ABSTRACT
Autographa californica multiple nucleopolyhedrovirus (AcMNPV) orf75 (ac75) is a highly conserved gene of unknown function. In this study, we constructed an ac75 knockout AcMNPV bacmid and investigated the role of ac75 in the baculovirus life cycle. The expression and distribution of the Ac75 protein were characterized, and its interaction with another viral protein was analyzed to further understand its function. Our data indicated that ac75 was required for the nuclear egress of nucleocapsids, intranuclear microvesicle formation, and subsequent budded virion (BV) formation, as well as occlusion-derived virion (ODV) envelopment and embedding of ODVs into polyhedra. Western blot analyses showed that two forms, of 18 and 15 kDa, of FLAG-tagged Ac75 protein were detected. Ac75 was associated with both nucleocapsid and envelope fractions of BVs but with only the nucleocapsid fraction of ODVs; the 18-kDa form was associated with only BVs, whereas the 15-kDa form was associated with both types of virion. Ac75 was localized predominantly in the intranuclear ring zone during infection and exhibited a nuclear rim distribution during the early phase of infection. A phase separation assay suggested that Ac75 was not an integral membrane protein. A coimmunoprecipitation assay revealed an interaction between Ac75 and the integral membrane protein Ac76, and bimolecular fluorescence complementation assays identified the sites of the interaction within the cytoplasm and at the nuclear membrane and ring zone in AcMNPV-infected cells. Our results have identified ac75 as a second gene that is required for both the nuclear egress of nucleocapsids and the formation of intranuclear microvesicles.
IMPORTANCE During the baculovirus life cycle, the morphogenesis of both budded virions (BVs) and occlusion-derived virions (ODVs) is proposed to involve a budding process at the nuclear membrane, which occurs while nucleocapsids egress from the nucleus or when intranuclear microvesicles are produced. However, the exact mechanism of virion morphogenesis remains unknown. In this study, we identified ac75 as a second gene, in addition to ac93, that is essential for the nuclear egress of nucleocapsids, intranuclear microvesicle formation, and subsequent BV formation, as well as ODV envelopment and embedding of ODVs into polyhedra. Ac75 is not an integral membrane protein. However, it interacts with an integral membrane protein (Ac76) and is associated with the nuclear membrane. These data enhance our understanding of the commonalities between nuclear egress of nucleocapsids and intranuclear microvesicle formation and may help to reveal insights into the mechanism of baculovirus virion morphogenesis.
KEYWORDS: Ac75, baculovirus, intranuclear microvesicle formation, nuclear egress of nucleocapsids, protein interaction, Ac76
INTRODUCTION
Baculoviridae is a family of insect-specific viruses with large, circular, double-stranded DNA genomes packaged within rod-shape nucleocapsids enclosed by lipid envelopes (1). Based on phylogenetic evidence and additional biological and morphological characteristics, the family Baculoviridae can be subdivided into four genera: Alphabaculovirus (lepidopteran nucleopolyhedroviruses [NPVs]), Betabaculovirus (lepidopteran granuloviruses [GVs]), Gammabaculovirus (hymenopteran NPVs), and Deltabaculovirus (dipteran NPVs) (2). Autographa californica multiple NPV (AcMNPV) is the most extensively studied baculovirus. A typical baculovirus infection produces two forms of virions: the budded virion (BV) and the occlusion-derived virion (ODV) (1, 3). BVs are highly infectious to most tissues of the host and in tissue culture and are thus required for spreading the infection within susceptible tissues or among cells in culture (4). ODVs initiate primary infection in the midgut epithelia of infected insects and thus are required for horizontal transmission among insect hosts (5–7). The major difference between BVs and ODVs is the origin of their envelopes (1, 6).
BVs obtain their envelopes from the plasma membrane during the early phase of infection via a strategy similar to that of other viruses that bud from the cell surface (4). Because baculoviruses replicate their DNA genomes and package these DNA molecules into nucleocapsids in the nuclei of host cells (8–10), progeny nucleocapsids must egress from the nucleus to gain access to the plasma membrane to form BVs. Although it has been suggested that baculovirus nucleocapsids exit from the nucleus via nuclear pores, the endoplasmic reticulum, and discontinuities in the nuclear membrane, the most common method of nuclear egress involves a budding process at the nuclear membrane, as documented by electron microscopy studies of NPVs (11–13). The mechanism for nuclear egress of herpesvirus nucleocapsids, which also gain access to the cytoplasm by budding at the nuclear membrane, has been well elucidated (14, 15), but the mechanism by which baculovirus nucleocapsids egress from the nucleus remains unknown. Several baculovirus-carried genes have been reported to affect the nuclear egress of nucleocapsids, including gp41, exon0, ac66, p48, ac93, and ac11 (16–21). Deletion of either exon0 or ac66 results in a significant reduction in the number of nucleocapsids that are transported from the nucleus to the cytoplasm of transfected cells (17, 18), while no nucleocapsids were observed to egress to the cytoplasm of transfected cells when gp41, p48, ac93, or ac11 was deleted or mutated (16, 19–21).
Later in infection, nucleocapsids are retained in mostly the nucleus and acquire envelopes from virus-induced intranuclear microvesicles to form ODVs. The morphogenesis of these intranuclear microvesicles remains unclear. Although there has been some controversy regarding the source of the intranuclear microvesicles, considerable evidence has been generated to support the hypothesis that these microvesicles are the result of budding of the nuclear membrane into the nucleoplasm (6). Several viral genes, including ac11, ac76, ac93, ac94 (odv-e25), ac103 (p48), and ac109, have been reported to be required for ODV envelopment (19–25). However, little is known regarding the genes that affect the morphogenesis of intranuclear microvesicles. Among the genes mentioned above, only ac76 and ac93 have been shown to be required for intranuclear microvesicle formation (20, 22). Notably, ac93 is essential for both the nuclear egress of nucleocapsids and the formation of intranuclear microvesicles (20), indicating that these two processes may share some common steps.
In this study, we identified ac75 as a second baculovirus gene that affects both the nuclear egress of nucleocapsids and the formation of intranuclear microvesicles. The ac75 gene is highly conserved and has been identified in all sequenced baculovirus genomes except for Culex nigripalpus NPV (CuniNPV) (1, 2). A recent transcriptomic study showed that there are three transcription start sites located 282, 93, and 14 nucleotides (nt) upstream of the predicted start codon of the ac75 open reading frame (ORF) (26). All three transcription initiation sites map to the first A of the canonical baculovirus late promoter motif TAAG (26). The transcription of ac75 terminates 42 nt downstream of the stop codon of the ac75 ORF (26). ac75 is predicted to encode a protein of 133 amino acids with a putative molecular mass of 15.5 kDa (27). InterProScan (28) and NCBI Conserved Domain Search (29) indicate that Ac75 homologs constitute a functionally unknown DUF1160 baculovirus protein family, and sequence-based queries showed that Ac75 does not contain any other domains or have any significant sequence similarity to any other proteins. Analyses of Ac75 using the TMpred server (30) and TMHMM server v. 2.0. (http://www.cbs.dtu.dk/services/TMHMM/) did not predict the presence of a transmembrane (TM) domain or TM helix. According to the SignalP 4.1 server (31), there is no signal peptide in Ac75. Motif Scan (32) predicted that Ac75 has an N-glycosylation site and six phosphorylation sites with questionable or weak matches. The ac75 homolog in Bombyx mori NPV (BmNPV), bm61, also encodes a gene product of 133 amino acids. The amino acid identity between BM61 and Ac75 is as high as 96%. Similar to the case for Ac75, BM61 is not predicted to contain any TM domain, TM helix, or signal peptide. bm61 is required in the BV pathway for the egress of nucleocapsids from the nucleus to the cytoplasm (33), and the BM61 protein has been identified as a structural component of both BVs and ODVs (34). However, the role of ac75 in AcMNPV replication remains unknown. In the present study, an ac75 knockout virus was generated to investigate the function of ac75 in the baculovirus life cycle. Our data showed that ac75 is required for the egress of nucleocapsids from the nucleus and intranuclear microvesicle formation. An analysis of the spatial-temporal expression of ac75 revealed that it is a late gene and that the Ac75 protein is localized predominantly in the intranuclear ring zone of infected cells late in infection, while also exhibiting a nuclear rim distribution during the early phase of infection. Ac75 was associated with the envelope and nucleocapsid fractions of BVs but only with the nucleocapsid fraction of ODVs. A phase separation assay showed that Ac75 is not an integral membrane protein. However, Ac75 interacts with the integral membrane protein Ac76, and this interaction occurs predominantly along the nuclear membrane, both in the absence of and during viral infection.
RESULTS
Construction of the ac75 knockout and repair AcMNPV bacmids.
To investigate the role of ac75 in the baculovirus life cycle, an AcMNPV ac75-null mutant, bMON14272-Ac75KO, was constructed via ET homologous recombination as previously described (35). To avoid affecting the transcription of ac74 and ac76, 228 bp of the 5′ end and 171 bp of the 3′ end of ac75 were retained, based on a transcriptomic study of AcMNPV (26); thus, only a 3-bp region of ac75 was deleted and replaced with a 1,040-bp chloramphenicol resistance (Cmr) gene cassette (Fig. 1). As a result, the remaining 3′ end of ac75 suffered a frameshift mutation because the number of nucleotides in the inserted Cmr cassette was not divisible by three. The insertion of the Cmr gene at the designated site within the ac75 locus was confirmed by restriction enzyme (REN) and PCR analyses, followed by sequencing (data not shown).
FIG 1.
Schematic diagram of the recombinant bacmids used in this study. The ac75 knockout mutant bMON14272-Ac75KO was constructed by replacing 3 bp of ac75 with a 1,040-bp Cmr cassette via ET homologous recombination. The ac75 knockout virus (vAc75KO) was constructed by inserting the GFP and polh genes into the polh locus of bMON14272-Ac75KO by Tn7-mediated transposition. The ac75 repair virus (vAc75:FLAG) was constructed by inserting the ac75 gene under the control of its native promoter and tagged at the C terminus with the 3×FLAG epitope sequence (black triangle), together with the GFP and polh genes, into the polh locus of bMON14272-Ac75KO. The pseudo-wild-type virus (vAcWT) was constructed by inserting the GFP and polh genes into the polh locus of bMON14272.
