Abstract
Management of cardiovascular risk factors in diabetes demands special attention due to their co-existence. Pioglitazone (PIO) and telmisartan (TLM) combination can be beneficial in effective control of cardiovascular complication in diabetes. In this research, we developed and validated a high throughput LC–MS/MS method for simultaneous quantitation of PIO and TLM in rat plasma. This developed method is more sensitive and can quantitate the analytes in relatively shorter period of time compared to the previously reported methods for their individual quantification. Moreover, till date, there is no bioanalytical method available to simultaneously quantitate PIO and TLM in a single run. The method was validated according to the USFDA guidelines for bioanalytical method validation. A linear response of the analytes was observed over the range of 0.005–10 µg/mL with satisfactory precision and accuracy. Accuracy at four quality control levels was within 94.27%–106.10%. The intra- and inter-day precision ranged from 2.32%–10.14 and 5.02%–8.12%, respectively. The method was reproducible and sensitive enough to quantitate PIO and TLM in rat plasma samples of a preclinical pharmacokinetic study. Due to the potential of PIO-TLM combination to be therapeutically explored, this method is expected to have significant usefulness in future.
Keywords: LC–MS/MS, Rat plasma, Pharmacokinetic applicability, Telmisartan, Pioglitazone, Pharmacokinetic application
1. Introduction
Metabolic syndrome is a disorder of collective factors which jointly contribute in progression of a series of detrimental chronic conditions including insulin resistance, diabetes and hypertension [1], [2], [3], [4], [5]. Statistically, 75% of people with type 2 diabetes are hypertensive and about 50% of hypertensive individuals are hyperinsulinemic [6]. Hypertension in diabetes mellitus thus is not rare and nowadays considered as common chronic co-existing condition [4]. Therefore, amelioration of one component of metabolic syndrome with single drug may not reduce overall cardiovascular risks and predominance of type 2 diabetes [7]. The occurrence of diabetes and its associated cardiovascular events has reached epidemic proportions, thus demanding urgent effort to target pathways and biomolecules involved in their pathogenesis [8]. It has been identified that in humans, mutation in the peroxisome proliferator–activated receptor (PPAR)-γ is responsible for developing the metabolic syndrome [9], [10]. Activation of PPAR ligands has attractive potential impact on metabolic disorder, diabetes and the related cardiovascular risk factors [2]. Pioglitazone (PIO), a thiazolidinediones antidiabetic, has high-affinity to PPAR-γ ligands and is reported to have blood pressure lowering effect and other cardiovascular benefits [8], [11], [12]. Telmisartan (TLM), an angiotensin type 1 receptor blocker (ARB) antihypertensive has been shown to have significant PPAR-γ receptor activation capacity [13], [14], [15]. Amongst all the tested antihypertensive, agents, the binding affinity for TLM to PPAR-γ has been reported to be the highest. Due to this uniqueness, TLM shows beneficial antidiabetic effects in diabetic patients independent of their antihypertensive, effects [16], [17].
Instead of using conventional single agents, combination of drugs that can stimulate PPAR-γ has the potential to provide distinguished opportunities for the prevention and treatment of diabetes and associated cardiovascular events in high-risk diabetic populations [2]. PIO is superior to TLM in controlling blood glucose level. In contrast, TLM is undoubtedly more effective than PIO in controlling blood pressure. When combined, they can provide better control of blood pressure, hyperglycaemia and insulin resistance as a whole compared to their individual use [18], [19], [20].
But, the in vivo safety of the drugs may get altered when they are combined. Therefore, before going forward with this combination, the potential drug interactions of PIO and TLM in terms of pharmacokinetic perspective need to be studied. There are several bioanalytical methods exist for analysis of PIO [21], [22], [23], [24], [25] and TLM [26], [27], [28] either alone or in combination with other analytes in different types of biological matrices. But, to the best of our knowledge, there was no reported validated bioanalytical method for their simultaneous quantitation in any type of biological matrix. This finding directed us to develop and validate a new LC–MS/MS method for easy and simultaneous determination of PIO and TLM from a single run. The effort was also to increase the sensitivity for detection of the analytes and minimize the run time compared to their existing quantitation methods. The common mass parameters for simultaneous detection of two analytes need to be developed and set critically. Individual existing method may not be suitable for simultaneous quantification of two analytes when they are combined. The common parameters should be suitable for both the analytes in their ionization; otherwise sensitivity of one or both the analytes will be lost. As there is a potential scope for this combination to be therapeutically explored in future, development of this method is expected to be highly useful for analysis of plasma samples of pharmacokinetic study of the combination.
The objective of this research was to develop and validate an LC–MS/MS method following the USFDA guidelines which can be used in simultaneous quantitation of PIO and TLM in the rat plasma samples of pharmacokinetic studies.
2. Materials and methods
2.1. Chemicals and reagents
Telmisartan, pioglitazone (Fig. 1) and rosiglitazone were obtained from Hangzhou Hyper Chemicals Limited (Zhejiang, China). Ammonium formate (analytical reagent grade), dichloromethane (HPLC grade) and methanol (HPLC grade) were purchased from Fisher Scientific, India. The HPLC grade water was used in developing and validating the analytical method.
Fig. 1.
