Abstract
Diets containing high proportions of fruits and vegetables reduce the risk of onset of chronic diseases. The role of herbal medicines in improving human health is gaining popularity over the years, which also increases the need for safety and efficiency of these products. Green leafy vegetables (GLVs) are the richest source of phenolic compounds with excellent antioxidant properties. Increased consumption of diets containing phenolic compounds may give positive and better results to human health and significantly improves the immune system. Highly selective, susceptible and versatile analytical techniques are necessary for extraction, identification, and quantification of phenolic compounds from plant extracts, which helps to utilize their important biological properties. Recent advances in the pre-treatment procedures, separation techniques and spectrometry methods are used for qualitative and quantitative analysis of phenolic compounds. The online coupling of liquid chromatography with mass spectrometry (LC–MS) has become a useful tool in the metabolic profiling of plant samples. In this review, the separation and identification of phenolic acids and flavonoids from GLVs by LC–MS have been discussed along with the general extraction procedures and other sources of mass spectrometer used. The review is devoted to the understanding of the structural configuration, nature and accumulation pattern of phenolic acids and flavonoids in plants and to highlighting the recent developments in the chemical investigation of these compounds by chromatographic and spectroscopic techniques. It concludes with the advantages of the combination of these two methods and prospects.
Keywords: Green leafy vegetables, Phenolic acids, Flavonoids, HPLC, ESI-MS
1. Introduction
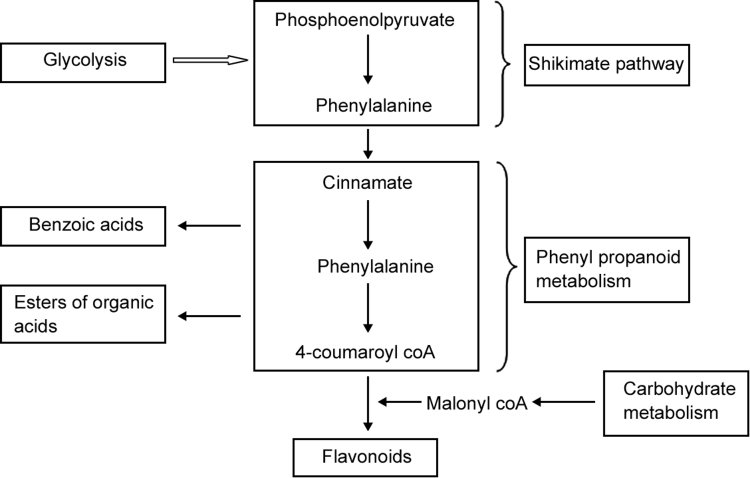
Plants are the important source for new natural products [1]. Natural products have gained popularity over the years due to their enormous potential as anti-oxidants, enzyme inhibitors, hypocholesterolemic agents, immunosuppressive agents, herbicides, anti-migraine and anti-parasitic agents [2]. Phytochemistry is the study of chemical or phytochemical compounds found in plants. These compounds mainly represent secondary metabolites in plants and are primarily produced due to biotic and abiotic stress factors. Oxidative stress is responsible for the pathogenesis of chronic diseases such as cancer, cardiovascular diseases (CVD) and diabetes [3]. Green vegetables are good source of antioxidants, but the exact mechanism in the prevention and treatment of chronic diseases by these vegetables is still not fully understood. It is assumed that the combination of antioxidants and phytochemicals found in vegetables may improve health by inhibiting the formation of free radicals responsible for early stage development of some chronic diseases [4]. Secondary metabolites exhibit significant pharmacological properties and play a crucial role in benefiting human health [1]. Phenolic compounds in plants are synthesized from shikimate and phenylpropanoid pathways represented in Fig. 1 [5]. Green leafy vegetables (GLVs) are the great source of plant foods as they contain nutrients, minerals, and antioxidants which are useful for maintaining human health as they occur naturally in whole foods. They play a crucial role in any well-balanced diets with significant nutritional value [6]. Regular intake of GLVs in diet reduces the risk of coronary heart diseases and pulmonary diseases and may inhibit various forms of cancer due to the presence of phenolic compounds [1].
Fig. 1.
Biosynthetic pathways involved in the formation of phenolic compounds [5].
Crude plant extracts usually contain complex mixture of different bioactive constituents. Among them, secondary metabolites are widely used for the preparation of herbal medicines and their derivative products. The herbs may provide a number of bioactive components [2]. Rapid and accurate detection of bioactive phytocompounds is crucial in the phytochemical investigation of crude plant samples [7]. Efficient systems should be available to screen those plant materials rapidly [1]. Chromatographic techniques contribute significantly in the area of natural products, especially regarding identification, separation, and characterization of bioactive compounds from plant sources [8]. Efficient screening of the plant extracts can be performed with both biological assays and chromatographic methods such as high-performance liquid chromatography (HPLC) in combination with various detection methods [7]. HPLC is a powerful technique for rapid analysis of bioactive constituents because it enables systematic profiling of the complex plant samples and specifically focuses on their identification and consistent evaluation of the identified compounds. However, a valuable and convincing chromatographic fingerprint must assign most of its peaks corresponding to the active constituents and toxic ingredients. The characteristic fragmentation patterns of the reference standards, the nature of analyzed samples (polar or non-polar) obtained from their retention time data, the online (UV) spectral information, literature information available and bio-sources of the compounds allow the identification of the complex bioactive phytochemical constituents in the plant samples. But HPLC could provide only limited structural information like UV spectrum, and tentative characterization of the eluted compound peaks in the samples by comparing with that of standard reference compounds. The interface of the chromatographic method with a mass spectrometer (MS) detection system is efficient. MS provides rapid qualitative determination and identification of unknown compounds from natural products extracts. Electro-spray ionization (ESI) is the most successful interface used in liquid chromatography–mass spectrometry (LC–MS) configuration. Therefore, LC–MS provides a new powerful approach to identifying the unknown constituents in plant extracts through efficient separation capabilities of HPLC and exact structural characterization by MS [2]. The online characterization of secondary metabolites from natural product extracts or fractions requires a high degree of sophistication, vast structural information, sensitivity, and selectivity. The introduction of hyphenated techniques enable the natural product researchers to use extremely powerful new tools to achieve excellent separation efficiency as well as to obtain online complementary spectroscopic data of the peak of interest within a complex sample mixture [9].
The present review will give a better understanding of the occurrence, distribution pattern, nature of phenolic acids and flavonoids and aims to highlight the significance and role of the hyphenated LC–MS methods in identifying, purifying and characterizing phenolic acids and flavonoids from GLVs. The article intends to summarize the advantages of the combination of this hyphenated technique and focus on the current state of the art of both the techniques for qualitative analysis of these compounds from GLVs. In this sense, the combination of LC–ESI-MS accurate mass measurements to generate empirical formula and to provide fragmentation data for structural confirmation represents a robust method for the analysis of complex systems [3].
2. Nomenclature of phenolic acids and flavonoids and their significance in GLVs
Phenolic compounds represent a large group of compounds (more than 8000) produced by the shikimic acid pathway. They are characterized by at least one aromatic ring attached with one or more hydroxyl groups [10]. Phenolic acids are found in the cell walls of plants as by-products of the monolignol pathway and the breakdown products of lignin. They are present either in free form or conjugated with sugar residue linked through hydroxyl groups or sometimes as conjugated esters [11]. Based on their carbon framework, they can be classified as hydroxy-cinnamic acids and hydroxy-benzoic acids. Benzoic and cinnamic acid derivatives accumulate in aerial parts (leaf, root, stem, and fruit) of GLVs. Hydroxy-cinnamic acid derived compounds are present as simple esters with sugar moieties such as glucose, while hydroxy-benzoic acids are usually present as glycosides [12]. Hydroxy-cinnamic acids with C6-C3 configuration are localized in plant cell wall and are involved in defense mechanisms of plants [10]. The major cinnamic acid derivatives found in plants are coumaric acid, sinapic acid, caffeic acid and ferulic acid. Hydroxy-benzoic acids have C6-C1 configuration with gallic acid, protocatechuic acid, syringic acid, and vanillic acid as major compounds [12]. Due to their enormous structural diversity, phenolic acids are associated with important functions in plants such as protein synthesis, nutrient uptake, enzyme activity, and allelopathy [13] along with other biological properties such as anti-pyretic, analgesic and anti-microbial activity [12].
Flavonoids have a C6-C3-C6 framework. They consist of fifteen carbon atoms with aromatic rings A and B joined by a heterocyclic C- ring. Based on the substitution pattern of the C-ring, they are classified into flavonols, flavones, isoflavones, anthocyanidins and flavanols [14]. They are found in plant cell vacuoles and undergo various chemical modifications such as hydroxylation, glycosylation, and methylation to form respective derivatives [15]. O-glycosides are common among a major group of leafy vegetables. The chemical structure of flavonoids is responsible for their hydrogen donating (radical scavenging) and metal-chelating properties [3]. Their main function is to protect plants against pathogen attack, UV light, and oxidative cell injury [14]. Flavonoids exhibit anti-fungal, anti-bacterial, anti-viral, anti-inflammatory and anti-ulcer activities [15]. Flavonoid conjugates act as UV protectants in plant cells [16].
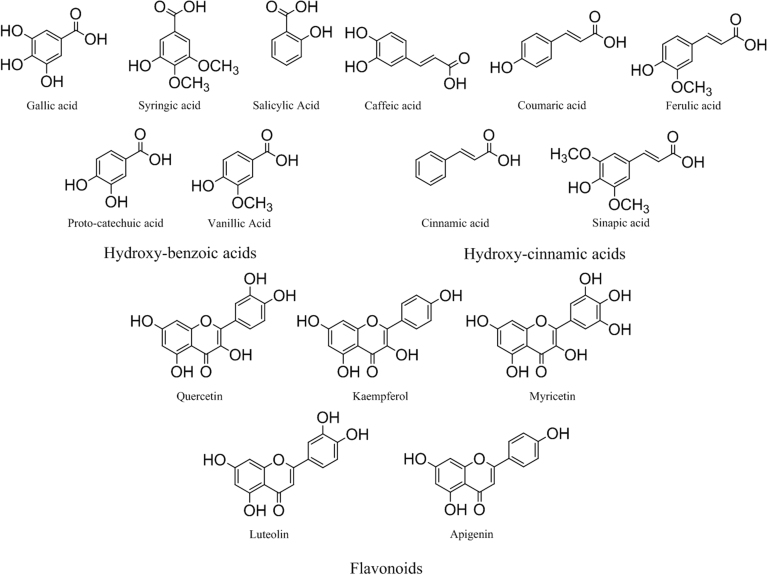
GLVs consist of polyphenolic compounds with diverse structural complexity. Some of the major phenolic acids and flavonoids found in GLVs are presented in Fig. 2. These compounds are mainly responsible for their anti-bacterial, anti-fungal, anti-inflammatory, and anti-proliferative activities [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29]. Table 1 summarizes significant biological properties of important GLV species.
Fig. 2.
Major phenolic acids and flavonoids in GLVs.
Table 1.
