Abstract
Purpose
Various hypoxia-related proteins are differentially expressed in the retina and secreted to the vitreous and/or aqueous humor of patients affected by dry or neovascular age-related macular degeneration (nAMD). To determine whether these conditions alter concentrations of cytokines also in the systemic circulation, we measured plasma levels of six hypoxia-related proteins.
Methods
Plasma was prepared from EDTA blood that was collected from patients affected by dry AMD (n = 5), nAMD (n = 11), proliferative diabetic retinopathy (PDR; n = 9), and patients with an epiretinal membrane (ERM; n = 11). ERM samples served as negative controls, PDR samples as positive controls. Protein concentrations of vascular endothelial growth factor (VEGF), erythropoietin (EPO), angiopoietin-like 4 (ANGPTL4), placental growth factor (PlGF), tumor necrosis factor alpha (TNF-α), and pigment epithelium-derived factor (PEDF) were determined by enzyme-linked immunosorbent assay (ELISA).
Results
The concentration of PlGF was significantly increased in plasma of patients affected by nAMD. Although no statistically significant differences were found for EPO, ANGPTL4, PlGF, TNF-α, and PEDF, the mean concentration of VEGF was lowest in the nAMD group. Plasma concentrations of the six factors did not correlate with gender or age of patients.
Conclusions
nAMD may increase plasma concentrations of PlGF, making it a candidate as a biomarker for the neovascular form of AMD. Other factors, however, were not differentially regulated, suggesting that their systemic concentrations are not generally increased in hypoxia-related retinal diseases.
Keywords: Age-related macular degeneration, Vascular endothelial growth factor, Cytokines, Plasma
Introduction
Age-related macular degeneration (AMD) is a leading cause of irreversible and progressive vision loss among the elderly in the Western world [1–3]. While deterioration of vision in dry AMD (geographic atrophy) occurs slowly, vision loss in neovascular AMD (nAMD) often happens within a few months [4, 5]. The severity and socio-economic impact of AMD combined with its increasing incidence makes an efficient treatment an urgent medical need. Several compounds that target vascular endothelial growth factor (VEGF) have been developed that demonstrate unprecedented results in randomized clinical trials preventing vision loss in the majority of patients with nAMD [6–9]. However, to date, no therapy has been approved for dry AMD.
Understanding the pathogenesis of dry AMD, i.e., the processes leading to the formation of geographic atrophy, is critical for the development of efficient therapies. Apart from known risk factors such as advanced age, cigarette smoke, high body mass index, and genetic variants [10–12], inflammation and oxidative damage have been implicated in disease induction and/or progression [13]. Recently, it was hypothesized that reduced tissue oxygenation (hypoxia) may be another contributing factor not only for the pathogenesis of nAMD but also of dry AMD [14, 15]. Reduced choroidal blood flow and tissue changes in the aging eye may reduce oxygen delivery to the retinal pigment epithelium (RPE) and the outer layer of the neuronal retina, potentially inducing mild but chronic hypoxia in photoreceptor cells and RPE. This may lead to the activation of hypoxia-inducible transcription factors (HIFs) [16, 17] and consequently to the induction of HIF target genes including VEGF [18, 19], a main factor for the development of choroidal neovascularization in nAMD [20–23]. In addition to VEGF, hypoxia increases expression of several additional factors such as angiopoietin like 4 (ANGPTL4), placental growth factor (PlGF), pigment epithelium derived factor (PEDF), tumor necrosis factor (TNF-α), erythropoietin (EPO), and others that have been implicated in angiogenesis, neovascularization, and/or inflammation [24–29]. To address whether patients suffering from retinal disease with a hypoxic component also show systemic changes of these hypoxia-regulated proteins, we studied plasma samples from patients with nAMD, dry AMD and patients with proliferative diabetic retinopathy (PDR). The non-hypoxic control group comprised of samples from patients with epiretinal membranes (ERM).
Materials and methods
Patients, collection of plasma, and ELISA
This non-interventional, single-center study at the Department of Ophthalmology at the University Hospital Zurich enrolled 36 participants and was approved by the human ethics committee of the Canton of Zurich, Switzerland. All study subjects were recruited among patients who were regularly scheduled for cataract surgery or vitrectomy. Informed consent was obtained from all participants prior to participation. The study adheres to the tenets of the Declaration of Helsinki. All participants underwent ophthalmologic examination 1 day before and 1 day after surgery as well as follow-up examinations after 1 month. The blood samples were collected by venous puncture prior to surgery, i.e., at a time when no additional oxygenation or medication was administered. At the time of taking blood samples, patients had been fasting for at least 6 h.
Exclusion criteria were: glaucoma, intraocular surgery within the last 6 months, ocular medications other than lubricants, intraocular inflammation, non-proliferative diabetic retinopathy, myopia of more than 6 diopters spherical equivalent, any other ocular vascular disease, previous retinal detachment, previous vitrectomy, retinal degenerative disease, and presence of any other retinal condition potentially affecting either function or oxygenation of the retina other than nAMD, dry AMD, or proliferative diabetic retinopathy (PDR). Patients with PDR or nAMD who received anti-VEGF treatments must have had a minimum interval of 2 weeks between sample collection and last intravitreal injection of an anti-VEGF drug.
Patients were assessed clinically during routine clinics and underwent slit-lamp examination, visual acuity testing, measurement of intraocular pressure, and optical coherence imaging.
