Abstract
A 61-year-old woman presented with a 1-month history of decreased activities of daily living. Magnetic resonance imaging revealed abnormal intensities of the bilateral frontal lobes and enhancement of the thickened dura matter. A biopsy of the dura mater revealed multinucleated giant cells. She had sinusitis and hematuria; she was diagnosed with granulomatosis with polyangiitis. Hypertrophic pachymeningitis (HPM) was considered to have interrupted the venous flow and caused vasogenic edema. Bilateral frontal lobe edema resulting from HPM due to granulomatosis with polyangiitis has not been reported. A biopsy and examination for other organ complications were useful for the diagnosis and treatment of our patient.
Keywords: pachymeningitis, vasogenic edema, granulomatosis with polyangiitis
Introduction
Hypertrophic pachymeningitis (HPM) is a rare inflammatory disease that causes thickening of the dura mater and can result from many causes, such as infection, autoimmune disease, and idiopathic disease. HPM generally causes headaches and cranial nerve palsy. We herein report a case of HPM that induced venous compression and congestion, resulting in bilateral frontal lobe vasogenic edema. Based on the biopsy results, we diagnosed the patient with granulomatosis with polyangiitis (GPA).
Case Report
A 61-year-old woman exhibited decreased activities of daily living and developed drowsiness over the course of 1 month. She had no headache. The physical and neurological findings indicated no abnormalities. Her body temperature was 36.8°C. She had no nasal obstruction or other upper respiratory symptoms. She had no skin rash. Her eyesight and vision were normal. Her pupil size, light reflex, and eye movements were normal. There was no motor weakness or sensory disturbance. She was suspected to have a mild decline in her cognitive function.
Serum biochemical and immunological examinations showed a C-reactive protein level of 4.49 mg/d and a leukocyte count of 9,790/μL. The creatinine level was 0.55 mg/dL (normal range: 0.4-0.7), but urinary blood was positive. The level of D-dimer was slightly elevated at 1.05 μg/mL (normal range: <1.0). Antineutrophil cytoplasmic antibody (ANCA) was negative. The patient's cerebrospinal fluid had a leukocyte count of 13/μL and an elevated protein level of 87 mg/dL. The cerebrospinal fluid opening pressure was not elevated. Chest X-ray and computed tomographic scans showed no abnormalities in her lung. On an ophthalmic examination, there were no findings suggestive of an intracranial pressure increase or inflammatory disease.
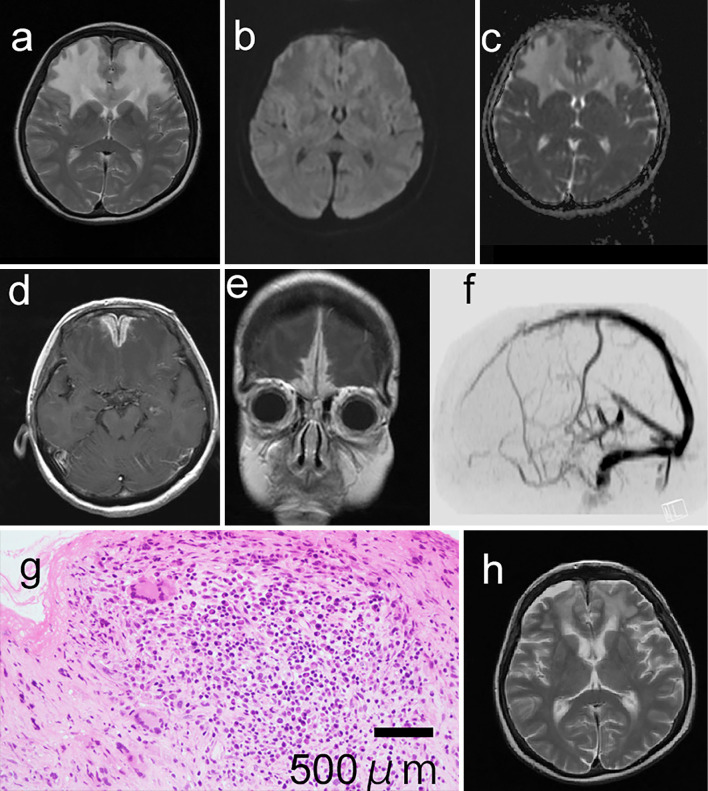
T2-weighted brain magnetic resonance imaging (MRI) revealed high-intensity areas in the bilateral frontal lobes (Figure a). Diffusion-weighted imaging of the same site demonstrated iso-signal intensity, and the apparent diffusion coefficient (ADC) value was increased (Figure b, c). Based on the high signal intensity on the T2-weighted image and the increased ADC value, angioedema was suspected. Thick, enhanced dura mater of the frontal lobe was observed on gadolinium-enhanced T1-weighted imaging (Figure d, e). We hypothesized that the thickened dura mater was compressing and obstructing the venous flow; however, magnetic resonance venography did not reveal any interruption of the superior sagittal sinus (Figure f). MRI also revealed sinusitis in the maxillary sinus.
