Abstract
Antidepressants remediate negative biases in emotional processing early in treatment, prior to mood improvement. However, the effects on reward processing potentially relevant to the treatment of anhedonia are less clear. Here we investigate the early and sustained effects of the dopamine and noradrenaline reuptake inhibitor bupropion on behavioural measures of emotional and reward processing in currently depressed individuals. Forty-six currently depressed patients and 42 healthy controls participated in a repeated measures study, during which open-label bupropion was administered to only the patient group over a six week period without a placebo group. All participants completed the Emotional Test Battery and a probabilistic instrumental learning task at week 0, week 2 and week 6. Currently depressed patients displayed negative biases in emotional processing and blunted response bias for high-probability wins compared to the healthy controls at baseline. Bupropion was found to reduce the negative biases in emotional processing early in treatment, including a significant decrease in the percentage misclassification of other face emotions as sad and the number of negative self-referent words falsely recalled between baseline and week 2. Conversely, bupropion was found to initially further reduce the response bias for high-probability wins between baseline and week 2. This effect reversed with six weeks' bupropion treatment and reward processing was normalized compared to the healthy controls. Early in treatment, bupropion acts to reduce negative biases in emotional processing but exacerbates impaired reward processing. The beneficial actions of bupropion on reward processing then occur later in treatment. Such dissociation in the temporal effects of bupropion on emotional and reward processing has implications for the treatment of the different symptom domains of negative affect and anhedonia in depression.
This article is part of a discussion meeting issue ‘Of mice and mental health: facilitating dialogue between basic and clinical neuroscientists’.
Keywords: depression, emotional processing, reward, dopamine, bupropion
1. Background
Cognitive theories of depression suggest that negative biases in emotional processing play an important role in the causation and maintenance of the persistent low mood characteristic of the disorder. Indeed, depressed patients, as well as those at risk of depression [1,2], display negative biases in emotional processing across a range of cognitive domains, including perception, attention and memory [3–6]. For example, they display increased perception of ambiguous face emotions as negative [7,8], an attentional bias towards negative face emotions and self-referent words [9] and increased recall of negative versus positive self-referent words [10] compared to healthy controls.
It has been hypothesized that antidepressants may act to restore the balance between positive and negative emotional processing early in treatment, prior to mood improvement [6,11–13]. Indeed, 7 day treatment or even an acute dose of the selective serotonin reuptake inhibitor (SSRI) citalopram or the noradrenaline reuptake inhibitor reboxetine was found to increase the perception of ambiguous faces as happy and the recall of positive self-referent words in both healthy volunteers [14–16] and depressed patients [17] compared to placebo. Importantly, these changes in emotional processing biases occurred in the absence of changes in subjective mood, suggesting that they may be a direct effect of antidepressant drug treatment rather than being a secondary consequence of changes in mood and affect.
However, depression is not only characterized by persistent low mood but also a loss of pleasure in response to receipt of reward, known as anhedonia. Depressed patients display blunted responsiveness to reward [18] and fail to develop a response bias towards high-probability win during probabilistic instrumental learning tasks [19–21]. These impairments correlate specifically with the severity of self-reported anhedonia, independently of overall depression severity [21–24]. Building on pre-clinical evidence for a role of dopamine in reward [25,26], it has been hypothesized that the anhedonia and underlying impaired reward processing in depression involve changes in the function of the dopamine system.
While serotonergic and/or noradrenergic antidepressants can alter emotional processing biases to improve mood, they do not appear to fully correct the experience of anhedonia [27] and may actually exacerbate the impaired reward processing [28]. It has therefore been suggested that antidepressants with an effect on the dopamine reward system may be better suited to treat anhedonia [27], but equally, their effects on emotional processing biases remain unclear. Bupropion is one of the few antidepressants that prevent the reuptake of dopamine (in addition to noradrenaline) and may be a potential treatment for patients displaying predominantly anhedonic symptoms [29]. We previously assessed the effect of a single dose of bupropion on emotional and reward processing in a different cohort of healthy volunteers compared to double blind administration of placebo. Bupropion was found to remediate negative biases in emotional processing but had unexpected detrimental effects on reward processing compared to placebo [30]. The current study aimed to assess whether this profile of effects of bupropion observed in healthy volunteers translated to depressed patients and longer-term treatment. If so, this would have implications for its potential for the treatment of anhedonia and the expected timescale for such effects. Therefore, here we investigate the early and sustained effects of bupropion on behavioural measures of emotional and reward processing in individuals suffering from depression.
2. Methods
This study was approved by the University of Oxford Clinical Trials and Research Governance Team (CTRG) and a NHS Research Ethics Committee (NRES Committee South Central – Berkshire B) (13/SC/0569). It was carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans. All participants provided written informed consent.
(a). Participant recruitment and screening
A total of 46 depressed patients and 42 healthy controls (HCs) were recruited from the general population. Via an in-depth psychiatric assessment including the Structured Clinical Interview (SCID) for DSM-IV [31], depressed patients were found to satisfy a diagnosis of a current episode of major depressive disorder (MDD) but no other Axis 1 DSM-V psychiatric disorder (apart from co-morbid anxiety disorders) and HCs were deemed to be free from either current or past history of any Axis 1 DSM-IV psychiatric disorders. Exclusion criteria included the use of any medication that could impact upon the safety or effect of bupropion within three weeks of the baseline assessments or psychological treatment within three months of the baseline assessments, drug or alcohol abuse in the past year, medical conditions judged to interfere with the safety of the participant or scientific assessments, smoking more than 10 cigarettes or equivalent a day and prior experience of the behavioural tasks used in the study. The MDD and HC groups were matched for gender, age and national adult reading test (NART)-derived verbal IQ [32].
(b). Study design and intervention
In a repeated-measures study design, open-label sustained release bupropion was administered to just the MDD group over a six week period but all participants completed the Emotional Test Battery (ETB) and a probabilistic instrumental learning task at week 0 (baseline), week 2 and week 6. The course of bupropion started the morning after baseline assessments' with the MDD group taking 150 mg once daily in the morning for the first 7–10 days, then 150 mg twice daily for the remaining five weeks. The HC group did not receive bupropion, placebo or any other intervention to control for the practice effects of repeated testing. Each visit also involved completion of a number of researcher-administered and self-report questionnaires.
(c). Questionnaire measures
The Hamilton Rating Scale for Depression (HAM-D) [33] was administered via a semi-structured interview with a trained experimenter. The rest of the questionnaires were self-report questionnaires and included the Adult Eysenck Personality Questionnaire (EPQ) [34], the Full Mood and Anxiety Symptom Questionnaire (MASQ) [35], the Positive and Negative Affect Schedule (PANAS) [36], the Snaith–Hamilton Pleasure Scale (SHAPS) [37] and the Oxford Questionnaire on the Emotional Side-effects of Antidepressants (OQuESA) [38]. For the SHAPS, in this study, the ratings of strongly agree, agree, disagree and strongly disagree were converted into a 4-point scale, with higher scores indicating higher anhedonia.