Patients with PDR are known to have a strong vascular response that involves activation of HIF transcription factors due to local hypoxia caused by reduced retinal perfusion and microvascular complications [30–35]. These samples served as a “positive control” for a hypoxic ocular response. All participants were ophthalmological patients referred to undergo intraocular surgery, thus we selected patients with ERM to serve as “negative control” because they are not known to have any hypoxia-related changes in the retina.
Based on available literature, we selected VEGF, EPO, ANGPTL4, PlGF, TNF-α, and PEDF as hypoxia-related factors [24, 25, 27, 36–39] to be tested in plasma as follows: Blood was collected in EDTA tubes and kept on ice until use. Plasma was prepared by centrifugation of the EDTA blood at 1300 × g for 15 min within 2 h after collection. Aliquots were prepared and stored in liquid nitrogen until analysis. Plasma concentrations of the six factors were determined by ELISA using the DuoSet (ANGPTL4 and PEDF) or the Quantikine format (VEGF, EPO, PlGF, TNF-α) according to manufacturer’s instructions (R & D Systems Inc., Minneapolis, Minnesota, USA). The minimal detectable dose (MDD) was defined as the value received by addition of two standard deviations to the mean optical density value of zero standard measurements. For the different factors the MDD was as follows: VEGF: 9 pg/ml; EPO: 6 mIU/ml; PlGF: 0.14 pg/ml; TNF-α: 0.12 pg/ml; ANGPTL4: 0.79 ng/ml; PEDF: 2.21 ng/ml.
Statistical analyses
Quantification of ELISA data was done by GraphPad Prism version 6.0f for Mac OS X (GraphPad Software, La Jolla, CA, USA) using Sigmoidal 4PL fit with 1/y2 correction for heteroscedasticity. Numbers were expressed as median (interquartile range (IQR)) Differences among groups were analyzed by one way analysis of variance (ANOVA), followed by Kruskal–Wallis rank sum test with Benjamini–Hochberg post-test for individual comparisons between groups. This statistical analysis was performed by R (Version 3.2.3) and R Studio (Version 0.99.887; R Core Team (2015), Vienna, Austria) with Ggplot2 (H. Wickham, 2009), dunn.test (A. Dinno, 2016) packages.
Results
Patients/demographics
Blood plasma samples were collected from 36 patients that were diagnosed with nAMD (11 patients), dry AMD (five patients) or PDR (nine patients). Eleven patients with an ERM served as controls. The mean age of all patients was 72 ± 14 years. Detailed demographic data are shown below in Table 1.
Table 1.
Patient groups and demographic data
| Disease group | No. of patients | Age (years); mean ± SE | Gender | |
|---|---|---|---|---|
| Male | Female | |||
| Dry AMD | 5 | 80 ± 7 | 1 | 4 |
| nAMD | 11 | 80 ± 5 | 4 | 7 |
| PDR | 9 | 59 ± 2 | 4 | 5 |
| ERM | 11 | 71 ± 4 | 5 | 6 |
All patients with nAMD received intravitreal injections of anti-VEGF drugs. Cataract surgeries were scheduled between two consecutive injections. Only three of the nine patients with PDR received intravitreal ranibizumab as only in those three a macular edema was diagnosed. All of the patients had undergone peripheral laser photocoagulation. Intraocular pressure was within normal ranges in all patient groups. Clinical information is detailed in Table 2.
Table 2.
Clinical data
| Disease group | Visual acuity | Intraocular pressure (mean) | Anti-VEGF therapy | Peripheral laser coagulation | |
|---|---|---|---|---|---|
| Min | Max | ||||
| Dry AMD | 20/60 | 20/40 | 14 mmHg | – | |
| nAMD | Hand movements | 20/60 | 14 mmHg | 5/11 ranibizumab and aflibercept | |
| 5/11 ranibizumab only | |||||
| 1/11 aflibercept only | |||||
| PDR | Counting fingers | 20/100 | 15 mmHg | 3/9 ranibizumab only | 9/9 |
| ERM | 20/200 | 20/30 | 16 mmHg | – | |
Plasma levels of hypoxia-related factors
Nearly all measured values were in the normal range reported for the factors in human plasma [40–48]. Plasma levels of PlGF were significantly higher in nAMD patients than in all other patient groups (Fig. 1).
Fig. 1.
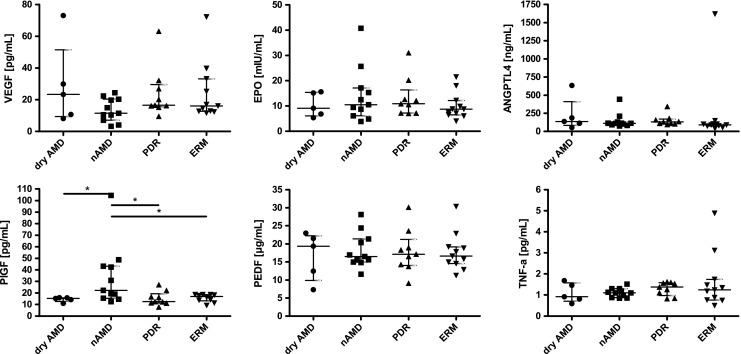
Concentrations of factors in plasma of patients. Shown are individual data points, as well as median (IQR). N = 5 (dry AMD), N = 11 (nAMD), N = 9 (PDR), N = 11 (ERM). *: P < 0.05
Although all other factors analyzed were statistically similarly expressed in all groups, VEGF showed a tendency towards lower levels in patients suffering from nAMD. As stated, all of these patients underwent intraocular anti-VEGF therapy 2–4 weeks before collection of plasma suggesting that the local treatment affected systemic VEGF levels. Mean levels of EPO were slightly elevated in patients of the nAMD and PDR groups (Fig. 1). Both nAMD and PDR have an established hypoxic component. Values outside IQR were from different patients, except for VEGF and EPO in the dry AMD group and PEDF and EPO in the ERM group. Notably, the two patients with VEGF values above average in the dry AMD group also had above-average levels for EPO, PEDF, and TNF. One of the two patients had also above-average levels of PlGF in addition. No correlation between plasma levels of the factors to sex or age of patients was found.