Pharmacological properties of important GLV species.
| Plant species | Pharmacological properties | References |
|---|---|---|
| Amaranthus hybridus | Antino-ciceptive | [17] |
| Amaranthus spinosus | Anti-malarial, Analgesic, Immuno-modulatory, Anti-fertility, Anti-diabetic, Anti-hyperlipidemic | [18] |
| Amaranthus spinosus L. | Anti-inflammatory | [19] |
| Amaranthus spinosus | Anti-diarrheal, Anti-ulcer | [20] |
| Amaranthus spinosus | Anti-depressant | [21] |
| Basella alba | Wound healing, Anti-viral, Anti-ulcer, Anti-inflammatory, Anti-depressant, Hepatoprotective, Anti-diabetic | [22] |
| Basella rubra | Anti-inflammatory | [23] |
| Brassica oleracea L. | Anti-inflammatory | [24] |
| Brassica oleracea | Anti-ulcerogenic | [25] |
| Brassica oleracea L. | Anti-ulcer | [26] |
| Brassica oleracea | Anti-hyperlipidemic | [27] |
| Spinacia oleracea | Hepatoprotective activity, Anti-cancer activity, CNS depressant effect, Anti-helmintic | [28] |
| Spinacia oleracea | Anti-inflammatory | [29] |
3. Sample preparation and extraction methods
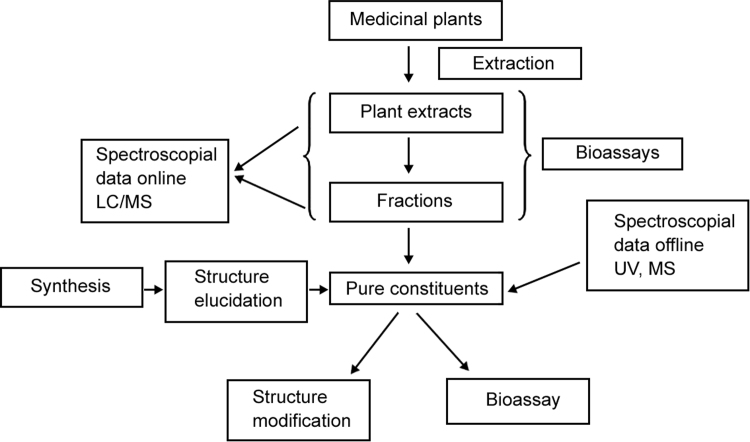
It is always better to know the exact structural entity of the bioactive compounds from plant samples at the initial stages of separation process, which helps to determine an efficient and selective isolation procedure. Chemical screening of samples from natural products extracts allows the localization and targeted isolation of novel bioactive phyto-constituents having significant biological properties [7]. The common method or procedure to obtain the pure form of isolated bioactive compounds from plants is shown in Fig. 3.
Fig. 3.
Procedure for obtaining the active principles from plants and use of LC hyphenated techniques as strategic analytical screening tools during the isolation process of a plant extract.
The concentration of secondary metabolites in GLVs depends on various external factors such as soil quality, irrigation methods used, cultivation process and climatic conditions [30]. External environmental factors and genetic factors are primarily responsible for the accumulation of these compounds in different parts of the plant. Various sample preparation methods such as drying, storage, lyophilization, and homogenization are used routinely during preparation of plant samples [31]. The total extraction yield of specific isolated compound(s) depends on the solvent systems used for extraction. Various factors influence the extraction procedure of phenolic compounds from plant materials such as the chemical nature of the isolated compounds, the methods employed for extraction, storage conditions, and finally the interfering compounds present [32]. Phenolic compounds interact with carbohydrate or proteins and form complexes which are not soluble in all solvents. Therefore, single solvents used may not be sufficient to isolate these compounds in the pure form [13].
Solid samples require pre-identification processes such as sieving, milling or grinding and homogenization whereas liquid samples can be filtered or centrifuged before extraction for successful analysis [13]. Plant extract usually contains a mixture of compounds with small amounts found in cell wall and vacuoles [32]. The solubility of these bioactive compounds depends on the nature of solvent (polar and non-polar or less polar) and degree of polymerization of phenolics [32]. Therefore, solvents with different polarities are important to separate these compounds from complex plant matrices. Generally, extraction procedures take longer time depending on the complexity of plant material, but longer durations may oxidize the phenolic compounds [32]. Polar and organic solvents such as water, methanol, ethanol, ethyl acetate and acetone are normally used for the extraction of phenolic acids and their derivatives [12]. Cell wall bound phenolics can be extracted with alkaline hydrolysis [32]. Further, the extracts concentrated under vacuum usually contain lipid fractions. Therefore, solvents like n-hexane, acetone, petroleum ether, and diethyl ether are used in common for lipid extraction [32]. Freezing the plant samples with liquid nitrogen is one of the convenient sample preparation methods to prevent or stop the further enzymatic activity. Then frozen samples can be ground to form the powder or may be extracted directly. This procedure is helpful for isolating compounds at endocellular level [33]. Leafy vegetables accumulate phenolic acids and flavonoids which are not uniformly distributed at cellular and sub-cellular levels. Plant cell wall contains insoluble phenolics which provide mechanical strength whereas plant vacuoles contain soluble phenolics. An aqueous solution of methanol, ethyl acetate and acetone is the most preferred solvent used for extraction of polyphenols from leafy vegetables [33].
The above sample preparation and extraction methods indicate that leafy vegetables contain a mixture of phenolic acid and flavonoids in significant amounts with varying proportion and a wide range of distribution patterns. The polar and organic solvents play a crucial role in establishing a suitable method to ease the extraction procedure of specific class of phytocompounds located within plant cell wall and vacuoles with highest purity. Analytical methods play a crucial role in identifying new sources of these phyto-compounds from plants [34]. Unwanted compounds or artifacts which affect the final recovery of the desired compounds may form by using several steps of extraction [13]. Therefore, to understand the mechanism and role of these phenolic compounds and their positive effect on human health, it is necessary to know their structure-activity relationship and the information of their exact chemical structure. The biological activity of a particular chemical entity in complex matrices can be established by identifying and purifying those bioactive compounds present in the plant extract samples [35].
4. HPLC
Two major factors play a vital role in natural product research. These are the separation and purification of bioactive constituents in crude plant samples or their fractions obtained from different natural sources, and their accurate structural identification [9]. HPLC is a robust analytical technique mainly used for the qualitative analysis of non-volatile classes of compounds such as phenolics, terpenoids, and alkaloids [36]. It is highly efficient and provides rapid and better analytical separation with higher sample loading capacity [37], [38]. The application of liquid chromatography is the qualitative or quantitative estimation of a particular composition of samples obtained from natural sources. The results of the qualitative analysis are evaluated based on the consistency in retention time of reference standards and the compounds in the analyzed sample. Quantitative estimation is done based on the standard curve generated after reference standards are injected at different concentration levels [39]. Sample derivatization is not required before analysis in this method [40].
The column is a major component of HPLC. The column contains the particles used as stationary phase. The stationary phases usually have a particle size ranging between 3 and 50 µm packing contained in a column with 2–5 mm bore size. Most of the separations are carried out on reversed-phase (RP) columns [31]. RP columns used in HPLC are more desirable and widely used for the analysis of multiple phyto-constituents [41]. Routine HPLC methods use RP octadecyl silica columns for phenolic compounds [33]. Silica-based C18 columns in RP-HPLC contain aliphatic C18 ligands, free silanols, water and mobile phase modifier [42].
Detectors play a significant role in maintaining accuracy and stability and are useful for reduceing the loss during the separation of a specific compound from complex plant samples [31]. Detectors most commonly used for separation of phenolic compounds are UV detectors, DAD and photodiode array detectors (PDA) [30].
Mobile phase selection depends on the type and nature of compounds to be separated by HPLC. Generally, water and organic solvents such as methanol and acetonitrile along with small concentrations of acetic acid, formic acid and tri-fluoroaceticacid (TFA) are used for the separation of phenolic compounds from plant samples in RP-HPLC.
It is a well-known fact that China and India are the major contributors of herbal preparations in the world owing to their enormous diversity and abundant resources of medicinal plants. Therefore, highly selective and sensitive methods are needed to examine the overall composition and to maintain their quality. HPLC is the method of choice and still is the ideal analytical separation technique used commonly for quantitative and qualitative analysis of natural products from crude plant samples or herbal preparations [43]. GLVs are the great source of phenolic compounds, and regular intake of leafy vegetables reduces the risk and may help in the prevention and treatment of chronic diseases. Therefore, to identify their potential value in healthcare, complete metabolic profiling of bioactive constituents is essential to determine the role of individual phenolic compound in GLVs and their associated effects. Various studies have reported the optimized chromatographic conditions used for the separation of phenolic acids and flavonoids from GLVs [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59]. Table 2 lists the compounds studied using HPLC method for qualitative and quantitative analysis. The information provided shows the diverse applications of HPLC technique in natural products analysis through the parameters evaluated and their optimized conditions.
Table 2.
Representative examples of HPLC profiling of phenolic acids and flavonoids in GLVs.
| Plant | Plant parts used | Solvent used for extraction | Stationary phase | Mobile phase composition | Flow-rate, wavelength (nm) settings | Phenolic acids | Flavonoids | References |
|---|---|---|---|---|---|---|---|---|
| Collard greens, Kale and Chinese broccoli ( Brassica oleracea) | Whole plant | Methanol : water (60:40 v/v). | C18 column (250 mm x 4.6 mm) | Solvent A- 0.1% formic acid in water. Solvent B- 0.1% formic acid in acetonitrile. | 1 mL/min, 190-650 nm | Caffeic acid, Coumaric acid, Sinapic acid and Ferulic acid derivatives. | Quercetin, Kaempferol and Isorhamnetin glycosides. | [44] |
| Merremia emarginata | Leaves | 70% aqueous ethanol and 5M HCl. | C18 column ( 4.6 mm x 250 mm) | Solvent A- water with 0.1 % formic acid. Solvent B- 100% ethanol. | 1 mL/min, 210-400 nm | Hydroxy-cinnamic acids, Hydroxy-benzoic acids and their derivatives. | Myricetin glucoside and galactoside. | [45] |
| Sarcocornia ambigua (Amaranthaceae) | Leaf and Stem | 100 % methanol. | C8 column (150 mm x 2.1 mm) | Solvent A- acetonitrile : water (95:5). Solvent B- 0.1 % formic acid in water. | 0.25 mL/min | p-Coumaric acid, Cinnamic acid, Vanillic acid, Ferulic acid, Caffeic acid, Syringic acid, Sinapic acid and Chlorogenic acid. | Galangin, Quercetin, Naringin, Kaempferol and Isoquercetin. | [46] |
| Ocimim gratissimum L. Vernonia amygdalina L. Corchorus olitorius L. Manihot utilissima L. | Whole plant | Methanol or ethanol/water (7:3 v/v) with formic acid (pH 2.5) and boiling water. | C12 column (150 mm x 4 mm) and (250 mm x 4.6 mm) | Solvent A- water with formic acid (pH 3.2). Solvent B- acetonitrile. | 0.4 mL/min and 0.8 mL/min, 254-358 nm | Rosmarinic acid, Caffeic acid and their derivatives, Chlorogenic acid, Ferulic acid and Cichoric acid. | Rutin, Luteolin and their glycosides, Kaempferol and their glycosides, Amentoflavone, Isoquercetin, Quercetin derivative, Cirsimaritin, Nevadensin, Cirsilol and Vicenin. | [47] |
| Spinach (Spinacia oleracea) | Leaves | Boiling water | C18 column (150 mm x 3.9 mm) | Methanol: water (50:50 v/v) adjusted to pH 2.4 by 0.07% formic acid. | 0.9 mL/min, 370 nm. | Not detected | Quercetin, Kaempferol, Apigenin, Luteolin and Myricetin. | [48] |
| Cabbage, Cauliflower & Spinach (Brassica oleraceae & Spinacia oleraceae) | Buds, Flowers and Leaves | Acidified methanol (1% HCl v/v), TBHQ and 1.2 M HCl. | C18 column (250 mm x 4.6 mm) | Solvent A- 3% TFA. Solvent B- acetonitrile: methanol (80:20 v/v). Finally mixture of Solvents A and B (50:50, v/v). | 1 mL/min, 360 nm. | Not detected | Kaempferol, Quercetin and Myricetin. | [49] |
