Abstract
Rationale
Adolescence is characterized by endocannabinoid (ECB)-dependent refinement of neural circuits underlying emotion, learning, and motivation. As a result, adolescent cannabinoid receptor stimulation (ACRS) with phytocannabinoids or synthetic agonists like “Spice” cause robust and persistent changes in both behavior and circuit architecture in rodents, including in reward-related regions like medial prefrontal cortex and nucleus accumbens (NAc).
Objectives and Methods
Here, we examine persistent effects of ACRS with the cannabinoid receptor 1/2 specific agonist WIN55-212,2 (1.2mg/kg/day, postnatal day (PD) 30-43), on natural reward seeking behaviors and ECB system function in adult male Long Evans rats (PD 60+).
Results
WIN ACRS increased palatable food intake, and altered attribution of incentive salience to food cues in a sign/goal tracking paradigm. ACRS also blunted hunger-induced sucrose intake, and resulted in increased anandamide and oleoylethanolamide levels in NAc after acute food restriction not seen in controls. ACRS did not affect novel food neophobia or locomotor response to a novel environment, but did increase preference for exploring a novel environment.
Conclusions
These results demonstrate that ACRS causes long-term increases in natural reward-seeking behaviors and ECB system function that persist into adulthood, potentially increasing liability to excessive natural reward seeking later in life.
Introduction
Adolescence is a dynamic period of neural circuit development, when subcortical emotion and motivation circuits mature, in part via activity-dependent endocannabinoid (ECB) signaling (Bossong and Niesink 2010; Brenhouse and Andersen 2011; Dow-Edwards and Silva 2017; Renard et al. 2014). Unfortunately, adolescence is also when many people first experiment with cannabis and synthetic cannabinoid agonist drugs like “Spice” or “K2” (Chen and Kandel 1995; Cuttler and Spradlin 2017; Mokrysz et al. 2016; Taylor et al. 2017). In humans, early use of these drugs is associated with cognitive and emotional deficits lasting into adulthood (Meier et al. 2012; Patton et al. 2002; Rubino et al. 2012; Scott et al. 2017), though causality is difficult to prove in these associational studies. In rodent models, exogenous adolescent cannabinoid receptor stimulation (ACRS) causes marked changes in neural circuit connectivity, gene expression, and other anatomical and biochemical features within cognition-, emotion-, and motivation-related brain circuits (Caballero and Tseng 2016; Hurd et al. 2014; Jager and Ramsey 2008; Lee and Gorzalka 2012; Renard et al. 2016b).
ACRS increases susceptibility to the rewarding effects of other drugs of abuse in adulthood (Doremus-Fitzwater and Spear 2016; Spear 2016), mediated by changes in brain reward systems including mesocorticolimbic dopamine pathways, endogenous opioids in nucleus accumbens (NAc), and the ECB system. ACRS with intermittent doses of Δ9-THC increase adulthood heroin (male rats) and cocaine (female rats) self-administration, stress-induced relapse of heroin seeking (Stopponi et al. 2014), as well as morphine conditioned place preference (Ellgren et al. 2007; Higuera-Matas et al. 2008; Tomasiewicz et al. 2012). In part, this may involve ACRS-induced persistent potentiation of mesocorticolimbic dopamine circuits, including increased basal (Behan et al. 2012; Gomes et al. 2015) and drug-induced activity in ventral tegmental area (VTA) dopamine neurons (Gomes et al. 2015; Pistis et al. 2004; Wegener and Koch 2009), and their forebrain projections (Renard et al. 2016a). Numerous changes in ECB signaling molecules and receptors have also been reported after ACRS (Caballero and Tseng 2012; Ellgren et al. 2008; Renard et al. 2016c), with likely consequences for conditioned and unconditioned drug and natural reward seeking that is mediated in part by corticolimbic cannabinoids (Achterberg et al. 2016; Laviolette and Grace 2006; Maldonado et al. 2006; Vlachou and Panagis 2014). Such ACRS-induced changes could put individuals at risk of developing psychiatric disorders including schizophrenia, depression, or addiction (Lubman et al. 2015; Renard et al. 2014; Renard et al. 2016b; Rubino and Parolaro 2015a; Spear 2016), and would also be expected to facilitate responsiveness to natural rewards like palatable foods or novelty.
Instead, several reports show decreases in seeking of natural rewards like palatable foods and social interaction after escalating dose adolescent THC exposure—an effect interpreted as depression-related anhedonia (Rubino et al. 2012). For example, social interaction is decreased after ACRS with THC, as is preference for a weak (1-2%) sucrose solution over water, intake of a salty, fatty snack in 4 repeated 20min intake tests, and stress-induced suppression of chow intake in food restricted rats (Bambico et al. 2010; Realini et al. 2011; Rubino et al. 2008). However, it is not clear to what extent these effects are mediated by alterations in learning, hedonic, appetitive, feeding-specific, or energy homeostasis processes, or instead by interactions between reward seeking and anxiety, or other emotional dysregulation caused by ACRS. Since dopamine and opioid circuits differentially mediate reward seeking and hedonics in mesolimbic regions like NAc (Baldo et al. 2013; Smith et al. 2010), and since ACRS affects both of these systems (Behan et al. 2012; Tomasiewicz et al. 2012), this begs the question of how ACRS affects specific behavioral assays of natural reward pursuit and consumption, and interactions between these processes and anxiety.
Here we examined how ACRS with the CB1/2 agonist WIN55, 212-2 (WIN) affects specific assays of reward cue learning, palatable food intake, hunger and satiety effects on feeding, responses to novelty, and anxiety, tested in adulthood after WIN washout. We also characterize ECB levels in medial prefrontal cortex (mPFC), NAc, and cerebellum following acute food restriction. Results suggest that ACRS with WIN causes major changes in natural reward seeking behavior that could indicate an addiction-prone phenotype later in life.
Methods
Subjects
Male Long Evans rats (n=50) were obtained from Harlan/Envigo (Indianapolis, IN, USA) post-weaning, arriving in our vivarium at postnatal day (PD) 21-22. Since sex differences in the effects of ACRS, and in the reward-related behaviors tested here are well-known (Becker 2009; King et al. 2016; Rubino et al. 2008; Silva et al. 2016; Wiley and Burston 2014), in the initial experiments reported here we restricted analyses to one sex only. They were subsequently housed in groups of 4 in wire-top polycarbonate tub-style cages (48×20×27cm) with bedding, paper nesting material, and ad libitum food and water in a climate controlled vivarium on a 12hr reverse light-dark cycle. At PD58, rats were separated into pairs ahead of behavioral testing. All procedures were approved by the University of California Irvine Institutional Animal Care and Use Committee, and are in accordance with the NIH Guide for the Care and Use of Laboratory Animals.
ACRS protocol
Starting at PD 30, rats were treated with 14 daily i.p. injections of WIN (0 or 1.2mg/kg), a moderate-dose chronic adolescent treatment protocol known to impact neural circuit development and behavior, followed by a homecage washout period of 14+d (Abboussi et al. 2014; Bambico et al. 2010; Schneider 2008) (Fig. 1A). Rats receiving WIN or vehicle were housed with cagemates receiving the same treatment. During ACRS treatment and subsequent washout rats were weighed daily. After the 14th injection (PD 43), >50g of standard rat chow was provided and intake (g consumed in 2.5hrs) was measured for each cage containing 4 rats receiving WIN or vehicle.
Figure 1. Adolescent Cannabinoid Receptor Stimulation Procedure.
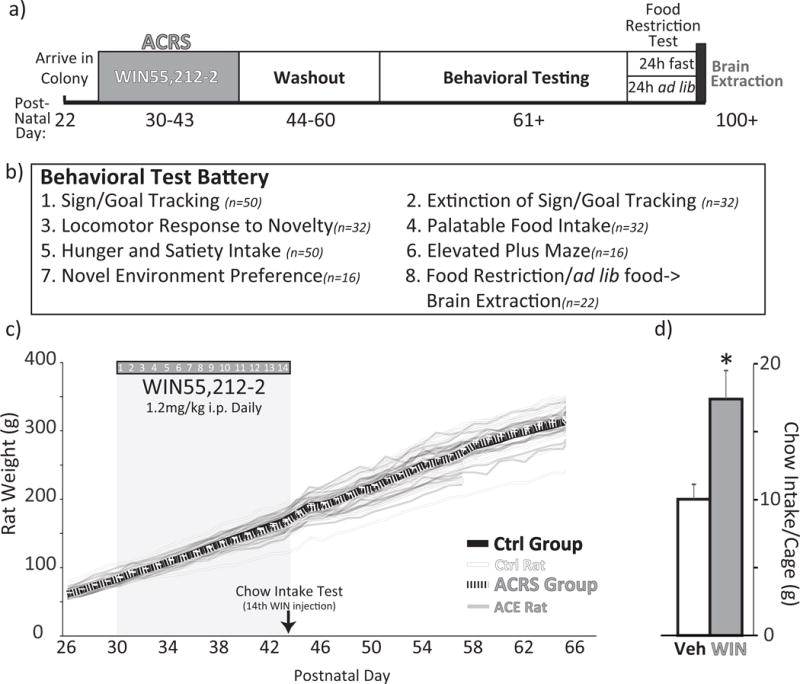
a) Timeline of experimental procedures is shown, with age (postnatal day) shown at bottom. b) List of behavioral tests, number of animals tested in each, and the order in which they were conducted. c) Body weights during WIN ACRS period (shaded box), and for 3 weeks thereafter. Group m+SEM displayed with solid black (ACRS) and grey (control) lines, and individual rats shown with semi-transparent lines of the same colors. d) Chow intake in the 2.5h after the 14th WIN injection (PD 43) is shown for vehicle (white bar) or WIN (grey bar) groups (m+SEM). * p<0.05 vehicle vs. WIN cages.