(d). Emotional Test Battery
The ETB (P1vital, Oxford, UK) comprises several validated, computerized cognitive tasks designed to assess emotional processing biases and has previously been described in full [17]. In brief, the Facial Expression Recognition Task (FERT) comprises a series of facial expressions associated with six basic emotions: anger, disgust, fear, happy, sad and surprise at a range of different intensity levels and participants are required to identify the emotion of the face. Signal detection theory is used to provide estimates of target sensitivity (d’) and beta. The Emotional Categorisation Task (ECAT) comprises a series of positive and negative self-referent words and participants are required to indicate whether they would like or dislike to be referred to as each word. In the Facial Dot-Probe Task (FDOT), the attentional vigilance to happy or fearful faces can be determined from participants' response latency to indicate the alignment of a dot probe appearing in the place of one of the faces. Finally, the Emotional Recall Task (EREC) is a surprise free recall task during which participants are required to remember as many of the self-referent words from the ECAT as they can in 2 min. Further details for each task are provided in the electronic supplementary material.
(e). Probabilistic instrumental learning task
The probabilistic instrumental learning task was adapted from that described by Pessiglione and colleagues [39] and has previously been described in full [30]. In brief, task stimuli consist of two pairs of symbols with one pair associated with win outcomes (win £1 or no change) and the other associated with loss outcomes (lose £1 or no change). Each symbol in the pair corresponds to reciprocal probabilities (0.7 or 0.3) of the associated outcomes occurring. Participants were required to choose between the two symbols in order to maximize their winnings. Once a choice was made, outcome feedback was provided. In order to maximize their winnings, participants used the outcome feedback to gradually learn the symbol–outcome associations over time, such that they consistently chose the symbol associated with high-probability win and avoided the symbol associated with high-probability loss. Note that different symbols were used at each visit to minimize learning effects confounding subsequent performance. Further details for this task are provided in the electronic supplementary material.
(f). Analysis
Reaction times were trimmed at the participant level: reaction times above 3 standard deviations from the mean or below 200 ms were excluded prior to calculating the mean. Each outcome measure from each task of the ETB was analysed using a repeated measures analysis of variance (ANOVA) with group (MDD or HC) as the between-subject factor and one within-subject factor depending on the task (e.g. face emotion, word valence) when assessing differences between groups at baseline. Analyses of how measures changed over time included study visit (baseline, week 2 and week 6) as an additional within-subject factor. For the probabilistic instrumental learning task, participants who performed worse than chance and completed the task with winnings of less than the initial £5 were assumed not to have understood the task and were excluded (8 HC, 7 MDD). Data were then averaged across the two runs and analysed as above. Significant visit by group interactions indicated changes that could not solely be explained by practice effects suggestive of an effect of bupropion and were followed up using appropriate post hoc t-tests.
3. Results
(a). Participant characterization
There were no significant differences between the MDD and HC groups with regards to gender, age or NART-derived verbal IQ. As would be expected, the MDD group had significantly higher HAM-D and EPQ neuroticism scores and significantly lower EPQ extroversion scores at baseline than the HC group (table 1). The majority of MDD patients displayed mild to moderate depression at baseline (figure 1).
Table 1.
Participant characterization. Values are reported as means ± standard deviation.
| HC | MDD | Sig./p | |
|---|---|---|---|
| n | 42 | 46 | |
| gender (male:female) | 13 : 29 | 13 : 33 | |
| age | 30.21 ± 8.13 | 29.52 ± 9.01 | 0.71 |
| NART | 116.31 ± 4.82 | 115.81 ± 4.34 | 0.48 |
| HAM-D | 0.86 ± 0.95 | 13.09 ± 3.03 | <0.001 |
| EPQ | |||
| neuroticism | 4.86 ± 3.25 | 18.98 ± 3.32 | <0.001 |
| psychoticism | 2.48 ± 2.32 | 4.72 ± 3.70 | <0.01 |
| lie | 10.00 ± 3.84 | 8.41 ± 4.31 | 0.07 |
| extraversion | 14.71 ± 4.27 | 7.54 ± 5.44 | <0.001 |
| clinical history of the MDD group | |||
| age of onset (years) | 17.85 ± 7.91 | ||
| number of previous episodes | 3.41 ± 6.58 | ||
| received previous treatment? | 36 Yes : 10 No | ||
| length of current episode (years) | 3.80 ± 4.46 | ||
Figure 1.
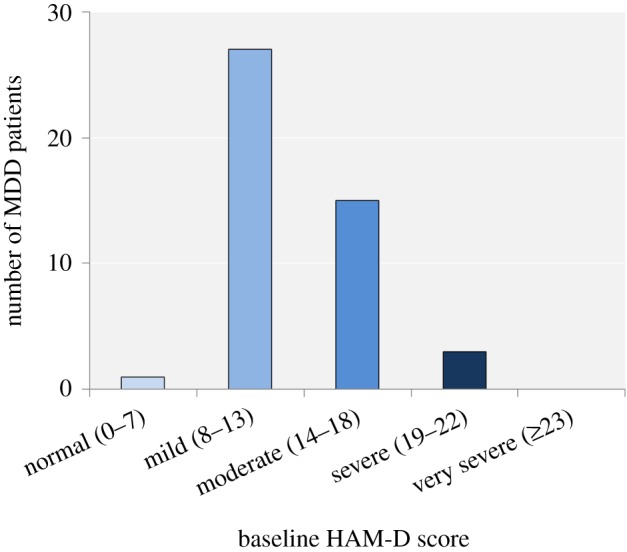
Number of MDD patients scoring within each category of symptom severity of the HAM-D. (Online version in colour.)
(b). Mood at baseline and change over time
Independent samples t-tests found the MDD group to be significantly different from the HC group (p < 0.001) for all remaining questionnaire measures at baseline. Paired t-tests found the MDD group to significantly improve on all questionnaire measures apart from the MASQ anxious arousal sub-scale with bupropion treatment (table 2).
Table 2.