To facilitate the observation of viral infection and to examine the effect of the deletion of ac75 on occlusion body (OB) morphogenesis, the enhanced green fluorescence protein (GFP)-encoding gene and the AcMNPV polyhedrin (polh) gene were inserted into the polh locus of bMON14272-Ac75KO via transposition to generate vAc75KO (Fig. 1). To ensure that any defective phenotype observed in vAc75KO was due to the deletion of ac75, an ac75 repair bacmid, vAc75:FLAG, was generated by inserting the ac75 ORF under the control of its native promoter and with 3×FLAG-encoding sequences at its 3′ terminus, along with the GFP and polh genes, into the polh locus of bMON14272-Ac75KO (Fig. 1). All constructs were confirmed by REN and PCR analyses, followed by sequencing (data not shown). The previously constructed pseudo-wild-type bacmid vAcWT (36) was used as a positive control (Fig. 1).
ac75 is required for BV production.
To study the effect of the deletion of ac75 on viral proliferation, Sf9 cells were transfected with vAc75KO, vAc75:FLAG, or vAcWT, and viral replication was monitored by fluorescence and OB formation. At 24 h posttransfection (p.t.), there were no obvious differences in the number of fluorescent cells among samples, indicating a comparable transfection efficiency (Fig. 2A). By 72 h p.t., fluorescence was observed in almost all vAcWT- and vAc75:FLAG-transfected cells. However, the number of fluorescent cells among the vAc75KO-transfected cells did not increase (Fig. 2A), suggesting that vAc75KO was unable to produce infectious BVs and spread the infection. Using light microscopy, OBs were observed in all three samples, and no obvious differences were observed in the numbers of cells containing OBs at 48 h p.t. (data not shown). By 96 h p.t., OBs were observed in the nuclei of most cells transfected with vAcWT or vAc75:FLAG, whereas the number of vAc75KO-transfected cells containing OBs did not increase. In addition, there appeared to be fewer intracellular OBs in vAc75KO-transfected cells than in the other two samples (Fig. 2B), suggesting that the deletion of ac75 might affect the normal levels of OB production. These results indicated that ac75 was important for infectious BV production in Sf9 cells.
FIG 2.
Analysis of viral replication in Sf9 cells. (A) Fluorescence microscopy of Sf9 cells transfected with vAcWT, vAc75KO, or vAc75:FLAG at 24 or 72 h p.t. (B) Light microscopy of Sf9 cells transfected with vAcWT, vAc75KO, or vAc75:FLAG at 96 h p.t. (C) Viral growth curves generated from Sf9 cells transfected or infected (MOI = 5 TCID50/cell) with the indicated viruses. The supernatants were harvested at the designated time points, and virus titers were determined by TCID50 endpoint dilution assays. Each data point represents the average from three independent transfections or infections. The error bars represent the standard deviations of the means. (D) Western blot analysis of cell extracts and purified BV particles. The transfected cells and the culture supernatants were harvested at 72 h p.t., and BVs were purified from the supernatants. Cell extracts (Cell) and purified BV particles (Sup), if any, were subjected to Western blot analysis with an anti-VP39 antibody as the primary antibody and a donkey anti-rabbit HRP-conjugated antibody as the secondary antibody to detect the major capsid protein VP39.
Viral growth curve analyses were performed to further evaluate the effect of the deletion of ac75 on viral replication. Sf9 cells were transfected with each bacmid, and BV titers were determined by a 50% tissue culture infective dose (TCID50) endpoint dilution assay at selected time points p.t. The repair virus vAc75:FLAG and the wild-type virus vAcWT showed comparable viral titer growth kinetics (Fig. 2C). However, no infectious virus was detected in the supernatants of vAc75KO-transfected cells at any time point up to 120 h p.t. (Fig. 2C), confirming that ac75 was essential for infectious BV production. The BV titers in supernatants prepared from vAc75:FLAG- or vAcWT-infected cells were determined to further assess the effect of ectopic expression of FLAG-tagged Ac75 on viral replication. A steady increase in virus production was observed for vAc75:FLAG, which was similar to the replication kinetics observed for vAcWT (Fig. 2C). These data confirmed that ectopically expressed, C-terminally FLAG-tagged Ac75 was able to rescue the viral replication defect in vAc75KO; thus, the defective phenotype was attributable to only the deletion of ac75.
To further determine whether vAc75KO produced any noninfectious BVs, Western blot analysis was performed to compare the levels of the major capsid protein VP39 in the supernatants of cells transfected with each bacmid (Fig. 2D). VP39 was detected in the cells transfected with all three bacmids, indicating that VP39 expression was not inhibited by the deletion of ac75 (Fig. 2D). However, while VP39 was detected in the supernatants of vAc75:FLAG- and vAcWT-transfected cells, no VP39 signal was detected in the supernatants of vAc75KO-transfected cells (Fig. 2D), even with a longer exposure time. This result indicated that the absence of ac75 led to a defect in BV production.
ac75 is not required for the synthesis of viral DNA.
To determine if the deletion of ac75 leads to a defect in viral DNA replication, the levels of viral DNA synthesis in ac75 knockout bacmid-transfected cells were measured by real-time quantitative PCR (qPCR) analyses. Since the deletion of gp64 prevents the propagation of infection from cell to cell but has no influence on viral DNA synthesis, a gp64 knockout bacmid (vGP64KO) was used as a noninfectious control (37, 38). Total intracellular DNA was isolated from cells transfected with vAc75KO or vGP64KO bacmid DNA at designated time points p.t. and treated with DpnI to eliminate all input bacmid DNA. Virus-specific DNA was quantified by qPCR. As shown in Fig. 3, the viral DNA replication levels of vAc75KO-transfected cells were comparable to those of vGP64KO-transfected cells throughout a 96-h time course. This result indicated that the deletion of ac75 did not affect the synthesis of viral DNA.
FIG 3.
qPCR analysis of viral DNA synthesis. Sf9 cells were transfected with vAc75KO or vGP64KO. At selected time points, total DNA was extracted, digested with DpnI to eliminate input bacmid DNA, and analyzed by qPCR. The values represent the means from three independent transfections. The error bars indicate standard deviations.
ac75 is required for the nuclear egress of nucleocapsids and intranuclear microvesicle formation.
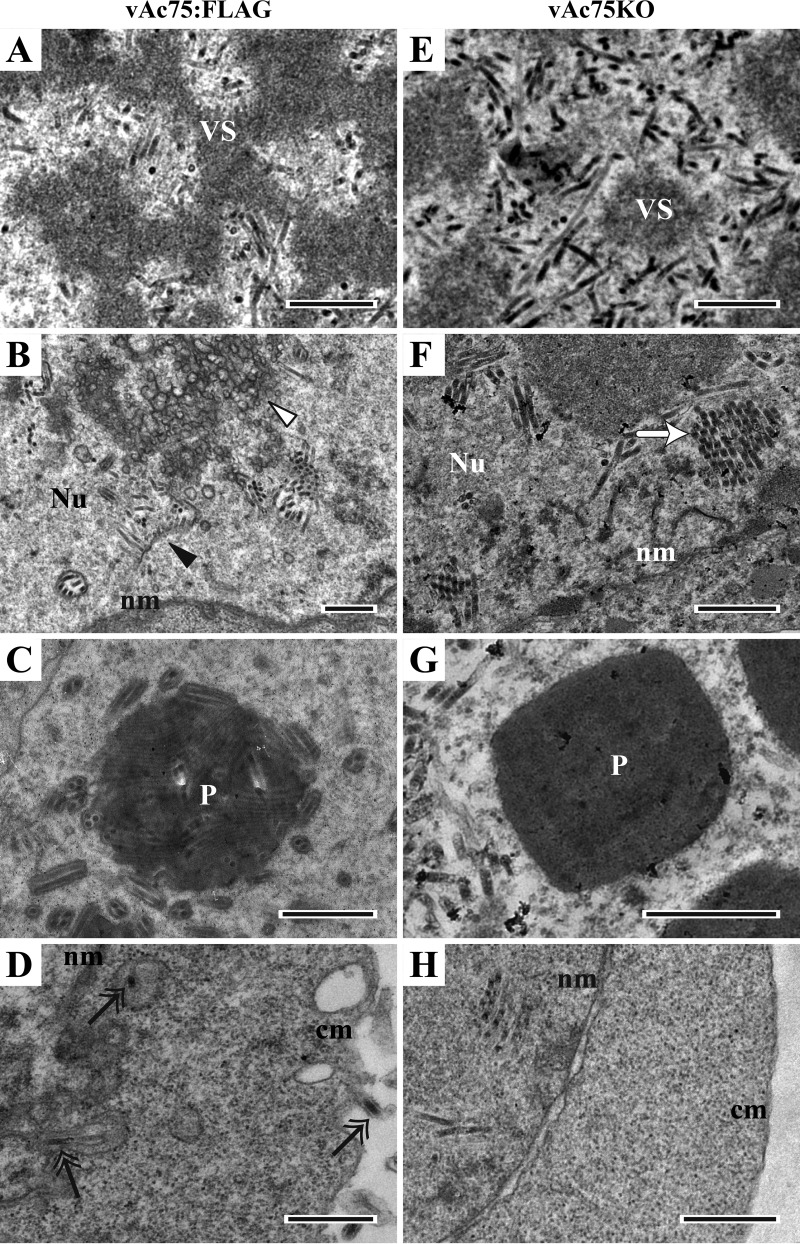
To further investigate the effect of the deletion of ac75 on virion morphogenesis, electron microscopy analyses were performed on vAc75KO-, vAc75:FLAG-, and vAcWT-transfected cells (Fig. 4). As expected, vAc75:FLAG-transfected cells (Fig. 4A) displayed typical characteristics of AcMNPV infection, such as a net-shaped virogenic stroma (VS) inundated with electron-dense rod-shaped nucleocapsids present in the centers of nuclei (Fig. 4A), clusters of virus-induced intranuclear microvesicles emerging (Fig. 4B, white triangle), nucleocapsids aligning with membranous profiles and acquiring their envelopes (Fig. 4B, black triangle), and mature ODVs being embedded into the developing OBs in the ring zone (Fig. 4C). In addition, nucleocapsids that were budding at the nuclear and cytoplasmic membranes and residing in the cytoplasm were easily observed in vAc75:FLAG-transfected cells (Fig. 4D, black double arrows). In vAcWT-transfected cells, characteristics similar to those of vAc75:FLAG-transfected cells were observed (data not shown).