| Red Amaranth (Amaranthus cruentus) | Seeds and Sprouts | Methanol: HCl (0.16 M): water (8:1:1 v/v) and 70 % acetone. | (250 mm x 4.6 mm) | Solvent A- acetonitrile. Solvent B- water. | 1 mL/min, 253 nm. | Gallic acid, p-hydroxy benzoic acid, Vanillic acid, p-Coumaric acid and Syringic acid. | Rutin, Vitexin and Isovitexin. | [50] |
| Komatsuna (Brassica rapa) Mizuna (Brassica rapa) Pok choi (Brassica rapa) Mitsuba (Cryptotaenia japonica) Horseno (Spinacea oleracea) Lettuce (Lactuca sativa) Red amaranth (Amaranthus tricolor) Green Amaranth (Amaranthus tricolor) | Leaves | 80% methanol containing 1% acetic acid and 90% methanol. | STR ODS-II (150 mm x 4.6 mm) | Solvent A- 1% acetic acid in water. (v/v) Solvent B- acetonitrile. | 0.7 mL/min, 254 nm, 280 nm and 360 nm. | Vanillic acid, Syringic acid, Caffeic acid, Chlorogenic acid, p-Coumaric acid, Ferulic acid, m-Coumaric acid and Sinapic acid. | Isoquercetin, Hyperoside and Rutin. | [51] |
| Kale (Brassica oleraceae) | Whole plant | Ethanol, methanol, 80% aqueous ethanol and 80% aqueous methanol. | C18 column (2.1 mm x 50 mm) | Solvent A- water containing 0.1 % formic acid. Solvent B- methanol containing 0.1% formic acid. | 0.4 mL/min | Proto-catechuic acid, 4-hydroxy benzoic acid, Vanillic acid, Salicyclic acid, 3-hydroxy cinnamic acid, cis and trans isomer derivatives of Caffeic acid, p-Coumaric acid, Ferulic acid and Sinapic acid. | Not detected | [52] |
| Amaranthus (Amaranthus spinosus L) | Stems | Water | C18 (250 mm x 4.6 mm) and a security guard C18 ODS and a C18 100 (4 mm x 3.0 mm) | Solvent A- 0.2% acetic acid in water (v/v). Solvent B- 0.5% acetic acid in water and acetonitrile (50:50,v/v). | 1 mL/min, 280 nm, 320 nm and 370 nm. | Caffeoyl quinic acid, Coumaroyl quinic acid and Feruloyl quinic acid. | Quercetin diglucoside, Quercetin-3-0-glucoside, Quercetin-3-0-rutinoside and Kaempferol diglycoside. | [53] |
| Spinach (Spinacia oleracea) | Leaves | Methanol : water: formic acid, (60:37:3 v/v/v) & 3% formic acid. | C18 column (4.6 mm x 250 mm) C18 guard column (3.9 mm x 20 mm) C18 semi-preparative column (7.8 mm x 300 mm) | Solvent A- 0.1 % aqueous formic acid. Solvent B- acetonitrile : methanol: 0.1 % aqueous formic acid (7:2:1,v:v:v). | 1.5 mL/min and 4.3 mL/min, 360 nm. | Not detected | O-methyl flavonol derivatives and flavones derivatives. | [54] |
| Broccoli (Brassica oleracea L.) | Heads | 62.5 % aqueous methanol, 2 M HCl and methanol. | C18 column (4.6 mm x 150 mm) | Methanol :water (60:40) acidified with 0.2 % orthophos-phoric acid. | 1 mL/min,370 nm. | Not detected | Quercetin and Kaempferol. | [55] |
| Kale (Brassica oleracea var.sabellica) | Leaves | 70% aqueous methanol, water, 90% aqueous methanol containing 5% acetic acid. | Semi-preparative luna column (250 mm x 10mm) with C18 security guard column (10 mm x 10mm) | Solvent A- 0.5% acetic acid in water. Solvent B- acetonitrile. | 2.7 mL/min, 280 nm. | Caffeoyl quinic acid, Sinapoyl glucosides. | Quercetin and Kaempferol glycosides. | [56] |
| Cabbage (Brassica oleracea) | Whole plant | 2.6 M NaOH in 53% aqueous methanol and HCl, 62.5% aqueous methanol containing TBHQ (2g/L) and 8 M HCl. | C18 column (3.9 mm x 150 mm) with C18 guard column (3.9 mm x 20 mm) | Solvent A- 0.1% formic acid in water. Solvent B-methanol. | 0.4 mL/min, 330 nm and 360 nm | Caffeic acid, p-Coumaric acid, Ferulic acid and Sinapic acid. | Quercetin and Kaempferol. | [57] |
| Pak choi (Brassica campestris L.) Chinese leaf mustard (Brassica juncea) | Whole plant | Acidic aqueous methanol containing 1% meta-phosphoric acid and 0.5 % oxalic acid dihydrate. | (250 mm x 4 mm) RP-18 Nucleodur column (8 mm x 4 mm) | Free phenolics-Solvent A- 0.15 % TFA in water. Solvent B- acetonitrile. Bound phenolics-Solvent A- 0.1% formic acid in water. Solvent B-acetonitrile. | 0.9 mL/min and 0.7 mL/min , 280 nm and 330 nm. | Vanillic acid, p-hydroxy-benzoic acid, Vanillin, p-Coumaric acid, Sinapic acid, trans-ferulic acid and cis-ferulic acid. | Kaempferol glycosides. | [58] |
| Indian spinach (Basella rubra) | Fruits | Water | C18 column (250 mm x 4.6 mm) | Solvent A- water with acetic acid (pH2.6) Solvent B- 80% acetonitrile. | 1.2 mL/min, 280 nm and 320 nm. | Generic acid, Chlorogenic acid, Sinapic acid, Ferulic acid and Coumaric acid. | Apigenin, Kaempferol, Luteolin, Myricetin and Quercetin. | [59] |
5. MS
MS is considered as a standard instrument in the analytical laboratory to characterize structurally and to calculate the m/z values of the known and unknown compounds present in plant samples. The qualitative analysis is done by calculating the mass and their relevant structural information whereas quantification is achieved by finding out the relationship between the peak and the compound content which the peak represents [39]. The molecular masses of large bio-molecules can be determined with an accuracy of 0.01% of the total molecular mass of the sample by this method [60].
MS can be divided into three steps: ionization, mass analysis, and detection. The sample gets ionized when introduced into the mass spectrometer and the molecular mass of the compound of interest is calculated based on m/z ratio. The resolution, mass range, scan rate and detection limit are the key components required for appropriate selection of the mass analyzer [33]. There are many types of mass analyzers routinely used such as magnetic (B)/electric (E) sector mass analyzer, time of flight (TOF) and quadrupole analyzers. Table 3 shows the type of mass analyzers commonly applied and their properties. Detectors are the important component of mass spectrometer that generates a signal from incident ions through secondary electrons or by the induced current. The detectors are used according to the nature of compounds to be characterized. MS detectors record either a current or charge produced by the ions [43]. Ion detector systems are classified into point detectors and array detectors. MS scans compounds within a particular mass range. The most common modes acquiring LC–MS data are as follows:
-
•
selected ion monitoring (SIM)
-
•
selected reaction monitoring (SRM) or multiple reaction monitoring (MRM)
Table 3.
Types of mass analyzers and their properties.
| Type | (m/z) range | Resolution | Operating pressure (torr) | Scanning speed | Adaptability to methods |
|---|---|---|---|---|---|
| Magnetic Sector | 104 | 105 | 10−6 | Slow | Nil |
| Time of Flight | 106 | 103–104 | 10−6 | Fast | Well suited for MALDI |
| Quadrupole ion trap | 104–105 | 103–104 | 10−3 | Moderate | Well suited for Electrospray |
Fragmentation patterns of the ions are mostly determined by electron spray ionization (ESI) and atmospheric pressure chemical ionization (APCI). Both ionization modes provide a rapid and complete fragmentation pattern with a comprehensive insight into their metabolite composition. ESI is the method of choice in natural products analysis. ESI mode is ideal for LC–MS analysis of secondary metabolites from plants. It is a soft ionization technique capable of producing small fragmentation patterns through electrical energy, which allows the ions to transfer from liquid to gaseous phase [43] before being analyzed in mass spectrometer [61]. Both positive and negative ionization modes are used in natural product analysis [15]. It is one of the successful ionization methods used to collect information on fragmentation patterns of plant secondary metabolites, mainly flavonoids. The transfer of analyte from solution to gaseous phase requires three major steps:
-
•
Formation of charged droplets from high voltage capillary tip where the analyte solution is injected.
-
•
The evaporation of solvent from the charged droplet.
-
•
Formation of gaseous phase ion which is maintained at a high voltage (2.5–6.0 kV) to the wall of the surrounding chamber [61], [62].
Ions formed through this process carry multiple charges, and the analytes remain intact when suitable instrumental conditions are used [62]. ESI (–ve mode) uses higher collision energy to produce adequate fragmentation than ESI (+ve mode) [15]. In positive ionization mode, the spraying nozzle is kept at the (+ve) potential, which causes charging either through protonation or sometimes due to metalation. During negative ionization mode of sample analysis, charging occurs via deprotonation of the analyte when spraying nozzle is kept at the (–ve) potential. The positive or negative charges would be repelled by high voltage capillary (same polarity), and finally, they would draft towards the liquid surface at the capillary outlet. Polar solvents like water, methanol, and acetonitrile are commonly used in ESI-MS analysis because these solvents readily undergo electrochemical reactions in the spraying nozzle [62].
6. Applications of LC–MS in the analysis of phenolic acids from GLVs
LC–MS is becoming a popular choice in natural products research because it can identify and predict exact molecular weight of compounds from a small amount of plant sample extracts with low concentrations [36].
Hydroxy-benzoic acids and hydroxy-cinnamic acids are the most common phenolic acids present in most of the plant species. Hydroxy-benzoic acids have the same basic structure, but the difference is in the number of hydroxyl groups attached to the benzene ring. The difference in hydroxyl groups and their positioning affects polarity and solubility of these compounds. Hydroxy-cinnamic acids are found in conjugation with amides, amines, amino acids, esters, sugar derivatives, and glycosides. Plant matrices contain these compounds with high structural similarities which make the separation difficult. Therefore, careful selection of stationary phase, mobile phase, and monitoring of wavelength parameters are required during separation process [63]. Routine HPLC methods use RP octadecyl silica columns for phenolic compounds. The elution of polar compounds (phenolic acids) is observed earlier in the gradient elution than the compounds of low polarity during RP-HPLC analysis. Therefore, the elution order will be in the following order: phenolic acids < cinnamic acids < flavonoids, but there is a definite chance of overlapping of the individual members of the different classes due to their enormous structural diversity [33]. Structural complexity and mobile phase affect elution time of compounds. RP-HPLC separation of the main phenolic acids such as ferulic acid, sinapic acid, and p-Coumaric acid is approximately 40–50 min, but some analysis may require 70–90 min [64]. The polarity of cinnamic and other phenolic acids is increased by the number of hydroxyl groups at the 4th position, followed by those at the 3rd and 2nd position. The compounds with more number of methoxy and acrylic groups increase the retention time and reduce polarity [33]. Specific lengths of alkyl chain are attached to the surface of silica. Retention differences and selectivity among columns are obtained through attached polar groups. They are good at retaining non-polar compounds but have limited retention time for polar compounds. The addition of polar end caps to the active sites of the silica surface or polar side chains to the alkyl attachment can increase polarity. The compounds which are separated during HPLC analysis differ in their polarities in a single run and require gradient elution with increased concentration of organic modifier [65]. Isocratic elution with the combination of water-methanol-acetic acid can separate free phenolic acids (caffeic acid, vanillic acid, syringic acid, ferulic acid, chlorogenic acid, protocatechuic acid, p-hydroxy benzoic acid and p-Coumaric acid). Maximum numbers of phenolic acids occur as trans-isomer in natural plants, but they are gradually converted to cis-isomer during UV-radiation. Cis-isomers are usually separated before trans-isomers using an RP-stationary phase, which contain an optically active molecule in the mobile phase [66]. Phenolic acids are mainly detected using UV–visible, DAD and fluorescence detectors. UV detection method is suitable for locating a phenol in the effluent of a column. Most of the benzoic acids have maximum absorption at 246–262 nm, but gallic acid and syringic acid have absorption maxima at 271 nm and 275 nm. Cinnamic acids have absorption maxima at two wavelength ranges: 225–235 nm and 290–330 nm. The most common detection range for phenolic compounds and their derivatives is 280 nm [13].
