ABSTRACT
Human parainfluenza virus type 3 (HPIV3) is a negative-sense single-stranded RNA virus belonging to the Paramyxoviridae family. HPIV3 is a lung-tropic virus causing airway diseases, including pneumonia, croup, and bronchiolitis, during infancy and childhood. The activation of the inflammasome by pathogens results in the production of proinflammatory cytokines such as interleukin-1β (IL-1β) during infection. Thus, the inflammasome-mediated proinflammatory response plays a critical role in regulating the immune response and virus clearance. The inflammasome is a multimeric protein complex triggering caspase-1 activation. Activated caspase-1 cleaves pro-IL-1β into its mature (and active) secretory form. Our study revealed inflammasome activation in macrophages following HPIV3 infection. Specifically, the activation of the NLRP3/ASC inflammasome resulted in the production of mature IL-1β from HPIV3-infected cells. Furthermore, Toll-like receptor 2 (TLR2) activation (first signal) and potassium efflux (second signal) constituted two cellular events mediating inflammasome activation following HPIV3 infection. During our studies, we surprisingly identified the HPIV3 C protein as an antagonist of inflammasome activation. The HPIV3 C protein is an accessory protein encoded by the open reading frame of the viral phosphoprotein (P) gene. The HPIV3 C protein interacted with the NLRP3 protein and blocked inflammasome activation by promoting the proteasomal degradation of the NLRP3 protein. Thus, our studies report NLRP3/ASC inflammasome activation by HPIV3 via TLR2 signaling and potassium efflux. Furthermore, we have identified HPIV3 C as a viral component involved in antagonizing inflammasome activation.
IMPORTANCE Human parainfluenza virus type 3 (HPIV3) is a paramyxovirus that causes respiratory tract diseases during infancy and childhood. Currently, there is no effective vaccine or antiviral therapy for HPIV3. Therefore, in order to develop anti-HPIV3 agents (therapeutics and vaccines), it is important to study the HPIV3-host interaction during the immune response. Inflammasomes play an important role in the immune response. Inflammasome activation by HPIV3 has not been previously reported. Our studies demonstrated inflammasome activation by HPIV3 in macrophages. Specifically, HPIV3 activated the NLRP3/ASC inflammasome by TLR2 activation and potassium efflux. C proteins of paramyxoviruses are accessory proteins encoded by the viral phosphoprotein gene. The role of the C protein in inflammasome regulation was unknown. Surprisingly, our studies revealed that the HPIV3 C protein antagonizes inflammasome activation. In addition, we highlighted for the first time a mechanism utilized by paramyxovirus accessory proteins to block inflammasome activation. The HPIV3 C protein interacted with the NLRP3 protein to trigger the proteasomal degradation of the NLRP3 protein.
KEYWORDS: inflammasome, innate immunity
INTRODUCTION
Nonsegmented negative-strand RNA (NNS) viruses belonging to the paramyxovirus family constitute highly pathogenic human viruses that cause high rates of mortality and morbidity (1–6). Viruses belonging to the paramyxovirus family include human parainfluenza viruses (types 1, 2, 3, 4a, and 4b), measles virus (MeV), mumps virus, Nipah virus, and Hendra virus. Within the paramyxovirus family, human parainfluenza virus type 3 (HPIV3) is one of the most important human respiratory tract pathogens during infancy and childhood, causing a spectrum of life-threatening respiratory tract diseases, including pneumonia, croup, and bronchiolitis (1–11). Although HPIV3 causes life-threatening respiratory tract diseases, there is currently no effective vaccine or antiviral therapy for HPIV3.
Inflammasome activation resulting in the production of the proinflammatory cytokine interleukin-1β (IL-1β) is a key cellular response that dictates the immune response and pathogen clearance (12–16). The inflammasome is a cytosolic platform essential for caspase-1 activation and the cleavage of pro-IL-1β to its mature secretory form. Although a wide spectrum of viruses activates the inflammasome, it is still unknown whether HPIV3 activates the inflammasome and the mechanism that regulates such activity during infection. Our present study demonstrates the activation of the NLRP3/ASC inflammasome by HPIV3. We furthermore show that the Toll-like receptor 2 (TLR2) pathway and potassium efflux constitute the first and second signals, respectively, conferring inflammasome activation during infection.
During our studies, we unexpectedly identified the HPIV3 C protein as a regulator of inflammasome activation. The expression of accessory proteins (e.g., C and V proteins) in infected cells is a hallmark of paramyxoviruses (17–19). C and V proteins play an important role in regulating the innate immune response and viral replication (20–32). However, there have been very limited studies on how accessory proteins may modulate the inflammasome during infection. Surprisingly, our studies revealed that the HPIV3 C protein antagonizes inflammasome activation. The expression of the HPIV3 C protein blocked inflammasome activation. Further mechanistic studies highlighted the interaction of the C protein with NLRP3 and the proteasomal degradation of NLRP3 in C protein-expressing cells. Thus, our study reports inflammasome activation by HPIV3, which is mediated by the TLR2 pathway and the potassium efflux-dependent activation of the NLRP3/ASC inflammasome. We furthermore identified the HPIV3 C protein as an inflammasome antagonist that blocked NLRP3/ASC inflammasome activation by promoting the proteasomal degradation of NLRP3.
RESULTS
HPIV3 activates the inflammasome.
RNA respiratory viruses such as influenza A virus and respiratory syncytial virus (RSV) infect human and mouse macrophages to trigger various innate immune responses, including inflammasome activation, the inflammatory response, and cell death (33–38). However, studies with HPIV3-infected macrophages are limited. For the present study, we utilized human macrophage THP-1 cells since HPIV3 infection in mouse cell lines is not well documented, and furthermore, HPIV3 does not infect nonhuman cells efficiently. In order to investigate inflammasome activation, we infected THP-1 cells with HPIV3. These cells support productive HPIV3 infection. Although we did not observe cellular cytotoxicity at 6 h postinfection (data not shown), plaque assays of the medium supernatant revealed the release of infectious HPIV3 (3.75 × 102 PFU/ml) from THP-1 cells at 6 h postinfection.
We performed an enzyme-linked immunosorbent assay (ELISA) to detect IL-1β in the medium supernatant to study inflammasome activation, since only mature IL-1β is released from inflammasome-activated cells. In addition, the supernatant was subjected to Western blotting to detect (i) the cleaved p10 domain of caspase-1, since the p10 portion of procaspase-1 is cleaved following inflammasome activation and is subsequently released from the cell (33, 39, 40), and (ii) the p17 portion of pro-IL-1β, which represents the cleaved, mature, secreted form of IL-1β (33, 39, 40). HPIV3 triggered IL-1β production (Fig. 1A), the maturation (i.e., cleavage) of procaspase-1, and pro-IL-1β cleavage (Fig. 1B). The inflammasome activates caspase-1, the enzyme that cleaves pro-IL-1β, thus promoting the release of mature IL-1β. HPIV3-mediated IL-1β production is caspase-1 dependent, since blocking caspase-1 [by using the caspase-1 inhibitor Ac-(NMe)Tyr-Val-Ala-Asp-CHO (Ac-YVAD-CHO)] abrogated IL-1β production (Fig. 1C). Inflammasome activation and IL-1β production required replication, since UV-inactivated HPIV3 failed to induce IL-1β production (Fig. 1D). These results demonstrated caspase-1-dependent inflammasome activation by HPIV3.
FIG 1.
Inflammasome activation by HPIV3. (A) THP-1 cells were infected with HPIV3. At 6 h, 12 h, and 24 h postinfection, the medium supernatant was assessed for IL-1β by an ELISA. (B) The TCA-precipitated supernatants derived from HPIV3-infected (8 h and 16 h) THP-1 cells were subjected to Western blotting with either IL-1β (p17) or caspase-1 (p10) antibody. Actin served as a loading control. (C) THP-1 cells were infected with HPIV3 for 6 h in the presence of either water (vehicle control) or a caspase-1 inhibitor (10 μM Ac-YVAD-CHO). The IL-1β level in the supernatant was assessed by an ELISA. (D) The IL-1β level was measured in the medium supernatant of THP-1 cells infected with either UV-irradiated HPIV3 (+ UV) or non-UV-irradiated HPIV3 (− UV). The ELISA values (A, C, and D) represent the means ± standard deviations (n = 8). *, P ≤ 0.05 by using Student's t test. The immunoblot (B) is representative of data from two independent experiments with similar results. UT, untreated.
HPIV3 activates the NLRP3/ASC inflammasome.