FIG 4.
Transmission electron microscopy of Sf9 cells transfected with either vAc75:FLAG (A to D) or vAc75KO (E to H) at 72 h p.t. (A and E) Electron-dense nucleocapsids in the intrastromal spaces of the VS. (B) Numerous intranuclear microvesicles (white triangle) and bundles of nucleocapsids aligning with membranous profiles (black triangle) in the ring zone. (C) Polyhedra with embedded or being-embedded virions. (D) Nucleocapsids (black double arrows) residing in the cytoplasm or budding from the nuclear or cytoplasmic membranes. (F) Nucleocapsids forming a bundle (white arrow) in the ring zone without evidence of intranuclear microvesicle or ODV presence. (G) Polyhedra devoid of embedded virions. (H) A portion of a cell showing a lack of nucleocapsids residing in the cytoplasm or budding at the nuclear or cytoplasmic membranes. Nu, nucleus; nm, nuclear membrane; P, polyhedron; cm, cytoplasmic membrane. Scale bars, 500 nm.
In vAc75KO bacmid-transfected cells, a typical VS and considerable electron-dense, rod-shaped nucleocapsids were also observed in the centers of nuclei (Fig. 4E), and the nucleocapsids were morphologically indistinguishable from those observed in vAc75:FLAG-transfected cells (Fig. 4A). However, no virus-induced intranuclear microvesicles were observed. As a result, even though nucleocapsids accumulated into bundles at the ring zone, they never became enveloped to form ODVs (Fig. 4F, white arrow). Consequently, the polyhedra observed in the vAc75KO bacmid-transfected cells did not appear to contain ODVs (Fig. 4G), although the size and shape of these polyhedra were similar to those in repair bacmid-transfected cells (Fig. 4G). Because no BVs were detected in the supernatants of vAc75KO-transfected cells, an accumulation of nucleocapsids would be observed in the perinuclear space or cytoplasm if they were able to egress from the nucleus. However, the nucleocapsids were observed only within the nuclei, and no nucleocapsids were observed in the cytoplasm or budding at the nuclear or cytoplasmic membrane (Fig. 4H). Thus, ac75 is required for the egress of nucleocapsids from the nucleus.
Taken together, these observations indicated that the deletion of ac75 did not affect nucleocapsid assembly or polyhedron formation. However, it did preclude intranuclear microvesicle formation and subsequent ODV envelopment and embedding of ODVs into polyhedra, as well as nuclear egress of nucleocapsids to form BVs.
ac75 is a late gene.
To determine whether the FLAG-tagged Ac75 was detectable and to analyze the temporal expression of Ac75, vAc75:FLAG-infected cells were collected at designated time points and analyzed by Western blotting with a mouse monoclonal anti-FLAG antibody (Fig. 5). A major immunoreactive band of approximately 18 kDa was first detected at low levels at 18 h postinfection (p.i.), increased in intensity by 24 h p.i., and persisted up to 96 h p.i. (Fig. 5). Given that the predicted molecular mass of Ac75 is 15.5 kDa and the 3×FLAG tag has a mass of approximately 2.7 kDa, the 18-kDa band should correspond to full-length Ac75 fused to the 3×FLAG-tag. At 24 h p.i., a second band of lower molecular mass (approximately 15 kDa) appeared and remained up to 96 h p.i., reaching peak abundance by 48 h p.i. and decreasing afterwards (Fig. 5). Because the 3×FLAG tag is at the C terminus of Ac75, the 15-kDa variant must have the same C terminus as the 18-kDa version. This band possibly represents a modified form, a degradation product, or an alternate translation product with the same stop codon as the full-length Ac75. The early-late gene product GP64 was probed as a control to verify the infection progress, and cellular actin staining was performed to confirm comparable protein loading (Fig. 5). These results indicated that ac75 is a late gene, which is consistent with the results of a recent transcriptomic study (26).
FIG 5.
Time course analysis of Ac75 expression. Sf9 cells were mock infected or infected with vAc75:FLAG at an MOI of 10 TCID50/cell. At the indicated time points, the cells were collected, resolved by SDS-12% PAGE, and analyzed by immunoblotting with a monoclonal anti-FLAG, -GP64, or -actin antibody to detect Ac75, GP64, or actin. Mi, mock-infected cells. The molecular masses (in kDa) of protein standards are indicated on the left.
Ac75 is localized predominantly to the intranuclear ring zone of AcMNPV-infected cells.
To further understand the functional role of Ac75 in the baculovirus life cycle, it was necessary to determine its subcellular localization. Cells infected with vAc75:FLAG were fixed and analyzed by immunofluorescence using a monoclonal anti-FLAG antibody (Fig. 6). Ac75 was distributed uniformly throughout the cell at 15 h p.i. By 18 h p.i., Ac75 was localized predominantly within the nucleus, with a slight distribution in the cytoplasm. Ac75 exhibited a nuclear rim distribution and became condensed at the periphery of the VS, which corresponds to the intranuclear ring zone, the site of several morphogenic processes associated with ODV development. The concentrated distribution of Ac75 in the ring zone was maintained up to 48 h p.i. By 72 h p.i., Ac75 was distributed evenly throughout the nucleus, excluding the polyhedra (Fig. 6). These findings suggested that Ac75 was distributed predominantly in the intranuclear ring zone during infection and exhibited a nuclear rim distribution during the early phase of infection.
FIG 6.
Subcellular localization of Ac75 as demonstrated by immunofluorescence. Sf9 cells were infected with vAc75:FLAG at an MOI of 10 TCID50/cell. At the indicated time points, the cells were fixed, incubated with a mouse monoclonal anti-FLAG antibody to detect Ac75, and visualized with a goat anti-mouse IgG conjugated to Alexa Fluor 555 (red) as the secondary antibody. Hoechst 33258 was used to identify the nucleus and DNA-rich regions (blue).
Ac75 is a structural protein of both BVs and ODVs.
Because Ac75 affected both BV and ODV morphogenesis, it was necessary to determine whether Ac75 was associated with virions. BVs and ODVs were purified from vAc75:FLAG-infected Sf9 cells and third-instar Spodoptera exigua larvae, respectively, and then were biochemically separated into envelope and nucleocapsid fractions. FLAG-tagged Ac75 was detected by Western blotting with a monoclonal anti-FLAG antibody. As shown in Fig. 7, Ac75 was present in both BVs and ODVs. More specifically, both the 18-kDa and 15-kDa forms of Ac75 were associated with the nucleocapsid fraction of BVs. However, in the envelope fraction of BVs, only the 18-kDa form was detected. Unlike the sublocalization to BVs, Ac75 was detected exclusively in the nucleocapsid fraction of ODVs, and while the 15-kDa form was detected, the high-molecular-mass form was absent (Fig. 7). The purity of the viral fractionations was verified by detecting control proteins, including the nucleocapsid protein VP39, the BV envelope-specific protein GP64, and the BV and ODV envelope protein ODV-E25. These control proteins were detected only in the expected fractions (Fig. 7), demonstrating that the fractionation was effective. These results indicated that Ac75 is a structural protein of both the nucleocapsid and envelope fractions of BVs but of only the nucleocapsid fraction of ODVs.
FIG 7.
Western blot analysis of Ac75 in purified and fractionated virions. BVs and ODVs were purified from vAc75:FLAG-infected cell supernatants and larvae, respectively, and were fractionated into envelope and nucleocapsid fractions. Proteins were detected by immunoblotting with an anti-FLAG antibody to detect FLAG-tagged Ac75, an anti-VP39 antibody to detect the major capsid protein VP39, an anti-GP64 antibody to detect the BV envelope-specific protein GP64, and an anti-ODV-E25 antibody to detect the BV/ODV envelope-associated protein ODV-E25. NC, nucleocapsid fraction; E, envelope fraction. The molecular masses (in kDa) of protein standards are noted on the left.
Ac75 is not an integral membrane protein.
Ac75 was localized at the nuclear membrane in virus-infected cells (Fig. 6) and was associated with the envelope of BVs (Fig. 7). However, bioinformatics analyses of Ac75 do not predict the existence of a TM domain or TM helix in this protein. To determine whether Ac75 is an integral membrane protein, vAc75:FLAG-infected cells collected at 72 h p.i. were fractionated into aqueous and detergent fractions with Triton X-114 and analyzed by Western blotting using a mouse monoclonal anti-FLAG antibody. Ac75 was detected only in the aqueous phase, and each of the two immunoreactive bands was easily visualized (Fig. 8). In contrast, GP64, an integral membrane protein, was detected exclusively in the detergent phase (Fig. 8). These results indicated that Ac75 is not an integral membrane protein.
FIG 8.

Distribution of Ac75 following phase separation. Sf9 cells were infected with vAc75:FLAG at an MOI of >10 TCID50/cell. At 72 h p.i., the cells were collected, extracted with 1% Triton X-114, and analyzed by Western blotting with a mouse monoclonal anti-FLAG or anti-GP64 antibody as the primary antibody and a goat anti-mouse HRP-conjugated antibody as the secondary antibody to detect Ac75 or GP64. Lanes: C, cell lysates; D, detergent phase; A, aqueous phase. The molecular masses (in kDa) of protein standards are noted on the left.
Ac75 interacts with Ac76 at the nuclear membrane.
Though not an integral membrane protein, Ac75 was distributed along the nuclear membrane at 18 h p.i. in vAc75:FLAG-infected cells and was required for the intranuclear microvesicle formation. Ac75 may associate with the nuclear membrane via interaction with an integral membrane protein. The only identified integral membrane protein that affects the formation of intranuclear microvesicles is Ac76 (22, 39). Therefore, we investigated its interaction with Ac75 using a coimmunoprecipitation assay. For this assay, vAc75:FLAG-infected cells were harvested at 72 h p.i., and the cell lysates were subjected to immunoprecipitation with protein A/G agarose beads conjugated with an anti-FLAG antibody. The presence of Ac75 and Ac76 in the cell lysates or immunocomplexes was assessed by Western blotting with an anti-FLAG or anti-Ac76 monoclonal antibody. As shown in Fig. 9A, both Ac75 and Ac76 were detected in the immunocomplexes precipitated by the anti-FLAG beads (lanes IP), indicating that Ac76 coimmunoprecipitated with Ac75, with vAcWT-infected cells used as a negative control. Both the 18-kDa and 15-kDa forms of Ac75 were detected in the cell lysates (lanes Input), but only the 18-kDa form was detected in the immunoprecipitation sample (lanes IP). These results suggested that Ac75 interacts with Ac76 in AcMNPV-infected cells.