MS is useful for determining the exact molecular masses of the separated phenolic acids from RP-HPLC. This method allows simultaneous monitoring of retention time of the isolated compound peak and determination of MS data during the analysis. The common fragment observed for most of the phenolic acids is [M-H-CO2]– in ESI (–ve) mode formed due to the elimination of carboxyl group from the deprotonated molecular ions. Standard fragmentation ions are also observed for some of the phenolic acids glycosides [67]. Therefore, LC–MS is established as a standard method for identification and characterization of phenolic acids. Various studies have been carried out on GLVs to identify their bioactive constituents using LC–MS methods [47], [51], [53], [57], [68]. Table 4 summarizes the applications of this hyphenated method to determine and characterize phenolic acids from GLVs using ESI as the source of ionization.
Table 4.
LC–MS identification of phenolic acids in GLVs.
| Plant | Compounds | Retention time (min) | Ionization method | Observed (M+H)+ | Observed (M-H)- | λmax | Scan range (m/z) | References |
|---|---|---|---|---|---|---|---|---|
| Spinacea oleracea | p-Coumaric acid | 16.4 | ESI | – | 163.2 | 225,310 | 50–1000 | [68] |
| Ferulic acid | 17.2 | 193 | 238, 295 | |||||
| o-Coumaric acid | 22.6 | 163.2 | 215, 277, 325 | |||||
| Sarcocornia ambigua | Cinnamic acid | 9.61 | ESI | 149 | – | – | – | [46] |
| p-Coumaric acid | 6.90 | 165 | ||||||
| Vanillic acid | 4.53 | 169 | ||||||
| Caffeic acid | 4.25 | 181 | ||||||
| Ferulic acid | 7.28 | 195 | ||||||
| Syringic acid | 4.53 | 199 | ||||||
| Sinapic acid | 7.18 | 225 | ||||||
| Chlorogenic acid | 2.64 | 355 | ||||||
| Komatsuna (Brassica rapa) | Vanillic acid | 30.60 | ESI | – | 167 | – | 0–1000 | [51] |
| Syringic acid | 34.79 | 197 | ||||||
| Mizuna (Brassica rapa) | Chlorogenic acid | 31.11 | 353 | |||||
| Caffeic acid | 32.00 | 179 | ||||||
| Pok choi (Brassica rapa) | p-Coumaric acid | 42.00 | 163 | |||||
| Ferulic acid | 47.90 | 193 | ||||||
| Mitsuba (Cryptotaenia japonica) | m-Coumaric acid | 49.60 | 163 | |||||
| Horseno (Spinacea oleracea) | Sinapic acid | 49.00 | 223 | |||||
| Ellagic acid | 52.52 | 301 | ||||||
| Lettuce (Lactuca sativa) | ||||||||
| Red amaranth (Amaranthus tricolor) | ||||||||
| Green Amaranth (Amaranthus tricolor) | ||||||||
| Cabbages (Brassica oleracea var.capitata) | Caffeic acid | 21.72 | ESI | – | 179.1 | – | 100–400 | [57] |
| p-Coumaric acid | 32.88 | 163.1 | ||||||
| Ferulic acid | 36.74 | 193.1 | ||||||
| Sinapic acid | 38.10 | 223.1 | ||||||
| Kale (Brassica oleracea L.var sabellica) | Protocatechuic acid | 2.1 | ESI | – | 152.9 | – | – | [52] |
| 4-hydroxy benzoic acid | 4.0 | 136.8 | ||||||
| Vanillic acid | 5.30 | 166.8 | ||||||
| trans-Caffeic acid | 5.65 | 178.7 | ||||||
| cis-Caffeic acid | 5.75 | 178.7 | ||||||
| trans-p-Coumaric acid | 7.38 | 162.7 | ||||||
| cis-p-Coumaric acid | 7.50 | 162.7 | ||||||
| trans-Ferulic acid | 7.95 | 192.8 | ||||||
| Salicyclic acid | 8.02 | 136.8 | ||||||
| 3-hydroxy cinnamic acid | 8.10 | 162.8 | ||||||
| cis-Ferulic acid | 8.12 | 192.8 | ||||||
| trans-Sinapic acid | 8.15 | 222.8 | ||||||
| cis-Sinapic acid | 8.30 | 222.8 | ||||||
| Amranthus spinosus | Caffeoyl-quinic acid | 15.6 | ESI | – | 353 | 243, 302,327 | 50–1000 | [53] |
| Caffeoyl-quinic acid | 16.1 | 353 | 234,314 | |||||
| Coumaroyl-quinic acid | 21.3 | 337 | 233,301,314 | |||||
| Coumaroyl-quinic acid | 22.6 | 337 | 232, 310 | |||||
| Feruloyl-quinic acid | 24.2 | 367 | 239,302,328 | |||||
| Feruloyl-quinic acid | 25.0 | 367 | 234,322 | |||||
| Vernonia amygdalina | Caffeoyl quinic acid | 11.4 | ESI | – | 353 | 330 | – | [47] |
| Chlorogenic acid | 12.0 | 353 | 330 | |||||
| Manihot utilissima | Ferulic acid | 19.2 | 193 | 330 | ||||
| Corchorus olitorius | Caffeoyl quinic derivative | 11.3 | 729 | 330 | ||||
| Chlorogenic acid | 12.0 | 353 | 326 | |||||
| 1,5-Dicaffeoylquinic acid | 19.1 | 515 | 328 | |||||
| Dicaffeoyl quinic acid | 19.7 | 515 | 326 | |||||
| Dicaffeoyl derivative | 20.7 | 515 | 328 | |||||
| Ocimum gratissimum | Caffeic acid | 12.3 | 179 | 330 | ||||
| Rosmarinic acid | 19.9 | 359 | 328 | |||||
| Cichoric acid | 26.4 | 473 | – | |||||
| Merremia emarginata | Vanillic acid | 2.0 | ESI | 168.31 | – | 259 | – | [45] |
| Quinic acid | 3.4 | – | 191.12 | – | ||||
| Trihydroxy(s) benzenpropanoic acid | 5.1 | – | 197.04 | 346 | ||||
| Caffeic acid hexose | 5.4 | – | 341.12 | 244, 324 | ||||
| 3-O-caffeoylquinic acid | 5.7 | – | 353.16 | 326 | ||||
| Protocatechuic acid | 6.0 | – | 153.19 | 218,260, 295 | ||||
| Caffeoyl glucose | 6.5 | – | 341.81 | – | ||||
| 4-O-caffeoyl quinic acid | 7.3 | – | 353.19 | 326 | ||||
| Rosmeric acid | 7.5 | – | 359 | 328 | ||||
| 5-O-feruloylquinic acid | 7.7 | 369.40 | 367.19 | 325 | ||||
| Caffeic acid | 8.6 | – | 179.13 | 328 | ||||
| Ferulic acid | 12.5 | – | 193 | 290,310 | ||||
| 1,3-dicaffeoylquinic acid | 13.0 | – | 515 | 333 | ||||
| 3,5-dicaffeoylquinic acid | 13.7 | – | 515 | 334 | ||||
| 4,5-dicaffeoylquinic acid | 14.6 | – | 515.36 | 334 | ||||
| 4-feruloyl-5-caffeoylquinic acid | 16.1 | – | 529 | 325 | ||||
| 5-O-coumaroylquinic acid | 24.8 | 339 | – | 325 | ||||
| Collard greens, Kale and Chinese broccoli (Brassica sps) | 3-caffeoyl quinic acid | 7.3 | ESI | – | 353 | 240,298,328 | 100–2000 | [44] |
| 3-p-coumarolyuinic acid | 10.3 | 337 | 310 | |||||
| 5-caffeoylquinic acid | 11.0 | 353 | 240,298,328 | |||||
| 4-caffeoylquinic acid | 12.2 | 353 | – | |||||
| 3-feruloylquinic acid | 12.3 | 367 | – | |||||
| 5-p-coumaroylquinic acid | 16.6 | 337 | 310 | |||||
| 5-feruloylquinic acid | 17.8 | 367 | – | |||||
| Caffeic acid | 14.2 | 179 | – | |||||
| Hydroxy-ferulic acid | 14.7 | 209 | – | |||||
| p-Coumaric acid | 21.4 | 163 | 310 | |||||
| Sinapic acid | 23.8 | 223 | 240,298,328 | |||||
| Ferulic acid | 24.2 | 193 | 240,298,328 |
7. Applications of LC–MS in the analysis of flavonoids from GLVs
Flavonoids separation in RP-HPLC is based on hydrophobicity. Selectivity differences are observed based on the nature of stationary phase, carbon loading, and polar attached groups. Different classes of flavonoids elute according to the following order: flavanols < flavanones < flavonols < flavones. Retention time of these compounds increases with a greater number of methoxy groups and decreases with a greater number of hydroxyl groups attached to the flavonoid backbone. Both the gradient elution systems (Binary and Isocratic) are used in flavonoids analysis, but isocratic gradient elution has limitations. It may not resolve compounds from different classes of flavonoids but favors separation with monolithic columns. Glycosylated flavonoids slightly decrease retention time whereas acylated compounds increase elution time during RP-HPLC analysis. Normal phase separation of flavonoids is not favourable due to their limited solubility in the mobile phase, and the analyte often retains on the column, except polymethoxylated flavones and acetylated flavonoids. Ionization of flavonoids occurs with a rise in pH, causing reduced retention time during RP separation. Therefore, to avoid ionization, small amounts of acetic acid (2%–5%) and trifluoroacetic acid (0.1%–1%) are added to the solvent mixture, which improves resolution and reproducibility of each separation [69].
Detection of flavonoids depends on their chemical properties and sensitivity of the analytes. Some compounds are identified based on the retention time of reference standards and UV absorption spectrum. Two compounds may be closely related and elute at the same time but are separated based on their differences in absorption spectra through multiple wavelength detection systems. Acetonitrile and methanol do not interfere during the detection of UV–Vis absorption bands at 240–285 nm and 300–560 nm of A and B rings of the flavonoid aglycones because of their low UV cut-off λmax values at 190 nm and 205 nm. In the case of flavones, the substitution patterns of hydroxy and methoxy groups and the types of glycosides (C or O-glycosides) result in small variation of the wavelength range of both the bands [69].