Discussion
The aim of our study was to evaluate whether plasma levels of hypoxia-related factors implicated in pathologic angiogenesis such as in nAMD, dry AMD, or PDR are significantly altered. To our knowledge, this is the first study simultaneously comparing plasma levels of factors implicated in hypoxia-related tissue responses in four different patient groups.
Although VEGF is considered to be the most important angiogenic factor for the development of retinal and choroidal neovascularization [49], additional factors such as EPO [50], ANGPTL4 [24], PlGF [51], and others may also contribute to disease development. TNF-α for example has been found in the ischemic retina [52], which implicates it in the response to hypoxia and in retinal angiogenesis, even though TNF-α has mostly been connected to inflammatory processes. PEDF is considered as an anti-angiogenic factor counteracting VEGF [53]. Misregulation in hypoxia may result in an imbalance between PEDF and VEGF potentially contributing to retinal neovascularization [54, 55]. Here we show that only PlGF was significantly increased in the plasma of nAMD patients whereas levels of VEGF, EPO, PEDF, ANGPTL4, and TNF-α did not significantly vary across patient groups. Although it has been reported that PlGF contributes to choroidal neovascularization [25], PlGF has not yet been determined in the circulation of AMD patients. We found that the plasma concentration of PlGF was significantly elevated in nAMD and differed from the other patient groups, as shown in Fig. 1. However, only about 50% of the nAMD patients presented plasma levels that were above the median of the control group. Clearly, a more detailed investigation with an increased cohort size and a careful correlation of PlGF levels with the severity of disease, status of therapy and response efficacy to anti-VEGF treatment is needed to identify the reason for this variability. At this point, it should be mentioned that apart from angiogenesis, PlGF has been associated with other systemic diseases including atherosclerosis [56], hypertension [57], and coronary artery disease [58]. Similarly, cardiovascular disease and hypertension have also been associated with nAMD [59]. On the other hand, despite the fact of a relatively high prevalence of these diseases in the elderly, they are not exclusive for a particular eye condition; thus it is likely that these effects would be leveled out among the different patient groups. However, since the present study was conducted without taking into consideration any systemic diseases, a possible influence of any systemic disease on the plasma levels of the studied factors cannot be neglected. However, PlGF is an interesting and promising candidate for further studies.
The lowest median plasma concentration of VEGF was unexpectedly found in nAMD patients (Fig. 1) where high intraocular levels of VEGF are causative for the neovascularization that characterizes the disease. The low plasma levels of VEGF were possibly related to the anti-VEGF treatment that all nAMD patients had received 2–4 weeks prior to sample collection. It is interesting to note that median VEGF levels in the dry AMD group tended to be elevated when compared to controls, implying a potential contribution of VEGF and/or hypoxia to the development or progression of this disease. Although earlier reports show elevated VEGF levels in plasma of PDR patients [60–62], VEGF was not above control in our PDR patient group (Fig. 1). All our PDR patients had received panretinal laser coagulation and three of them at least once an anti-VEGF therapy prior to surgery, potentially explaining this difference. Alternatively, the discrepancy may be based on the generally good control of blood sugar levels in Swiss diabetics [63]: it has been reported that plasma levels of VEGF were elevated in diabetics with poor blood sugar control but dropped to the normal range after patients had achieved better control [64]. In addition, the studies reporting higher VEGF levels were conducted in non-Caucasians [60, 65], which may point to potential differences between patient cohorts and/or ethnic groups.
The median plasma concentrations of ANGPTL4, TNF-α, PEDF, and EPO were not statistically different from their respective controls. Nevertheless, some variations were apparent. Although not reaching significance, EPO was high in the nAMD and PDR group where increased levels have been reported before [38, 66]. Both groups have a strong vascular phenotype as a result of hypoxic insults. As a classical hypoxia-regulated protein, increased EPO levels in these patients may thus reflect the hypoxic state of the ocular tissue that led to the vascular phenotype. This is also reflected in median levels of ANGPTL4, which are elevated in all groups compared to non-hypoxic controls (ERM). Elevated levels of ANGPTL4 in PDR in patients were already reported before and the factor is discussed as potential therapeutic target for patients suffering from diabetic retinopathy [24]. Elevated plasma levels of PEDF and TNF-α were demonstrated in PDR patients [67–69]. PEDF was also increased in nAMD when compared to dry AMD [70]. The authors also reported a positive correlation between VEGF and PEDF in the nAMD group. These data were not corroborated in our study.
