Abstract
Fear conditioning is widely employed to examine the mechanisms that underlie dysregulations of the fear system. Various manipulations are often used following fear acquisition to attenuate fear memories. In rodent studies, freezing is often the main output measure to quantify ‘fear’. Here, we developed data-driven criteria for defining a standard benchmark that indicates remission from conditioned fear and for identifying subgroups with differential treatment responses. These analyses will enable a better understanding of individual differences in treatment responding.
This article is part of a discussion meeting issue ‘Of mice and mental health: facilitating dialogue between basic and clinical neuroscientists’.
Keywords: fear learning, extinction, reinstatement, remission, individual differences
1. Introduction
Freezing, or becoming motionless in the presence of fear-evoking stimuli, is one of the innate defensive reactions of rats and other animals, and is widely used as an outcome measure in studies of conditioned fear. In fear conditioning (table 1), the pairing of an initial neutral stimulus (conditional stimulus, CS) to an unconditional aversive stimulus (US) leads to associative learning, such that the previously neutral stimulus comes to elicit a conditioned response (CR). The subsequent presentation of the CS in the absence of the US leads to a progressive decrease in conditioned responding, a phenomenon called extinction. Impaired extinction of conditioned freezing has been suggested as a model of human anxiety disorders such as post-traumatic stress disorder (PTSD), and has been used to develop novel treatments aimed at enhancing fear extinction or disrupting fear memories. Thus, experimental therapies aimed at treating human anxiety disorders are often developed in non-human animal models, with long-term reductions in conditioned freezing (e.g. prevention of spontaneous recovery, renewal or fear reinstatement) used as a primary outcome measure. Such studies have traditionally relied on significant mean differences in freezing between experimental groups to gauge treatment efficacy. However, there are two limitations to this approach: (i) the ultimate objective when treating human illness is not just to reduce symptoms but to restore patients to good health, with clear benchmarks defining remission. For example, remission from PTSD has been defined as no longer meeting DSM or ICD criteria for a PTSD diagnosis [1]. The percentage of patients who remit under treatment provides important additional information when evaluating the efficacy of a treatment, but ‘remission’ in terms of rodent freezing behaviour has not been operationalized, much less routinely assessed; (ii) just as with human patients, there may be extensive response heterogeneity to a treatment, with some subjects responding well and others responding poorly, which is not captured by a simple analysis of means.
Table 1.
Glossary of key terms and definitions.
| key term | definition |
|---|---|
| fear conditioning | experimental paradigm in which the pairing of an initially neutral stimulus (conditional stimulus; CS) to an aversive unconditional stimulus (US) leads to associative learning, such that subsequent presentations of the CS elicit a conditioned response (CR) |
| extinction | repeated presentations of the conditioned stimulus (CS) in the absence of an unconditioned stimulus (US) lead to a progressive decrease in conditioned responding |
| reinstatement | return of conditioned responding (return of fear) after exposure to an unsignalled stressor (e.g. a shock) |
| renewal | return of conditioned responding (return of fear) when the conditioned stimulus (CS) is presented in a context other than the extinction context |
| spontaneous recovery | return of conditioned responding (return of fear) after the passage of time following extinction |
In this paper, our objective is to develop data-driven criteria for defining a standard benchmark that indicates remission from conditioned fear and for identifying subgroups with differential treatment responses. We present our methods and results in three parts. Study 1: To identify an objective criterion for remission, we use logistic regression to obtain the freezing point at which there is an equal probability of belonging to a population of rats that never underwent fear conditioning versus a population of rats that did undergo fear conditioning. Study 2: To identify subgroups that are homogeneous in terms of treatment response, we use agglomerative hierarchical clustering to group subjects according to their patterns of freezing across acquisition, extinction and reinstatement conditions. Study 3: We apply the criteria for remission and homogeneous subgroups established in Studies 1 and 2 to the reanalysis of an experiment evaluating the impact of extinction training and pharmacological interventions on the prevention of fear reinstatement.
2. Study 1
(a). Methods
The data used for analysis in this study were aggregated from two published studies [2,3] and three additional, unpublished, studies using the fear-conditioning-by-proxy (FCbP) triadic design [2–5], which includes both an experimental group that receives fear conditioning (FC) and one with no fear conditioning (No-FC). This analysis was limited to data from rats that had no prior experience with fear conditioning, resulting in a sample size of N = 113 for the No-FC group and N = 117 for the FC group for Study 1a (Males) and N = 142 for the No-FC group and the FC group in Study 1b (Females).
(i). Subjects
In Study 1a, subjects were male Sprague–Dawley rats bred at the University of Texas at Austin from breeder pairs that consisted of males acquired from Harlan (now Envigo) (275–300 g) and females acquired from Charles River (215–275 g). Rats were weaned into same sex triads with littermates on postnatal day 21. In Study 1b, subjects were the female littermates of the Sprague–Dawley males used for Study 1a and female littermates of the Long Evans males bred for extinction behavioural phenotypes at the University of Texas at Austin (see Shumake et al. [6] for a detailed description of selection criteria and breeding procedures).
All subjects were housed in clear plastic cages and maintained on a 12 h–12 h light–dark cycle (lights on at 07.00) with ad libitum access to food and water. In Study 1a, all animals were fear conditioned at approximately 100 days of age. In Study 1b, subjects were fear conditioned at approximately 80 days of age.
Procedures were conducted in compliance with the National Institutes of Health Guide for the Care and Use of Experimental Animals and were approved by the Institutional Animal Care and Use Committee at the University of Texas at Austin, under protocol number AUP-2015-00114.
(ii). Apparatus and stimuli
Study 1a. In approximately half of the animals tested (n = 124), fear conditioning and/or testing took place in standard conditioning chambers equipped with two Plexiglas walls, two metal walls and stainless-steel rod floors connected to a shock generator (Coulbourn Instruments). Behaviour was recorded with digital cameras mounted on the top of each unit. The conditioned stimulus (CS) was an 80 dB, 5 kHz tone, 20 s in duration and the unconditioned stimulus (US) was a 0.7 mA footshock, 500 ms in duration. Stimulus delivery was controlled using Freeze Frame software (Coulbourn Instruments).
The other half of testing (n = 106) took place in the left portion of a closed shuttle box equipped with two black and white striped walls, two metal walls and stainless-steel rod floors connected to a shock generator (Coulbourn Instruments). Stimulus delivery was controlled using Graphic State 2 software (Coulbourn Instruments). The CS was an 80 dB white noise, 20 s in duration and the US was a 0.6 mA footshock, 500 ms in duration (when applicable).
Study 1b. In Study 1b, all animals were fear conditioned in standard conditioning chambers equipped with two Plexiglas walls, two metal walls and stainless-steel rod floors connected to a shock generator (Coulbourn Instruments). The CS was an 80 dB, 5 kHz tone, 20 s in duration and the US was a 0.7 mA footshock, 500 ms in duration. Stimulus delivery was controlled using Freeze Frame software (Coulbourn Instruments).