FIG 9.
Interaction between Ac75 and Ac76 and location of the interaction. (A) Immunoprecipitation assay. Sf9 cells were infected with vAcWT (WT) or vAc75:FLAG (Rep). At 72 h p.i., the cells were collected, lysed for immunoprecipitation with protein A/G-agarose beads conjugated with an anti-FLAG antibody, and subjected to Western blot analysis. Ac75 expression and immunoprecipitation were detected with a mouse monoclonal anti-FLAG antibody, and Ac76 expression and coimmunoprecipitation were detected with the mouse monoclonal anti-Ac76 antibody. A goat anti-mouse HRP-conjugated antibody was used as the secondary antibody. Input, cell lysates; IP, immunoprecipitation with protein A/G-agarose beads conjugated with an anti-FLAG antibody. (B) BiFC assay. Sf9 cells cotransfected or coinfected with the indicated plasmids or viruses were collected at the designated time points, fixed, probed with a mouse monoclonal anti-lamin B antibody (ADL67), and visualized using a donkey anti-mouse IgG conjugated to Alexa Fluor 647 (magenta) as the secondary antibody. The interactions of proteins were detected by visualizing the green fluorescence (BiFC). Hoechst 33258 was used to identify the nucleus and DNA-rich regions (blue). Panels i and ii, cells cotransfected with plasmids expressing split fluorescent protein-fused Ac75 and Ac76 at 36 h p.t. Panels iii and iv, cells infected with AcMNPV at an MOI of >10 TCID50/cell after cotransfection with plasmids expressing split fluorescent protein-fused Ac75 and Ac76 for 12 h at 24 h p.i. Panels v and vi, cells coinfected with the indicated viruses at an MOI of >10 TCID50/cell at 24 h p.i.
To determine the cellular location of the interaction, a bimolecular fluorescence complementation (BiFC) assay (40, 41) was performed using the N-terminal fragment of yellow fluorescent protein (YFP) (YN) and the C-terminal fragment of Venus (VC). Green fluorescence is observed if the two proteins fused with YN or VC interact. The nuclear membrane was observed by staining with an antibody (ADL67) against lamin B, which underlies the nucleoplasmic face of the inner nuclear membrane (INM). Plasmids expressing Ac75 or Ac76 with C-terminally fused YN or VC were constructed and cotransfected into Sf9 cells. Green fluorescence was observed in the cytoplasm, and some was colocalized with the inner nuclear marker when Ac75 and Ac76 were transiently expressed (Fig. 9B, panels i and ii), which is consistent with the localization of each protein. During transient expression, Ac75 was distributed throughout the cell (data not shown), while Ac76 was localized in the cytoplasm and nuclear membrane (39). Therefore, their interaction occurred only in the cytoplasm and at the nuclear membrane. The BiFC fluorescence of transiently expressed Ac75 and Ac76 became more concentrated at the nuclear membrane during AcMNPV infection (Fig. 9B, panels iii and iv), suggesting that these two proteins interact at the nuclear membrane during viral infection. To determine the actual subcellular location of the interaction between Ac75 and Ac76 during viral infection, we constructed pseudo-wild-type viruses expressing the fusion proteins Ac75YN, Ac75VC, Ac76YN, and Ac76VC under the control of the native promoters of these viral proteins. Sf9 cells were coinfected with the selected viruses indicated in Fig. 9B, panels v and vi, and the fluorescent signal of BiFC was detected at 24 h p.i. The green fluorescence was distributed in the cytoplasm and ring zone, along with a clear accumulation at the nuclear membrane (Fig. 9B, panels v and vi). The results indicated that in AcMNPV-infected cells, Ac75 interacts with Ac76 in the cytoplasm and at the nuclear ring zone and nuclear membrane.
DISCUSSION
The ac75 gene is highly conserved, as its homologs are found in all sequenced lepidopteran NPV, GV, and hymenopteran NPV genomes, suggesting that it may play a fundamental role in the baculovirus life cycle. The ac75 homolog in BmNPV, bm61, is a late gene, and its gene product is associated with both BVs and ODVs and is localized to the nuclear membrane and intranuclear ring zone of BmNPV-infected cells (34). bm61 has been identified as an essential gene that is involved in the egress of nucleocapsids from the nucleus (33). However, the function of ac75 in AcMNPV replication remained unknown. In this study, we generated an ac75 knockout AcMNPV bacmid via homologous recombination and investigated the role of ac75 in the AcMNPV life cycle. Our data indicated that ac75 was required for the nuclear egress of nucleocapsids and that the Ac75 protein was localized to the nuclear membrane and intranuclear ring zone and associated with both BVs and ODVs, similar to the results described for bm61 and its gene product. In addition, we demonstrated that ac75 was required for the formation of virus-induced intranuclear microvesicles and thus played an essential role in ODV morphogenesis and subsequent embedding of ODVs into polyhedra. We also analyzed the exact distribution of Ac75 in virion fractions (capsid and envelope) by viral fractionation studies. The results showed that Ac75 was associated with both the nucleocapsid and envelope fractions of BVs but with only the nucleocapsid fraction of ODVs. The interaction of Ac75 with the only identified integral membrane protein that affects the formation of intranuclear microvesicles, Ac76, was analyzed to further understand its function, and the result revealed an interaction between these two proteins within the cytoplasm and at the nuclear membrane and ring zone in AcMNPV-infected cells.
Western blot analyses of purified virions showed that Ac75 was a component of both BVs and ODVs (Fig. 7), which is consistent with the distribution of BM61, an Ac75 homolog in BmNPV (34). Braunagel et al. (42) and Wang et al. (43) performed extensive proteomic analyses of AcMNPV ODVs and BVs and produced a list of 44 ODV-associated and 34 BV-associated proteins, respectively. However, Ac75 was not included in these lists. It is possible that Ac75 is produced in too low an abundance and may not be easily detected or that it is not amenable to the proteomic techniques used in these studies. Further analysis by viral fractionation showed that Ac75 was associated with both the envelope and nucleocapsid of BVs but with only the nucleocapsid of ODVs. The distribution of Ac75 in virion fractions was slightly different from that of Ha69, the Ac75 homolog in Helicoverpa armigera NPV (HearNPV), which was identified by proteomic analysis as a component of both the nucleocapsid and envelope of BVs but of only the envelope of ODVs (44). It cannot be ruled out that the amount of Ac75 associated with the ODV envelope was too small to be detected by immunoblotting. The divergence in various virus species should also be taken into consideration to explain this difference, as there are some other baculovirus proteins that present different distribution patterns in virions between homologs. For example, AcMNPV VP80 was detected in only the nucleocapsid components of BVs and ODVs (45), while its homolog in HearNPV is associated with the nucleocapsid of BVs and both the nucleocapsid and envelope of ODVs (44). P33 is BV and ODV associated in AcMNPV (46, 47), while it was detected only in ODVs in HearNPV (44).
Using an anti-FLAG antibody, we detected two immunoreactive bands in whole-cell lysates of vAc75:FLAG-infected Sf9 cells (Fig. 5). The predominant band of approximately 18 kDa corresponded to the predicted molecular mass for the FLAG-tagged Ac75. A second band of approximately 15 kDa appeared at 24 h p.i. Common reasons for the appearance of an immunoreactive band with a molecular mass smaller than the predicted value include the existence of alternative splicing, translation of the internal ORF, and posttranslational processing, such as proteolytic cleavage. No spliced form of the ac75 transcript was identified in the transcriptomic study (26). Furthermore, the predicted internal ORF starting at nt 157 of ac75 encodes a putative 9.5-kDa protein, which will be approximately 12.5 kDa with the 3×FLAG tag and thus does not correspond to the 15-kDa band. Thus, the presence of the low-molecular-mass variant of Ac75 may be due to posttranslational processing. Because the 3×FLAG tag is at the C terminus of Ac75, the 15-kDa variant may be a product of proteolytic cleavage in the N-terminal region. Notably, the 18-kDa form of Ac75 was present only in BV preparations and in both nucleocapsid and envelope fractions, while the 15-kDa form was exclusively associated with the nucleocapsid fraction in both BVs and ODVs. Therefore, the 15-kDa form of Ac75 is probably a functional form that was generated or accumulated in the nucleus and was then assembled into the nucleocapsid and thus became associated with the nucleocapsids of both BVs and ODVs. This explanation is also consistent with the absence of the 15-kDa variant in the immunoprecipitation sample (Fig. 9A). The 15-kDa protein is likely to be associated mostly with the nucleocapsids, hindering its immunoprecipitation by the antibody-conjugated beads. With regard to the larger form of Ac75, because it was BV specific, we speculate that it is localized predominantly out of the nucleus and is incorporated into the nucleocapsid and envelope of BVs when nucleocapsids are transported through the cytoplasm and bud at the cytoplasmic membrane. It is not unusual for baculovirus proteins to be expressed as variants of different sizes from a single gene. For instance, two immunoreactive bands of 42 and 30 kDa were detected by antisera to BV/ODV-C42 in AcMNPV-infected cells. Similarly, these two forms show different distribution patterns in virions. The 42-kDa protein is present in both BVs and ODVs, while the 30-kDa protein is associated only with ODVs (48). Two forms of FP25K are present during AcMNPV infection, and both are associated with BVs and ODVs (49). BV/ODV-E26 exhibits at least two forms in AcMNPV-infected cells. One form migrates at 26 kDa, is palmitoylated, and readily associates with cellular membranes. A second form migrates at 33 kDa, accumulates within the nucleus, and is colocalized with IE1 (50). However, the functional difference between the different forms of baculovirus proteins derived from one single gene have rarely been investigated, and the mechanism and the biological significance of a baculovirus gene producing different protein forms remain unknown. Ac75 appears to be a good candidate for such studies.