Mood at baseline and change over time. Values are reported as means ± standard deviation.
| HC |
MDD |
|||||||
|---|---|---|---|---|---|---|---|---|
| questionnaire | test visit 1 (baseline)* |
test visit 2 | test visit 3 | effect of time | test visit 1 (baseline)* |
test visit 2 | test visit 3 | effect of time |
| n | 42 | 40 | 40 | 46 | 43 | 41 | ||
| HAM-D | 0.86 ± 0.95 | 0.83 ± 1.17 | 0.88 ± 1.56 | no change p = 0.89 | 13.09 ± 3.03 | 10.37 ± 3.22 | 6.05 ± 3.35 | decrease p < 0.001 |
| SHAPS | 20.02 ± 4.41 | 20.25 ± 4.24 | 19.93 ± 5.08 | no change p = 1.00 | 34.09 ± 5.30 | 31.60 ± 5.31 | 26.56 ± 6.37 | decrease p < 0.001 |
| MASQ | ||||||||
| general distress: mixed | 21.76 ± 5.25 | 21.18 ± 5.43 | 21.33 ± 6.37 | no change p = 0.63 | 46.48 ± 8.60 | 40.77 ± 9.44 | 33.02 ± 8.52 | decrease p < 0.001 |
| general distress: anxious | 14.21 ± 3.78 | 13.73 ± 3.15 | 13.35 ± 2.82 | no change p = 0.17 | 26.24 ± 6.43 | 22.23 ± 6.72 | 19.34 ± 5.48 | decrease p < 0.001 |
| anxious arousal | 19.29 ± 3.95 | 18.08 ± 1.42 | 17.98 ± 1.29 | decrease p < 0.05 | 27.85 ± 8.00 | 26.63 ± 7.90 | 25.00 ± 7.06 | no change p = 0.07 |
| general distress: depressive | 16.17 ± 5.23 | 15.45 ± 4.64 | 16.15 ± 6.97 | no change p = 0.92 | 41.00 ± 8.84 | 32.63 ± 10.53 | 24.71 ± 8.44 | decrease p < 0.001 |
| anhedonic depression | 45.10 ± 7.44 | 44.15 ± 8.89 | 45.23 ± 13.54 | no change p = 0.97 | 84.15 ± 10.27 | 74.42 ± 14.10 | 61.83 ± 15.80 | decrease p < 0.001 |
| PANAS | ||||||||
| positive affect | 33.55 ± 7.18 | 32.15 ± 7.24 | 33.05 ± 8.63 | no change p = 0.19 | 17.98 ± 5.49 | 20.70 ± 8.18 | 25.17 ± 9.22 | increase p < 0.001 |
| negative affect | 11.48 ± 2.54 | 11.38 ± 2.25 | 11.45 ± 2.49 | no change p = 0.92 | 21.28 ± 6.77 | 20.28 ± 6.90 | 16.17 ± 5.71 | decrease p < 0.001 |
| OQuESA | ||||||||
| section 1 | 15.36 ± 3.97 | 15.53 ± 4.26 | 14.88 ± 4.34 | no change p = 0.30 | 42.41 ± 8.28 | 37.56 ± 10.18 | 30.66 ± 11.53 | decrease p < 0.001 |
| section 2 | 9.07 ± 3.54 | 8.63 ± 1.41 | 8.63 ± 1.92 | no change p = 0.43 | 32.33 ± 5.60 | 28.26 ± 7.53 | 21.76 ± 9.01 | decrease p < 0.001 |
| section 3 | 10.56 ± 4.57 | 9.95 ± 5.63 | no change p = 0.42 | |||||
*Independent samples t-tests found MDDs to be significantly different from HCs (p < 0.001) for all baseline questionnaire scores.
(c). Emotional Test Battery
(i). Facial Expression Recognition Task
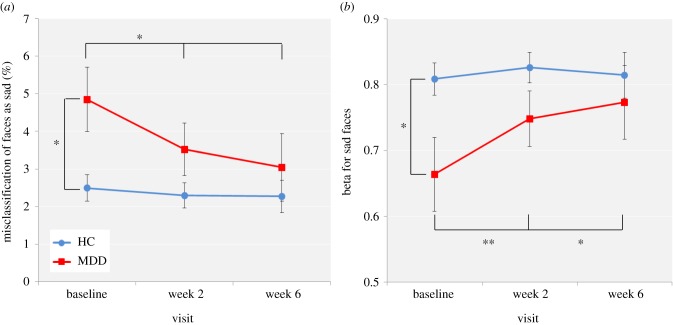
Baseline differences between the MDD and HC groups. For percentage accuracy or d’, there was no significant main effect of group (F1,85 = 0.43, p = 0.51; F1,85 = 1.15, p = 0.29) or face emotion by group interaction (F5,425 = 0.47, p = 0.80; F5,425 = 0.25, p = 0.94). There was a significant face emotion by group interaction for both percentage misclassification (F5,425 = 3.16, p < 0.01) and beta measure of response bias (F5,420 = 3.23, p < 0.01). Post hoc independent-samples t-tests found that the MDD group misclassified significantly more other face emotions as sad compared with the HC group (t85 = 2.32, p < 0.05). Note that increased sad per cent misclassification refers to increased percentage misclassification of other face emotions as sad and not increased percentage misclassification of sad faces as other face emotions. This was reflected in significantly reduced beta compared with the HC group (t85 = 3.32, p < 0.05), indicating increased response bias towards sad faces (figure 2). No significant differences were found for any other face emotion. For reaction time, there was a significant main effect of group (F1,83 = 5.15, p < 0.05), with the MDD group responding significantly more slowly than the HC group across all face emotions.
Figure 2.
FERT (a) percentage misclassification of other face emotions as sad and (b) beta for sad faces for the HC and MDD groups across the three visits. Values are reported as means ± standard error of the mean. Asterisks denote the degree of significance obtained for planned comparisons (*p < 0.05; **p < 0.01). (Online version in colour.)
Effect of bupropion. There was no significant face emotion by visit by group interaction for percentage accuracy (F10,780 = 0.76, p = 0.67) or d’ (F10,780 = 1.40, p = 0.18). There was a significant visit by group interaction for percentage misclassification (F2,156 = 3.12, p < 0.05). Post hoc analyses found a marginally significant visit by group interaction for percentage misclassification of other face emotions as sad (F2,156 = 2.87, p = 0.06), with only the bupropion-treated MDD group displaying a significant decrease in the percentage misclassification of other face emotions as sad between baseline and week 2 (t41 = 2.72, p < 0.05) or baseline and week 6 (t39 = 2.21, p < 0.05), such that the group difference at baseline (t85 = −2.47, p < 0.05) was no longer significant at week 2 (t81 = −1.54, p = 0.13) or week 6 (t79 = −0.77, p = 0.45) (figure 2a). A similar pattern of results was observed for beta for sad faces, with a trend towards significance for a visit by group interaction (F2,156 = 2.47, p = 0.09) and only the bupropion-treated MDD group displaying a significant increase in beta for sad faces between baseline and week 2 (t41 = −2.85, p < 0.01) or baseline and week 6 (t39 = −2.05, p < 0.05), such that the group difference at baseline (t85 = 2.32, p < 0.05) was no longer significant at week 2 (t81 = 1.58, p = 0.12) or week 6 (t79 = 0.62, p = 0.54) (figure 2b). No significant differences were found for any other face emotion.