(iii). Testing procedure
Study 1a. A subset of animals (N = 46) were part of a control group in a developmental experiment examining early life experiences [2]. On post-natal day 17, these animals were removed from their mother, placed in a clean cage and transported to a different room for 10 min. They were not exposed to any stimuli during this time. Another subset of animals (n = 79) were weighed and received a single injection of saline (intraperitoneal) on post-natal day 45. These animals were equally distributed across FC and No-FC groups. Fear conditioning (day 1): After a 7-min habituation period one rat per triad (FC rat) received three presentations of the CS (duration = 20 s; inter-trial interval (ITI) = 180 s on average, variable), each co-terminating with the US. No-FC rats remained in their home cage.
Fear conditioning by proxy (day 2): One day after conditioning, the fear conditioned rat was returned to the chamber accompanied by a cagemate. Three CSs were played in the absence of the US and rats could freely interact. The third rat (No-FC rat) remained in the home cage and was not exposed to any stimuli.
Fear test (day 3): 48 h after FC, each animal was presented the CS in the absence of the US three times (ITI = 180 s on average, variable). Fear retention was analysed as an average (the mean) of freezing during the three CS presentations for each rat.
Study 1b. All subjects were experimentally naive prior to the fear conditioning procedure which was identical to the procedure used in Study 1a. All females had their oestrous cycles tracked for one to two weeks prior to fear conditioning (or no fear conditioning). Vaginal smears were taken daily between 09.30 and 11.00 h and samples were observed under a light microscope at 10× magnification. The phase of oestrous (proestrus, oestrus, dioestrus 1 or dioestrus 2) was determined from a description of their vaginal cytology (see [7]). In N = 72 animals, fear retention was timed to occur when the FC rat was in proestrus. We have previously found no effect of oestrous cycle on fear retention in this paradigm [5].
(iv). Data scoring
Videos were watched by a trained observer blind to experimental condition, and freezing was scored during each CS presentation. Freezing was defined as the absence of any movement, excluding breathing and whisker twitching. The total number of seconds spent freezing throughout the CS presentation was expressed as a percentage of CS duration (20 s) for analysis during the fear retention test on day 3.
(v). Fear classification cut-off analysis
To develop a probabilistic model relating observed freezing scores to the likelihood of belonging to the FC versus No-FC population, a logistic regression was performed using the mean freezing scores from three tone presentations to predict the probability that an animal has been conditioned to fear the tone. This probability is given by the following expression:
where β0 is the intercept and β1 is the slope from a generalized linear model using a logit link function in which the binomial variable of whether or not a subject has undergone fear conditioning was predicted by freezing scores. β1 is multiplied by the freezing score expressed on a percentage scale (0–100). Conceptually, if one were to know nothing about an animal other than its freezing behaviour, this function would allow one to calculate the odds that the animal has undergone fear conditioning. The percentage of freezing at which the odds are 50/50 is then given by the negative ratio of the intercept to the slope:
We aimed to use this quantity as our benchmark for remission from conditioned fear, and we computed a 95% confidence interval for this value by resampling it with 10 000 bootstrap replicates, using the adjusted bootstrap percentile (BCa) method as implemented in the R package ‘boot’ [8]. If freezing scores are reduced to less than this quantity, an observer blind to the training history of a fear conditioned animal could objectively conclude that this animal bears a stronger resemblance to a population that has never undergone fear conditioning than to a population that has. To evaluate the utility of this criterion, we graphed the probability density functions for each population and evaluated the degree to which these functions overlap.
(b). Results
(i). Conditioned freezing versus spontaneous freezing
As figure 1 illustrates, there were large individual differences in both tone–shock conditioned freezing (FC group) and spontaneous freezing (No-FC group) for both males and females. Both distributions departed extensively from the normal distribution. For Study 1a (Males), logistic regression found that the optimal point for minimizing overlap between the two distributions was at 37.3% freezing, 95% CI [32.5, 41.8]. At this cut-off, there were only three rats from the FC group misclassified as belonging to the No-FC group, and only four rats from the No-FC group misclassified as belonging to the FC group. The remaining 223 rats were correctly classified. In summary, there was very little overlap between the distributions: 97.3% of animals with a mean freezing score less than 37.3% belong to the No-FC group, and 96.6% of animals with a mean freezing score greater than 37.3% belong to the FC group (figure 1a).
Figure 1.
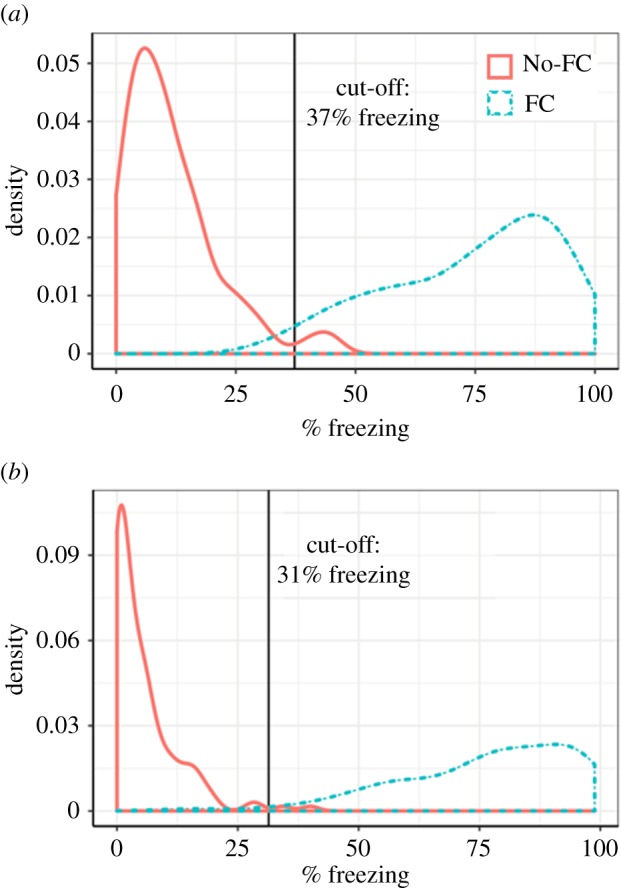
Freezing distributions for (a) males and (b) females below and above cut-off criteria for fear responding. There were large individual differences in tone–shock conditioned freezing (FC group) and spontaneous freezing (No-FC group) for both males and females. (a) For males, logistic regression found that the optimal point for minimizing overlap between the two distributions was at 37.3% freezing. (b) For females, the optimal point for minimizing overlap between the two distributions was at 31.4% freezing. (Online version in colour.)
For Study 1b (Females), logistic regression found that the optimal point for minimizing overlap between the two distributions was at 31.4% freezing, 95% CI [24.9, 37.9]. At this cut-off, there were only two rats from the FC group misclassified as belonging to the No-FC group, and only two rats from the No-FC group misclassified as belonging to the FC group. The remaining 138 rats were correctly classified. In summary, for the Females, much like was the case for the Males, there was very little overlap between the distributions (figure 1b).
The coefficients and standard errors for the logistic equation (given in the above methods) for calculating probability of belonging to the fear conditioned population are as follows: β0 = −9.1 (s.e. = 2.1) and β1 = 0.24 (s.e. = 0.055). Figure 2 shows graphs of the expected probability of having undergone fear conditioning as a function of freezing for each individual, with the actual history of fear conditioning indicated by colour and shape of symbols.