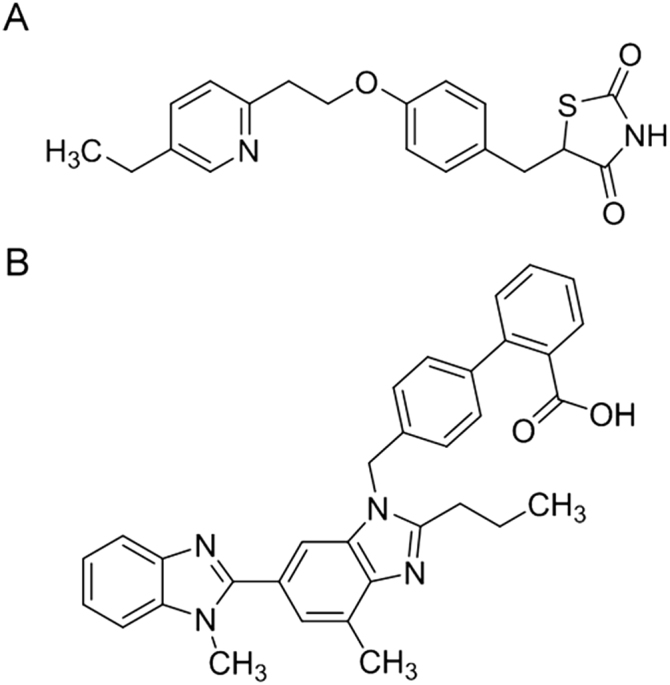
Structural representation of (A) pioglitazone and (B) telmisartan.
2.2. Instrument and chromatography
The Shimadzu (Kyoto, Japan) liquid chromatographic system used was assembled with solvent pump (LC-20 AD), controller (LC-20 AD) and column oven (CTO-10ASvp). SIL-20A autosampler was used and 10 μL of processed samples was injected to a Phenomenex Kinetex C18 (50 mm × 4.6 mm, 5 µm) column kept at ambient atmosphere. A blend of methanol-10 mM ammonium formate in water (9:1, v/v) was used as the isocratic mobile phase. Selection of mobile phase was based on effect on sensitivity of the method, suitability for mass detector, total time required for the analysis and ease of its preparation. The flow rate was fixed at 0.5 mL/min. The quantitation for both of the analytes and internal standard (IS) rosiglitazone was achieved by detecting in MS/MS with a triple quadrupole mass spectrometer (API 2000, AB Sciex Instruments, Canada). Sensitivity was found maximum in positive ion mode, when turboionspray interface temperature and ion spray voltage were set at 400 °C and 5500 V, respectively. Nebulizer gas and auxillary gas (common parameters) were set at 50 psi. The curtain gas and collision gas were set at 20 and 4 on an arbitrary scale. The optimized compound parameters viz., declustering potential (DP), focusing potential (FP), entrance potential (EP), collision cell entrance potential (CEP), collision energy (CE) and collision cell exit potential (CXP), for the analytes and IS are summarized in Table 1.
Table 1.
Optimized LC–MS/MS voltages.
| Parameters | Telmisartan | Pioglitazone | Rosiglitazone |
|---|---|---|---|
| Declusturing potential (V) | 50 | 62 | 30 |
| Focusing potential (V) | 400 | 310 | 400 |
| Entrance potential (V) | 10 | 5.4 | 4 |
| Collision cell entrance potential (V) | 20 | 17.45 | 29.3 |
| Collision energy (eV) | 40 | 40 | 46 |
| Collision cell exit potential (V) | 18 | 2 | 15 |
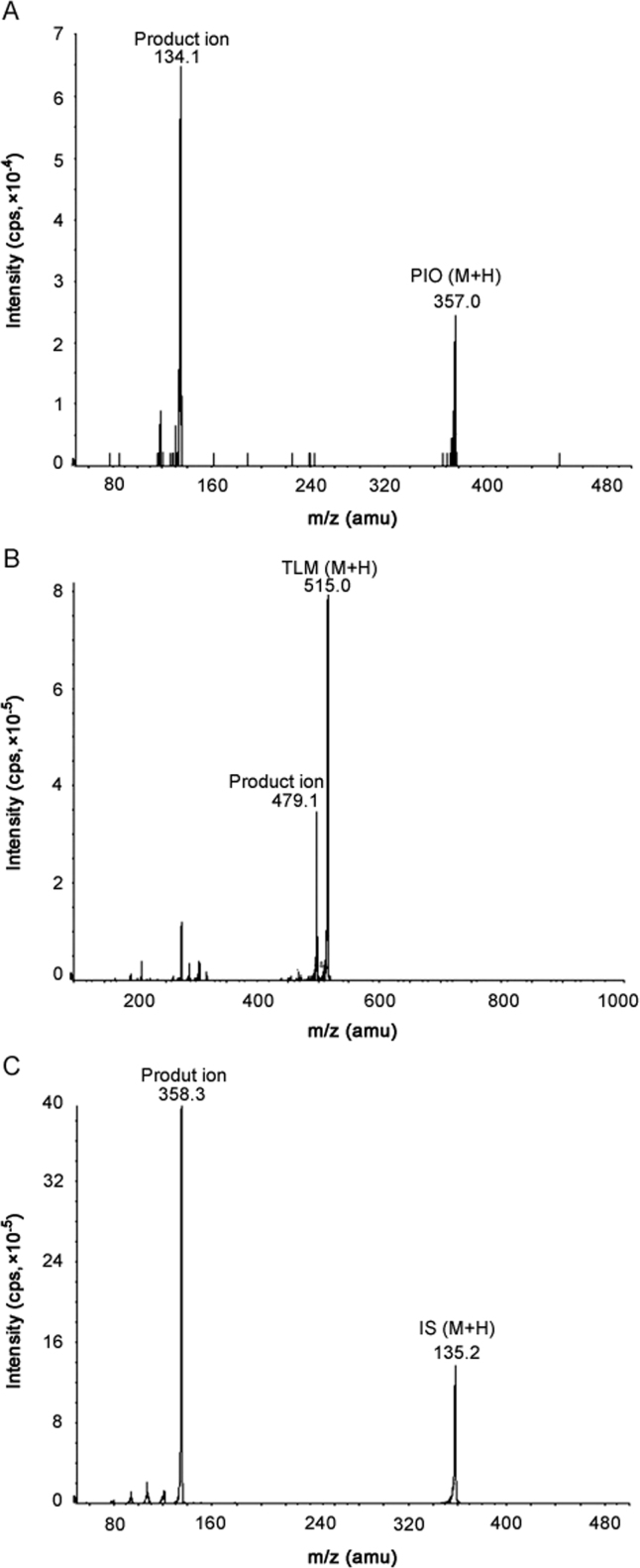
Detection of the ion pairs were performed by multiple reaction monitoring (MRM) mode. The m/z transition pairs (precursor ion/product ion) were 515.0/497.1 for TLM, 357.0/134.1 for PIO and 358.3/135.2 for IS (Fig. 2). TLM (C33H30N4O2) shows its protonated parent ion at 515 (M+H). The product ion (C33H29N4O+, 497.1) for TLM forms after removal of one OH- group. PIO (C19H20N2O3S) shows its protonated parent ion at 357 (M+H). The product ion (C9H12N+, 134.1) for PIO forms after complete cleavage of the molecule at its ester bond. Rosiglitazone (C18H19N3O3S) shows its protonated parent ion at 358.3 (M+H). The product ion (C8H11N2+, 135.2) forms in a similar way after cleavage of its ester bond. The quadrupoles were set at unit resolution. The analytical data were processed by Analyst software (version 1.4.1).