MS provides structural information of phenolic compounds by identifying the distribution pattern of substituents between A and B rings [11]. LC–MS is found to be an efficient method in flavonoid analysis, particularly for their glycosidic derivatives and acylated conjugates. The identification of these compounds is achieved through structural characterization by calculating their mass to charge (m/z) ratio [70] with accuracy and in smaller quantities [13]. ESI and APCI are the ideal ionization sources used in flavonoid analysis. The coupling of HPLC with MS is possible through these ionization sources [71]. Flavonoids are found in their glycosidic form in most of the plants and are mainly classified into two types: O-glycosides and C-glycosides. The normal mass spectra of flavonoid glycosides with different ionization techniques result in a similar kind of fragmentation pathways. In many cases, the presence of impurities in the sample or the analyzed compounds may not yield the corresponding fragment ion. In such cases, collision induced dissociation (CID) MS/MS methods are useful for generating the appropriate fragment ion of the compounds [72]. m/z modification is possible through CID, in which precursor ions undergo collisional activation with the neutral atoms or molecules (such as inert gas) in the gaseous phase. It involves the addition of energy to already vibrationally excited ions [12]. The structural identification of acylated flavonoid glycosides is difficult, as these compounds are commonly found to be acylated with aliphatic (acetic acid) or aromatic acids directly to the glycosidic part or its aglycone part [73]. The common acyl groups mostly seen in conjugation with a flavonoid and their probable characteristic fragment ions are presented in Table 5. Structure-specific information about the acyl related product ions is observed in the [M+H]+ and [M+Na]+ low energy CID spectra and radical acid-related product ions at high energy CID spectra. It gives relevant information on the positioning of the acyl group at the exact location of the flavonoid backbone and confirms its identity. Caffeoyl or cinnamoyl groups can occur in the glycosyl part of flavonoids. The exact location of the acyl group on the glycosidic part is difficult to determine, but acyl groups are supposed to appear at the 6th position of a hexose moiety although other positions cannot be excluded [15]. ESI spectra of glycosidic compounds typically show a pseudo-molecular ion (e.g. [M+H]), aglycone ion and ions associated with the solvent even though their fragmentation can often be induced by raising the cone voltage [5]. Fragmentation of flavonoids in their first-order mass spectra gives further details about their structures. For example, the cleavage of O-glycosidic bonds between the flavonoid and the sugars can be used to elucidate the sugar residues. The cleavage of a hexose sugar is detected as an ion that has a 162 Da smaller m/z value than the molecular ion, whereas decrease in m/z value of 132 Da than the molecular ion detected as pentose sugar [74]. C-glycosidic bonds are more stable than O-glycosidic bonds. The cleavage of sugar moieties takes place in those compounds which have more than one sugar moiety attached to an aglycone. The number of sugar moieties bound to an aglycone may vary from one to five units [73]. O-glycosides are labile in nature and cleaved under low collision energy conditions. This causes the loss of hexosides (162 amu), deoxy-hexosides (142 amu) and pentosides (132 amu). In some cases, m/z at 163 and 147 are observed for hexoses and pentoses, respectively. Protonated [M+H] + and deprotonated [M–H]– ions are observed with low cone voltages during flavonoid analysis with small fragmentation patterns. Adducts such as [M-H+ACONa] –, [M+HSO4]–, and [M-H+ACONa+MeOH] – are commonly detected based on the composition of the mobile phase used [71]. Sodium and potassium adducts are often seen during flavonoids analysis in ESI (+ve mode). These are formed during storage of the sample in glass solution and are detected during the analysis of flavanol-3-O-glycosides and isoflavones [15]. Loss of water (18 Da), CO (28 Da) and C2H2O (42 Da) and combined loss of H2O and CO (46 Da) are commonly observed in less characterized flavonoids with little fragmentation patterns. O-methylated isoflavones, flavones, and flavonols show the loss of methyl radical with product ion [M+H-15] +. Further, the combined loss of methyl group and water [M+H-33] + is also observed during analysis of flavonoids [70].
Table 5.
Most common acyl groups found in the glycosyl part of flavonoids and their characteristic product ions [15].
| Acyl groups | Characteristic fragments |
|---|---|
| Acetyl | [M+H-acetylhexose]+: −204 u |
| Malonyl | [M+H-malonylhexose]+: −248 u; [M+H-malonyl]+: −86 u; [M+H-CO2]: −44 u |
| Coumaroyl | [M+H-coumaroyl]+: −146 u; [M+H-coumaroylhexose]+: −308 u |
| Galloyl | [M+H-galloyl]: −152 u; [M+H-galloylhexose]+:−314 u; [M-H-gallic acid]-: −170 u |
| Benzoyl | [M+H-benzoylhexose]+: −266 u |
| Feruloyl | [M+H-feruloylhexose]+: −338 u; [M+H-feruloyl]+: −176 u |
| Sinapoyl | [M+H-sinapoyl]+: −206 u; [M+H-sinapoylhexose]+: −368 u |
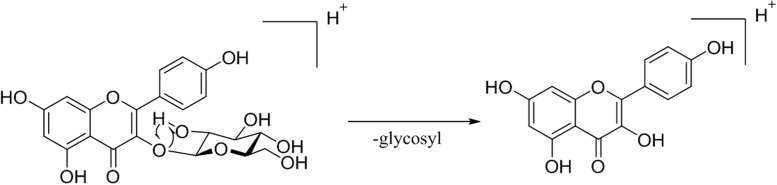
Fragmentation pathway of O-glycosylated flavonoids begins with the cleavage of glycosidic bonds and removal of the sugar moieties [72]. In mass spectrometer, a rearrangement reaction involves the cleavage of a glycosidic moiety from a flavonoid glycoside, which is presented [15].
In C-glycosylated flavonoid, complete removal of the sugar moiety is not observed rather than the elimination of fragments or several fragments of the sugar ring [73]. Intensities of the observed protonated molecule ions [M+H] + in the mass spectrum are dependent on the number of sugar moieties attached to aglycone moiety. The more are the number of sugar rings, less is the intensity of [M+H] + ions. Sugars are attached to flavonoid ring via an acid resistant C-C bond. The suitable use of high and low energy collision experiment may improve results [70]. At high collision energies, 6-C glycosyl flavonoids lose water molecules more significantly than 8-C glycosyl flavonoids for [M+H] + and [M-H] – fragmentation pattern of ions. During the identification of flavonol-3,7-di-O-glycosides and their glycan substituents, the differentiation becomes easier as their protonated molecules lose a glycan more readily at the 3rd position than the 7th position [15].
GLVs accumulate tremendous amounts of flavonoids, especially in their leaves and fruits. They mainly occur as glycosidic derivatives and are responsible for their various biological applications. Table 6 summarizes the role of LC–MS in identifying and characterizing these flavonoids from important GLVs to exploit their significance in food and pharmaceutical industries.
Table 6.
LC–MS identification of flavonoids in GLVs.
| Plant | Compounds | Retention time (min) | Ionization method | Observed (M+H)+ | Observed (M-H)- | λmax (nm) | Scan range (m/z) | References |
|---|---|---|---|---|---|---|---|---|
| Merremia emarginata | p-hydroxybenzoyl-quercetin-3-O-galactoside | 8.2 | ESI | – | 583 | – | – | [45] |
| Myricetin hexoside | 24.1 | 479.18 | 218, 520 | |||||
| 4-myricetin-3-O-galactoside | 24.8 | 479.18 | – | |||||
| Sarcocornia ambigua (Amaranthaceae) | Kaempferol | 9.72 | ESI | 287 | – | – | – | [46] |
| Galangin | 11.32 | 271 | ||||||
| Quercetin | 8.93 | 303 | ||||||
| Isoquercetin | 6.85 | 465 | ||||||
| Spinach (Spinacia oleracea) | Luteolin | – | ESI & MRM | 286.99, 153 | – | – | 50–350 | [48] |
| Myricetin | 318.94, 153 | |||||||
| Kaempferol | 286.92, 153 | |||||||
| Quercetin | 302.93, 153 | |||||||
| Apigenin | 271.02,153 | |||||||
| (Brassica oleracea) | Quercetin | 30.22 | ESI | – | 301 | 360 | 100–400 | [57] |
| Kaempferol | 43.52 | 285 | ||||||
| Amranthus spinosus | Quercetin-diglycoside | 25.9 | ESI | – | 609 | 231, 257, 264,300, 357 | 50–1000 | [53] |
| Quercetin-3-O-rutinoside | 26.6 | 609 | 231, 256, 264,302, 354 | |||||
| Quercetin-3-O-glucoside | 28.0 | 463 | 231, 256, 263,302, 354 | |||||
| Kaempferol diglycoside | 33.5 | 593 | 231,265,300, 348 | |||||
| Vernonia amygdalina | Rutin | 14.6 | ESI | – | 609 | 256/354 | – | [47] |
| Luteolin-7-O-rutinoside | 14.7 | 593 | 264/348 | |||||
| Luteolin-7-O-glucoside | 15.6 | 447 | 260/348 | |||||
| Luteolin-4’-O-rutinoside | 16.1 | 593 | 260/336 | |||||
| Luteolin-7-O- glucuronide | 18.6 | 461 | 254/348 | |||||
| Apigenin-6-O or 7-O-glucuronide | 20.0 | 445 | 268/334 | |||||
| Luteolin | 28.5 | 285 | 264/348 | |||||
| Manihot utilissima | Rutin | 14.6 | 609 | 356 | ||||
| Kaempferol-4’-O-rutinoside | 15.6 | 593 | 260/348 | |||||
| Kaempferol-3-O-rutinoside | 16.2 | 593 | 260/348 | |||||
| Amentoflavone | 38.1 | 537 | 268/336 | |||||
| Corchorus olitorius | Isoquercetin | 15.6 | 463 | 256/358 | ||||
| Quercetin derivative | 22.1 | 533 | 354 | |||||
| Hyperoside | 15.4 | 463 | 256/358 | |||||
| Ocimum gratissimum | Vicenin-2 | 11.2 | 593 | 270/336 | ||||
| Rutin | 14.6 | 609 | 256/356 | |||||
| Luteolin-7-O-glucoside | 15.6 | 447 | 260/348 | |||||
| Kaempferol-3-O- rutinoside | 16.2 | 593 | – | |||||
| Cirsilol | 34.3 | 329 | 273/344 | |||||
| Cirsimaritin | 38.8 | 313 | 276/334 | |||||
| Nevadensin | 41.1 | 343 | 284/332 | |||||
| Komatsuna (Brassica rapa) | Isoquercetin (Quercetin-3-glucoside) | 54.26 | ESI | – | 463 | 360 | – | [51] |
| Mizuna (Brassica rapa) | Hyperoside (Quercetin-3-galactoside) | 53.25 | 463 | |||||
| Pok choi (Brassica rapa) | Rutin (Quercetin-3-rutinoside) | 53.00 | 609 | |||||
| Mitsuba (Cryptotaenia japonica) | ||||||||
| Horseno (Spinacea oleracea) | ||||||||
| Lettuce (Lactuca sativa) | ||||||||
| Red amaranth (Amaranthus tricolor) | ||||||||
| Green Amaranth (Amaranthus tricolor) | ||||||||
| Kale (Brassica oleracea var.sabellica) | Kaempferol-3-O-hydroxyferuloyl-sophorosie-7-O-glucoside | – | ESI | – | 353.0 | – | – | [56] |
| Quercetin-3-O-sinapoyl-sophoroside-7-O-glucoside | 993.1 | |||||||
| Kaempferol-3-O-sinapoyl-sophoroside-7-O-diglucoside | 1139.4 | |||||||
| Kaempferol-3-O-sinapoyl-sophoroside-7-O-glucoside | 977.1 | |||||||
| Kaempferol-3-O-feruloyl-sophoroside-7-O-diglucoside | 1109.9 | |||||||
| Kaempferol-3-O-feruloyl-sophoroside-7-O-glucoside | 947.1 | |||||||
| Kaempferol-3-O-disinapoyl-triglucoside-7-O-glucoside | 1344.4 |
8. Advantages of LC–MS method
The method offers various advantages over other chromatographic methods, which are described below:
-
a)
Selectivity: Co-eluting peaks can be isolated by mass selectivity and are not constrained by chromatographic resolution.
-
b)
Peak assignment: A molecular fingerprint for the compound under study is generated, which ensures correct peak assignment in the presence of complex matrices.
-
c)
Molecular weight information: Confirmation and identification of both known and unknown compounds even with low concentrations of the sample.
-
d)
Structural information: Controlled fragmentation pattern enables structural elucidation of a chemical compound.
-
e)
Rapid method development: Providing easy identification of eluted analytes without retention time validation.
-
f)
Sample matrix adaptability: Decreasing sample preparation time and hence is less time-consuming.
-
g)
Quantitation: Quantitative and qualitative data can be obtained simultaneously with limited instrument optimization.
-
h)
Cost effective: Another advantage of LC–MS method is the capacity to multiplex several analytes within a single analytical run with minimal incremental cost. This method has the potential to simplify laboratory set-up (e.g. creation of test panels) and provides additional useful information (e.g. metabolite profiles) [75], [76].
