Abstract
Impairments in response inhibition and salience attribution (iRISA) have been proposed to underlie the clinical symptoms of drug addiction as mediated by cortico-striatal-thalamo-cortical networks. The bulk of evidence supporting the iRISA model comes from neuroimaging research that has focused on cortical and striatal influences with less emphasis on the role of the thalamus. Here, we highlight the importance of the thalamus in drug addiction, focusing on animal literature findings on thalamic nuclei in the context of drug-seeking, structural and functional changes of the thalamus as measured by imaging studies in human drug addiction, particularly during drug cue and non-drug reward processing, and response inhibition tasks. Findings from the animal literature suggest that the paraventricular nucleus of the thalamus, the lateral habenula and the mediodorsal nucleus may be involved in the reinstatement, extinction and expression of drug-seeking behaviours. In support of the iRISA model, the human addiction imaging literature demonstrates enhanced thalamus activation when reacting to drug cues and reduced thalamus activation during response inhibition. This pattern of response was further associated with the severity of, and relapse in, drug addiction. Future animal studies could widen their field of focus by investigating the specific role(s) of different thalamic nuclei in different phases of the addiction cycle. Similarly, future human imaging studies should aim to specifically delineate the structure and function of different thalamic nuclei, for example, through the application of advanced imaging protocols at higher magnetic fields (7 Tesla).
This article is part of a discussion meeting issue ‘Of mice and mental health: facilitating dialogue between basic and clinical neuroscientists’.
Keywords: thalamus, functional MRI, substance use disorders, drug self-administration, response inhibition, cue reactivity
1. Introduction
The Impaired Response Inhibition and Salience Attribution (iRISA) model of drug addiction proposes that reduced response inhibition and excessive salience attribution to drug cues, along with reduced salience attributed to non-drug rewards, are core clinical symptoms observed in drug addiction in human populations [1,2]. These impairments have been found to map onto dysfunctions of both the prefrontal cortex (PFC) and the striatum. The PFC shows enhanced activation with acute drug intake and exposure to drug-associated cues, but reduction in activation during higher-order emotional and cognitive processing, especially during protracted abstinence. The thalamus is integral to the circuits that underlie the reward [3] and response inhibition processes mediating salience and control processes in goal-oriented behaviours [4–7].
This review aims to highlight the importance of the thalamus as a central structure within the cortico-striato-thalamo-cortical loops that are central to addiction and study the fit of the thalamus into the iRISA model of addiction. After providing a brief overview of the cortico-striato-thalamo-cortical reward circuit and the pertinent anatomical connections of the thalamus within this circuit, this review highlights animal research investigating the role of the thalamus in drug-seeking behaviours, focusing on the paraventricular nucleus of the thalamus (PVT), the lateral habenula (LHb; a part of the epithalamus) and the mediodorsal (MD) nucleus, followed by reviewing structural and functional changes observed in the thalamus based on human imaging studies in drug addiction, with a particular focus on thalamic responses to drug cues, non-drug reward processing and response inhibition. Animal and human studies have inherent differences in the approaches used, with animal studies most often precisely targeting thalamic nuclei, whereas human studies are looking at thalamus activation more globally. Animal studies are further able to investigate the behavioural effects of disruption to thalamus activity using lesion and deep brain stimulation methods. Human studies are able to better examine the relationship between the thalamus and systems level changes across the whole brain in addiction. Detailed study selection criteria are presented in electronic supplementary material, table S1 (for animal studies) and electronic supplementary material, table S2 (for human imaging studies).
2. Addiction circuitry and the thalamus
Drugs of abuse increase dopamine in the reward circuit (which is important for initiating and maintaining motivated behaviours) comprise the midbrain dopamine areas (including the ventral tegmental area (VTA) and substantia nigra), the ventral (including the nucleus accumbens (NAc)) and dorsal striatum (including the caudate and putamen) and the PFC (including the medial PFC, orbitofrontal cortex and lateral PFC). Both the neuroanatomy of the reward circuit and the involvement of reward circuit regions in addiction have been extensively reviewed elsewhere [1,2,8–11]. Here, we focus specifically on the connections and the role of the thalamus within this circuit (figure 1).
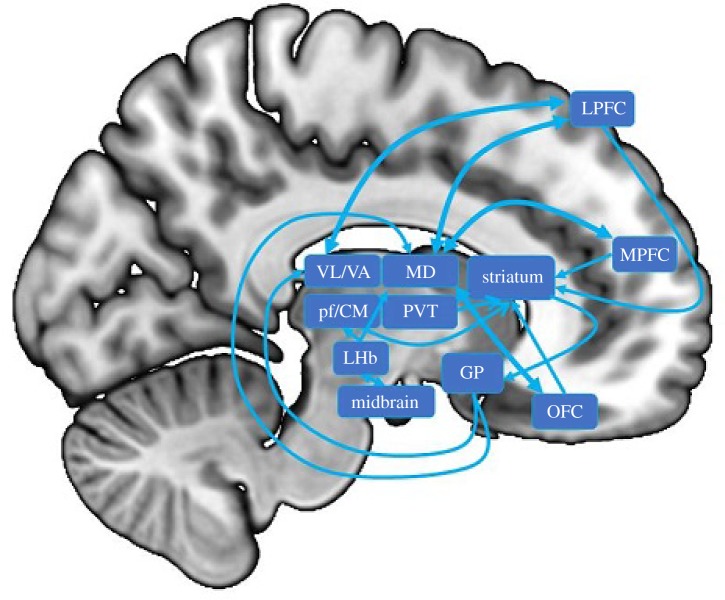
Figure 1.
A simplified schematic of thalamus connections relevant to drug addiction. LHb, lateral habenula; MD, mediodorsal nucleus; PVT, paraventricular nucleus of the thalamus; Pf/CM, parafascicular/centromedian nuclei; VL/VA, ventral lateral/ventral anterior nuclei; GP, globus pallidus; LPFC, lateral prefrontal cortex; MPFC, medial prefrontal cortex; OFC, orbitofrontal cortex.
The striatum receives major dopaminergic projections from the midbrain, as well as afferent connections from the cortex, primarily the frontal cortex. The striatum projects via the globus pallidus to the thalamus, which then projects back to the frontal cortex. Nuclei within the thalamus function as both input and output structures within this circuit, with the MD nucleus and ventral lateral thalamic complex (including the ventral lateral and ventral anterior nuclei) serving as the final subcortical structures of this circuit, prior to projecting back to the frontal cortex [12].
The majority of projections from the globus pallidus to the thalamus land in the ventral lateral thalamic complex and the MD nucleus. The ventral and dorsal striatum receive projections from midline (including the PVT) and intralaminar (including the centromedian and parafascicular nuclei) thalamic nuclei. In primates, the parafascicular nucleus projects to the ventral and dorsal striatum and the centromedian nucleus projects to the dorsal striatum [13–18], allowing these nuclei to mediate both the motivational and goal-oriented behaviour supported by the ventral striatum, and cognitive processes thought to involve the dorsal striatum, such as response and motor inhibition [9,19–22]. Recent rodent studies have focused on the role of the PVT in addiction, probably due to its extensive connections with the ventral and dorsal striatum [23–27]. Although not a part of the thalamus proper, nor an integral part of cortico-striato-thalamo-cortical loops, the LHb has also become a major focus of investigation in the rodent addiction literature due to its connections with the VTA. The VTA sends dopaminergic projections to the LHb, while the LHb sends inhibitory connections back to the VTA [28–30]. This inhibitory projection to the VTA allows the LHb to attenuate responses within the VTA and reduce the potency of reward (including drug cue) signals. Unlike the thalamus proper, which does not show evidence of having connections between thalamic nuclei, the LHb sends efferent connections to other thalamic nuclei, including the centromedian, parafascicular and MD nuclei [31].
Most connections between the frontal cortex and the thalamus are reciprocal, allowing them to maintain the flow of information between these regions. Nevertheless, there are more extensive projections from the cortex to the thalamus than those projecting back, as well as non-reciprocal cortico-thalamic components, together supporting the notion that the thalamus has a role in integrating information that goes beyond functioning as a simple relay [3,32–36]. The ventral lateral thalamic complex projects primarily to motor and premotor regions of the PFC, as well as to the dorsolateral PFC [37–40]. Compared with the PVT and LHb, there has been less focus on the MD nucleus of the thalamus in the rodent addiction literature, probably due to its extensive connections with the PFC, which are less apparent in rodents than primates. Nevertheless, the MD nucleus has a role in goal-directed behaviours and is likely to play a part in the human addiction circuitry [4,41–44]. The medial portions of the primate MD nucleus connect primarily with the medial PFC and orbitofrontal regions and the lateral portions of the MD connect primarily with the lateral PFC [39,45,46]. This connectivity pattern of the MD nucleus allows it to mediate activity within the PFC, as well as integrate incoming information from the basal ganglia. Therefore, the MD nucleus may have a role in the cognitive and emotional processes supported by the PFC, such as higher-order valuation/motivation and cognitive control.
3. Animal studies of drug seeking
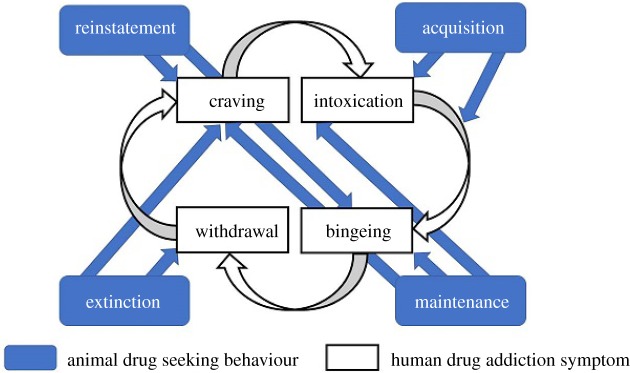
Our review of animal studies of drug-seeking behaviour is restricted to studies that used drug conditioned place preference (CPP) or drug self-administration paradigms, which elicit behaviours in animals that have some correspondence to clinical symptoms seen in human drug addiction (figure 2). These paradigms produce four distinct stages of behaviour: acquisition, maintenance, extinction and reinstatement. Acquisition refers to the ability to acquire CPP or drug self-administration behaviour and would be the equivalent of initiating the iRISA cycle in humans. Maintenance is the period after acquisition when behavioural response has become stabilized and can roughly correspond to the compulsive drug-administration/bingeing stage observed in clinical manifestations of human drug addiction, although it also probably involves craving/intoxication. Despite being distinct concepts, acquisition and maintenance are often tested together in drug self-administration paradigms as reduced acquisition also results in lower maintenance levels of drug self-administration. Extinction training involves systematically reducing drug-seeking behaviours by pairing a drug cue with a context signalling lack of drug and would correspond to the withdrawal (and craving) stage of iRISA. Finally, reinstatement refers to a return of drug-seeking behaviours by reintroducing the animal to the drug or a drug (or stress)-associated cue or context. Reinstatement might correspond to craving/bingeing/relapse, and thalamus activation associated with increased reinstatement behaviours may indicate enhanced salience associated with drug cues.
Figure 2.
Schematic of the relationship between animal drug-seeking behaviours in drug self-administration and conditioned place preference paradigms and clinical symptoms of human drug addiction.