(ii). Emotional Categorisation Task
A large proportion of individuals had near 100% accuracy for the ECAT, therefore in order to generate more normally distributed data, an arcsine transformation was first applied to the ECAT percentage data prior to analysis.
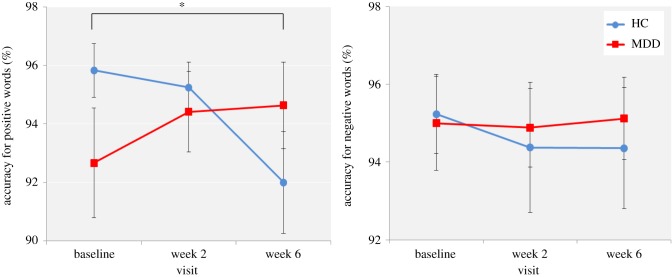
Baseline differences between the MDD and HC groups. For percentage accuracy, there was a significant word valence by group interaction (F1,85 = 4.12, p < 0.05), driven by reduced accuracy for positive versus negative self-referent word classification in the MDD group. The post hoc tests for each emotion separately, however, were not significant (positive: t85 = 1.46, p = 0.15; negative: t85 = −0.33, p = 0.75) (figure 3). For reaction time, there was a significant main effect of group (F1,85 = 6.25, p < 0.05), with the MDD group responding significantly more slowly than the HC group for both negative and positive self-referent words.
Figure 3.
ECAT arcsine percentage accuracy for positive self-referent words for the HC and MDD groups across the three visits. Values are reported as means ± standard error of the mean. (Online version in colour.)
Effect of bupropion. A significant visit by group interaction was found for percentage accuracy in positive self-referent word classification (F2,156 = 3.94, p < 0.05). It appeared that while the HC group displayed a decrease in the percentage accuracy for positive self-referent words, the bupropion-treated MDD group displayed an increase in the percentage accuracy for positive self-referent words across the three visits; however, post hoc paired t-tests only found the decrease in percentage accuracy for positive self-referent words in the HC group between baseline and week 6 to be significant (t39 = 2.64, p < 0.05) (figure 3).
(iii). Facial Dot-Probe Task
Baseline differences between the MDD and HC groups. A significant main effect of group was found for reaction time only (F1,85 = 8.95, p < 0.01), with the MDD group responding significantly more slowly than the HC group across all of the task conditions.
Effect of bupropion. There was also a significant visit by group interaction for reaction time (F2,156 = 12.55, p < 0.001), with the bupropion-treated MDD group displaying a greater reduction in reaction time than the HC group between baseline and week 2, likely as a result of their higher reaction time at baseline.
(iv). Emotional Recall Task
Baseline differences between the MDD and HC groups. There was no significant main effect of group or word valence by group interaction for either correctly (F1,86 = 0.08, p = 0.77; F1,86 = 1.07, p = 0.31) or falsely (F1,86 = 1.09, p = 0.30; F1,86 = 1.52, p = 0.22) recalled self-referent words.
Effect of bupropion. As illustrated in figure 4, the bupropion-treated MDD group displayed a decrease in the number of negative self-referent words falsely recalled between baseline and week 2 that was not observed in the HC group. A visit by group interaction was not found with three levels of visit (baseline, week 2 and week 6) (F2,158 = 2.75, p = 0.07) but was found with two levels of visit (baseline and week 2) (F1,81 = 5.73, p < 0.05) that remained when considering just negative falsely recalled self-referent words (F1,81 = 4.60, p < 0.05) and was confirmed by post hoc paired t-tests (t42 = 2.12, p < 0.05). There was no difference between the HC and MDD groups in the number of self-referent words correctly recalled across the three visits.
Figure 4.
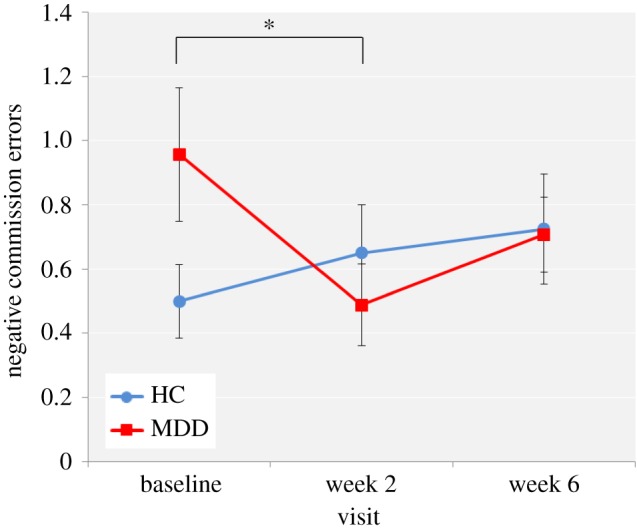
EREC number of negative self-referent words falsely recalled for the HC and MDD groups across the three visits. Values are reported as means ± standard error of the mean. Asterisk denotes the degree of significance obtained for planned comparisons (*p < 0.05). (Online version in colour.)
(v). Probabilistic instrumental learning task
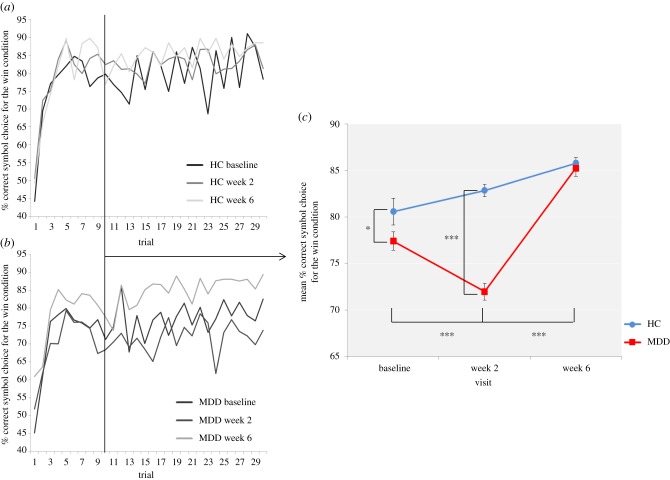
Baseline differences between the MDD and HC groups. Independent samples t-tests did not find a significant difference between the MDD and HC groups at baseline for the total monetary amount at the end of the task (t76 = −0.42, p = 0.67), amount won (t76 = 0.69, p = 0.49) or amount lost (t76 = −1.26, p = 0.21). In order to provide more temporal information about reward learning differences between the MDD and HC groups, learning curves were produced for each group depicting trial-by-trial the proportion of participants who chose the correct symbol in the win condition, associated with high-probability win (figure 5a,b). Both groups learnt to choose the high-probability win by about trial 10. To assess reward sensitivity after learning, the proportion of participants choosing the correct symbol in the win condition was averaged over the last 20 trials of the task [40] and the MDD group was found to be significantly less likely to choose the correct symbol in the win condition compared with the HC group (t38 = 2.03, p = 0.05), suggesting possible insensitivity to high-probability win. There were no baseline group differences in the likelihood of avoiding the incorrect symbol in the loss condition.
