Abstract
A 49-year-old woman with a history of heavy alcohol drinking was admitted to our hospital due to jaundice and abdominal distention. A blood test showed leukophilia, mild hypoalbuminemia, hyperbilirubinemia, hepatobiliary injury and coagulopathy. Image studies showed an extremely enlarged fatty liver and splenomegaly. The Japan alcoholic hepatitis score and Maddrey's discriminant function were 10 and 54 points, respectively. We diagnosed her with severe alcoholic hepatitis and treated her with corticosteroids, but her liver function did not improve. We therefore administered the vitamin E product tochopheryl acetate (150 mg/day) as an add-on therapy, after which her leukophilia, liver enzymes and coagulopathy improved immediately.
Keywords: severe alcoholic hepatitis, vitamin E, oxidative stress
Introduction
Alcoholic liver disease (ALD) is classified into three histological stages: fatty liver or simple steatosis, alcoholic hepatitis (AH) and chronic hepatitis with hepatic fibrosis or cirrhosis (1). These stages are not distinguished according to disease progression but rather exist in the individual liver at the same time. AH is a serious form of ALD. It is characterized by the acute onset of a fever, jaundice, hepatomegaly, leukophilia and liver failure in heavy drinkers of alcohol (2). The prognosis of patients with severe AH is very poor. The standard therapy for severe AH is the administration of corticosteroids. Recently, antioxidants such as N-acetylcysteine (NAC) and metadoxine have been shown to decrease the mortality in patients with severe AH (3). However, these drugs have not been approved for ALD in Japan.
Oxidative stress is considered to be an important factor in the development and progression of ALD as well as nonalcoholic steatohepatitis (NASH) (4-6). Vitamin E is known to be an antioxidant that prevents liver damage induced by oxygen free radicals. It was extensively evaluated in the Placebo for Nonalcoholic Steatohepatitis (PIVENS) trial as an option for NASH treatment (4). We herein report a patient with severe AH successfully treated by vitamin E as an add-on to corticosteroid therapy.
Case Report
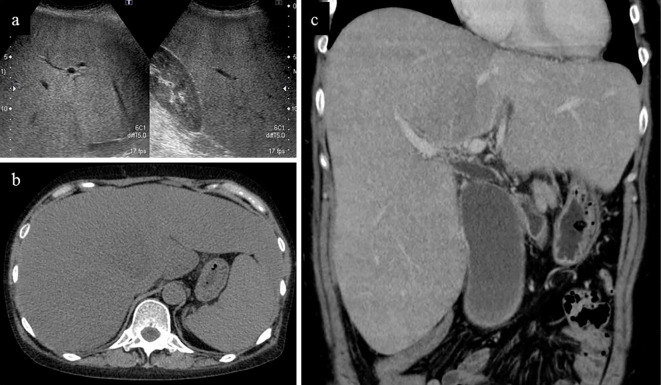
A 49-year-old woman was admitted to our hospital due to jaundice and abdominal distention. She had not received hepatotoxic chemicals or drugs. She had started drinking alcohol at 42 years of age. She had consumed approximately 30 g of ethanol per day between the ages of 42 and 47 and 170 g per day since the age of 47. Her lifetime cumulative alcohol consumption was about 195 kg of ethanol. She had suffered from anorexia for the previous 3 months and had lost 10 kg in weight in the previous 2 months. She had noticed dark urine, jaundice and general fatigue for the previous month. She became febrile and felt abdominal distention one week prior to the admission. A physical examination revealed icteric skin and an enlarged hard liver on palpation. Blood tests showed leukophilia, hypoalbuminemia, hyperbilirubinemia, hepatobiliary injury and coagulopathy, but her viral hepatitis markers, antinuclear antibody and anti-mitochondrial antibody were negative (Table). Ultrasonography and contrast-enhanced computed tomography (CT) showed an extremely enlarged fatty liver and splenomegaly (Figure). Maddrey's discriminant function (MDF) (5) and the Japan alcoholic hepatitis score (JAS) (7) were 54 and 10 points, respectively. She was diagnosed with severe AH.
Table.