(a). Paraventricular nucleus of the thalamus involvement in reinstatement of drug-seeking behaviours
The PVT has been targeted in the reinstatement of drug-seeking behaviours and in mediating associations between drug and drug-related cues. Neural activation (measured as c-Fos expression) has been found in the PVT after the reinstatement of drug-seeking behaviour in rats, although this activation was not seen immediately after the acquisition of alcohol (and sucrose) and cocaine self-administration behaviour [47,48]. This suggests that, while the PVT appears to be involved in reinstatement behaviours, it may have less involvement in the acquisition of drug habits. The PVT also showed differences in neuronal activation between rats with more versus fewer reinstatement behaviours, and a positive correlation between c-Fos expression and reinstatement behaviour in the more severe group [49]. It was further found that greater neuronal activation (measured as c-Fos expression) within the PVT was correlated with more cocaine-seeking behaviour after reinstatement following a drug cue, but not after the reinstatement of behaviour conditioned to a palatable food reward [50], suggesting that the degree of neuronal activation within the PVT is behaviourally relevant.
Disruption of neural activity in the PVT (with a low-dose baclofen/muscimol solution that disrupts neuronal firing, or tetrodotoxin—a sodium channel blocker) attenuated drug, cue- or context-induced reinstatement, while further disrupting neural activity in the PVT with a higher solution dose completely abolished reinstatement of drug-seeking behaviours in rats previously trained to self-administer cocaine [51–53]. Similarly, disruption of the posterior PVT abolished reinstatement of cocaine-seeking behaviour, but only attenuated renewal of food-seeking behaviour in rats [54], suggesting that PVT neurons may preferentially mediate behaviours associated with drugs of abuse, although drugs of abuse recruit the reward system, which also mediates food rewards [55–57].
One mechanism in the PVT that may have a role in mediating reinstatement of drug seeking appears to act through orexin/hypocretin signalling [58]. Orexin/hypocretin (and cocaine- and amphetamine-regulated transcript (CART)) are neuropeptides associated with appetite control-, reward- and motivation-related behaviours [59]. Neurons within the PVT that were activated after the reinstatement of ethanol-seeking behaviour appeared to be surrounded by CART or orexin immunoreactive terminals [60]. The CART and orexin fibres within the PVT also exist in close proximity to neurons that project to the NAc, thereby allowing these neuropeptides to mediate the PVT-NAc projections [61]. Importantly, injection of orexin/hypocretin peptides into the PVT reinstated cocaine self-administration behaviours after extinction, similar to an acute injection of cocaine [62].
Neurons within the PVT that project to the NAc may constitute another mechanism underlying the reinstatement of drug-seeking behaviours, by mediating drug self-administration and withdrawal. Reinstatement of ethanol-seeking behaviours in rats led to greater activation of neurons within the PVT that project to the NAc [51]. Optogenetic activation, which involves using light to trigger neuronal firing after genetically modifying neurons to express light-sensitive ion channels, of the PVT-NAc projection has been shown to be aversive [63]. Selective disruption of PVT neurons projecting to the NAc attenuated acquisition of cocaine self-administration behaviour, but had no effect on incubation of craving [64], an effect whereby drug-seeking behaviour increases during extinction [65]. Similarly, optogenetic disruption of PVT neurons projecting to the NAc reduced withdrawal-induced place aversion and somatic signs of opiate withdrawal [63]. Taken together, disruption of the PVT-NAc projection appears to attenuate drug self-administration and the aversive effect of drug withdrawal (but not craving-induced drug-seeking behaviour), suggesting that PVT neurons that project to the NAc may mediate reinstatement by influencing aversive processing (e.g. the expression of withdrawal symptoms during abstinence).
(b). Lateral habenula involvement in extinction and reinstatement of drug-seeking behaviour
The role of the habenula in drug addiction has recently been extensively reviewed [66]. Here, we focus on studies investigating the relationship between the LHb and drug-seeking behaviours. In rodents, the LHb (in addition to the PVT) has shown more neuronal activation (measured as c-Fos expression) after cue- and drug-induced reinstatement of drug-seeking behaviours [67,68]. Lesioning the LHb increased ethanol-seeking behaviour [69] during the acquisition/maintenance phase of a self-administration task, but had no effect on heroin- [70] or cocaine-seeking behaviour in rats, although it did disrupt the ability of rats to correctly inhibit behaviour based on a cue indicating cocaine absence [71]. By contrast, lesioning the LHb reduced combined cue and yohimbine (a stimulant) induced reinstatement of ethanol and cocaine-seeking behaviours [69,72]. Lesioning the LHb appeared to have different effects on extinction behaviours depending on when the lesion occurred. While lesioning the LHb prior to extinction training prevented complete extinction of cocaine-seeking behaviours in rats [73], behaviour did not change when the lesion was made after extinction training was complete [72]. Lesion studies suggest that the involvement of the LHb in drug seeking is complex, with results depending on which phase of the self-administration cycle the lesion occurred.
This complexity further extends into deep brain stimulation studies of the LHb, which showed some results that were consistent, and others that were inconsistent with lesion studies. During the acquisition/maintenance phase, low-frequency deep brain stimulation increased, while combined high- and low-frequency deep brain stimulation reduced drug self-administration behaviours [73]. Combined frequency deep brain stimulation in the LHb in rats during extinction showed enhancement (faster time to reach extinction threshold) of extinction effects, a result that was inconsistent with lesion studies, while deep brain stimulation reduced drug-induced reinstatement of cocaine-seeking behaviours, which was consistent with lesion effects [73,74]. Disruption of the LHb may impact drug-seeking behaviour via its dopaminergic connections. Injection of a D3 receptor antagonist into the LHb showed a dose-dependent reduction in cue-induced reinstatement of nicotine-seeking behaviours in rats [75]. LHb involvement in drug seeking appears to be complex, with lesion and deep brain stimulation studies showing differing effects during acquisition/maintenance and extinction. These differences may be attributable to when lesions are applied, and on frequencies used during deep brain stimulation. Nevertheless, both deep brain stimulation and lesion studies suggest that interrupting LHb function reduced pharmacologically induced reinstatement of drug-seeking behaviours.
(c). Disruption of the mediodorsal nucleus attenuates expression of drug-seeking behaviours
Rats with lesions to the MD nucleus, compared with sham lesions, showed attenuation of cocaine self-administration behaviour, expressed as less frequent responding, and self-administration of lower doses of cocaine after reaching maintenance levels [76]. Furthermore, in rats already trained to prefer drug-associated locations, reversible inactivation of the MD nucleus (with lidocaine), which allows temporally sensitive disruption of neurons, prior to testing (but not before or right after training) disrupted CPP for a methamphetamine-paired location, although CPP recovered after 24 h [77]. This finding suggests that the MD nucleus may be preferentially involved in the expression of habitual drug-taking behaviours, which may translate to the compulsive drug intake as seen during bingeing in humans.
(d). Widespread thalamic involvement during drug-seeking behaviour in primates
Compared with rodents, primates have a more complex cortex, including PFC, with more structurally and functionally distinct subregions [78]. As the thalamus has extensive, reciprocal connections with the cortex, there are likely differences in how the thalamus responds to drug self-administration between rodents and primates. To date, relatively few studies have examined thalamic effects of drug self-administration in primates, although studies examining glucose utilization (measured with 2-[14C]deoxyglucose) find widespread thalamic activation, as has been suggested by rodent studies that examined c-Fos expression throughout the thalamus [48,49]. In rhesus monkeys trained to self-administer cocaine, exposure to cocaine cues after the acquisition of drug-seeking behaviours was associated with higher metabolism in multiple regions of the thalamus, including the ventral anterior thalamus, MD thalamus, midline thalamic regions, parafascicular/centromedian nuclei and habenula [79]. Nevertheless, it is clear that this relationship is not linear as suggested by studies using exposure to cocaine cues where 30-day abstinence was associated with higher metabolism in one study [80] and decreased metabolism in another study that also showed decreased metabolism after exposure to cocaine cues after a more protracted 90-day abstinence [81].
4. Thalamus in human drug addiction
Both rodent and non-human primate literature suggests that the thalamus, as a structure within the reward circuit, has an important role in drug self-administration behaviours. Indeed, animal studies investigating thalamic involvement in reinstatement of drug-seeking behaviours showed increased thalamic activation with reinstatement, which may translate to increased response to salient drug cues in the human literature. The more widespread involvement of the thalamus seen in non-human primates is likely to extend to the human neuroimaging literature, although these studies could be confounded by the comparatively lower spatial resolution used to investigate brain structure and function in humans. In addition, animal studies typically focus on a single region within the thalamus (e.g. the PVT), while human imaging studies more often use whole brain analyses. While focusing on a single region of interest (e.g. one thalamic nuclei) (known as region of interest (ROI) analyses in neuroimaging studies) allows for the discovery of more subtle effects, and are often necessary for the investigations conducted in animal research (e.g. lesion studies), investigating whole brain changes allows for the discovery of systems level effects, such as investigating brain regions that co-activate, or are correlated, with the thalamus. The human neuroimaging literature also allows us to better investigate the role of the thalamus in specific cognitive processes underlying clinical symptoms observed in drug addiction.
(a). Structural and functional integrity of the thalamus in addiction
Neuroimaging methods allows us to measure both the structural and functional integrity of the whole brain, providing important insight into the effects of drug addiction on different brain regions at once (without the need to preselect and exclusively focus on a priori regions of interest). Grey matter volume measures the amount of grey matter between the pia mater and grey–white matter interface of the brain and is an in vivo measure of grey matter structural integrity [82]. Diffusion tensor imaging captures structural connectivity in vivo using water diffusion to measure white matter tracts (composed of axonal projections) that connect brain regions to one another [83]. Resting state connectivity measures the integrity of functional networks at rest and uses co-activation between brain regions as an indicator of shared neuronal activity [84]. Finally, task-related functional neuroimaging measures brain responses to behavioural manipulations. While disagreements between structural and functional neuroimaging methods exist [85,86], structural and functional neuroimaging results are associated [87–91].
Despite some negative findings [92–95], lower thalamic grey matter volumes in drug-dependent individuals, across drugs of abuse including alcohol, cocaine, nicotine, methamphetamine, opioids, cannabis and synthetic cannabinoids [95–107], have been reported. Such decreased grey matter volume in the thalamus (as well as the amygdala and cortical regions including the PFC) is associated, for example, with increased craving for methamphetamine [102], more lifetime tobacco use [108], more years of substance use in polysubstance users [109], and shorter abstinence length in alcohol users [110]. A recent meta-analysis supported the conclusion that chronic cigarette smoking was associated with grey matter volume decreases in the thalamus (as well as the insula, cerebellum, parahippocampal gyrus and multiple PFC regions) [111].
When examined using diffusion tensor imaging, reduced white matter integrity (as measured by fractional anisotropy where greater water diffusion within white matter bundles suggests lower white matter integrity) in tracts through, and around, the thalamus have been reported in addiction to alcohol, cocaine, methamphetamine, opioids and cannabis [112–117]. A recent systematic review of white matter microstructure in adolescent substance users reported that out of 10 studies, five showed reduced fractional anisotropy in white matter projections from the thalamus [118]. Of these, two studies found lower fractional anisotropy with higher alcohol use. A recent longitudinal study showed that cannabis using adolescents had a less positive fractional anisotropy change at a 2-year follow-up in anterior thalamic radiations when compared with non-cannabis using controls [115]. These studies indicate that substance use during adolescence is likely to influence (and possibly predate abnormalities in) white matter development, which may lead to functional changes in thalamo-cortical circuits associated with an enhanced predisposition to transition from substance use and abuse to drug addiction in these vulnerable individuals.