Drugs
WIN55-212,2 (mesylate) (#10009023, Cayman Chemical. Ann Arbor, MI, USA) was dissolved in 5% polyethylene glycol, 5% Tween 80 and physiological saline daily prior to injection.
Behavioral Testing
Staring at PD 60 (17+ days after the final WIN injection, at the onset of young adulthood (Schneider and Koch, 2003)), a battery of behavioral tests occurred (order of testing shown in Fig. 1B). Three cohorts of animals were tested, with all undergoing sign/goal tracking training, and subsets undergoing other behavioral tests (detailed below). All behavioral testing was conducted during the dark phase of the light cycle.
Sign/goal tracking
We used a sign/goal tracking task in which a discrete cue was repeatedly paired with delivery of a palatable food pellet into an adjacent food cup. Over repeated training, rats develop conditioned approach responses to the lever (sign-tracking) and/or food cup magazine (goal-tracking)—an individual difference that predicts a variety of other reward seeking behaviors for natural and drug rewards (Robinson et al. 2014; Saunders and Robinson 2013). Individual rat bias toward sign or goal tracking is operationalized with a Pavlovian Conditioned Approach (PCA) score, which ranges from +1 (exclusive sign tracking) to −1 (exclusive goal tracking)(Meyer et al. 2012). This score is computed from the average of three behavioral variables indicating cue preference: 1) response bias (number of lever deflections minus number of cup entries/total interactions), 2) probability bias (per session probability of at least one deflection of the CS+ lever during a cue presentation, minus probability of cup entry), and 3) latency bias (average latency to deflect the lever minus average latency to enter the food cup). We defined PCA scores between −1 and −0.3 as reflecting predominant goal tracking, −0.3 to +0.3 as “intermediate” behavior, and +0.3 to +1 as predominant sign tracking. PCA scores were computed for each rat on each training day.
Following procedures described in (Mahler and Berridge 2009), we trained ad lib fed rats on the sign/goal tracking task for 8 consecutive days. Rats were first habituated to 45mg banana-flavored palatable food pellets (containing carbohydrate (52%), fat (6.3%) and protein (20.2%; Dustless Precision Pellets, #F0059; Bio-Serv, Flemington NJ, USA)) in their home cages, then magazine trained in 1-2 sessions, where 25 pellets were individually delivered into a food cup on a 30sec variable interval schedule, until >90% of pellets were retrieved by all rats. On the next 8 days, rats received training sessions on which extension of an illuminated lever, accompanied by a tone emitted from a speaker at the top of the box, was presented for 8sec, followed immediately by delivery of a palatable banana-flavored pellet into a food cup positioned adjacent to the lever cue. 25 such cue/reward pairings were presented on each 35-45min training session. Importantly, pressing the CS+ lever had no effect on the timing or probability of reward delivery. An inactive control lever was continually available throughout each session, and while interactions with this lever were recorded, they also did not affect reward delivery. All training sessions were video recorded for subsequent coding of rears and CS+ lever, food cup, and inactive lever interactions during CS+ periods on acquisition days 1-3 (early), and 7-8 (late).
Extinction of sign/goal tracking
On the day after the 8th sign/goal tracking training session, 2 cohorts of rats (control n=17, ACRS n=17) rats underwent a final ~2hr session on which they received 75 presentations of the CS+ cue on the same 90sec variable interval schedule without delivery of palatable food in order to examine within-session extinction of sign/goal tracking behavior.
Locomotor response to novelty
48hrs after the sign/goal tracking extinction test, the same rats (control n=17, ACRS n=17) were placed into a novel environment; a 43×43×30.5cm Med Associates locomotor testing chamber without bedding or food/water. Distance travelled was automatically scored by infrared beam breaks, and analyzed in 15min bins throughout the 120min session.
Familiar and novel palatable food intake
The same rats (control n=17, ACRS n=17) were habituated to the same locomotor testing box on the following day for 90min, were then given 30min access to a plastic weigh boat filled with pre-weighed 45mg banana-flavored palatable food pellets used previously in sign/goal tracking training. Food intake was recorded by weight. On the following day, 30min intake of a novel chocolate food (M&Ms brand candy) was similarly tested after a 90min baseline habituation period to the same chamber, followed by an identical final test on the following day, with the now-familiar chocolate reward.
Hunger and satiety modulation of sucrose intake
Next, all three cohorts of rats (control n=25, ACRS n=25 were habituated for 1hr/day for 6 days to a clear 48×20×27cm polycarbonate tub cage with bedding and two bottles containing water or 15% sucrose solution, with intake of water and sucrose recorded daily by weight. On the following day, they were acutely food restricted (chow removed) 6hrs prior to another 1hr test, with water intake recorded over this homecage restriction period, and water and sucrose intake measured during the 1hr two-bottle ‘acute hunger’ test. Two additional habituation sessions without restriction were conducted on the next two days to re-stabilize intake, then a similar procedure was employed to acutely satiate rats with sucrose, by allowing them 6hrs access to sucrose (without food or water) in their homecage prior to the 1hr ‘acute satiety’ test.
Elevated Plus Maze
In one cohort of rats (control n=8, ACRS n=8), an elevated plus maze (arms: 50.8×12.7cm, closed arm wall height: 30.5cm, elevated 73.7cm above the floor) was used to examine anxiety-related behavior in ACRS and controls. Rats were placed in the closed center compartment, and entries into, and time spent (all four paws) on open and closed arms was measured in the 5min test via offline hand scoring.
Novel Place Preference
48hrs after elevated plus maze testing, the same rats (control n=8, ACRS n=8) were trained on 2 daily 30min sessions to familiarize them with one side of a three chamber Med Associates rat conditioned place preference box. On the next day, they were allowed to explore all three chambers of the box in a 15min test. Animal position was scored automatically by infrared beam breaks, and time spent in the familiar or novel compartments was quantified.
Quantification of video recorded behavior
Behavior in sign/goal tracking, and hunger/satiety regulation of food intake was coded by observers blind to ACRS history. For the sign/goal tracking experiment, bouts and duration of contact with the lever CS+ cue, and the always-present control lever and food cup, as well as rears, were quantified in the 8sec prior to and during cue presentations on training days 1-3. For the sucrose acute hunger/satiety intake experiment, latency to first drink from the sucrose or water bottles on each session was also recorded.
Acute food restriction and tissue collection
Following all behavioral tests, twenty-two rats from two cohorts were habituated to wire bottom cages to prevent coprophagia for 48hrs. Control and ACRS rats were randomly assigned to 24hr food restriction, or no restriction (not restricted: control n=4, ACRS n=8; restricted: control n=6, ACRS n=4) (Kirkham et al. 2002). Rats were isoflurane anesthetized, and brains extracted and rapidly flash frozen in liquid nitrogen. Frozen brains were sectioned into 1mm coronal sections, and discrete 2-10mg samples of brain regions of interest were scalpel dissected for analysis of ECB content. Samples with ECB levels more than 2SD from group means were excluded from analyses (4 total, group sizes shown in Fig. 6).
Endocannabinoid analysis
Procedures were previously described (Astarita et al. 2009; Wei et al. 2015). Tissue samples were homogenized in methanol containing internal stands for 2H4-anandamide (2H4-AEA), 2H4-oleoylethanolamide (2H4-OEA), and 2H8-2-arachidonoyal-snglycerol (2H8-2-AG). Lipids were separated by a modified Folch-Pi method using chloroform/methanol/water (2:1:1) and open-bed silica column chromatography. For liquid chromatography/mass spectrometry (LC/MS) analyses, we used an Agilent 1200 LC system coupled to a 6410 triple quadrupole MS system (Agilent Technologies, Palo Alto, CA). The column was a ZORBAX Eclipse XDB-C18 (4.6 × 50 mm, 1.8 μm, Agilent Technologies). We used a gradient elution method as follows: solvent A consisted of methanol with 0.25 % acetic acid and 5 mM ammonium acetate, and solvent B consisted of water with 0.25 % acetic acid and 5 mM ammonium acetate. Lipids were eluted with a gradient of methanol in water (from 90 % to 100 % in 5 min, to 100 % in 7 min, and to 90 % in 8 min) at a flow rate of 1 mL/min. Column temperature was held at 40 °C. MS detection was in electrospray ionization (ESI) and positive ionization mode, with capillary voltage at 3.5 kV and fragmentor voltage at 135 V. N2 was used as drying gas at a flow rate of 12 L/min and temperature of 350 °C. Nebulizer pressure was set at 50 psi. Quantifications were conducted by an isotope dilution method, monitoring [M+H]+ in the selected ion monitoring (SIM) mode. The multiple reaction transitions monitored were as follows: anandamide, m/z 348→62; 2H4-anandamide, m/z 352→66; OEA, m/z 326→62; 2H4-OEA, m/z 330→66; 2-AG, m/z 379→287; 2H8-2-AG, m/z 387→295 (m/z, mass-to-charge ratio). Detection and analysis were performed using Mass Hunter Workstation software (Agilent).
