Abstract
An accurate, sensitive and selective method is developed for determination of ergocalciferol (vitamin D2) in human plasma using LC–MS/MS. After liquid-liquid extraction with n-hexane, ergocalciferol was derivatized by reacting with 4-phenyl-1,2,4-triazoline-3,5-dione (PTAD), a strong dienophile based on Diels-Alder reaction. Ergocalciferol and its deuterated internal standard, ergocalciferol-d6, were analyzed on X Select CSH C18 (100 mm×4.6 mm, 2.5 µm) column using acetonitrile and 0.1% (v/v) formic acid in water containing 0.14% methylamine within 6.0 min under gradient elution mode. Tandem mass spectrometry in positive ionization mode was used to quantify ergocalciferol by multiple reaction monitoring (MRM). Entire data processing was done using Watson LIMS™ software which provided excellent data integrity and high throughput with improved operational efficiency. The major advantage of this method includes higher sensitivity (0.10 ng/mL), superior extraction efficiency (≥83%) and small sample volume (100 µL) for processing. The method was linear in the concentration range of 0.10–100 ng/mL for ergocalciferol. The intra-batch and inter-batch accuracy and precision (% CV) values varied from 97.3% to 109.0% and 1.01% to 5.16%, respectively. The method was successfully applied to support a bioequivalence study of 1.25 mg ergocalciferol capsules in 12 healthy subjects.
Keywords: Ergocalciferol; Diels-Alder reaction; 4-phenyl-1,2,4-triazoline-3,5-dione; LC–MS/MS; Human plasma
1. Introduction
Vitamin D is a fat-soluble vitamin and plays a critical role in calcium and phosphorus metabolism, and bone health. Additionally, vitamin D deficiency is associated with several diseases such as diabetes, cardiovascular disorders, cancer, multiple sclerosis, and immune system functions [1], [2], [3]. The levels of vitamin D in human body are maintained via exposure to the sun, dietary uptake and nutritional supplements. The two common forms of vitamin D are vitamin D2 (ergocalciferol, ERG) and vitamin D3 (cholecalciferol). The synthetic D2 form can be used as a tracer to study vitamin D metabolism. Vitamin D2 is mainly derived from plant sources and is formed by irradiation of ergosterol produced by yeasts, while vitamin D3 is biosynthesized in the skin of vertebrates upon irradiation of 7-dehydrocholesterol by ultraviolet (UV) radiation, and is also absorbed from food [4]. Structurally they are quite similar except that the side chain in vitamin D2 contains a double bond between carbons 22 and 23, and a methyl group on carbon 24, which is not present in vitamin D3. Both these forms have similar metabolic pathways and undergo hydroxylation in the liver by 25-hydroxylase to form 25-hydroxyvitamin D2 (25OH D2) and 25-hydroxyvitamin D3 (25OH D3), which are the major circulating forms in blood. They are further hydroxylated in the kidney or placenta to form their biologically active forms 1, 25-dihydroxyvitamin D2/3 (1,25OH D2/3) [5]. The biotransformation of vitamin D is shown in Fig. 1.
Fig. 1.
Biotransformation of two common forms of vitamin D, vitamin D2 (ergocalciferol) and vitamin D3 (cholecalciferol).
Several methods are available in literature for the determination of 25OH D2/3 [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19] and 1,25OH D2/3 metabolites [9], [12], [13], [17], [20], [21], which are potential markers for vitamin D deficiency and function. However, few methods are reported for the determination of ERG (vitamin D2) in biological samples [18], [19]. Shah et al. [18] developed a method for the simultaneous analysis of eight analogues of vitamin D using 1.0 mL serum samples by liquid chromatography-tandem mass spectrometry (LC–MS/MS). The calibration established for ERG was in the range of 0.20–40 ng/mL. Similarly, an ultra-high performance supercritical fluid chromatography-mass spectrometry method was developed to determine ERG with a sensitivity of 10 ng/mL using 200 µL plasma samples [19].
