Abstract
A sensitive, rapid, simple and economical ultra-performance liquid chromatography–tandem mass spectrometric method (UPLC–MS/MS) was developed and validated for simultaneous determination of imatinib, dasatinib and nilotinib in human plasma using gliquidone as internal standard (IS). Liquid-liquid extraction method with ethyl acetate was used for sample pre-treatment. The separation was performed on an Xtimate Phenyl column using isocratic mobile phase consisting of A (aqueous phase: 0.15% formic acid and 0.05% ammonium acetate) and B (organic phase: acetonitrile) (A:B=40:60, v/v). The flow rate was 0.25 mL/min and the total run time was 6 min. The multiple reaction monitoring (MRM) transitions, m/z 494.5→394.5 for imatinib, 488.7→401.5 for dasatinib, 530.7→289.5 for nilotinib and 528.5→403.4 for IS, were chosen to achieve high selectivity in the simultaneous analyses. The method exhibited great improvement in sensitivity and good linearity over the concentration range of 2.6–5250.0 ng/mL for imatinib, 2.0–490.0 ng/mL for dasatinib, and 2.4–4700.0 ng/mL for nilotinib. The method showed acceptable results on sensitivity, specificity, recovery, precision, accuracy and stability tests. This UPLC–MS/MS assay was successfully used for human plasma samples analysis and no significant differences were found in imatinib steady-state trough concentrations among the SLC22A5 −1889T>C or SLCO1B3 699G>A genotypes (P>0.05). This validated method can provide support for clinical therapeutic drug monitoring and pharmacokinetic investigations of these three tyrosine kinase inhibitors (TKIs).
Keywords: UPLC–MS/MS, Imatinib, Dasatinib, Nilotinib, Polymorphism
1. Introduction
Tyrosine kinase inhibitors (TKIs) are oral small molecule drugs for the treatment of various cancers. The successful use of TKIs has observably improved the long-term survival outcome of patients [1]. However, suboptimal response and response failure to such therapy are still observed in chronic myeloid leukemia (CML) patients [2]. Moreover, most of the patients experience intolerable adverse reactions [3]. Imatinib, dasatinib and nilotinib are the first-line treatment of CML. The relationship between imatinib steady-state trough concentrations and clinical response has been established in CML patients [4]. Moreover, evaluation of the concentrations of dasatinib and nilotinib may also be beneficial to clinical therapy [5]. For example, Wang et al. [6] have reported that the major cytogenetic response is significantly associated with weighted average steady-state dasatinib plasma concentrations, and pleural effusion is significantly associated with its trough concentrations. Besides, a higher nilotinib exposure is found to be associated with higher incidence of bilirubin elevation [7]. Therefore, therapeutic drug monitoring (TDM) of this category of drugs potentially provides a useful tool for individualizing treatment and improving clinical response by dose-adjustment.
It is worth noting that interpatient variability is high (60%) in imatinib steady-state trough concentrations [8]. Gene polymorphisms of transporters may affect imatinib steady-state trough concentrations [9]. Imatinib is a substrate for organic cation transporter 2 (OCTN2) encoded by SLC22A5 and organic anion transporting polypeptide 1B3 (OATP1B3) encoded by SLCO1B3 [10], [11]. However, it is unknown whether polymorphisms in these two genes are related to interpatient variability in imatinib steady-state trough concentrations.
To date, some methods using liquid chromatography–tandem mass spectrometry (LC–MS/MS) for simultaneous determination of several TKIs have been published [12], [13], [14], [15], [16], [17], [18]. However, as previously reported, isotope-labeled ISs, such as [2H8]-imatinib, [2H8]-dasatinib and [13C, 2H3]-nilotinib, were chosen. While isotope-labeled ISs are not quite fit for the conventional TDM in most hospitals’ labs in China because of their difficult availability and high price. Furthermore, one of these assays is developed using online, automated sample pre-treatment and TurboFlow HPLC system, which is limited for routine TDM purposes [16]. Another assay selects solid phase extraction (SPE), a more costly method compared with liquid-liquid extraction, as sample preparation [17]. In the aspect of elution modes, researchers in these studies prefer to use gradient elution which is more complex and slower than isocratic elution [19].
From the point of view of TDM in clinical practice, a rapid, simple and economical method is particularly important for determining a large number of human plasma samples in hospitals. As a result, we mainly aimed to develop and validate a sensitive, rapid and simple UPLC–MS/MS method with minor costs for simultaneous analysis of imatinib, dasatinib and nilotinib, which can be easily applied to conventional TDM in clinical practice in China. This method has been used for quantification of these three TKIs in human plasma samples obtained from a cohort of CML patients. In addition, the application of this method to a pilot study investigating the relationship between imatinib steady-state trough concentrations and different gene polymorphisms of transporters may be of interest to researchers in the pharmacokinetic field.