Figure 2.
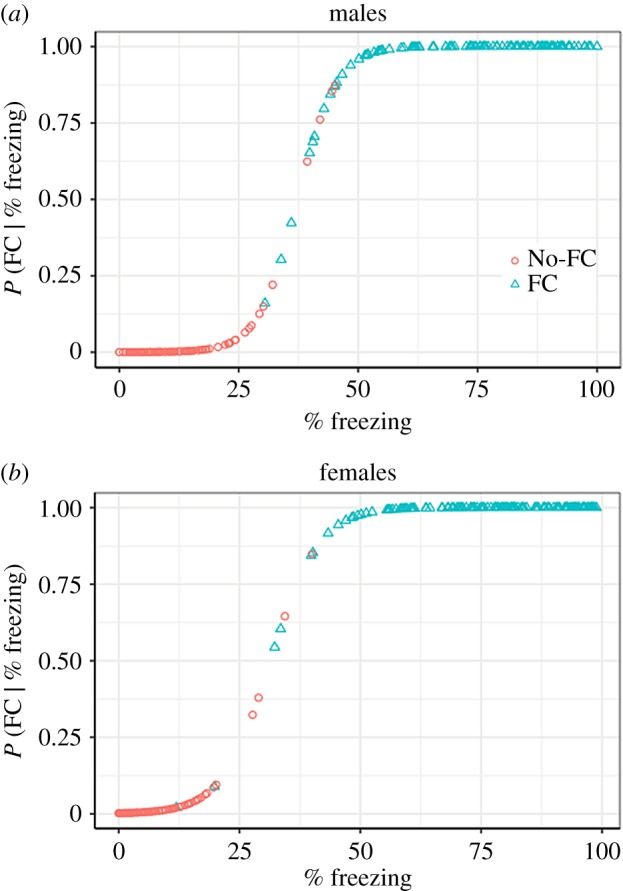
Expected probability of having undergone fear conditioning as a function of freezing for each individual. (a) The shape of the function for males shows that the probability of fear conditioning (Y-axis) changes rapidly as one moves away from the 37.3% freezing mark (X-axis). (b) For females, the optimal point for minimizing overlap between the two distributions was at 31.4% freezing. For the females, much like was the case for the males, there was very little overlap between the distributions. These graphs show the predictions of the logistic models described in Study 1, with the actual history of fear conditioning indicated by colour and shape. (Online version in colour.)
The shape of the function in figure 2a (males) shows that, owing to the minimal overlap of the distributions (3%), the probability changes rapidly as one moves away from the 37.3% freezing mark: the presence of conditioned fear becomes a virtual certainty when freezing is greater than 50%, and the absence of conditioned fear becomes a virtual certainty when freezing is less than 25%. This suggests that, in the context of evaluating treatments to reduce conditioned fear, freezing levels that remain above 50% should indicate non-remission, and freezing levels that fall below 25% should indicate full remission. Freezing levels that fall between 25% and 50% should be considered a partial response to treatment. We suggest using the midpoint between these two values, 37.5%, as the upper bound for indicating probable remission. This value is close to the empirically derived value of 37.3%—well within the 95% CI—and can easily be remembered as the midpoint between 25 and 50. This value of 37.5% is also very much in line with the criterion established by the individual differences analysis of Galatzer-Levy et al. [9], who determined, through an analysis of their own samples, and a methodology different from ours, that their ‘Failure to Extinguish’ group showed freezing levels greater than 37.03% during their last three trials of extinction. The function in figure 2b (females) also shows a very similar pattern of data, with a slight shift down to a cut-off criterion of 31.4%.
3. Study 2
(a). Methods
(i). Subjects
Study 2 used data from 215 subjects from two published studies [10,11]. Auchter et al. [10] included 124 male Sprague-Dawley rats, acquired from Harlan (now Envigo) (250–300 g). Auchter et al. [11] included 91 male Long-Evans rats, selected from the third and fourth generations of a line bred selectively for extinction phenotype (see [6] for a detailed description of selection criteria and breeding procedures). Briefly, breeders for a low extinguisher (LE) and high extinguisher (HE) line were chosen by a two-step procedure that selected for differences in extinction in the absence of differences in acquisition. A randomly bred (RB) control line was started from a random selection of males and females from the sample that were not chosen for the LE or HE lines. All subjects were housed in clear plastic cages and maintained on a 12 h–12 h light–dark cycle (lights on at 07.00) with ad libitum access to food and water.
Procedures were conducted in compliance with the National Institutes of Health Guide for the Care and Use of Experimental Animals and were approved by the Institutional Animal Care and Use Committee at the University of Texas at Austin.
(ii). Fear conditioning, extinction and reinstatement procedures
All testing took place in standard conditioning chambers equipped with two Plexiglas walls, two metal walls and stainless-steel rod floors connected to a shock generator (Coulbourn Instruments). Behaviour was recorded with digital cameras mounted on the top of each unit. The conditioned stimulus (CS) was an 80 dB, 5 kHz tone, 20 s in duration and the unconditioned stimulus (US) was a 0.7 mA footshock, 500 ms in duration. Stimulus delivery was controlled using Freeze Frame software (Coulbourn Instruments). Subjects underwent fear conditioning, extinction and reinstatement as described in two published studies [10,11]. Briefly, rats were habituated to the context for 10 min [10] or 15 min [11] the day prior to fear conditioning. Fear conditioning consisted of three 20 s presentations of a tone CS co-terminating with a 0.7 mA footshock.
On the following day, subjects were placed back into the same context, given 3 min to acclimate, and then received either extinction training alone (no-retrieval condition), or a single retrieval CS followed 60 min later by extinction (retrieval condition). Extinction consisted of 18 (retrieval condition) or 19 (no-retrieval condition) CSs. In Auchter et al. [10], the timing of the extinction CSs was manipulated such that the inter-trial intervals (ITIs) were either fixed (i.e. the same ITI between each CS) or variable (i.e. a different ITI between each CS), and the average ITI was either 1 min or 2 min. In Auchter et al. [11], all subjects received variable extinction ITIs, and the average ITI was 2 min in all groups. However, following extinction, half of the subjects were given an injection of mitochondrial enhancer USP methylene blue, while the other half received saline. Twenty-four hours after extinction, subjects were placed back into the chambers, allowed to habituate for 5 min, followed by two exposures to the US alone. The following day, subjects were placed back in the context and given three presentations of the CS alone to probe for reinstatement-induced return of fear. Freezing was scored the same way as Study 1.
(iii). Subgroup phenotype cluster analysis
As noted above, subjects underwent a variety of differential conditioning parameters that may have impacted fear reinstatement, and we analyse the impact of some of these factors in Study 3. However, for the purposes of Study 2, these factors are ignored because we wish to establish whether subjects can be clustered into homogeneous subgroups based solely on their freezing profiles across three time points: (i) the beginning of the extinction session, (ii) the end of the extinction session, and (iii) following reinstatement. To classify animals, we used agglomerative hierarchical clustering analysis. With this procedure, groups of subjects that merge at high values relative to the merge points of their subgroups are considered candidates for natural subgroups [12]. Hierarchical clustering was conducted according to the Ward.D2 agglomeration method in R on a subject dissimilarity matrix defined by the Manhattan distance. The resulting dendrogram was then pruned using the Dynamic Tree Cut package [13] using the hybrid method.