LC–MS/MS has become the preferred technique for rapid quantification of vitamin D and its metabolites [22]. In the present work a highly rugged, selective and rapid LC–MS/MS method has been developed and fully validated as per the USFDA guidelines for determination of ERG in human plasma using deuterated internal standard (IS). The method offers small turnaround time for analysis, high sensitivity (0.10 ng/mL) and utilizes only 100 µL human plasma for sample processing. ERG was derivatized with 4-phenyl-1,2,4-triazoline-3,5-dione (PTAD) to improve the ionization efficiency and thereby the sensitivity of the method. The method is free from endogenous matrix interference and was successfully applied to a bioequivalence study with 1.25 mg ERG capsules in healthy subjects. The reproducibility in the measurement of study data has been demonstrated by reanalysis of incurred samples.
2. Experimental
2.1. Chemicals and materials
The details regarding reference standards and other materials used in the study are given in Supplementary Material.
2.2. Instrumentation and analysis conditions
A Shimadzu UFLC XR Prominence (Kyoto, Japan) system interfaced with MDS SCIEX API 5500 (Toronto, Canada) triple quadrupole mass spectrometer and equipped with electro spray ionization was used in the present work. Chromatographic analysis of ERG was carried out on Waters X Select CSH C18 (100 mm×4.6 mm, 2.5 µm) column maintained at 40 °C using acetonitrile (solvent A) and 0.1% formic acid in water containing 0.14% methylamine (solvent B) as the mobile phase in a gradient mode at a flow rate of 0.8 mL/min. The optimized chromatographic conditions and mass parameters are summarized in Supplementary Material.
2.3. Calibration (CSs) and quality control samples (QCs)
CSs were made at 0.10, 0.20, 0.50, 1.00, 2.00, 5.00, 10.0, 20.0, 50.0 and 100.0 ng/mL concentrations, while QCs were prepared at 80.0 ng/mL (HQC, high quality control), 40.0/3.00 ng/mL (MQC-1/2, medium quality control), 0.300 ng/mL (LQC, low quality control) and 0.100 ng/mL (LLOQ QC, lower limit of quantification quality control). All details about solution preparation are given in Supplementary Material.
2.4. Plasma sample preparation after derivatization
Prior to analysis, all frozen subject samples, CSs and QCs were thawed and allowed to equilibrate at room temperature. To an aliquot of 100 µL of spiked plasma/subject sample, 50 µL of 20.0 ng/mL solution of ERG-d6, 100 µL of 2.0 % (v/v) ammonia solution in water was added and vortexed for 20 s. Extraction of ERG and ERG-d6 was done with 2.5 mL of n-hexane on a rotary mixer for 10 min at 18g, followed by centrifugation at 2147g for 5 min at 10 °C. The organic layer was separated and evaporated to dryness in a thermostatically controlled water-bath maintained at 40 °C under a gentle stream of nitrogen. Further, 20 µL, 100 µg/mL of PTAD solution in acetonitrile was added to the dried sample, vortexed for 20 s and kept for 20 min at room temperature for complete derivatization. Thereafter, the sample was reconstituted with 80 µL of 0.1% (v/v) formic acid in water and acetonitrile (20:80, v/v), briefly vortexed and 10 µL was used for injection in the chromatographic system.
2.5. Method validation procedures
The method was validated as per the USFDA guidelines [23] and the procedures followed were similar to our previous work [24]. The detailed procedures are described in Supplementary material.
2.6. Bioequivalence study and incurred sample reanalysis
The bioequivalence study was carried out using a single dose of 1.25 mg ergocalciferol capsule (Generic Company) with a reference capsule, DRISDOL (50,000 IU, equivalent to 1.25 mg of ergocalciferol) from Validus Pharmaceuticals LLC, Parsippany, New Jersey, USA in 12 healthy Indian subjects under fasting. The study was executed following International Conference on Harmonization, E6 Good Clinical Practice guidelines [25]. To prove the method reproducibility, incurred sample reanalysis (ISR) was performed as reported previously [26]. The details of both the experiments are given in Supplementary material.