2. Experimental
2.1. Reagents and chemicals
Imatinib mesylate (Batch number: 085M4727V, purity: 100%) was purchased from SIGMA-ALDRICH, Saint Louis, USA. Dasatinib (Lot number: 10-ABY-13-1, purity: 98%) was obtained from Toronto Research Chemicals Inc., Canada. Nilotinib (E1610009, purity >99%) was obtained from Aladdin®, Shanghai, China. Gliquidone standard was purchased from National Institute for Food and Drug Control, China. Acetonitrile, methanol and ethyl acetate were of HPLC grade, and other chemicals and solvents were all of the highest analytical grade.
2.2. Preparation of stock solutions, calibration standards, and quality control samples
Stock solutions of imatinib, dasatinib and nilotinib standards were prepared in methanol at concentrations of 1.05 mg/mL, 0.98 mg/mL, and 0.94 mg/mL, respectively. A stock solution of gliquidone (IS) was prepared at 1.04 mg/mL in methanol. All stock solutions were stored at −20 ℃. Working solutions were prepared by diluting these stock solutions with 60% acetonitrile, and the working solution of IS for analysis was about 50 μg/mL. Calibration standards were prepared from these working solutions by diluting with blank plasma to achieve concentrations of 2.6 – 5250.0 ng/mL for imatinib, 2.0–490.0 ng/mL for dasatinib, and 2.4–4700.0 ng/mL for nilotinib. Similarly, quality control (QC) samples run in each assay were also prepared.
2.3. Chromatographic and mass spectrometric conditions
UPLC analysis was performed by a Waters Acquity Ultra Performance LC system (Waters, USA). Chromatographic separation was achieved on an Xtimate Phenyl column (2.1 mm×150 mm, 3 µm, Welch Materials, Inc., USA) using isocratic mobile phase. The column temperature was maintained at 40 ℃, and the auto-sampler temperature was set at 10 ℃. The mobile phase consisted of A (aqueous phase: 0.15% formic acid and 0.05% ammonium acetate) and B (organic phase: acetonitrile) (A:B=40:60,v/v). The flow rate was 0.25 mL/min. The total run time was 6 min. The UPLC system was connected to the mass spectrometer Quattro Premier XE MICROMASS (Waters, USA). The analytes and IS quantifications were achieved by operating the mass spectrometer in positive ion electrospray ionization (ESI) source with MRM mode.
The solution of each TKI at a concentration of 1 μg/mL was injected for testing under the isocratic elution with the mobile phase (A:B=40:60, v/v). Take imatinib for example, the Mass Scan function and Daughter Scan function were used to find the precursor ion and product ion of imatinib, respectively. According to the scanning results, the most prominent precursor ion and product ion of imatinib were at m/z 494.5 and m/z 394.5, respectively. So, the MRM transition m/z 494.5→394.5 was created for imatinib. The influential parameter such as collision energy (CE) was optimized step by step during repeated injections. We started from 10 V to 40 V when we optimized the CE for m/z 494.5→394.5, with an interval of 5 V between each injection. When the maximal point was found, we would further optimize the parameter delicately. By doing so, we could find that the strongest signal for the channel m/z 494.5→394.5 was acquired at 28 V. The same strategy was also applied to the tuning of dasatinib, nilotinib and IS. Characteristic transitions were 488.7→401.5 for dasatinib, 530.7→289.5 for nilotinib, and 528.5→403.4 for IS. The optimum CE was 32 V, 30 V and 15 V for dasatinib, nilitonib and IS, respectively. The ion source and desolvation temperatures were 120 ℃ and 350 ℃, respectively. The optimum cone voltages were 45 V, 60 V, 50 V and 30 V for imatinib, dasatinib, nilitonib and IS, respectively. The cone gas flow was 50 L/h and the desolvation gas flow was 750 L/h. Masslynx Sofware (version 4.1, Waters) was used for data acquisition and processing.
2.4. Sample preparation
The liquid-liquid extraction method was used for plasma sample pre-treatment. 60 μL of IS working solution (50 μg/mL) and 50 μL of sodium hydroxide solution (0.1 mol/L) were added into 300 μL of plasma sample. After vortex mixing for about 2 min, ethyl acetate (1.2 mL) was added and vortex-mixed thoroughly for 5 min, then the mixture was centrifuged at 15,000 rpm at 4 ℃ for 10 min. The upper organic phase of the extraction was transferred to another clean tube and evaporated to dryness using a Thermo Electron (USA) RVT4104 refrigerated vapor trap at 35 ℃. Afterwards, the residue was dissolved with 100 μL of 60% acetonitrile and centrifuged at 15,000rpm at 4 ℃ for 3 min. The clear supernatant (20 μL) was injected to the column for analysis.
2.5. Method validation
2.5.1. Specificity and selectivity
The specificity was tested by analyzing six different batches of blank human plasma so as to ensure that no endogenous substances interfere with analytes and IS existed in plasma. The selectivity of the method was demonstrated by comparing chromatograms obtained from blank plasma and spiked plasma.