Fig. 2.
Product ion mass spectra of (A) pioglitazone, (B) telmisartan, and (C) rosiglitazone (IS).
2.3. Standard solutions
Separate stock solutions (1.0 mg/mL) of TLM, PIO and rosiglitazone were prepared in methanol for subsequent working solution preparation. Calibration curve (CC) and quality control (QC) standards were prepared by diluting the individual solutions. Required amount of working solutions was spiked to the blank rat plasma to prepare a combined eight-point calibration curve with the concentrations of 0.005, 0.01, 0.05, 0.1, 0.5, 1, 5 and 10 μg/mL for individual analytes. QC standards were prepared separately by spiking the analyte solution to blank rat plasma to achieve the final concentrations of 0.015 (low QC (LQC), 4.5 (medium QC (MQC)) and 8 (high QC (HQC)) µg/mL. The working IS solution of 30 µg/mL of rosiglitazone was prepared in methanol. Polypropylene vials were used to store the solutions at –20℃.
2.4. Preparation of calibration and QC samples
Calibration samples were prepared by spiking 10 μL of individual analyte stock solution to 90 μL of blank rat plasma and adding 10 μL of IS solution. Recovery, precision and accuracy samples were prepared by spiking blank rat plasma with PIO and TLM at lower limit of quantitation (LLOQ), LQC, MQC and HQC levels. Stability study samples were also prepared in the same procedure at LQC and HQC levels and kept at −20 °C until analysis.
2.5. Sample preparation
To the 100 μL of rat plasma sample, 10 μL of IS solution (30 µg/mL) equivalent to 0.30 µg was spiked and vortex mixed for 1 min. The samples were extracted using 2 mL of dichloromethane. The extraction was facilitated by hand mixing for 10 min after addition of the extracting solvent to the plasma and subjected to centrifugation at 4000 rpm for 15 min. The dichloromethane layer (1.8 mL) was pipetted out and subjected to evaporation at 40 °C in nitrogen evaporator. The dried residue was re-dissolved in 100 μL of mobile phase and 10 μL of was injected into the LC–MS system.
2.6. Method validation
The method was validated for the fundamental validation parameters following the USFDA guidelines for the bioanalytical method validation [29].
2.6.1. Selectivity and specificity
Blank plasma of six different rats was collected to determine the selectivity and specificity of the method. Any interference from unwanted plasma components at the elution time of PIO, TLM and IS was evaluated.
2.6.2. Recovery
Recovery experiment was carried out at LQC, MQC and HQC levels for PIO and TLM, whereas recovery of IS was performed at the concentration of 3 μg/mL. Response of PIO and TLM extracted from the QC samples (n=5, each level) was compared to their corresponding response after post extracted standard plasma sample at similar level for determination of the extraction recovery [21].
2.6.3. Matrix effect
The similar QC levels as used in recovery experiment were used for matrix effect determination. The effects of plasma components on the ionization of the analytes were determined after comparison of the response from post extracted QC samples (n=5, each level) with that from the neat samples at similar concentrations [29].
2.6.4. Calibration curve
The chromatographic area ratio of MRM pair of PIO and TLM to the IS against the theoretical concentration of calibration standards was plotted to construct the calibration curve of the individual analytes. The resulted values were used in linear regression analysis. The acceptance criteria for the back-calculated standard concentration were ±15% deviation from the theoretical value. For LLOQ this criteria was set to ±20% of coefficient of variation (CV) [29].
2.6.5. Accuracy and precision
The closeness of mean results determined by the method to the actual concentration of the analyte including the repeatability was evaluated. Five sets of four different QC levels (LLOQ, LQC, MQC and HQC) were injected on three separate days to determine the inter- and intra- day precision and accuracy of the method. The acceptance criteria of the data included as the accuracy should be within ±15%. For LLOQ, accuracy acceptable limit of deviation was ±20%. The precision around the theoretical value should not cross 15% of the CV. For LLOQ, the same criteria were set as in calibration points [29].