Statistics
For analysis of ACRS effects on sign/goal tracking behavior and locomotor response to novelty, mixed model ANOVAs with day as within subjects, and ACRS group as between subjects variables were used, along with Tukey posthoc analyses, and Greenhouse-Geisser correction for sphericity as needed. Initial sign/goal tracking acquisition was separately analyzed with χ2 tests comparing frequency of behaviors of each scored type emitted during initial training trials, and mixed model ANOVAs with ACRS group × trial block (average of cues 1-5 versus 21-25 on days 1, 2, or 3) factors. Late-training (average of days 7-8) Pavlovian conditioned approach (PCA) score, palatable familiar, novel, or habituated food intake, 2-bottle sucrose or water intake and latency to drink (after ad-lib food, acute hunger or acute satiety), elevated plus maze percentage open arm time, and novelty preference percent session time on novel side were tested with independent samples t-tests comparing ACRS to control rats. For ECB analyses, 2-AG and AEA levels were tested with 2 way ANOVAs with food restriction and ACRS group as factors.
Results
Acute WIN effects
Daily WIN treatment did not affect body weight during either the 14d exposure period (no main effect of adolescent treatment) or in the 14d washout period (Fig. 1C). On the 14th daily WIN treatment (PD 43), WIN treated rats ate more chow in the 2.5hrs following injection than vehicle injected rats (t6=3.13, p=0.02, Fig. 1D).
Sign/goal tracking acquisition
On an initial magazine training day, where food pellets were dispensed on a 30sec variable interval schedule, ACRS and control rats entered the food cup to a similar extent, and left a similar number of pellets uneaten during this 20-30min habituation session. ACRS and control rats did, however, differ in their response to the initial presentation of the novel lever/tone cue on sign/goal tracking training day 1, with most control rats (56%) investigating the novel (and as yet unpaired with food) lighted lever and none entering the food cup during this initial 8sec cue period. In contrast, only 44% of ACRS rats investigated the novel lever, and 32% entered the food cup during the first ever cue presentation (20% interacted with both lever and food cup; difference in likelihood of food cup entry on first ever cue χ2=38.72, p<0.001; Fig. 2A). Accordingly, ACRS rats had more cup entries during the initial 5 cue presentations on day 1 (t32=3.48, p=0.001), but by the last 5 cues of the day (cues 21-25), groups approached the cup similarly (interaction of group × cue block; F1,32=4.96, p=0.033; Fig. 2B). No other differences between ACRS and controls in cue period CS+ lever interactions, control lever interactions, food cup entries, or rearing was observed over the first 3 training days, when behavior was being acquired (no group effect, or group × day interaction; Fig. 2B–D).
Figure 2. Acquisition of Sign and Goal Tracking to Food-Predictive Cues.
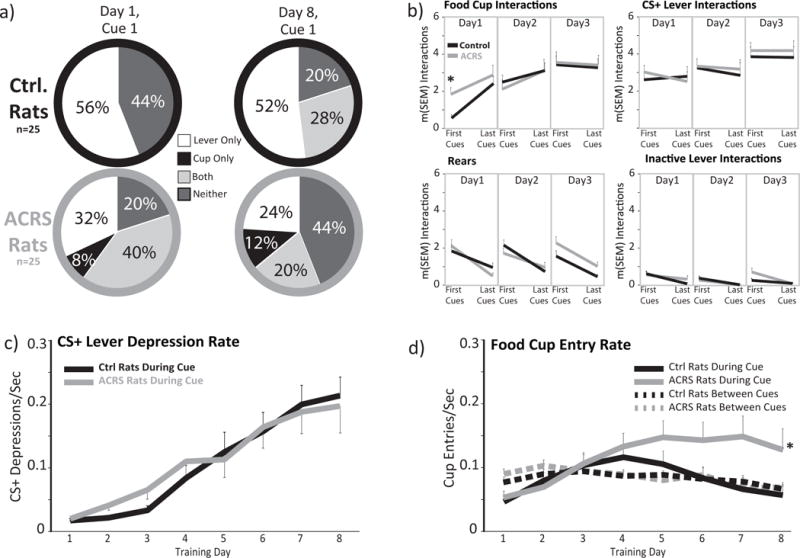
a) the percentage of rats in control (top row) and ACRS (bottom row) that interacted with the lever cue only (white), cup only (black), both lever and cup (light grey), or neither stimulus (dark grey) on the first ever lever extension on sign/goal tracking training day 1 (left column), or the first cue presentation on the 8th training day (right column). b) In each panel, m+SEM hand-scored interactions with the food cup (top left), cue lever (top right), or inactive lever (bottom right), or rears (bottom left) are shown during the first 5, or last 5 cue presentations on sign/goal tracking training days 1, 2, and 3. *p<0.05, interaction of ACRS × cue block. c) m+SEM rate of CS+ lever depressions (depressions/sec) on each training day in control (black line) and ACRS rats (grey line). d) m+SEM rate of food cup entries during cues (solid black, grey lines) or in non-cue periods (dashed black, grey lines). *p<0.05, ACRS group × day interaction.
Established sign/goal tracking
As expected, all control rats show a clear bias toward preferential conditioned approach and interaction with either the CS+ lever (i.e. sign tracking; PCA score >0.3) or the food-delivering cup (i.e. goal tracking; PCA score <−0.3; Fig. 3A–C) (Peterson et al. 1972; Robinson et al. 2014). In contrast, over a third of ACRS rats developed cue approach that was intermediate between sign and goal tracking over the 8 days of training (group × day interaction; F7,336=2.44, p=0.019)(Fig. 3A). ACRS similarly affected each of the cue bias metrics composing the PCA score, including probability bias (interaction of group × day; F7,336=2.71, p=0.01), response bias (F7,336=2.14, p=0.039), and trended for latency bias (F7,336=1.91, p=0.067). In the last two days of training (days 7&8), three quarters (76%) of control rats came to predominantly sign track during CS+ periods (PCA score m(SEM)=0.61(0.03)), and the remainder goal tracked (PCA: m=−0.55(0.08); overall control group PCA score m(SEM)=0.332(0.11)). In contrast, 36% of ACRS rats (compared with 0% of control rats) approached both cues indiscriminately by days 7&8 (PCA score >−0.3, and <0.3), and were therefore classified as “intermediate” in phenotype (intermediate group m(SEM)=−0.07(0.06)). The remaining ACRS rats either sign tracked (44%; PCA m=0.56(0.04)), or goal tracked (20%; PCA m=−0.63(0.1); Fig. 3C).
Figure 3. Cue Preference in ACRS and Control Rats.
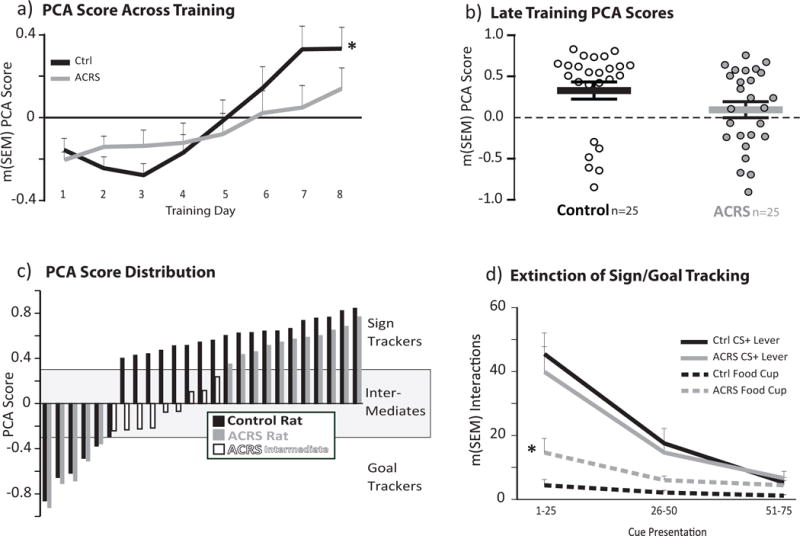
a) Pavlovian Conditioned Approach (PCA) score across training days. Black line=control, grey line=ACRS. Positive values indicate bias toward the CS+ lever (sign tracking), negative values indicated bias toward food cup (goal tracking) *p<0.05, group × day interaction. b) Mean+SEM PCA Score on training days 7&8 show with black (control) or grey (ACRS) horizontal lines and error bars. Individual rats’ data are represented with white or grey circles. c) Rat-by-rat PCA scores, ranked from most negative to most positive. Control rats=black bars, ACRS rats=grey bars, ACRS rats defined as intermediates (PCA<0.3 and >−0.3)=white bars). d) CS+ lever (solid black, grey lines) and during cue food cup entries (dashed black, grey lines) emitted during the first, second, or third 25-cue blocks on sign/goal tracking extinction training test. * p<0.05, Main effect of ACRS on food cup entries.