2.5.2. Recovery and matrix effect
The recovery of analytes and IS was obtained by comparing processed QC samples (low, medium and high concentrations, n=5 for each concentration level) with post-extracted spiked samples at the same concentration levels. The matrix effect was determined by comparing the post-extracted spiked samples with non-extracted samples reconstituted in 60% acetonitrile.
2.5.3. The lower limit of quantification (LLOQ)
LLOQ was obtained based on a signal-to-noise ratio of 10:1. LLOQ was defined as the lowest concentration where the percentage coefficient of variation (CV) of replicates should be less than 20% and the accuracy of the measurement should be within ±20% (n=5).
2.5.4. The intra- and inter-day accuracy and precision
The intra- and inter-day accuracy and precision were obtained by analysis of five replicates of each QC sample (low, medium and high concentrations levels) on three separate days. The percentage coefficient of variation (CV) and relative standard error (RE) were used to evaluate the intra- and inter-day precision and accuracy.
2.5.5. Stability
Stability was also assessed at three QC concentration levels. It included bench-top stability, auto-sampler stability, freeze–thaw stability and long-term storage stability. The bench-top stability was assessed by keeping samples at ambient temperature for 5 h. The auto-sampler stability was determined by re-analyzing the processed QC samples stored at the auto-sampler at 10 ℃ for 8 h. For freeze–thaw stability, QC samples were stored at −70 ℃ for more than 3 h and thawed unassisted at room temperature, and then frozen again at −70 ℃. The cycle was repeated three times. Long-term storage stability was determined by storing QC samples at –70 ℃ for 3 months.
2.6. Method application
The UPLC–MS/MS assay was applied to determine concentrations of imatinib, dasatinib or nilotinib in a cohort of CML patients at steady-state (at least one month of treatment). Sampling time points were recorded. Blood samples were centrifuged as soon as possible at 3000 rpm at 4 ℃ for 5 min. Plasma supernatant was stored at −70 ℃ until analysis. On the day of analysis, samples were thawed at room temperature and processed as described in section 2.4. Furthermore, relationship between imatinib steady-state trough concentrations and polymorphisms of SLC22A5 and SLCO1B3 was assessed among CML patients. The study was approved by the local ethics committee. Informed consent was obtained from all individual participants included in the study.
3. Results and discussion
3.1. Sample pre-treatment
The sample pre-treatment method was tested initially. Protein precipitation with acetonitrile or methyl alcohol and liquid-liquid extraction with diethyl ether, dichloromethane, ethyl acetate or tert-Butyl methyl were tested. It turned out that the signal response and peak shape were better after the liquid-liquid extraction with ethyl acetate than other methods. By the way, ethyl acetate has lower toxicity than by other extraction agents. It is noteworthy that ion suppression and matrix effects are severe issues in ESI [20]. Ionic species, like salts in biologic samples, have high ionization efficiency or surface activity, which competes with analytes during evaporation. Thus, improving the sample cleaning procedure is important. For these reasons, protein precipitation method should be avoided. So, the liquid-liquid extraction with ethyl acetate was chosen as the sample pre-treatment method. Additionally, due to the basicity of analytes, we added sodium hydroxide solution (0.1 mol/L) into the extraction to improve the analytes' solubility and the extraction rate.
3.2. Chromatographic and mass spectrometric conditions
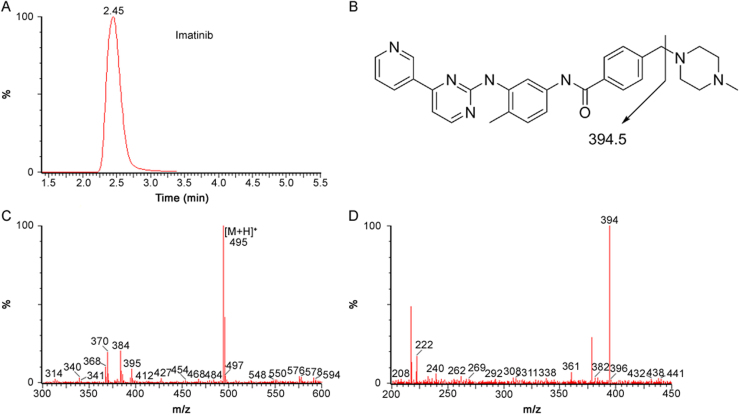
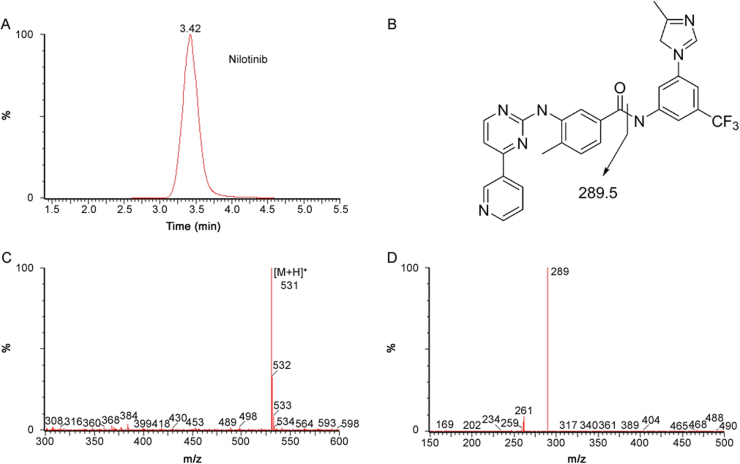
The analytes were ionized with an ESI source operating in the positive ion mode. In order to maximize sensitivity and achieve high specificity, the MRM mode was used in this method. Characteristic transitions (precursor ion→product ion) were m/z 494.5→394.5 for imatinib (Fig. 1), 488.7→401.5 for dasatinib (Fig. 2), and 530.7→289.5 for nilotinib (Fig. 3).