Although significant changes in intraocular levels of the investigated factors have been reported for pathologies involving hypoxic conditions [71–76], most changes in the circulation of our patient groups were only minor, the exception being PlGF. Why only some of the hypoxia-regulated factors that were found to be increased in the eye were elevated in plasma of patients while others were not is unclear. Possible explanations may include different stabilities of the proteins in ocular fluids and plasma, differences in the cell types producing the factors, differential secretion, or others. Additionally, differences in patient care, e.g., variability in the control of blood sugar levels, potential differences between ethnic groups, and the size of the cohorts tested may influence data outcome and explain differences to published data. It seems likely, however, that ocular concentrations of specific factors in the diseased eye do not necessarily correlate with factor concentrations in plasma. Therefore, our data suggest that the response to hypoxic events in retinal pathologies remains mostly local, even though many factors produced by such conditions are secreted proteins. The confinement of hypoxia-induced angiogenic factors to the local environment of the eye might thus help to prevent potentially dangerous systemic neo-angiogenesis in patients. This possibility needs to be taken into consideration when searching for circulating biomarkers for eye pathologies.
Strengths/limitations
The strength of our study is that we used a protocol ensuring that all procedures were performed the same way in all patients. Furthermore, we studied and compared for the first time to our knowledge three different groups with the following retinal hypoxic diseases: nAMD, dry AMD, and PDR as well ERM as a non-hypoxic control group. This simultaneous analysis allows for a direct comparison of values measured.
However, the limitation of the study is that the number of the enrolled patients was very small and not equal for all groups, making it difficult to demonstrate comparability among the groups. Since only very few cytokine studies have been conducted so far, the availability of commercial kits was limited and their use needed some tweaking to work efficiently. Moreover, we restricted the exclusion criteria only to eye conditions without referring to any systemic diseases, which could also have an influence on plasma levels of the studied factors.
Conclusions
Based on the current results, we hypothesize that local intraocular regulatory mechanisms regarding cytokine secretion under hypoxic conditions exist [50, 66] that are unrelated to systemic regulation. Trends in the different cytokines such as elevated levels of ANGPTL4 in the dry AMD group suggest new avenues for therapeutic targets in dry AMD. Since our study included only a small number of patients, further studies with larger patient groups are needed to verify a possible systemic expression of factors related to hypoxia-induced angiogenesis in the eye.
Acknowledgements
The authors thank Elena Lüssi and Sarah Steinmann for the administrative support and help in preparing the blood samples.
Funding
Novartis Schweiz AG provided financial support in the form of a research agreement.
The sponsor had no role in the design or conduct of this research.
Compliance with ethical standards
Conflict of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Financial disclosure
None.
Footnotes
Zygoula Ioanna and Schori Christian has Equal contribution, shared first authorship
A correction to this article is available online at https://doi.org/10.1007/s00417-017-3861-0.
References
- 1.Klein R, Klein BE, Linton KL. Prevalence of age-related maculopathy. Beaver Dam Eye Study Ophthalmol. 1992;99:933–943. doi: 10.1016/s0161-6420(92)31871-8. [DOI] [PubMed] [Google Scholar]
- 2.Mitchell P, Smith W, Attebo K, Wang JJ. Prevalence of age-related maculopathy in Australia. Blue Mountains Eye Study Ophthalmol. 1995;102:1450–1460. doi: 10.1016/s0161-6420(95)30846-9. [DOI] [PubMed] [Google Scholar]
- 3.Vingerling JR, Dielemans I, Hofman A, Grobbee DE, Hijmering M, Kramer CF, de Jong PT. The prevalence of age-related maculopathy in the Rotterdam study. Ophthalmology. 1995;102:205–210. doi: 10.1016/S0161-6420(95)31034-2. [DOI] [PubMed] [Google Scholar]
- 4.Group MPS Krypton laser photocoagulation for neovascular lesions of age-related macular degeneration. Results of a randomized clinical trial. Macular photocoagulation study group. Arch Ophthalmol. 1990;108:816–824. doi: 10.1001/archopht.1990.01070080058036. [DOI] [PubMed] [Google Scholar]
- 5.(1993) Laser photocoagulation of subfoveal neovascular lesions of age-related macular degeneration. Updated findings from two clinical trials. Macular Photocoagulation Study Group. Arch Ophthalmol 111: 1200-1209 [DOI] [PubMed]
- 6.Brown DM, Kaiser PK, Michels M, Soubrane G, Heier JS, Kim RY, Sy JP, Schneider S. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1432–1444. doi: 10.1056/NEJMoa062655. [DOI] [PubMed] [Google Scholar]