ACRS did not affect food cup entry rate during non-cue periods (Fig. 2D), rearing during cues on the first 3d of training, or on the last day (Fig. 2B), or pressing of the control inactive lever on any training day during cue periods or non-cue periods (Fig. 2B). In addition, when effects of ACRS was examined only in rats that came to preferentially sign track (control n=19; ACRS n=11) or goal track (control n=6; ACRS n=5) by late training, no effects of ACRS were present (no main effect of ACRS in sign trackers or goal trackers, or group × day interactions). Therefore, the main effect of ACRS was to create a relatively unusual behavioral phenotype, in which some rats approached both the CS+ lever and the food cup during most cue presentations.
Sign/goal tracking extinction
Following the 8th day of cue training, we subjected two cohorts of rats (n=17/group) to a single extinction session, in which 75 unrewarded cue presentations occurred on a VI-90sec schedule. ACRS and control rats extinguished their CS+ lever interactions similarly across this session [Main effect of cue block (three 25 cue blocks): F2,64=54.26, p<0.001, no interaction of group × cue block, Fig. 3D]. However, ACRS rats entered the cup more than controls during extinction (main effect of ACRS; F1,32=5.77, p=0.022; group × block interaction: F2,64=2.93, p=0.061; Fig. 3D), likely related to the more frequent cup entries made by ACRS rats on prior (rewarded) training days.
Familiar and novel palatable food intake
Next, a subset of ACRS and control rats were presented with the opportunity to freely consume palatable foods for 30min, following a 90min habituation period on the same day, and the 120min novel environment locomotion test in the same box on the prior day. ACRS rats ate more familiar banana-flavored palatable pellets than controls on the first test day (t32=2.06, p=0.048, and more of a novel candy-coated chocolate reward (M&Ms) on the second day (t32=2.49, p=0.018), but did not eat more than controls on test day 3, when again offered the now familiar chocolate reward (Fig. 4A). All rats ate significantly less of the novel chocolate reward than the familiar banana pellet reward, and this neophobia was equivalent in ACRS and control rats (main effect of group: F1,32=6.83, p=0.014; no interaction of group × food type).
Figure 4. ACRS Effects on Palatable Food Intake.
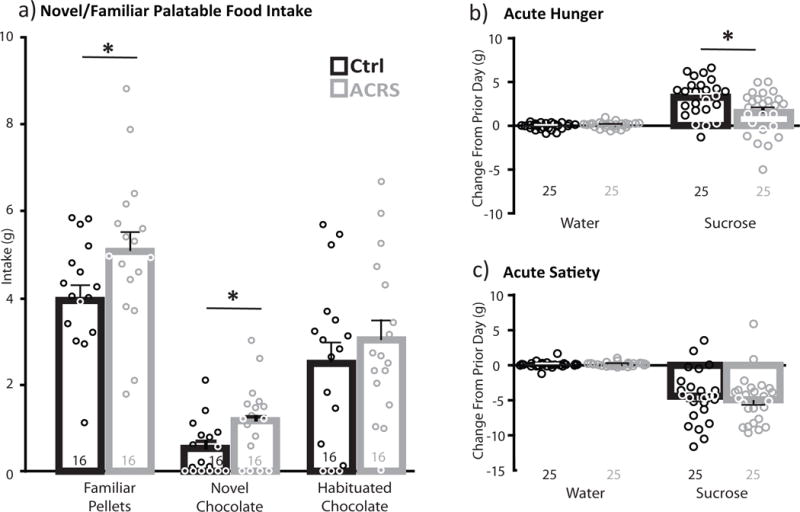
a) In 30min intake tests held separate days, consumption of familiar banana-flavored pellets, a novel chocolate reward, or the same chocolate reward, now habituated, is shown for each group (black bars=control m+SEM), grey bars=ACRS m+SEM, circles represent individual control (black) or ACRS rats (grey). *p<0.05, control versus ACRS. b) Intake of water (left) or 15% sucrose solution (right) after acute food restriction in a 2-bottle choice test. *p<0.05, control versus ACRS. c) intake of water or sucrose after acute sucrose satiety. Group ns shown below bars.
Hunger/satiety modulation of sucrose intake
Next, we examined effects of prior ACRS on hunger and satiety regulation of food intake, by examining modulation of voluntary sucrose solution intake. Intake of 15% sucrose, water, or the ratio of sucrose/water was not significantly different in ACRS and control rats during the initial 6d habituation and intake stabilization period (average daily ratio of sucrose/water: control m=23.4(1.77), ACRS m=26.7(2.26)). During this habituation period, latency to sample the sucrose bottle decreased from day 1 to day 6 of habituation (F1,32=27.97, p<0.001), to a similar extent in ACRS and controls (no group × day interaction). Next, 1hr sucrose and water intake was measured after acute 6hr food restriction (access to water only in home cage), or acute sucrose satiety (access to 15% sucrose in homecage). Intake of water or sucrose in this 6hr pre-testing period was similar in ACRS and controls [water: control m=6.8(1.0), ACRS m=7.3(0.7); sucrose: control m=45.1(3.2), ACRS m=48.3(2.7)]. In control rats, acute food restriction resulted in increased intake of sucrose, but not water, compared to stable performance over the prior 2 days (increased sucrose intake during hunger compared to baseline: F1,48=53.73, p<0.001; no similar effect on water; Fig. 4B). No such hunger-induced increase in sucrose intake was observed in ACRS rats (group × liquid interaction: F1,48=8.53, p=0.005; sucrose intake: t48=2.66, p=0.011; Fig. 4B). Latency to first drink sucrose was very short, but was statistically unaffected by either acute hunger or ACRS history (no change in latency to drink sucrose from baseline to hunger day, no interaction of day × group, or main effect of ACRS). Both ACRS and control rats showed normal sucrose satiety-induced suppression of feeding (decreased sucrose intake during satiety compared to baseline: F1,48=119.38, p<0.001, increased water intake compared to baseline: F1,48=4.76, p=0.034; no group × liquid interaction; Fig. 4C). Acute satiety resulted in increased latency to drink sucrose, but did so similarly in both groups (no group × liquid interaction).
Locomotor response to novelty
When rats were tested for locomotor activity in a novel testing environment (Bardo et al. 2013; Belin et al. 2011; Deminiere et al. 1989), ACRS and controls were nearly identical (Fig. 5A).
Figure 5. ACRS Effects on Novelty Induced Locomotion and Novel Environment Preference.
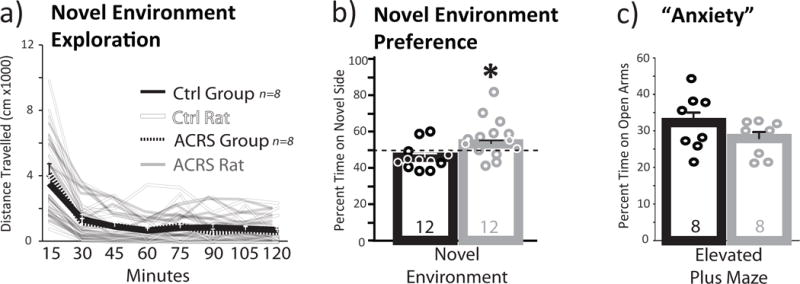
a) m+SEM Distance traveled on a 1h novel environment locomotion test is shown in 15min bins for control (black line; individual rats shown with faded black lines) and ACRS rats (m+SEM=dashed line, individual rats shown with faded white lines). b) In a novel environment preference test, ACRS rats (grey bar; circles represent individual rats) spent a greater percentage of the session time on the novel side than controls (black bar; circles represent individual rats). c) In an elevated plus maze task, percent of session time spent on the open arms of the apparatus, an index of anxiety, is shown. Group size shown in bars. *p<0.05, vehicle versus ACRS. Group ns shown in bars.
Novel place preference
ACRS and control rats showed similar locomotor responses during habituation to the familiar, black-walled chamber. On the novelty preference test, rats were allowed to spend time in this familiar chamber, or explore the novel grey-walled center area, and white-walled chamber environments. ACRS rats spent more time in the novel, white-walled chamber (53.1(0.02)% of the test session) than controls (45.9(0.02)%; group × side interaction: F1,21=5.07, p=0.035; percent session time on novel side: t21=2.26, p=0.034; Fig. 5B).
Elevated Plus Maze
No difference in anxiety, as measured in a plus maze task, tested in well-handled rats, was observed after ACRS, relative to controls (Fig. 5C).
Endocannabinoid response to food restriction
ECB levels in gross forebrain dissections are dynamically altered by food restriction (Kirkham et al. 2002), and we found that ACRS decreases hunger-induced intake of sucrose solution. Therefore, we examined whether ACRS and/or acute food restriction alters levels of the ECBs AEA, 2-AG, or OEA in specific dissections of mPFC, NAc, or cerebellum. ACRS increased NAc AEA (F1,15=7.4, p=0.016) and NAc OEA (F1,15=5.2, p=0.037), and decreased cerebellum AEA (F1,15=6.6, p=0.021). Food restriction increased NAc OEA (F1,15=10.5, p=0.006), and decreased cerebellum AEA (F1,15=14.6, p=0.002). An interaction between ACRS and restriction was seen on NAc OEA (F1,15=6.4, p=0.024), and a trend toward interaction was seen in NAc AEA (F1,15=4.3, p=0.056). No other ACRS or restriction effects on ECB levels in the measured regions were observed (Fig. 6).
