther. Drug. Monit. 2004;26:200–205. doi: 10.1097/00007691-200404000-00020. [DOI] [PubMed] [Google Scholar]
- 37.Sergi M., Bafile E., Compagnone Curini D.R. Multiclass analysis of illicit drugs in plasma and oral fluids by LC-MS/MS. Anal. Bioanal. Chem. 2009;393:709–711. doi: 10.1007/s00216-008-2456-3. [DOI] [PubMed] [Google Scholar]
- 38.Kazanga I., Tameni S., Piccinotti A. Psychoactive substances in seriously injured drivers in Denmark. Forensic Sci. Int. 2012;215:46–49. [Google Scholar]
- 39.Conde J., Dias J.T., Grazú V. Revisiting 30 years of biofunctionalization and surface chemistry of inorganic nanoparticles for nanomedicine. Front. Chem. 2014;2:48–50. doi: 10.3389/fchem.2014.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Buzea C., Ivan I., Blandino P. Nanomaterials and nanoparticles: sources and toxicity. Bio. Inter. 2007;2:171–175. doi: 10.1116/1.2815690. [DOI] [PubMed] [Google Scholar]
- 41.Ruzilawati A.B., Wan Yusuf W.N., Ramli N. Determination of Morphine in Human Urine by A Simple Reverse Phase High-Performance Liquid Chromatography Method with UV Detection. Int. J. Pharm. Sci. Drug Res. 2013;5:18–22. [Google Scholar]
- 42.Coles R., Mark M., Kushnir I. Surveillance of emerging drugs of abuse in Hong Kong: validation of an analytical tool. J. Anal. Toxico. 2007;31:13–15. doi: 10.12809/hkmj144398. [DOI] [PubMed] [Google Scholar]
- 43.Rezaei B., Foroughi-Dehnavi Sh. Fabrication of electrochemical sensor based on molecularly imprinted polymer and nanoparticles for determination trace amounts of morphine. Ionics. 2015;13:12–15. [Google Scholar]
- 44.Chericoni S., Stefanelli F., Iannella V., Giusiani M. Simultaneous determination of morphine, codeine and 6-acetyl morphine in human urine and blood samples using direct aqueous derivatisation: validation and application to real cases. J. Chroma. B. 2014;949:127–130. doi: 10.1016/j.jchromb.2013.09.034. [DOI] [PubMed] [Google Scholar]
- 45.Zhang X., Cao M. Chen Gaozhong, Hu G. Determination of Morphine and Codeine in Human Urine by Gas Chromatography-Mass Spectrometry. J. Anal. Methods Chem. 2013;15:6–10. doi: 10.1155/2013/151934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bashir M., Mohammadi M.A., Alkazemi B. analgesic effect of intraarticular morphine, after arthroscopic knee surgery Int. J. Pharm. Pharm. Sci. 2013;5:92–95. [Google Scholar]
- 47.Bakar Ruzilawati A., Miran H. Validated high performance liquid chromatography method for analysis of methamphetamine in human urine using liquid- liquid extraction, Asian. J. Pharm. Clin. Res. 2015;8:199–201. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material