Electron microscopy analysis demonstrated that Ac75 affected the nuclear egress of nucleocapsids and the formation of virus-induced intranuclear microvesicles and thus was essential for both BV production and ODV envelopment, which is also true for Ac93 (20). Our previous studies showed that two additional viral proteins, P48 and Ac76, are also involved in both BV production and ODV envelopment but do not interfere with nucleocapsid formation (19, 22). P48 is required for the nuclear egress of nucleocapsids and ODV envelopment and thus affects both BV and ODV production (19). Ac76 was demonstrated to be essential for BV production and the formation of intranuclear microvesicles and subsequent envelopment of ODVs (22). Recently, Tao et al. (21) reported that the deletion of ac11 blocks the nuclear egress of nucleocapsids and ODV envelopment. The identification of increasing numbers of genes involved in both BV production and ODV envelopment indicates that the two processes may share some common steps. The nuclear egress of nucleocapsids is indispensable for BV production, and the most common method of NPV egress observed in electron microscopy studies involves a budding process at the nuclear membrane (11, 13). Intranuclear microvesicles are the precursors of the ODV envelope, and their formation is generally hypothesized to be the result of budding of discrete regions of the INM into the nucleoplasm (6). Therefore, we speculate that budding of the nuclear membrane may be the common event. The budding may consist of a series of steps. First, the nuclear lamina underlying the nucleoplasmic face of the INM needs to be disrupted, because the nucleocapsids are too large to pass through the crossover spacing of the nuclear lamina (5, 51), while the formation of the intranuclear microvesicles requires a more fluid nuclear membrane (6, 11). Second, membrane curvature will be generated at the budding sites (52), despite the inverse directions of budding in these two process. Finally, budding is complete after membrane scission at the bud neck (52). Viral proteins involved in both nuclear egress of nucleocapsids and intranuclear microvesicle formation are likely to function in these common steps. These proteins could participate in the regional disruption of the nuclear lamina or modify the nuclear membrane to promote bending or membrane scission. Further study on the molecular mechanisms of Ac75 or Ac93 could likely reveal the shared mechanisms of nuclear egress of nucleocapsids and intranuclear microvesicle formation during baculovirus infection. Because the nuclear membrane is heavily utilized and induced to bud toward the cytoplasm and nucleoplasm during infection, NPV-infected cells could be an ideal model for investigating nuclear membrane-mediated vesicle transport.
Although Ac75 is required for intranuclear microvesicle formation, to our surprise, it was not detected in the envelope fraction of ODVs. This result is quite similar to that for Ac93, which is also involved in intranuclear microvesicle formation but is absent in the ODV envelope (20). As mentioned above, low abundance may be a reason for this observation. It is also probable that Ac75 does not function as a structural component in ODV envelopment. Ac75 was observed to be associated with the nuclear membrane by immunofluorescence analysis (Fig. 6). However, bioinformatics analysis (data not shown) and a Triton X-114 detergent phase partition assay suggested that Ac75 is not an integral membrane protein (Fig. 8). Therefore, it is possible that Ac75 is associated with the nuclear membrane via an interaction with an integral membrane protein. To address this possibility, the interaction between Ac75 and Ac76, the only integral membrane protein (39) that is also required for intranuclear microvesicle formation (22), was examined by coimmunoprecipitation and BiFC. As expected, the results showed that Ac75 interacts with Ac76 in the cytoplasm and at the nuclear membrane and ring zone (Fig. 9). Based on these data, we speculate that Ac75 is associated with the nuclear membrane via an interaction with Ac76 and participates in the nuclear egress of nucleocapsids and the formation of intranuclear microvesicles. However, Ac75 then separates from the membrane after fulfilling its role and is subsequently absent in the ODV envelope.
MATERIALS AND METHODS
Cells, insect, viruses, and antibodies.
Spodoptera frugiperda IPLB-Sf21-AE clonal isolate 9 (Sf9) insect cells were cultured at 27°C in Grace's insect medium (Thermo Fisher Scientific) supplemented with 10% fetal bovine serum, 100 μg/ml penicillin, and 30 μg/ml streptomycin. Larvae of Spodoptera exigua were reared on an artificial diet at 28°C (53). The bacmid bMON14272, which contains an AcMNPV genome, was propagated in Escherichia coli strain DH10B as previously described (54). The pseudo-wild-type AcMNPV vAcWT was constructed by the insertion of the polh and GFP genes into the polh locus of the bacmid bMON14272 (36). The BV titers were determined by a TCID50 endpoint dilution assay in Sf9 cells (55).
A peptide-specific monoclonal antibody against AcMNPV Ac76 (amino acids 49 to 58) was generated in mice by Abmart. Polyclonal anti-AcMNPV ODV-E25 antibody was a gift from Z. H. Hu (Wuhan Institute of Virology, China) (43). The polyclonal antibody against AcMNPV VP39 was generated by Li et al. (56). Mouse monoclonal anti-FLAG antibody, mouse monoclonal anti-actin antibody, mouse monoclonal anti-GP64 AcC6 antibody, and mouse monoclonal anti-lamin B antibody ADL67 (57) were purchased from Abmart, Proteintech, eBioscience, and Developmental Studies Hybridoma Bank, respectively. Alexa Fluor 555-conjugated goat anti-mouse antibody, Alexa Fluor 647-conjugated donkey anti-mouse antibody, and goat anti-mouse horseradish peroxidase (HRP)-conjugated antibody were purchased from Thermo Fisher Scientific. Donkey anti-rabbit HRP-conjugated antibody was purchased from GE Healthcare.
Construction of recombinant AcMNPV bacmids.
An ac75-null AcMNPV bacmid, in which the ac75 gene was disrupted by insertion of a Cmr gene cassette for antibiotic selection, was generated via ET homologous recombination in E. coli by employing the bacmid bMON14272 as previously described (22). Briefly, a 473-bp fragment homologous to the 5′ region of the ac75 ORF (AcMNPV nt 63300 to 63772) was PCR amplified from bMON14272 using primers ac75-US1 and ac75-US2 (the primers used in this study are listed in Table 1). A 380-bp fragment homologous to the 3′ region of the ac75 ORF (AcMNPV nt 62917 to 63296) was PCR amplified from bMON14272 using primers ac75-DS1 and ac75-DS2.
TABLE 1.
Primer sequences used in this study
| Primer | Sequencea (5′ to 3′) |
|---|---|
| ac75-US1 | ATAAGCTTCACTGGCCATATTTAGCCTAGTG |
| ac75-US2 | ATCTGCAGTTTATTAGAAGAGTCGACCGTTTC |
| ac75-DS1 | TAGGATCCAGTAGGCGACAGGTTGATTTTTTAATAC |
| ac75-DS2 | ATGGTACCTATCGAGGAGTTGTCGTCCGTG |
| 5′ac74sp2 | CTTTTTGCTTTGGCGTGGTCAATGG |
| 3′ac76sp1 | ATATTTGTTGTTGGGCGCACTGG |
| 3FLAG-SV40-F | TCTAGAGACTACAAAGACCATGACGGTGATTATAAAGATCATGATATCGATTACAAGGATGACGATGACAAGTGAGATCATAATCAGCCATACCACATT |
| SV40-R | AAACTGCAGGATCCAGACATGATAAGATACATTGATGA |
| ac75-F | GAATTCGCCATATTTAGCCTAGTGTATGAC |
| ac75-R | TCTAGAATACGCTGGCAGTTGGTATGC |
| YN-F | CCGCTCGAGAGATCCATCGCCACCGTGAGCAAGGGCGAGGAG |
| YN-R | TCCCCGCGGCTAGGCCATGATATAGACGTTGTGG |
| VC-F | CCGCTCGAGCGTCCGGCGTGCAAAATC |
| VC-R | TCCCCGCGGTTACTTGTACAGCTCGTCCATGC |
| Ac75-BAMHI-F | GGATCCATGTCCAATTTAATGAAAAACTTTTTC |
| Ac75-XHOI-R | CTCGAGATACGCTGGCAGTTGGTATGCT |
| Ac76-BamHI-F | GGATCCATGAATTTATATTTGTTGTTGGGCG |
| Ac76-XhoI-R | CTCGAGATCTATTGAGCTGGTATTTTTGTTTAGA |
| YN-3-SV40-F | ACGTCTATATCATGGCCTAGGATCATAATCAGCCATACCA |
| PC-XbaI-SV40-R | ACCTACCTGCAGGCTCTAGATCTAGAGATCCAGACATGATAA |
| SV40-5-VC-R | TGGTATGGCTGATTATGATCTTACTTGTACAGCTCGTCCA |
| VC-3-SV40-F | TGGACGAGCTGTACAAGTAAGATCATAATCAGCCATACCA |
| 75-3-VC-F | CATACCAACTGCCAGCGTATCGTCCGGCGTGCAAAATCCC |
| 76-3-VC-F | AAAATACCAGCTCAATAGATCGTCCGGCGTGCAAAATCCC |
| SV40-5-YN-R | TGGTATGGCTGATTATGATCCTAGGCCATGATATAGACGT |
| 75-3-YN-F | CATACCAACTGCCAGCGTATAGATCCATCGCCACCGTGAG |
| 76-3-YN-F | AAAATACCAGCTCAATAGATAGATCCATCGCCACCGTGAG |
| PC-EcoRI-75P-F | TACAATGGCTGTTAGAATTCGAATTCGCCATATTTAGCCTAG |
| YN-5-75-R | CTCACGGTGGCGATGGATCTATACGCTGGCAGTTGGTATG |
| VC-5-75-R | GGGATTTTGCACGCCGGACGATACGCTGGCAGTTGGTATG |
| PC-EcoRI-76P-F | TACAATGGCTGTTAGAATTCGAATTCACGCCCTTCAAAGATT |
| YN-5-76-R | CTCACGGTGGCGATGGATCTATCTATTGAGCTGGTATTTT |
| VC-5-76-R | GGGATTTTGCACGCCGGACGATCTATTGAGCTGGTATTTT |
The underlined sequences are restriction enzyme sites.
The products were cloned into pUC18-CmR (58) to generate pUC18-US-CmR-DS, and this plasmid was digested to obtain a linear fragment that contained the Cmr gene flanked by the two homologous regions. The linear fragment was electrotransformed into DH10Bac (Thermo Fisher Scientific) to obtain an ac75-null bacmid, bMON14272-Ac75KO. The insertion of the Cmr gene cassette was confirmed by PCR analysis with primers 5′ac74sp2 and 3′ac76sp1 and by sequencing.