(b). Results
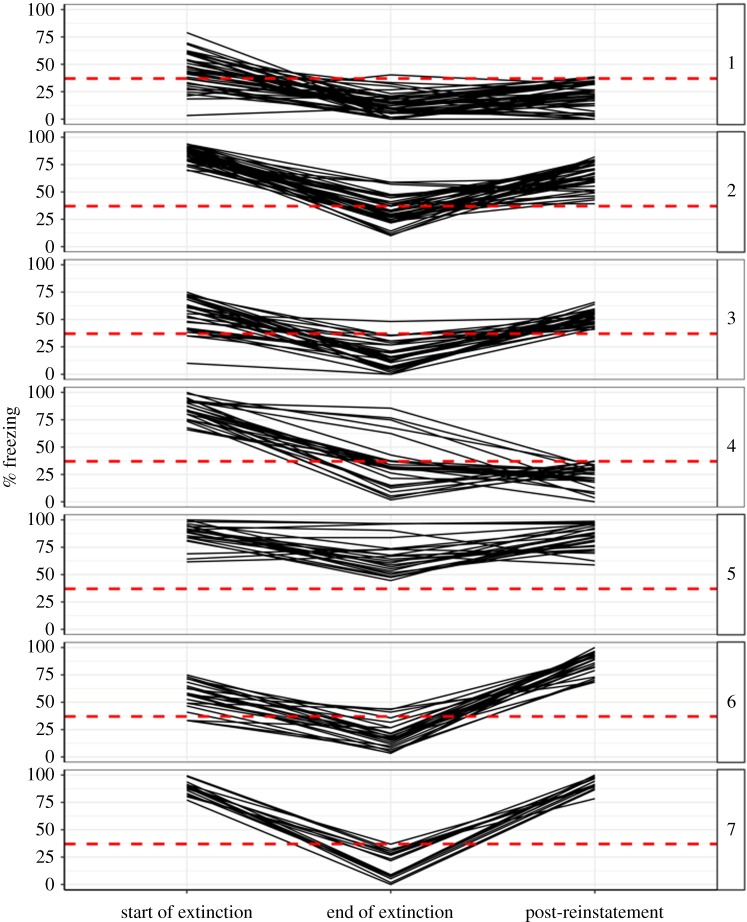
The cluster analysis identified seven homogeneous subgroups in terms of patterns of freezing 24 h after acquisition, at the end of extinction, and 24 h after reinstatement. The individual freezing profiles for all 215 subjects are graphed for each of these groups in figure 3, with panels numbered 1–7 in order of largest to smallest group size.
Figure 3.
Phenotypic extinction subgroups identified by cluster analysis. Each solid black line graph shows the individual longitudinal trajectory of freezing behaviour (Y-axis) for each subject across three critical phases of training (labelled on the X-axis). The horizontal dashed red line is a reference line indicating criterion for remission (37.5% freezing). Each panel indicates a homogeneous subgroup showing similar freezing trajectories. Subgroup 1: Mild initial fear with successful extinction and long-term fear reduction (n = 44). Subgroup 2: Severe initial fear with moderately successful extinction followed by return of fear (n = 42). Subgroup 3: Mild initial fear with largely successful extinction followed by return of fear (n = 33). Subgroup 4: Severe initial fear with largely successful extinction and long-term fear reduction (n = 27). Subgroup 5: Severe, persistent fear (n = 27). Subgroup 6: Fear incubation (n = 25). Subgroup 7: A more extreme version of Subgroup 2 (n = 17). (Online version in colour.)
Subgroup 1: Mild initial fear with successful extinction and long-term fear reduction (n = 44). The most common subgroup identified, characterizing 20% of the sample, consisted of subjects who showed relatively low levels of freezing following acquisition (median = 43%, range = 3–79%) and low levels of freezing following extinction (median = 11%, range = 0–40%) that were maintained following reinstatement (median = 23%, range = 0–39%). This represents a group that experienced a successful long-term reduction in fear, and the maximum freezing score observed following reinstatement for this group, 39%, is very close to the criterion of remission (37.5%) that we established using a completely different approach and sample in Study 1. However, the fact that the majority of these subjects were freezing less than 50% of the time even prior to extinction suggests that the success of this subgroup, like that of many human patients, may depend on having less severe baseline symptoms.
Subgroup 2: Severe initial fear with moderately successful extinction followed by return of fear (n = 42). The next most common subgroup, also characterizing 20% of the sample, consisted of subjects who showed high levels of freezing following acquisition (median = 85%, range = 70–94%) and moderate to low levels of freezing following extinction (median = 32%, range = 10–59%) that were mostly lost following reinstatement (median = 64%, range = 39–82%). This represents a non-responding subgroup, and note that the minimum freezing score observed for this group following reinstatement was 39%, once again very close to the previously derived criterion of remission (37.5%).
Subgroup 3: Mild initial fear with largely successful extinction followed by return of fear (n = 33). The third most common subgroup identified, characterizing 15% of the sample, appeared similar to Subgroup 1 in terms of fear acquisition (median = 58%, range = 10–75%) and extinction (median = 16%, range = 0–48%), except these subjects returned to their baseline level of fear following reinstatement (median = 52%, range = 41–66%). This represents a subgroup with less baseline severity that nonetheless did not respond to extinction and/or other manipulations aimed at reducing fear. The minimum freezing score observed for this group, 41%, is again just above the 37.5% threshold for remission from fear.
Subgroup 4: Severe initial fear with largely successful extinction and long-term fear reduction (n = 27). The fourth most common subgroup, characterizing 13% of the sample, represents the ideal treatment response group: despite high initial levels of freezing (median = 84%, range = 66–100%), most subjects experienced substantially less freezing following extinction (median = 32%, range = 2–86%), and all experienced a long-term reduction in freezing following reinstatement (median = 27%, range = 0–37%). Interestingly, this group contained a small number of subjects who experienced a long-term reduction in fear despite showing little reduction in fear during the extinction session itself, but this pattern was too rare to support clustering them as a separate subgroup. Note that the maximum final level of freezing observed for this subgroup was 37%, corresponding exactly to the criterion of remission established in Study 1.
Subgroup 5: Severe, persistent fear (n = 27). Another 13% of the sample showed high initial levels of freezing (median = 90%, range = 62–100%) that showed little or no response to extinction (median = 67%, range = 45–97%) which, not surprisingly, also translated into high levels of freezing following reinstatement (median = 85%, range = 59–99%).
Subgroup 6: Fear incubation (n = 25). Another 12% of the sample showed evidence of fear incubation; despite relatively low initial levels of freezing following acquisition (median = 58%, range = 33–75%) and extinction (median = 18%, range = 3–44%), these subjects showed very high levels of freezing following reinstatement (median = 89%, range = 68–100%).