Figure 5.
Learning curves depicting trial-by-trial the proportion of participants who chose the correct symbol in the win condition associated with high-probability win at baseline, week 2 and week 6 for the (a) HC group and (b) MDD group. (c) Proportion of participants choosing the correct symbol in the win condition averaged over the last 20 trials of the probabilistic instrumental learning task at baseline, week 2 and week 6. Asterisks denote the degree of significance obtained for planned comparisons (*p < 0.05, ***p < 0.001). (Online version in colour.)
Effect of bupropion. There was no significant visit by group interaction for the total monetary amount at the end of the task (F2,108 = 0.07, p = 0.93), amount won (F2,108 = 0.51, p = 0.60) or amount lost (F2,108 = 1.24, p = 0.29). Regarding reward sensitivity after learning, a visit by group interaction was found for the win condition (F2,76 = 20.15, p < 0.001). Post hoc t-tests found only the bupropion-treated MDD group to display a significant reduction in the likelihood of choosing the correct symbol in the win condition between baseline and week 2 (t19 = 4.70, p < 0.001), such that the group difference observed at baseline was even greater at week 2 (t38 = 9.67, p < 0.001). The bupropion-treated MDD group then displayed a significant increase in the likelihood of choosing the correct symbol in the win condition between week 2 and week 6 (t19 = −11.20, p < 0.001), such that the group difference observed at baseline and week 2 was no longer significant (t38 = 0.51, p = 0.61) (figure 5c). No relationships were found between the likelihood of choosing the correct symbol in the win condition and anhedonia. There were no group differences in the change in the likelihood of avoiding the incorrect symbol in the loss condition.
4. Discussion
The present study aimed to investigate the early and sustained effects of bupropion on behavioural measures of emotional and reward processing in individuals suffering from depression in a repeated measures study design with an additional group of HCs not receiving any intervention. At baseline, the MDD group displayed the expected negative biases in emotional processing and impaired reward processing, as well as an overall non-valence-specific slowing of reaction times across all tasks compared with the HC group. Bupropion was found to reduce such negative biases in emotional processing early in treatment, including a significant decrease in the percentage misclassification of other face emotions as sad and the number of negative self-referent words falsely recalled between baseline and week 2. Conversely, bupropion was found to exacerbate impaired reward processing, with a significant decrease in the likelihood of choosing high-probability wins between baseline and week 2 prior to normalization to HC levels after the full six week treatment.
(a). Emotional processing
At baseline, the MDD group was found to display significantly increased misclassification of other face emotions as sad, reflected in decreased beta or increased response bias for sad faces, compared to the HC group. This is consistent with previous research that suggests depressed patients display negative biases in emotional processing [3–6], including increased perception of ambiguous face emotions as negative [7,8]. The MDD group also displayed an overall non-valence-specific slowing of reaction times across all tasks at baseline, consistent with psychomotor slowing that is also observed in depression.
Bupropion was found to significantly decrease the misclassification of other face emotions as sad or response bias for sad faces, as well as reduce the number of negative self-referent words falsely recalled between baseline and week 2. These results replicate those found in our previous study where an acute dose of bupropion was found to decrease the misclassification of other face emotions as sad and decrease negative self-referent recognition memory compared with placebo in healthy volunteers [30]. Therefore, bupropion may act to remediate the negative biases in emotional processing early in treatment, similarly to other antidepressants [6,11–13].
This provides further support for the hypothesis that antidepressants may act to restore the balance between positive and negative emotional processing early in treatment prior to mood improvement and may be related to the extent of later clinical improvement [41]. The similarity in effects on emotional processing across antidepressants acting on a range of neurotransmitters, including serotonin, noradrenaline and dopamine, and across healthy control and patient participant groups validates the use of this translational model both to help understand the effects of established treatments but also for the initial screening of the therapeutic potential of novel treatments for depression.
(b). Reward processing
At baseline, the MDD group also displayed expected abnormal response selection during the probabilistic instrumental learning task. In such tasks, HCs typically develop a reward response bias, learning to choose the stimulus associated with high-probability win and avoid the stimulus associated with high-probability loss in order to maximize payoffs. MDD patients fail to develop a reward response bias, resulting in reduced likelihood of choosing the stimulus associated with high-probability win [19–21]. The MDD group was found to be significantly less likely to choose the correct symbol in the win condition associated with high-probability win compared with the HC group at baseline in the present study, again consistent with previous research.
Building on pre-clinical evidence for a role of dopamine in reward [25,26], it has been suggested that such impaired reward processing involves changes in the dopamine system and may be particularly affected by drugs with an effect on this system. It has previously been found that administration of L-DOPA, the metabolic precursor of dopamine, significantly increases the likelihood of choosing the stimulus associated with high-probability win and subsequently the amount of money won during a probabilistic instrumental learning task, compared with the dopaminergic receptor antagonist haloperidol, although not compared with placebo [39]. Given that bupropion is one of the few antidepressants with an effect on dopamine function and has previously been shown to increase reward-related neural activity [42], it could be expected that treatment with the drug would remediate the impaired reward processing observed in the MDD group, acting to reduce the insensitivity to high-probability wins. Instead, bupropion was found to exacerbate impaired reward processing, with a significant decrease in the likelihood of choosing the correct symbol in the win condition associated with high-probability win between baseline and week 2. While this seems paradoxical, our previous study also found an acute dose of bupropion to have the same detrimental effects on reward processing compared with placebo in healthy volunteers [30]. It was suggested that bupropion may act to exacerbate impaired reward processing early in treatment, with the beneficial effects occurring later in treatment. Indeed, bupropion was found to normalize reward processing to HC levels following the full six week treatment. These are similar effects to those of both SSRIs [28,43] and amisulpride. While amisulpride is an antagonist at the presynaptic D2 receptor, acting to increase levels of dopamine in the synapse and increase reward-related neural activity, it has also been found to have no positive behavioural effects on reward learning [44]. It may be that acute administration of medication acting on dopamine function normalizes neural dysfunction but more chronic exposure is required for the positive behavioural effects on reward learning to occur [45,46].