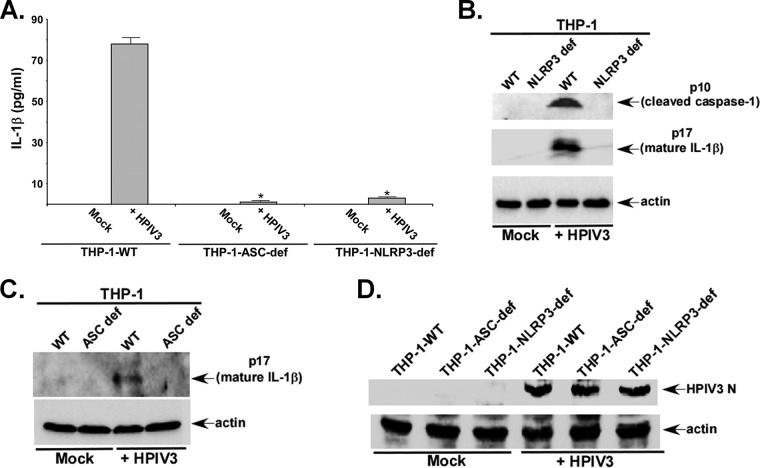
In order to identify the specific inflammasome complex activated by HPIV3, we infected ASC-deficient THP-1 (THP-1-ASC-def) cells, NLRP3-deficient THP-1 (THP-1-NLRP3-def) cells, and control wild-type (WT) THP-1 (THP-1-WT) cells with HPIV3. THP-1-NLRP3-def and THP-1-ASC-def cells are devoid of NLRP3 and ASC proteins, respectively. HPIV3 activated the NLRP3/ASC inflammasome since IL-1β production was drastically reduced following infection of ASC-deficient and NLRP3-deficient macrophages (Fig. 2A). Concomitantly, caspase-1 cleavage and pro-IL-1β maturation were abolished in HPIV3-infected cells lacking NLRP3 (Fig. 2B). As expected, we failed to detect mature (cleaved) IL-1β (i.e., p17) in HPIV3-infected ASC-deficient THP-1 cells (Fig. 2C). We detected similar levels of HPIV3 protein (HPIV3 nucleocapsid or N protein) expression in control and deficient THP-1 cells (Fig. 2D), and thus, the loss of inflammasome activation in deficient cells is not due to inefficient HPIV3 infection. Note that at this time, we do not know why we observed reduced IL-1β production from HPIV3-infected THP-1-WT cells (i.e., the cells that served as a positive control for ASC- and NLRP3-deficient cells) compared to parental wild-type THP-1 cells. Thus, our studies demonstrated that HPIV3 activates the NLRP3/ASC inflammasome.
FIG 2.
HPIV3 activates the NLRP3/ASC inflammasome. (A) THP-1-WT (control), NLRP3-deficient THP-1 (THP-1-NLRP3-def), and ASC-deficient THP-1 (THP-1-ASC-def) cells were infected with HPIV3 for 6 h. IL-1β levels in the supernatant were assessed by an ELISA. (B) Detection of the cleaved caspase-1 p10 subunit and the mature p17 subunit of IL-1β in the supernatant of HPIV3-infected THP-1-WT and THP-1-NLRP3-def cells by performing Western blotting with p10- and p17-specific antibodies. Actin served as a loading control. (C) Detection of the mature p17 subunit of IL-1β in the supernatant of HPIV3-infected THP-1-WT and THP-1-ASC-def cells by performing Western blotting with p17-specific antibody. Actin served as a loading control. The ELISA values (A, C, and D) represent the means ± standard deviations. *, P ≤ 0.05 by using Student's t test. UT, untreated. (D) THP-1-WT (control), THP-1-NLRP3-def, and THP-1-ASC-def cells were infected with HPIV3 for 6 h. The cell lysate from infected cells was subjected to Western blotting with anti-HPIV3 RNP antibody to detect the HPIV3 nucleocapsid (N) protein. The ELISA values (A) represent the means ± standard deviations (n = 8). *, P ≤ 0.05 by using Student's t test. Immunoblots (B and C) are representative of data from two independent experiments with similar results.
The TLR2 pathway is required for inflammasome activation during HPIV3 infection, the first signal for inflammasome activation.
Two signals are required for inflammasome activation in macrophages (12–16, 41). The first signal is mediated by pattern recognition receptors (PRRs). PRRs are activated upon the detection of pathogen-associated molecular patters (PAMPs). Among various PRRs, activated TLRs serve as the first signal for various pathogens (42–48). Furthermore, we have shown the activation of the TLR-MyD88 pathway by HPIV3 (49). Therefore, we examined whether activated TLRs also relay the first signal during HPIV3 infection. The role of TLRs was investigated by transfecting WT and dominant negative (DN) TLRs (49, 50) in THP-1 cells. Specifically, we chose to focus on TLR4, TLR9, and TLR2 since respiratory RNA viruses such as RSV and influenza A virus activate TLR2 and TLR4 in both human and mouse macrophages (33, 35); on the other hand, we utilized TLR9 as a negative control because TLR9 is activated by bacteria. Although TLR4 was efficiently expressed in THP-1 cells (Fig. 3A), TLR4 does not play a role in HPIV3-mediated inflammasome activation. Compared to WT TLR4-expressing cells, the expression of DN TLR4 in THP-1 cells did not alter IL-1β production from HPIV3-infected cells (Fig. 3B). Likewise, HPIV3 activated the inflammasome via a TLR9-independent mechanism, since the levels of IL-1β production from HPIV3-infected cells were similar following the expression of WT TLR9 and DN TLR9 (Fig. 3C and D). However, our study revealed a role of TLR2 in mediating the first signal during HPIV3 infection. WT TLR2 and DN TLR2 were efficiently expressed in THP-1 cells (Fig. 3E). Compared to cells expressing WT TLR2, a significant loss of IL-1β production was noted for DN TLR2-expressing cells (Fig. 3F). Furthermore, we observed reduced levels of mature IL-1β (p17) in the supernatant of DN TLR2-expressing cells infected with HPIV3 (Fig. 3G). In order to further validate our results, we next silenced TLR2 in THP-1 cells by using TLR2-specific small interfering RNA (siRNA) (Fig. 3H). In accord with data from our DN TLR2 studies, TLR2 silencing led to diminished IL-1β production following HPIV3 infection (Fig. 3I). Thus, activated TLR2 constitutes the first signal required for inflammasome activation during HPIV3 infection.
FIG 3.
The TLR2 pathway confers the first signal during HPIV3-mediated inflammasome activation. (A) THP-1 cells were transfected with plasmids expressing either WT TLR4 or DN TLR4. At 16 h posttransfection, TLR4 expression was analyzed by RT-PCR. NT, nontransfected. (B) THP-1 cells expressing either WT TLR4 or DN TLR4 were infected with HPIV3 (4 h), and the medium supernatant was assayed for IL-1β production by an ELISA. (C) THP-1 cells were transfected with plasmids expressing either WT TLR9 or DN TLR9. At 16 h posttransfection, TLR9 expression was analyzed by RT-PCR. (D) THP-1 cells expressing either WT TLR9 or DN TLR9 were infected with HPIV3 (4 h), and the supernatant was assayed for IL-1β production by an ELISA. (E) THP-1 cells were transfected with plasmids expressing either WT TLR2 or DN TLR2. At 16 h posttransfection, TLR2 expression was analyzed by RT-PCR. (F) THP-1 cells expressing either WT TLR2 or DN TLR2 were infected with HPIV3 (6 h), and the supernatant was assayed for IL-1β production by an ELISA. (G) Western blotting with p17-specific antibody was performed to detect the mature p17 subunit of IL-1β in the supernatant of HPIV3-infected THP-1 cells expressing either WT TLR2 or DN TLR2. Actin served as a loading control. (H) THP-1 cells were transfected with either human TLR2 siRNA (40 and 100 pmol) or control siRNA (100 pmol). TLR2 expression was analyzed by RT-PCR. (I) THP-1 cells transfected with either control siRNA or TLR2 siRNA were infected with HPIV3. At 6 h postinfection, IL-1β levels in the supernatant were measured by an ELISA. The ELISA values (B, D, F, and I) represent the means ± standard deviations (n = 8). *, P ≤ 0.05 by using Student's t test. The immunoblot (G) is representative of data from two independent experiments with similar results. Con siRNA, control siRNA.
Inflammasome activation during HPIV3 infection requires potassium efflux by the ATP-sensitive potassium channel, the second signal for inflammasome activation.
Next, we focused our study on identifying the second signal that initiates inflammasome activation and mature IL-1β release during HPIV3 infection. Potassium efflux serves as a second signal for inflammasome activation by several pathogens (51–56), including RSV and NNS viruses belonging to the Paramyxoviridae family (33). Therefore, we examined the role of potassium efflux by the ATP-sensitive potassium channel in triggering NLRP3/ASC inflammasome activation during HPIV3 infection.
In order to study the role of potassium efflux during HPIV3 infection, the ATP-sensitive potassium channel was inhibited with glibenclamide. Based on data from previous reports (33, 53–55), we used a nontoxic concentration (50 μM) of glibenclamide. This concentration was used previously to study the role of the macrophage ATP-sensitive potassium channel in inflammasome activation (33, 53–55). THP-1 cells pretreated with glibenclamide (50 μM) for 2 h were infected with HPIV3 in the presence of glibenclamide or dimethyl sulfoxide (DMSO) (vehicle control). Glibenclamide treatment drastically blocked IL-1β release from HPIV3-infected cells (Fig. 4A). In addition, glibenclamide treatment abrogated pro-IL-1β maturation (Fig. 4B) and procaspase-1 cleavage (data not shown). We previously treated macrophages with 50 μM glibenclamide for 12 h during RSV infection (33). Such treatment did not alter the expression of pro-IL-1β, NLRP3, and ASC (33). Therefore, the loss of IL-1β production from glibenclamide-treated HPIV3-infected cells is not due to enhanced cellular toxicity or the reduced expression of inflammasome components (i.e., NLRP3, pro-IL-1β, and ASC).
FIG 4.