Figure.
T2-weighted brain magnetic resonance imaging (MRI) demonstrating high-signal-intensity areas in the bilateral frontal lobes (a). The corresponding areas exhibited slightly low intensity on diffusion-weighted imaging (b), and the apparent diffusion coefficient (ADC) values were increased (c). Axial (d) and coronal (e) gadolinium-enhanced MRI demonstrating diffuse dural enhancement and thickening consistent with hypertrophic pachymeningitis. Contrast-enhanced magnetic resonance venography did not reveal the interruption of the superior sagittal sinuses (f). Hematoxylin and Eosin staining tissue biopsy sections of the dura mater (×200) show the infiltration of inflammatory cells and multinucleated giant cells (g). The high signal intensity of the frontal lobes had disappeared (h) three months after starting therapy.
An open biopsy of the dura mater revealed inflammatory cell infiltration with a few multinucleated giant cells (Figure g). The biopsy tissue of the frontal cerebral parenchyma was edematous without inflammatory changes. Based on the findings of sinusitis, hematuria, and multinucleated giant cells of the dura mater, we diagnosed this patient with HPM with GPA. Oral corticosteroid and methotrexate therapy improved the symptoms and MRI abnormalities (Figure h).
Discussion
A few cases of HPM inducing cerebral venous sinus thrombosis have been reported previously (1-3); however, bilateral frontal lobe edema resulting from HPM due to GPA has not been described. We believe that HPM interrupted the venous flow and caused bilateral frontal lobe edema in the present patient.
A high signal intensity on T2-weighted imaging and an increase in the ADC value suggested vasogenic edema of the bilateral frontal lobes. A biopsy of the frontal cerebral parenchyma also uncovered edema without inflammation and malignancy. In the case of cerebral venous thrombosis, bilateral brain involvement is not rare because of the anatomy of cerebral venous drainage (4). Although interruption of the flow of the superior sagittal sinus was not demonstrated in our case, we believe that reflux failure of the venous, superior sagittal sinus, and other small vessels explained the bilateral involvement.
In the present case, although serum ANCA was negative, a dura mater biopsy and examination for other organ complications were useful for achieving an accurate diagnosis. In a review of 48 cases of GPA with meningeal manifestations, the onset of HPM preceded other signs of GPA in 13 patients (27%). In 13 of 35 patients (37.2%), serum ANCA was negative (5). Even if serum ANCA is negative, it is important to pay attention to the possibility of GPA. An accurate diagnosis led to effective treatment in our patient.
In conclusion, to our knowledge, this is the first case report of HPM due to GPA inducing bilateral frontal lobe edema. If serum ANCA is negative, then clinicians should check for other organ complications.
The authors state that they have no Conflict of Interest (COI).
References
- 1.Xia Z, Chen-Plotkin A, Schmahmann JD. Hypertrophic pachymeningitis and cerebral venous sinus thrombosis in inflammatory bowel disease. J Clin Neurosci 17: 1454-1456, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saito T, Fujimori J, Yoshida S, Kaneko K, Kodera T. Case of cerebral venous thrombosis caused by MPO-ANCA associated hypertrophic pachymeningitis. Rinsho Shinkeigaku (Clin Neurol) 54: 827-830, 2014. (in Japanese, Abstract in English). [DOI] [PubMed] [Google Scholar]
- 3.Bhatia R, Tripathi M, Srivastava A, et al. Idiopathic hypertrophic cranial pachymeningitis and dural sinus occlusion: two patients with long-term follow up. J Clin Neurosci 16: 937-942, 2009. [DOI] [PubMed] [Google Scholar]
- 4.Saposnik G, Barinagarrementeria F, Brown RD Jr, et al. Diagnosis and management of cerebral venous thrombosis: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 42: 1158-1192, 2014. [DOI] [PubMed] [Google Scholar]
- 5.Di Comite G, Bozzolo EP, Praderio L, Tresoldi M, Sabbadini MG. Meningeal involvement in Wegener's granulomatosis is associated with localized disease. Clin Exp Rheumatol 24: S60-S64, 2006. [PubMed] [Google Scholar]