3. Results and discussion
3.1. Method development
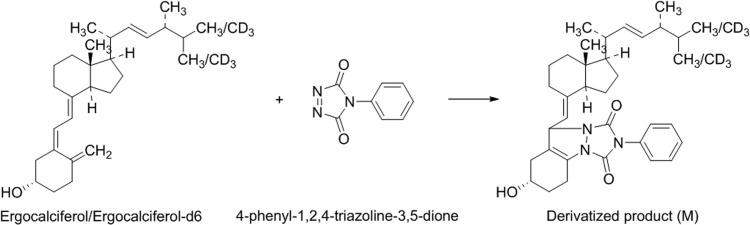
In general, the analysis of various forms of vitamin D and its metabolites is quite challenging due to their low ionization efficiency in mass spectrometry. Therefore, it is difficult to attain higher sensitivity as their expected concentration levels found in biological matrices is extremely low. Further, their structures indicate limited possibility for ionization during ESI. One approach is to use alternative ionization sources like atmospheric pressure chemical ionization (APCI) [7], [10], [14] or atmospheric pressure photo ionization (APPI) [6] to increase the sensitivity or through pre-column or post-column derivatization of the analytes with suitable dienophiles like PTAD, 4-[2-(6,7-dimethoxy-4-methyl-3-oxo-3,4-dihydroquinoxalyl)ethyl]-1,2,4-triazoline-3,5-dione (DMEQTAD), 4-(4-nitro phenyl)-1,2,4-triazoline-3,5-dione (NPTAD) and 4-ferrocenyl methyl-1,2,4-triazoline-3,5-dione (FMTAD) [11], which can help to improve the sensitivity by 10–100 folds [12]. To develop a method with a sensitivity of 0.10 ng/mL in human plasma, ERG was first derivatized prior to LC–MS/MS analysis. Higashi et al. [6] have reported use of PTAD, an azodicarbonyl compound for derivatization of salivary 25OH D3. PTAD is one of the strongest dienophiles, which reacts rapidly with dienes in Diels-Alder reactions. Thus, in the present work PTAD derivative of ERG was prepared as shown in Fig. 2, by suitably optimizing the derivatization conditions.
Fig. 2.
Derivatized product (M) of ergocalciferol/ergocalciferol-d6 with 4-phenyl-1,2,4-triazoline-3,5-dione.
After the extraction of ERG and ERG-d6 in n-hexane under alkaline conditions, different concentrations of PTAD (100–500 µg/mL) in acetonitrile were investigated to achieve highest signal-to-noise (S/N) ratio in the mass spectra. Similarly, the incubation time (10–60 min) for completion of the derivatization reaction was also examined. The optimum conditions for derivatization established were 20 µL, 100 µg/mL of PTAD solution and an incubation time of 20 min at room temperature, which is much less compared to previous reports [9], [11]. The derivatized product was easily ionized in the positive ESI-MS due to higher proton affinitive tendency of triazoline moiety and thus increased the ionization efficiency. Further, augmentation in the efficiency was achieved by fortifying the mobile phase with methylamine, which promotes adduct ion [M + CH3NH3]+ formation. Apart from the enhancement in ionization efficiency, the derivatization of ERG and its adduct formation shifted the m/z of the precursor ion by about 200 amu, in the region of m/z 600 amu. This change helped in minimizing interference from the low m/z background fragment ions. The Q3 mass spectra of ERG and ERG-d6 exhibited one major fragment at m/z 298.2 amu, which was useful to sensitive MRM analysis (Fig. S1). A dwell time of 200 ms afforded at least 25 data points for ERG and ERG-d6 and no cross talk was observed between their MRMs due to identical products ions.