2.6.6. Stability experiments
Five replicates of three different QC levels (LQC, MQC and HQC) were injected to determine the stability of the analytes in various predetermined conditions. The post-preparative stability of the sample was determined after comparison of the response of immediate injection of the extracted samples to that of the re-injected samples after keeping in the autosampler for 6 h. The relative stability was calculated by considering the initial area of the peak of PIO and TLM as 100%. The post-preparative stability of the drugs and the IS was assessed based on the batch size in validation samples over the anticipated run time which will not exceed 6 h. Short-term and long-term stability were determined after placing the QC samples for 8 h on bench top at room temperature and for 7 days at −20 °C. The time period for short-term stability study was determined based on the possible duration that the samples may require to be maintained at room temperature for future pharmacokinetic study. The long-term stability evaluation for 7 days was expected to be sufficient between the first sample collection and the last sample analysis. The stability of analytes in the plasma following consecutive three freeze-thaw cycles (−20 °C storage temperature) was determined. The processing of the samples was performed following the method as mentioned in Section 2.5. Sample stability was confirmed based on the stability analysis results where the values for accuracy (±15%) and precision (±15%) found were within the acceptable limits [29].
2.6.7. Preclinical pharmacokinetic experiment
A preclinical pharmacokinetic study of PIO and TLM combination in rats (six) was performed. The study was approved by Institutional Ethical Committee, Jadavpur University, India. The rats were administered 2 mg/kg of TLM and 3 mg/kg of PIO orally. Rat blood samples were collected at pre-dose, 0.5, 1.5, 2, 3, 5, 8, 12, 24 and 36 h into the tubes with EDTA as anti-coagulant. The blood samples were subjected to centrifuging for plasma separation and stored frozen at −20 °C until analysis. Before analysis, 10 μL of IS solution was spiked with the plasma (100 μL) samples and processed. The data analysis was performed following non-compartmental method with WinNonlin Version 5.1 software.
3. Results
3.1. Method validation
3.1.1. Selectivity and specificity
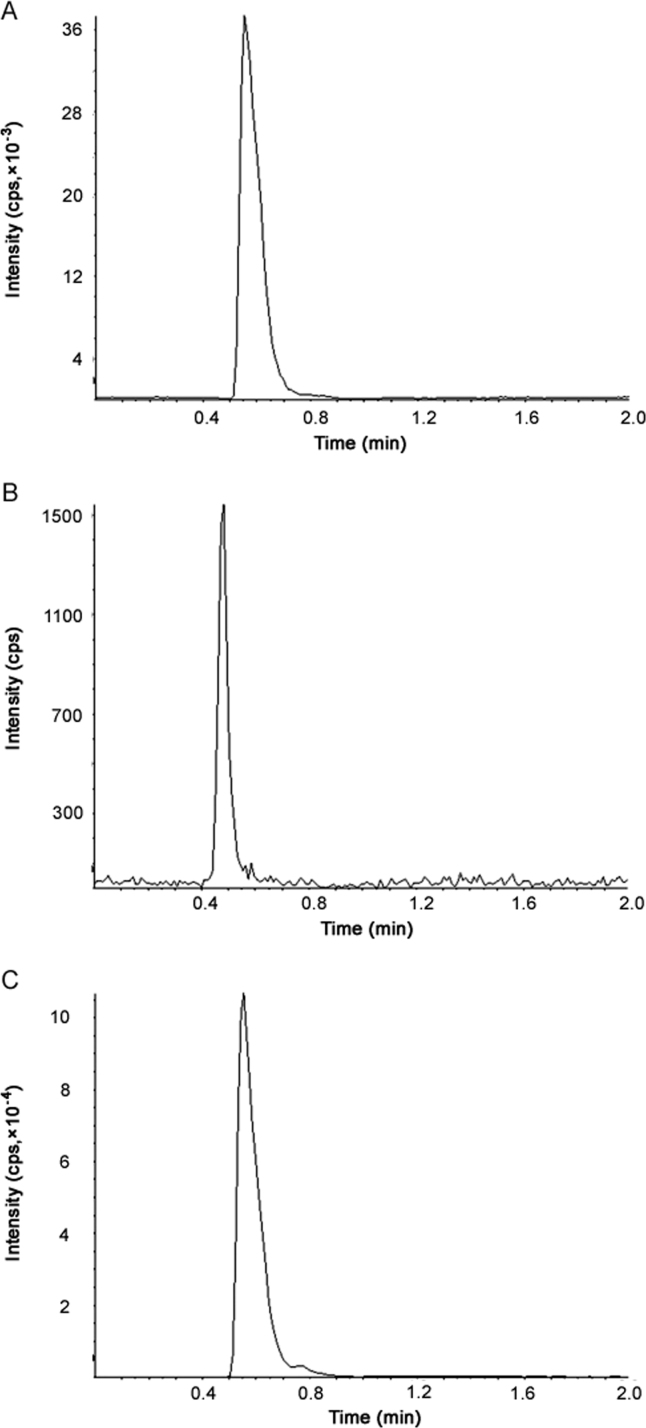
The retention time for TLM, PIO and IS was 0.47, 0.56 and 0.55 min, respectively. We did not find any plasma component to interfere at the elution time of PIO, TLM and IS. Total time required to complete a run was 2 min. Typical MRM chromatograms of PIO and TLM at LLOQ, as well as IS in rat plasma are shown in Fig. 3. The method was found capable to separate and quantify PIO and TLM in the presence of other plasma components.
Fig. 3.
Typical MRM chromatograms of (A) PIO spiked in rat plasma at LLOQ, (B) TLM spiked in rat plasma at LLOQ, (C) IS spiked in rat plasma at 3 µg/mL.