During LC analysis of phenolic compounds, UV detectors have more advantages than other detectors. UV detectors are used for identification because of their high sensitivity and low UV absorbance for the separated compounds [30]. The UV detection system gets saturated if large amounts of high UV absorbing compounds are analyzed by HPLC. The UV detection method can be more useful if the desired compounds to be separated are in small quantities within a sample [77]. Preparative scale detectors are less sensitive than analytical detectors used in HPLC [77]. Monochromatic detectors can not quantify unseparated compounds because of the overlapping of the absorption spectra by chromatographic conditions. In such cases, DAD can quantify these compounds [31], [78]. Further, they are involved in purity check of the isolated compound peaks. Modern DADs have the sensitivity level of 1 × 10−5 AU [31]. HPLC has overcome the difficulties in separation methods by optimizing the time for analysis and increasing resolution. Generally, in routine environments where a large number of samples are analyzed daily, HPLC plays a crucial role in separating complex mixtures within a short period provided the conditions are maintained and facilitates acquisition of analytical data quickly. Depending on the nature of isolated compounds and gradient elution, successful resolution of complex phenolic compounds is achieved by altering the mobile phase. This particular situation arises due to the same molecular weight and the absorption spectrum of the isolated compound peak which cannot be differentiated by the detectors used. The mobile phase, concentration, type of modifier (an organic component of the mobile phase) and pH of the buffer are the main factors causing differences in the separation of compounds with high variability. If the solutes are weak electrolytes in nature, then mobile phase pH should be maintained. Variation in pH will not change the selectivity if solute doesn’t dissociate [42]. The extent or degree of separation is mostly determined by the selection of stationary phase and mobile phase [79]. In recent time, apart from efficiency and resolution, peak capacity has evolved as a major parameter which evaluates the separation capability of HPLC. Peak capacity represents the maximum number of compounds separated under defined experimental conditions such as column, gradient, and flow-rate [71]. HPLC columns with small diameter show higher sensitivity when combined with ESI-MS due to the elution of the concentrated analyte in a smaller volume. But these columns have an advantage as they provide little scope for the buffers and contaminants to enter the mass spectrometer thus maintaining the ionization source for longer periods of higher sensitivity [72]. The guard columns do play a crucial role in increasing the lifespan and performance of the analytical columns because they safeguard columns by removing the strong binding components such as chlorophyll and other endogenous materials commonly observed during analysis of crude plant samples [79]. Advanced HPLC techniques like high temperature liquid chromatography (HTLC), ultra-high pressure chromatography (UHPLC) and two-dimensional liquid chromatography (LC × LC) use new types of columns in detection of phenolic compounds. These columns are monolithic and seem to contain porous particles of size about 1.7–10 µm, 3–25 cm length and 1–4.6 mm column inner diameter. UHPLC applies pressure (up to 1300 bars) and uses small particle size (sub-2 μm particles) in the stationary phase and short columns. This causes the faster resolution of fine chromatographic peaks and apparently reduces scan time of 10 min or less [80].
Hydrophilic interaction liquid chromatography (HILIC) and 2-dimensional liquid chromatography (2-D LC) are becoming popular choice for plant sample analysis due to their recent developments in instrumentation. HILIC can be more effective when linked to MS, because the mobile phase used for separation has higher compatibility. 2-D LC is a latest and rapidly growing LC technique which provides separation and identification of structurally similar and minor compounds from complex plant samples enhancing peak capacity and selectivity. A successful combination of 2-D LC × HILIC and 2-D LC × RP-LC has been used to detect polar and semi-polar fractions in traditional Chinese medicine [81].
The development of an accurate LC-retention prediction system augments MS information, increasing the scope for compound identification [82]. The online coupling of LC technique with a high-resolution MS enables the easy identification of unknown contaminants from biological samples over the last two decades. LC techniques offer many advantages and have replaced gas chromatography (GC) in the analysis of plant samples due to their low sample pre-treatment and their ability to determine polar or thermally stabile compounds [80]. LC–MS method is very compatible with solvents used as mobile phase in RP- HPLC [83]. LC–MS provides accurate structural data which is helpful for determining the novelty or utility of identified constituents from complex plant matrices and further the biological and pharmacological testing of those samples. This procedure allows avoiding the unnecessary isolation of some minor compounds of interest which may be biologically active [7]. LC–MS system has been mainly improved in two aspects: developments in the LC part are beneficial for throughput and resolution, while MS detection enhancement has a positive impact on sensitivity and selectivity [84]. The miniaturization is an important factor considered for all analytical methods and also during the hyphenation of LC and MS [82]. While interfacing HPLC to MS, two major problems arise and have to be overcome:
-
•
The vacuum system of the MS has to handle the large amounts of solvents eluting from the HPLC.
-
•
New methods for ionization of non-volatile and polar compounds (present in solution) have to be developed.
The inlet of the mobile phase of the HPLC into the MS ion source as a spray, where only a small fraction of this solvent enters the MS analyzers and the development of sophisticated differential pumping system which enables handling pressure differences of 8 orders of magnitudes together solves the vacuum problem. The recent advances in the ionization methods allow differentiating in the mechanisms involved in ion formation [85].
MS is a versatile technique with described applications from astronomical studies and geophysics to biology and medicine [43]. The use of ESI method as ionization source in the analysis of phenolic compounds has enhanced the efficiency of MS regarding their identification and characterization of molecular masses of unknown compounds from crude or purified samples. The online coupling of HPLC and MS using ESI enables high resolution and characterization of a wide range of polar compounds [86]. Good sensitivity and high throughput are the key factors responsible for enhancing the separation and identification capabilities of this hyphenated technique [87]. The main advantages of ESI method include ionization of large, fragile biomolecules (MW>70000) within a small m/z range (< 2000 m/z) with precision and appropriate fragmentation patterns and easy interface with HPLC [62]. In ESI (–ve mode) the molecule gets deprotonated because of the analysis which takes place above the isoelectric point of a molecule [88]. Negative ionization is more sensitive than positive ionization due to increased chemical noise [52]. Due to its sensitivity, it gives additional and complete fragmentation information (molecular weight, substitution pattern and elemental formula) of the compounds detected [15]. In ESI (+ve mode), the number of charged species observed is reflected in the number of basic sites on a molecule that can be protonated at low pH [88]. [M+ NH4]+, [M+Na]+ in positive mode and [M+Cl]–, [M+OAc]– in negative mode are detected in the mass spectrum without the addition of these modifiers to the analyte solution. Positive ionization gives great fragmentation patterns and is more useful for identification of flavonoid-C-glycosides [52]. ESI method has certain limitations such as the incapability to analyze non-polar compounds with accuracy, incompatibility to some solvents, sensitivity to salts (buffers) and the analysis of polyphenolic flavonoids due to their high polarity and low volatility [15], [88]. Lots of recent developments have been carried out to maximize the applications of ESI methods such as a number of sprayer modifications (pneumatically assisted electrospray, ultrasonic nebulizer electrospray and nanoelectrospray) [62]. Combined ion sources can be considered as a useful option because it offers the advantage of possible detection of both polar and non-polar analytes in one single run, which aids to identify more compounds from complex matrices [82]. The use of lower liquid flows is among the recent developments in ESI methods. Micro-electrospray method uses flow rate of μL/min. Nanoelectrospray method uses flow rates at nL/min with a 1–2 µm i.d of capillaries [83].
HPLC and MS in combination provide easy separation with qualitative identification or qualitative analysis of crude plant samples or any other biological fluids such as plasma, urine, and blood [89]. Significantly, LC–MS is more readily used to analyze or identify polar compounds in plant samples with high accuracy and multiplexing options [90]. Higher sensitivity and specificity can be achieved by LC–MS in comparison to direct injection methods [91]. LC–MS is one of the major untargeted analytical techniques to determine total metabolite profiles, which helps in the identification and relative quantification of all peaks in the chromatogram as ions that are initially defined by retention time and molecular mass [75].
The mass spectrum combined with a similar retention time of reference compounds can provide information to identify the unknown peaks unambiguously. The overestimation of concentration caused by co-elution can be eliminated by the selected ion monitor mode [40]. UV–Vis detector is a good option to estimate and identify impurities of unknown samples, when used in conjugation with MS. This allows monitoring of the absorption and response factor of related identified compounds which are almost the same, but MS ionization efficiencies can be significantly different [83]. Mass accuracies of 1.0–0.1 Da are often seen during the LC–MS analysis of samples using quadrupole ion-traps or linear quadrupole mass analyzers. But this level of accuracy is not sufficient to differentiate many compounds having same molecular masses. For example, isoflavones (genistein and medicarpin) have the same molecular mass of 270, but have different chemical compositions, 270.2390 (C15H10O5) and 270.2830 (C16H14O4), which result in different accurate masses. The accurate measurement of the masses of the compounds allows differentiating these compounds in the mass domain even if they cannot be structurally resolved or separated in the chromatographic domain. However, if the chromatography step is avoided or compressed significantly, then ion suppression, competitive ionization, and other matrix effects become increasingly more influential [92]. During LC–MS quantification of identified compounds, ion suppression plays a crucial role whose effects need to be quantitatively estimated. The interference of the ion suppression and matrix effects is observed during quantitative analysis of samples. The use of the internal standard is preferred during the mass analysis and ionization process to remove any possible differences such as contamination of the ion source or mobile phase and ion suppression/enhancement [80]. Recently LC gradients used in LC–MS analysis have shorter analysis time and use short columns of length (3–5 cm) which are comparatively smaller than the length of previous columns approximately 25 cm with longer gradient time (30–60 min). But there are certain limitations of using shorter columns with faster gradient elution methods as these may result in incomplete chromatographic peak separation. Flow injection analysis (FIA) can be used to directly inject the sample into MS without any separation process, but again the ionization suppression effects can be observed and will thus intervene during sample analysis [83].
With new advancement in LC–MS methods, it is obvious that sensitivity and selectivity offered by this method have contributed significantly to chemical screening of unknown constituents from plant samples and their quantitative determination [82].
9. Conclusion and prospective
Some phenolic compounds are present in low concentrations in plants, which are difficult or may not be identified but can have high significance. Extraction and structural characterization of the bioactive compounds from crude plant samples are the primary steps to understand their importance and mechanism of action. The critical understanding of the biosynthetic pathways is vital for evaluating the role of plants and their interaction with the environment. Thus it will be useful for genetic manipulation (both qualitative and quantitative) of the phenolic content of plants.
The methods used by LC–MS allow the easy and rapid screening of phenolic acids and flavonoids from GLVs used in traditional medicines and their pharmaceutical preparations. Apparently, antioxidants from plant sources have become an important component of human health care. During the phytochemical analysis of GLVs using this hyphenated technique, the relationship between structural characteristics and fragmentation patterns of the identified compounds should be more systematically investigated. During the literature search, a number of studies investigated phenolic compounds from GLVs using LC–MS methods. However, a majority of the papers cited in the present article are just meagre attempts. Further efforts are required to investigate the distribution pattern of phytocompounds in GLVs by these methods to understand the structural-functional relationship and their role in food and pharmaceutical industries. The tentative identification or characterization of the unknown phyto-constituents from plant samples is only possible when characteristic fragmentation behaviors of the internal reference standards, nature of the eluted compounds (polar or non-polar) acquired from the retention time data, exact UV spectral information and data from the previous studies are available. The selection of an analytical method depends on the nature of the compounds to be identified or characterized and the rationale of the complete analysis. LC–MS has become an indispensable tool in natural products analysis and has gained considerable interest in complete profiling of secondary metabolites because of their unique features such as chemical identification, high resolution and sensitivity with reduced cost. This method can effectively detect compounds with low concentrations in the plant samples while providing high resolution, high reproducibility, and accuracy. A large amount of preliminary information about the constituents of an unknown plant extract sample before the isolation step can be obtained by combining these two methods. The ESI used for analysis further broadens the spectrum of identified analytes and their possible applications.