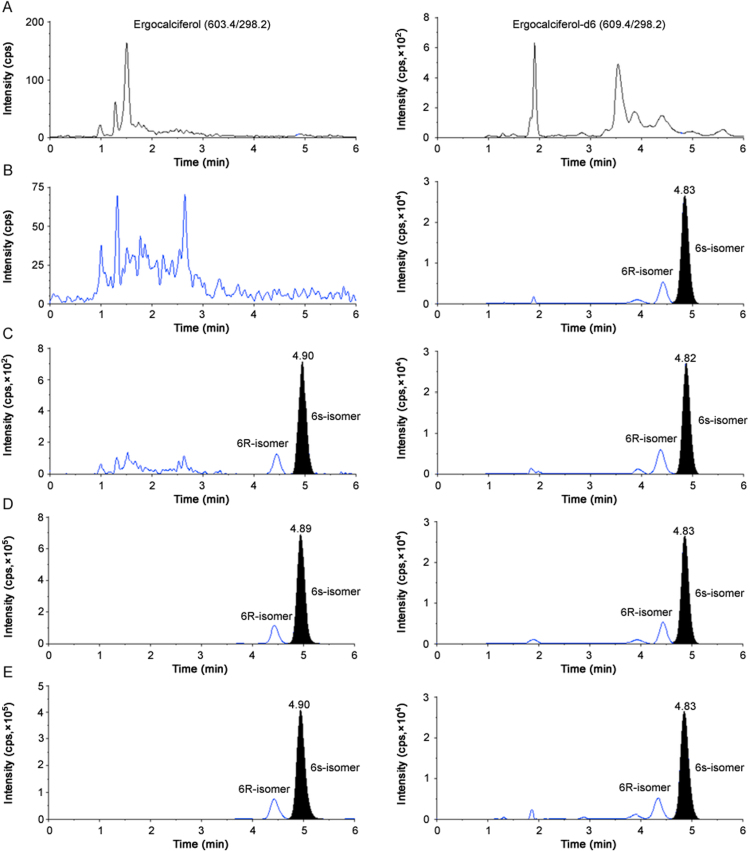
To develop an efficient chromatography free from endogenous matrix components, different compositions (aqueous and organic) of the mobile phase were studied on four reversed phase columns, BDS Hypersil C18 (100 mm×4.6 mm, 5 µm), Kinetex C18 (100 mm×4.6 mm, 5 µm), X Select CSH C18 (100 mm×4.6 mm, 2.5 µm) and Gemini C18 (100 mm×4.6 mm, 5 µm). It has been reported earlier that two epimers, 6 S and 6 R, are formed when vitamin D analogues are derivatized with PTAD as it reacts with s-cis-diene moiety from the α- and β-sides. 6S-isomer is observed in greater proportion compared to 6R-isomer and the ratio of 6S/6R is ~5.0:1.0 [9]. Thus, two peaks are generally observed in the MRM ion chromatograms if the two epimers are well resolved under the optimized chromatographic conditions, and the major peak is used for quantitation purpose. Mobile phase comprising acetonitrile/methanol and 0.1%–0.5% formic acid/0.1%–0.5% methylamine solution/5–10 mM ammonium formate was tried under gradient elution conditions. On BDS Hypersil C18 column, ERG was adequately retained but the base line was very unstable with a noticeable peak tailing, while Kinetex C18 and Gemini C18 columns offered better peak shape but the response was not adequate to achieve the desired sensitivity. Moreover, it was not possible to resolve the epimers completely on all the three columns. Nonetheless, the best chromatographic condition with high signal intensity, baseline resolution of the epimers, minimum matrix interference, good peak shape and acceptable retention was achieved on X Select CSH C18 column using acetonitrile and 0.1% formic acid in water containing 0.14% methylamine, as the mobile phase under gradient conditions. The retention time of ERG was 4.9 min in a total analysis time of was 6.0 min. The reinjection reproducibility assessed as % CV was ≤0.65% for 100 injections on the same column. The sensitivity achieved for ERG was 0.10 ng/mL at S/N ≥42, which was higher than that of all other methods reported in biological samples. Use of labeled internal standard, ERG-d6, allowed counteracting any target ion suppression caused by the sample matrix and adequately compensated for any variability during extraction. The representative chromatograms shown in Fig. 3 of double blank plasma (without ERG and ERG-d6), blank plasma spiked with ERG-d6, ERG at LLOQ and ULOQ, and subject sample at Cmax after administration of 1.25 mg of ERG demonstrates the selectivity of the method. Moreover, none of the commonly used medications by human volunteers interfered at the retention time of ERG and ERG-d6.
Fig. 3.
Representative ion-chromatograms of (A) double blank plasma (without ergocalciferol and ergocalciferol-d6) (B) blank plasma with ergocalciferol-d6, (C) ergocalciferol at LLOQ and ergocalciferol-d6, (D) ergocalciferol at ULOQ and ergocalciferol-d6, (E) subject sample at Cmax after administration of 1.25 mg dose of ergocalciferol.
3.2. Assay performance and validation results
No significant carryover (column and autosampler) was observed from ULOQ samples in subsequent blank plasma samples (≤0.87% of LLOQ sample). Selectivity of the assay was assessed in ten different batches of blank plasma to establish that quantitation of ERG is unaffected by the presence of biological matrix and other interfering components. There was no apparent interference at the retention time of ERG in any of the batches.