3.1.2. Recovery
Comparison of analyte response in the standard following normal extraction with the standard response in which the analytes were mixed after extraction was performed to determine the extraction recovery of the method. Average extraction recovery for TLM was 93.35% −94.10% whereas for PIO it was 90.29% −95.70%. The extraction recovery of rosiglitazone determined at 3 µg/mL was 88%. The extent of recovery of PIO, TLM and IS was consistent, precise, and reproducible. So, the extraction efficiency of the method within the limits of requirement has been established.
3.1.3. Matrix effect
The plasma components did not change or deteriorate the behavior of the developed method as the % CV of five replicates at three QC levels was found to be below 3% for PIO and TLM (data not shown). The interference from the plasma was insignificant.
3.1.4. Calibration curve
The correlation between instrumental response with known concentrations of PIO and TLM was established by evaluating the best fit of area ratio of the peak (analyte to IS) against concentration. The mean regression (n=3) for TLM and PIO was >0.999. The calibration curves were linear in their concentrations range of 0.005–10 µg/mL. As the injection volume was 10 μL, the actual amount of injected analyte ranged from 0.05 ng to 100 ng. Reproducibility of the calibration curve across the calibration points was established. The lowest concentrations with the CV <20% was taken as LLOQ and found to be 0.005 µg/mL. The accuracy found for the mean of back-calculated concentration for three linearity experiments was within 96.54%–110.87% and 90.87%–109.27% for TLM and PIO, respectively. The precision (% CV) values were within 1.41%–9.67% and 1.35%–6.12% for TLM and PIO, respectively. The number of standards used in constructing the standard curve was sufficient to establish the analyte/response relationship.
3.1.5. Accuracy and precision
The data from this experiment confirmed the accuracy and repeatability of the developed method within the calibration concentration range. The results of intra- and inter-day accuracy and precision determined by analyzing five replicates of QC samples at four levels on three separate days are summarized in Tables 2 and 3.
Table 2.
Intra-day accuracy and precision data of the analytes.
| Qualitycontrol | Run | PIO |
TLM |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean found (ng/mL) | SD | % CV | Accuracy % | Mean found (ng/mL) | SD | % CV | Accuracy % | ||
| LLOQ | 1 | 5.15 | 0.19 | 3.71 | 102.92 | 4.90 | 0.50 | 10.14 | 97.92 |
| 2 | 5.20 | 0.23 | 4.33 | 103.96 | 5.09 | 0.46 | 9.13 | 101.76 | |
| 3 | 5.27 | 0.17 | 3.19 | 105.44 | 4.86 | 0.40 | 8.21 | 97.24 | |
| 4 | 4.79 | 0.32 | 6.74 | 95.84 | 5.18 | 0.27 | 5.30 | 103.68 | |
| LQC | 1 | 15.24 | 0.93 | 6.11 | 101.60 | 14.46 | 0.83 | 5.72 | 96.40 |
| 2 | 14.32 | 1.00 | 7.01 | 95.47 | 14.46 | 1.07 | 7.39 | 96.40 | |
| 3 | 14.52 | 1.04 | 7.15 | 96.80 | 14.14 | 0.98 | 6.92 | 94.27 | |
| 4 | 14.72 | 0.53 | 3.58 | 98.13 | 14.86 | 0.57 | 3.82 | 99.07 | |
| MQC | 1 | 4404.00 | 168.02 | 3.82 | 97.87 | 4538.00 | 302.27 | 6.66 | 100.84 |
| 2 | 4644.00 | 254.13 | 5.47 | 103.20 | 4734.00 | 309.24 | 6.53 | 105.20 | |
| 3 | 4302.00 | 149.23 | 3.47 | 95.60 | 4616.00 | 275.92 | 5.98 | 102.58 | |
| 4 | 4388.00 | 199.67 | 4.55 | 97.51 | 4630.00 | 107.47 | 2.32 | 102.89 | |
| HQC | 1 | 8072.00 | 687.95 | 8.52 | 100.90 | 7650.00 | 491.07 | 6.42 | 95.63 |
| 2 | 7782.00 | 654.46 | 8.41 | 97.28 | 7918.00 | 442.12 | 5.58 | 98.98 | |
| 3 | 8488.00 | 422.46 | 4.98 | 106.10 | 8076.00 | 706.14 | 8.74 | 100.95 | |
| 4 | 7742.00 | 455.16 | 5.88 | 96.78 | 7868.00 | 463.86 | 5.90 | 98.35 | |
% CV: coefficient of variation. Accuracy: (Mean assayed concentration – nominal concentration)/(nominal concentration) × 100.
Table 3.
Inter-day accuracy and precision data of the analytes.
| Qualitycontrol | PIO |
TLM |
||||||
|---|---|---|---|---|---|---|---|---|
| Mean found (ng/mL) | SD | % CV | Accuracy % | Mean found (ng/mL) | SD | % CV | Accuracy % | |
| LLOQ | 5.10 | 0.29 | 5.62 | 102.04 | 5.01 | 0.41 | 8.12 | 100.15 |
| LQC | 14.70 | 0.90 | 6.10 | 98.00 | 14.48 | 0.85 | 5.87 | 96.53 |
| MQC | 4434.50 | 222.72 | 5.02 | 98.54 | 4629.50 | 250.90 | 5.42 | 102.88 |
| HQC | 8021.00 | 603.81 | 7.53 | 100.26 | 7878.00 | 516.37 | 6.55 | 98.48 |
% CV: coefficient of variation. Accuracy: (Mean assayed concentration – nominal concentration)/(nominal concentration) × 100.