Laboratory Findings on Admission.
| Hematology | Serology | ||||
| WBC | 21,700 | /µL | CRP | 10.64 | |
| Neutro. | 89.7 | % | IgG | 1,557 | mg/dL |
| RBC | 227×104 | /µL | IgA | 322 | mg/dL |
| Hb | 9.1 | g/dL | IgM | 109 | mg/dL |
| Ht | 28.0 | % | ANA | (-) | |
| MCV | 32.5 | fL | AMA | (-) | |
| Plt | 28.1×104 | /µL | HAV-IgM Ab | (-) | |
| HBsAg | (-) | ||||
| Biochemistry | HBcAb | (-) | |||
| TP | 7.0 | g/dL | HCV-RNA | (-) | |
| Alb | 3.2 | g/dL | HCV-Ab | (-) | |
| T-bil | 21.8 | mg/dL | HEV-IgA | (-) | |
| D-bil | 16.6 | mg/dL | CMV-IgM | (-) | |
| AST | 220 | IU/L | CMV-IgG | (+) | |
| ALT | 27 | IU/L | EBV-IgM (VCA) | (-) | |
| LDH | 324 | IU/L | EBV-IgG (VCA) | (+) | |
| ALP | 669 | IU/L | Anti EBNA Ab | (+) | |
| γGTP | 693 | IU/L | HGF | 2.66 | ng/mL |
| T-cho | 244 | mg/dL | AFP | 5.2 | ng/mL |
| TG | 379 | mg/dL | |||
| FPG | 107 | mg/dL | Coagulation | ||
| BUN | 11 | mg/dL | PT% | 39.6 | % |
| Cre | 0.37 | mg/dL | PT-INR | 1.61 | |
| Na | 137 | Meq/L | APTT | 56.4 | sec. |
| K | 3.9 | Meq/L | Fibrinogen | 344 | mg/dL |
| Cl | 99 | Meq/L | FDP | 4.6 | µg/mL |
| Fe | 48 | µg/dL | |||
| Ferritin | 829 | ng/mL | Fibrotic marker | ||
| NH3 | 62 | µg/dL | Hyaluronic acid | 14,300 | ng/mL |
| Vitamin B12 | 1,090 | pg/mL | Type IV collagen | 1,936 | ng/mL |
| Folic acid | 2.4 | ng/mL | M2BPGi | >20 | C.O.I. |
| Endotoxin | <3.0 | pg/mL | |||
AFP: alpha fetoprotein, Alb: albumin, ALP: alkaline phosphatase, ALT: alanine aminotransferase, AMA: anti-mitochondrial antibody, ANA: antinuclear antibody, APTT: activated partial thromboplastin time, AST: aspartate aminotransferase, BUN: blood urea nitrogen, CMV: cytomegalovirus, CRP: C-reactive protein, Cre: creatinine, D-bil: direct bilirubin, EBNA: EBV nuclear antigen, EBV: Epstein-Barr virus, FDP: fibrin degradation product, γGTP: gamma glutamiltranspeptidase, HA: hepatitis A, Hb: hemoglobin, HCV: hepatitis C virus, HEV: hepatitis E virus, HGF: hepatocyte growth factor, Ht: hematocrit, LDH: lactate dehydrogenase, MCV: mean corpuslar volume, M2BPGi: Mac-2 binding protein, Plt: platelet, PT: prothrombin time, RBC: red blood cell, T-bil: total bilirubin, T-cho: total cholesterol, TG: triglyceride, TP: total protein, WBC: white blood cell
Figure 1.
Image findings on admission. a) Ultrasonography showed hepatorenal contrast. b) Plain CT and c) contrast-enhanced CT revealed an enlarged liver and spleen.
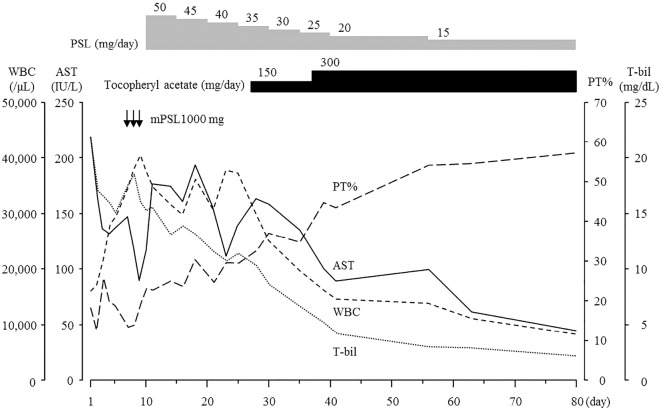
Figure 2.