A potential mechanism for the exacerbation of impaired reward learning may involve presynaptic autoreceptors that are activated with acute inhibition of dopamine reuptake leading to a paradoxical decrease in levels of dopamine in the synapse, but are desensitized with repeated treatment [47]. Alternatively, inhibition of dopamine reuptake may decrease the sensitivity of the dopamine reward system. Dopamine neurons exhibit either tonic or phasic firing. It has been suggested that the phasic firing of dopamine neurons may encode the difference between the actual occurrence of a reward and the predicted occurrence of a reward, known as the reward prediction error (RPE), providing a neural mechanism for instrumental learning [25]. If an unexpected reward occurs (positive surprise), the phasic firing of dopamine neurons increases to encode a positive RPE and the predicted occurrence of that reward is updated [25]. Inhibition of dopamine reuptake may act to increase tonic levels of dopamine, which in turn would decrease the phasic firing of dopamine neurons and the sensitivity of the dopamine reward system [48]. Such reduced sensitivity of the dopamine reward system may disrupt RPEs and subsequent instrumental learning. Psychologically, this may have the effect of reducing reward discriminability such that both outcomes in the win condition seem of similar magnitude [49].
In summary, bupropion appears to have distinct temporal effects on emotional and reward processing, acting to remediate negative biases in emotional processing but exacerbate impaired reward processing early in treatment, with the beneficial effects of bupropion on reward processing only occurring later in treatment. Therefore, despite the hypothesis that bupropion may be better suited to treat anhedonia, the timing of effects on reward processing suggests that anhedonia may also initially worsen prior to clinical improvement and needs to be further explored in more fine-grained clinical studies.
It is important to highlight a limitation of this study. Ideally, the study would have employed a double-blind design involving a separate control group of MDD patients receiving placebo for six weeks. However, we judged six weeks of no treatment in an acutely depressed group to be ethically and medically challenging outside of a randomized controlled trial for a novel compound. Instead, an open-label, repeated measures design was used and a separate group of HCs were recruited who did not receive bupropion, placebo or any other intervention to allow for comparison at baseline and to control for practice effects, similarly to other studies with the same ethical considerations, for example [50,51]. This does introduce a confound between treatment and group such that changes over time may be the result of regression to the mean of a spurious initial difference between groups rather than effects of bupropion treatment. However, the correspondence in effects using repeated measures in depressed patients here to a between-groups comparison with placebo in healthy volunteers provides reassurance that these effects are related to bupropion treatment and not simply an effect of expectation, changes in depression or repeat testing.
5. Conclusion
Overall, despite its alternative mechanism of action involving noradrenaline and dopamine, bupropion appears to have a similar profile of effects on emotional and reward processing to other antidepressants. Bupropion was found to remediate negative biases in emotional processing early in treatment, which may be instrumental in its therapeutic effect as an antidepressant as described by current theories of antidepressant action. Bupropion was also found to exacerbate reward deficits early in treatment prior to normalization following longer-term treatment. Such dissociation in the temporal effects of bupropion on emotional and reward processing has clinical implications for the use of bupropion in targeting anhedonia early versus late in treatment.
Supplementary Material
Data accessibility
See [52] for additional data.
Authors' contributions
A.E.L.W. carried out the data collection and statistical analysis and drafted the manuscript. M.B. designed the reward task and aided statistical analysis. C.J.H., W.C.D., M.F. and M.B. conceived of the study, designed the study, coordinated the study and helped draft the manuscript. All authors gave final approval for publication.
Competing interests
C.J.H. has received consultancy fees from P1vital, Servier and Lundbeck. She is director of Oxford Psychologists Ltd. and holds shares in the same company. She has received grant income from Lundbeck, Servier, Sunovion, Johnson & Johnson and UCB. M.B. is employed part time by P1vital Ltd and has received travel expenses from Lundbeck. W.C.D. and M.F. are employed by Janssen Research & Development, LLC of Johnson & Johnson, and hold equity in Johnson & Johnson, Inc.
Funding
This research was funded by Janssen Research & Development, limited liability company (LLC), of Johnson & Johnson and the Medical Research Council and supported by the NIHR Oxford Health Biomedical Research Centre. The views expressed are those of the authors and not necessarily those of Janssen R&D, the NHS, the NIHR or the Department of Health. We are grateful to the Royal Society for their support of the costs of attending this meeting ‘of mice and mental health: facilitating dialogue between basic and clinical neuroscientists’ convened by Amy Milton and Emily A. Holmes.