3.1.6. Stability
The data for in-injector stability (6 h), short-term stability (8 h) at room temperatures freeze/thaw stability (three freeze/thaw cycles) and long-term stability at −20 °C for 7 days indicated that observed experimental concentration for PIO and TLM at LQC, MQC and HQC levels was within the acceptable limits (±15%) of the theoretical value (Table 4).
Table 4.
Stability data of the analytes in rat plasma.
| Stability | Quality control | PIO |
TLM |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Meanfound(ng/mL) | SD | % CV | Accuracy % | Mean found (ng/mL) | SD | % CV | Accuracy% | ||
| Immediate injection | LQC | 14.84 | 0.46 | 3.11 | 98.93 | 14.76 | 0.80 | 5.43 | 98.40 |
| MQC | 4542.00 | 197.66 | 4.35 | 100.93 | 4716.00 | 192.69 | 4.09 | 104.80 | |
| HQC | 7988.00 | 404.31 | 5.06 | 99.85 | 7876.00 | 404.51 | 5.14 | 98.45 | |
| In-injector stability (6 h) | LQC | 14.36 | 0.32 | 2.23 | 95.73 | 14.10 | 0.67 | 4.78 | 94.00 |
| MQC | 4300.00 | 132.48 | 3.08 | 95.56 | 4584.00 | 182.02 | 3.97 | 101.87 | |
| HQC | 7626.00 | 418.49 | 5.49 | 95.33 | 7472.00 | 326.53 | 4.37 | 93.40 | |
| Short-term stability (8 h at room temperature) | LQC | 14.48 | 0.48 | 3.29 | 96.53 | 15.00 | 0.91 | 6.06 | 100.00 |
| MQC | 4156.00 | 244.70 | 5.89 | 92.36 | 4178.00 | 144.98 | 3.47 | 92.84 | |
| HQC | 7914.00 | 345.59 | 4.37 | 98.93 | 7788.00 | 457.02 | 5.87 | 97.35 | |
| Freeze-thaw (three freeze-thaw cycles) | LQC | 14.84 | 0.66 | 4.46 | 98.93 | 14.42 | 1.06 | 7.33 | 96.13 |
| MQC | 4346.00 | 142.93 | 3.29 | 96.58 | 4462.00 | 135.54 | 3.04 | 99.16 | |
| HQC | 7666.00 | 220.64 | 2.88 | 95.83 | 7976.00 | 428.05 | 5.37 | 99.70 | |
| Long-term stability (7 days at −20 °C) | LQC | 13.80 | 0.64 | 4.67 | 92.00 | 14.28 | 1.28 | 8.93 | 95.20 |
| MQC | 4054.00 | 252.15 | 6.22 | 90.09 | 3992.00 | 196.39 | 4.92 | 88.71 | |
| HQC | 7724.00 | 429.57 | 5.56 | 96.55 | 7586.00 | 293.48 | 3.87 | 94.83 | |
% CV: coefficient of variation. Accuracy: (Mean assayed concentration – nominal concentration)/(nominal concentration) ×100.
3.2. Preclinical pharmacokinetics
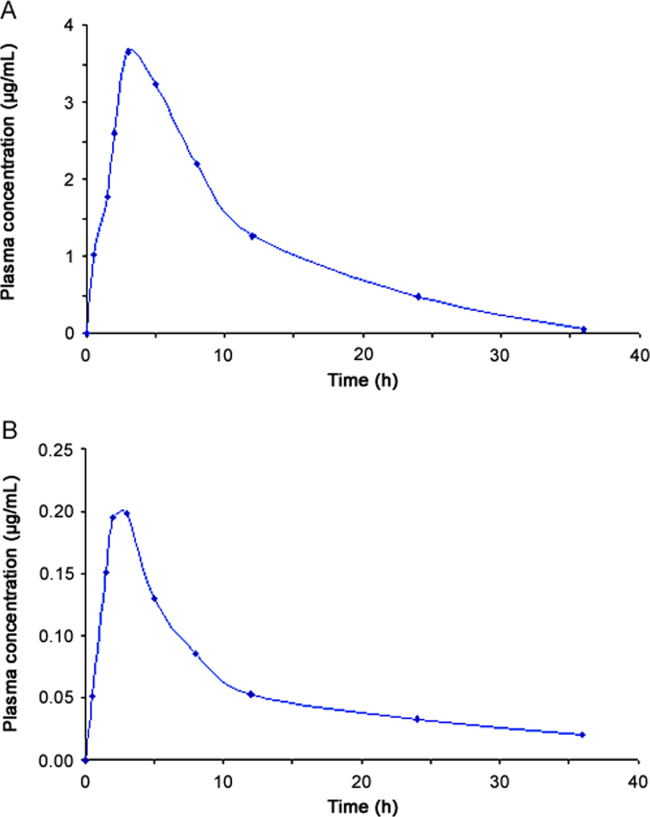
The validated method was sensitive enough to accurately quantify both the analytes in plasma samples of a single dose pharmacokinetic study of TLM and PIO in experimental rats. The area under the curve (plasma concentration) from initial time to 36 h (AUC0–36) was 2.16±0.19 µg h/mL for TLM and 41.58±3.23 µg h/mL for PIO. The maximum plasma concentration (Cmax) values were 0.21±0.02 µg/mL and 3.87±0.15 µg/mL at the time (tmax) 2.67±0.52 h and 3.67±1.03 h, for TLM and PIO, respectively. The area under the curve (plasma concentration) from time zero to infinity (AUC0-α) was 2.56±0.22 µg h/mL and 42.05±3.33 µg h/mL for TLM and PIO, respectively. The mean plasma concentration (±SD) of TLM and PIO vs time profile is shown in Fig. 4.
Fig. 4.
Plasma concentration–time profile of (A) PIO, and (B) TLM in rat plasma following oral administration to healthy rats.