Figure 6. ACRS and Food Restriction Effects on Brain Endocannabinoid Levels.
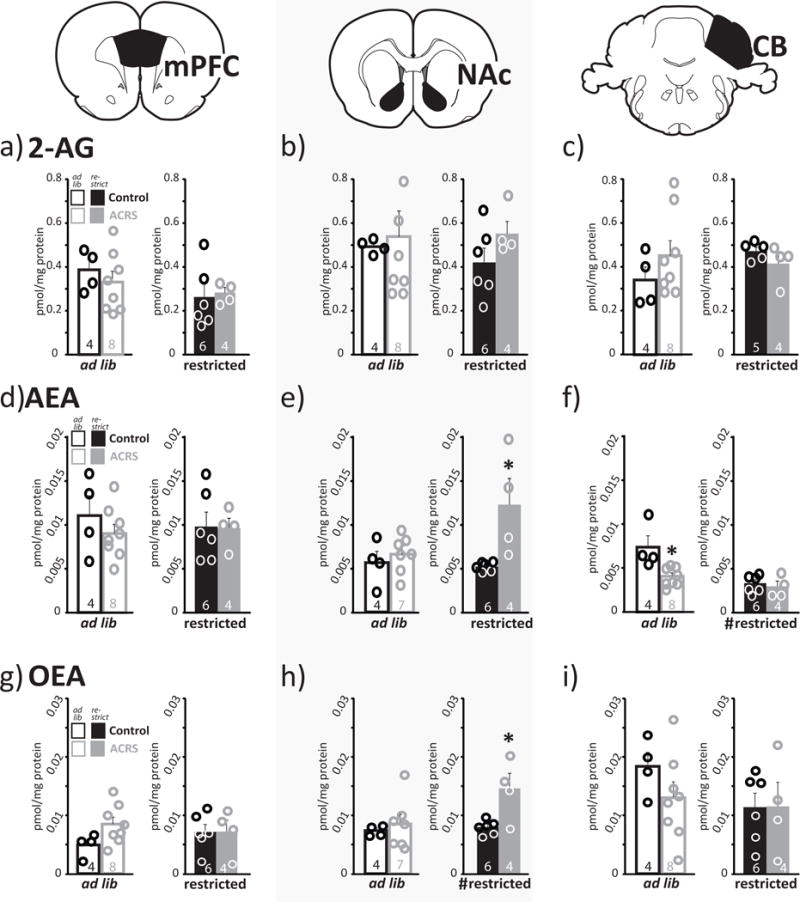
m+SEM levels of a–c) 2-AG, d–f) AEA, or g–i) OEA observed in a,d,g) medial prefrontal cortex, b,e,h) nucleus accumbens, or c,f,i) cerebellum are shown in ad libitum fed rats (left panels; white bars; circles represent individual rats) or food restricted rats (right panels; solid bars; circles represent individual rats). Control rats are represented with black borders or filled bars, ACRS rats represented with grey bordered or filled bars. ECB levels in dissected samples normalized by protein content in sample. Sample size shown in bars. *p<0.05, main effect of ACRS/control. #p<0.05, main effect of restriction
Discussion
We found that chronic adolescent CB1/2 receptor stimulation with WIN in male rats causes persistent changes in conditioned and unconditioned natural reward seeking behavior. Alterations in food cue learning, increased binge-like intake of palatable food, suppression of hunger-induced sucrose intake, and increased preference for a novel environment are suggestive of a behavioral phenotype that could put individuals at risk of developing compulsive appetitive disorders like obesity or addiction later in life. These results show that ACRS with WIN causes nuanced changes in natural reward processing, and contribute to the expanding list of behavioral phenotypes observed after ACRS in rodents.
ACRS with WIN robustly altered sign and goal tracking behavior, an assay of Pavlovian conditioned approach to reward cues that is thought to represent attribution and targeting of incentive salience (Mahler and Berridge 2009; Robinson et al. 2014). Rats spontaneously vary in their propensity to assign incentive salience to reward-predictive cues (i.e. sign track) in this task (Jenkins and Moore 1973; Saunders and Robinson 2013), and sign tracking behavior is associated with greater susceptibility to the relapse-promoting properties of response-contingent drug cues (Saunders and Robinson 2011; Saunders et al. 2013), and to phasic responses of VTA dopamine neurons to food-predictive cues (Flagel et al. 2011). In contrast, goal tracking behavior instead predicts reactivity to multimodal contextual drug cues, and motivational gating effects of drug discriminative stimuli (Pitchers et al. 2017; Robinson et al. 2014). The main effect of WIN ACRS here was to increase goal tracking behavior in rats that also exhibited significant sign tracking, creating in over one third of ACRS rats (but no control rats) a relatively unusual “intermediate” phenotype, where rats approach and interact with both the reward-predicting lever cue, and the reward-delivering (but always present) food cup. This could indicate ACRS-induced changes in attention, reward expectation, or incentive salience targeting, all processes linked with individual differences in sign/goal tracking (Lovic et al. 2011; Robinson et al. 2014). Further study using more specific behavioral tasks is needed to disentangle these (non-mutually-exclusive) psychological changes due to adolescent exposure to WIN.
We also observed consistent changes in unconditioned palatable food intake in ACRS rats, relative to controls. WIN itself increased chow intake acutely in adolescents, but body weight was not affected during or after 14d WIN treatment, demonstrating overt integrity of energy homeostasis mechanisms. In adulthood, ACRS rats consumed 130% of control levels of familiar palatable food (banana-flavored pellets used in sign/goal tracking tests containing sugar, fat, and protein), when tested in a habituated environment in a 30min test. When a novel chocolate reward was introduced on the following day, both ACRS and control rats showed the expected neophobia of the new reward, decreasing their intake far below that of the familiar pellet reward offered on the prior day. However, even in the face of this neophobia, ACRS rats ate more chocolate than controls, consistent with enhancement of hedonic reward-based feeding mechanisms. Interestingly, increased intake was not observed under all conditions, since ACRS and controls ate similar amounts of chocolate upon a second exposure to this food, and drank similar amounts of 15% sucrose in daily 1 hour water/sucrose preference tests, and a 6hr homecage access test. In contrast, THC and WIN in adolescence decreased homecage 1-2% sucrose preference (Bambico et al. 2010; Rubino et al. 2008), and escalating-dose THC increased neophobia for a salty, fatty snack food (Realini et al. 2011), and reduced intake of chow presented to hungry rats in a stressful environment (Bambico et al. 2010), potentially indicating a difference between WIN ACRS and adolescent THC exposure, and/or methodological differences like testing environment, nutrient composition (sugar, fat, protein, etc), or restricted/continuous access conditions. Regardless, our data confirm that WIN ACRS effects on adulthood food intake depend on an interaction between increased palatability and other factors like novelty, expectancy, and food type (e.g. solid versus liquid, presence or absence of fat). Notably, anxiety on the elevated plus maze task was not altered in ACRS rats here, as previously reported (Bambico et al. 2010; Biscaia et al. 2003; Rubino et al. 2008).
In contrast to increased palatable food intake, hunger-induced drinking of a 15% sucrose solution was attenuated in ACRS rats. Control rats appropriately increased their intake after 6hr food restriction (6h water pre-exposure), while ACRS rats did not. This could indicate altered hunger-induced feeding in ACRS rats, which may be mediated by changes in central and/or peripheral sensors of physiological calorie need, which are regulated importantly by ECBs (Di Marzo et al. 2009; Lau et al. 2017; Solinas et al. 2008), and are altered by ACRS (Llorente-Berzal et al. 2011). In contrast, ACRS and control rats showed similar sucrose satiety-induced suppression of intake. Therefore, ACRS-induced dysregulation of food intake and seeking is therefore far more nuanced than previously realized—with increases in palatability- and incentive motivation-related behaviors, and decreases in hunger-induced sucrose drinking.
To ask whether ECB system dysregulation within brain reward circuits might underlie altered hunger-induced food intake behavior in ACRS rats, we examined levels of AEA, OEA and 2-AG in mPFC, NAc, and cerebellum of ACRS and control rats. Following acute 24hr food restriction or ad lib food, precise scalpel dissections of ACRS and control rat mPFC, NAc, and cerebellum were collected, and analyzed via LC/MS for ECB content. We modeled our protocol based on (Kirkham et al. 2002), which showed increased AEA and 2-AG after food restriction in much less specific dissections of the limbic forebrain, a sample containing mPFC, NAc, and several other reward circuit structures (but not cerebellum). Here, we did not find changes in 2-AG, AEA, or OEA in the mPFC, NAc, or cerebellum of acutely food-restricted control rats, relative to ad lib fed controls. In contrast, in ACRS rats food restriction did increase NAc AEA and OEA levels in ACRS rats, relative to control restricted rats, and to unrestricted ACRS rats. Neither AEA in mPFC and cerebellum, nor 2-AG in any measured region was affected by food restriction or ACRS. It is not clear whether hunger-associated NAc AEA/OEA recruitment in ACRS rats is related to the failure of these rats to appropriately increase sucrose intake when hungry. Since AEA in NAc has been linked specifically to assigning palatability (or ‘liking’) to food rewards (Mahler et al. 2007; Shinohara et al. 2009), it is also possible that altered NAc AEA signaling during hunger in ACRS rats relates to the increased palatable food intake/goal tracking phenotype we observed in these animals.