The method was shown to be linear over the concentration range of 0.10–100 ng/mL. All calibration lines prepared in ERG depleted blank plasma samples were linear with correlation coefficient (r2) ≥0.9988. The mean regression line obtained from the combination of five calibration curves was y=(0.2446±0.0117) x – (0.0004±0.0001). The regression was performed using Watson LIMS™ software which provided excellent data integrity and high throughput with improved operational efficiency. The accuracy and precision (% CV) observed for the CSs ranged from 98.2% to 102.4% and 2.82% to 6.16%, respectively. The limit of detection (LOD) and limit of quantitation (LOQ) of the method for ERG was 0.04 ng/mL and 0.10 ng/mL at S/N ratio of 10 and 42, respectively.
The intra-batch and inter-batch precision and accuracy results for ERG across five QC levels are shown in Table S1. The precision (% CV) determined at each concentration level was ≤5.16% and the accuracy was within 97.3%–109.0% for intra-batch and inter-batch experiments. The extraction recovery was examined by utilizing stable isotope derivative, ERG-d6. The method showed highly consistent and quantitative recovery ranging from 83.0% to 85.4% across QC levels (Table 1). Co-extracted and co-eluting matrix components can significantly influence the method accuracy and reproducibility. The matrix effect expressed as IS-normalized matrix factors was in the range of 1.04–1.05 at all QC levels as shown in Table 2. Relative matrix effect in different plasma sources including haemolysed, heparinized and lipemic was studied at LQC and HQC levels in duplicate. The mean precision (% CV) values observed were 3.95 and 2.63, respectively at both these concentrations (Table S2).
Table 1.
Extraction recovery of ergocalciferol and ergocalciferol-d6.
| QC level (ng/mL) | Ergocalciferol |
Ergocalciferol-d6 |
||||
|---|---|---|---|---|---|---|
| Mean area response |
Extraction recovery (%) | Mean area response |
Extraction recovery (%) | |||
| Post-extraction spiking | Pre-extraction spiking | Post-extraction spiking | Pre-extraction spiking | |||
| HQC (80.0) | 7006545 | 5818432 | 83.0 | 493724 | 427070 | 86.5 |
| MQC-1 (40.0) | 3578144 | 3001422 | 83.9 | 483473 | 419236 | 86.7 |
| MQC-2 (3.00) | 287385 | 240530 | 83.7 | 493898 | 431564 | 85.4 |
| LQC (0.300) | 29514 | 25201 | 85.4 | 489605 | 427845 | 87.4 |
HQC: high quality control; MQC: medium quality control; LQC: low quality control.
Extraction recovery: (pre-extraction spiking/post-extraction spiking) × 100.
Table 2.
Matrix factors for ergocalciferol and ergocalciferol-d6.
| QC level (ng/mL) | Ergocalciferol |
Ergocalciferol-d6 |
IS-normalized matrix factor | ||||
|---|---|---|---|---|---|---|---|
| Mean area response |
Matrix factor | Mean area response |
Matrix factor | ||||
| Post-extraction spiking | Spiking in mobile phase | Post-extraction spiking | Spiking in mobile phase | ||||
| HQC (80.0) | 7006545 | 6502566 | 1.08 | 493724 | 472568 | 1.04 | 1.04 |
| MQC-1 (40.0) | 3578144 | 3295690 | 1.09 | 483473 | 465896 | 1.04 | 1.05 |
| MQC-2 (3.00) | 287385 | 269564 | 1.07 | 493898 | 478521 | 1.03 | 1.04 |
| LQC (0.300) | 29514 | 27546 | 1.07 | 489605 | 479624 | 1.02 | 1.05 |
HQC: high quality control; MQC: medium quality control; LQC: low quality control.
Matrix factor: post-extraction spiking/spiking in mobile phase.
Samples kept for short-term and long-term stock solution stability remained unchanged up to 72 h and 14 days, respectively, for ERG and ERG-d6. Bench top stability of ERG in plasma was established up to 10 h and for minimum of five freeze and thaw cycles at –20 °C and –70 °C. Processed sample stability and auto sampler stability (wet extract) were determined up to 48 h and dry extract up to 24 h without significant loss of ERG. Spiked plasma samples stored at –20 °C and –70 °C for long-term stability experiment were found stable for a minimum period of 160 days. The detailed results for stability study are presented in Table S3.