Fig. 1.
Typical MRM chromatograms of human plasma spiked with imatinib (A), fragmentation pathway of imatinib (B), and its positive product ion mass spectrum (C: precursor ion, D: product ion).
Fig. 2.
Typical MRM chromatograms of human plasma spiked with dasatinib (A), fragmentation pathway of dasatinb (B), and its positive product ion mass spectrum (C: precursor ion, D: product ion).
Fig. 3.
Typical MRM chromatograms of human plasma spiked with nilotinib (A), fragmentation pathway of nilotinb (B), and its positive product ion mass spectrum (C: precursor ion, D: product ion).
As previously reported, isotope-labeled ISs, such as [2H8]-imatinib, [2H8]-dasatinib and [13C, 2H3]-nilotinib, were chosen. However, there are some disadvantages of isotope-labeled ISs. Single isotope-labeled ISs usually suffer from “cross-talk” between MRM channels due to the very similarities of their m/z to the original analyte. Thus, multiple isotope-labeled ISs are usually used for simultaneous determination of several drugs. However, since they are difficult to obtain (must purchase from overseas companies) and very costly, multiple isotope-labeled ISs are not quite fit for the conventional TDM in most hospitals labs in China. Therefore, gliquidone was chosen as the IS because of its similar skeleton structure and chromatographic compared to the analytes. Moreover, gliquidone is economical and easy to access from National Institute for Food and Drug Control in China.
The mobile phase should be chosen to achieve the best chromatographic peak shape and mass spectrometric resolution for analytes and IS with a minimum run time. In this study, a mobile phase consisting of A (aqueous phase: 0.15% formic acid and 0.05% ammonium acetate) and B (organic phase: acetonitrile) (60:40, v/v) was used with an isocratic elution mode. In order to obtain better symmetrical peak shapes and ionization efficiency, formic acid was added into the mobile phase. The ammonium acetate was also added because of its function of enhancing the retention of the analytes [21]. After choosing the optimistic eluent system, the next important step is to select the elution mode. Gradient elution and isocratic elution were both tested in this study. However, it turned out that the chromatographic peak shape and separation effect were not improved significantly in the gradient elution mode (Results were not shown). Besides, optimization of the gradient elution is more complex and separation is slower than isocratic elution. Furthermore, it is reported that a number of chromatographers had a phobia of “ghost” peaks, baseline noise and other disturbances (e.g. eluent mixing) in gradient elution [19]. Therefore, isocratic elution was selected as elution mode in this study.
3.3. Method validation
3.3.1. Specificity, selectivity, matrix effect and recovery
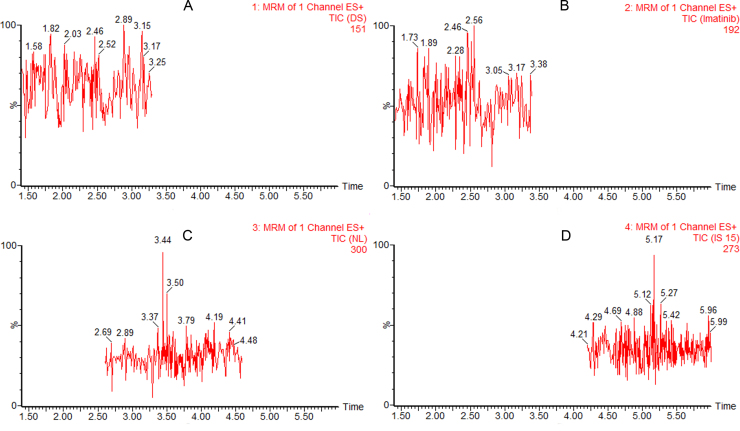
The specificity was tested by analyzing six different batches of blank human plasma to ensure that no endogenous substances interfere with analytes and IS existed in plasma. The selectivity of the method was demonstrated by comparing chromatograms obtained from blank plasma and spiked plasma. The typical MRM chromatogram of blank plasma is shown in Fig. S1. The typical MRM chromatograms of human plasma spiked with imatinib, dasatinib and nilotinib are shown in Fig. 1A, Fig. 2A and Fig. 3A, respectively. The retention time was about 2.4 min for imatinib, 2.3 min for dasatinib, 3.5 min for nilotinib and 5.2 min for IS. No significant interference was observed at the retention time of analytes and IS in the blank human plasma. QC samples (n=5) at each of the three concentration levels were assayed for recovery. The mean extraction recovery was stable and ranged from 53.2% to 67.9% for imatinib (RSD<9%), 63.7%–71.8% for dasaitinb (RSD<13%), 82.6%–103.4% for nilotinib (RSD<20%) (Table 1). Extraction recovery was considered acceptable according to the results.