Clinical course from days 1 to 80. The patient was treated with methylprednisolone 1,000 mg/day from days 7 to 9. Subsequently, oral prednisolone was started at 50 mg/day and tapered by 5 mg every 5 days. Although the T-bil levels decreased gradually, the WBC count, AST and PT% were not improved after initiating steroid therapy. Oral tochopheryl acetate (150 mg/day) was started at day 27. The laboratory data improved immediately. Tochopheryl acetate was increased to 300 mg/day at day 37. AST: aspartate aminotransferase, mPSL: methylprednisolone, PSL: prednisolone, PT: prothrombin time, T-bil: total bilirubin, WBC: white blood cell
She was treated with lactulose and proton pump inhibitor. Her laboratory data improved gradually, and the data at day 5 showed a total bilirubin (T-bil) level of 15.2 mg/dL, aspartate aminotransferase (AST) of 138 IU/L and prothrombin activity (PT%) of 40.0%. However, these data worsened at day 7. Therefore, we performed steroid pulse therapy with methylprednisolone 1,000 mg/day from days 7 to 9. Subsequently, oral prednisolone was started at 50 mg/day and tapered by 5 mg every 5 days. Although the serum level of T-bil was decreased to 10.4 mg/day at day 28, her AST and PT% did not improve after the initiation of steroid therapy. We began administering the oral vitamin E product tochopheryl acetate (150 mg/day) at day 27. Her leukophilia, hyperbilirubinemia, hepatobiliary injury and coagulopathy immediately improved. We increased tochopheryl acetate to 300 mg/day at day 37. CT showed improvement of fatty liver and hepatomegaly at day 39 (Figure). After discharge at day 43, she continued a medium dose of prednisolone and 300 mg/day of tochopheryl acetate. Laboratory data at day 210 showed T-bil of 3.5 mg/dL, AST of 64 IU/L and PT% of 56.1%. The serum levels of fibrotic markers were decreased to 745 ng/mL of hyaluronic acid, 448 ng/mL of type IV collagen and 3.65 C.O.I of M2BPGi at day 105.
Figure 3.
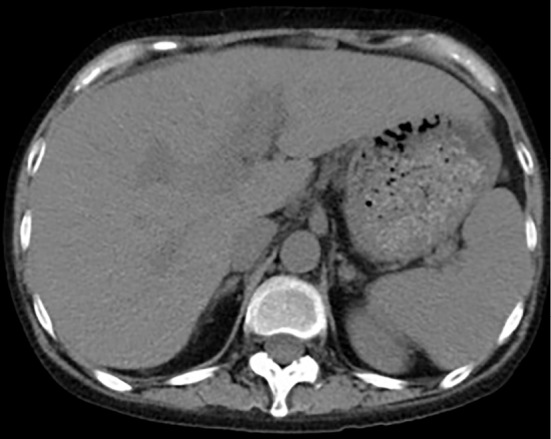
CT at day 39 showed improvement in the fatty liver and hepatomegaly.
Discussion
AH is an acute hepatic manifestation caused by heavy alcohol drinking. There are several scoring systems to assess the severity and guide treatment of AH (5). Among them, MDF is most often used clinically to predict the prognosis of AH (5). Severe AH is usually defined as MDF ≥32. It is associated with high short-term morbidity and mortality (20-50% in one month) (6). The JAS was developed to predict the 100-day mortality in Japanese patients (7). It is calculated by age, presence of gastrointestinal bleeding or disseminated intravascular coagulation, white blood cell count, serum creatinine concentration and prothrombin time and has a higher area under the receiver operating characteristic curve than the Glasgow alcoholic hepatitis score in Japanese patients. The present case was diagnosed with severe AH based on an MDF of 52 and JAS of 10 points.
The first-line treatment for severe AH is the administration of corticosteroids (6). Corticosteroids suppress the inflammatory process by inhibiting the action of transcription factors such as activator protein 1 (AP-1) and nuclear factor κB (NF-κB) (8). In AH, this effect is manifested as a reduction in proinflammatory cytokines, such as interleukin-8 (IL-8) and tumor necrosis factor α (TNF-α) (9,10). A recent network meta-analysis revealed that corticosteroids reduced the short-term mortality compared with placebo (11). However, some patients with severe AH are refractory to corticosteroids. The present case also did not show a sufficient response to corticosteroids.