Subgroup 7: A more extreme version of Subgroup 2 (n = 17). The final subgroup, comprising 8% of the sample, showed the same pattern as Subgroup 2, which is the classic ‘V’-shaped response of reduction in fear followed by return of fear. They share similar levels of initial freezing to Subgroup 2 (median = 88%, range = 77–99%), but differ in that they show somewhat better extinction (median = 22%, range = 0 = 37%) and much worse return of fear (median = 91%, range = 78–100%). Whereas Subgroup 2 might be considered as showing a partial long-term response to fear-reduction interventions, Subgroup 7 showed no long-term response whatsoever.
4. Study 3
(a). Methods
Fear classification and phenotype criteria established in Studies 1 and 2 were employed to revisit the results of a previously published study from our laboratory [11]. Please see this study and its associated supplemental section for a more conventional analysis that models freezing as a continuous outcome measure using hierarchical linear and mixed effects regressions.
In this new analysis, the primary outcome measure for each treatment condition is the rate of fear remission, defined as the percentage of subjects meeting the criterion established in Study 1 (fewer than 37.5% freezing), following an attempt to reinstate fear as described in the Methods for Study 2. A 95% confidence interval for each remission rate was estimated using 10 000 bootstrap replicates and the BCa method in the R package ‘boot’ [8].
(b). Results
(i). Remission rates under various extinction manipulations
As shown in figure 4, under standard extinction training with saline injections, the rate of long-term remission was only 18%, 95% CI [4%, 35%]. The rate of remission nearly doubled with either the addition of a retrieval trial prior to extinction (33%, 95% CI [13%, 54%]) or MB injection after extinction (35%, 95% CI [17%, 55%]). However, all remission rates lie within each other's 95% CIs, so we cannot reject the null hypothesis: there is insufficient evidence that either of these manipulations on its own has an impact on long-term fear remission. Even if this is a Type II error, a full two-thirds of the sample fail to achieve remission under either manipulation alone. However, if the manipulations are combined, 50% of the subjects achieve remission status, 95% CI [27%, 68%], demonstrating significant superiority over standard extinction with saline (figure 4). Collapsing across all interventions, we observed a significantly lower rate of remission for the LE line (14%, 95% CI [3%, 30%]) than we observed for either the RB line (35%, 95% CI [21%, 50%]) or the HE line (50%, 95% CI [29%, 67%]). We are underpowered to statistically test the hypothesis that lines might have differentially responded to particular interventions, but the majority of LE rats failed to remit under any intervention. In contrast, the majority of HE rats achieved remission under every intervention except for standard extinction, but only the combination of Ret+Ext (Retrieval+Extinction) and MB resulted in a majority remission for RB rats. The difference was especially stark for the combined intervention, with close to 2/3 of RB and HE rats but fewer than 1/5 LE rats remitting.
Figure 4.
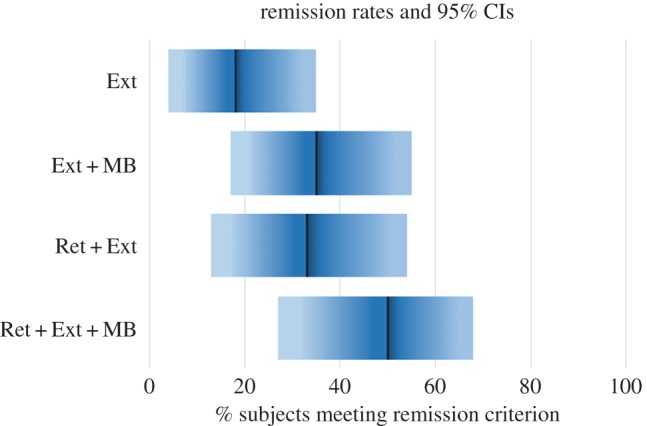
Remission rates under various extinction manipulations. Group remission rate is shown as a dark bar. Confidence intervals are shown in blue. Remission is defined as less than 37.5% freezing following reinstatement. Note that the percentages here refer to proportion of subjects achieving remission, not freezing scores. Under standard extinction training with saline injections, the rate of long-term remission was only 18%, 95% CI [4%, 35%]. The rate of remission almost doubled with either the addition of a retrieval trial prior to extinction (33%, 95% CI [13%, 54%]) or MB injection after extinction (35%, 95% CI [17%, 55%]). When the manipulations are combined, 50% of the subjects achieve remission status, 95% CI [27%, 68%], demonstrating significant superiority over standard extinction with saline. Ext, Extinction; MB, Methylene Blue; Ret, Retrieval. (Online version in colour.)
(ii). Association of treatment condition with responder subgroups
Study 2 identified seven candidate subgroups that clustered together in terms of their pattern of freezing changes over the course of acquisition, extinction and reinstatement. We can now ask whether the MB and Retrieval interventions had any impact on these response patterns. Owing to the small n when the various experimental conditions are parsed across seven subgroups, we must necessarily limit this analysis to a few key comparisons of interest. Also, we are unable to estimate confidence intervals (the incidence for some of the cross-tabulations is zero), and instead rely on permutation tests to assess the probability of obtaining the observed difference under the condition that the null hypothesis is true (that subgroup membership is independent of each experimental condition). To simulate this condition, we randomly shuffled the subgroup labels 10 000 times and recomputed the difference in membership rates for each class across the following three comparisons: Ret+Ext versus Ext, MB versus saline, and LE versus HE. For each resample, we retained the largest observed difference due to chance, and the family-wise-error corrected p-values are given by the proportion of chance maximal differences that were equal to or larger than the observed differences.
(iii). Retrieval+Extinction versus Extinction
There was no evidence to support that the Ret+Ext manipulation influenced subgroup membership (table 2).
Table 2.
Representation of Extinction versus Retrieval+Extinction (Ret+Ext) in phenotypic subgroups. There was no evidence to support that the Ret+Ext manipulation influenced subgroup membership.
| subgroup phenotype | Ext | Ret+Ext | p-value |
|---|---|---|---|
| 1 | 0.08 | 0.18 | 0.72 |
| 2 | 0.31 | 0.29 | 1.00 |
| 3 | 0.06 | 0.07 | 1.00 |
| 4 | 0.19 | 0.24 | 0.97 |
| 5 | 0.23 | 0.18 | 0.98 |
| 6 | 0.04 | 0.02 | 1.00 |
| 7 | 0.08 | 0.02 | 0.96 |
(iv). Methylene Blue versus Saline
Rats receiving MB were 11 times more likely to belong to Subgroup 1 (mild initial fear, successful extinction and minimal reinstatement) than rats that received saline (p = 0.08). As MB was given following the end of extinction (and could play no causal role in determining initial fear or extinction success), this suggests that MB may be particularly well-suited at preventing return of fear for individuals with mild baseline symptoms who respond well to extinction training. There was no evidence of over- or under-representation of MB-treated subjects in any of the other subgroups (p ≥ 0.39) (table 3).
Table 3.