Potassium efflux serves as a second signal during inflammasome activation by HPIV3. (A) THP-1 cells were pretreated with either DMSO (vehicle control) or 50 μM the ATP-sensitive potassium channel inhibitor glibenclamide (Gly) for 2 h prior to HPIV3 infection for 6 h in the presence of DMSO or glibenclamide. The medium supernatant was assayed for IL-1β production by an ELISA. (B) Western blotting with p17-specific antibody was performed to detect the mature p17 subunit of IL-1β in the supernatant of HPIV3-infected THP-1 cells treated with either DMSO (vehicle control) or glibenclamide. Actin served as a loading control. (C) THP-1 cells treated with buffer containing either 150 mM KCl or 150 mM NaCl (control) were infected with HPIV3. At 6 h postinfection, the IL-1β level in the supernatant was analyzed by an ELISA. The ELISA values (A and C) represent the means ± standard deviations (n = 8). *, P ≤ 0.05 by using Student's t test. The immunoblot (B) is representative of data from two independent experiments with similar results. UT, untreated (i.e., not treated with glibenclamide).
The role of potassium efflux in inflammasome activation during HPIV3 infection was further validated by infecting THP-1 cells with HPIV3 in the presence of buffer containing either 150 mM NaCl (control) or 150 mM KCl (56). Incubation with excess potassium (i.e., KCl treatment) blocks potassium efflux, and this method was previously utilized to demonstrate the role of potassium efflux during inflammasome activation (56). KCl treatment during HPIV3 infection significantly reduced IL-1β release from infected THP-1 cells (Fig. 4C). Note that for these experiments, we infected cells for 4 h to avoid cellular toxicity due to prolonged exposure to NaCl and KCl. These studies demonstrated that potassium efflux by the ATP-sensitive potassium channel serves as a second signal to activate the inflammasome during HPIV3 infection.
HPIV3 C protein antagonizes inflammasome activation.
The paramyxovirus C protein antagonizes the innate antiviral response by blocking beta interferon (IFN-β) production and inhibiting IFN-β signaling via the JAK/STAT pathway (17–19, 27–30). However, it is still unknown whether the C protein can antagonize additional innate immune pathways. Surprisingly, we have identified the HPIV3 C protein as an inflammasome pathway antagonist.
We reconstituted the inflammasome in HEK293 cells by expressing pro-IL-1β, procaspase-1, NLRP3, and ASC in these cells, as reported by us previously (33, 39). We expressed the FLAG-tagged C protein (FLAG-C) and empty FLAG (control) in these reconstituted HEK293 cells. Robust C protein expression in HEK293 cells was noted (Fig. 5A). Reverse transcription-PCR (RT-PCR) analysis revealed similar mRNA expression levels of pro-IL-1β, procaspase-1, NLRP3, and ASC in control and in C protein-expressing cells (data not shown). A significant reduction in inflammasome activation (as deduced from IL-1β release) was observed for FLAG-C-expressing HEK293 cells (Fig. 5B). IL-1β release was diminished by ∼57% following the expression of the HPIV3 C protein (Fig. 5B). In order to investigate whether the inhibition of inflammasome activation by the C protein is specific for HPIV3, we examined IL-1β production from inflammasome-reconstituted HEK293 cells expressing the HPIV1 C protein. Although the HPIV1 C protein was expressed efficiently in HEK293 cells (Fig. 5C), it did not block IL-1β production (Fig. 5D). Thus, the inflammasome-blocking function is specific for the HPIV3 C protein because the HPIV1 C protein does not possess such activity.
FIG 5.
HPIV3 C protein inhibits inflammasome activation. (A) Cell lysates obtained from nontransfected (NT), empty FLAG-transfected, and FLAG-C protein-transfected HEK293 cells were subjected to Western blotting with anti-FLAG antibody. (B) Inflammasome-reconstituted HEK293 cells (i.e., cells expressing ASC, NLRP3, pro-IL-1β, and procaspase-1) expressing either empty FLAG or the FLAG-C protein were treated with nigericin (15 μM) for 30 min. IL-1β production was measured by performing an ELISA with the medium supernatant. (C) Cell lysates obtained from empty FLAG-transfected and FLAG-HPIV1 C protein-transfected HEK293 cells were subjected to Western blotting with anti-FLAG antibody. (D) Inflammasome-reconstituted HEK293 cells (i.e., cells expressing ASC, NLRP3, pro-IL-1β, and procaspase-1) expressing either empty FLAG or the FLAG-HPIV1 C protein were treated with nigericin (15 μM) for 30 min. IL-1β production was measured by performing an ELISA with the supernatant. (E) RT-PCR analysis of C protein expression in NT and FLAG-C protein-transfected THP-1 cells. (F) THP-1 cells expressing either empty FLAG or the FLAG-C protein were treated with LPS (4 h) and nigericin (30 min). IL-1β levels in the supernatant were analyzed by an ELISA. (G) Western blotting with p17 (to detect the mature p17 subunit of IL-1β)- and p10 (to detect the cleaved caspase-1 p10 subunit)-specific antibodies was performed with the supernatant of THP-1 cells (expressing either empty FLAG or the FLAG-C protein) treated with LPS alone or LPS and nigericin. Actin served as a loading control. (H) THP-1 cells expressing either empty FLAG or the FLAG-HPIV1 C protein were treated with LPS (1 h) and nigericin (30 min). IL-1β levels in the supernatant were analyzed by an ELISA. The ELISA values (B, D, F, and H) represent the means ± standard deviations (n = 8). *, P ≤ 0.05 by using Student's t test. Immunoblots (A, C, and G) are representative of data from two independent experiments with similar results. UT, untreated.
The anti-inflammasome activity of the HPIV3 C protein was further validated by using THP-1 cells. We expressed FLAG-C and empty FLAG (control) in THP-1 cells. The expression of the C protein was detected in THP-1 cells (Fig. 5E). C protein-expressing THP-1 cells were treated with lipopolysaccharide (LPS) (the first signal for inflammasome activation) and nigericin (the second signal for inflammasome activation) to trigger NLRP3/ASC inflammasome activation. The expression of the C protein led to a significant reduction in IL-1β production (Fig. 5F). IL-1β production was reduced by ∼40% following the expression of the HPIV3 C protein in THP-1 cells (Fig. 5F). Furthermore, we noted the loss of caspase-1 cleavage and pro-IL-β maturation in C protein-expressing THP-1 cells (Fig. 5G). To demonstrate specificity, we expressed the FLAG-tagged HPIV1 C protein in THP-1 cells. High levels of the FLAG-HPIV1 C protein were detected in THP-1 cells (data not shown). The loss of inflammasome activation in THP-1 cells was once again specific for the HPIV3 C protein since the expression of the HPIV1 C protein in THP-1 cells did not result in reduced IL-1β production following treatment with LPS and nigericin (Fig. 5H). These results demonstrated that the HPIV3 C protein possesses an anti-inflammasome property, and it specifically blocks NLRP3/ASC inflammasome activation.
HPIV3 C protein triggers NLRP3 degradation via the ubiquitin-proteasome system.
In an effort to understand the mechanism by which the C protein inhibits inflammasome activation, we focused on the ability of the C protein to degrade inflammasome components, since the Sendai virus C protein was previously shown to degrade cellular factors like STAT1 (57). Indeed, during our studies, we noticed reduced expression levels of the NLRP3 protein in HEK293 cells expressing the C protein. Western blotting with an anti-Myc antibody revealed the loss of NLRP3 protein levels in HEK293 cells coexpressing Myc-tagged NLRP3 (Myc-NLRP3) and FLAG-C (Fig. 6A). Based on the quantification of the protein bands, we observed an ∼70% reduction in NLRP3 protein levels following HPIV3 C protein expression (Fig. 6A). RT-PCR analysis revealed no change in NLRP3 mRNA levels in control versus FLAG-C-expressing cells (Fig. 6B). The effect of the HPIV3 C protein on NLRP3 was specific, since FLAG-C expression did not alter ASC protein levels following the coexpression of Myc-ASC and FLAG-C proteins in HEK293 cells (Fig. 6C). Furthermore, the coexpression of the FLAG-HPIV1 C protein and Myc-NLRP3 did not diminish NLRP3 protein expression (Fig. 6D). This result demonstrated that the HPIV3 C protein specifically targets the NLRP3 protein for degradation.
FIG 6.
HPIV3 C protein promotes NLRP3 degradation. (A) Cell lysates collected from HEK293 cells coexpressing Myc-NLRP3 and FLAG-C were subjected to Western blotting with anti-Myc and anti-FLAG antibodies. (B) RT-PCR analysis of NLRP3 expression in HEK293 cells coexpressing either Myc-NLRP3 and FLAG-C or Myc-NLRP3 and empty FLAG. (C) Cell lysates collected from HEK293 cells coexpressing Myc-ASC and FLAG-C were subjected to Western blotting with anti-Myc antibody. (D) Cell lysates collected from HEK293 cells coexpressing Myc-NLRP3 and the FLAG-HPIV1 C protein were subjected to Western blotting with anti-Myc and anti-FLAG antibodies. In panels A and D, protein bands corresponding to NLRP3 and actin were quantified to calculate the NLRP3/actin ratio (relative intensity). The immunoblot (A) is representative of data from three independent experiments with similar results. NT, not transfected; FLAG, empty FLAG.