Drug addiction is also associated with local functional changes within the thalamus at rest. Along with changes in local metabolism and cerebral blood flow, local thalamus activation during rest can be measured with magnetic resonance imaging using fractional amplitude of low-frequency fluctuations (fALFF), which has been suggested as a measure related to spontaneous neuronal activity [119], and regional homogeneity, which is a measure of the coherence of local spontaneous fluctuations [120]. Using a more objective measure of neural activity, the thalamus showed lower glucose metabolism (as measured by [18F]deoxyglucose positron emission tomography (PET)) in individuals with both past and current opiate dependence undergoing methadone maintenance treatment compared with healthy individuals, with the lowest metabolism observed in the currently dependent individuals [121]. Compared with healthy individuals, cannabis-dependent individuals showed lower increases in thalamus glucose metabolism (measured with [18F]deoxyglucose PET) after acute methylphenidate administration [122]. In addition, acute nicotine administration in short-term abstinent cigarette smokers decreased regional cerebral blood flow (measured with [11C]H2O PET) within the thalamus compared with placebo [123]. But, acute administration of lorazepam (a benzodiazepine), which has a calming, rather than stimulating effect increased glucose metabolism (measured with [18F]deoxyglucose PET) [124]. Examining thalamus function with magnetic resonance imaging techniques showed that in short-term abstinent cigarette smokers, increased thalamic regional cerebral blood flow was associated with increased subjective nicotine craving in short-term abstinent cigarette smokers (measured with arterial spin labelling) [125]. In studies examining thalamus function during resting state, cocaine-dependent individuals showed increased thalamus function compared with healthy individuals, measured as fALFF [100]. Heroin administration in treatment-seeking heroin-dependent individuals also increased fALFF and regional homogeneity in the thalamus compared with placebo [126]. By contrast, heroin-dependent individuals on methadone maintenance treatment showed decreased regional homogeneity within the thalamus compared with healthy individuals [127]. Objective measures of thalamus metabolism (measured with PET) suggest decreased thalamus activation in addicted individuals that can be further decreased with acute administration of stimulants. In comparison, investigations using magnetic resonance imaging methods found increased thalamus function in addicted individuals, suggesting that associations between the magnetic resonance imaging measures and metabolic function within the thalamus require further investigation.
Thalamic resting state connectivity is also altered in drug-addicted individuals [128]. Compared with healthy individuals, alcohol-dependent individuals showed decreased connectivity between seeds in the NAc and subgenual anterior cingulate cortex, and the thalamus [129]. Cocaine-addicted individuals also showed decreased connectivity between the thalamus and anterior cingulate cortex [130], as well as the midbrain [131]. The latter result was corroborated by a more recent study reporting reduced connectivity between the thalamus and both the putamen and the midbrain's VTA, with the strength of the VTA-thalamus connectivity being negatively associated with years of cocaine use in cocaine-addicted individuals [132]. Cigarette smokers similarly showed reduced connectivity between the thalamus and the anterior cingulate cortex, with findings also showing reduced connectivity with the caudate and the dorsolateral PFC; the strength of the dorsolateral PFC-thalamus connectivity was negatively associated with severity of nicotine dependence [133]. Counterintuitively, a positive correlation between dorsal anterior cingulate cortex-thalamus connectivity strength with both nicotine dependence and risk taking behaviour in a Balloon Analogue Risk Task was reported in cigarette smokers, though this study used ROI to ROI rather than seed to whole brain connectivity as most other studies did, which may bias the results based on ROI selection [134]. Compared with healthy individuals, ketamine-dependent individuals showed reduced connectivity between the PFC, the motor/supplementary motor area, and posterior parietal regions and the thalamus, with connectivity between the posterior parietal cortex and the lateral dorsal thalamus correlated negatively with ketamine craving [135]. A study found that, compared with healthy individuals, abstinent heroin-dependent individuals showed increased connectivity between the amygdala and the thalamus, though this study used ROI to ROI rather than seed to whole brain analyses [136]. By contrast, acute heroin administration in addicted individuals reduced connectivity between the thalamus and frontal, parietal and temporal cortices compared with placebo [126]. Consistent with the findings of reduced grey and white matter integrity, most of these studies indicate that the thalamus shows reduced resting state functional connectivity with frontal and striatal regions in drug addiction. Furthermore, several of these studies showed that reduced thalamic connectivity was associated with increased craving and other measures of severity of drug dependence.
The thalamus shows both reduced structural grey and white matter integrity, reduced thalamus metabolism and reduced functional connectivity with striatal and frontal regions. This reduction in structural and functional integrity of the thalamus and its connectivity was associated with greater severity of addiction.
(b). The impairments in response inhibition and salience attribution model
At the core of drug addiction in humans is a conditioned response to stimuli associated with drugs that develop in habitual users along with an inability to inhibit these responses, despite adverse consequences and the decrease in the pleasure that is derived from the drug. These processes and their association with the clinical symptoms of drug addiction are encompassed by the iRISA model [1,2]. The iRISA model proposes that in drug addiction, attribution of primary salience to drug cues occurs at the expense of salience attributed to other reinforcers (such as food or money). The thalamus is a part of the cortico-striato-thalamo-cortical circuits underlying both reward and motivated behaviours [9] and cognitive control processes [137]. Here, we review studies with 15 or more subjects per group that show thalamic response in drug-dependent compared with healthy individuals to salient drug cues and non-drug reward, and during tasks requiring response inhibition. In addition, we review studies that did not include a healthy control group if they examine associations between task-related thalamus activation and severity of addiction, relapse, abstinence or stress. Figure 3 and table 1 show the distribution of peaks of activation in the thalamus from imaging studies comparing addicted and healthy individuals using drug cue exposure, non-drug reward and response inhibition paradigms in human addiction.
Figure 3.
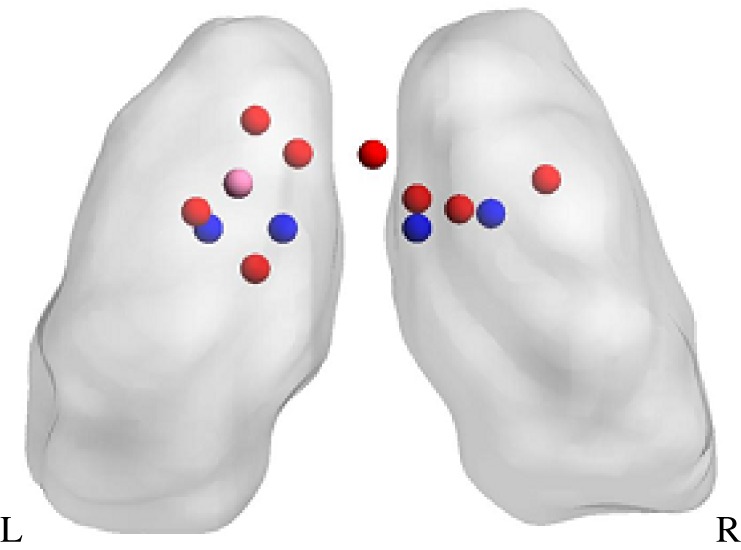
Peak thalamic coordinates from fMRI and PET studies using drug cue (red) and non-drug reward (pink) paradigms and response inhibition tasks (blue). See table 1 for list of papers included.
Table 1.
Table showing coordinates of peak activation for thalamic clusters in papers finding thalamic activation in addicted compared with healthy individuals in drug cue, non-drug reward and response inhibition tasks. Only studies reporting thalamus coordinates in MNI or Talairach space significant at cluster level (converted to MNI for presentation purposes) and located within the thalamus were included. Abbreviations are as follows: IAPS, international affective picture system; MID, monetary incentive delay; dwStroop, drug word stroop task; cwStroop, colour word stroop task; MNI, Montreal Neurological Institute coordinate system.
| MNI |
||||||
|---|---|---|---|---|---|---|
| paper | addiction | paradigm | x | y | z | thalamus activation |
| drug cues | ||||||
| Li et al. [138] | heroin | cue reactivity | −3 | −9 | 3 | ↑ |
| 3 | −12 | 3 | ↑ | |||
| Wang et al. [139] | heroin | cue reactivity | 0 | −9 | 0 | ↑ |
| Filbey et al. [140] | cannabis | cue reactivity | 5.87 | −12.7 | 6.71 | ↑ |
| 11.74 | −10.7 | 6.84 | ↑ | |||
| −5.85 | −16.69 | 6.45 | ↑ | |||
| −5.84 | −6.78 | 4.77 | ↑ | |||
| −9.81 | −12.89 | 10.51 | ↑ | |||
| non-drug reward | ||||||
| Asensio et al. [141] | cocaine | IAPS | −7 | −11 | 19 | ↓ |
| inhibition | ||||||
| Tomasi et al. [130] | cocaine | dwStroop | −4 | −14 | 7 | ↓ |
| 3 | −14 | 7 | ↓ | |||
| Mitchell et al. [142] | cocaine | cwStroop | 8 | −13 | 7 | ↓ |
| −9 | −14 | 8 | ↓ | |||
(c). Salience attribution
Although negative results have been reported (in a study that included both moderate and heavy smokers) [143], in comparison to healthy individuals, increased drug-cue-related thalamic activation is commonly reported (in conjunction with activation in both ventral and dorsal striatum, as well as the PFC) in drug-addicted individuals encompassing chronic cocaine smokers [144], treatment-seeking heroin-dependent individuals [138], methadone-treated heroin-dependent individuals [139] and regular cannabis users [140] (see electronic supplementary material, table S2).
Stress may enhance the salience of drug cues in addicted individuals. In studies examining only addicted individuals, cigarette smokers showed greater MD thalamus activation and greater anti-correlation between ventral striatum and the thalamus to drug compared with neutral cues after being exposed to psychosocial stress induced by a difficult mental arithmetic task [145]. Along with acutely induced stress, studies find that individual differences in lifetime stressors also result in greater thalamic responding to drug cues. Compared with addicted individuals without a history of abuse, cocaine-addicted individuals with a history of physical, sexual or emotional abuse showed greater MD thalamus activation to drug compared with neutral cues [146]. Alcohol-dependent individuals showed a positive association between thalamic activation and drug cues with both the Beck Depression Inventory and Beck Anxiety Inventory scores [147], suggesting that individual differences in depression and anxiety enhance thalamic drug cue reactivity in susceptible individuals.
Abstinence may also influence thalamic responding to drug cues in addicted individuals. The thalamus showed greater activation in short-term abstinent compared with satiated cigarette smokers [148]. In regular cannabis users, the thalamus showed an association between greater activation and higher cue-induced craving [140]. The thalamus showed different effects depending on the length of abstinence, with heroin-addicted individuals having greater drug-related thalamic activation after short-term abstinence (mean one month) but lower activation after a longer abstinence period (mean 13 months) [149], supporting the notion of cue-induced incubation of craving [150–153]. Thus, long-term abstinence may allow normalization of thalamic response to drug cues, possibly associated with a decrease in the salience attributed to drug cues in addicted individuals.