References
- 1.Chan SW, Goodwin GM, Harmer CJ. 2017. Highly neurotic never-depressed students have negative biases in information processing. Psychol. Med. 37, 1281–1291. ( 10.1017/S0033291707000669) [DOI] [PubMed] [Google Scholar]
- 2.Joormann J, Talbot L, Gotlib IH. 2007. Biased processing of emotional information in girls at risk for depression. J. Abnorm. Psychol. 116, 135–143. ( 10.1037/0021-843X.116.1.135) [DOI] [PubMed] [Google Scholar]
- 3.Mathews A, Macleod C. 2005. Cognitive vulnerability to emotional disorders. Annu. Rev. Clin. Psychol. 1, 167–195. ( 10.1146/annurev.clinpsy.1.102803.143916) [DOI] [PubMed] [Google Scholar]
- 4.Leppanen JM. 2006. Emotional information processing in mood disorders: a review of behavioral and neuroimaging findings. Curr. Opin Psychiatry 19, 34–39. ( 10.1097/01.yco.0000191500.46411.00) [DOI] [PubMed] [Google Scholar]
- 5.Elliott R, Zahn R, Deakin JFW, Anderson IM. 2011. Affective cognition and its disruption in mood disorders. Neuropsychopharmacology 36, 153–182. ( 10.1038/npp.2010.77) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roiser JP, Elliott R, Sahakian BJ. 2012. Cognitive mechanisms of treatment in depression. Neuropsychopharmacology 37, 117–136. ( 10.1038/npp.2011.183) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gur RC, Erwin RJ, Gur RE, Zwil AS, Heimberg C, Kraemer HC. 1992. Facial emotion discrimination: II. Behavioral findings in depression. Psychiatry Res. 42, 241–251. ( 10.1016/0165-1781(92)90116-K) [DOI] [PubMed] [Google Scholar]
- 8.Bouhuys AL, Geerts E, Gordijn MC. 1999. Depressed patients’ perceptions of facial emotions in depressed and remitted states are associated with relapse: a longitudinal study. J. Nerv. Ment. Dis. 187, 595–602. ( 10.1097/00005053-199910000-00002) [DOI] [PubMed] [Google Scholar]
- 9.Mogg K, Bradley BP, Williams R. 1995. Attentional bias in anxiety and depression: the role of awareness. Br. J. Clin. Psychol. 34, 17–36. ( 10.1111/j.2044-8260.1995.tb01434.x) [DOI] [PubMed] [Google Scholar]
- 10.Matt GE, Vázquez C, Campbell WK. 1992. Mood-congruent recall of affectively toned stimuli: a meta-analytic review. Clin. Psychol. Rev. 12, 227–255. ( 10.1016/0272-7358(92)90116-P) [DOI] [Google Scholar]
- 11.Harmer CJ, Goodwin GM, Cowen PJ. 2009. Why do antidepressants take so long to work? A cognitive neuropsychological model of antidepressant drug action. Br. J. Psychiatry 195, 102–108. ( 10.1192/bjp.bp.108.051193) [DOI] [PubMed] [Google Scholar]
- 12.Pringle A, Browning M, Cowen PJ, Harmer CJ. 2011. A cognitive neuropsychological model of antidepressant drug action. Prog. Neuropsychopharmacol. Biol. Psychiatry 35, 1586–1592. ( 10.1016/j.pnpbp.2010.07.022) [DOI] [PubMed] [Google Scholar]
- 13.Harmer CJ, Cowen PJ. 2013. ‘It's the way that you look at it’—a cognitive neuropsychological account of SSRI action in depression. Phil. Trans. R. Soc. B 368, 20120407 ( 10.1098/rstb.2012.0407) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harmer CJ, Bhagwagar Z, Perrett DI, Völlm BA, Cowen PJ, Goodwin GM. 2003. Acute SSRI administration affects the processing of social cues in healthy volunteers. Neuropsychopharmacology 28, 148–152. ( 10.1038/sj.npp.1300004) [DOI] [PubMed] [Google Scholar]
- 15.Harmer CJ, Hill SA, Taylor MJ, Cowen PJ, Goodwin GM. 2003. Toward a neuropsychological theory of antidepressant drug action: increase in positive emotional bias after potentiation of norepinephrine activity. Am. J. Psychiatry 160, 990–992. ( 10.1176/appi.ajp.160.5.990) [DOI] [PubMed] [Google Scholar]
- 16.Harmer CJ, Shelley NC, Cowen PJ, Goodwin GM. 2004. Increased positive versus negative affective perception and memory in healthy volunteers following selective serotonin and norepinephrine reuptake inhibition. Am. J. Psychiatry 161, 1256–1263. ( 10.1176/appi.ajp.161.7.1256) [DOI] [PubMed] [Google Scholar]
- 17.Harmer CJ, O'Sullivan U, Favaron E, Massey-Chase R, Ayres R, Reinecke A, Goodwin GM, Cowen PJ. 2009. Effect of acute antidepressant administration on negative affective bias in depressed patients. Am. J. Psychiatry 166, 1178–1184. ( 10.1176/appi.ajp.2009.09020149) [DOI] [PubMed] [Google Scholar]
- 18.Mcfarland BR, Klein DN. 2009. Emotional reactivity in depression: diminished responsiveness to anticipated reward but not to anticipated punishment or to nonreward or avoidance. Depress. Anxiety 26, 117–122. ( 10.1002/da.20513) [DOI] [PubMed] [Google Scholar]
- 19.Henriques JB, Davidson RJ. 2000. Decreased responsiveness to reward in depression. Cogn. Emot. 14, 711–724. ( 10.1080/02699930050117684) [DOI] [Google Scholar]
- 20.Pizzagalli DA, Iosifescu D, Hallett LA, Ratner KG, Fava M. 2008. Reduced hedonic capacity in major depressive disorder: evidence from a probabilistic reward task. J. Psychiatr. Res. 43, 76–87. ( 10.1016/j.jpsychires.2008.03.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vrieze E, Pizzagalli DA, Demyttenaere K, Hompes T, Sienaert P, De Boer P, Schmidt M, Claes S. 2013. Reduced reward learning predicts outcome in major depressive disorder. Biol. Psychiatry 73, 639–645. ( 10.1016/j.biopsych.2012.10.014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Steele JD, Kumar P, Ebmeier KP. 2007. Blunted response to feedback information in depressive illness. Brain 130, 2367–2374. ( 10.1093/brain/awm150) [DOI] [PubMed] [Google Scholar]
- 23.Chase HW, Frank MJ, Michael A, Bullmore ET, Sahakian BJ, Robbins TW. 2010. Approach and avoidance learning in patients with major depression and healthy controls: relation to anhedonia. Psychol. Med. 40, 433–440. ( 10.1017/S0033291709990468) [DOI] [PubMed] [Google Scholar]
- 24.Steele JD, Meyer M, Ebmeier KP. 2004. Neural predictive error signal correlates with depressive illness severity in a game paradigm. Neuroimage 23, 269–280. ( 10.1016/j.neuroimage.2004.04.023) [DOI] [PubMed] [Google Scholar]
- 25.Schultz W, Dayan P, Montague PR. 1997. A neural substrate of prediction and reward. Science 275, 1593–1599. ( 10.1126/science.275.5306.1593) [DOI] [PubMed] [Google Scholar]
- 26.Everitt BJ, Robbins TW. 2005. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat. Neurosci. 8, 1481–1489. ( 10.1038/nn1579) [DOI] [PubMed] [Google Scholar]
- 27.Nutt D, Demyttenaere K, Janka Z, Aarre T, Bourin M, Canonico PL, Carrasco JL, Stahl S. 2007. The other face of depression, reduced positive affect: the role of catecholamines in causation and cure. J. Psychopharmacol. 21, 461–471. ( 10.1177/0269881106069938) [DOI] [PubMed] [Google Scholar]
- 28.McCabe C, Mishor Z, Cowen PJ, Harmer CJ. 2010. Diminished neural processing of aversive and rewarding stimuli during selective serotonin reuptake inhibitor treatment. Biol. Psychiatry 67, 439–445. ( 10.1016/j.biopsych.2009.11.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tomarken AJ, Dichter GS, Freid C, Addington S, Shelton RC. 2004. Assessing the effects of bupropion SR on mood dimensions of depression. J. Affect. Disord. 78, 235–241. ( 10.1016/S0165-0327(02)00306-3) [DOI] [PubMed] [Google Scholar]
- 30.Walsh AEL, Huneke N, Brown R, Browning M, Cowen PJ, Harmer CJ. Submitted. A dissociation of the acute effects of bupropion on positive emotional processing and reward processing in healthy volunteers, Psychological Medicine. [DOI] [PMC free article] [PubMed]
- 31.First MB. 1997. User's guide for the structured clinical interview for DSM-IV Axis 1 disorders : SCID-I clinician version. Washington, DC: American Psychiatric Press. [Google Scholar]
- 32.Nelson HE, Willison J.1991. National adult reading test (NART). 2nd edn (eds Hazel E. Nelson with Jonathan Willison.) Windsor, UK: NFER-Nelson.