- 7.Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY, Kim RY. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1419–1431. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- 8.Heier JS, Antoszyk AN, Pavan PR, Leff SR, Rosenfeld PJ, Ciulla TA, Dreyer RF, Gentile RC, Sy JP, Hantsbarger G, Shams N. Ranibizumab for treatment of neovascular age-related macular degeneration: a phase I/II multicenter, controlled, multidose study. Ophthalmology. 2006;113(633):e631–e634. doi: 10.1016/j.ophtha.2005.10.052. [DOI] [PubMed] [Google Scholar]
- 9.Martin DF, Maguire MG, Ying GS, Grunwald JE, Fine SL, Jaffe GJ. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med. 2011;364:1897–1908. doi: 10.1056/NEJMicm1005605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khan JC, Thurlby DA, Shahid H, Clayton DG, Yates JR, Bradley M, Moore AT, Bird AC. Smoking and age related macular degeneration: the number of pack years of cigarette smoking is a major determinant of risk for both geographic atrophy and choroidal neovascularisation. Br J Ophthalmol. 2006;90:75–80. doi: 10.1136/bjo.2005.073643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seddon JM, Cote J, Davis N, Rosner B. Progression of age-related macular degeneration: association with body mass index, waist circumference, and waist-hip ratio. Arch Ophthalmol. 2003;121:785–792. doi: 10.1001/archopht.121.6.785. [DOI] [PubMed] [Google Scholar]
- 12.Haines JL, Hauser MA, Schmidt S, Scott WK, Olson LM, Gallins P, Spencer KL, Kwan SY, Noureddine M, Gilbert JR, Schnetz-Boutaud N, Agarwal A, Postel EA, Pericak-Vance MA. Complement factor H variant increases the risk of age-related macular degeneration. Science. 2005;308:419–421. doi: 10.1126/science.1110359. [DOI] [PubMed] [Google Scholar]
- 13.Cao S, Ko A, Partanen M, Pakzad-Vaezi K, Merkur AB, Albiani DA, Kirker AW, Wang A, Cui JZ, Forooghian F, Matsubara JA. Relationship between systemic cytokines and complement factor H Y402H polymorphism in patients with dry age-related macular degeneration. Am J Ophthalmol. 2013;156:1176–1183. doi: 10.1016/j.ajo.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DaCosta J. Minocycline protects retinal pigment epithelial cells from hypoxia. ARVO Meeting Abstr. 2013;54:4108. [Google Scholar]
- 15.McLeod DS, Grebe R, Bhutto I, Merges C, Baba T, Lutty GA. Relationship between RPE and Choriocapillaris in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2009;50:4982–4991. doi: 10.1167/iovs.09-3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci U S A. 1995;92:5510–5514. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang GL, Semenza GL. General involvement of hypoxia-inducible factor 1 in transcriptional response to hypoxia. Proc Natl Acad Sci U S A. 1993;90:4304–4308. doi: 10.1073/pnas.90.9.4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vadlapatla RK, Vadlapudi AD, Pal D, Mukherji M, Mitra A. Ritonavir inhibits HIF-1{alpha} mediated VEGF expression in retinal pigment epithelial cells. ARVO Meeting Abstr. 2013;54:3275. doi: 10.1038/eye.2013.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ozaki H, Yu AY, Della N, Ozaki K, Luna JD, Yamada H, Hackett SF, Okamoto N, Zack DJ, Semenza GL, Campochiaro PA. Hypoxia inducible factor-1alpha is increased in ischemic retina: temporal and spatial correlation with VEGF expression. Invest Ophthalmol Vis Sci. 1999;40:182–189. [PubMed] [Google Scholar]
- 20.Kliffen M, Sharma HS, Mooy CM, Kerkvliet S, de Jong PT. Increased expression of angiogenic growth factors in age-related maculopathy. Br J Ophthalmol. 1997;81:154–162. doi: 10.1136/bjo.81.2.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lopez PF, Sippy BD, Lambert HM, Thach AB, Hinton DR. Transdifferentiated retinal pigment epithelial cells are immunoreactive for vascular endothelial growth factor in surgically excised age-related macular degeneration-related choroidal neovascular membranes. Invest Ophthalmol Vis Sci. 1996;37:855–868. [PubMed] [Google Scholar]
- 22.Tong JP, Chan WM, Liu DT, Lai TY, Choy KW, Pang CP, Lam DS. Aqueous humor levels of vascular endothelial growth factor and pigment epithelium-derived factor in polypoidal choroidal vasculopathy and choroidal neovascularization. Am J Ophthalmol. 2006;141:456–462. doi: 10.1016/j.ajo.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 23.Wang F, Rendahl KG, Manning WC, Quiroz D, Coyne M, Miller SS. AAV-mediated expression of vascular endothelial growth factor induces choroidal neovascularization in rat. Invest Ophthalmol Vis Sci. 2003;44:781–790. doi: 10.1167/iovs.02-0281. [DOI] [PubMed] [Google Scholar]