Contrary to expectations, few neuroimaging studies find significant thalamus activation differences between addicted and healthy individuals. One study found that cocaine-dependent individuals showed reduced thalamic activation to pleasant versus neutral cues compared with healthy individuals [141]. Two other studies have found associations between thalamus activation and treatment effects, though neither of these studies found group differences between addicted and healthy individuals at baseline. Treatment-seeking cocaine-dependent individuals showed increased thalamic activation to the anticipation of a monetary reward 1 year after the initiation of cognitive behavioural therapy treatment compared with baseline [154], indicating that there is enhancement of thalamic responses to non-drug rewards with long-term abstinence. Contrary to iRISA model predictions, in treatment-seeking individuals with cocaine dependence, lower thalamic response to the anticipation and delivery of a monetary reward at the onset of treatment was associated with greater abstinence [155].
In summary, thalamic responses to drug cues were consistent with the iRISA model, with drug-dependent individuals showing greater activation to salient drug cues compared with healthy individuals. However, evidence of how thalamic activation to non-drug rewards differs in drug addiction is sparse, with all studies focusing on cocaine-dependent populations. Short-term abstinence and stress may increase drug-related thalamus response, while long-term abstinence appeared to normalize thalamic response to drug cues, and possibly non-drug rewards.
(d). Response inhibition
The thalamus has a role in mediating cognitive control through its connectivity with the PFC [156], suggesting that it may be involved in the abnormalities seen in response inhibition as predicted by the iRISA model. Cognitive control, or response inhibition, refers to the ability to stop behaviour that has become habitual or to stop one's behaviour after initiation. Tasks commonly used to measure response inhibition are the Stroop task, the go/no-go task and the stop signal task (SST). In the Stroop task, conflict is created between an automatic/habitual response (e.g. reading colour words) and a slower response (e.g. naming of the colour in which the colour words are printed), with both competing for the same processing resources; the common comparison is between responses and incongruent (e.g. the word blue printed in red colour) versus congruent words (e.g. the word blue printed in blue colour) (for both accuracy and reaction time). In the go/no-go task, participants respond rapidly to a frequent ‘go’ signal and withhold responses to a less frequent ‘no-go’ signal; accurate no-go signals reflect an individual's ability to exert inhibitory control. The SST measures the ability to stop a response that has already been initiated, with successful stopping and the stop signal reaction time (SSRT) both measuring response inhibition. Stop signal anticipation (p[stop]) is a measure of the likelihood of a stop coming up, and can be estimated based on Bayesian modelling of stop trial probabilities.
Compared with studies of non-drug reward, more studies have examined thalamic responses during response inhibition tasks. One study found that, compared with healthy individuals, cocaine-dependent individuals showed lower Stroop-related thalamus (as well as the striatum and PFC) activation to the incongruency effect on a colour word Stroop task [157]. Other studies examined overall task activation during drug and colour word Stroop tasks and found lower thalamus (along with PFC) activation, as well as lower connectivity between the midbrain and the thalamus [130], and lower intrinsic connectivity (a measure of connectivity between each voxel and every other voxel in the brain) in the thalamus (along with the dorsal striatum and PFC) [142]. In alcohol-dependent individuals only, the thalamus showed increased activation during successful response inhibition after acute alcohol administration [158]. Acute modafinil (a wakefulness-promoting drug) administration in alcohol-dependent individuals resulted in greater increases in thalamic activation in poor performing individuals (with lower SSRT) on the SST [159]. These studies suggest that, in conjunction with striatal and PFC regions, there is decreased thalamus activation during response inhibition tasks in drug-addicted individuals, and that acute drug administration increased this activation.
Connectivity between the thalamus and PFC regions is altered during tasks requiring response inhibition. Cocaine-dependent individuals showed greater thalamic connectivity with the ventromedial PFC compared with healthy individuals during failed response inhibition, and this increased connectivity was associated with inferior response inhibition (longer SSRT) [160]. In a follow-up study by the same group, a larger sample of cocaine-dependent compared with healthy individuals showed increased frontoparietal network connectivity with the MD thalamus and decreased functional connectivity with the ventrolateral thalamus while performing the SST, with the former associated with inferior response inhibition (longer SSRT) in the cocaine-dependent, but not healthy, individuals [161]. These studies suggest that the connectivity between the thalamus and cortex is disrupted in addiction and that this difference in connectivity is linked to behavioural manifestations of response inhibition.
Several lines of evidence suggest that response inhibition-related activations within the thalamus are associated with severity of substance abuse. Greater thalamus connectivity with the midbrain during a drug word Stroop task was associated with fewer years of cocaine use in cocaine-dependent individuals [130], and greater thalamic activation to successful response inhibition on the SST was associated with fewer years of dependence in alcohol-dependent individuals [162]. By contrast, in alcohol-dependent individuals, more thalamic activation to stop signal anticipation (greater p[stop]) was associated with greater alcohol consumption in the preceding month (which is a more acute measure of alcohol consumption), though this study did not find group differences to stop signal anticipation in the thalamus [163]. One study found that thalamic activation to prediction error on the SST contributed to a model predicting individuals who progressed from occasional stimulant use to problematic stimulant use 3 years later [164]. These studies suggest that lower thalamic activation during response inhibition is associated with more severe drug dependence.
Thalamic activation to inhibitory processes has also been shown to be associated with relapse and abstinence. In a prospective study examining only treatment-seeking cocaine-dependent individuals, lower thalamic response to failed response inhibition during the SST predicted an earlier time to relapse [165]. By contrast, decreased intrinsic connectivity within the thalamus (in a colour word Stroop task) prior to treatment initiation was associated with greater abstinence during treatment, though in this study, they examined the overall task effect rather than specifically the inhibition effect [142]. Prolonged abstinence has also been shown to alter thalamus activation during response inhibition tasks. The thalamus showed lower activation to failed response inhibition during a go/no-go task in former compared with current cigarette smokers [166], and during failed response inhibition in the SST in long-term compared to short-term abstinent cocaine-dependent individuals [167]. These studies suggest that thalamus activation during response inhibition processes both predicts relapse, and is changed with long-term abstinence, though how the thalamus might mediate these processes requires further investigation.
In summary, as predicted by the iRISA model, the thalamus showed lower activation during response inhibition tasks in addicted individuals, with decreased thalamic activation during inhibitory processes associated with more severe addiction. Thalamus activation during response inhibition both predict relapse and differ by length of abstinence.
5. Conclusion
Studies in non-human animals showed that different thalamic nuclei may be involved in different aspects of drug addiction. There is evidence to suggest that the PVT and LHb are involved in reinstatement of drug-seeking behaviours, which may mimic increased salience of drug cues in abstinent humans. The MD nucleus may be preferentially involved in the expression of drug-seeking behaviours in animals conditioned to self-administer drugs, further indicating that the thalamus mediates the association between drug cues (or context) and drug-seeking behaviours. Interestingly, the LHb shows some evidence of mediating inhibition of drug-seeking behaviours in contexts that predict lack of drug availability, supporting its role in the behavioural inhibition aspect of drug addiction.
In the human neuroimaging literature, the thalamus showed a baseline reduction in structural grey matter volume and white matter integrity coupled with a reduction in baseline thalamus metabolism and functional connectivity at rest in addicted individuals. However, despite this baseline reduction in activation, thalamus hyperactivity was observed when exposed to drug cues, indicating that the thalamic response to salient drug cues overcame a general reduction in thalamic activation. This finding is predicted by the iRISA model of addiction, which posits that the salience of drug cues to addicted individuals is greatly heightened. Also consistent with the iRISA model, the thalamus showed reduced activation during response inhibition. In addition, thalamic response to drug cues and response inhibition were associated with the severity of drug addiction and showed reduced activation to failed response inhibition after long-term abstinence, suggesting that this change is behaviourally relevant. In addition, the human neuroimaging literature showed that the thalamus had similar responses to those observed in the striatum and PFC to salient drug cues and response inhibition in human drug addiction. The iRISA model also predicts that in drug-addicted individuals, salience of non-drug rewards is reduced. However, few studies found thalamus activation differences between addicted and non-addicted individuals, suggesting that the involvement of the thalamus in non-drug reward processes requires further investigation.
Animal studies could broaden their field of focus by studying the role of different thalamic nuclei in the different phases of the addiction cycle. Future studies in humans should seek to better delineate the roles of different thalamic regions in the cognitive processes underlying iRISA through the application of advanced imaging protocols at higher magnetic fields (i.e. 7 Tesla). Both approaches may allow more direct across-species comparisons.
This review highlights the importance of more investigation into the role of the thalamus, as an integral structure within the cortico-striato-thalamo-cortical loops that are central to drug addiction. Furthermore, both animal and human research suggest that the thalamus may be involved in enhanced salience of drug-associated cues in addicted individuals (though based on current animal research, this association can only be inferred from the involvement of the thalamus in reinstatement behaviours).
Supplementary Material
Supplementary Material
Acknowledgements
We thank Anna Zilverstrand for her helpful suggestions and insightful comments on the manuscript. We are grateful to the Royal Society for their support of the costs of attending the meeting ‘Of mice and mental health: facilitating dialogue between basic and clinical neuroscientists' convened by Amy Milton and Emily A. Holmes.
Data accessibility
This article has no additional data.
Authors' contributions
N.A.-K., R.Z.G., J.A.M. and S.N.H. contributed ideas and conceptualization, A.S.H. wrote the manuscript.
Competing interests
We declare we have no competing interests.
Funding
This research was funded by NIH grant R21DA040046.