We further established the involvement of the C protein in regulating NLRP3 protein expression by investigating the status of endogenous NLRP3 protein in HPIV3 C protein-expressing THP-1 cells. The expression of the FLAG-HPIV3 C protein in THP-1 cells resulted in the reduced expression of endogenous NLRP3 protein (Fig. 7A). Based on the quantification of the protein bands, we observed an ∼60% reduction in NLRP3 protein levels following HPIV3 C protein expression (Fig. 7A). In contrast, the expression of the FLAG-HPIV1 C protein in THP-1 cells did not result in a loss of NLRP3 protein levels (Fig. 7B). These studies demonstrated that the HPIV3 C protein promotes NLRP3 protein degradation.
FIG 7.
HPIV3 C protein promotes NLRP3 degradation via the proteasome. (A) Cell lysates collected from THP-1 cells expressing FLAG-C were subjected to Western blotting with anti-NLRP3 antibody to examine the status of endogenous NLRP3 protein expression. The blot was also probed with anti-FLAG antibody to detect the expression of the FLAG-C protein. (B) Cell lysates collected from THP-1 cells expressing FLAG-HPIV1 C were subjected to Western blotting with anti-NLRP3 antibody to examine the status of endogenous NLRP3 protein expression. The blot was also probed with anti-FLAG antibody to detect the expression of the FLAG-HPIV1 C protein. (C) Cell lysates collected from HEK293 cells coexpressing Myc-NLRP3 and FLAG-C in the presence of the vehicle (DMSO) or the proteasome inhibitor MG132 (10 μM) were subjected to Western blotting with anti-Myc antibody. In panels A to C, protein bands corresponding to NLRP3 and actin were quantified to calculate the NLRP3/actin ratio (relative intensity). Immunoblots (A and C) are representative of data from two independent experiments with similar results. FLAG, empty FLAG.
Next, we examined whether the C protein triggers the ubiquitin-mediated proteasomal degradation of the NLRP3 protein. For these studies, we treated cells with proteasomal inhibitors. HEK293 cells coexpressing Myc-NLRP3 and FLAG-C proteins were treated with either DMSO (vehicle control) or MG132. Western blot analysis with anti-Myc antibody revealed higher levels of the NLRP3 protein in MG132-treated C protein-expressing cells than in DMSO-treated C protein-expressing cells (Fig. 7C). Quantification of the protein bands revealed that although the expression of the HPIV3 C protein diminished NLRP3 protein levels by ∼70%, treatment of cells with MG132 resulted in a reduction of NLRP3 protein levels by ∼40% (Fig. 7C). Thus, our studies provide mechanistic insight into the anti-inflammasome activity of the HPIV3 C protein. The HPIV3 C protein acts as an inflammasome antagonist by promoting NLRP3 protein degradation via proteasomal machinery.
HPIV3 C protein interacts with NLRP3.
In an effort to further understand the mechanism that promotes C protein-mediated NLRP3 degradation, we performed coimmunoprecipitation (co-IP) analysis to examine the possible interaction of the C protein with NLRP3. We conducted our co-IP studies in the presence of MG132 to prevent NLRP3 degradation, thus maximizing our effort to detect the interaction of NLRP3 and C proteins. We coexpressed Myc-NLRP3 and FLAG-C in HEK293 cells in the presence of MG132, followed by immunoprecipitation (IP) of cell lysates with anti-FLAG antibody and immunoblotting (IB) with anti-Myc antibody. Our co-IP studies revealed an interaction of the C protein with NLRP3 (Fig. 8A). We further validated our results by conducting coimmunofluorescence analysis (co-IFA). Co-IFA was performed by using HEK293 cells coexpressing Myc-NLRP3 and FLAG-C proteins. These cells were labeled with anti-Myc antibodies (and Texas Red-labeled secondary antibody) and anti-FLAG antibodies (and fluorescein isothiocyanate [FITC]-labeled secondary antibody) to detect NLRP3 (Fig. 8B, red) and the C protein (green), respectively. Confocal microscopic analysis of merged (yellow) images demonstrated the colocalization of NLRP3 with the C protein (Fig. 8B). Note that the effect of the C protein on NLRP3 protein levels is evident from the co-IFA images. One can visualize enhanced NLRP3 protein levels in two cells not expressing the C protein versus one cell that is expressing the C protein (Fig. 8B, Myc-NLRP3 + FLAG-C protein panel). We next utilized a fluorescence intensity measurement tool to quantify the fluorescence intensity of NLRP3 in the same visual field with cells expressing either high or low (or no) levels of the C protein. Data were obtained following the visualization of 15 different fields. Compared to cells expressing low levels of or no C protein, a significant loss of the NLRP3-specific fluorescence intensity was observed in cells expressing high C protein levels (Fig. 8C). This observation corroborated the results obtained with HEK293 cells (Fig. 6A).
FIG 8.
Interaction of HPIV3 C protein with NLRP3. (A) HEK293 cells were cotransfected with FLAG-C and Myc-NLRP3 in the presence of MG132. Cells lysates were immunoprecipitated (IP) with anti-FLAG antibody and subsequently immunoblotted (IB) with anti-Myc and anti-FLAG antibodies. Total cell lysates were also subjected to Western blotting with anti-FLAG and anti-Myc antibodies. (B) HEK293 cells coexpressing FLAG-C and Myc-NLRP3 were subjected to coimmunofluorescence analysis. Merged images (yellow) depict the colocalization of the C protein (green) and NLRP3 (red). Nuclei were stained with DAPI (blue). (C) Quantification of Myc-NLRP3 protein-specific fluorescence intensity from cells (present in the same visual field) with either high or low (or no) C protein expression levels. Quantification was performed with 15 different visual fields (n = 15). (D) THP-1 cells were transfected with FLAG-C in the presence of MG132. Lysates from these cells were immunoprecipitated with anti-FLAG antibody and subsequently immunoblotted with anti-NLRP3 and anti-FLAG antibodies. Total cell lysates were also subjected to Western blotting with anti-NLRP3 and anti-FLAG antibodies. FLAG, empty FLAG. The immunoblot (A) is representative of data from two independent experiments with similar results. The fluorescence values (C) represent the means ± standard deviations (n = 15). *, P ≤ 0.05 by using Student's t test.
In order to evaluate the interaction of the C protein with endogenous NLRP3, we expressed the FLAG-C protein in THP-1 cells in the presence of MG132. The cell lysate was then subjected to IP with anti-FLAG antibody and IB with anti-NLRP3 antibody. We observed an interaction of the C protein with endogenous NLRP3 in THP-1 cells (Fig. 8D). Thus, our study revealed the interaction of the C protein with NLRP3.
HPIV3 C protein promotes ubiquitination of NLRP3 protein.
Proteasomal degradation occurs due to the covalent modification of targeted proteins with ubiquitin. Since the C protein triggered NLRP3 proteasomal degradation, we speculated that the C protein can promote the ubiquitination of the NLRP3 protein. Therefore, we examined the ubiquitination status of the NLRP3 protein in THP-1 cells during inflammasome activation in the absence and presence of the HPIV3 C protein. We specifically evaluated the NLRP3 ubiquitination status during the first signal (i.e., LPS treatment) and the first and second signals (i.e., treatment with LPS and nigericin) of inflammasome activation. FLAG (control)- or FLAG-C protein-expressing THP-1 cells were treated with either LPS or LPS plus nigericin. The ubiquitination status of NLRP3 in these cells was assessed by IP of the cell lysate with an antiubiquitin antibody and IB with an anti-NLRP3 antibody. As expected, lower levels of the NLRP3 protein were visualized in the cell lysate of C protein-expressing cells (Fig. 9A). Interestingly, enhanced ubiquitination of the NLRP3 protein was observed in cells expressing the HPIV3 C protein (Fig. 9A). Note that although the C protein promoted NLRP3 ubiquitination during the first signal (i.e., LPS treatment), the C protein accelerated NLRP3 ubiquitination during actual inflammasome activation (i.e., treatment with LPS plus nigericin). In contrast to the HPIV3 C protein, the HPIV1 C protein did not trigger NLRP3 protein ubiquitination. Although we detected ubiquitinated NLRP3 protein in HPIV3 C protein-expressing inflammasome-activated cells (i.e., cells treated with LPS plus nigericin), such ubiquitinated NLRP3 protein was not observed in inflammasome-activated cells expressing the HPIV1 C protein (Fig. 9B). Thus, our studies demonstrate an interaction of the HPIV3 C protein with NLRP3, which may lead to NLRP3 ubiquitination and the subsequent degradation of the NLRP3 protein via proteasomal machinery.
FIG 9.
HPIV3 C protein promotes ubiquitination of NLRP3 protein. (A) THP-1 cells expressing either empty FLAG or the FLAG-C protein were treated with either LPS or LPS and nigericin. Lysates from treated THP-1 cells were immunoprecipitated (IP) with antiubiquitin antibody and subsequently immunoblotted (IB) with anti-NLRP3 antibody. Total cell lysates were also subjected to Western blotting with anti-NLRP3 antibody. NLRP3-specific bands are indicated by red dots. Both high and lower exposures of the same gel are shown. (B) THP-1 cells expressing either the FLAG-HPIV1 C protein or the FLAG-HPIV3 C protein were treated with LPS and nigericin. Lysates from treated THP-1 cells were immunoprecipitated with antiubiquitin antibody and subsequently immunoblotted with anti-NLRP3 antibody. Total cells lysates were also subjected to Western blotting with anti-NLRP3 antibody. Immunoblots are representative of data from two independent experiments with similar results. Ub, ubiquitin.