4. Discussion
For a preclinical study, the plasma samples are required to be analyzed using an accurate and validated bioanalytical method to ensure the reliability of the outcome of the study. In the present study, an LC–MS/MS method was developed for quantitation of PIO and TLM in rat plasma which will be useful for analysis of the samples of a preclinical pharmacokinetic study. This method needs only 100 μL of plasma samples for a rat pharmacokinetic study, which can be considered as highly advantageous because of the difficulty of withdrawing high amount of blood sample for several times per day from such small animals. The outcome of the validation experiment supports the specificity, accuracy, precision and stability of the method. With this developed method, PIO and TLM can be quantitated in a single run using a single IS within a very short period of time (2 min). The selection of quicker elution is quite normal for LC–MS analysis, provided that all the eluted analytes get sufficiently ionized and one analyte does not affect the ionization of others. For this analysis, a good linearity was observed for both the analytes. Therefore, we found it advantageous due to the high throughputness of the method. Shorter retention time and co-elution of the analytes did not show any disadvantage during validation of the method. Hence, the method can be concluded as a high throughput method and definitely will have economic benefit over other existing methods. The method thus has distinctive advantage and added ethical value towards animal welfare. The economic benefit is justified as the requirement of analytical solvents, reagents and analysis time as a whole has been minimized. The simplicity of the method in terms of sample processing is another added advantage. The sample preparation procedure involves simple liquid-liquid extraction followed by separation of supernatant, evaporation and reconstitution before injecting onto the LC–MS/MS system. The extraction recovery following this procedure was satisfactory for both the analytes and rosiglitazone (>90%). The inter-day and intra-day precision study confirmed the reproducibility of the method independent of time (CV within ±15%). The experimental data for precision, accuracy, recovery and stability studies were found acceptable as specified in FDA guideline for bioanalytical method validation (CV within ±15%). The preclinical pharmacokinetic experiment result supports the efficiency and enough sensitivity of the method to quantitate TLM and PIO in combination from rat plasma. The calibration range of the method is expected to cover the required concentration range desired for a pharmacokinetic study in rat.
5. Conclusions
A high throughput, highly sensitive and reproducible LC–MS/MS method has been developed and validated for the simultaneous quantitation of PIO and TLM in rat plasma using relatively small sample volumes. The method was established as precise and suitable to determine TLM and PIO in the plasma samples of preclinical pharmacokinetic study in rats. The novelty of the method can be justified by unavailability of any reported bioanalytical method for this combination in any type of biological matrix before this study. Again, the sensitivity for detection of PIO and TLM has been improved compared to the existing quantification methods. The method can quantitate them even if they are at a level of 5 ng/mL. Minimization of total chromatographic run time is another advantage of this method. The run time of this method is shorter than the previously reported methods for their individual quantitation. This method can be fabricated easily to make it applicable for other types of biological matrices for preclinical or clinical use in future.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Acknowledgments
We are grateful to Bioequivalence Study Centre, Jadavpur University, India, for providing the necessary instrumental facilities.
References
- 1.Han J.Y., Kim Y.J., Kim L. PPAR- γ agonist and angiotensin II receptor antagonist ameliorate renal tubulointerstitial fibrosis. Korean Med. Sci. 2010;25:35–41. doi: 10.3346/jkms.2010.25.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mollie A.J., Jun R. Peroxisome proliferator-activated receptor (PPAR) in metabolic syndrome and type 2 diabetes mellitus. Curr. Diabetes Rev. 2007;3:33–39. doi: 10.2174/157339907779802067. [DOI] [PubMed] [Google Scholar]
- 3.Varga T., Czimmerer Z., Nagy L. PPARs are a unique set of fatty acid regulated transcription factors controlling both lipid metabolism and inflammation. Biochim. Biophys. Acta. 1812;2011:1007–1022. doi: 10.1016/j.bbadis.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee T.I., Kao Y.H., Chen Y.C. Cardiac peroxisome-proliferator-activated receptor expression in hypertension co-existing with diabetes. Clin. Sci. 2011;121:305–312. doi: 10.1042/CS20100529. [DOI] [PubMed] [Google Scholar]
- 5.Christiane M., Alcione L., Mariana C. Glucose and fatty acid metabolism in infarcted heart from streptozotocin-induced diabetic rats after 2 weeks of tissue remodeling Irigoyen. Cardiovas. Diabetol. 2015;14:149–159. doi: 10.1186/s12933-015-0308-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amanda N.L., Samuel D.J. Comorbidities of diabetes and hypertension: mechanisms and approach to target organ protection. J. Clin. Hypertens. 2011;13:244–251. doi: 10.1111/j.1751-7176.2011.00434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iciar M.T., Cristina S.C., Amparo S.G. Type 2 diabetes and cardiovascular disease: have all risk factors the same strength?, World. J. Diabetes. 2014;5:444–470. doi: 10.4239/wjd.v5.i4.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Halabi C.M., Beyer A.M., Lange W.J. Interference with PPARg function in smooth muscle causes vascular dysfunction and hypertension. Cell. Metab. 2008;7:215–226. doi: 10.1016/j.cmet.2007.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lime W., Birgit W., Eva-Maria P.W. Natural product agonists of peroxisome proliferator-activated receptor gamma (PPARγ): a review. Biochem. Pharmacol. 2014;92:73–89. doi: 10.1016/j.bcp.2014.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maji D., Samanta S. A review on the role of peroxisome prolifertor-activated receptor-γ agonists and hybrids in type 2 diabetes and cardiomyopathy. Asian J. Pharm. Clin. Res. 2015;8:26–31. [Google Scholar]