The pathophysiology of severe AH consists of a complex interaction between ethanol metabolism, inflammation and the immune response (12,13). Kupffer cell activation and the production of pro-inflammatory cytokines and reactive oxygen species (ROS) are implied to be involved in liver damage (14,15). Furthermore, mitochondrial glutathione (mGSH), which is an antioxidant agent, is severely decreased in hepatocytes. Oxidative stress is a key factor in the pathogenesis of severe AH. Therefore, many studies have used antioxidants to treat AH, and some have shown favorable effects in severe cases (16). Antioxidant monotherapy for severe AH has not shown a survival benefit compared with placebo. Vitamin E treatment for three months improved serum hyaluronic acid levels but had no beneficial effects on the liver function in patients with mild to moderate AH (17). A broad antioxidant cocktail was equivalent in effect to a placebo in the six-month mortality and was inferior to corticosteroids in the one-month mortality (18,19). However, antioxidants such as NAC, metadoxine and pentoxifylline (PTX) as an add-on to corticosteroids have shown favorable effects compared with corticosteroids alone. NAC reduces the levels of free radicals and reconstitutes the glutathione stocks in hepatocytes (16). NAC monotherapy did not increase the survival rate compared with a placebo in severe AH, but the combination of NAC and corticosteroids was superior to the effect of corticosteroids alone in reducing the rates of short-term mortality, infection and hepatorenal syndrome (11,16). Metadoxine is an oral antioxidant that has an effect on mitochondria. Patients with severe AH receiving a combination therapy of metadoxine and corticosteroids or PTX showed a significantly higher survival at six months than patients receiving prednisolone or PTX alone (16). PTX is a nonselective phosphodiesterase inhibitor that blocks the transcription of TNF-α to reduce the serum level of the gene product (20,21). It also decreases lipid peroxidation end products by increasing cyclic adenosine monophosphate (cAMP) and cyclic guanosine monophosphate (cGMP) and reduces the cellular injury by free radicals (22). PTX in combination with corticosteroids improved the short-term mortality in cases of severe AH (11).
NASH is a condition associated with obesity and insulin resistance. NASH and AH share many histologic findings, including ballooned hepatocytes, steatosis, Mallory-Denk bodies, inflammation, intrasinusoidal collagen and fibrosis or cirrhosis (6). Vitamin E is a free radical scavenger that acts on transforming growth factor (TGF)-β1, peroxisome proliferator-activated receptors, and apoptosis-regulating genes (23). The PIVENS trial showed that vitamin E significantly improved the histological findings compared with a placebo in patients with NASH (4). Oxidative stress is considered to be one of the main common pathophysiological factors in both AH and NASH in the development and progression of the disease (24-26). It has been well documented that ALD patients are deficient in vitamin E (27). Therefore, we tried treating the present case with vitamin E as an add-on to corticosteroids, although vitamin E does not have an effect on all cases of severe AH (17). In addition, we selected treatment with vitamin E because it was less expensive than other therapies, such as granulocytapheresis and plasma exchange. Granulocytapheresis and plasma exchange are also rather invasive. After the initiation of vitamin E, the white blood cell count, AST and T-bil immediately decreased, and the PT% increased subsequently.
We herein report a patient with severe AH successfully treated with vitamin E as an add-on to corticosteroids. Severe AH has a very poor prognosis and is often refractory to corticosteroid treatment. Therefore, vitamin E add-on therapy may be a new therapeutic option in patients with severe AH refractory to corticosteroids.
The authors state that they have no Conflict of Interest (COI).
References
- 1.O'Shea RS, Dasarathy S, McCullough AJ. Alcoholic liver disease. Hepatology 51: 307-328, 2010. [DOI] [PubMed] [Google Scholar]
- 2.Naveau S, Giraud V, Borotto E, et al. Excess weight risk factor for alcoholic liver disease. Hepatology 25: 108-111, 1997. [DOI] [PubMed] [Google Scholar]