References
- 1.Goldstein RZ, Volkow ND. 2002. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am. J. Psychiatry 159, 1642–1652. ( 10.1176/appi.ajp.159.10.1642) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goldstein RZ, Volkow ND. 2011. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat. Rev. Neurosci. 12, 652–669. ( 10.1038/nrn3119) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haber SN, Calzavara R. 2009. The cortico-basal ganglia integrative network: the role of the thalamus. Brain Res. Bull. 78, 69–74. ( 10.1016/j.brainresbull.2008.09.013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parnaudeau S, Taylor K, Bolkan SS, Ward RD, Balsam PD, Kellendonk C. 2015. Mediodorsal thalamus hypofunction impairs flexible goal-directed behavior. Biol. Psychiatry 77, 445–453. ( 10.1016/j.biopsych.2014.03.020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Bourbon-Teles J, Bentley P, Koshino S, Shah K, Dutta A, Malhotra P, Egner T, Husain M, Soto D. 2014. Thalamic control of human attention driven by memory and learning. Curr. Biol. 24, 993–999. ( 10.1016/j.cub.2014.03.024) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Phillips JM, Kambi NA, Saalmann YB. 2016. A subcortical pathway for rapid, goal-driven, attentional filtering. Trends Neurosci. 39, 49–51. ( 10.1016/j.tins.2015.12.003) [DOI] [PubMed] [Google Scholar]
- 7.Saalmann YB. 2014. Intralaminar and medial thalamic influence on cortical synchrony, information transmission and cognition. Front. Syst. Neurosci. 8, 83 ( 10.3389/fnsys.2014.00083) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Volkow ND, Fowler JS. 2000. Addiction, a disease of compulsion and drive: involvement of the orbitofrontal cortex. Cereb. Cortex 10, 318–325. ( 10.1093/cercor/10.3.318) [DOI] [PubMed] [Google Scholar]
- 9.Haber SN, Knutson B. 2010. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology 35, 4–26. ( 10.1038/npp.2009.129) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koob GF, Volkow ND. 2010. Neurocircuitry of addiction. Neuropsychopharmacology 35, 217–238. ( 10.1038/npp.2009.110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Volkow ND, Wang G-J, Fowler JS, Tomasi D. 2012. Addiction circuitry in the human brain. Annu. Rev. Pharmacol. Toxicol. 52, 321–336. ( 10.1146/annurev-pharmtox-010611-134625) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haber S, McFarland NR. 2001. The place of the thalamus in frontal cortical-basal ganglia circuits. Neuroscientist 7, 315–324. ( 10.1177/107385840100700408) [DOI] [PubMed] [Google Scholar]
- 13.Berendse HW, Groenewegen HJ. 1990. Organization of the thalamostriatal projections in the rat, with special emphasis on the ventral striatum. J. Comp. Neurol. 299, 187–228. ( 10.1002/cne.902990206) [DOI] [PubMed] [Google Scholar]
- 14.Fenelon G, Francois C, Percheron G, Yelnik J. 1991. Topographic distribution of the neurons of the central complex (centre median-parafascicular complex) and of other thalamic neurons projecting to the striatum in macaques. Neuroscience 45, 495–510. ( 10.1016/0306-4522(91)90244-I) [DOI] [PubMed] [Google Scholar]
- 15.Francois C, Percheron G, Parent A, Sadikot AF, Fenelon G, Yelnik J. 1991. Topography of the projection from the central complex of the thalamus to the sensorimotor striatal territory in monkeys. J. Comp. Neurol. 305, 17–34. ( 10.1002/cne.903050104) [DOI] [PubMed] [Google Scholar]
- 16.Sadikot AF, Parent A, François C. 1992. Efferent connections of the centromedian and parafascicular thalamic nuclei in the squirrel monkey: a PHA-L study of subcortical projections. J. Comp. Neurol. 315, 137–159. ( 10.1002/cne.903150203) [DOI] [PubMed] [Google Scholar]
- 17.Giménez-Amaya JM, McFarland NR, de las Heras S, Haber SN. 1995. Organization of thalamic projections to the ventral striatum in the primate. J. Comp. Neurol. 354, 127–149. ( 10.1002/cne.903540109) [DOI] [PubMed] [Google Scholar]
- 18.Sidibé M, Paré J-F, Smith Y. 2002. Nigral and pallidal inputs to functionally segregated thalamostriatal neurons in the centromedian/parafascicular intralaminar nuclear complex in monkey. J. Comp. Neurol. 447, 286–299. ( 10.1002/cne.10247) [DOI] [PubMed] [Google Scholar]
- 19.Alexander GE, DeLong MR, Strick PL. 1986. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu. Rev. Neurosci. 9, 357–381. ( 10.1146/annurev.ne.09.030186.002041) [DOI] [PubMed] [Google Scholar]
- 20.Middleton FA, Strick PL. 2000. Basal ganglia output and cognition: evidence from anatomical, behavioral, and clinical studies. Brain Cogn. 42, 183–200. ( 10.1006/brcg.1999.1099) [DOI] [PubMed] [Google Scholar]
- 21.Frank MJ, Loughry B, O'Reilly RC. 2001. Interactions between frontal cortex and basal ganglia in working memory: a computational model. Cogn. Affect. Behav. Neurosci. 1, 137–160. ( 10.3758/CABN.1.2.137) [DOI] [PubMed] [Google Scholar]
- 22.Haber SN. 2003. The primate basal ganglia: parallel and integrative networks. J. Chem. Neuroanat. 26, 317–330. ( 10.1016/j.jchemneu.2003.10.003) [DOI] [PubMed] [Google Scholar]
- 23.Moga MM, Weis RP, Moore RY. 1995. Efferent projections of the paraventricular thalamic nucleus in the rat. J. Comp. Neurol. 359, 221–238. ( 10.1002/cne.903590204) [DOI] [PubMed] [Google Scholar]
- 24.Wise RA. 1996. Neurobiology of addiction. Curr. Opin. Neurobiol. 6, 243–251. ( 10.1016/S0959-4388(96)80079-1) [DOI] [PubMed] [Google Scholar]
- 25.Everitt BJ, Dickinson A, Robbins TW. 2001. The neuropsychological basis of addictive behaviour. Brain Res. Brain Res. Rev. 36, 129–138. ( 10.1016/S0165-0173(01)00088-1) [DOI] [PubMed] [Google Scholar]
- 26.Li S, Kirouac GJ. 2008. Projections from the paraventricular nucleus of the thalamus to the forebrain, with special emphasis on the extended amygdala. J. Comp. Neurol. 506, 263–287. ( 10.1002/cne.21502) [DOI] [PubMed] [Google Scholar]
- 27.Vertes RP, Hoover WB. 2008. Projections of the paraventricular and paratenial nuclei of the dorsal midline thalamus in the rat. J. Comp. Neurol. 508, 212–237. ( 10.1002/cne.21679) [DOI] [PubMed] [Google Scholar]
- 28.Phillipson OT, Pycock CJ. 1982. Dopamine neurones of the ventral tegmentum project to both medial and lateral habenula. Some implications for habenular function. Exp. Brain Res. 45, 89–94. [DOI] [PubMed] [Google Scholar]
- 29.Araki M, McGeer PL, Kimura H. 1988. The efferent projections of the rat lateral habenular nucleus revealed by the PHA-L anterograde tracing method. Brain Res. 441, 319–330. ( 10.1016/0006-8993(88)91410-2) [DOI] [PubMed] [Google Scholar]
- 30.Gruber C, Kahl A, Lebenheim L, Kowski A, Dittgen A, Veh RW. 2007. Dopaminergic projections from the VTA substantially contribute to the mesohabenular pathway in the rat. Neurosci. Lett. 427, 165–170. ( 10.1016/j.neulet.2007.09.016) [DOI] [PubMed] [Google Scholar]
- 31.Herkenham M, Nauta WJ. 1979. Efferent connections of the habenular nuclei in the rat. J. Comp. Neurol. 187, 19–47. ( 10.1002/cne.901870103) [DOI] [PubMed] [Google Scholar]
- 32.Giguere M, Goldman-Rakic PS. 1988. Mediodorsal nucleus: areal, laminar, and tangential distribution of afferents and efferents in the frontal lobe of rhesus monkeys. J. Comp. Neurol. 277, 195–213. ( 10.1002/cne.902770204) [DOI] [PubMed] [Google Scholar]
- 33.Sherman SM, Guillery RW. 1996. Functional organization of thalamocortical relays. J. Neurophysiol. 76, 1367–1395. [DOI] [PubMed] [Google Scholar]
- 34.McFarland NR, Haber SN. 2002. Thalamic relay nuclei of the basal ganglia form both reciprocal and nonreciprocal cortical connections, linking multiple frontal cortical areas. J. Neurosci. 22, 8117–8132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Erickson SL, Lewis DA. 2004. Cortical connections of the lateral mediodorsal thalamus in cynomolgus monkeys. J. Comp. Neurol. 473, 107–127. ( 10.1002/cne.20084) [DOI] [PubMed] [Google Scholar]
- 36.Zikopoulos B, Barbas H.. 2007. Parallel driving and modulatory pathways link the prefrontal cortex and thalamus. PLoS ONE 2, e848 ( 10.1371/journal.pone.0000848) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schell GR, Strick PL. 1984. The origin of thalamic inputs to the arcuate premotor and supplementary motor areas. J. Neurosci. 4, 539–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wiesendanger R, Wiesendanger M. 1985. The thalamic connections with medial area 6 (supplementary motor cortex) in the monkey (macaca fascicularis). Exp. Brain Res. 59, 91–104. [DOI] [PubMed] [Google Scholar]
- 39.Goldman-Rakic PS, Porrino LJ. 1985. The primate mediodorsal (MD) nucleus and its projection to the frontal lobe. J. Comp. Neurol. 242, 535–560. ( 10.1002/cne.902420406) [DOI] [PubMed] [Google Scholar]
- 40.Matelli M, Luppino G. 1996. Thalamic input to mesial and superior area 6 in the macaque monkey. J. Comp. Neurol. 372, 59–87. ( 10.1002/(SICI)1096-9861(19960812)372:1%3C59::AID-CNE6%3E3.0.CO;2-L) [DOI] [PubMed] [Google Scholar]
- 41.Corbit LH, Muir JL, Balleine BW. 2003. Lesions of mediodorsal thalamus and anterior thalamic nuclei produce dissociable effects on instrumental conditioning in rats. Eur. J. Neurosci. 18, 1286–1294. ( 10.1046/j.1460-9568.2003.02833.x) [DOI] [PubMed] [Google Scholar]
- 42.Mitchell AS, Browning PGF, Baxter MG. 2007. Neurotoxic lesions of the medial mediodorsal nucleus of the thalamus disrupt reinforcer devaluation effects in rhesus monkeys. J. Neurosci. 27, 11 289–11 295. ( 10.1523/JNEUROSCI.1914-07.2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chakraborty S, Kolling N, Walton ME, Mitchell AS. 2016. Critical role for the mediodorsal thalamus in permitting rapid reward-guided updating in stochastic reward environments. Elife 5, e13588 ( 10.7554/eLife.13588) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alcaraz F, Naneix F, Desfosses E, Marchand AR, Wolff M, Coutureau E. 2016. Dissociable effects of anterior and mediodorsal thalamic lesions on spatial goal-directed behavior. Brain Struct. Funct. 221, 79–89. ( 10.1007/s00429-014-0893-7) [DOI] [PubMed] [Google Scholar]
- 45.Ray JP, Price JL. 1992. The organization of the thalamocortical connections of the mediodorsal thalamic nucleus in the rat, related to the ventral forebrain-prefrontal cortex topography. J. Comp. Neurol. 323, 167–197. ( 10.1002/cne.903230204) [DOI] [PubMed] [Google Scholar]
- 46.Ray JP, Price JL. 1993. The organization of projections from the mediodorsal nucleus of the thalamus to orbital and medial prefrontal cortex in macaque monkeys. J. Comp. Neurol. 337, 1–31. ( 10.1002/cne.903370102) [DOI] [PubMed] [Google Scholar]
- 47.Wedzony K, et al. 2003. Different pattern of brain c-Fos expression following re-exposure to ethanol or sucrose self-administration environment. Naunyn. Schmiedebergs. Arch. Pharmacol. 368, 331–341. ( 10.1007/s00210-003-0811-7) [DOI] [PubMed] [Google Scholar]