The ac75 knockout and the repair AcMNPV bacmids containing the polh and GFP genes were constructed by site-specific transposition, as previously described (19). The pFB1-PH-GFP construct, containing the AcMNPV polh and GFP genes (35), was transformed into electrocompetent DH10B cells (Thermo Fisher Scientific) containing the pMON7124 helper plasmid and bMON14272-Ac75KO to generate the ac75 knockout bacmid vAc75KO.
To generate a C-terminal FLAG-tagged ac75 repair bacmid, vAc75:FLAG, a donor plasmid (pFB1-Ac75:3F-PH-GFP) was constructed. The DNA fragment containing the 3×FLAG-coding sequence and a simian virus 40 (SV40) poly(A) signal was PCR amplified from pFastBac1 using primers 3FLAG-SV40-F and SV40-R and cloned into pUC18 to generate pUC18-3F-SV40. The ac75 gene fragment, including its native promoter region but lacking a termination codon, was PCR amplified from bMON14272 using primers ac75-F and ac75-R and cloned into pUC18-3F-SV40 to generate the plasmid pUC18-Ac75-3F-SV40. The Ac75-3F-SV40 fragment was digested from pUC18-Ac75-3F-SV40 and subcloned into pFB1-PH-GFP to generate the donor plasmid, pFB1-Ac75:3F-PH-GFP. The donor plasmid was then transformed into electrocompetent DH10B cells harboring the pMON7124 helper plasmid and bMON14272-ac75KO to generate the ac75 repair bacmid, vAc75:FLAG.
The recombination products were confirmed by PCR and REN analyses, and the correct recombinant bacmids were electroporated into E. coli DH10B cells and screened for tetracycline sensitivity to ensure that the isolated bacmids were free of helper plasmids. Bacmid DNA was extracted and purified using a Qiagen large-construct kit (Qiagen) and quantified by determining the optical density.
Analysis of BV propagation.
For viral growth curves, Sf9 cells (1.0 × 106 cells/35-mm-diameter dish) were transfected in triplicate with 1.0 μg of each bacmid construct (vAc75KO, vAc75:FLAG, or vAcWT) or infected in triplicate with vAc75:FLAG or vAcWT at a multiplicity of infection (MOI) of 5 TCID50/cell. At the indicated time points, the supernatants containing the BVs were harvested, and cell debris was removed by centrifugation at 3,000 × g for 10 min. The BV titers were determined by a TCID50 endpoint dilution assay in Sf9 cells (55).
To detect whether there were noninfectious progeny BVs produced in vAc75KO-transfected cells, 2.0 × 106 cells were transfected with 2.0 μg of each bacmid construct (vAc75KO, vAc75:FLAG, or vAcWT). The supernatants were harvested at 72 h p.t., purified, and processed for immunoblot analysis as previously described (19). Cell pellets were collected as a control. A polyclonal anti-AcMNPV VP39 antibody (1:1,000) was used as the primary antibody to detect the presence of the major capsid protein VP39. A donkey anti-rabbit HRP-conjugated antibody (1:10,000) was used as the secondary antibody. According to the transfection efficiency, one-fifth of the vAc75:FLAG and vAcWT BV samples and all of the vAc75KO and mock BV samples were used for Western blotting. Therefore, the amount of vAc75KO- and mock-transfected cells used for immunoblotting was five times that of vAc75:FLAG- and vAcWT-transfected cells.
Quantitative analysis of viral DNA synthesis.
qPCR was performed to assess viral DNA synthesis as previously described, with some modifications (38). Briefly, 1 × 106 Sf9 cells were transfected in triplicate with 1 μg of vAc75KO or vGP64KO bacmid DNA, and cells were collected at selected time points p.t. Total DNA from each sample was isolated with a Universal genomic DNA extraction kit (TaKaRa) and resuspended in 150 μl of sterile water. Prior to PCR, 5 μl of total DNA from each sample was digested with 20 units of DpnI restriction enzyme (NEB) overnight in a 50-μl reaction volume to eliminate input bacmid DNA. qPCR was performed with 8 μl of the digested DNA using the SYBR Premix Ex Taq II (TaKaRa) and primers targeting a 100-bp region of the AcMNPV gp41 gene under conditions described previously (59).
Transmission electron microscopy.
Sf9 cells (1 × 106 cells/35-mm-diameter dish) were transfected with each bacmid (vAcWT, vAc75KO, or vAc75:FLAG). At 72 h p.t., the cells were dislodged with a rubber policeman, pelleted by centrifugation at 500 × g for 10 min, and then fixed, dehydrated, embedded, sectioned, and stained as previously described (60). The samples were examined with a JEM-100CX/II transmission electron microscope (JEOL, Japan) at an accelerating voltage of 80 kV.
Time course analysis of Ac75 expression.
Sf9 cells (1 × 106 cells/35-mm-diameter dish) were infected with vAc75:FLAG at an MOI of 5 TCID50/cell. At selected time points p.i., the cells were collected and centrifuged at 3,000 × g for 10 min at room temperature. Immunoblotting was performed with a mouse monoclonal anti-FLAG antibody (1:2,000) as the primary antibody to detect FLAG-tagged Ac75. As a control, the baculovirus early-late gene product GP64 was detected with a monoclonal anti-GP64 AcC6 antibody (1:3,000), and the cellular actin was detected with a monoclonal anti-actin antibody (1:2,000).
Immunofluorescence microscopy.
Cells were processed for immunofluorescence microscopy as previously described (39). Briefly, Sf9 cells on a 35-mm glass-bottom culture dish (MatTek, USA) were infected with vAc75:FLAG at an MOI of 10 TCID50/cell. At the indicated time points p.i., cells were fixed, permeabilized, and immunostained with a mouse monoclonal anti-FLAG primary antibody (1:200) to detect FLAG-tagged Ac75. An Alexa Fluor 555-conjugated goat anti-mouse antibody (1:200) was used as the secondary antibody. Prior to analysis, the labeled cells were stained with Hoechst 33258 (Thermo Fisher Scientific) to identify the nucleus and DNA-rich regions. Images were captured using a Zeiss LSM 780 confocal laser scanning microscope (Carl Zeiss; Germany).
Purification and fractionation of BVs and ODVs.
BVs were purified from vAc75:FLAG-infected Sf9 cells, and ODVs were purified from vAc75:FLAG-infected third-instar S. exigua larvae, which were then fractionated into envelope and nucleocapsid fractions as previously described (20, 61). Immunoblotting was performed with a mouse monoclonal anti-FLAG antibody (1:1,000) or with one of the following primary antibodies: monoclonal anti-GP64 AcC6 antibody (1:3,000), polyclonal anti-AcMNPV ODV-E25 antibody (1:2,000), or polyclonal antibody against AcMNPV VP39 (1:1,000). A donkey anti-rabbit HRP-conjugated antibody (1:10,000) or goat anti-mouse HRP-conjugated antibody (1:5,000) was used as the secondary antibody.
Fractionation of integral membrane proteins with Triton X-114.
Sf9 cells (3 × 106) were infected with vAc75:FLAG at an MOI of >10 TCID50/cell and collected at 60 h p.i. Detergent fractionations with Triton X-114 were performed as previously described (62). The resulting detergent phase and aqueous phase were subjected to Western blot analysis with a mouse monoclonal anti-FLAG antibody or a monoclonal anti-GP64 AcC6 antibody as described above.
Coimmunoprecipitation.
Sf9 cells (1 × 107) were infected with vAc75:FLAG at an MOI of >10 TCID50/cell. At 72 h p.i., the cells were harvested, washed once with phosphate-buffered saline (PBS), and lysed in cell lysis buffer for Western blotting and immunoprecipitation (20 mM Tris [pH 7.5], 150 mM NaCl, and 1% Triton X-100; Beyotime) supplemented with 2 μg/ml cOmplete EDTA-free protease inhibitor cocktail (Roche). Immunoprecipitation was performed with protein A/G-agarose beads conjugated to an anti-FLAG antibody (Abmart) as previously described (39). The immunocomplexes were resolved by SDS-12% PAGE and analyzed by Western blotting with a mouse monoclonal anti-FLAG antibody (1:2,000; Abmart) or a mouse monoclonal anti-Ac76 antibody (1:1,000). Sf9 cells infected with vAcWT (MOI, >10 TCID50/cell) were used as a negative control.
Construction of transient-expression plasmids and recombinant viruses and BiFC assays.
To examine the intracellular localization of the interaction of Ac75 and Ac76, four transient-expression plasmids and four recombinant viruses were generated. The 5′ region of the YFP gene, encoding amino acids 2 to 155 of YFP, was PCR amplified from pIB-YFP (donated by Qili Feng, South China Normal University, China) with primers YN-F and YN-R and cloned into pIB/V5-His (Thermo Fisher Scientific) to generate pIB-YN. The sequence encoding a C-terminal fragment of Venus, along with a linker fragment, was PCR amplified from pBiFC-VC155 (donated by Qing Zhang, Sun Yat-sen University, China) with primers VC-F and VC-R and cloned into pIB/V5-His to generate pIB-VC. The ac75 and the ac76 ORFs without the stop codons were PCR amplified from bMON14272 using primer pairs Ac75-BAMHI-F/Ac75-XHOI-R and Ac76-BamHI-F/Ac76-XhoI-R. The products were cloned into pIB-YN or pIB-VC to generate the transient-expression plasmids pIB-Ac75YN, pIB-Ac75VC, pIB-Ac76YN, and pIB-Ac76VC.