The precision (% CV) and accuracy for method ruggedness with different columns and analysts ranged from 2.60% to 6.58% and 98.6% to 101.7%, respectively, across five QC levels. The precision (% CV) for dilution reliability of ¼th and 1/10th dilutions was between 2.07% and 5.92%, while the accuracy results were within 99.3% −108.8%, respectively, which is within the acceptance limit of 15% for precision (% CV) and 85%–115% for accuracy.
3.3. Application to a bioequivalence study and incurred sample reanalysis (ISR)
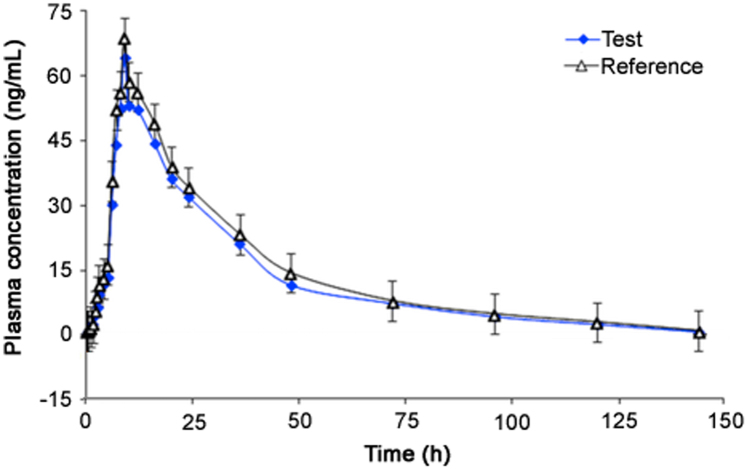
Currently there are no reports on the pharmacokinetics of ERG in human plasma, especially in the Indian subjects. The developed method was successfully used to quantify ERG concentration in human plasma samples after oral administration of a single 1.25 mg dose of test and reference formulations. Fig. 4 shows the mean plasma concentration vs. time profile of ERG in healthy subjects under fasting condition. The method was adequately sensitive in measuring ERG plasma concentration up to 144 h. The mean pharmacokinetic parameters (Cmax, AUC0–144 h, AUC0-inf, Tmax, t1/2 and Kel) obtained for the test and reference formulations are presented in Table 3. All the parameters of the test formulation correlated well with the reference formulation. The 90% confidence interval of individual ratio geometric mean for test/reference was within 85.69% −101.96% for Cmax, AUC0–144 h and AUC0-inf. Moreover, the intra-subject variation (% CV) found was in the range of 11.02%–13.46%. This confirms that the test and reference formulations were pharmacokinetically equivalent in terms of rate and extent of drug absorption. ISR study was performed by reanalyzing 56 incurred samples and the results showed acceptable method reproducibity with % change within ±12% between the original and repeat results for all the samples.
Fig. 4.
Pharmacokinetic profiles of ergocalciferol after oral administration of 1.25 mg dose of test and reference formulation in 12 healthy subjects.
Table 3.
Mean pharmacokinetic parameters (±SD) following oral administration of 1.25 mg test and reference capsule formulations of ergocalciferol in 12 healthy Indian subjects.
| Parameter | Test | Reference | Ratio (Test/Reference, %) |
|---|---|---|---|
| Cmax (ng/mL) | 64.03 ± 16.42 | 68.83 ± 17.24 | 93.51 |
| AUC0 – 144 (h·ng/mL) | 2573.9 ± 439.2 | 2690.1 ± 466.2 | 96.36 |
| AUC0-inf (h·ng/mL) | 2804.5 ± 476.3 | 2921.2 ± 502.7 | 96.70 |
| Tmax (h) | 9.1 ± 3.5 | 8.9 ± 3.9 | – |
| t1/2 (h) | 32.75 ± 3.71 | 31.69 ± 4.31 | – |
| Kel ( h–1) | 0.021 ± 0.003 | 0.022 ± 0.003 | – |
SD: standard deviation.