ACRS also selectively increased preference for a novel, slightly stressful environment—a behavior linked both to sensation seeking in humans, and to propensity to develop addiction-like compulsive seeking of cocaine (Belin et al. 2011). When rats were allowed to choose to spend time in either a familiar habituated chamber or a novel one, ACRS rats spent more time exploring the novel environment. Such propensity to explore a novel environment predicts the subsequent transition of cocaine self-administration from goal-directed (recreational drug taking) to compulsive and punishment-resistant (addiction-like seeking) (Belin and Deroche-Gamonet 2012). Another commonly tested measure of rat novelty response is locomotion in a novel environment. Rats showing the most locomotor activity in this test (high responders) acquire cocaine self-administration faster than low responders (Cain et al. 2005; Wingo et al. 2016), but ACRS and control rats here did not differ on this measure. Since novelty-induced locomotion is uncorrelated with novel environment preference when tested in the same animals (Belin et al. 2011; Cain et al. 2005), we propose that the specific increase in preference for novelty after ACRS reveals a specific enhancement of sensation-seeking-like behavior (Belin and Deroche-Gamonet 2012), a trait related to addiction vulnerability in humans.
This report has several limitations that require additional study to address. First, only one dosage of WIN was tested here, chosen based on prior reports of persistent cognitive and emotional effects after adolescent administration (Abboussi et al. 2014; Bambico et al. 2010; Schneider 2008). Persistent effects of adolescent exposure to cannabinoid drugs are known to be dependent upon dose, sex, and cannabinoid agonist used [importantly, agonists vary in their affinity for cannabinoid CB1 and CB2 receptors, and non-cannabinoid receptors (De Petrocellis et al. 2012; Lowin et al. 2016)]. Therefore, interactions between these factors and persistent effects of ACRS on reward seeking are likely (Rubino and Parolaro 2015b; Schneider et al. 2008). In addition, it is possible that WIN ACRS effects may in part involve acute behavioral effects of adolescent cannabinoid agonists on sociality, memory, food intake, or other behavioral factors (Blanco-Gandia et al. 2015; Renard et al. 2016b; Rubino and Parolaro 2008; Trezza et al. 2014)—calling attention to the need for further characterization of such effects in ACRS studies.
In sum, these results indicate that chronic ACRS with the cannabinoid receptor direct agonist WIN yields robust alterations in natural reward-seeking behaviors in rats, including increased palatable food intake, altered targeting of incentive salience to food cues, and decreased hunger-induced sucrose intake accompanied by upregulation of AEA and OEA in NAc. These findings suggest that adolescent exposure to moderate dose WIN causes a behavioral phenotype that could put ACRS individuals at risk of later-life appetitive disorders including obesity, eating disorders, or addiction, and may have implications for understanding roles of cannabinoid receptor signaling in adolescent development of mesocorticolimbic reward circuits.
Acknowledgments
We thank Erik Castillo, Jenny Cevallos, Stephanie Lenogue, Iohana Pagnoncelli, Gagandeep Lal, Christopher Cross, and Richard Dang for assistance in treating adolescent rats, behavioral testing, behavioral scoring, and sample preparation.
Funding and Disclosures: These studies were funded by R00 DA035251, the UCI Office of Research, and the Irvine Center for Addiction Neuroscience.
Footnotes
No financial conflicts of interest exist.
References
- Abboussi O, Tazi A, Paizanis E, El Ganouni S. Chronic exposure to WIN55,212-2 affects more potently spatial learning and memory in adolescents than in adult rats via a negative action on dorsal hippocampal neurogenesis. Pharmacol Biochem Behav. 2014;120:95–102. doi: 10.1016/j.pbb.2014.02.014. [DOI] [PubMed] [Google Scholar]
- Achterberg EJM, van Swieten MMH, Driel NV, Trezza V, Vanderschuren L. Dissociating the role of endocannabinoids in the pleasurable and motivational properties of social play behaviour in rats. Pharmacol Res. 2016;110:151–158. doi: 10.1016/j.phrs.2016.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astarita G, Ahmed F, Piomelli D. Lipidomic analysis of biological samples by liquid chromatography coupled to mass spectrometry. Methods in molecular biology. 2009;579:201–19. doi: 10.1007/978-1-60761-322-0_10. [DOI] [PubMed] [Google Scholar]
- Baldo BA, Pratt WE, Will MJ, Hanlon EC, Bakshi VP, Cador M. Principles of motivation revealed by the diverse functions of neuropharmacological and neuroanatomical substrates underlying feeding behavior. Neurosci Biobehav Rev. 2013;37:1985–98. doi: 10.1016/j.neubiorev.2013.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bambico FR, Nguyen NT, Katz N, Gobbi G. Chronic exposure to cannabinoids during adolescence but not during adulthood impairs emotional behaviour and monoaminergic neurotransmission. Neurobiol Dis. 2010;37:641–55. doi: 10.1016/j.nbd.2009.11.020. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Neisewander JL, Kelly TH. Individual differences and social influences on the neurobehavioral pharmacology of abused drugs. Pharmacol Rev. 2013;65:255–90. doi: 10.1124/pr.111.005124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB. Sexual differentiation of motivation: a novel mechanism? Horm Behav. 2009;55:646–54. doi: 10.1016/j.yhbeh.2009.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behan AT, Hryniewiecka M, O’Tuathaigh CM, Kinsella A, Cannon M, Karayiorgou M, Gogos JA, Waddington JL, Cotter DR. Chronic adolescent exposure to delta-9-tetrahydrocannabinol in COMT mutant mice: impact on indices of dopaminergic, endocannabinoid and GABAergic pathways. Neuropsychopharmacology. 2012;37:1773–83. doi: 10.1038/npp.2012.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belin D, Berson N, Balado E, Piazza PV, Deroche-Gamonet V. High-novelty-preference rats are predisposed to compulsive cocaine self-administration. Neuropsychopharmacology. 2011;36:569–79. doi: 10.1038/npp.2010.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belin D, Deroche-Gamonet V. Responses to novelty and vulnerability to cocaine addiction: contribution of a multi-symptomatic animal model. Cold Spring Harb Perspect Med. 2012;2 doi: 10.1101/cshperspect.a011940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biscaia M, Marin S, Fernandez B, Marco EM, Rubio M, Guaza C, Ambrosio E, Viveros MP. Chronic treatment with CP 55,940 during the peri-adolescent period differentially affects the behavioural responses of male and female rats in adulthood. Psychopharmacology (Berl) 2003;170:301–8. doi: 10.1007/s00213-003-1550-7. [DOI] [PubMed] [Google Scholar]
- Blanco-Gandia MC, Mateos-Garcia A, Garcia-Pardo MP, Montagud-Romero S, Rodriguez-Arias M, Minarro J, Aguilar MA. Effect of drugs of abuse on social behaviour: a review of animal models. Behav Pharmacol. 2015;26:541–70. doi: 10.1097/FBP.0000000000000162. [DOI] [PubMed] [Google Scholar]
- Bossong MG, Niesink RJ. Adolescent brain maturation, the endogenous cannabinoid system and the neurobiology of cannabis-induced schizophrenia. Progress in neurobiology. 2010;92:370–85. doi: 10.1016/j.pneurobio.2010.06.010. [DOI] [PubMed] [Google Scholar]
- Brenhouse HC, Andersen SL. Developmental trajectories during adolescence in males and females: a cross-species understanding of underlying brain changes. Neurosci Biobehav Rev. 2011;35:1687–703. doi: 10.1016/j.neubiorev.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caballero A, Tseng KY. Association of Cannabis Use during Adolescence, Prefrontal CB1 Receptor Signaling, and Schizophrenia. Front Pharmacol. 2012;3:101. doi: 10.3389/fphar.2012.00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caballero A, Tseng KY. GABAergic Function as a Limiting Factor for Prefrontal Maturation during Adolescence. Trends Neurosci. 2016;39:441–8. doi: 10.1016/j.tins.2016.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain ME, Saucier DA, Bardo MT. Novelty seeking and drug use: contribution of an animal model. Exp Clin Psychopharmacol. 2005;13:367–75. doi: 10.1037/1064-1297.13.4.367. [DOI] [PubMed] [Google Scholar]