The wild-type virus expressing the fusion protein Ac75YN, Ac75VC, Ac76YN, or Ac76VC under the control of the native promoter of ac75 or ac76 was constructed by site-specific transposition as mentioned above. The donor plasmids pFBPC-Ac75YN, pFBPC-Ac75VC, pFBPC-Ac76YN, and pFBPC-Ac76VC were generated by overlap PCR and seamless cloning. The construction of pFBPC-Ac75YN is described as an example as follows. The fragment YN-SV40 with 20-bp flanking sequences homologous to the 3′ region of ac75 or the sequence downstream of the XbaI site of the plasmid pFB1-PH-mCherry (51) at its 5′ and 3′ termini was amplified from plasmids pIB-YN and pFastBac1 with primers 75-3-YN-F, YN-3-SV40-F, SV40-5-YN-R, and PC-XbaI-SV40-R via overlap PCR. The promoter and ORF of ac75 (without a stop codon) with the 20-bp fragment homologous to the sequence upstream of the EcoRI site of plasmid pFB1-PH-mCherry at its 5′ terminus and a 20-bp fragment homologous to the 5′ region of YN at its 3′ terminus were amplified from bMON14272 with primer pair PC-EcoRI-75P-F/YN-5-75-R. Next, the fragment Ac75-YN-SV40 was amplified with primer pair PC-EcoRI-75P-F/PC-XbaI-SV40-R via overlap PCR using the above-described amplified fragments as templates and was cloned into pFB1-PH-mCherry via seamless cloning using a Trelief SoSoo cloning kit (Tsingke) to generate pFBPC-Ac75YN according to the manufacturer's instructions. Primers PC-EcoRI-75P-F, VC-5-75-R, 75-3-VC-F, SV40-5-VC-R, VC-3-SV40-F, and PC-XbaI-SV40-R were used to generated plasmid pFBPC-Ac75VC; primers PC-EcoRI-76P-F, YN-5-76-R, SV40-5-YN-R, YN-3-SV40-F, 76-3-YN-F, and PC-XbaI-SV40-R were used to generate plasmid pFBPC-Ac76YN; and primers PC-EcoRI-76P-F, VC-5-76-R, 76-3-VC-F, SV40-5-VC-R, VC-3-SV40-F, and PC-XbaI-SV40-R were used to generate plasmid pFBPC-Ac76VC. All four donor plasmids were confirmed by sequencing and then transformed into electrocompetent DH10B cells harboring the pMON7124 helper plasmid and bMON14272 to generate vAc75YN, vAc75VC, vAc76YN, or vAc76VC. The recombination products were confirmed by PCR analyses.
A series of BiFC assays was performed. For the cotransfection experiment, Sf9 cells were cotransfected with the plasmid pIB-Ac75YN/pIB-Ac76VC or pIB-Ac75VC/pIB-Ac76YN (2 μg of each plasmid) expressing split fluorescent protein-fused Ac75 and Ac76. For the cotransfection-infection experiment, Sf9 cells were infected with AcMNPV at an MOI of >10 TCID50/cell after cotransfection with the above-mentioned plasmids for 12 h. For the coinfection experiment, Sf9 cells were coinfected with the virus vAc75YN/vAc76VC or vAc75VC/vAc76YN (each at MOI of >10 TCID50/cell) expressing split fluorescent protein-fused Ac75 and Ac76. Cells were fixed at 36 h p.t. or 24 h p.i. and processed for immunofluorescence microscopy as described above using the mouse monoclonal anti-lamin B antibody ADL67 (1:200) as the primary antibody and an Alexa Fluor 647-conjugated donkey anti-mouse antibody (1:200) as the secondary antibody to observe the nuclear membrane.
ACKNOWLEDGMENTS
We thank Zhihong Hu (Wuhan Institute of Virology) for her generous gift of the E25 polyclonal antiserum.
This research was supported by the National Natural Science Foundation of China (31572056 and 31270039) and the Guangzhou Science and Technology Project (201707020003).
REFERENCES
- 1.Rohrmann GF. 2013. Baculovirus molecular biology: 3rd ed National Center for Biotechnology Information, Bethesda, MD. [PubMed] [Google Scholar]
- 2.Jehle JA, Blissard GW, Bonning BC, Cory JS, Herniou EA, Rohrmann GF, Theilmann DA, Thiem SM, Vlak JM. 2006. On the classification and nomenclature of baculoviruses: a proposal for revision. Arch Virol 151:1257–1266. doi: 10.1007/s00705-006-0763-6. [DOI] [PubMed] [Google Scholar]
- 3.van Oers MM, Vlak JM. 2007. Baculovirus genomics. Curr Drug Targets 8:1051–1068. doi: 10.2174/138945007782151333. [DOI] [PubMed] [Google Scholar]
- 4.Blissard GW, Wenz JR. 1992. Baculovirus gp64 envelope glycoprotein is sufficient to mediate pH-dependent membrane fusion. J Virol 66:6829–6835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Slack J, Arif BM. 2007. The baculoviruses occlusion-derived virus: virion structure and function. Adv Virus Res 69:99–165. doi: 10.1016/S0065-3527(06)69003-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Braunagel SC, Summers MD. 2007. Molecular biology of the baculovirus occlusion-derived virus envelope. Curr Drug Targets 8:1084–1095. doi: 10.2174/138945007782151315. [DOI] [PubMed] [Google Scholar]
- 7.Hodgson JJ, Arif BM, Krell PJ. 2007. Reprogramming the chiA expression profile of Autographa californica multiple nucleopolyhedrovirus. J Gen Virol 88:2479–2487. doi: 10.1099/vir.0.82863-0. [DOI] [PubMed] [Google Scholar]
- 8.Fraser MJ. 1986. Ultrastructural observations of virion maturation in Autographa californica nuclear polyhedrosis virus infected Spodoptera frugiperda cell cultures. J Ultrastruct Mol Struct Res 95:189–195. doi: 10.1016/0889-1605(86)90040-6. [DOI] [Google Scholar]
- 9.Young JC, MacKinnon EA, Faulkner P. 1993. The architecture of the virogenic stroma in isolated nuclei of Spodoptera frugiperda cells in vitro infected by Autographa californica nuclear polyhedrosis virus. J Struct Biol 110:141–153. doi: 10.1006/jsbi.1993.1015. [DOI] [Google Scholar]
- 10.Harrap KA. 1972. The structure of nuclear polyhedrosis viruses. 3. Virus assembly. Virology 50:133–139. doi: 10.1016/0042-6822(72)90353-4. [DOI] [PubMed] [Google Scholar]
- 11.Williams GV, Faulkner P. 1997. Cytological changes and viral morphogenesis during baculovirus infection, p 61–107. In Miller LK. (ed), The baculoviruses. Springer, Boston, MA. [Google Scholar]
- 12.Ge JQ, Yang ZN, Tang XD, Xu HJ, Hong J, Chen JG, Zhang CX. 2008. Characterization of a nucleopolyhedrovirus with a deletion of the baculovirus core gene Bm67. J Gen Virol 89:766–774. doi: 10.1099/vir.0.83398-0. [DOI] [PubMed] [Google Scholar]
- 13.Knudson DL, Harrap KA. 1975. Replication of nuclear polyhedrosis virus in a continuous cell culture of Spodoptera frugiperda: microscopy study of the sequence of events of the virus infection. J Virol 17:254–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson DC, Baines JD. 2011. Herpesviruses remodel host membranes for virus egress. Nat Rev Microbiol 9:382–394. doi: 10.1038/nrmicro2559. [DOI] [PubMed] [Google Scholar]
- 15.Hellberg T, Passvogel L, Schulz KS, Klupp BG, Mettenleiter TC. 2016. Nuclear egress of herpesviruses: the prototypic vesicular nucleocytoplasmic transport. Adv Virus Res 94:81–140. doi: 10.1016/bs.aivir.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 16.Olszewski J, Miller LK. 1997. A role for baculovirus GP41 in budded virus production. Virology 233:292–301. doi: 10.1006/viro.1997.8612. [DOI] [PubMed] [Google Scholar]
- 17.Fang M, Dai X, Theilmann DA. 2007. Autographa californica multiple nucleopolyhedrovirus EXON0 (ORF141) is required for efficient egress of nucleocapsids from the nucleus. J Virol 81:9859–9869. doi: 10.1128/JVI.00588-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ke J, Wang J, Deng R, Wang X. 2008. Autographa californica multiple nucleopolyhedrovirus ac66 is required for the efficient egress of nucleocapsids from the nucleus, general synthesis of preoccluded virions and occlusion body formation. Virology 374:421–431. doi: 10.1016/j.virol.2007.12.033. [DOI] [PubMed] [Google Scholar]
- 19.Yuan M, Wu W, Liu C, Wang Y, Hu Z, Yang K, Pang Y. 2008. A highly conserved baculovirus gene p48 (ac103) is essential for BV production and ODV envelopment. Virology 379:87–96. doi: 10.1016/j.virol.2008.06.015. [DOI] [PubMed] [Google Scholar]
- 20.Yuan M, Huang Z, Wei D, Hu Z, Yang K, Pang Y. 2011. Identification of Autographa californica nucleopolyhedrovirus ac93 as a core gene and its requirement for intranuclear microvesicle formation and nuclear egress of nucleocapsids. J Virol 85:11664–11674. doi: 10.1128/JVI.05275-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tao XY, Choi JY, Kim WJ, An SB, Liu Q, Kim SE, Lee SH, Kim JH, Woo SD, Jin BR, Je YH. 2015. Autographa californica multiple nucleopolyhedrovirus ORF11 is essential for budded-virus production and occlusion-derived-virus envelopment. J Virol 89:373–383. doi: 10.1128/JVI.01742-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu Z, Yuan M, Wu W, Liu C, Yang K, Pang Y. 2010. Autographa californica multiple nucleopolyhedrovirus ac76 is involved in intranuclear microvesicle formation. J Virol 84:7437–7447. doi: 10.1128/JVI.02103-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen L, Hu X, Xiang X, Yu S, Yang R, Wu X. 2012. Autographa californica multiple nucleopolyhedrovirus odv-e25 (Ac94) is required for budded virus infectivity and occlusion-derived virus formation. Arch Virol 157:617–625. doi: 10.1007/s00705-011-1211-9. [DOI] [PubMed] [Google Scholar]
- 24.Lehiy CJ, Wu W, Berretta MF, Passarelli AL. 2013. Autographa californica M nucleopolyhedrovirus open reading frame 109 affects infectious budded virus production and nucleocapsid envelopment in the nucleus of cells. Virology 435:442–452. doi: 10.1016/j.virol.2012.10.015. [DOI] [PubMed] [Google Scholar]
- 25.Alfonso V, Maroniche GA, Reca SR, Lopez MG, del Vas M, Taboga O. 2012. AcMNPV core gene ac109 is required for budded virion transport to the nucleus and for occlusion of viral progeny. PLoS One 7:e46146. doi: 10.1371/journal.pone.0046146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen YR, Zhong S, Fei Z, Hashimoto Y, Xiang JZ, Zhang S, Blissard GW. 2013. The transcriptome of the baculovirus Autographa californica multiple nucleopolyhedrovirus in Trichoplusia ni cells. J Virol 87:6391–6405. doi: 10.1128/JVI.00194-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ayres MD, Howard SC, Kuzio J, Lopez-Ferber M, Possee RD. 1994. The complete DNA sequence of Autographa californica nuclear polyhedrosis virus. Virology 202:586–605. doi: 10.1006/viro.1994.1380. [DOI] [PubMed] [Google Scholar]