Table 1.
Matrix effect and extraction recovery of imatinib, dasatinib and nilotinib.
| Drug | Concentration (ng/mL) | Matrix effect (%) | RSD (%) | Extraction recovery (%) | RSD (%) |
|---|---|---|---|---|---|
| Imatinib | 5.3 | 110.0 | 9.1 | 62.5 | 7.2 |
| 525.0 | 112.4 | 6.6 | 53.2 | 10.0 | |
| 4725.0 | 113.8 | 1.7 | 67.9 | 4.8 | |
| Dasatinib | 4.9 | 110.9 | 4.6 | 64.2 | 13.0 |
| 147.0 | 108.8 | 6.0 | 63.7 | 12.8 | |
| 392.0 | 105.5 | 3.0 | 71.8 | 5.8 | |
| Nilotinib | 4.7 | 110.8 | 8.3 | 103.4 | 18.2 |
| 470.0 | 110.1 | 4.1 | 94.9 | 5.6 | |
| 4230.0 | 96.2 | 2.4 | 82.6 | 2.6 |
3.3.2. Linearity, LLOQ, accuracy and precision
Calibration curves were obtained using the peak area ratios of drugs to the IS by weighed least square linear regression (weighting factor=1/x for imatinib and dasatinib, 1/x2 for nilotinib). Selection of the best weighting factor is according to the RE, which compares the regressed concentrations obtained from each weighting factor with the nominal standard concentrations. The weighting factor of 1/x for imatinib and dasatinib and 1/x2 for nilotinib were chosen as the best weighting factors because they gave the least sum of RE across the whole concentration range of each drug. LLOQ is 2.6 ng/mL, 2.0 ng/mL and 2.4 ng/mL for imatinib, dasatinib and nilotinib, respectively. Results indicate that this method was more sensitive than some published methods [12], [14], [22].
The intra- and inter-day accuracy and precision were determined at three QC concentrations with five replicates for each concentration level. Accuracy is expressed as deviations from the nominal concentrations and precision is expressed as CV values [12]. The detailed results are summarized in Table 2. The intra- and inter-day accuracy values were all within ±15% and precision values were all less than 15%. Results indicate that the accuracy and precision met the requirement of determination.
Table 2.
Precision and accuracy results of QC samples of imatinib, dasatinib and nilotinib.
| Drug | Conc. (ng/mL) | Intra-day |
Inter-day |
||||
|---|---|---|---|---|---|---|---|
| Mean±SD(ng/mL) | CV (%) | RE(%)a | Mean±SD (ng/mL) | CV (%) | RE (%)a | ||
| Imatinib | 5.3 | 5.4±0.4 | 7.9 | 1.5 | 5.4±0.7 | 13.3 | 1.3 |
| 525.0 | 584.0±8.8 | 1.5 | 11.2 | 563.0±45.9 | 8.2 | 7.2 | |
| 4725.0 | 4848.2±596.4 | 12.3 | 2.6 | 4580.3±204.1 | 4.5 | −3.1 | |
| Dasatinib | 4.9 | 4.9±0.6 | 12.2 | 0.4 | 4.3±0.3 | 8.3 | −12.6 |
| 147.0 | 139.3±5.8 | 4.2 | −5.2 | 149.7±11.0 | 7.3 | 1.8 | |
| 392.0 | 384.0±34.6 | 9.0 | −2.0 | 387.3±21.2 | 5.5 | −1.2 | |
| Nilotinib | 4.7 | 4.4±0.5 | 10.6 | −5.7 | 5.2±0.7 | 13.9 | 9.9 |
| 470.0 | 411.3±11.2 | 2.7 | −12.5 | 537.1±26.6 | 4.9 | 14.3 | |
| 4230.0 | 4724.5±179.0 | 3.8 | −11.7 | 4510.4±302.2 | 6.7 | 6.6 | |
RE=[(mean measured concentration)/(nominal concentration)−1]×100.