DISCUSSION
HPIV-3 is an important human pathogen causing respiratory tract diseases, including pneumonia, croup, and bronchiolitis, in infants and children (1–11). Although HPIV3 causes life-threatening lung diseases, there is currently no effective vaccine or antiviral therapy for HPIV3. Therefore, it is imperative to study the immune response during HPIV3 infection for the development of antiviral therapeutics and vaccines to combat infection. However, immune-related studies with HPIV3 are limited. We previously showed that HPIV3 activates TLR-MyD88 and RIGI pathways for the innate immune response (49, 58). A recent study implicated a role of autophagy in regulating HPIV3 infection (59). It was also reported that HPIV3 infects monocytes and dendritic cells (DCs) (60), and HPIV3-infected DCs trigger CD4 T cell activity (61). HPIV3 infection also results in the production of the immune cytokine IFN-γ and the chemokine RANTES during nasal epithelial cell infection (62).
IL-1β is a proinflammatory cytokine shaping the protective immune response during infection. IL-1β plays a vital role in orchestrating an optimal immune response for efficient RNA virus (e.g., RSV and influenza A virus) clearance from an infected host (33, 34, 63). IL-1β is released from cells following the cleavage of inactive pro-IL-1β into its mature form by active caspase-1. Caspase-1 is activated by the inflammasome complex (e.g., the NLRP3/ASC inflammasome complex) (12–16).
The paramyxovirus (Paramyxovirinae) subfamily of single-stranded RNA viruses consists of several genera, including respiroviruses (e.g., Sendai virus, HPIV1, HPIV3, and bovine parainfluenzavirus type 3), rubulaviruses (e.g., simian virus 5 and mumps virus), morbilliviruses (e.g., MeV), and henipaviruses (e.g., Nipah and Hendra viruses). Nipah and Hendra virus infections resulted in IL-1β release (64, 65). However, the role of the inflammasome during this process is still unknown. Although inflammasome activation by two nonhuman paramyxoviruses, Sendai virus (a mouse virus) (66, 67) and Newcastle disease virus (NDV) (an avian virus) (68) have been documented, studies on inflammasome activation by human paramyxoviruses are limited. There is only one study showing NLRP3 inflammasome activation by measles virus (69). However, that study did not examine the mechanism by which measles virus activates the inflammasome. In this regard, we now report that another human paramyxovirus, HPIV3, activates the inflammasome. Furthermore, we also elucidate the mechanism triggering inflammasome activation during HPIV3 infection. Our results demonstrate that the TLR2 pathway (first signal) and potassium efflux (second signal) promote NLRP3/ASC inflammasome activation during HPIV3 infection.
Paramyxoviruses express unique accessory proteins such as the C and V proteins, which originate from the open reading frame of the viral phosphoprotein (P) gene (17–32). The C protein is expressed by respiroviruses, morbilliviruses, and henipaviruses. It is not expressed by rubulaviruses. The V protein is expressed by “specific” paramyxoviruses, including Sendai virus, NDV, and MeV. HPIV1 and HPIV3 do not express the V protein. V proteins are known antagonists of the innate immune response (17–26, 29, 70–72). For example, the V protein blocks the type I IFN response (17–19, 21, 23–26, 29, 32). Similar to the V protein, the C protein also antagonizes the IFN response by several mechanisms (17–19, 27–29, 73, 74). Interestingly, the HPIV3 C protein evades the IFN response by blocking STAT1 phosphorylation and thus preventing its translocation to the nucleus to induce antiviral gene expression (28). In addition, the paramyxovirus C protein also regulates viral gene expression by virtue of inhibiting viral RNA synthesis (31, 75).
A role of the MeV V protein in blocking inflammasome activation was reported previously (69). The MeV V protein diminished NLRP3-driven IL-1β secretion from THP-1 macrophages (69). Following inflammasome activation by MeV, the interaction of NLRP3 with the V protein was visualized in the perinuclear region of THP-1 macrophages (69). However, the exact mechanism conferring the inflammasome antagonist activity of the V protein is still unknown. The role of the paramyxovirus C protein in antagonizing IFN-independent innate immune responses has not been reported yet. In this study, we have identified the HPIV3 C protein as an inflammasome antagonist that prevented IL-1β production. Mechanistically, we demonstrated the interaction of the C protein with NLRP3 and the loss of the NLRP3 protein in C protein-expressing cells due to proteasomal degradation. Therefore, our study for the first time illustrates a mechanism of inflammasome antagonism by a paramyxovirus accessory protein. We show that the HPIV3 C protein triggers NLRP3 proteasomal degradation that culminates in the loss of NLRP3/ASC inflammasome activation. Interestingly, our study demonstrates that the C protein of HPIV1 (another member of the paramyxovirus subfamily) did not promote NLRP3 degradation or block inflammasome activation. Thus, the inflammasome antagonism function of the C protein is specific for HPIV3 and is not conserved among other members of the paramyxovirus subfamily. Among the parainfluenza viruses, HPIV3 is the most clinically important human pathogen (1–11), since it is second only to RSV in terms of causing pneumonia and bronchiolitis in children and infants (9). Thus, we postulate that HPIV3 may have evolved several antagonism mechanisms to dampen the innate immune response for its advantage to cause severe disease in humans. In this regard, the C protein of HPIV3 is unique compared to the C and V proteins of other paramyxoviruses: (i) the HPIV3 C protein shares only 30% to 40% amino acid identity with other paramyxovirus C proteins, and (ii) HPIV3 is the only paramyxovirus that does not express a truncated version of the C protein (e.g., C′, Y1, or Y2) (18, 19, 29). Thus, it is possible that the inflammasome-antagonizing function of the HPIV3 C protein is a unique characteristic of HPIV3.
In summary, our study demonstrates NLRP3/ASC inflammasome activation by HPIV3 in macrophages via the TLR2 pathway and potassium efflux. Furthermore, we have identified the HPIV3 C protein as an inflammasome antagonist, since it blocked inflammasome activation and subsequent IL-1β release. Mechanistically, the C protein interacted with NLRP3 to trigger NLRP3 protein degradation via ubiquitin-proteasome machinery.
MATERIALS AND METHODS
Virus and cell culture.
HPIV3 (strain NIH 47885) was propagated in CV-1 cells and purified by centrifugation twice on a discontinuous sucrose gradient, as described previously (36, 49, 58, 76). HEK293 cells were maintained in Dulbecco's modified Eagle medium (DMEM) supplemented with 10% fetal bovine (FBS), 100 IU/ml penicillin, and 100 μg/ml streptomycin. Human THP-1 cells (ATCC) were differentiated by culturing in complete medium (RPMI 1640 medium supplemented with 10% FBS, 10 mM HEPES, 1 mM sodium pyruvate, 100 IU/ml penicillin, 100 μg/ml streptomycin [Life Technologies], and 0.05 mM β-mercaptoethanol) containing 100 nM phorbol 12-myristate 13-acetate (PMA) for 24 h. Culture medium containing PMA was replaced with fresh complete medium, and cells were maintained for another 24 h before treatment and infection. ASC-deficient THP-1 (THP-1-ASC-def) cells (catalog number thp-dasc), NLRP3-deficient THP-1 (THP-1-NLRP3-def) cells (catalog number thp-dnlp), and positive-control THP-1 (THP-1-WT) cells (catalog number thp-null) were purchased from InvivoGen.
Virus infection, transfections, and treatments.
Differentiated human macrophages were infected with HPIV3 at a multiplicity of infection (MOI) of 1 to 2 in serum-free, antibiotic-free Opti-MEM medium (Life Technologies). Virus adsorption was performed for 1.5 h at 37°C. Following adsorption, cells were washed twice with phosphate-buffered saline (PBS) (pH 7.4), and infection was continued in the presence of serum or low-serum-containing complete medium for 6 h if not otherwise stated in the figure legends. HEK293 cells and THP-1 cells were transfected with plasmids expressing the HPIV3 C protein (Uniprot accession number P06165), the HPIV1 C protein (GenBank accession number NC_003461), human procaspase-1, pro-IL-1β, ASC, NLRP3, WT TLRs (WT-TLR2, WT-TLR4, and WT-TLR9), and DN TLRs (DN-TLR2, DN-TLR4, and DN-TLR9) and with the corresponding empty plasmids (with the corresponding tags only). Lipofectamine 2000 (Invitrogen) and Lipofectamine LTX (Invitrogen) were used to transfect HEK293 and THP-1 cells, respectively. Transfected cells were either treated with 100 ng/ml LPS (InvivoGen) for 1 h to 4 h followed by treatment with 15 μM nigericin (InvivoGen) for 30 min or infected with HPIV3. In several experiments, transfected cells were also treated with the proteasome inhibitor MG132 (10 to 50 μM) (InvivoGen).
Human TLR2 was silenced in THP-1 cells by transfecting cells with 40 to 100 pmol of siRNAs using Lipofectamine 2000 for 24 h. Control and human TLR2 siRNAs were obtained from Santa Cruz Biotechnology.
In some experiments, THP-1 cells were pretreated for 2 h with either a caspase-1 inhibitor (Ac-YVAD-CHO) (10 μM) (AnaSpec) or the ATP-sensitive potassium channel inhibitor glibenclamide (50 μM) (Sigma-Aldrich) prior to HPIV3 infection. In addition, THP-1 cells were pretreated for 30 min with 150 mM KCl (10 mM HEPES, 5 mM NaH2PO4, 150 mM KCl, 1 mM MgCl2, 1 mM CaCl2, 1% bovine serum albumin [BSA] [pH 7.4]) or 150 mM NaCl (10 mM HEPES, 5 mM KH2PO4, 150 mM NaCl, 1 mM MgCl2, 1 mM CaCl2, 1% BSA [pH 7.4]) prior to HPIV3 infection. After pretreatment, cells were infected with HPIV3 in the absence or presence of the corresponding inhibitors or treatments (i.e., KCl and NaCl treatments).