LC–MS technique is developing rapidly due to its ability to structurally characterize compounds based on various C-ring cleavages and perhaps the reliability of the acquired data. Ionization modes and HPLC conditions affect fragmentation patterns. Special attention is required during the analysis of conjugated derivatives of phenolic acids and especially flavonoids as a small error may lead to the wrong identification of the desired compound. However, it is obvious that increasing applications of this hyphenated technique will benefit future research on natural products from plants. The present article summarizing the role of LC–MS methods for separation and characterization of phenolic acids and flavonoids from GLVs will surely be helpful in future examination and finding of novel compounds from medicinal plants with high sensitivity, selectivity, and accuracy.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Acknowledgments
The author acknowledges the funding support provided by Ministry of Human Resource Development (MHRD/RTV-5/2012) New Delhi, India. The author would also like to extend sincere gratitude to Dr. M. Padmavati, RGSOIPL, Indian Institute of Technology Kharagpur, for her moral support and inspiring thoughts.
Footnotes
Peer review under responsibility of Xi'an Jiaotong University.
References
- 1.Boligon A.A., Athyade M.L. Importance of HPLC in analysis of plant extracts. Austin Chromatogr. 2014;1:1–2. [Google Scholar]
- 2.Yang M., Sun J., Lu Z. Phytochemical analysis of traditional Chinese medicine using liquid chromatography coupled with mass spectrometry. J. Chromatogr. A. 2009;1216:2045–2062. doi: 10.1016/j.chroma.2008.08.097. [DOI] [PubMed] [Google Scholar]
- 3.Martin M.A., Ramos S. Cocoa polyphenols in oxidative stress: potential health applications. J. Funct. Foods. 2016;27:570–588. [Google Scholar]
- 4.Carter P., Gray L.J., Troughton J. Fruit and vegetable intake and incidence of type 2 diabetes mellitus: systematic review and meta-analysis. BMJ. 2010:4229. doi: 10.1136/bmj.c4229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ryan D., Robards K., Prenzler P. Applications of mass spectrometry to plant phenols. Trends Anal. Chem. 1999;18:362–372. [Google Scholar]
- 6.Esmail Al-Snafi Ali. The pharmacological importance of Brassica nigra and Brassica rapa grown in Iraq. J. Pharm. Biol. 2015;5:240–253. [Google Scholar]
- 7.Wolfender J.L., Rodriguez S., Hostettmann K. Liquid chromatography coupled to mass spectrometry and nuclear magnetic resonance spectroscopy for the screening of plant constituents. J. Chromatogr. A. 1998;794:299–316. [Google Scholar]
- 8.Costa D.C., Costa H.S., Albuquerque T.G. Advances in phenolic compounds analysis of aromatic plants and their potential applications. Trends Food Sci. Technol. 2015;45:336–354. [Google Scholar]
- 9.Patel K.N., Patel J.K., Patel M.P. Introduction to hyphenated techniques and their applications in pharmacy. Pharm. Methods. 2010;1:2–13. doi: 10.4103/2229-4708.72222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cartea M.E., Francisco M., Soengas P. Phenolic compounds in Brassica vegetables. Molecules. 2011;16:251–280. doi: 10.3390/molecules16010251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mandal S.M., Chakraborty D., Dey S. Phenolic acids as a signalling molecule in plant-microbe symbioses. Plant Signal Behav. 2010;5:359–368. doi: 10.4161/psb.5.4.10871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robbins R.J. Phenolic acids in foods: an overview of analytical methodology. J. Agric. Food Chem. 2003;51:2866–2887. doi: 10.1021/jf026182t. [DOI] [PubMed] [Google Scholar]
- 13.Stalikas C.D. Phenolic acids and flavonoids: occurrence and analytical methods. In: Uppu R.M., editor. Free Radicals and Antioxidant Protocols. Springer Science+Business Media LLC; 2010. pp. 65–90. [Google Scholar]
- 14.Ignat I., Volf I., Popa V.I. A critical review of methods for characterization of polyphenolic compounds in fruits and vegetables. Food Chem. 2011;126:1821–1835. doi: 10.1016/j.foodchem.2010.12.026. [DOI] [PubMed] [Google Scholar]
- 15.Cuyckens F., Claeys M. Mass spectrometry in the structural analysis of flavonoids. J. Mass. Spectrom. 2004;39:1–15. doi: 10.1002/jms.585. [DOI] [PubMed] [Google Scholar]
- 16.Shirley W.B. Biosynthesis of flavonoid and effects of stress. Curr. Opin. Plant Biol. 2002;5:218–223. doi: 10.1016/s1369-5266(02)00256-x. [DOI] [PubMed] [Google Scholar]
- 17.Singh S., Sheoran S.S. Evaluation of the antinoceptive activity of Amaranthus hybridus Linn root extracts. Acta Pol. Pharm. 2011;68:255–259. [PubMed] [Google Scholar]
- 18.Kawade R. A pharmacognostic and pharmacological review: Amaranthus spinosus. World J. Pharm. Res. 2013;2:2099–2110. [Google Scholar]
- 19.Olajide O.A., Ogunleye B.R., Erinle T.O. Anti-inflammatory properties of Amaranthus spinosus leaf extract. Pharm. Biol. 2004;42:521–525. [Google Scholar]
- 20.Hussain Z., Amresh G., Singh S. Antidiarrheal and antiulcer activity of Amaranthus spinosus in experimental animals. Pharm. Biol. 2009;47:932–939. [Google Scholar]
- 21.Ashok Kumar B.S., Lakshman K., Velmurugan C. Antidepressant activity of methanolic extract of amaranthus spinosus. Basic Clin. Neurosci. 2014;5:11–17. [PMC free article] [PubMed] [Google Scholar]
- 22.Deshmukh S.A., Gaikwad D.K. A review of the taxonomy, ethnobotany, phytochemistry and pharmacology of Basella alba (Basellaceae) J. Appl. Pharm. Sci. 2014;4:153–165. [Google Scholar]
- 23.Josthna P., Bhanupriya K., Kotakadi V.S. Anti-inflammatory effect of Basella rubra on Oxazolone-induced colitis in rat. AJPCT. 2014;2:832–841. [Google Scholar]
- 24.Rokayya S., Li C.J., Zhao Y. Cabbage (Brassica oleracea L.var capitata) phytochemicals with antioxidant and anti-inflammatory potential. Asian Pac. J. Cancer Prev. 2014;14:6657–6672. doi: 10.7314/apjcp.2013.14.11.6657. [DOI] [PubMed] [Google Scholar]
- 25.Carvalho C.A., Fernandes K.M., Matta S.L. Evaluation of antiulcerogenic activity of aqueous extract of Brassica oleracea var.capitata (Cabbage) on Wistar rat gastric ulceration. Arq. De. Gastroenterol. 2011;48:276–282. doi: 10.1590/s0004-28032011000400011. [DOI] [PubMed] [Google Scholar]
- 26.Agbaje, Oluwatoyin E., Okpara Antiulcer activity of aqueous extract of fresh leaf of Brassica oleraceae Linn.var.Acephala (D.C.) Alef (Brassicaceae) Int. Res. J. Pharm. 2013;4:107–113. [Google Scholar]
- 27.Ji C., Li C., Gong W. Hypolipidemic action of hydroxycinnamic acids from Cabbage (Brassica oleracea L.var.capitata) on hypercholesterolaemic rat in relation to its antioxidant activity. J. Food Nutr. Res. 2015;3:317–324. [Google Scholar]
- 28.Subhash G.P., Virbhadrappa S.R., Vasant O.K. Spinacia oleracea Linn: a pharmacognostic and pharmacological overview. Int. J. Res. Ayurveda Pharm. 2010;1:78–84. [Google Scholar]
- 29.Jaime L., Vazquez E., Fornari T. Extraction of functional ingredients from spinach (Spinacia oleracea L.) using liquid solvent and supercritical CO2 extraction. J. Sci. Food Agr. 2015;95:722–729. doi: 10.1002/jsfa.6788. [DOI] [PubMed] [Google Scholar]
- 30.Chandra S., Khan S., Avula B. Assessment of total phenolic and flavonoid content, antioxidant properties and yield of aeroponically and conventionally grown leafy vegetables and fruit crops: a comparative study. Evid. Based Complement Altern. Med. 2014;2014:253875. doi: 10.1155/2014/253875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oniszczuck A., Hawryl A. Sample preparation of plant material. In: Hajnos M.W., Sherma J., editors. High Performance Liquid Chromatography in Phytochemical Analysis. CRC Press, Taylor & Francis Group; Boca Raton: 2011. pp. 107–142. [Google Scholar]
- 32.Naczk M., Shahidi F. Extraction and analysis of phenolics in food. J. Chromatogr. A. 2004;1054:95–111. [PubMed] [Google Scholar]
- 33.Robards K. Strategies for the determination of bioactive phenols in plants, fruit and vegetables. J. Chromatogr. A. 2003;1000:657–691. doi: 10.1016/s0021-9673(03)00058-x. [DOI] [PubMed] [Google Scholar]
- 34.Gouveia S., Castilho P.C. Characterization of phenolic acid derivatives and flavonoids from different morphological parts of Helichrysum obconicum by a RP-HPLC-DAD-(-)-ESI-MS method. Food Chem. 2011;129:333–344. doi: 10.1016/j.foodchem.2011.04.078. [DOI] [PubMed] [Google Scholar]
- 35.Chen X.F., Wu H.T., Tan G.G. Liquid chromatography coupled with time of flight and ion trap mass spectrometry for qualitative analysis of herbal medicines. J. Pharm. Anal. 2011;1:235–245. doi: 10.1016/j.jpha.2011.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harborne J.B. Chapman & Hall; London: 1998. Phytochemical Methods: a Guide to Modern Techniques of Plant Analysis. [Google Scholar]
- 37.Long Y., Zhang W., Wang F. Simultaneous determination of three curcuminoids in Curcuma longa L. by high performance liquid chromatography coupled with electrochemical detection. J. Pharm. Anal. 2014;4:325–330. doi: 10.1016/j.jpha.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen Q., Li Y., Chen Z. Separation, identification and quantification of active constituents in Fructus Psoraleae by high performance liquid chromatography with UV, ion trap mass spectrometry and electrochemical detection. J. Pharm. Anal. 2012;2:143–151. doi: 10.1016/j.jpha.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pang B., Zhu Y., Lu L. The applications and features of liquid chromatography-mass spectrometry in the analysis of traditional chinese medicine. Evid. Based Complement. Altern. Med. 2016;2016:3837270. doi: 10.1155/2016/3837270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang Z., Wang B., Eaves D.H. Phenolic compound profile of selected vegetables frequently consumed by Africans Americans in the Southeast United States. Food Chem. 2007;103:1395–1402. [Google Scholar]
- 41.Patel R.K., Patel V.R., Patel M.G. Development and validation of a RP-HPLC method for the simultaneous determination of Embelin, Rottlerin and Ellagic acid in Vidangadi Churna. J. Pharm. Anal. 2012;2:366–371. doi: 10.1016/j.jpha.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Turek K., Dzido T.H. Separation selectivity of some phenolic acids in RP-HPLC systems with binary mobile phase comprises various modifiers. Adsorption. 2010;16:287–294. [Google Scholar]
- 43.Steinmann D., Ganzera M. Recent advances on HPLC/MS in medicinal plant analysis. J. Pharm. Biomed. Anal. 2011;55:744–757. doi: 10.1016/j.jpba.2010.11.015. [DOI] [PubMed] [Google Scholar]
- 44.Lin L.Z., Harnly J.M. Identification of phenolic components of collard greens, kale and Chinese broccoli. J. Agric. Food Chem. 2009;57:7401–7408. doi: 10.1021/jf901121v. [DOI] [PubMed] [Google Scholar]