- 24.Babapoor-Farrokhran S, Jee K, Puchner B, Hassan SJ, Xin X, Rodrigues M, Kashiwabuchi F, Ma T, Hu K, Deshpande M, Daoud Y, Solomon S, Wenick A, Lutty GA, Semenza GL, Montaner S, Sodhi A. Angiopoietin-like 4 is a potent angiogenic factor and a novel therapeutic target for patients with proliferative diabetic retinopathy. Proc Natl Acad Sci U S A. 2015;112:E3030–E3039. doi: 10.1073/pnas.1423765112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rakic JM, Lambert V, Devy L, Luttun A, Carmeliet P, Claes C, Nguyen L, Foidart JM, Noel A, Munaut C. Placental growth factor, a member of the VEGF family, contributes to the development of choroidal neovascularization. Invest Ophthalmol Vis Sci. 2003;44:3186–3193. doi: 10.1167/iovs.02-1092. [DOI] [PubMed] [Google Scholar]
- 26.He X, Cheng R, Benyajati S, Ma JX. PEDF and its roles in physiological and pathological conditions: implication in diabetic and hypoxia-induced angiogenic diseases. Clin Sci (Lond) 2015;128:805–823. doi: 10.1042/CS20130463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li S, Fu XA, Zhou XF, Chen YY, Chen WQ. Angiogenesis-related cytokines in serum of proliferative diabetic retinopathy patients before and after vitrectomy. Int J Ophthalmol. 2012;5:726–730. doi: 10.3980/j.issn.2222-3959.2012.06.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou J, Wang S, Xia X. Role of intravitreal inflammatory cytokines and angiogenic factors in proliferative diabetic retinopathy. Curr Eye Res. 2012;37:416–420. doi: 10.3109/02713683.2012.661114. [DOI] [PubMed] [Google Scholar]
- 29.Mirshahi A, Hoehn R, Lorenz K, Kramann C, Baatz H. Anti-tumor necrosis factor alpha for retinal diseases: current knowledge and future concepts. J Ophthalmic Vis Res. 2012;7:39–44. [PMC free article] [PubMed] [Google Scholar]
- 30.Lim JI, Spee C, Hinton DR. A comparison of hypoxia-inducible factor-alpha in surgically excised neovascular membranes of patients with diabetes compared with idiopathic epiretinal membranes in nondiabetic patients. Retina. 2010;30:1472–1478. doi: 10.1097/IAE.0b013e3181d6df09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stitt AW, Gardiner TA, Archer DB. Histological and ultrastructural investigation of retinal microaneurysm development in diabetic patients. Br J Ophthalmol. 1995;79:362–367. doi: 10.1136/bjo.79.4.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boeri D, Maiello M, Lorenzi M. Increased prevalence of microthromboses in retinal capillaries of diabetic individuals. Diabetes. 2001;50:1432–1439. doi: 10.2337/diabetes.50.6.1432. [DOI] [PubMed] [Google Scholar]
- 33.Joussen AM, Murata T, Tsujikawa A, Kirchhof B, Bursell SE, Adamis AP. Leukocyte-mediated endothelial cell injury and death in the diabetic retina. Am J Pathol. 2001;158:147–152. doi: 10.1016/S0002-9440(10)63952-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jiang F, Tang YT, Guo L, Jiao XY. The role of insulin-like growth factor I and hypoxia inducible factor 1alpha in vascular endothelial growth factor expression in type 2 diabetes. Ann Clin Lab Sci. 2013;43:37–44. [PubMed] [Google Scholar]
- 35.Wang X, Wang G, Wang Y. Intravitreous vascular endothelial growth factor and hypoxia-inducible factor 1a in patients with proliferative diabetic retinopathy. Am J Ophthalmol. 2009;148:883–889. doi: 10.1016/j.ajo.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 36.Zehetner C, Kirchmair R, Kralinger M, Kieselbach G. Correlation of vascular endothelial growth factor plasma levels and glycemic control in patients with diabetic retinopathy. Acta Ophthalmol. 2013;91:e470–e473. doi: 10.1111/aos.12081. [DOI] [PubMed] [Google Scholar]
- 37.Lip PL, Chatterjee S, Caine GJ, Hope-Ross M, Gibson J, Blann AD, Lip GY. Plasma vascular endothelial growth factor, angiopoietin-2, and soluble angiopoietin receptor tie-2 in diabetic retinopathy: effects of laser photocoagulation and angiotensin receptor blockade. Br J Ophthalmol. 2004;88:1543–1546. doi: 10.1136/bjo.2004.048587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gholamhossein Y, Behrouz H, Asghar Z. Diabetic retinopathy risk factors: plasma erythropoietin as a risk factor for proliferative diabetic retinopathy. Korean J Ophthalmol. 2014;28:373–378. doi: 10.3341/kjo.2014.28.5.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dawson DW, Volpert OV, Gillis P, Crawford SE, Xu H, Benedict W, Bouck NP. Pigment epithelium-derived factor: a potent inhibitor of angiogenesis. Science. 1999;285:245–248. doi: 10.1126/science.285.5425.245. [DOI] [PubMed] [Google Scholar]
- 40.Larsson A, Skoldenberg E, Ericson H. Serum and plasma levels of FGF-2 and VEGF in healthy blood donors. Angiogenesis. 2002;5:107–110. doi: 10.1023/A:1021588227705. [DOI] [PubMed] [Google Scholar]
- 41.Wognum AW, Lam V, Goudsmit R, Krystal G. A specific in vitro bioassay for measuring erythropoietin levels in human serum and plasma. Blood. 1990;76:1323–1329. [PubMed] [Google Scholar]
- 42.Bruno CM, Neri S, Sciacca C, Bertino G, Di Prima P, Cilio D, Pellicano R, Caruso L, Cristaldi R. Plasma erythropoietin levels in anaemic and non-anaemic patients with chronic liver diseases. World J Gastroenterol. 2004;10:1353–1356. doi: 10.3748/wjg.v10.i9.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jonker JT, Smit JW, Hammer S, Snel M, van der Meer RW, Lamb HJ, Mattijssen F, Mudde K, Jazet IM, Dekkers OM, de Roos A, Romijn JA, Kersten S, Rensen PC. Dietary modulation of plasma angiopoietin-like protein 4 concentrations in healthy volunteers and in patients with type 2 diabetes. Am J Clin Nutr. 2013;97:255–260. doi: 10.3945/ajcn.112.043687. [DOI] [PubMed] [Google Scholar]