3.3.3. Stability
Three QC concentration levels were applied to evaluate the stability of each analyte under a variety of conditions. Bench-top stability test aims to ensure that analytes are not degraded at ambient temperature during the sample preparation time. Results indicate that blood samples stored at ambient temperature for 5 h are still stable. Results from auto-sampler stability suggest that reconstituted samples stored at auto-sampler vials for 8 h have no significant degradation. The effect of three freeze/thaw cycles was tested for each analyte, and CV values were all less than 9%. During the long-term storage of samples at −70 ℃ for three months, no significant degradation of the analytes was observed. Results are all summarized in Table 3, and show that the stability of analytes is acceptable under all stability tests.
Table 3.
Stability tests results.
| Drug | Conc. (ng/mL) | Bench-topstabilitya |
Autos-samplerstabilityb |
Freeze–thawstabilityc |
Long-term storage stabilityd |
||||
|---|---|---|---|---|---|---|---|---|---|
| Mean±SD (ng/mL) | CV (%) | Mean±SD (ng/mL) | CV (%) | Mean±SD (ng/mL) | CV (%) | Mean±SD (ng/mL) | CV (%) | ||
| Imatinib | 5.3 | 5.3±0.7 | 13.1 | 5.3±0.5 | 9.0 | 5.5±0.5 | 9.0 | 5.3±0.5 | 9.7 |
| 525.0 | 537.4±38.0 | 7.1 | 528.4±18.7 | 3.5 | 524.6±45.4 | 8.6 | 504.9±37.7 | 7.5 | |
| 4725.0 | 4668.5±370.4 | 7.9 | 4763.8±260.4 | 5.5 | 4715.5±297.2 | 6.3 | 4674.2±168.6 | 3.6 | |
| Dasatinib | 4.9 | 4.3±0.6 | 14.8 | 4.2±0.4 | 10.3 | 4.2±0.3 | 6.3 | 4.0±0.4 | 9.9 |
| 147.0 | 151.7±12.8 | 8.4 | 158.3±5.3 | 3.4 | 146.3±10.0 | 6.8 | 143.1±10.2 | 7.1 | |
| 392.0 | 402.2±12.0 | 3.0 | 400.0±17.8 | 4.5 | 407.9±24.3 | 6.0 | 386.9±22.4 | 5.8 | |
| Nilotinib | 4.7 | 5.1±0.4 | 7.4 | 4.5±0.4 | 8.2 | 5.0±0.2 | 4.7 | 4.9±0.4 | 8.8 |
| 470.0 | 477.5±47.0 | 9.8 | 536.5±23.0 | 4.3 | 494.7±39.5 | 8.0 | 417.1±17.1 | 4.1 | |
| 4230.0 | 3843.5±93.7 | 2.4 | 4286.0±136.5 | 3.2 | 4383.2±370.5 | 8.4 | 4051.4±163.0 | 4.0 | |
Stored at ambient temperature (25 ℃) for 5 h.
Stored at auto-sampler's temperature (10 ℃) for 8 h.
After three freeze-thaw cycles.
at –70℃ for 3 months.
3.4. Method application
The validated UPLC–MS/MS assay was used to determine concentrations of imatinib, dasatinib or nilotinib in a cohort of CML patients at steady-state (at least one month of treatment) (Table 4). The method is sensitive in determining these three drugs and all concentrations are above LLOQ. Moreover, it has been reported that polymorphisms of SLC22A5 and SLCO1B3 can affect transport of endogenous and xenobiotic substances [23]. However, it is unknown whether polymorphisms in these two genes are related to interpatient variability in imatinib steady-state trough concentrations. Therefore, the influence of two single nucleotide polymorphisms (SNPs) of these two genes on imatinib steady-state trough concentrations was evaluated in 25 CML patients (Table 5). Results showed that there were no significant differences in imatinib steady-state trough concentrations among the SLC22A5 −1889T>C or SLCO1B3 699 G>A genotypes (P>0.05). This is the first study to evaluate the relationship between imatinib steady-state trough concentrations and SLC22A5 −1889T>C or SLCO1B3 699 G>A genotypes in Chinese CML patients. Even so, further studies with a larger patient population are needed to confirm this result.
Table 4.
Concentrations of imatinib, dasatinib or nilotinib in human plasma of CML patients (n=18).
| Drug | Sample ID | Time interval (h)a | Dose (mg/d) | Conc. (ng/mL) |
|---|---|---|---|---|
| Imatinib | 1 | 2.7 | 400 | 2899.6 |
| 2 | 6.6 | 400 | 2517.2 | |
| 3 | 8.7 | 400 | 2366.2 | |
| 4 | 14.9 | 400 | 2196.0 | |
| 5 | 15.2 | 400 | 1931.5 | |
| 6 | 16.2 | 400 | 1194.1 | |
| 7 | 22.2 | 400 | 664.3 | |
| 8 | 25.7 | 400 | 400.1 | |
| Dasatinib | 1 | 7.8 | 100 | 16.8 |
| 2 | 5.3 | 100 | 94.5 | |
| 3 | 1.2 | 100 | 157.6 | |
| 4 | 3.6 | 100 | 132.9 | |
| 5 | 12.1 | 100 | 13.2 | |
| Nilotinib | 1 | 1.4 | 600 | 2535.3 |
| 2 | 1.8 | 600 | 3130.4 | |
| 3 | 4.4 | 600 | 2437.6 | |
| 4 | 5.0 | 600 | 1920.8 | |
| 5 | 6.3 | 600 | 1495.8 |
The time interval between the last medication and sampling.