- 28.Jones P, Binns D, Chang HY, Fraser M, Li W, McAnulla C, McWilliam H, Maslen J, Mitchell A, Nuka G, Pesseat S, Quinn AF, Sangrador-Vegas A, Scheremetjew M, Yong SY, Lopez R, Hunter S. 2014. InterProScan 5: genome-scale protein function classification. Bioinformatics 30:1236–1240. doi: 10.1093/bioinformatics/btu031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marchler-Bauer A, Bo Y, Han L, He J, Lanczycki CJ, Lu S, Chitsaz F, Derbyshire MK, Geer RC, Gonzales NR, Gwadz M, Hurwitz DI, Lu F, Marchler GH, Song JS, Thanki N, Wang Z, Yamashita RA, Zhang D, Zheng C, Geer LY, Bryant SH. 2017. CDD/SPARCLE: functional classification of proteins via subfamily domain architectures. Nucleic Acids Res 45:D200–D203. doi: 10.1093/nar/gkw1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hofmann K, Stoffel W. 1993. A database of membrane spanning proteins segments. Biol Chem Hoppe-Seyler 374:166. [Google Scholar]
- 31.Petersen TN, Brunak S, von Heijne G, Nielsen H. 2011. SignalP 4.0: discriminating signal peptides from transmembrane regions. Nat Methods 8:785–786. doi: 10.1038/nmeth.1701. [DOI] [PubMed] [Google Scholar]
- 32.Sigrist CJ, Cerutti L, de Castro E, Langendijk-Genevaux PS, Bulliard V, Bairoch A, Hulo N. 2010. PROSITE, a protein domain database for functional characterization and annotation. Nucleic Acids Res 38:D161–D166. doi: 10.1093/nar/gkp885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shen H, Chen K. 2012. BM61 of Bombyx mori nucleopolyhedrovirus: Its involvement in the egress of nucleocapsids from the nucleus. FEBS Lett 586:990–995. doi: 10.1016/j.febslet.2011.12.040. [DOI] [PubMed] [Google Scholar]
- 34.Shen H, Chen K, Yao Q, Zhou Y. 2009. Characterization of the Bm61 of the Bombyx mori nucleopolyhedrovirus. Curr Microbiol 59:65–70. doi: 10.1007/s00284-009-9399-6. [DOI] [PubMed] [Google Scholar]
- 35.Wu W, Lin T, Pan L, Yu M, Li Z, Pang Y, Yang K. 2006. Autographa californica multiple nucleopolyhedrovirus nucleocapsid assembly is interrupted upon deletion of the 38K gene. J Virol 80:11475–11485. doi: 10.1128/JVI.01155-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cai Y, Long Z, Qiu J, Yuan M, Li G, Yang K. 2012. An ac34 deletion mutant of Autographa californica nucleopolyhedrovirus exhibits delayed late gene expression and a lack of virulence in vivo. J Virol 86:10432–10443. doi: 10.1128/JVI.00779-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Monsma SA, Oomens AG, Blissard GW. 1996. The GP64 envelope fusion protein is an essential baculovirus protein required for cell-to-cell transmission of infection. J Virol 70:4607–4616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vanarsdall AL, Okano K, Rohrmann GF. 2006. Characterization of the role of very late expression factor 1 in baculovirus capsid structure and DNA processing. J Virol 80:1724–1733. doi: 10.1128/JVI.80.4.1724-1733.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wei D, Wang Y, Zhang X, Hu Z, Yuan M, Yang K. 2014. Autographa californica nucleopolyhedrovirus Ac76: a dimeric type II integral membrane protein that contains an inner nuclear membrane-sorting motif. J Virol 88:1090–1103. doi: 10.1128/JVI.02392-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kerppola TK. 2006. Design and implementation of bimolecular fluorescence complementation (BiFC) assays for the visualization of protein interactions in living cells. Nat Protoc 1:1278–1286. doi: 10.1038/nprot.2006.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hodgson JJ, Arif BM, Krell PJ. 2011. Interaction of Autographa californica multiple nucleopolyhedrovirus cathepsin protease progenitor (proV-CATH) with insect baculovirus chitinase as a mechanism for proV-CATH cellular retention. J Virol 85:3918–3929. doi: 10.1128/JVI.02165-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Braunagel SC, Russell WK, Rosas-Acosta G, Russell DH, Summers MD. 2003. Determination of the protein composition of the occlusion-derived virus of Autographa californica nucleopolyhedrovirus. Proc Natl Acad Sci U S A 100:9797–9802. doi: 10.1073/pnas.1733972100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang R, Deng F, Hou D, Zhao Y, Guo L, Wang H, Hu Z. 2010. Proteomics of the Autographa californica nucleopolyhedrovirus budded virions. J Virol 84:7233–7242. doi: 10.1128/JVI.00040-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hou D, Zhang L, Deng F, Fang W, Wang R, Liu X, Guo L, Rayner S, Chen X, Wang H, Hu Z. 2013. Comparative proteomics reveal fundamental structural and functional differences between the two progeny phenotypes of a baculovirus. J Virol 87:829–839. doi: 10.1128/JVI.02329-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marek M, Merten OW, Galibert L, Vlak JM, van Oers MM. 2011. Baculovirus VP80 protein and the F-actin cytoskeleton interact and connect the viral replication factory with the nuclear periphery. J Virol 85:5350–5362. doi: 10.1128/JVI.00035-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu W, Passarelli AL. 2010. Autographa californica multiple nucleopolyhedrovirus Ac92 (ORF92, P33) is required for budded virus production and multiply enveloped occlusion-derived virus formation. J Virol 84:12351–12361. doi: 10.1128/JVI.01598-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nie Y, Fang M, Theilmann DA. 2011. Autographa californica multiple nucleopolyhedrovirus core gene ac92 (p33) is required for efficient budded virus production. Virology 409:38–45. doi: 10.1016/j.virol.2010.09.023. [DOI] [PubMed] [Google Scholar]
- 48.Braunagel SC, Guidry PA, Rosas-Acosta G, Engelking L, Summers MD. 2001. Identification of BV/ODV-C42, an Autographa californica nucleopolyhedrovirus orf101-encoded structural protein detected in infected-cell complexes with ODV-EC27 and p78/83. J Virol 75:12331–12338. doi: 10.1128/JVI.75.24.12331-12338.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Braunagel SC, Burks JK, Rosas-Acosta G, Harrison RL, Ma H, Summers MD. 1999. Mutations within the Autographa californica nucleopolyhedrovirus FP25K gene decrease the accumulation of ODV-E66 and alter its intranuclear transport. J Virol 73:8559–8570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Burks JK, Summers MD, Braunagel SC. 2007. BV/ODV-E26: a palmitoylated, multifunctional structural protein of Autographa californica nucleopolyhedrovirus. Virology 361:194–203. doi: 10.1016/j.virol.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 51.Zhang X, Xu K, Wei D, Wu W, Yang K, Yuan M. 2017. Baculovirus infection induces disruption of the nuclear lamina. Sci Rep 7:7823. doi: 10.1038/s41598-017-08437-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bonifacino JS, Glick BS. 2004. The mechanisms of vesicle budding and fusion. Cell 116:153–166. doi: 10.1016/S0092-8674(03)01079-1. [DOI] [PubMed] [Google Scholar]
- 53.Li G, Pang Y, Chen Q, Su Z, Wen X. 2002. Studies on the artificial diet for beet armyworm Spodoptera exigua. Chin J Biol Control 18:132–134. [Google Scholar]
- 54.Luckow VA, Lee SC, Barry GF, Olins PO. 1993. Efficient generation of infectious recombinant baculoviruses by site-specific transposon-mediated insertion of foreign genes into a baculovirus genome propagated in Escherichia coli. J Virol 67:4566–4579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.O'Reilly DR, Miller LK, Luckow VA. 1992. Baculovirus expression vectors: a laboratory manual. Oxford University Press, New York, NY. [Google Scholar]
- 56.Li L, Li Z, Chen W, Pang Y. 2007. Cloning, expression of Autographa californica nucleopolyhedrovirus vp39 gene in Escherichia coli and preparation of its antibody. Biotechnology 17:3–5. [Google Scholar]
- 57.Stuurman N, Maus N, Fisher PA. 1995. Interphase phosphorylation of the Drosophila nuclear lamin: site-mapping using a monoclonal antibody. J Cell Sci 108:3137–3144. [DOI] [PubMed] [Google Scholar]
- 58.Li A, Zhao H, Lai Q, Huang Z, Yuan M, Yang K. 2015. Posttranslational modifications of baculovirus protamine-like protein P6.9 and the significance of its hyperphosphorylation for viral very late gene hyperexpression. J Virol 89:7646–7659. doi: 10.1128/JVI.00333-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vanarsdall AL, Okano K, Rohrmann GF. 2005. Characterization of the replication of a baculovirus mutant lacking the DNA polymerase gene. Virology 331:175–180. doi: 10.1016/j.virol.2004.10.024. [DOI] [PubMed] [Google Scholar]
- 60.Li Y, Wang J, Deng R, Zhang Q, Yang K, Wang X. 2005. vlf-1 deletion brought AcMNPV to defect in nucleocapsid formation. Virus Genes 31:275–284. doi: 10.1007/s11262-005-3242-3. [DOI] [PubMed] [Google Scholar]
- 61.Wu W, Liang H, Kan J, Liu C, Yuan M, Liang C, Yang K, Pang Y. 2008. Autographa californica multiple nucleopolyhedrovirus 38K is a novel nucleocapsid protein that interacts with VP1054, VP39, VP80, and itself. J Virol 82:12356–12364. doi: 10.1128/JVI.00948-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rosas-Acosta G, Braunagel SC, Summers MD. 2001. Effects of deletion and overexpression of the Autographa californica nuclear polyhedrosis virus FP25K gene on synthesis of two occlusion-derived virus envelope proteins and their transport into virus-induced intranuclear membranes. J Virol 75:10829–10842. doi: 10.1128/JVI.75.22.10829-10842.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]