- 45.Ramesh Kumar A., Sivasudha T., Jeyadevi R. Profiling of phenolic compounds using UPLC-Q-TOF-MS/MS and nephroprotective activity of Indian green leafy vegetable Merremia emarginata (Burm.f.) Food Res. Int. 2013;50:94–101. [Google Scholar]
- 46.Bertin R.L., Gonzaga L.V., Graciele da Silva C.B. Nutrient composition and identification/quantification of major phenolic compounds in Sarcocornia ambigua (Amaranthaceae) using HPLC-ESI-MS/MS. Food Res. Int. 2014;55:404–411. [Google Scholar]
- 47.Ola S.S., Catia G., Marzia I. HPLC/DAD/MS characterization and analysis of flavonoids and cynnamoil derivatives in four Nigerian green leafy vegetables. Food Chem. 2009;115:1568–1574. [Google Scholar]
- 48.Dehkharghanian M., Adenier H., Vijayalakshmi M.A. Study of flavonoids in aqueous spinach extract using positive electrospray ionisation tandem quadrupole mass spectrometry. Food Chem. 2010;121:863–870. [Google Scholar]
- 49.Sultana B., Anwar F. Flavonols (kaempferol, quercetin, myricetin) contents of selected fruits, vegetables and medicinal plants. Food Chem. 2008;108:879–884. doi: 10.1016/j.foodchem.2007.11.053. [DOI] [PubMed] [Google Scholar]
- 50.Pasko P., Sajewicz M., Gorinstein S. Analysis of selected phenolic acids and flavonoids in Amaranthus and Chenopodium quinoa seeds and sprouts by HPLC. Acta Chromatogr. 2008;20:661–672. [Google Scholar]
- 51.Khanam U.K.S., Oba S., Yanase E. Phenolic acids, flavonoids and total antioxidant capacity of selected leafy vegetables. J. Funct. Foods. 2012;4:979–987. [Google Scholar]
- 52.Oniszczuk A., Olech M. Optimization of ultrasound-assisted extraction and LC-ESI-MS/MS analysis of phenolic acids from Brassica oleracea L.var.sabellica. Ind. Crops Prod. 2016;83:359–363. [Google Scholar]
- 53.Stintzing F.C., Kammerer D., Schieber A. Betacyanins and phenolic compounds from Amaranthus spinosus L. and Boerhavia erecta L. Z. Nat. 2004;59:1–8. doi: 10.1515/znc-2004-1-201. [DOI] [PubMed] [Google Scholar]
- 54.Cho M.J., Howard L.R., Prior R.L. Flavonoid content and antioxidant capacity of spinach genotypes determined by high performance liquid chromatography/mass spectrometry. J. Sci. Food Agr. 2008;88:1099–1106. [Google Scholar]
- 55.Tuszynska M. Validation of the analytical method for the determination of flavonoids in broccoli. J. Hort. Res. 2014;22:131–140. [Google Scholar]
- 56.Fiol M., Adermann S., Neugart S. Highly glycosylated and acylated flavonols isolated from Kale (Brassica oleracea var.sabellica)-structure-antioxidant activity relationship. Food Res. Int. 2012;47:80–89. [Google Scholar]
- 57.Park S., Arasu M.V., Jiang N. Metabolite profiling of phenolics, anthocyanins and flavonols in Cabbage (Brassica oleracea var.capitata) Ind. Crops Prod. 2014;60:8–14. [Google Scholar]
- 58.Harbaum B., Hubbermann E.M., Zhu Z. Free and bound phenolic compounds in leaves of pak choi (Brassica Campestris L.ssp.Chinensis var Communis) and Chinese leaf mustard (Brassica juncea Coss) Food Chem. 2008;110:838–846. doi: 10.1016/j.foodchem.2008.02.069. [DOI] [PubMed] [Google Scholar]
- 59.Kumar S.S., Manoj P., Giridhar P. Fruit extracts of Basella rubra that are rich in bioactives and betalains exhibit antioxidant activity and cytotoxicity against human cervical carcinoma cells. J. Funct. Foods. 2015;15:509–515. [Google Scholar]
- 60.Devanshu S., Rahul M., Annu G. Quantitative bioanalysis by LC-MS/MS: a review. J. Pharm. Biomed. Sci. 2010;7:1–9. [Google Scholar]
- 61.Ho C.S., Lam C.W.K., Chan M.H.M. Electrospray ionization mass spectrometry: principles and clinical applications. Clin. Biochem. Rev. 2003;24:3–10. [PMC free article] [PubMed] [Google Scholar]
- 62.Banerjee S., Mazumdar S. Electrospray ionization mass spectrometry: a technique to access the information beyond the molecular weight of the analyte. Int. J. Anal. Chem. 2012;2012:282574. doi: 10.1155/2012/282574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Teixeira J., Gaspan A., Garrido E.M. Hydroxycinnamic acid antioxidants: an electrochemical overview. Biomed. Res. Int. 2013;2013:251754. doi: 10.1155/2013/251754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Olkowski A.A., Amarowicz R., Peiquiang Y. A rapid HPLC method for the determination of phenolic acids in plant material. Pol. J. Food Nutr. Sci. 2003;12:53–57. [Google Scholar]
- 65.Jandera P., Skerikova V., Rehova L. RP-HPLC analysis of phenolic compounds and flavonoids in beverages and plant extracts using a Coul Array detector. J. Sep Sci. 2005;28:1005–1022. doi: 10.1002/jssc.200500003. [DOI] [PubMed] [Google Scholar]
- 66.Kumar B.R. A review on metabolic engineering approaches for enrichment and production of new secondary metabolites in Basella species. World J. Pharm. Pharm. Sci. 2016;5:652–671. [Google Scholar]
- 67.Santi M.M., Dipjyoti C. Mass spectrometric detection of phenolic acids. In: Ramawat K.G., Merillion J.M., editors. Natural Products: Phytochemistry, Botany and Metabolism of Alkaloids, Phenolics and Terpenes. Springer-Verlag; Berlin Heidelberg, New York: 2013. pp. 2047–2057. [Google Scholar]
- 68.Bunea A., Andjelkovic M., Socaciu C. Total and individual carotenoids and phenolic acids content in fresh, refrigerated and processed spinach (Spinacia oleracea L) Food Chem. 2008;108:649–656. doi: 10.1016/j.foodchem.2007.11.056. [DOI] [PubMed] [Google Scholar]
- 69.Annie Bligh S.W., Ogegbo O., Wang Z.T. Flavonoids by HPLC. In: Ramawat K.G., Merillon J.M., editors. Natural Products. Springer-Verlag; Berlin Heidelberg: 2013. pp. 2107–2144. [Google Scholar]
- 70.deRijke E., Out P., Niessen W.M.A. Analytical separation and detection methods for flavonoids. J. Chromatogr. A. 2006;1112:31–63. doi: 10.1016/j.chroma.2006.01.019. [DOI] [PubMed] [Google Scholar]
- 71.de Villiers A., Venter P., Pasch H. Recent advances and trends in the liquid chromatography-mass spectrometry analysis of flavonoids. J. Chromatogr. A. 2016;1430:16–78. doi: 10.1016/j.chroma.2015.11.077. [DOI] [PubMed] [Google Scholar]
- 72.Stobiecki M. Application of mass spectrometry for the identification and structural studies of flavonoid glycosides. Phytochemistry. 2000;54:237–256. doi: 10.1016/s0031-9422(00)00091-1. [DOI] [PubMed] [Google Scholar]
- 73.Stobiecki M., Kachlicki P., Wojakowaska A. Application of LC/MS system to structural characterization of flavonoid glycoconjugates. Phytochem. Lett. 2015;11:358–367. [Google Scholar]
- 74.Tian Q., Li D., Patil B.S. Identification and determination of flavonoids in buckwheat (Fagopyrum esculentum Moench, Polygonaceae) by high-performance liquid chromatography with electrospray ionisation mass spectrometry and photodiode array ultraviolet detection. Phytochem. Anal. 2002;13:251–256. doi: 10.1002/pca.649. [DOI] [PubMed] [Google Scholar]
- 75.Kumar P.R., Dinesh S.R., Rini R. LC-MS – a review and recent update. World J. Pharm. Pharm. Sci. 2016;5:377–391. [Google Scholar]
- 76.Pitt J.J. Principles and applications of liquid chromatography-mass spectrometry in clinical biochemistry. Clin. Biochem. Rev. 2009;30:19–34. [PMC free article] [PubMed] [Google Scholar]
- 77.Stead P. Isolation by preparative HPLC. In: Cannell R.J.P., editor. Methods in Biotechnology, Natural Products Isolation. Humana Press Inc; Totowa, New Jersey: 1998. pp. 165–208. [Google Scholar]
- 78.Costa D.C., Costa H.S., Albuquerque T.G. Advances in phenolic compound analysis of aromatic plants and their potential applications. Trends Food Sci. Technol. 2015;45:336–354. [Google Scholar]
- 79.Sasidharan S., Chen Y., Saravanan D. Extraction, isolation and characterization of bioactive compounds from plants' extracts. Afr. J. Tradit. Complement. Altern. Med. 2011;8:1–10. [PMC free article] [PubMed] [Google Scholar]
- 80.Sosa-Ferrera Z., Mahugo-Santana C., Santana-Rodriguez J.J. New developments in liquid chromatography-mass spectrometry for the determination of micropollutants. Chromatogr. Res. Int. 2012;2012:748989. [Google Scholar]
- 81.Khoddami A., Wilkes M.A., Roberts T.H. Techniques for analysis of plant phenolic compounds. Molecules. 2013;18:2328–2375. doi: 10.3390/molecules18022328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hird S.J., Lau B.P.-Y., Schuhmacher R., Krska R. Liquid chromatography-mass spectrometry for the determination of chemical contaminants in food. Trends Anal. Chem. 2014;59:59–72. [Google Scholar]
- 83.Chang-Kee L., Lord G. Current developments in LC-MS for pharmaceutical analysis. Biol. Pharm. Bull. 2002;25:547–557. doi: 10.1248/bpb.25.547. [DOI] [PubMed] [Google Scholar]
- 84.Zhao J., Ge L.Y., Xiong W. Advanced development in phytochemicals analysis of medicine and food dual purposes plants used in China (2011–2014) J. Chromatogr. A. 2016;1428:39–54. doi: 10.1016/j.chroma.2015.09.006. [DOI] [PubMed] [Google Scholar]
- 85.Levsen K., Preiss A., Spraul M. Springer-Verlag; Berlin, Germany: 2002. Proceedings of 11th International Symposium on Nondestructive characterization of Materials X1; pp. 151–180. [Google Scholar]
- 86.Toth G., Alberti A., Solyomvary A. Phenolic profiling of various olive bark-types and leaves: HPLC-ESI/MS study. Ind. Crops Prod. 2015;67:432–438. [Google Scholar]
- 87.Haneef J., Shaharyar M., Hussain A. Application of LC-MS/MS for quantitative analysis of glucocorticoids and stimulants in biological fluids. J. Pharm. Anal. 2013;3:341–348. doi: 10.1016/j.jpha.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Manners G.D., Breska A.P., III, Schoch T.K. Analysis of bitter limonoids in citrus juices by atmospheric pressure chemical ionization and electrospray ionization liquid chromatography-mass spectrometry. J. Agric. Food Chem. 2003;51:3709–3714. doi: 10.1021/jf021124t. [DOI] [PubMed] [Google Scholar]
- 89.Li M., Hou X.F., Zhang J. Application of HPLC/MS in the analysis of traditional Chinese medicines. J. Pharm. Anal. 2011;2:81–91. doi: 10.1016/S2095-1779(11)70015-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wang J., Sun J.Y., Sha C.T. Optimization, validation and application of an assay for the activity of HMG-CoA reductase in-vitro by LC-MS/MS. J. Pharm. Anal. 2015;5:383–388. doi: 10.1016/j.jpha.2015.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kamboj A. Analytical evaluation of herbal drugs. In: Vallisuta Omboon., editor. Drug Discovery Research in Pharmacognosy. In Tech; Croatia: 2012. pp. 23–60. [Google Scholar]
- 92.Sumner L.W., Huhman D.V., Wochniak E.U. Methods, applications and concepts of metabolic profiling: secondary metabolism. In: Baginsky S., Fernie A.R., editors. Plant Systems Biology. Springer; Birkhauser Verlag: 2007. pp. 195–212. [Google Scholar]