Table 5.
Relationship between imatinib steady-state trough concentrations and polymorphisms of SLC22A5 and SLCO1B3 (n=25, dose=400 mg).
| Genotype | n | Steady-state trough conc. (ng/mL) |
P-valuea | |
|---|---|---|---|---|
| Mean | SD | |||
| SLC22A5 −1889T>C | ||||
| TT | 3 | 1285.8 | 960.4 | 0.36 |
| TC | 11 | 1745.6 | 708.8 | |
| CC | 11 | 2081.3 | 711.9 | |
| SLCO1B3 699 G>A | ||||
| GG | 2 | 2331.9 | 291.6 | 0.54 |
| GA | 6 | 1804.9 | 641.5 | |
| AA | 17 | 1791.8 | 826.3 | |
Kruskal–Wallis test was used. P-values of <0.05 were considered statistical significance.
4. Conclusions
A sensitive and selective UPLC–MS/MS assay was developed and validated for simultaneous determination of imatinib, dasatinib and nilotinib in human plasma. The method shows advantages in the aspect of economical IS, simple sample preparation, and short run time, which make it capable of analyzing a large number of human plasma samples in clinical practice in China. The application of this method to a pilot study investigating the relationship between imatinib steady-state trough concentrations and different gene polymorphisms of transporters may be of interest to researchers in the pharmacokinetic field.
Conflicts of interest statement
All authors declared that there are no conflicts of interest.
Footnotes
Peer review under responsibility of Xi'an Jiaotong University.
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.jpha.2017.07.009.
Appendix A. Supplementary material
Fig. S1.
Typical MRM chromatograms of blank plasma samples (A: dasatinib, B: imatinib, C: nilotinib, D: IS).
References
- 1.Ali M.A. Chronic myeloid leukemia in the era of tyrosine kinase inhibitors: an evolving paradigm of molecularly targeted therapy. Mol. Diagn. Ther. 2016;20:315–333. doi: 10.1007/s40291-016-0208-1. [DOI] [PubMed] [Google Scholar]
- 2.Diamond J.M., Melo J.V. Mechanisms of resistance to BCR-ABL kinase inhibitors. Leuk. Lymphoma. 2011;52(Suppl 1):12–22. doi: 10.3109/10428194.2010.546920. [DOI] [PubMed] [Google Scholar]
- 3.Lahaye T., Riehm B., Berger U. Response and resistance in 300 patients with BCR-ABL-positive leukemias treated with imatinib in a single center: a 4.5-year follow-up. Cancer. 2005;103:1659–1669. doi: 10.1002/cncr.20922. [DOI] [PubMed] [Google Scholar]
- 4.Miura M. Therapeutic drug monitoring of imatinib, nilotinib, and dasatinib for patients with chronic myeloid leukemia. Biol. Pharm. Bull. 2015;38:645–654. doi: 10.1248/bpb.b15-00103. [DOI] [PubMed] [Google Scholar]
- 5.Yu H.X., Steeghs N., Nijenhuis C.M. Practical guidelines for therapeutic drug monitoring of anticancer tyrosine kinase inhibitors: focus on the pharmacokinetic targets. Clin. Pharmacokinet. 2014;53:305–325. doi: 10.1007/s40262-014-0137-2. [DOI] [PubMed] [Google Scholar]
- 6.Wang X., Roy A., Hochhaus A. Differential effects of dosing regimen on the safety and efficacy of dasatinib: retrospective exposure-response analysis of a Phase III study. Clin. Pharmacol. 2013;5:85–97. doi: 10.2147/CPAA.S42796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Larson R.A., Yin O.Q., Hochhaus A. Population pharmacokinetic and exposure-response analysis of nilotinib in patients with newly diagnosed Ph+ chronic myeloid leukemia in chronic phase. Eur. J. Clin. Pharmacol. 2012;68:723–733. doi: 10.1007/s00228-011-1200-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bouchet S., Titier K., Moore N. Therapeutic drug monitoring of imatinib in chronic myeloid leukemia: experience from 1216 patients at a centralized laboratory. Fundam. Clin. Pharmacol. 2013;27:690–697. doi: 10.1111/fcp.12007. [DOI] [PubMed] [Google Scholar]
- 9.Takahashi N., Miura M., Scott S.A. Influence of CYP3A5 and drug transporter polymorphisms on imatinib trough concentration and clinical response among patients with chronic phase chronic myeloid leukemia. J. Hum. Genet. 2010;55:731–737. doi: 10.1038/jhg.2010.98. [DOI] [PubMed] [Google Scholar]