- 44.Jeevaratnam K, Nadarajah VD, Judson JP, Nalliah S, Abdullah MF. Periodic assessment of plasma sFlt-1 and PlGF concentrations and its association with placental morphometry in gestational hypertension (GH) - a prospective follow-up study. BMC Pregnancy Childbirth. 2010;10:58. doi: 10.1186/1471-2393-10-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Krauss T, Pauer HU, Augustin HG. Prospective analysis of placenta growth factor (PlGF) concentrations in the plasma of women with normal pregnancy and pregnancies complicated by preeclampsia. Hypertens Pregnancy. 2004;23:101–111. doi: 10.1081/PRG-120028286. [DOI] [PubMed] [Google Scholar]
- 46.Himmerich H, Fulda S, Linseisen J, Seiler H, Wolfram G, Himmerich S, Gedrich K, Pollmacher T. TNF-alpha, soluble TNF receptor and interleukin-6 plasma levels in the general population. Eur Cytokine Netw. 2006;17:196–201. [PubMed] [Google Scholar]
- 47.Petersen SV, Valnickova Z, Enghild JJ. Pigment-epithelium-derived factor (PEDF) occurs at a physiologically relevant concentration in human blood: purification and characterization. Biochem J. 2003;374:199–206. doi: 10.1042/bj20030313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li X, Wang T, Yang T, Shen Y, An J, Liu L, Dong J, Guo L, Li D, Zhang X, Chen L, Xu D, Wen F. Elevated plasma levels of pigment epithelium-derived factor correlated with inflammation and lung function in COPD patients. Int J Chron Obstruct Pulmon Dis. 2015;10:587–594. doi: 10.2147/COPD.S78546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Witmer AN, Vrensen GF, Van Noorden CJ, Schlingemann RO. Vascular endothelial growth factors and angiogenesis in eye disease. Prog Retin Eye Res. 2003;22:1–29. doi: 10.1016/S1350-9462(02)00043-5. [DOI] [PubMed] [Google Scholar]
- 50.Watanabe D, Suzuma K, Matsui S, Kurimoto M, Kiryu J, Kita M, Suzuma I, Ohashi H, Ojima T, Murakami T, Kobayashi T, Masuda S, Nagao M, Yoshimura N, Takagi H. Erythropoietin as a retinal angiogenic factor in proliferative diabetic retinopathy. N Engl J Med. 2005;353:782–792. doi: 10.1056/NEJMoa041773. [DOI] [PubMed] [Google Scholar]
- 51.Yao YG, Yang HS, Cao Z, Danielsson J, Duh EJ. Upregulation of placental growth factor by vascular endothelial growth factor via a post-transcriptional mechanism. FEBS Lett. 2005;579:1227–1234. doi: 10.1016/j.febslet.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 52.Zheng L, Gong B, Hatala DA, Kern TS. Retinal ischemia and reperfusion causes capillary degeneration: similarities to diabetes. Invest Ophthalmol Vis Sci. 2007;48:361–367. doi: 10.1167/iovs.06-0510. [DOI] [PubMed] [Google Scholar]
- 53.Zhang SX, Wang JJ, Gao G, Parke K, Ma JX. Pigment epithelium-derived factor downregulates vascular endothelial growth factor (VEGF) expression and inhibits VEGF-VEGF receptor 2 binding in diabetic retinopathy. J Mol Endocrinol. 2006;37:1–12. doi: 10.1677/jme.1.02008. [DOI] [PubMed] [Google Scholar]
- 54.Gao G, Li Y, Zhang D, Gee S, Crosson C, Ma J. Unbalanced expression of VEGF and PEDF in ischemia-induced retinal neovascularization. FEBS Lett. 2001;489:270–276. doi: 10.1016/S0014-5793(01)02110-X. [DOI] [PubMed] [Google Scholar]
- 55.Ogata N, Nishikawa M, Nishimura T, Mitsuma Y, Matsumura M. Unbalanced vitreous levels of pigment epithelium-derived factor and vascular endothelial growth factor in diabetic retinopathy. Am J Ophthalmol. 2002;134:348–353. doi: 10.1016/S0002-9394(02)01568-4. [DOI] [PubMed] [Google Scholar]
- 56.Khurana R, Moons L, Shafi S, Luttun A, Collen D, Martin JF, Carmeliet P, Zachary IC. Placental growth factor promotes atherosclerotic intimal thickening and macrophage accumulation. Circulation. 2005;111:2828–2836. doi: 10.1161/CIRCULATIONAHA.104.495887. [DOI] [PubMed] [Google Scholar]
- 57.Carnevale D, Lembo G. PlGF, immune system and hypertension. Oncotarget. 2015;6:18246–18247. doi: 10.18632/oncotarget.4914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fong SW, Few LL, See Too WC, Khoo BY, Nik Ibrahim NN, Yahaya SA, Yusof Z, Mohd Ali R, Abdul Rahman AR, Yvonne-Tee GB. Systemic and coronary levels of CRP, MPO, sCD40L and PlGF in patients with coronary artery disease. BMC Res Notes. 2015;8:679. doi: 10.1186/s13104-015-1677-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hogg RE, Woodside JV, Gilchrist SE, Graydon R, Fletcher AE, Chan W, Knox A, Cartmill B, Chakravarthy U. Cardiovascular disease and hypertension are strong risk factors for choroidal neovascularization. Ophthalmology. 2008;115:1046–1052. doi: 10.1016/j.ophtha.2007.07.031. [DOI] [PubMed] [Google Scholar]