Representation of saline versus methylene blue (MB) in phenotypic subgroups. Rats receiving MB were 11 times more likely to belong to Subgroup 1 (mild initial fear, successful extinction and minimal reinstatement) than rats that received saline (p = 0.08). As MB was given following the end of extinction (and could play no causal role in determining initial fear or extinction success), this suggests that MB may be particularly well-suited at preventing return of fear for individuals with mild baseline symptoms who respond well to extinction training. There was no evidence of over- or under-representation of MB-treated subjects in any of the other subgroups (p ≥ 0.39).
| subgroup phenotype | Ext+saline | Ext+MB | p-value |
|---|---|---|---|
| 1 | 0.02 | 0.22 | 0.08 |
| 2 | 0.37 | 0.24 | 0.39 |
| 3 | 0.07 | 0.06 | 1.00 |
| 4 | 0.23 | 0.20 | 1.00 |
| 5 | 0.16 | 0.24 | 0.84 |
| 6 | 0.07 | 0.00 | 0.90 |
| 7 | 0.07 | 0.04 | 1.00 |
(v). High extinguishers versus low extinguishers
LE rats were strikingly over-represented in Subgroup 5; nearly one out of every two LE rats was characterized by severe, persistent fear, whereas only one out of 25 HE rats fitted this profile, an improbable difference (p < 0.01) under the null hypothesis. There was no evidence of over- or under-representation of HE or LE subjects in any of the other subgroups (p ≥ 0.21) (table 4).
Table 4.
Representation of High Extinguishers (HEs) versus Low Extinguishers (LEs) in phenotypic subgroups. LE rats were strikingly over-represented in Subgroup 5; nearly one out of every two LE rats was characterized by severe, persistent fear, whereas only one out of 25 HE rats fitted this profile, an improbable difference (p < 0.01) under the null hypothesis. There was no evidence of over- or under-representation of HE or LE subjects in any of the other subgroups (p ≥ 0.21).
| subgroup phenotype | LE | HE | p-value |
|---|---|---|---|
| 1 | 0.00 | 0.14 | 0.61 |
| 2 | 0.32 | 0.32 | 1.00 |
| 3 | 0.09 | 0.04 | 0.99 |
| 4 | 0.14 | 0.36 | 0.21 |
| 5 | 0.41 | 0.04 | 0.01 |
| 6 | 0.05 | 0.04 | 1.00 |
| 7 | 0.00 | 0.07 | 0.97 |
5. Discussion
Fear conditioning is widely employed to examine the mechanisms that underlie dysregulations of the fear system. Various manipulations are often used following fear acquisition, to identify avenues to attenuate fear memories. In rodent studies, freezing is often quantified as the predominant dependent variable to determine outcome. Most fear conditioning studies, including many of our own, simply rely on examining mean group differences to evaluate whether a manipulation was a significant improvement over a control group. In many respects, this approach falls short of delivering the most useful data. Ultimately, while the means' comparison offers a useful assessment of overall treatment efficacy, it is also important to determine the proportion of individuals within a group that show significant improvement in terms of clear bench-marks that establish a return to normal functioning. Additionally, the current standard of analysis in most fear conditioning studies generally does not capture the spectrum of individual differences that may contribute to different outcome trajectories. Here, we developed criteria for a standard operationalized benchmark for return of fear after treatment, using logistic regression analysis applied to freezing data from a moderately large sample of rats that either underwent fear conditioning or did not. Next, we used cluster analysis together with our established criteria to identify homogeneous subgroups of rats that fell into specific acquisition/extinction/recovery phenotypes. Finally, we applied our remission criteria and subgroup phenotypes to a re-analysis of previous published experiments from our laboratory, to evaluate the broader usefulness of those combined analytical approaches.
(a). Applying fear classification criteria to determine remission rates
Under Study 1, we established data-driven criteria for remission, partial remission and no remission (<25%; 25–50%; >50%), with the midpoint (37.5%) being the best dichotomous cut-off for remission versus non-remission. An important ensuing question is: how broadly applicable are the criteria identified? Our re-analysis of previous experiments in Study 3 suggests that the criteria established under Study 1 are in line with the conclusions reached when employing a more standard means of analysing the data. Effectively, we found that the remission rates under Retrieval+Extinction or Methylene Blue (MB)+Extinction were roughly twice that of extinction alone. When the manipulations were combined (Retrieval+Extinction+MB), 50% of individuals achieved remission. This is consistent with our previous findings using a linear modelling approach that controlled for individual differences in acquisition and extinction learning and demonstrated that the retrieval+extinction paradigm and MB have significant independent and additive effects on preventing fear reinstatement [10,11]; moreover, the present analysis underscores this previous finding of statistical significance with a finding of practical significance: in other words, half of all subjects are restored to pre-conditioning levels of freezing by combining these approaches and, importantly, half maintain some level of residual fear. These are important metrics to consider when translating these interventions to the clinic. It is also evident that response to these interventions is genetically constrained given that the majority of rats from our low extinction line (LE) failed to remit under any intervention, whereas the majority of rats from our high extinction (HE) line achieved remission under every intervention, except standard extinction. The ability to determine, on a subject-to-subject basis, whether remission was achieved, as well as the overall remission rate for each experimental group, is a powerful addition offered by the present analysis.
A perusal of the fear conditioning/extinction literature also suggests that our fear classification criteria are very much in line with the mean data for a number of studies (the cited studies are representative examples; the list is not exhaustive) from different laboratories, in which fear extinction, and some form of return of fear were reported [9,14–20]. Notably, reported means that were determined to be indicative of a significant return of fear were above 37%, and the means that were determined to indicate a significant fear reduction were below 30% (in males) [9,14–20].
(b). Clusters of homogeneous subgroup phenotypes emerge from a heterogeneous population
Rats, and other rodents, are often thought to be part of a fairly homogeneous population, especially when compared with their human counterparts. Still, as behavioural testing increases in complexity, there is often evidence of within-group variability, which increases the range of data, without necessarily impacting between-group comparisons. An important question arises regarding whether such individual differences merely reflect the presence of a few outliers, or are representative of subcategories of behavioural phenotypes that could provide helpful explanatory power in present or future studies. In the majority of behavioural neuroscience studies, the n per group is approximately 8–12 (see [21–28] for recent examples; see [29] for review of small sample sizes in behavioural neuroscience research). As such, we are rarely sufficiently powered to determine whether there is a pattern that explains individual differences. Here, we performed analyses on a moderately-sized sample (215 rats), and classified the rats' behavioural responding (freezing) using agglomerative hierarchical clustering analysis. The cluster analysis identified seven homogeneous subgroups in terms of patterns of freezing 24 h after acquisition, at the end of extinction, and 24 h after reinstatement: Subgroup 1: Mild initial fear with successful extinction and long-term fear reduction (20%); Subgroup 2: Severe initial fear with moderately successful extinction followed by return of fear (20%); Subgroup 3: Mild initial fear with largely successful extinction followed by return of fear (15%); Subgroup 4: Severe initial fear with largely successful extinction and long-term fear reduction (13%); Subgroup 5: Severe, persistent fear (13%); Subgroup 6: Fear incubation (12%); Subgroup 7: A more extreme version of Subgroup 2 (8%).