- 48.Pelloux Y, Hoots JK, Cifani C, Adhikary S, Martin J, Minier-Toribio A, Bossert JM, Shaham Y. 2017. Context-induced relapse to cocaine seeking after punishment-imposed abstinence is associated with activation of cortical and subcortical brain regions. Addict. Biol. ( 10.1111/adb.12527) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.James MH, Charnley JL, Flynn JR, Smith DW, Dayas CV. 2011. Propensity to ‘relapse’ following exposure to cocaine cues is associated with the recruitment of specific thalamic and epithalamic nuclei. Neuroscience 199, 235–242. ( 10.1016/j.neuroscience.2011.09.047) [DOI] [PubMed] [Google Scholar]
- 50.Matzeu A, Cauvi G, Kerr TM, Weiss F, Martin-Fardon R. 2015. The paraventricular nucleus of the thalamus is differentially recruited by stimuli conditioned to the availability of cocaine versus palatable food. Addict. Biol. 22, 70–77. ( 10.1111/adb.12280) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hamlin AS, Clemens KJ, Choi EA, McNally GP. 2009. Paraventricular thalamus mediates context-induced reinstatement (renewal) of extinguished reward seeking. Eur. J. Neurosci. 29, 802–812. ( 10.1111/j.1460-9568.2009.06623.x) [DOI] [PubMed] [Google Scholar]
- 52.James MH, Charnley JL, Jones E, Levi EM, Yeoh JW, Flynn JR, Smith DW, Dayas CV. 2010. Cocaine- and amphetamine-regulated transcript (CART) signaling within the paraventricular thalamus modulates cocaine-seeking behaviour. PLoS ONE 5, e12980 ( 10.1371/journal.pone.0012980) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Browning JR, Jansen HT, Sorg BA. 2014. Inactivation of the paraventricular thalamus abolishes the expression of cocaine conditioned place preference in rats. Drug Alcohol. Depend. 134, 387–390. ( 10.1016/j.drugalcdep.2013.09.021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Matzeu A, Weiss F, Martin-Fardon R. 2015. Transient inactivation of the posterior paraventricular nucleus of the thalamus blocks cocaine-seeking behavior. Neurosci. Lett. 608, 34–39. ( 10.1016/j.neulet.2015.10.016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Choi DL, Davis JF, Magrisso IJ, Fitzgerald ME, Lipton JW, Benoit SC. 2012. Orexin signaling in the paraventricular thalamic nucleus modulates mesolimbic dopamine and hedonic feeding in the rat. Neuroscience 210, 243–248. ( 10.1016/j.neuroscience.2012.02.036) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Labouèbe G, Boutrel B, Tarussio D, Thorens B. 2016. Glucose-responsive neurons of the paraventricular thalamus control sucrose-seeking behavior. Nat. Neurosci. 19, 999–1002. ( 10.1038/nn.4331) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang X, van den Pol AN. 2017. Rapid binge-like eating and body weight gain driven by zona incerta GABA neuron activation. Science 356, 853–859. ( 10.1126/science.aam7100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Matzeu A, Zamora-Martinez ER, Martin-Fardon R. 2014. The paraventricular nucleus of the thalamus is recruited by both natural rewards and drugs of abuse: recent evidence of a pivotal role for orexin/hypocretin signaling in this thalamic nucleus in drug-seeking behavior. Front. Behav. Neurosci. 8, 117 ( 10.3389/fnbeh.2014.00117) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vicentic A, Jones DC. 2007. The CART (cocaine- and amphetamine-regulated transcript) system in appetite and drug addiction. J. Pharmacol. Exp. Ther. 320, 499–506. ( 10.1124/jpet.105.091512) [DOI] [PubMed] [Google Scholar]
- 60.Dayas CV, McGranahan TM, Martin-Fardon R, Weiss F. 2008. Stimuli linked to ethanol availability activate hypothalamic CART and orexin neurons in a reinstatement model of relapse. Biol. Psychiatry 63, 152–157. ( 10.1016/j.biopsych.2007.02.002) [DOI] [PubMed] [Google Scholar]
- 61.Parsons MP, Li S, Kirouac GJ. 2006. The paraventricular nucleus of the thalamus as an interface between the orexin and CART peptides and the shell of the nucleus accumbens. Synapse 59, 480–490. ( 10.1002/syn.20264) [DOI] [PubMed] [Google Scholar]
- 62.Matzeu A, Kerr TM, Weiss F, Martin-Fardon R. 2016. Orexin-A/Hypocretin-1 mediates cocaine-seeking behavior in the posterior paraventricular nucleus of the thalamus via Orexin/Hypocretin receptor-2. J. Pharmacol. Exp. Ther. 359, 273–279. ( 10.1124/jpet.116.235945) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhu Y, Wienecke CFR, Nachtrab G, Chen X. 2016. A thalamic input to the nucleus accumbens mediates opiate dependence. Nature 530, 219–222. ( 10.1038/nature16954) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Neumann PA, et al. 2016. Cocaine-induced synaptic alterations in thalamus to nucleus accumbens projection. Neuropsychopharmacology 41, 2399–2410. ( 10.1038/npp.2016.52) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Grimm JW, Hope BT, Wise RA, Shaham Y. 2001. Neuroadaptation. Incubation of cocaine craving after withdrawal. Nature 412, 141–142. ( 10.1038/35084134) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Velasquez KM, Molfese DL, Salas R. 2014. The role of the habenula in drug addiction. Front. Hum. Neurosci. 8, 174 ( 10.3389/fnhum.2014.00174) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang F, Zhou W, Liu H, Zhu H, Tang S, Lai M, Yang G. 2005. Increased c-Fos expression in the medial part of the lateral habenula during cue-evoked heroin-seeking in rats. Neurosci. Lett. 386, 133–137. ( 10.1016/j.neulet.2005.06.008) [DOI] [PubMed] [Google Scholar]
- 68.Brown RM, Short JL, Lawrence AJ. 2010. Identification of brain nuclei implicated in cocaine-primed reinstatement of conditioned place preference: a behaviour dissociable from sensitization. PLoS ONE 5, e15889 ( 10.1371/journal.pone.0015889) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Haack AK, Sheth C, Schwager AL, Sinclair MS, Tandon S, Taha SA. 2014. Lesions of the lateral habenula increase voluntary ethanol consumption and operant self-administration, block yohimbine-induced reinstatement of ethanol seeking, and attenuate ethanol-induced conditioned taste aversion. PLoS ONE 9, e92701 ( 10.1371/journal.pone.0092701) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang Y, Zhang F, Tang S, Lai M, Hao W, Zhang Y, Yang J, Zhou W. 2009. Lack of effect of habenula lesion on heroin self-administration in rats. Neurosci. Lett. 461, 167–171. ( 10.1016/j.neulet.2009.06.029) [DOI] [PubMed] [Google Scholar]
- 71.Zapata A, Hwang E-K, Lupica CR. 2017. Lateral habenula involvement in impulsive cocaine seeking. Neuropsychopharmacology 42, 1103–1112. ( 10.1038/npp.2016.286) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gill MJ, Ghee SM, Harper SM, See RE. 2013. Inactivation of the lateral habenula reduces anxiogenic behavior and cocaine seeking under conditions of heightened stress. Pharmacol. Biochem. Behav. 111, 24–29. ( 10.1016/j.pbb.2013.08.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Friedman A, et al. 2010. Electrical stimulation of the lateral habenula produces enduring inhibitory effect on cocaine seeking behavior. Neuropharmacology 59, 452–459. ( 10.1016/j.neuropharm.2010.06.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lax E, et al. 2013. Neurodegeneration of lateral habenula efferent fibers after intermittent cocaine administration: implications for deep brain stimulation. Neuropharmacology 75, 246–254. ( 10.1016/j.neuropharm.2013.06.034) [DOI] [PubMed] [Google Scholar]
- 75.Khaled MATM, Pushparaj A, Di Ciano P, Diaz J, Le Foll B. 2014. Dopamine D3 receptors in the basolateral amygdala and the lateral habenula modulate cue-induced reinstatement of nicotine seeking. Neuropsychopharmacology 39, 3049–3058. ( 10.1038/npp.2014.158) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Weissenborn R, Whitelaw RB, Robbins TW, Everitt BJ. 1998. Excitotoxic lesions of the mediodorsal thalamic nucleus attenuate intravenous cocaine self-administration. Psychopharmacology 140, 225–232. ( 10.1007/s002130050761) [DOI] [PubMed] [Google Scholar]
- 77.Kuo C-S, Chai S-C, Chen H-H. 2011. Mediodorsal nucleus of the thalamus is critical for the expression of memory of methamphetamine-produced conditioned place preference in rats. Neuroscience 178, 138–146. ( 10.1016/j.neuroscience.2010.12.021) [DOI] [PubMed] [Google Scholar]
- 78.Öngür D, Price JL. 2000. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb. Cortex 10, 206–219. ( 10.1093/cercor/10.3.206) [DOI] [PubMed] [Google Scholar]
- 79.Porrino LJ, Lyons D, Miller MD, Smith HR, Friedman DP, Daunais JB, Nader MA. 2002. Metabolic mapping of the effects of cocaine during the initial phases of self-administration in the nonhuman primate. J. Neurosci. 22, 7687–7694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Porrino LJ, Beveridge TJR, Smith HR, Nader MA. 2016. Functional consequences of cocaine expectation: findings in a non-human primate model of cocaine self-administration. Addict. Biol. 21, 519–529. ( 10.1111/adb.12231) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Beveridge TJR, Smith HR, Nader SH, Nader MA, Porrino LJ. 2014. Functional consequences of cocaine re-exposure after discontinuation of cocaine availability. Neuropharmacology 85, 528–537. ( 10.1016/j.neuropharm.2014.06.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ashburner J, Friston KJ. 2000. Voxel-based morphometry–the methods. Neuroimage 11, 805–821. ( 10.1006/nimg.2000.0582) [DOI] [PubMed] [Google Scholar]
- 83.Le Bihan D, Mangin JF, Poupon C, Clark CA, Pappata S, Molko N, Chabriat H. 2001. Diffusion tensor imaging: concepts and applications. J. Magn. Reson. Imaging 13, 534–546. ( 10.1002/jmri.1076) [DOI] [PubMed] [Google Scholar]
- 84.Shmuel A, Leopold DA. 2008. Neuronal correlates of spontaneous fluctuations in fMRI signals in monkey visual cortex: Implications for functional connectivity at rest. Hum. Brain Mapp. 29, 751–761. ( 10.1002/hbm.20580) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Honey CJ, Sporns O, Cammoun L, Gigandet X, Thiran JP, Meuli R, Hagmann P. 2009. Predicting human resting-state functional connectivity from structural connectivity. Proc. Natl Acad. Sci. USA 106, 2035–2040. ( 10.1073/pnas.0811168106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Damoiseaux JS, Greicius MD. 2009. Greater than the sum of its parts: a review of studies combining structural connectivity and resting-state functional connectivity. Brain Struct. Funct. 213, 525–533. ( 10.1007/s00429-009-0208-6) [DOI] [PubMed] [Google Scholar]