4. Conclusions
An efficient, accurate and precise LC–MS/MS method is established for the analysis of ERG in human plasma. The major features of the proposed method include higher sensitivity compared to all existing procedures for the analysis of ERG in biological fluids, low imprecision, low sample volume needed for processing and a wide analytical measurement range. Data processing by Watson LIMS software provided superior data integrity during method validation and sample analysis. It offers accelerated laboratory turnaround time, reduces cost associated with sample management, and improves operational efficiency. The method yielded complete separation of 6S- and 6R-epimers of the derivatized product and had no endogenous peaks that may interfere with the quantitation process. Moreover, this is the first report on the pharmacokinetics of ERG in healthy Indian subjects.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Acknowledgments
The authors are thankful to Directors, Mr. Apurva Shah, Mr. Binoy Gardi and Chief Operating Officer Mr. Venu Madhav of Veeda Clinical Research (India) for providing infrastructure facilities to carry out this work.
Footnotes
Peer review under responsibility of Xi'an Jiaotong University.
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.jpha.2017.05.006.
Appendix A. Supplementary material
Supplementary material
.
References
- 1.Thacher T.D., Clarke B.L. Vitamin D insufficiency. Mayo Clin. Proc. 2011;86:50–60. doi: 10.4065/mcp.2010.0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hewison M. Vitamin D and immune function: an overview. Proc. Nutr. Soc. 2012;71:50–61. doi: 10.1017/S0029665111001650. [DOI] [PubMed] [Google Scholar]
- 3.Lee J.H., O’Keefe J.H., Bell D. Vitamin D deficiency an important, common, and easily treatable cardiovascular risk factor? J. Am. Coll. Cardiol. 2008;52:1949–1956. doi: 10.1016/j.jacc.2008.08.050. [DOI] [PubMed] [Google Scholar]
- 4.Holick M.F. Vitamin D status: measurement, interpretation, and clinical application. Ann. Epidemiol. 2009;19:73–78. doi: 10.1016/j.annepidem.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Su Z., Narla S.N., Zhu Y. 25-Hydroxyvitamin D: analysis and clinical application. Clin. Chim. Acta. 2014;433:200–205. doi: 10.1016/j.cca.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 6.Higashi T., Shibayama Y., Fuji M. Liquid chromatography–tandem mass spectrometric method for the determination of salivary 25-hydroxyvitamin D3: a noninvasive tool for the assessment of vitamin D status. Anal. Bioanal. Chem. 2008;391:229–238. doi: 10.1007/s00216-007-1780-3. [DOI] [PubMed] [Google Scholar]
- 7.Bogusz M.J., Enazi E.A., Tahtamoni M. Determination of serum vitamins 25-OH-D2 and 25-OH-D3 with liquid chromatography-tandem mass spectrometry using atmospheric pressure chemical ionization or electrospray source and core-shell or sub-2 µm particle columns: a comparative study. Clin. Biochem. 2011;44:1329–1337. doi: 10.1016/j.clinbiochem.2011.08.1134. [DOI] [PubMed] [Google Scholar]
- 8.Bedner M., Phinney K.W. Development and comparison of three liquid chromatography-atmospheric chemical ionization/mass spectrometry methods for determining vitamin D metabolites in human serum. J. Chromatogr. A. 2012;1240:132–139. doi: 10.1016/j.chroma.2012.03.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garg U., Munar A., Frazee C., III A simple, rapid atmospheric pressure chemical ionization liquid chromatography tandem mass spectrometry method for the determination of 25-hydroxyvitamin D2 and D3. J. Clin. Lab. Anal. 2012;26:349–357. doi: 10.1002/jcla.21530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thibeault D., Caron N., Djiana R. Development and optimization of simplified LC-MS/MS quantification of 25-hydroxyvitamin D using protein precipitation combined with on-line solid phase extraction (SPE) J. Chromatogr. B. 2012;883–884:120–127. doi: 10.1016/j.jchromb.2011.12.021. [DOI] [PubMed] [Google Scholar]