- 3.Thursz M, Morgan TR. Treatment of severe alcoholic hepatitis. Gastroenterology 150: 1823-1834, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sanyal AJ, Chalasani N, Kowdley KV, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med 36: 1675-1685, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crabb DW, Bataller R, Chalasani NP, et al. Standard definitions and common data elements for clinical trials in patients with alcoholic hepatitis: recommendation from the NIAAA alcoholic hepatitis consortia. Gastroenterology 150: 785-790, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lucey MR, Mathurin P, Morgan TR. Alcoholic hepatitis. N Engl J Med 360: 2758-2769, 2009. [DOI] [PubMed] [Google Scholar]
- 7.Horie Y, Yamagishi Y, Ebinuma H, et al. A new scoring system for prognostic stratification in patients with alcoholic hepatitis in Japan. Kanzo 53: 429-431, 2012. (in Japanese, Abstract in English). [Google Scholar]
- 8.Barnes PJ, Karin M. Nuclear factor-kappaB: a pivotal transcription factor in chronic inflammatory diseases. N Engl J Med 336: 1066-1071, 1997. [DOI] [PubMed] [Google Scholar]
- 9.Spahr L, Rubbia-Brandt L, Pugin J, et al. Rapid changes in alcoholic hepatitis histology under steroids: correlation with soluble intercellular adhesion molecule-1 in hepatic venous blood. J Hepatol 35: 582-589, 2001. [DOI] [PubMed] [Google Scholar]
- 10.Taieb J, Mathurin P, Elbim C, et al. Blood neutrophil functions and cytokine release in severe alcoholic hepatitis: effect of corticosteroids. J Hepatol 32: 579-586, 2000. [DOI] [PubMed] [Google Scholar]
- 11.Singh S, Murad MH, Chandar AK, et al. Comparative effectiveness of pharmacological interventions for severe alcoholic hepatitis: a systematic review and network meta-analysis. Gastroenterology 149: 958-970, 2015. [DOI] [PubMed] [Google Scholar]
- 12.Gao B, Bataller R. Alcoholic liver disease: pathogenesis and new therapeutic targets. Gastroenterology 141: 1572-1585, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Higuera-de la Tijera F, Servin-Caamano AI, Cruz-Herrera J, et al. Treatment with metadoxine and its impact on early mortality in patients with severe alcoholic hepatitis. Ann Hepatol 13: 343-352, 2014. [PubMed] [Google Scholar]
- 14.Sass DA, Shaikh OS. Alcoholic hepatitis. Clin Liver Dis 10: 219-237, 2006. [DOI] [PubMed] [Google Scholar]
- 15.Voican CS, Perlemuter G, Naveau S. Mechanisms of the inflammatory reaction implicated in alcoholic hepatitis: 2011 update. Clin Res Hepatol Gastroenterol 35: 465-474, 2011. [DOI] [PubMed] [Google Scholar]
- 16.Nguyen-Khac E, Thevenot T, Piquet MA, et al. Glucocorticoids plus N-acetylcysteine in severe alcoholic hepatitis. N Engl J Med 365: 1781-1789, 2011. [DOI] [PubMed] [Google Scholar]
- 17.Mezey E, Potter JJ, Rennie-Tankersley L, et al. A randomized placebo controlled trial of vitamin E for alcoholic hepatitis. J Hepatol 40: 40-46, 2004. [DOI] [PubMed] [Google Scholar]
- 18.Stewart S, Prince M, Bassendine M, et al. A randomized trial of antioxidant therapy alone or with corticosteroids in acute alcoholic hepatitis. J Hepatol 47: 277-283, 2007. [DOI] [PubMed] [Google Scholar]
- 19.Phillips M, Curtis H, Portmann B, et al. Antioxidants versus corticosteroids in the treatment of severe alcoholic hepatitis-a randomised clinical trial. J Hepatol 44: 784-790, 2006. [DOI] [PubMed] [Google Scholar]
- 20.Radfar M, Larijani B, Hadjibabaie M, et al. Effects of pentoxifylline on oxidative stress and levels of EGF and NO in blood of diabetic type-2 patients; a randomized, double-blind placebo-controlled clinical trial. Biomed Pharmacother 59: 302-306, 2005. [DOI] [PubMed] [Google Scholar]
- 21.Abenavoli L, Milic N, Rouabhia S, et al. Pharmacotherapy of acute alcoholic hepatitis in clinical practice. World J Gastroenterol 20: 2159-2167, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abdollahi M, Chan TS, Subrahmanyam V, et al. Effects of phosphodiesterase 3,4,5 inhibitors on hepatocyte cAMP levels, glycogenolysis, gluconeogenesis and susceptibility to a mitochondrial toxin. Mol Cell Biochem 252: 205-211, 2003. [DOI] [PubMed] [Google Scholar]
- 23.Yoneda M, Hasegawa T, Sato K. Vitamin E therapy for NAFLD/NASH. Nutrition 31: 898-899, 2015. [DOI] [PubMed] [Google Scholar]
- 24.Day CP. Pathogenesis of steatohepatitis. Best Pract Res Clin Gastroenterol 16: 663-678, 2002. [DOI] [PubMed] [Google Scholar]
- 25.Tilg H, Moschen AR. Evolution of inflammation in nonalcoholic fatty liver disease: the multiple parallel hits hypothesis. Hepatology 52: 1836-1846, 2010. [DOI] [PubMed] [Google Scholar]
- 26.Levene AP, Goldin RD. The epidemiology, pathogenesis and histopathology of fatty liver disease. Histopathology 61: 141-152, 2012. [DOI] [PubMed] [Google Scholar]
- 27.Arteel G, Marsano L, Mendez C, et al. Advances in alcoholic liver disease. Best Pract Res Clin Gastroenterol 17: 625-647, 2003. [DOI] [PubMed] [Google Scholar]