- 10.Yamakawa Y., Hamada A., Nakashima R. Association of genetic polymorphisms in the influx transporter SLCO1B3 and the efflux transporter ABCB1 with imatinib pharmacokinetics in patients with chronic myeloid leukemia. Ther. Drug Monit. 2011;33:244–250. doi: 10.1097/FTD.0b013e31820beb02. [DOI] [PubMed] [Google Scholar]
- 11.Angelini S., Pantaleo M.A., Ravegnini G. Polymorphisms in OCTN1 and OCTN2 transporters genes are associated with prolonged time to progression in unresectable gastrointestinal stromal tumours treated with imatinib therapy. Pharmacol. Res. 2013;68:1–6. doi: 10.1016/j.phrs.2012.10.015. [DOI] [PubMed] [Google Scholar]
- 12.van Erp N.P., de Wit D., Guchelaar H. A validated assay for the simultaneous quantification of six tyrosine kinase inhibitors and two active metabolites in human serum using liquid chromatography coupled with tandem mass spectrometry. J. Chromatogr. B. 2013;937:33–43. doi: 10.1016/j.jchromb.2013.08.013. [DOI] [PubMed] [Google Scholar]
- 13.Lankheet N.A.G., Hillebrand M.J.X., Rosing H. Method development and validation for the quantification of dasatinib, erlotinib, gefitinib, imatinib, lapatinib, nilotinib, sorafenib and sunitinib in human plasma by liquid chromatography coupled with tandem mass spectrometry. Biomed. Chromatogr. 2013;27:466–476. doi: 10.1002/bmc.2814. [DOI] [PubMed] [Google Scholar]
- 14.Andriamanana I., Gana I., Duretz B. Simultaneous analysis of anticancer agents bortezomib, imatinib, nilotinib, dasatinib, erlotinib, lapatinib, sorafenib, sunitinib and vandetanib in human plasma using LC/MS/MS. J. Chromatogr. B. 2013;926:83–91. doi: 10.1016/j.jchromb.2013.01.037. [DOI] [PubMed] [Google Scholar]
- 15.Götze L., Hegele A., Metzelder S.K. Development and clinical application of a LC-MS/MS method for simultaneous determination of various tyrosine kinase inhibitors in human plasma. Clin. Chim. Acta. 2012;413:143–149. doi: 10.1016/j.cca.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 16.Couchman L., Birch M., Ireland R. An automated method for the measurement of a range of tyrosine kinase inhibitors in human plasma or serum using turbulent flow liquid chromatography–tandem mass spectrometry. Anal. Bioanal. Chem. 2012;403:1685–1695. doi: 10.1007/s00216-012-5970-2. [DOI] [PubMed] [Google Scholar]
- 17.Bouchet S., Chauzit E., Ducint D. Simultaneous determination of nine tyrosine kinase inhibitors by 96-well solid-phase extraction and ultra performance LC/MS-MS. Clin. Chim. Acta. 2011;412:1060–1067. doi: 10.1016/j.cca.2011.02.023. [DOI] [PubMed] [Google Scholar]
- 18.Haouala A., Zanolari B., Rochat B. Therapeutic drug monitoring of the new targeted anticancer agents imatinib, nilotinib, dasatinib, sunitinib, sorafenib and lapatinib by LC tandem mass spectrometry. J. Chromatogr. B. 2009;877:1982–1996. doi: 10.1016/j.jchromb.2009.04.045. [DOI] [PubMed] [Google Scholar]
- 19.Schellinger A.P., Carr P.W. Isocratic and gradient elution chromatography: a comparison in terms of speed, retention reproducibility and quantitation. J. Chromatogr. A. 2006;1109:253–266. doi: 10.1016/j.chroma.2006.01.047. [DOI] [PubMed] [Google Scholar]
- 20.King R., Bonfiglio R., Fernandez-Metzler C. Mechanistic investigation of ionization suppression in electrospray ionization. J. Am. Soc. Mass Spectrom. 2000;11:942–950. doi: 10.1016/S1044-0305(00)00163-X. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Y., Qiang S., Yu Z. LC-MS-MS determination of imatinib and N-desmethyl imatinib in human plasma. J. Chromatogr. Sci. 2014;52:344–350. doi: 10.1093/chromsci/bmt037. [DOI] [PubMed] [Google Scholar]
- 22.De Francia S., D'Avolio A., Martino F. De. New HPLC-MS method for the simultaneous quantification of the antileukemia drugs imatinib, dasatinib, and nilotinib in human plasma. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2009;877:1721–1726. doi: 10.1016/j.jchromb.2009.04.028. [DOI] [PubMed] [Google Scholar]
- 23.Zhou F., Zhu L., Wang K. Recent advance in the pharmacogenomics of human Solute Carrier Transporters (SLCs) in drug disposition. Adv. Drug Deliv. Rev. 2017;116:21–36. doi: 10.1016/j.addr.2016.06.004. [DOI] [PubMed] [Google Scholar]