- 11.Sengupta P., Das A., Pal T.K. Stability-indicating RP-HPLC method for simultaneous determination of olmesartan medoxomil and pioglitazone in fixed dose combination tablet dosage form. Asian J. Chem. 2010;22:6471–6479. [Google Scholar]
- 12.Kaur N., Kaur S., Singh P. The effect of pioglitazone, a PPAR-γ agonist, on cardiovascular risk factors in type 2 diabetics. Int. J. Biol. Med. Res. 2012;3:1558–1560. [Google Scholar]
- 13.Pang T., Benicky J., Wang J. Telmisartan ameliorates lipopolysaccharide-induced innate immune response through peroxisome proliferator-activated receptor-γ activation in human monocytes. J. Hypertens. 2012;30:87–96. doi: 10.1097/HJH.0b013e32834dde5f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fujimura A., Ushijima K., Ando H. Does the PPAR-γ-activating property of telmisartan provide a benefit in clinical practice? Hyperten. Res. 2013;36:183. doi: 10.1038/hr.2012.189. [DOI] [PubMed] [Google Scholar]
- 15.Attia Y.M., Elalkamy E.F., Hammam O.A. Telmisartan, an AT1 receptor blocker and a PPAR gamma activator, alleviates liver fibrosis induced experimentally by Schistosoma mansoni infection. Parasit. Vectors. 2013;6:199–211. doi: 10.1186/1756-3305-6-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hsueh W., Davidai G., Henry R. Telmisartan effects on insulin resistance in obese or overweight adults without diabetes or hypertension. J. Clin. Hypertens. 2010;12:746–752. doi: 10.1111/j.1751-7176.2010.00335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goyal S.N., Bharti S., Bhatia J. Telmisartan, a dual ARB/partial PPAR-γ agonist, protects myocardium from ischaemic reperfusion injury in experimental diabetes. Diabetes Obes. Metab. 2011;13:533–541. doi: 10.1111/j.1463-1326.2011.01377.x. [DOI] [PubMed] [Google Scholar]
- 18.Sengupta P., Nandi U., Pal T.K. Development of safety profile evaluating pharmacokinetics, pharmacodynamics and toxicity of a combination of pioglitazone and olmesartan medoxomil in Wistar albino rats. Regul. Toxicol. Pharmacol. 2012;62:7–15. doi: 10.1016/j.yrtph.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 19.Shahataa M.G., Mostafa-Hedeab G., Ali E.F. Effects of telmisartan and pioglitazone on high fructose induced metabolic syndrome in rats. Can. J. Physiol. Pharmacol. 2016;94:907–917. doi: 10.1139/cjpp-2016-0090. [DOI] [PubMed] [Google Scholar]
- 20.Sengupta P., Das A., Ibrahim F. Safety profiling of pioglitazone and telmisartan combination by sub-chronic toxicity study in rat. Regul. Toxicol. Pharmacol. 2016;81:155–161. doi: 10.1016/j.yrtph.2016.08.009. [DOI] [PubMed] [Google Scholar]
- 21.Sengupta P., Sarkar A.K., Bhaumik U. Development and validation of a LC-ESI-MS/MS method for simultaneous quantitation of olmesartan and pioglitazone in rat plasma and its pharmacokinetic application. Biomed. Chromatogr. 2010;24:1342–1349. doi: 10.1002/bmc.1447. [DOI] [PubMed] [Google Scholar]
- 22.Karra V.K., Pilli N.R., Inamadugu J.K. Simultaneous determination of pioglitazone and candesartan in human plasma by LC-MS/MS and its application to a human pharmacokinetic study. J. Pharm. Anal. 2012;2:167–173. doi: 10.1016/j.jpha.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sharma R.N., Pancholi S.S., Rajaram S.P. HPLC-DAD method for the pharmacokinetic interaction study of atorvastatin with pioglitazone and cholestyramine in wistar rats. Sci. Pharm. 2014;82:555–570. doi: 10.3797/scipharm.1401-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Samanthula G., Vaddepally L., Saladi S. Rapid LC-ESI-MS-MS method for the simultaneous determination of sitagliptin and pioglitazone in rat plasma and its application to pharmacokinetic study. AJAC. 2012;3:849–858. [Google Scholar]
- 25.Sengupta P., Bhaumik U., Ghosh A. LC-MS/MS Development and validation for simultaneous quantitation of metformin, pioglitazone and glimepiride in human plasma and its application to a bioequivalence study. Chromatographia. 2009;69:1243–1250. [Google Scholar]
- 26.Chen X., Xu B., Yang J. Simultaneous determination of telmisartan and pitavastatin in rat plasma by UPLC-MS/MS: application to pharmacokinetic interaction study. J. Pharm. Biomed. Anal. 2016;131:373–379. doi: 10.1016/j.jpba.2016.09.006. [DOI] [PubMed] [Google Scholar]
- 27.Vasu B.R., Jaswanth K.I., Nageswara R.P. Simultaneous determination of telmisartan and amlodipine in human plasma by LC–MS/MS and its application in a human pharmacokinetic study. J. Pharm. Anal. 2012;2:319–326. doi: 10.1016/j.jpha.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raghunadha R.S., Sarath C., Koteswara R. Development and validation of high-performance liquid chromatography-tandem mass spectrometric method for simultaneous quantification of telmisartin in human plasma. Int. J. Pharma. Sci. Drug Res. 2010;2:188–192. [Google Scholar]
- 29.Food and Drug Administration of the United States, Guidance for industry—bioanalytical method validation. US department of health and human services, center for drug evaluation and research, center for veterinary medicine, 2001. (http://www/fda.gov/ cder/guidance/index.htm).