- Chen K, Kandel DB. The natural history of drug use from adolescence to the mid-thirties in a general population sample. Am J Public Health. 1995;85:41–7. doi: 10.2105/ajph.85.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuttler C, Spradlin A. Measuring cannabis consumption: Psychometric properties of the Daily Sessions, Frequency, Age of Onset, and Quantity of Cannabis Use Inventory (DFAQ-CU) PLoS One. 2017;12:e0178194. doi: 10.1371/journal.pone.0178194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Petrocellis L, Orlando P, Moriello AS, Aviello G, Stott C, Izzo AA, Di Marzo V. Cannabinoid actions at TRPV channels: effects on TRPV3 and TRPV4 and their potential relevance to gastrointestinal inflammation. Acta physiologica. 2012;204:255–66. doi: 10.1111/j.1748-1716.2011.02338.x. [DOI] [PubMed] [Google Scholar]
- Deminiere JM, Piazza PV, Le Moal M, Simon H. Experimental approach to individual vulnerability to psychostimulant addiction. Neurosci Biobehav Rev. 1989;13:141–7. doi: 10.1016/s0149-7634(89)80023-5. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Ligresti A, Cristino L. The endocannabinoid system as a link between homoeostatic and hedonic pathways involved in energy balance regulation. Int J Obes (Lond) 2009;33(Suppl 2):S18–24. doi: 10.1038/ijo.2009.67. [DOI] [PubMed] [Google Scholar]
- Doremus-Fitzwater TL, Spear LP. Reward-centricity and attenuated aversions: An adolescent phenotype emerging from studies in laboratory animals. Neurosci Biobehav Rev. 2016;70:121–134. doi: 10.1016/j.neubiorev.2016.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dow-Edwards D, Silva L. Endocannabinoids in brain plasticity: Cortical maturation, HPA axis function and behavior. Brain Res. 2017;1654:157–164. doi: 10.1016/j.brainres.2016.08.037. [DOI] [PubMed] [Google Scholar]
- Ellgren M, Artmann A, Tkalych O, Gupta A, Hansen HS, Hansen SH, Devi LA, Hurd YL. Dynamic changes of the endogenous cannabinoid and opioid mesocorticolimbic systems during adolescence: THC effects. Eur Neuropsychopharmacol. 2008;18:826–34. doi: 10.1016/j.euroneuro.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellgren M, Spano SM, Hurd YL. Adolescent cannabis exposure alters opiate intake and opioid limbic neuronal populations in adult rats. Neuropsychopharmacology. 2007;32:607–15. doi: 10.1038/sj.npp.1301127. [DOI] [PubMed] [Google Scholar]
- Flagel SB, Clark JJ, Robinson TE, Mayo L, Czuj A, Willuhn I, Akers CA, Clinton SM, Phillips PE, Akil H. A selective role for dopamine in stimulus-reward learning. Nature. 2011;469:53–7. doi: 10.1038/nature09588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes FV, Guimaraes FS, Grace AA. Effects of pubertal cannabinoid administration on attentional set-shifting and dopaminergic hyper-responsivity in a developmental disruption model of schizophrenia. Int J Neuropsychopharmacol. 2015;18 doi: 10.1093/ijnp/pyu018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuera-Matas A, Soto-Montenegro ML, del Olmo N, Miguens M, Torres I, Vaquero JJ, Sanchez J, Garcia-Lecumberri C, Desco M, Ambrosio E. Augmented acquisition of cocaine self-administration and altered brain glucose metabolism in adult female but not male rats exposed to a cannabinoid agonist during adolescence. Neuropsychopharmacology. 2008;33:806–13. doi: 10.1038/sj.npp.1301467. [DOI] [PubMed] [Google Scholar]
- Hurd YL, Michaelides M, Miller ML, Jutras-Aswad D. Trajectory of adolescent cannabis use on addiction vulnerability. Neuropharmacology. 2014;76(Pt B):416–24. doi: 10.1016/j.neuropharm.2013.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jager G, Ramsey NF. Long-term consequences of adolescent cannabis exposure on the development of cognition, brain structure and function: an overview of animal and human research. Curr Drug Abuse Rev. 2008;1:114–23. doi: 10.2174/1874473710801020114. [DOI] [PubMed] [Google Scholar]
- Jenkins HM, Moore BR. The form of the auto-shaped response with food or water reinforcers. Journal of the Experimental Analysis of Behavior. 1973;20:163–181. doi: 10.1901/jeab.1973.20-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King CP, Palmer AA, Woods LC, Hawk LW, Richards JB, Meyer PJ. Premature responding is associated with approach to a food cue in male and female heterogeneous stock rats. Psychopharmacology (Berl) 2016;233:2593–605. doi: 10.1007/s00213-016-4306-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkham TC, Williams CM, Fezza F, Di Marzo V. Endocannabinoid levels in rat limbic forebrain and hypothalamus in relation to fasting, feeding and satiation: stimulation of eating by 2-arachidonoyl glycerol. Br J Pharmacol. 2002;136:550–7. doi: 10.1038/sj.bjp.0704767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau BK, Cota D, Cristino L, Borgland SL. Endocannabinoid modulation of homeostatic and non-homeostatic feeding circuits. Neuropharmacology. 2017 doi: 10.1016/j.neuropharm.2017.05.033. [DOI] [PubMed] [Google Scholar]
- Laviolette SR, Grace AA. The roles of cannabinoid and dopamine receptor systems in neural emotional learning circuits: implications for schizophrenia and addiction. Cellular and molecular life sciences : CMLS. 2006;63:1597–613. doi: 10.1007/s00018-006-6027-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee TT, Gorzalka BB. Timing is everything: evidence for a role of corticolimbic endocannabinoids in modulating hypothalamic-pituitary-adrenal axis activity across developmental periods. Neuroscience. 2012;204:17–30. doi: 10.1016/j.neuroscience.2011.10.006. [DOI] [PubMed] [Google Scholar]
- Llorente-Berzal A, Fuentes S, Gagliano H, Lopez-Gallardo M, Armario A, Viveros MP, Nadal R. Sex-dependent effects of maternal deprivation and adolescent cannabinoid treatment on adult rat behaviour. Addict Biol. 2011;16:624–37. doi: 10.1111/j.1369-1600.2011.00318.x. [DOI] [PubMed] [Google Scholar]
- Lovic V, Saunders BT, Yager LM, Robinson TE. Rats prone to attribute incentive salience to reward cues are also prone to impulsive action. Behav Brain Res. 2011;223:255–61. doi: 10.1016/j.bbr.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowin T, Pongratz G, Straub RH. The synthetic cannabinoid WIN55,212-2 mesylate decreases the production of inflammatory mediators in rheumatoid arthritis synovial fibroblasts by activating CB2, TRPV1, TRPA1 and yet unidentified receptor targets. J Inflamm (Lond) 2016;13:15. doi: 10.1186/s12950-016-0114-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubman DI, Cheetham A, Yucel M. Cannabis and adolescent brain development. Pharmacol Ther. 2015;148:1–16. doi: 10.1016/j.pharmthera.2014.11.009. [DOI] [PubMed] [Google Scholar]
- Mahler SV, Berridge KC. Which cue to “want?” Central amygdala opioid activation enhances and focuses incentive salience on a prepotent reward cue. J Neurosci. 2009;29:6500–13. doi: 10.1523/JNEUROSCI.3875-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler SV, Smith KS, Berridge KC. Endocannabinoid hedonic hotspot for sensory pleasure: anandamide in nucleus accumbens shell enhances ‘liking’ of a sweet reward. Neuropsychopharmacology. 2007;32:2267–78. doi: 10.1038/sj.npp.1301376. [DOI] [PubMed] [Google Scholar]
- Maldonado R, Valverde O, Berrendero F. Involvement of the endocannabinoid system in drug addiction. Trends Neurosci. 2006;29:225–32. doi: 10.1016/j.tins.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Meier MH, Caspi A, Ambler A, Harrington H, Houts R, Keefe RS, McDonald K, Ward A, Poulton R, Moffitt TE. Persistent cannabis users show neuropsychological decline from childhood to midlife. Proc Natl Acad Sci U S A. 2012;109:E2657–64. doi: 10.1073/pnas.1206820109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer PJ, Lovic V, Saunders BT, Yager LM, Flagel SB, Morrow JD, Robinson TE. Quantifying individual variation in the propensity to attribute incentive salience to reward cues. PLoS One. 2012;7:e38987. doi: 10.1371/journal.pone.0038987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mokrysz C, Landy R, Gage SH, Munafo MR, Roiser JP, Curran HV. Are IQ and educational outcomes in teenagers related to their cannabis use? A prospective cohort study. J Psychopharmacol. 2016;30:159–68. doi: 10.1177/0269881115622241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton GC, Coffey C, Carlin JB, Degenhardt L, Lynskey M, Hall W. Cannabis use and mental health in young people: cohort study. BMJ. 2002;325:1195–8. doi: 10.1136/bmj.325.7374.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson GB, Ackilt JE, Frommer GP, Hearst ES. Conditioned Approach and Contact Behavior toward Signals for Food or Brain-Stimulation Reinforcement. Science. 1972;177:1009–1011. doi: 10.1126/science.177.4053.1009. [DOI] [PubMed] [Google Scholar]