- 33.Hamilton M. 1960. A rating scale for depression. J. Neurol. Neurosurg. Psychiatry 23, 56–62. ( 10.1136/jnnp.23.1.56) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eysenck HJ, Eysenck SBG. 1975. Manual of the Eysenck personality questionnaire (junior and adult). Sevenoaks, UK: Hodder and Stoughton. [Google Scholar]
- 35.Watson D, Clark LA, Weber K, Assenheimer JS, Strauss ME, McCormick RA. 1995. Testing a tripartite model: I. Evaluating the convergent and discriminant validity of anxiety and depression symptom scales. J. Abnormal Psychol. 104, 3–14 ( 10.1037/0021-843X.104.1.3) [DOI] [PubMed] [Google Scholar]
- 36.Watson D, Clark LA, Tellegen A. 1998. Development and validation of brief measures of positive and negative affect: the PANAS scales. J. Pers. Soc. Psychol. 54, 1063–1070. ( 10.1037/0022-3514.54.6.1063) [DOI] [PubMed] [Google Scholar]
- 37.Snaith RP, Hamilton M, Morley S, Humayan A, Hargreaves D, Trigwell P. 1995. A scale for the assessment of hedonic tone the Snaith–Hamilton Pleasure Scale. Br. J. Psychiatry 167, 99–103. ( 10.1192/bjp.167.1.99) [DOI] [PubMed] [Google Scholar]
- 38.Price J, Cole V, Doll H, Goodwin GM. 2012. The Oxford Questionnaire on the Emotional Side-effects of Antidepressants (OQuESA): development, validity, reliability and sensitivity to change. J. Affect. Disord. 140, 66–74. ( 10.1016/j.jad.2012.01.030) [DOI] [PubMed] [Google Scholar]
- 39.Pessiglione M, Seymour B, Flandin G, Dolan RJ, Frith CD. 2006. Dopamine-dependent prediction errors underpin reward-seeking behaviour in humans. Nature 442, 1042–1045. ( 10.1038/nature05051) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harrison NA, Voon V, Cercignani M, Cooper EA, Pessiglione M, Critchley HD. 2016. A neurocomputational account of how inflammation enhances sensitivity to punishments versus rewards. Biol. Psychiatry 80, 73–81. ( 10.1016/j.biopsych.2015.07.018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tranter R, Bell D, Gutting P, Harmer C, Healy D, Anderson IM. 2009. The effect of serotonergic and noradrenergic antidepressants on face emotion processing in depressed patients. J. Affect. Disord. 118, 87–93. ( 10.1016/j.jad.2009.01.028) [DOI] [PubMed] [Google Scholar]
- 42.Dean Z, Horndasch S, Giannopoulos P, Mccabe C. 2016. Enhanced neural response to anticipation, effort and consummation of reward and aversion during bupropion treatment. Psychol. Med. 46, 2263–2274. ( 10.1017/S003329171600088X) [DOI] [PubMed] [Google Scholar]
- 43.Scholl J, Kolling N, Nelissen N, Browning M, Rushworth MFS, Harmer CJ. 2017. Beyond negative valence: 2-week administration of a serotonergic antidepressant enhances both reward and effort learning signals. PLoS Biol. 15, e2000756 ( 10.1371/journal.pbio.2000756) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Admon R, Kaiser RH, Dillon DG, Beltzer M, Goer F, Olson DP, Vitaliano G, Pizzagalli DA. 2017. Dopaminergic enhancement of striatal response to reward in major depression. Am. J. Psychiatry 174, 378–386. ( 10.1176/appi.ajp.2016.16010111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Amore M, Jori MC. 2001. Faster response on amisulpride 50 mg versus sertraline 50–100 mg in patients with dysthymia or double depression: a randomized, double-blind, parallel group study. Int. Clin. Psychopharmacol. 16, 317–324. ( 10.1097/00004850-200111000-00001) [DOI] [PubMed] [Google Scholar]
- 46.Cassano GB, Jori MC. 2002. Efficacy and safety of amisulpride 50 mg versus paroxetine 20 mg in major depression: a randomized, double-blind, parallel group study. Int. Clin. Psychopharmacol. 17, 27–32. ( 10.1097/00004850-200201000-00004) [DOI] [PubMed] [Google Scholar]
- 47.Ford CP. 2014. The role of D2-autoreceptors in regulating dopamine neuron activity and transmission. Neuroscience 282, 13–22. ( 10.1016/j.neuroscience.2014.01.025) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Grace AA. 1991. Phasic versus tonic dopamine release and the modulation of dopamine system responsivity: a hypothesis for the etiology of schizophrenia. Neuroscience 41, 1–24. ( 10.1016/0306-4522(91)90196-U) [DOI] [PubMed] [Google Scholar]
- 49.Rutledge RB, Skandali N, Dayan P, Dolan RJ. 2015. Dopaminergic modulation of decision making and subjective well-being. J. Neurosci. 35, 9811–9822. ( 10.1523/JNEUROSCI.0702-15.2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fu CHY, et al. 2004. Attenuation of the neural response to sad faces in major depression by antidepressant treatment: a prospective, event-related functional magnetic resonance imaging study. Arch. Gen. Psychiatry 61, 877–889. ( 10.1001/archpsyc.61.9.877) [DOI] [PubMed] [Google Scholar]
- 51.Fu CHY, et al. 2007. Neural responses to happy facial expressions in major depression following antidepressant treatment. Am. J. Psychiatry 164, 599–607. ( 10.1176/ajp.2007.164.4.599) [DOI] [PubMed] [Google Scholar]
- 52.Walsh AEL, Browning M, Drevets WC, Furey M, Harmer CJ. 2017. Data from: Dissociable temporal effects of bupropion on behavioural measures of emotional and reward processing in depression. Dryad Digital Repository ( 10.5061/dryad.17296) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Walsh AEL, Browning M, Drevets WC, Furey M, Harmer CJ. 2017. Data from: Dissociable temporal effects of bupropion on behavioural measures of emotional and reward processing in depression. Dryad Digital Repository ( 10.5061/dryad.17296) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
See [52] for additional data.