- 87.Hagmann P, Cammoun L, Gigandet X, Meuli R, Honey CJ, Wedeen VJ, Sporns O. 2008. Mapping the structural core of human cerebral cortex. PLoS Biol. 6, e159 ( 10.1371/journal.pbio.0060159) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Skudlarski P, Jagannathan K, Calhoun VD, Hampson M, Skudlarska BA, Pearlson G. 2008. Measuring brain connectivity: diffusion tensor imaging validates resting state temporal correlations. Neuroimage 43, 554–561. ( 10.1016/j.neuroimage.2008.07.063) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hermundstad AM, et al. 2013. Structural foundations of resting-state and task-based functional connectivity in the human brain. Proc. Natl Acad. Sci. USA 110, 6169–6174. ( 10.1073/pnas.1219562110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shen K, Hutchison RM, Bezgin G, Everling S, McIntosh AR. 2015. Network structure shapes spontaneous functional connectivity dynamics. J. Neurosci. 35, 5579–5588. ( 10.1523/JNEUROSCI.4903-14.2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mišić B, Betzel RF, de Reus MA, van den Heuvel MP, Berman MG, McIntosh AR, Sporns O. 2016. Network-level structure-function relationships in human neocortex. Cereb. Cortex 26, 3285–3296. ( 10.1093/cercor/bhw089) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Segobin SH, Chételat G, Le Berre A-P, Lannuzel C, Boudehent C, Vabret F, Eustache F, Beaunieux H, Pitel A-L. 2014. Relationship between brain volumetric changes and interim drinking at six months in alcohol-dependent patients. Alcohol. Clin. Exp. Res. 38, 739–748. ( 10.1111/acer.12300) [DOI] [PubMed] [Google Scholar]
- 93.Koenders L, et al. 2016. Grey matter changes associated with heavy cannabis use: a longitudinal sMRI study. PLoS ONE 11, e0152482 ( 10.1371/journal.pone.0152482) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cardenas VA, Durazzo TC, Gazdzinski S, Mon A, Studholme C, Meyerhoff DJ. 2011. Brain morphology at entry into treatment for alcohol dependence is related to relapse propensity. Biol. Psychiatry 70, 561–567. ( 10.1016/j.biopsych.2011.04.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chanraud S, Martelli C, Delain F, Kostogianni N, Douaud G, Aubin H-J, Reynaud M, Martinot J-L. 2007. Brain morphometry and cognitive performance in detoxified alcohol-dependents with preserved psychosocial functioning. Neuropsychopharmacology 32, 429–438. ( 10.1038/sj.npp.1301219) [DOI] [PubMed] [Google Scholar]
- 96.Nurmedov S, Metin B, Ekmen S, Noyan O, Yilmaz O, Darcin A, Dilbaz N. 2015. Thalamic and cerebellar gray matter volume reduction in synthetic cannabinoids users. Eur. Addict. Res. 21, 315–320. ( 10.1159/000430437) [DOI] [PubMed] [Google Scholar]
- 97.Mechtcheriakov S, Brenneis C, Egger K, Koppelstaetter F, Schocke M, Marksteiner J. 2007. A widespread distinct pattern of cerebral atrophy in patients with alcohol addiction revealed by voxel-based morphometry. J. Neurol. Neurosurg. Psychiatry 78, 610–614. ( 10.1136/jnnp.2006.095869) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.van Holst RJ, de Ruiter MB, van den Brink W, Veltman DJ, Goudriaan AE. 2012. A voxel-based morphometry study comparing problem gamblers, alcohol abusers, and healthy controls. Drug Alcohol. Depend. 124, 142–148. ( 10.1016/j.drugalcdep.2011.12.025) [DOI] [PubMed] [Google Scholar]
- 99.Wetherill RR, Jagannathan K, Hager N, Childress AR, Rao H, Franklin TR. 2015. Cannabis, cigarettes, and their co-occurring use: disentangling differences in gray matter volume. Int. J. Neuropsychopharmacol. 18, pyv061 ( 10.1093/ijnp/pyv061) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ide JS, Zhang S, Hu S, Sinha R, Mazure CM, Li C-SR. 2014. Cerebral gray matter volumes and low-frequency fluctuation of BOLD signals in cocaine dependence: duration of use and gender difference. Drug Alcohol. Depend. 134, 51–62. ( 10.1016/j.drugalcdep.2013.09.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Liao Y, Tang J, Liu T, Chen X, Hao W. 2012. Differences between smokers and non-smokers in regional gray matter volumes: a voxel-based morphometry study. Addict. Biol. 17, 977–980. ( 10.1111/j.1369-1600.2010.00250.x) [DOI] [PubMed] [Google Scholar]
- 102.Morales AM, Kohno M, Robertson CL, Dean AC, Mandelkern MA, London ED. 2015. Gray-matter volume, midbrain dopamine D2/D3 receptors and drug craving in methamphetamine users. Mol. Psychiatry 20, 764–771. ( 10.1038/mp.2015.47) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ritz L, Segobin S, Lannuzel C, Boudehent C, Vabret F, Eustache F, Beaunieux H, Pitel AL. 2016. Direct voxel-based comparisons between grey matter shrinkage and glucose hypometabolism in chronic alcoholism. J. Cereb. Blood Flow Metab. 36, 1625–1640. ( 10.1177/0271678X15611136) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sim ME, et al. 2007. Cerebellar gray matter volume correlates with duration of cocaine use in cocaine-dependent subjects. Neuropsychopharmacology 32, 2229–2237. ( 10.1038/sj.npp.1301346) [DOI] [PubMed] [Google Scholar]
- 105.Mashhoon Y, Sava S, Sneider JT, Nickerson LD, Silveri MM. 2015. Cortical thinness and volume differences associated with marijuana abuse in emerging adults. Drug Alcohol. Depend. 155, 275–283. ( 10.1016/j.drugalcdep.2015.06.016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hanlon CA, Owens MM, Joseph JE, Zhu X, George MS, Brady KT, Hartwell KJ. 2016. Lower subcortical gray matter volume in both younger smokers and established smokers relative to non-smokers. Addict. Biol. 21, 185–195. ( 10.1111/adb.12171) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mon A, Durazzo TC, Abe C, Gazdzinski S, Pennington D, Schmidt T, Meyerhoff DJ. 2014. Structural brain differences in alcohol-dependent individuals with and without comorbid substance dependence. Drug Alcohol. Depend. 144, 170–177. ( 10.1016/j.drugalcdep.2014.09.010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Peng P, Wang Z, Jiang T, Chu S, Wang S, Xiao D. 2015. Brain-volume changes in young and middle-aged smokers: a DARTEL-based voxel-based morphometry study. Clin. Respir. J. 11, 621–631. ( 10.1111/crj.12393) [DOI] [PubMed] [Google Scholar]
- 109.Noyan CO, Kose S, Nurmedov S, Metin B, Darcin AE, Dilbaz N. 2016. Volumetric brain abnormalities in polysubstance use disorder patients. Neuropsychiatr. Dis. Treat. 12, 1355–1363. ( 10.2147/NDT.S107733) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Durazzo TC, Mon A, Gazdzinski S, Yeh P-H, Meyerhoff DJ. 2015. Serial longitudinal magnetic resonance imaging data indicate non-linear regional gray matter volume recovery in abstinent alcohol-dependent individuals. Addict. Biol. 20, 956–967. ( 10.1111/adb.12180) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sutherland MT, Riedel MC, Flannery JS, Yanes JA, Fox PT, Stein EA, Laird AR. 2016. Chronic cigarette smoking is linked with structural alterations in brain regions showing acute nicotinic drug-induced functional modulations. Behav. Brain Funct. 12, 16 ( 10.1186/s12993-016-0100-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bora E, Yücel M, Fornito A, Pantelis C, Harrison BJ, Cocchi L, Pell G, Lubman DI. 2012. White matter microstructure in opiate addiction. Addict. Biol. 17, 141–148. ( 10.1111/j.1369-1600.2010.00266.x) [DOI] [PubMed] [Google Scholar]
- 113.Bell RP, Foxe JJ, Nierenberg J, Hoptman MJ, Garavan H. 2011. Assessing white matter integrity as a function of abstinence duration in former cocaine-dependent individuals. Drug Alcohol. Depend. 114, 159–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bava S, Jacobus J, Thayer RE, Tapert SF. 2013. Longitudinal changes in white matter integrity among adolescent substance users. Alcohol. Clin. Exp. Res. 37(Suppl 1), E181–E189. ( 10.1111/j.1530-0277.2012.01920.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Becker MP, Collins PF, Lim KO, Muetzel RL, Luciana M. 2015. Longitudinal changes in white matter microstructure after heavy cannabis use. Dev. Cogn. Neurosci. 16, 23–35. ( 10.1016/j.dcn.2015.10.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Li Y, Dong H, Li F, Wang G, Zhou W, Yu R, Zhang L. 2017. Microstructures in striato-thalamo-orbitofrontal circuit in methamphetamine users. Acta Radiol. 58, 1378–1385. ( 10.1177/0284185117692170) [DOI] [PubMed] [Google Scholar]
- 117.Yip SW, Morie KP, Xu J, Constable RT, Malison RT, Carroll KM, Potenza MN. 2017. Shared microstructural features of behavioral and substance addictions revealed in areas of crossing fibers. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2, 188–195. ( 10.1016/j.bpsc.2016.03.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Baker STE, Yücel M, Fornito A, Allen NB, Lubman DI. 2013. A systematic review of diffusion weighted MRI studies of white matter microstructure in adolescent substance users. Neurosci. Biobehav. Rev. 37, 1713–1723. ( 10.1016/j.neubiorev.2013.06.015) [DOI] [PubMed] [Google Scholar]
- 119.Zou Q-H, Zhu C-Z, Yang Y, Zuo X-N, Long X-Y, Cao Q-J, Wang Y-F, Zang Y-F. 2008. An improved approach to detection of amplitude of low-frequency fluctuation (ALFF) for resting-state fMRI: fractional ALFF. J. Neurosci. Methods 172, 137–141. ( 10.1016/j.jneumeth.2008.04.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zang Y, Jiang T, Lu Y, He Y, Tian L. 2004. Regional homogeneity approach to fMRI data analysis. Neuroimage 22, 394–400. ( 10.1016/j.neuroimage.2003.12.030) [DOI] [PubMed] [Google Scholar]
- 121.Prosser J, London ED, Galynker II. 2009. Sustained attention in patients receiving and abstinent following methadone maintenance treatment for opiate dependence: performance and neuroimaging results. Drug Alcohol. Depend. 104, 228–240. ( 10.1016/j.drugalcdep.2009.04.022) [DOI] [PubMed] [Google Scholar]
- 122.Wiers CE, Shokri-Kojori E, Wong CT, Abi-Dargham A, Demiral ŞB, Tomasi D, Wang G-J, Volkow ND. 2016. Cannabis abusers show hypofrontality and blunted brain responses to a stimulant challenge in females but not in males. Neuropsychopharmacology 41, 2596–2605. ( 10.1038/npp.2016.67) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zubieta J, Lombardi U, Minoshima S, Guthrie S, Ni L, Ohl LE, Koeppe RA, Domino EF. 2001. Regional cerebral blood flow effects of nicotine in overnight abstinent smokers. Biol. Psychiatry 49, 906–913. ( 10.1016/S0006-3223(00)01070-2) [DOI] [PubMed] [Google Scholar]
- 124.Volkow ND, Wang GJ, Fowler JS, Hitzemann R, Gatley SJ, Dewey SS, Pappas N. 1998. Enhanced sensitivity to benzodiazepines in active cocaine-abusing subjects: a PET study. Am. J. Psychiatry 155, 200–206. ( 10.1176/ajp.155.10.1325) [DOI] [PubMed] [Google Scholar]
- 125.Wang Z, Faith M, Patterson F, Tang K, Kerrin K, Wileyto EP, Detre JA, Lerman C. 2007. Neural substrates of abstinence-induced cigarette cravings in chronic smokers. J. Neurosci. 27, 14 035–14 040. ( 10.1523/JNEUROSCI.2966-07.2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Denier N, et al. 2015. Abnormal functional integration of thalamic low frequency oscillation in the BOLD signal after acute heroin treatment. Hum. Brain Mapp. 36, 5287–5300. ( 10.1002/hbm.23011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Qiu Y-W, Han L-J, Lv X-F, Jiang G-H, Tian J-Z, Zhuo F-Z, Su H-H, Lin C-L, Zhang X-L. 2011. Regional homogeneity changes in heroin-dependent individuals: resting-state functional MR imaging study. Radiology 261, 551–559. ( 10.1148/radiol.11102466) [DOI] [PubMed] [Google Scholar]