RNA isolation and RT-PCR.
Total RNA was isolated by using TRIzol reagent (Life Technologies) according to the manufacturer's instructions. Reverse transcription of the isolated RNA was performed by using MultiScribe reverse transcriptase (Applied Biosystems) to generate template cDNA. PCR was carried out in a 25-μl reaction mixture by using Apex 2× Taq Red master mix (Genesee Scientific). Amplification conditions for the PCR were as follows: an initial denaturation step (95°C for 3 to 5 min) was followed by 28 to 35 cycles of denaturation (94°C for 30 s), annealing (60°C for 30 s), and extension (72°C for 30 s), followed by either 5 or 10 min at 72°C for elongation. Amplified PCR products were analyzed on 1% agarose gels, and DNA bands were visualized by using the ChemiDoc XRS+ system (Bio-Rad). Amplification of the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a loading control. The primers used for the amplification of the indicated genes are as follows: forward primer 5′-GTCAGTGGTGGACCTGACCT and reverse primer 5′-AGGGGTCTACATGGCAACTG-3′ for human GAPDH, forward primer 5′-AAACAGATGAAGTGCTCCTTCCAG-3′ and reverse primer 5′-TGGAGAACACCACTTGTTGCTCCA-3′ for human pro-IL-1β, forward primer 5′-GGTCCTGAAGGAGAAGAGAA-3′ and reverse primer 5′-AGGCCTGGATGATGATCACC-3′ for human procaspase-1, forward primer 5′-TTCTTTCTGTTTGCTGAGTTTTTG-3′ and reverse primer 5′-TTCCTGGCATATCACAGTGG-3′ for human NLRP3, forward primer 5′-GGACGCCTTGGCCCTCACCG-3′ and reverse primer 5′-GGCGCGGCTCCAGAGCCCTG-3′ for human ASC, forward primer 5′-ATGCTAAAAACTATCAAATC-3′ and reverse primer 5′-TCATTGATCTTGTATCAGGTG-3′ for the HPIV3 C protein, forward primer 5′-GCCAAAGTCTTGATTGATTGG-3′ and reverse primer 5′-TTGAAGTTCTCCAGCTCCTG-3′ for human TLR2, forward primer 5′-GCTTCTTGCTGGCTGCATAA-3′ and reverse primer 5′-GAAATGGAGGCACCCCTTC-3′ for human TLR4, and forward primer 5′-GCATCTTCTTCCGCTCACTC-3′ and reverse primer 5′-AGCCACGAAGCTGAAGTTGAT-3′ for human TLR9.
Western blotting and immunoprecipitation.
THP-1 and HEK293 cell lysates were prepared by using 1% Triton X-100 in PBS (pH 7.4) and 1× Complete Mini EDTA-free protease inhibitor cocktail (Roche Diagnostics). Clarified cell lysates (centrifuged at 13,000 rpm for 10 min at 4°C) were subjected to 10 to 15% SDS-PAGE, and separated proteins were transferred onto an Amersham Protran 0.2-μm nitrocellulose blotting membrane. Western blotting was performed by probing the separated proteins with specific primary antibodies and horseradish peroxidase (HRP)-conjugated secondary antibodies.
To detect proteins (e.g., the p10 portion of procaspase-1 and the p17 portion of pro-IL-1β) in the medium supernatant (reduced-serum-supplemented medium), proteins from the medium supernatant were precipitated with trichloroacetic acid (TCA) and washed with acetone (33, 39) before Western blotting with either p10 or p17 antibodies. Human caspase-1 p10 antibody and human ubiquitin antibodies were obtained from Santa Cruz Biotechnology. Human IL-1β antibody (for the detection of p17) was obtained from the NCI Frederick Cancer Research and Development Center. Actin antibody was purchased from Bethyl Laboratories. Polyclonal HPIV3 RNP antibody (76) was used to detect the HPIV3 N protein. Anti-FLAG and anti-Myc antibodies were obtained from Clontech and the University of Iowa Developmental Studies Hybridoma Bank, respectively. NLRP3 antibody was purchased from Adipogene.
For IP assays, HEK293 and THP-1 cell lysates derived from FLAG-C-expressing cells were harvested as described above. For IP, supernatants obtained from clarified cell lysates were incubated with anti-FLAG agarose beads (Origene) for 12 h at 4°C. After incubation, the mixture of the lysate and beads was centrifuged at 800 rpm for 1 min and washed five times with wash buffer (1× PBS, 10 mM Tris HCl [pH 7.4], and 1× protease inhibitor). The protein complex was eluted from the beads by boiling the beads in the presence of 2× sample buffer. The sample was subjected to Western blot analysis (immunoblotting) with anti-FLAG, anti-Myc, or anti-NLRP3 antibodies. In another experiment, FLAG-C-expressing THP-1 cells were treated with either LPS alone or LPS and nigericin. Cell lysates collected from these cells were immunoprecipitated with antiubiquitin antibody and subsequently immunoblotted with anti-NLRP3 antibody.
In some experiments, specific protein bands from the Western blots were quantified by using the ChemiDoc XRS+ software Image Lab 5.1 (Bio-Rad).
ELISA.
Levels of IL-1β in the medium supernatant of virus-infected or LPS- and nigericin-treated cells were assayed by using a human IL-1β ELISA kit (eBioscience).
Immunofluorescence.
HEK293 cells were cultured in Biocoat 12-mm-round coverslips (Corning) and transfected as described above. The cells were cotransfected with Myc-NLRP3 and FLAG-C expression plasmids. At 16 h posttransfection, cells were washed twice with cold PBS, fixed with 4% formaldehyde in PBS for 10 min, permeabilized with 0.2% Triton X-100 for 10 min, blocked with 1% BSA for 1 h, probed with anti-Myc and anti-FLAG antibodies (12 h at 4°C), washed 3 times for 5 min each, probed with Alexa Fluor 594- and FITC-labeled secondary antibodies (Jackson Laboratories), washed 3 times, and finally mounted onto glass slides with Prolong Gold antifade reagent with DAPI (4′,6-diamidino-2-phenylindole) (Life Technologies). Glass slides were then visualized by using a Zeiss 510 Meta confocal laser scanning microscope.
In some experiments, the resultant microscopic pictures were analyzed on a region of interest (ROI) to calculate the fluorescence color intensity (pixels) of specific proteins by using ImageJ software (https://imagej.nih.gov/ij/). Specifically, in the same visual field, we quantified the Myc-NLRP3-specific fluorescence intensity in three different cell types: (i) cells expressing high levels of C protein, (ii) cells expressing low levels of C protein, and (iii) cells lacking C protein expression. Fifteen different fields were analyzed to derive the data.
Statistical approach.
All data were analyzed by using GraphPad Prism software (version 6.0), and a significance test was carried out by using Student's t test with a two-tailed P value and a 95% confidence interval. A P value of ≤0.05 was considered significant.
ACKNOWLEDGMENTS
This work was supported by NIH grant AI083387 (to S.B.).
We thank David J. Prieur (WSU) for critically reading the manuscript.