- Pistis M, Perra S, Pillolla G, Melis M, Muntoni AL, Gessa GL. Adolescent exposure to cannabinoids induces long-lasting changes in the response to drugs of abuse of rat midbrain dopamine neurons. Biol Psychiatry. 2004;56:86–94. doi: 10.1016/j.biopsych.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Pitchers KK, Phillips KB, Jones JL, Robinson TE, Sarter M. Diverse roads to relapse: A discriminative cue signaling cocaine availability is more effective in renewing cocaine-seeking in goal-trackers than sign-trackers, and depends on basal forebrain cholinergic activity. J Neurosci. 2017 doi: 10.1523/JNEUROSCI.0990-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Realini N, Vigano D, Guidali C, Zamberletti E, Rubino T, Parolaro D. Chronic URB597 treatment at adulthood reverted most depressive-like symptoms induced by adolescent exposure to THC in female rats. Neuropharmacology. 2011;60:235–43. doi: 10.1016/j.neuropharm.2010.09.003. [DOI] [PubMed] [Google Scholar]
- Renard J, Krebs MO, Le Pen G, Jay TM. Long-term consequences of adolescent cannabinoid exposure in adult psychopathology. Frontiers in neuroscience. 2014;8:361. doi: 10.3389/fnins.2014.00361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renard J, Rosen LG, Loureiro M, De Oliveira C, Schmid S, Rushlow WJ, Laviolette SR. Adolescent Cannabinoid Exposure Induces a Persistent Sub-Cortical Hyper-Dopaminergic State and Associated Molecular Adaptations in the Prefrontal Cortex. Cereb Cortex. 2016a doi: 10.1093/cercor/bhv335. [DOI] [PubMed] [Google Scholar]
- Renard J, Rushlow WJ, Laviolette SR. What Can Rats Tell Us about Adolescent Cannabis Exposure? Insights from Preclinical Research. Canadian journal of psychiatry Revue canadienne de psychiatrie. 2016b;61:328–34. doi: 10.1177/0706743716645288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renard J, Vitalis T, Rame M, Krebs MO, Lenkei Z, Le Pen G, Jay TM. Chronic cannabinoid exposure during adolescence leads to long-term structural and functional changes in the prefrontal cortex. Eur Neuropsychopharmacol. 2016c;26:55–64. doi: 10.1016/j.euroneuro.2015.11.005. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Yager LM, Cogan ES, Saunders BT. On the motivational properties of reward cues: Individual differences. Neuropharmacology. 2014;76(Pt B):450–9. doi: 10.1016/j.neuropharm.2013.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubino T, Parolaro D. Long lasting consequences of cannabis exposure in adolescence. Molecular and cellular endocrinology. 2008;286:S108–13. doi: 10.1016/j.mce.2008.02.003. [DOI] [PubMed] [Google Scholar]
- Rubino T, Parolaro D. The Impact of Exposure to Cannabinoids in Adolescence: Insights from Animal Models. Biol Psychiatry. 2015a doi: 10.1016/j.biopsych.2015.07.024. [DOI] [PubMed] [Google Scholar]
- Rubino T, Parolaro D. Sex-dependent vulnerability to cannabis abuse in adolescence. Front Psychiatry. 2015b;6:56. doi: 10.3389/fpsyt.2015.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubino T, Vigano D, Realini N, Guidali C, Braida D, Capurro V, Castiglioni C, Cherubino F, Romualdi P, Candeletti S, Sala M, Parolaro D. Chronic delta 9-tetrahydrocannabinol during adolescence provokes sex-dependent changes in the emotional profile in adult rats: behavioral and biochemical correlates. Neuropsychopharmacology. 2008;33:2760–71. doi: 10.1038/sj.npp.1301664. [DOI] [PubMed] [Google Scholar]
- Rubino T, Zamberletti E, Parolaro D. Adolescent exposure to cannabis as a risk factor for psychiatric disorders. J Psychopharmacol. 2012;26:177–88. doi: 10.1177/0269881111405362. [DOI] [PubMed] [Google Scholar]
- Saunders BT, Robinson TE. Individual variation in the motivational properties of cocaine. Neuropsychopharmacology. 2011;36:1668–76. doi: 10.1038/npp.2011.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders BT, Robinson TE. Individual variation in resisting temptation: implications for addiction. Neurosci Biobehav Rev. 2013;37:1955–75. doi: 10.1016/j.neubiorev.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders BT, Yager LM, Robinson TE. Cue-evoked cocaine “craving”: role of dopamine in the accumbens core. J Neurosci. 2013;33:13989–4000. doi: 10.1523/JNEUROSCI.0450-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider M. Puberty as a highly vulnerable developmental period for the consequences of cannabis exposure. Addict Biol. 2008;13:253–63. doi: 10.1111/j.1369-1600.2008.00110.x. [DOI] [PubMed] [Google Scholar]
- Schneider M, Schomig E, Leweke FM. Acute and chronic cannabinoid treatment differentially affects recognition memory and social behavior in pubertal and adult rats. Addict Biol. 2008;13:345–57. doi: 10.1111/j.1369-1600.2008.00117.x. [DOI] [PubMed] [Google Scholar]
- Scott JC, Wolf DH, Calkins ME, Bach EC, Weidner J, Ruparel K, Moore TM, Jones JD, Jackson CT, Gur RE, Gur RC. Cognitive functioning of adolescent and young adult cannabis users in the Philadelphia Neurodevelopmental Cohort. Psychol Addict Behav. 2017;31:423–434. doi: 10.1037/adb0000268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinohara Y, Inui T, Yamamoto T, Shimura T. Cannabinoid in the nucleus accumbens enhances the intake of palatable solution. Neuroreport. 2009;20:1382–5. doi: 10.1097/WNR.0b013e3283318010. [DOI] [PubMed] [Google Scholar]
- Silva L, Black R, Michaelides M, Hurd YL, Dow-Edwards D. Sex and age specific effects of delta-9-tetrahydrocannabinol during the periadolescent period in the rat: The unique susceptibility of the prepubescent animal. Neurotoxicol Teratol. 2016;58:88–100. doi: 10.1016/j.ntt.2016.02.005. [DOI] [PubMed] [Google Scholar]
- Smith KS, Mahler SV, Pecina S, Berridge KC. Hedonic Hotspots: Generating Sensory Pleasure in the Brain. In: Berridge KC, Kringelbach ML, editors. Pleasures of the Brain. Oxford University Press; 2010. pp. 27–49. [Google Scholar]
- Solinas M, Goldberg SR, Piomelli D. The endocannabinoid system in brain reward processes. Br J Pharmacol. 2008;154:369–83. doi: 10.1038/bjp.2008.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP. Consequences of adolescent use of alcohol and other drugs: Studies using rodent models. Neurosci Biobehav Rev. 2016;70:228–243. doi: 10.1016/j.neubiorev.2016.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stopponi S, Soverchia L, Ubaldi M, Cippitelli A, Serpelloni G, Ciccocioppo R. Chronic THC during adolescence increases the vulnerability to stress-induced relapse to heroin seeking in adult rats. Eur Neuropsychopharmacol. 2014;24:1037–45. doi: 10.1016/j.euroneuro.2013.12.012. [DOI] [PubMed] [Google Scholar]
- Taylor M, Collin SM, Munafo MR, MacLeod J, Hickman M, Heron J. Patterns of cannabis use during adolescence and their association with harmful substance use behaviour: findings from a UK birth cohort. J Epidemiol Community Health. 2017 doi: 10.1136/jech-2016-208503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasiewicz HC, Jacobs MM, Wilkinson MB, Wilson SP, Nestler EJ, Hurd YL. Proenkephalin mediates the enduring effects of adolescent cannabis exposure associated with adult opiate vulnerability. Biol Psychiatry. 2012;72:803–10. doi: 10.1016/j.biopsych.2012.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trezza V, Baarendse PJ, Vanderschuren LJ. On the interaction between drugs of abuse and adolescent social behavior. Psychopharmacology (Berl) 2014;231:1715–29. doi: 10.1007/s00213-014-3471-z. [DOI] [PubMed] [Google Scholar]
- Vlachou S, Panagis G. Regulation of brain reward by the endocannabinoid system: a critical review of behavioral studies in animals. Curr Pharm Des. 2014;20:2072–88. doi: 10.2174/13816128113199990433. [DOI] [PubMed] [Google Scholar]
- Wegener N, Koch M. Behavioural disturbances and altered Fos protein expression in adult rats after chronic pubertal cannabinoid treatment. Brain Res. 2009;1253:81–91. doi: 10.1016/j.brainres.2008.11.081. [DOI] [PubMed] [Google Scholar]
- Wei D, Lee D, Cox CD, Karsten CA, Penagarikano O, Geschwind DH, Gall CM, Piomelli D. Endocannabinoid signaling mediates oxytocin-driven social reward. Proc Natl Acad Sci U S A. 2015;112:14084–9. doi: 10.1073/pnas.1509795112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley JL, Burston JJ. Sex differences in Delta(9)-tetrahydrocannabinol metabolism and in vivo pharmacology following acute and repeated dosing in adolescent rats. Neurosci Lett. 2014;576:51–5. doi: 10.1016/j.neulet.2014.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingo T, Nesil T, Choi JS, Li MD. Novelty Seeking and Drug Addiction in Humans and Animals: From Behavior to Molecules. Journal of neuroimmune pharmacology : the official journal of the Society on NeuroImmune Pharmacology. 2016;11:456–70. doi: 10.1007/s11481-015-9636-7. [DOI] [PMC free article] [PubMed] [Google Scholar]