- 60.Ma Y, Zhang Y, Zhao T, Jiang YR. Vascular endothelial growth factor in plasma and vitreous fluid of patients with proliferative diabetic retinopathy patients after intravitreal injection of bevacizumab. Am J Ophthalmol. 2012;153:307–313. doi: 10.1016/j.ajo.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 61.Lip PL, Belgore F, Blann AD, Hope-Ross MW, Gibson JM, Lip GY. Plasma VEGF and soluble VEGF receptor FLT-1 in proliferative retinopathy: relationship to endothelial dysfunction and laser treatment. Invest Ophthalmol Vis Sci. 2000;41:2115–2119. [PubMed] [Google Scholar]
- 62.Wang J, Chen S, Jiang F, You C, Mao C, Yu J, Han J, Zhang Z, Yan H. Vitreous and plasma VEGF levels as predictive factors in the progression of proliferative diabetic retinopathy after vitrectomy. PLoS One. 2014;9:e110531. doi: 10.1371/journal.pone.0110531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kaiser A, Vollenweider P, Waeber G, Marques-Vidal P. Prevalence, awareness and treatment of type 2 diabetes mellitus in Switzerland: the CoLaus study. Diabet Med. 2012;29:190–197. doi: 10.1111/j.1464-5491.2011.03422.x. [DOI] [PubMed] [Google Scholar]
- 64.Kakizawa H, Itoh M, Itoh Y, Imamura S, Ishiwata Y, Matsumoto T, Yamamoto K, Kato T, Ono Y, Nagata M, Hayakawa N, Suzuki A, Goto Y, Oda N. The relationship between glycemic control and plasma vascular endothelial growth factor and endothelin-1 concentration in diabetic patients. Metabolism. 2004;53:550–555. doi: 10.1016/j.metabol.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 65.Tsai DC, Charng MJ, Lee FL, Hsu WM, Chen SJ. Different plasma levels of vascular endothelial growth factor and nitric oxide between patients with choroidal and retinal neovascularization. Ophthalmologica. 2006;220:246–251. doi: 10.1159/000093079. [DOI] [PubMed] [Google Scholar]
- 66.Mohan N, Monickaraj F, Balasubramanyam M, Rema M, Mohan V. Imbalanced levels of angiogenic and angiostatic factors in vitreous, plasma and postmortem retinal tissue of patients with proliferative diabetic retinopathy. J Diabetes Complicat. 2012;26:435–441. doi: 10.1016/j.jdiacomp.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 67.Ogata N, Matsuoka M, Matsuyama K, Shima C, Tajika A, Nishiyama T, Wada M, Jo N, Higuchi A, Minamino K, Matsunaga H, Takeda T, Matsumura M. Plasma concentration of pigment epithelium-derived factor in patients with diabetic retinopathy. J Clin Endocrinol Metab. 2007;92:1176–1179. doi: 10.1210/jc.2006-2249. [DOI] [PubMed] [Google Scholar]
- 68.Doganay S, Evereklioglu C, Er H, Turkoz Y, Sevinc A, Mehmet N, Savli H. Comparison of serum NO, TNF-alpha, IL-1beta, sIL-2R, IL-6 and IL-8 levels with grades of retinopathy in patients with diabetes mellitus. Eye (Lond) 2002;16:163–170. doi: 10.1038/sj/eye/6700095. [DOI] [PubMed] [Google Scholar]
- 69.Demircan N, Safran BG, Soylu M, Ozcan AA, Sizmaz S. Determination of vitreous interleukin-1 (IL-1) and tumour necrosis factor (TNF) levels in proliferative diabetic retinopathy. Eye (Lond) 2006;20:1366–1369. doi: 10.1038/sj.eye.6702138. [DOI] [PubMed] [Google Scholar]
- 70.Machalinska A, Safranow K, Mozolewska-Piotrowska K, Dziedziejko V, Karczewicz D. PEDF and VEGF plasma level alterations in patients with dry form of age-related degeneration--a possible link to the development of the disease. Klin Ocz. 2012;114:115–120. [PubMed] [Google Scholar]
- 71.Aiello LP, Northrup JM, Keyt BA, Takagi H, Iwamoto MA. Hypoxic regulation of vascular endothelial growth factor in retinal cells. Arch Ophthalmol. 1995;113:1538–1544. doi: 10.1001/archopht.1995.01100120068012. [DOI] [PubMed] [Google Scholar]
- 72.Grimm C, Wenzel A, Groszer M, Mayser H, Seeliger M, Samardzija M, Bauer C, Gassmann M, Reme CE. HIF-1-induced erythropoietin in the hypoxic retina protects against light-induced retinal degeneration. Nat Med. 2002;8:718–724. doi: 10.1038/nm723. [DOI] [PubMed] [Google Scholar]
- 73.Xin X, Rodrigues M, Umapathi M, Kashiwabuchi F, Ma T, Babapoor-Farrokhran S, Wang S, Hu J, Bhutto I, Welsbie DS, Duh EJ, Handa JT, Eberhart CG, Lutty G, Semenza GL, Montaner S, Sodhi A. Hypoxic retinal Muller cells promote vascular permeability by HIF-1-dependent up-regulation of angiopoietin-like 4. Proc Natl Acad Sci U S A. 2013;110:E3425–E3434. doi: 10.1073/pnas.1217091110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tudisco L, Della Ragione F, Tarallo V, Apicella I, D'Esposito M, Matarazzo MR, De Falco S. Epigenetic control of hypoxia inducible factor-1alpha-dependent expression of placental growth factor in hypoxic conditions. Epigenetics. 2014;9:600–610. doi: 10.4161/epi.27835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hempel SL, Monick MM, Hunninghake GW. Effect of hypoxia on release of IL-1 and TNF by human alveolar macrophages. Am J Respir Cell Mol Biol. 1996;14:170–176. doi: 10.1165/ajrcmb.14.2.8630267. [DOI] [PubMed] [Google Scholar]
- 76.Yang XM, Yafai Y, Wiedemann P, Kuhrt H, Wang YS, Reichenbach A, Eichler W. Hypoxia-induced upregulation of pigment epithelium-derived factor by retinal glial (Muller) cells. J Neurosci Res. 2012;90:257–266. doi: 10.1002/jnr.22732. [DOI] [PubMed] [Google Scholar]