- 11.Zhang S., Jian W., Sullivan S. Development and validation of an LC-MS/MS based method for quantification of 25 hydroxyvitamin D2 and 25 hydroxyvitamin D3 in human serum and plasma. J. Chromatogr. B. 2014;961:62–70. doi: 10.1016/j.jchromb.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 12.Mineva E.M., Schleicher R.L., Chaudhary-Webb M. A candidate reference measurement procedure for quantifying serum concentrations of 25-hydroxyvitamin D3 and 25-hydroxyvitamin D2 using isotope-dilution liquid chromatography-tandem mass spectrometry. Anal. Bioanal. Chem. 2015;407:5615–5624. doi: 10.1007/s00216-015-8733-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee D., Garrett T.J., Goldberger B.A. Quantitation of 25-hydroxyvitamin D2 and D3 in serum and plasma by LC-MS/MS. Bioanalysis. 2015;7:167–178. doi: 10.4155/bio.14.229. [DOI] [PubMed] [Google Scholar]
- 14.Adamec J., Jannasch A., Huang J. Development and optimization of an LC-MS/MS-based method for simultaneous quantification of vitamin D2, vitamin D3, 25-hydroxyvitamin D2 and 25-hydroxyvitamin D3. J. Sep. Sci. 2011;34:11–20. doi: 10.1002/jssc.201000410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lipkie T.E., Janasch A., Cooper B.R. Quantification of vitamin D and 25-hydroxyvitamin D in soft tissues by liquid chromatography-tandem mass spectrometry. J. Chromatogr. B. 2013;932:6–11. doi: 10.1016/j.jchromb.2013.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ding S., Schoenmakers I., Jones K. Quantitative determination of vitamin D metabolites in plasma using UHPLC-MS/MS. Anal. Bioanal. Chem. 2010;398:779–789. doi: 10.1007/s00216-010-3993-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones G., Kaufmann M. Vitamin D metabolite profiling using liquid chromatography-tandem mass spectrometry (LC-MS/MS) J. Steroid Biochem. Mol. Biol. 2016;164:110–114. doi: 10.1016/j.jsbmb.2015.09.026. [DOI] [PubMed] [Google Scholar]
- 18.Shah I., Petroczi A., Naughton D.P. Method for simultaneous analysis of eight analogues of vitamin D using liquid chromatography tandem mass spectrometry. Chem. Cent. J. 2012;6:1–8. doi: 10.1186/1752-153X-6-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jumaah F., Larrsson S., Essen S. A rapid method for the separation of vitamin D and its metabolites by ultra-high performance supercritical fluid chromatography-mass spectrometry. J. Chromatogr. A. 2016;1440:191–200. doi: 10.1016/j.chroma.2016.02.043. [DOI] [PubMed] [Google Scholar]
- 20.Hedman C.J., Wiebe D.A., Dey S. Development of a sensitive LC/MS/MS method for vitamin D metabolites: 1,25-dihydroxyvitamin D2&3 measurement using a novel derivatizing agent. J. Chromatogr. B. 2014;953–954:62–67. doi: 10.1016/j.jchromb.2014.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fang H., Yu S., Cheng Q. Determination of 1,25-dihydroxyvitamin D2 and 1,25-dihydroxyvitamin D3 in human serum using liquid chromatography with tandem mass spectrometry. J. Chromatogr. B. 2016;1027:19–26. doi: 10.1016/j.jchromb.2016.04.034. [DOI] [PubMed] [Google Scholar]
- 22.van den Ouweland J.M.W., Vogeser M., Bächer S. Vitamin D and metabolites measurement by tandem mass spectrometry. Rev. Endocr. Metab. Disord. 2013;14:159–184. doi: 10.1007/s11154-013-9241-0. [DOI] [PubMed] [Google Scholar]
- 23.Guidance for Industry, Bioanalytical Method Validation, US Department of Health and Human Services, Food and Drug Administration Centre for Drug Evaluation and Research (CDER), Centre for Veterinary Medicine (CVM), May 2001.
- 24.Shah P.A., Shah J.V., Sanyal M. LC–tandem mass spectrometry method for the simultaneous determination of metformin and sitagliptin in human plasma after ion-pair solid phase extraction. J. Pharm. Biomed. Anal. 2016;131:64–70. doi: 10.1016/j.jpba.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 25.Guidance for Industry: ICH E6 Good Clinical Practice, U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER), Centre for Biologics Evaluation and Research (CBER), April 1996.
- 26.Yadav M., Shrivastav P.S. Incurred sample reanalysis: a decisive tool in bioanalytical research. Bioanalysis. 2011;3:1007–1024. doi: 10.4155/bio.11.76. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material