It is important to note that the ‘treatment’ employed to reduce fear was not entirely independent of the outcome, and indeed had some impact in shaping the clustering. Our data suggest that whereas there was no evidence to suggest that the Retrieval+Extinction manipulation influenced subgroup membership, rats that received the MB treatment were 11 times more likely to belong to Subgroup 1 than rats that received saline. Since MB was given following the end of extinction, it suggests that this form of treatment may be particularly well-suited at preventing return of fear in individuals with a priori mild fear who respond well to extinction treatment. Given the similarities between Subgroups 1 and 3 for initial fear and extinction, it is possible that Subgroup 1 was essentially created by MB acting on Subgroup 3. There was also an over-representation of the LE rats in Subgroup 5 (nearly 50% of LE rats displayed severe, persistent fear, whereas only 1 out of 25 HE rats fitted this profile). We believe there is value in including our breeding lines in this analysis, since there is undoubtedly the presence of subgroups in clinical samples that show very low rates of remission [30]. In this effort, a number of different studies have been conducted to examine whether extinction-based treatments could be used to target stronger fear memories [31,32].
(c). Is there any useful/added value in having the ability to determine the subgroup phenotypes?
One might argue that outliers are already often present in datasets, and that our cluster analysis would allow us to better identify and define the outliers. Such outliers are handled differently depending on the studies. In some, they are identified, and the analyses are performed with them included. Others perform the analyses with and without the outliers; yet others simply remove the outliers, and run the analyses without them. We would contend that it is generally advisable to at least include available details about the outliers. Having the ability to better understand and explain the extremes and their estimated occurrence may be valuable information. Still, caution should be exercised when applying standards derived from approximately 200 individuals to a sample of approximately 8–12. Future work could potentially examine the possible impact of having individuals from specific phenotypic characteristics in selective experimental groups, and could help determine appropriate sample sizes for specific subgroups and questions of interest.
Subgroup phenotypic information could also be used as inclusion/exclusion criteria in future studies, or retrospectively applied to theoretically expand on published findings that employed specific inclusion/exclusion criteria. Another important aspect of being able to define subcategories of behavioural phenotypes is that they will enable us to better determine the clinical subgroup for which a certain manipulation may be best suited.
(d). Emerging questions and conclusions
It is important to bear in mind that freezing, the dependent variable used for the present analyses, is only one metric assessment of ‘fear’ expression (albeit a very useful one, and the most commonly employed in rodent studies of fear conditioning). Still, a valid limitation of the present work is that it may rely too heavily on this one measure to render judgement on rates of remission. It is important to emphasize that different training (conditioning) and different subjective experiences may contribute to differential degrees in freezing. The cut-off threshold in males and females, for example, could conceivably be explained by weight differences (and potentially different subjective experiences, as a result). Along similar lines, one might argue that there is a subjective element to human fear that is not readily captured in rats (for example, an individual who shows extreme subjective fear at baseline, and shows reduction in subjective fear that might still be above threshold for an established criterion of remission might feel as though he/she nevertheless qualitatively benefitted from treatment). There are, however, indices of behavioural assessment that can be employed in rodent studies that can indirectly convey gradients of subjective fear, and may be indicative of increased behavioural flexibility. A reduction in freezing in a rodent experiment, for example, may or may not be correlated with an increase in approach behaviour and/or reduced avoidance. In turn, reduction in avoidance behaviour could, in and of itself, contribute to an improved treatment outcome. Expanding the behavioural repertoire when testing for treatment success will improve our ability to disambiguate qualitative differences in outcome (e.g. [18,33–35]).
As a final point to ponder, it is possible that some of the rats that were assigned to one treatment group might have responded differently (better/worse) had they been in another. We certainly cannot rule out this possibility in the present set of studies. Moving forward, it would be of great value to identify a means of determining, at the very least, whether an individual might be predicted to be a good or poor extinguisher prior to administering treatment. Efforts in that direction will be crucial in developing tailored interventions for anxiety-related disorders.
Ethics
Procedures were conducted in compliance with the National Institutes of Health Guide for the Care and Use of Experimental Animals and were approved by the Institutional Animal Care and Use Committee at the University of Texas at Austin, under protocol number AUP-2015-00114.
Data accessibility
All data presented in this article may be found in the Texas Data Repository under the following link: (http://dx.doi.org/10.18738/T8/HKWKVK) [36].
Authors' contributions
J.S. carried out data analysis, participated in the design of the study, conceived the study and drafted the manuscript; M.L.-H.M. participated in the design of the study, conceived the study and drafted the manuscript; C.J. contributed data to the study, and edited the manuscript; A.A. contributed data to the study, and edited the manuscript. All authors gave final approval for submission.
Competing interests
We have no competing interests.
Funding
This project was funded by grants from the National Institute of Mental Health (1R21MH086805 and 1R01MH091147 to M.-H.M.). We are grateful to the Royal Society for their support of the costs of attending the meeting ‘Of mice and mental health: facilitating dialogue between basic and clinical neuroscientists’ convened by Amy L. Milton and Emily A. Holmes.
References
- 1.Morina N, Wicherts JM, Lobbrecht J, Priebe S. 2014. Remission from post-traumatic stress disorder in adults: a systematic review and meta-analysis of long term outcome studies. Clin. Psychol. Rev. 34, 249–255. ( 10.1016/j.cpr.2014.03.002) [DOI] [PubMed] [Google Scholar]
- 2.Jones CE, Monfils MH. 2016. Post-retrieval extinction in adolescence prevents return of juvenile fear. Learn. Mem. 23, 567–575. ( 10.1101/lm.043281.116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jones CE, Monfils MH. 2016. Dominance status predicts social fear transmission in laboratory rats. Anim. Cogn. 19, 1051–1069. ( 10.1007/s10071-016-1013-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bruchey AK, Jones CE, Monfils MH. 2010. Fear conditioning by-proxy: social transmission of fear during memory retrieval. Behav. Brain Res. 214, 80–84. ( 10.1016/j.bbr.2010.04.047) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jones CE, Riha PD, Gore AC, Monfils MH. 2014. Social transmission of Pavlovian fear: fear conditioning by-proxy in related female rats . Anim. Cogn. 17, 827–834. ( 10.1007/s10071-013-0711-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shumake J, Furgeson-Moreira S, Monfils MH. 2014. Predictability and heritability of individual differences in fear learning. Anim. Cogn. 17, 1207–1221. ( 10.1007/s10071-014-0752-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marcondes FK, Bianchi FJ, Tanno AP. 2002. Determination of the estrous cycle phases of rats: some helpful considerations. Braz. J. Biol. 62, 609–614. ( 10.1590/S1519-69842002000400008) [DOI] [PubMed] [Google Scholar]
- 8.Canty A, Ripley B.2017. https://cran.r-project.org/web/packages/boot/citation.html boot: Bootstrap R (S-Plus) functions. R package version 1.3-19.