- 128.Zilverstand A, O'Halloran R, Goldstein RZ. 2017 Resting-state and structural brain connectivity in individuals with stimulant addiction. A systematic review. In The Routledge handbook of the philosophy and science of addiction (eds Ahmed SH, Pickard H) London, UK: Routledge. [Google Scholar]
- 129.Camchong J, Stenger A, Fein G. 2013. Resting-state synchrony in long-term abstinent alcoholics. Alcohol. Clin. Exp. Res. 37, 75–85. ( 10.1111/j.1530-0277.2012.01859.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Tomasi D, Volkow ND, Wang R, Carrillo JH, Maloney T, Alia-Klein N, Woicik PA, Telang F, Goldstein RZ.. 2010. Disrupted functional connectivity with dopaminergic midbrain in cocaine abusers. PLoS ONE 5, e10815 ( 10.1371/journal.pone.0010815) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Verdejo-Garcia A, et al. 2014. Functional alteration in frontolimbic systems relevant to moral judgment in cocaine-dependent subjects. Addict. Biol. 19, 272–281. ( 10.1111/j.1369-1600.2012.00472.x) [DOI] [PubMed] [Google Scholar]
- 132.Gu H, Salmeron BJ, Ross TJ, Geng X, Zhan W, Stein EA, Yang Y. 2010. Mesocorticolimbic circuits are impaired in chronic cocaine users as demonstrated by resting-state functional connectivity. Neuroimage 53, 593–601. ( 10.1016/j.neuroimage.2010.06.066) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wang C, Bai J, Wang C, von Deneen KM, Yuan K, Cheng J. 2017. Altered thalamo-cortical resting state functional connectivity in smokers. Neurosci. Lett. 653, 120–125. ( 10.1016/j.neulet.2017.05.038) [DOI] [PubMed] [Google Scholar]
- 134.Wei Z, et al. 2016Resting-state functional connectivity between the dorsal anterior cingulate cortex and thalamus is associated with risky decision-making in nicotine addicts. Sci. Rep. 6, 21778 ( 10.1038/srep21778). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Liao Y, et al. 2016. Decreased thalamocortical connectivity in chronic ketamine users. PLoS ONE 11, e0167381 ( 10.1371/journal.pone.0167381) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Xie C, et al. 2011. Identification of hyperactive intrinsic amygdala network connectivity associated with impulsivity in abstinent heroin addicts. Behav. Brain Res. 216, 639–646. ( 10.1016/j.bbr.2010.09.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Aron AR. 2007. The neural basis of inhibition in cognitive control. Neuroscientist 13, 214–228. ( 10.1177/1073858407299288) [DOI] [PubMed] [Google Scholar]
- 138.Li Q, et al. 2012. Craving correlates with mesolimbic responses to heroin-related cues in short-term abstinence from heroin: an event-related fMRI study. Brain Res. 1469, 63–72. ( 10.1016/j.brainres.2012.06.024) [DOI] [PubMed] [Google Scholar]
- 139.Wang Y, et al. 2014. Reduced responses to heroin-cue-induced craving in the dorsal striatum: effects of long-term methadone maintenance treatment. Neurosci. Lett. 581, 120–124. ( 10.1016/j.neulet.2014.08.026) [DOI] [PubMed] [Google Scholar]
- 140.Filbey FM, Dunlop J, Ketcherside A, Baine J, Rhinehardt T, Kuhn B, DeWitt S, Alvi T. 2016. fMRI study of neural sensitization to hedonic stimuli in long-term, daily cannabis users. Hum. Brain Mapp. 37, 3431–3443. ( 10.1002/hbm.23250) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Asensio S, Romero MJ, Palau C, Sanchez A, Senabre I, Morales JL, Carcelen R, Romero FJ. 2010. Altered neural response of the appetitive emotional system in cocaine addiction: an fMRI Study. Addict. Biol. 15, 504–516. ( 10.1111/j.1369-1600.2010.00230.x) [DOI] [PubMed] [Google Scholar]
- 142.Mitchell MR, Balodis IM, Devito EE, Lacadie CM, Yeston J, Scheinost D, Constable RT, Carroll KM, Potenza MN. 2013. A preliminary investigation of Stroop-related intrinsic connectivity in cocaine dependence: associations with treatment outcomes. Am. J. Drug Alcohol. Abuse 39, 392–402. ( 10.3109/00952990.2013.841711) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Vollstädt-Klein S, Kobiella A, Bühler M, Graf C, Fehr C, Mann K, Smolka MN. 2011. Severity of dependence modulates smokers’ neuronal cue reactivity and cigarette craving elicited by tobacco advertisement. Addict. Biol. 16, 166–175. ( 10.1111/j.1369-1600.2010.00207.x) [DOI] [PubMed] [Google Scholar]
- 144.Ray S, Haney M, Hanson C, Biswal B, Hanson SJ. 2015. Modeling causal relationship between brain regions within the drug-cue processing network in chronic cocaine smokers. Neuropsychopharmacology 40, 2960–2968. ( 10.1038/npp.2015.150) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Dagher A, Tannenbaum B, Hayashi T, Pruessner JC, McBride D. 2009. An acute psychosocial stress enhances the neural response to smoking cues. Brain Res. 1293, 40–48. ( 10.1016/j.brainres.2009.07.048) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Regier PS, et al. 2016. Emotional, physical and sexual abuse are associated with a heightened limbic response to cocaine cues. Addict. Biol. 22, 1768–1777. ( 10.1111/adb.12445) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Feldstein Ewing SW, Filbey FM, Chandler LD, Hutchison KE. 2010. Exploring the relationship between depressive and anxiety symptoms and neuronal response to alcohol cues. Alcohol. Clin. Exp. Res. 34, 396–403. ( 10.1111/j.1530-0277.2009.01104.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.McClernon FJ, Kozink RV, Lutz AM, Rose JE. 2009. 24-h smoking abstinence potentiates fMRI-BOLD activation to smoking cues in cerebral cortex and dorsal striatum. Psychopharmacology 204, 25–35. ( 10.1007/s00213-008-1436-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Lou M, Wang E, Shen Y, Wang J. 2012. Cue-elicited craving in heroin addicts at different abstinent time: an fMRI pilot study. Subst. Use Misuse 47, 631–639. ( 10.3109/10826084.2011.646381) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Bedi G, Preston KL, Epstein DH, Heishman SJ, Marrone GF, Shaham Y, de Wit H. 2011. Incubation of cue-induced cigarette craving during abstinence in human smokers. Biol. Psychiatry 69, 708–711. ( 10.1016/j.biopsych.2010.07.014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Wang G, Shi J, Chen N, Xu L, Li J, Li P, Sun Y, Lu L. 2013. Effects of length of abstinence on decision-making and craving in methamphetamine abusers. PLoS ONE 8, e68791 ( 10.1371/journal.pone.0068791) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Li P, et al. 2015. Incubation of alcohol craving during abstinence in patients with alcohol dependence. Addict. Biol. 20, 513–522. ( 10.1111/adb.12140) [DOI] [PubMed] [Google Scholar]
- 153.Parvaz MA, Moeller SJ, Goldstein RZ. 2016. Incubation of cue-induced craving in adults addicted to cocaine measured by electroencephalography. JAMA Psychiatry 73, 1127–1134. ( 10.1001/jamapsychiatry.2016.2181) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Balodis IM, Kober H, Worhunsky PD, Stevens MC, Pearlson GD, Carroll KM, Potenza MN. 2016. Neurofunctional reward processing changes in cocaine dependence during recovery. Neuropsychopharmacology 41, 2112–2121. ( 10.1038/npp.2016.11) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Jia Z, Worhunsky PD, Carroll KM, Rounsaville BJ, Stevens MC, Pearlson GD, Potenza MN. 2011. An initial study of neural responses to monetary incentives as related to treatment outcome in cocaine dependence. Biol. Psychiatry 70, 553–560. ( 10.1016/j.biopsych.2011.05.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Wimmer RD, Schmitt LI, Davidson TJ, Nakajima M, Deisseroth K, Halassa MM. 2015. Thalamic control of sensory selection in divided attention. Nature 526, 705–709. ( 10.1038/nature15398) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kober H, DeVito EE, DeLeone CM, Carroll KM, Potenza MN. 2014. Cannabis abstinence during treatment and one-year follow-up: relationship to neural activity in men. Neuropsychopharmacology 39, 2288–2298. ( 10.1038/npp.2014.82) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Nikolaou K, Critchley H, Duka T. 2013. Alcohol affects neuronal substrates of response inhibition but not of perceptual processing of stimuli signalling a stop response. PLoS ONE 8, e76649 ( 10.1371/journal.pone.0076649) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Schmaal L, Joos L, Koeleman M, Veltman DJ, van den Brink W, Goudriaan AE. 2013. Effects of modafinil on neural correlates of response inhibition in alcohol-dependent patients. Biol. Psychiatry 73, 211–218. ( 10.1016/j.biopsych.2012.06.032) [DOI] [PubMed] [Google Scholar]
- 160.Zhang S, Hu S, Bednarski SR, Erdman E, Li C-SR. 2014. Error-related functional connectivity of the thalamus in cocaine dependence. Neuroimage Clin. 4, 585–592. ( 10.1016/j.nicl.2014.01.015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Zhang S, Hu S, Sinha R, Potenza MN, Malison RT, Li C-SR. 2016. Cocaine dependence and thalamic functional connectivity: a multivariate pattern analysis. Neuroimage Clin. 12, 348–358. ( 10.1016/j.nicl.2016.08.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Sjoerds Z, van den Brink W, Beekman ATF, Penninx BWJH, Veltman DJ. 2014. Response inhibition in alcohol-dependent patients and patients with depression/anxiety: a functional magnetic resonance imaging study. Psychol. Med. 44, 1713–1725. ( 10.1017/S0033291713002274) [DOI] [PubMed] [Google Scholar]
- 163.Hu S, Ide JS, Zhang S, Sinha R, Li C-SR. 2015. Conflict anticipation in alcohol dependence—a model-based fMRI study of stop signal task. Neuroimage Clin. 8, 39–50. ( 10.1016/j.nicl.2015.03.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Harlé KM, Stewart JL, Zhang S, Tapert SF, Yu AJ, Paulus MP. 2015. Bayesian neural adjustment of inhibitory control predicts emergence of problem stimulant use. Brain 138, 3413–3426. ( 10.1093/brain/awv246) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Luo X, et al. 2013. Error processing and gender-shared and -specific neural predictors of relapse in cocaine dependence. Brain 136, 1231–1244. ( 10.1093/brain/awt040) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Weywadt CR, Kiehl KA, Claus ED. 2017. Neural correlates of response inhibition in current and former smokers. Behav. Brain Res. 319, 207–218. ( 10.1016/j.bbr.2016.11.030) [DOI] [PubMed] [Google Scholar]
- 167.Li C-SR, Luo X, Sinha R, Rounsaville BJ, Carroll KM, Malison RT, Ding Y-S, Zhang S, Ide JS. 2010. Increased error-related thalamic activity during early compared to late cocaine abstinence. Drug Alcohol. Depend. 109, 181–189. ( 10.1016/j.drugalcdep.2010.01.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This article has no additional data.