REFERENCES
- 1.Karron RA, Collins PL. 2013. Parainfluenza viruses, p 996–1023. In Knipe DM, Howley PM, Cohen JI, Griffin DE, Lamb RA, Martin MA, Racaniello VR, Roizman B (ed), Fields virology, 6th ed Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 2.Fox TG, Christenson JC. 2014. Influenza and parainfluenza viral infections in children. Pediatr Rev 35:217–227, quiz 228. doi: 10.1542/pir.35-6-217. [DOI] [PubMed] [Google Scholar]
- 3.Lamb RA, Parks GD. 2013. Paramyxoviridae, p 957–995. In Knipe DM, Howley PM, Cohen JI, Griffin DE, Lamb RA, Martin MA, Racaniello VR, Roizman B (ed), Fields virology, 6th ed Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 4.Bjornson CL, Johnson DW. 2008. Croup. Lancet 371:329–339. doi: 10.1016/S0140-6736(08)60170-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Glezen WP, Frank AL, Taber LH, Kasel JA. 1984. Parainfluenza virus type 3: seasonality and risk of infection and reinfection in young children. J Infect Dis 150:851–857. doi: 10.1093/infdis/150.6.851. [DOI] [PubMed] [Google Scholar]
- 6.Jalal H, Bibby DF, Bennett J, Sampson RE, Brink NS, MacKinnon S, Tedder RS, Ward KN. 2007. Molecular investigations of an outbreak of parainfluenza virus type 3 and respiratory syncytial virus infections in a hematology unit. J Clin Microbiol 45:1690–1696. doi: 10.1128/JCM.01912-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schmidt AC, Schaap-Nutt A, Bartlett EJ, Schomacker H, Boonyaratanakornkit J, Karron RA, Collins PL. 2011. Progress in the development of human parainfluenza virus vaccines. Expert Rev Respir Med 5:515–526. doi: 10.1586/ers.11.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weinberg GA, Hall CB, Iwane MK, Poehling KA, Edwards KM, Griffin MR, Staat MA, Curns AT, Erdman DD, Szilagyi PG, New Vaccine Surveillance Network. 2009. Parainfluenza virus infection of young children: estimates of the population-based burden of hospitalization. J Pediatr 154:694–699. doi: 10.1016/j.jpeds.2008.11.034. [DOI] [PubMed] [Google Scholar]
- 9.Henrickson KJ. 2003. Parainfluenza viruses. Clin Microbiol Rev 16:242–264. doi: 10.1128/CMR.16.2.242-264.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reed G, Jewett PH, Thompson J, Tollefson S, Wright PF. 1997. Epidemiology and clinical impact of parainfluenza virus infections in otherwise healthy infants and young children <5 years old. J Infect Dis 175:807–813. doi: 10.1086/513975. [DOI] [PubMed] [Google Scholar]
- 11.Moscona A. 2005. Entry of parainfluenza virus into cells as a target for interrupting childhood respiratory disease. J Clin Invest 115:1688–1698. doi: 10.1172/JCI25669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Franchi L, Eigenbrod T, Munoz-Planillo R, Nunez G. 2009. The inflammasome: a caspase-1-activation platform that regulates immune responses and disease pathogenesis. Nat Immunol 10:241–247. doi: 10.1038/ni.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lamkanfi M, Dixit VM. 2009. The inflammasomes. PLoS Pathog 5:e1000510. doi: 10.1371/journal.ppat.1000510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kanneganti TD. 2010. Central roles of NLRs and inflammasomes in viral infection. Nat Rev Immunol 10:688–698. doi: 10.1038/nri2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davis BK, Wen H, Ting JP. 2011. The inflammasome NLRs in immunity, inflammation, and associated diseases. Annu Rev Immunol 29:707–735. doi: 10.1146/annurev-immunol-031210-101405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rathinam VA, Vanaja SK, Fitzgerald KA. 2012. Regulation of inflammasome signaling. Nat Immunol 13:333–342. doi: 10.1038/ni.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chambers R, Takimoto T. 2009. Antagonism of innate immunity by paramyxovirus accessory proteins. Viruses 1:574–593. doi: 10.3390/v1030574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gotoh B, Komatsu T, Takeuchi K, Yokoo J. 2002. Paramyxovirus strategies for evading the interferon response. Rev Med Virol 12:337–357. doi: 10.1002/rmv.357. [DOI] [PubMed] [Google Scholar]
- 19.Bose S, Banerjee AK. 2003. Innate immune response against nonsegmented negative strand RNA viruses. J Interferon Cytokine Res 23:401–412. doi: 10.1089/107999003322277810. [DOI] [PubMed] [Google Scholar]
- 20.Davis ME, Wang MK, Rennick LJ, Full F, Gableske S, Mesman AW, Gringhuis SI, Geijtenbeek TBH, Duprex WP, Gack MU. 2014. Antagonism of the phosphatase PP1 by the measles virus V protein is required for innate immune escape of MDA5. Cell Host Microbe 16:19–30. doi: 10.1016/j.chom.2014.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ramachandran A, Horvath CM. 2010. Dissociation of paramyxovirus interferon evasion activities: universal and virus-specific requirements for conserved V protein amino acids in MDA5 interference. J Virol 84:11152–11163. doi: 10.1128/JVI.01375-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parisien JP, Bamming D, Komuro A, Ramachandran A, Rodriguez JJ, Barber G, Wojahn RD, Horvath CM. 2009. A shared interface mediates paramyxovirus interference with antiviral RNA helicases MDA5 and LGP2. J Virol 83:7252–7260. doi: 10.1128/JVI.00153-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ulane CM, Kentsis A, Cruz CD, Parisien JP, Schneider KL, Horvath CM. 2005. Composition and assembly of STAT-targeting ubiquitin ligase complexes: paramyxovirus V protein carboxyl terminus is an oligomerization domain. J Virol 79:10180–10189. doi: 10.1128/JVI.79.16.10180-10189.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Palosaari H, Parisien JP, Rodriguez JJ, Ulane CM, Horvath CM. 2003. STAT protein interference and suppression of cytokine signal transduction by measles virus V protein. J Virol 77:7635–7644. doi: 10.1128/JVI.77.13.7635-7644.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Poole E, He B, Lamb RA, Randall RE, Goodbourn S. 2002. The V proteins of simian virus 5 and other paramyxoviruses inhibit induction of interferon-beta. Virology 303:33–46. doi: 10.1006/viro.2002.1737. [DOI] [PubMed] [Google Scholar]
- 26.He B, Paterson RG, Stock N, Durbin JE, Durbin RK, Goodbourn S, Randall RE, Lamb RA. 2002. Recovery of paramyxovirus simian virus 5 with a V protein lacking the conserved cysteine-rich domain: the multifunctional V protein blocks both interferon-beta induction and interferon signaling. Virology 303:15–32. doi: 10.1006/viro.2002.1738. [DOI] [PubMed] [Google Scholar]
- 27.McAllister CS, Toth AM, Zhang P, Devaux P, Cattaneo R, Samuel CE. 2010. Mechanisms of protein kinase PKR-mediated amplification of beta interferon induction by C protein-deficient measles virus. J Virol 84:380–386. doi: 10.1128/JVI.02630-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Malur AG, Chattopadhyay S, Maitra RK, Banerjee AK. 2005. Inhibition of STAT 1 phosphorylation by human parainfluenza virus type 3 C protein. J Virol 79:7877–7882. doi: 10.1128/JVI.79.12.7877-7882.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gotoh B, Komatsu T, Takeuchi K, Yokoo J. 2001. Paramyxovirus accessory proteins as interferon antagonists. Microbiol Immunol 45:787–800. doi: 10.1111/j.1348-0421.2001.tb01315.x. [DOI] [PubMed] [Google Scholar]
- 30.Wells G, Addington-Hall M, Malur AG. 2012. Mutations within the human parainfluenza virus type 3 (HPIV 3) C protein affect viral replication and host interferon induction. Virus Res 167:385–390. doi: 10.1016/j.virusres.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 31.Malur AG, Hoffman MA, Banerjee AK. 2004. The human parainfluenza virus type 3 (HPIV 3) C protein inhibits viral transcription. Virus Res 99:199–204. doi: 10.1016/j.virusres.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 32.Ludlow LE, Lo MK, Rodriguez JJ, Rota PA, Horvath CM. 2008. Henipavirus V protein association with Polo-like kinase reveals functional overlap with STAT1 binding and interferon evasion. J Virol 82:6259–6271. doi: 10.1128/JVI.00409-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Segovia J, Sabbah A, Mgbemena V, Tsai SY, Chang TH, Berton MT, Morris IR, Allen IC, Ting JP, Bose S. 2012. TLR2/MyD88/NF-kappaB pathway, reactive oxygen species, potassium efflux activates NLRP3/ASC inflammasome during respiratory syncytial virus infection. PLoS One 7:e29695. doi: 10.1371/journal.pone.0029695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Allen IC, Scull MA, Moore CB, Holl EK, McElvania-TeKippe E, Taxman DJ, Guthrie EH, Pickles RJ, Ting JPY. 2009. The NLRP3 inflammasome mediates in vivo innate immunity to influenza A virus through recognition of viral RNA. Immunity 30:556–565. doi: 10.1016/j.immuni.