- 9.Galatzer-Levy IR, Bonanno GA, Bush DE, Ledoux JE. 2013. Heterogeneity in threat extinction learning: substantive and methodological considerations for identifying individual difference in response to stress. Front. Behav. Neurosci. 7, 55 ( 10.3389/fnbeh.2013.00055) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Auchter A, Cormack LK, Niv Y, Gonzalez-Lima F, Monfils MH. 2017. Reconsolidation-extinction interactions in fear memory attenuation: the role of inter-trial interval variability. Front. Behav. Neurosci. 11, 2 ( 10.3389/fnbeh.2017.00002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Auchter AM, Shumake J, Gonzalez-Lima F, Monfils MH. 2017. Preventing the return of fear using reconsolidation updating and methylene blue is differentially dependent on extinction learning. Sci. Rep. 7, 46071 ( 10.1038/srep46071) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tibshirani R, Walther G, Hastie T. 2001. Estimating the number of clusters in a data set via the gap statistic. J. R. Statist. Soc. B 63, 411–423. ( 10.1111/1467-9868.00293) [DOI] [Google Scholar]
- 13.Langfelder P, Zhang B, Horvath S. 2016. Defining clusters from a hierarchical cluster tree: the Dynamic Tree Cut package for R. Bioinformatics 24, 719–720. ( 10.1093/bioinformatics/btm563) [DOI] [PubMed] [Google Scholar]
- 14.Bravo-Rivera C, Roman-Ortiz C, Brignoni-Perez E, Sotres-Bayon F, Quirk GJ. 2014. Neural structures mediating expression and extinction of platform-mediated avoidance. J. Neurosci. 34, 9736–9742. ( 10.1523/JNEUROSCI.0191-14.2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Halladay LR, Zelikowsky M, Blair HT, Fanselow MS. 2012. Reinstatement of extinguished fear by an unextinguished conditional stimulus. Front. Behav. Neurosci. 6, 18 ( 10.3389/fnbeh.2012.00018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zelikowsky M, Pham DL, Fanselow MS. 2012. Temporal factors control hippocampal contributions to fear renewal after extinction. Hippocampus 22, 1096–1106. ( 10.1002/hipo.20954) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schiller D, Cain CK, Curley NG, Schwartz JS, Stern SA, Ledoux JE, Phelps EA. 2008. Evidence for recovery of fear following immediate extinction in rats and humans. Learn. Mem. 15, 394–402. ( 10.1101/lm.909208) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bouton ME, Vurbic D, Woods AM. 2008. d-cycloserine facilitates context-specific fear extinction learning. Neurobiol. Learn. Mem. 90, 504–510. ( 10.1016/j.nlm.2008.07.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gruene TM, Roberts E, Thomas V, Ronzio A, Shansky RM. 2015. Sex-specific neuroanatomical correlates of fear expression in prefrontal-amygdala circuits. Biol. Psychiatry 78, 186–193. ( 10.1016/j.biopsych.2014.11.014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gruene TM, Flick K, Stefano A, Shea SD, Shansky RM. 2015. Sexually divergent expression of active and passive conditioned fear responses in rats. eLife 4, e11352 ( 10.7554/eLife.11352) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burgos-Robles A, et al. 2017. Amygdala inputs to prefrontal cortex guide behavior amid conflicting cues of reward and punishment. Nat. Neurosci. 20, 824–835. ( 10.1038/nn.4553) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bernier BE, Lacagnina AF, Ayoub A, Shue F, Zemelman BV, Krasne FB, Drew MR. 2017. Dentate gyrus contributes to retrieval as well as encoding: evidence from context fear conditioning, recall, and extinction. J. Neurosci. 37, 6359–6371. ( 10.1523/JNEUROSCI.3029-16.2017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vollmer LL, Schmeltzer S, Schurdak J, Ahlbrand R, Rush J, Dolgas CM, Baccei ML, Sah R. 2016. Neuropeptide Y impairs retrieval of extinguished fear and modulates excitability of neurons in the infralimbic prefrontal cortex. J. Neurosci. 36, 1306–1315. ( 10.1523/JNEUROSCI.4955-13.2016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yau JO, McNally GP. 2015. Pharmacogenetic excitation of dorsomedial prefrontal cortex restores fear prediction error. J. Neurosci. 35, 74–83. ( 10.1523/JNEUROSCI.3777-14.2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lovett-Barron M, et al. 2014. Dendritic inhibition in the hippocampus supports fear learning. Science 343, 857–863. ( 10.1126/science.1247485) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bukalo O, et al. 2015. Prefrontal inputs to the amygdala instruct fear extinction memory formation. Sci. Adv. 1, e1500251 ( 10.1126/sciadv.1500251) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schmidt SD, Furini CRG, Zinn CG, Cavalcante LE, Ferreira FF, Behling JAK, Myskiw JC, Izquierdo I. 2017. Modulation of the consolidation and reconsolidation of fear memory by three different serotonin receptors in hippocampus . Neurobiol. Learn. Mem. 142, 48–54. ( 10.1016/j.nlm.2016.12.017) [DOI] [PubMed] [Google Scholar]
- 28.Do-Monte FH, Quiñones-Laracuente K, Quirk GJ. 2015. A temporal shift in the circuits mediating retrieval of fear memory. Nature 519, 460–463. ( 10.1038/nature14030) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Button KS, Ioannidis JPA, Mokrysz C, Nosek BA, Flint J, Robinson ESJ, Munafo MR. 2013. Power failure: why small sample size undermines the reliability of neuroscience. Nat. Rev. Neurosci. 14, 365–376. ( 10.1038/nrn3475) [DOI] [PubMed] [Google Scholar]
- 30.Cusack K, et al. 2016. Psychological treatments for adults with posttraumatic stress disorder: a systematic review and meta-analysis. Clin. Psychol. Rev. 43, 128–141. ( 10.1016/j.cpr.2015.10.003) [DOI] [PubMed] [Google Scholar]
- 31.Rau V, DeCola JP, Fanselow MS. 2005. Stress-induced enhancement of fear learning: an animal model of posttraumatic stress disorder. Neurosci. Biobehav. Rev. 29, 1207–1223. ( 10.1016/j.neubiorev.2005.04.010) [DOI] [PubMed] [Google Scholar]
- 32.Finsterwald C, Steinmetz AB, Travaglia A, Alberini CM. 2015. From memory impairment to posttraumatic stress disorder-like phenotypes: the critical role of an unpredictable second traumatic experience. J. Neurosci. 35, 15 903–15 915. ( 10.1523/JNEUROSCI.0771-15.2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bravo-Rivera C, Roman-Ortiz C, Montesinos-Cartagena M, Quirk GJ. 2015. Persistent active avoidance correlates with activity in prelimbic cortex and ventral striatum. Front. Behav. Neurosci. 9, 184 ( 10.3389/fnbeh.2015.00184) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shumake J, Monfils MH. 2015. Assessing fear following retrieval + extinction through suppression of baseline reward seeking vs. freezing. Front. Behav. Neurosci. 9, 355 ( 10.3389/fnbeh.2015.00355) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gruene TM, Flick K, Stefano A, Shea SD, Shansky RM. 2015. Sexually divergent expression of active and passive conditioned fear responses in rats. eLife 4, e11352 ( 10.7554/eLife.11352) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shumake J, Jones C, Auchter A, Monfils M-H. 2017. Data from: Data-driven criteria to assess fear remission and phenotypic variability of extinction in rats Texas Data Repository. ( 10.18738/T8/HKWKVK) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Shumake J, Jones C, Auchter A, Monfils M-H. 2017. Data from: Data-driven criteria to assess fear remission and phenotypic variability of extinction in rats Texas Data Repository. ( 10.18738/T8/HKWKVK) [DOI] [PMC free article] [PubMed]
Data Availability Statement
All data presented in this article may be found in the Texas Data Repository under the following link: (http://dx.doi.org/10.18738/T8/HKWKVK) [36].