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tsai SY, Segovia JA, Chang TH, Morris IR, Berton MT, Tessier PA, Tardif MR, Cesaro A, Bose S. 2014. DAMP molecule S100A9 acts as a molecular pattern to enhance inflammation during influenza A virus infection: role of DDX21-TRIF-TLR4-MyD88 pathway. PLoS Pathog 10:e1003848. doi: 10.1371/journal.ppat.1003848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sabbah A, Chang TH, Harnack R, Frohlich V, Tominaga K, Dube PH, Xiang Y, Bose S. 2009. Activation of innate immune antiviral responses by Nod2. Nat Immunol 10:1073–1080. doi: 10.1038/ni.1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mgbemena V, Segovia JA, Chang T-H, Tsai S-Y, Cole GT, Hung C-Y, Bose S. 2012. Transactivation of inducible nitric oxide synthase gene by Kruppel-like factor 6 regulates apoptosis during influenza A virus infection. J Immunol 189:606–615. doi: 10.4049/jimmunol.1102742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsai SY, Segovia JA, Chang TH, Shil NK, Pokharel SM, Kannan TR, Baseman JB, Defrene J, Page N, Cesaro A, Tessier PA, Bose S. 2015. Regulation of TLR3 activation by S100A9. J Immunol 195:4426–4437. doi: 10.4049/jimmunol.1500378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Segovia JA, Tsai SY, Chang TH, Shil NK, Weintraub ST, Short JD, Bose S. 2015. Nedd8 regulates inflammasome-dependent caspase-1 activation. Mol Cell Biol 35:582–597. doi: 10.1128/MCB.00775-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bose S, Segovia JA, Somarajan SR, Chang T-H, Kannan T, Baseman JB. 2014. ADP-ribosylation of NLRP3 by Mycoplasma pneumoniae CARDS toxin regulates inflammasome activity. mBio 5:e02186-14. doi: 10.1128/mBio.02186-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Netea MG, Nold-Petry CA, Nold MF, Joosten LA, Opitz B, van der Meer JH, van de Veerdonk FL, Ferwerda G, Heinhuis B, Devesa I, Funk CJ, Mason RJ, Kullberg BJ, Rubartelli A, van der Meer JW, Dinarello CA. 2009. Differential requirement for the activation of the inflammasome for processing and release of IL-1beta in monocytes and macrophages. Blood 113:2324–2335. doi: 10.1182/blood-2008-03-146720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.He Y, Hara H, Nunez G. 2016. Mechanism and regulation of NLRP3 inflammasome activation. Trends Biochem Sci 41:1012–1021. doi: 10.1016/j.tibs.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Anand PK, Malireddi RK, Kanneganti TD. 2011. Role of the nlrp3 inflammasome in microbial infection. Front Microbiol 2:12. doi: 10.3389/fmicb.2011.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Toma C, Higa N, Koizumi Y, Nakasone N, Ogura Y, McCoy AJ, Franchi L, Uematsu S, Sagara J, Taniguchi Si. 2010. Pathogenic Vibrio activate NLRP3 inflammasome via cytotoxins and TLR/nucleotide-binding oligomerization domain-mediated NF-κB signaling. J Immunol 184:5287–5297. doi: 10.4049/jimmunol.0903536. [DOI] [PubMed] [Google Scholar]
- 45.Lamkanfi M, Malireddi RK, Kanneganti TD. 2009. Fungal zymosan and mannan activate the cryopyrin inflammasome. J Biol Chem 284:20574–20581. doi: 10.1074/jbc.M109.023689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Babelova A, Moreth K, Tsalastra-Greul W, Zeng-Brouwers J, Eickelberg O, Young MF, Bruckner P, Pfeilschifter J, Schaefer RM, Grone HJ, Schaefer L. 2009. Biglycan, a danger signal that activates the NLRP3 inflammasome via Toll-like and P2X receptors. J Biol Chem 284:24035–24048. doi: 10.1074/jbc.M109.014266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Delaloye J, Roger T, Steiner-Tardivel QG, Le Roy D, Reymond MK, Akira S, Petrilli V, Gomez CE, Perdiguero B, Tschopp J, Pantaleo G, Esteban M, Calandra T. 2009. Innate immune sensing of modified vaccinia virus Ankara (MVA) is mediated by TLR2-TLR6, MDA-5 and the NALP3 inflammasome. PLoS Pathog 5:e1000480. doi: 10.1371/journal.ppat.1000480. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 48.van de Veerdonk FL, Joosten LA, Devesa I, Mora-Montes HM, Kanneganti T-D, Dinarello CA, van der Meer JW, Gow NA, Kullberg BJ, Netea MG. 2009. Bypassing pathogen-induced inflammasome activation for the regulation of interleukin-1β production by the fungal pathogen Candida albicans. J Infect Dis 199:1087–1096. doi: 10.1086/597274. [DOI] [PubMed] [Google Scholar]
- 49.Bose S, Kar N, Maitra R, DiDonato JA, Banerjee AK. 2003. Temporal activation of NF-κB regulates an interferon-independent innate antiviral response against cytoplasmic RNA viruses. Proc Natl Acad Sci U S A 100:10890–10895. doi: 10.1073/pnas.1832775100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Smith MF, Mitchell A, Li GL, Ding S, Fitzmaurice AM, Ryan K, Crowe S, Goldberg JB. 2003. Toll-like receptor (TLR) 2 and TLR5, but not TLR4, are required for Helicobacter pylori-induced NF-kappa B activation and chemokine expression by epithelial cells. J Biol Chem 278:32552–32560. doi: 10.1074/jbc.M305536200. [DOI] [PubMed] [Google Scholar]
- 51.Nour AM, Reichelt M, Ku CC, Ho MY, Heineman TC, Arvin AM. 2011. Varicella-zoster virus infection triggers formation of an interleukin-1beta (IL-1beta)-processing inflammasome complex. J Biol Chem 286:17921–17933. doi: 10.1074/jbc.M110.210575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang Y, Gao B, Xiong S. 2014. Involvement of NLRP3 inflammasome in CVB3-induced viral myocarditis. Am J Physiol Heart Circ Physiol 307:H1438–H1447. doi: 10.1152/ajpheart.00441.2014. [DOI] [PubMed] [Google Scholar]
- 53.Masters SL, Dunne A, Subramanian SL, Hull RL, Tannahill GM, Sharp FA, Becker C, Franchi L, Yoshihara E, Chen Z, Mullooly N, Mielke LA, Harris J, Coll RC, Mills KHG, Mok KH, Newsholme P, Nunez G, Yodoi J, Kahn SE, Lavelle EC, O'Neill LAJ. 2010. Activation of the NLRP3 inflammasome by islet amyloid polypeptide provides a mechanism for enhanced IL-1 beta in type 2 diabetes. Nat Immunol 11:897–904. doi: 10.1038/ni.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Abdul-Sater AA, Koo E, Hacker G, Ojcius DM. 2009. Inflammasome-dependent caspase-1 activation in cervical epithelial cells stimulates growth of the intracellular pathogen Chlamydia trachomatis. J Biol Chem 284:26789–26796. doi: 10.1074/jbc.M109.026823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Qu Y, Franchi L, Nunez G, Dubyak GR. 2007. Nonclassical IL-1β secretion stimulated by P2X7 receptors is dependent on inflammasome activation and correlated with exosome release in murine macrophages. J Immunol 179:1913–1925. doi: 10.4049/jimmunol.179.3.1913. [DOI] [PubMed] [Google Scholar]
- 56.Cassel SL, Eisenbarth SC, Iyer SS, Sadler JJ, Colegio OR, Tephly LA, Carter AB, Rothman PB, Flavell RA, Sutterwala FS. 2008. The Nalp3 inflammasome is essential for the development of silicosis. Proc Natl Acad Sci U S A 105:9035–9040. doi: 10.1073/pnas.0803933105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Garcin D, Marq JB, Strahle L, le Mercier P, Kolakofsky D. 2002. All four Sendai virus C proteins bind Stat1, but only the larger forms also induce its mono-ubiquitination and degradation. Virology 295:256–265. doi: 10.1006/viro.2001.1342. [DOI] [PubMed] [Google Scholar]
- 58.Sabbah A, Bose S. 2009. Retinoic acid inducible gene I activates innate antiviral response against human parainfluenza virus type 3. Virol J 6:200. doi: 10.1186/1743-422X-6-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ding B, Zhang G, Yang X, Zhang S, Chen L, Yan Q, Xu M, Banerjee AK, Chen M. 2014. Phosphoprotein of human parainfluenza virus type 3 blocks autophagosome-lysosome fusion to increase virus production. Cell Host Microbe 15:564–577. doi: 10.1016/j.chom.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 60.Plotnicky-Gilquin H, Cyblat D, Aubry JP, Delneste Y, Blaecke A, Bonnefoy JY, Corvaia N, Jeannin P. 2001. Differential effects of parainfluenza virus type 3 on human monocytes and dendritic cells. Virology 285:82–90. doi: 10.1006/viro.2001.0933. [DOI] [PubMed] [Google Scholar]
- 61.Le Nouen C, Hillyer P, Munir S, Winter CC, McCarty T, Bukreyev A, Collins PL, Rabin RL, Buchholz UJ. 2010. Effects of human respiratory syncytial virus, metapneumovirus, parainfluenza virus 3 and influenza virus on CD4+ T cell activation by dendritic cells. PLoS One 5:e15017. doi: 10.1371/journal.pone.0015017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lewandowska-Polak A, Brauncajs M, Paradowska E, Jarzêbska M, Kurowski M, Moskwa S, Leœnikowski ZJ, Kowalski ML. 2015. Human parainfluenza virus type 3 (HPIV3) induces production of IFNγ and RANTES in human nasal epithelial cells (HNECs). J Inflammation 12:16. doi: 10.1186/s12950-015-0054-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Huang H, Saravia J, You D, Shaw AJ, Cormier SA. 2015. Impaired gamma delta T cell-derived IL-17A and inflammasome activation during early respiratory syncytial virus infection in infants. Immunol Cell Biol 93:126–135. doi: 10.1038/icb.2014.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rockx B, Brining D, Kramer J, Callison J, Ebihara H, Mansfield K, Feldmann H. 2011. Clinical outcome of henipavirus infection in hamsters is determined by the route and dose of infection. J Virol 85:7658–7671. doi: 10.1128/JVI.00473-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mathieu C, Guillaume V, Volchkova VA, Pohl C, Jacquot F, Looi RY, Wong KT, Legras-Lachuer C, Volchkov VE, Lachuer J, Horvat B. 2012. Nonstructural Nipah virus C protein regulates both the early host proinflammatory response and viral virulence. J Virol 86:10766–10775. doi: 10.1128/JVI.01203-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kanneganti TD, Body-Malapel M, Amer A, Park JH, Whitfield J, Franchi L, Taraporewala ZF, Miller D, Patton JT, Inohara N, Nunez G. 2006. Critical role for Cryopyrin/Nalp3 in activation of caspase-1 in response to viral infection and double-stranded RNA. J Biol Chem 281:36560–36568. doi: 10.1074/jbc.M607594200. [DOI] [PubMed] [Google Scholar]
- 67.Park S, Juliana C, Hong S, Datta P, Hwang I, Fernandes-Alnemri T, Yu JW, Alnemri ES. 2013. The mitochondrial antiviral protein MAVS associates with NLRP3 and regulates its inflammasome activity. J Immunol 191:4358–4366. doi: 10.4049/jimmunol.1301170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang B, Zhu J, Li D, Wang Y, Zhan Y, Tan L, Qiu X, Sun Y, Song C, Meng C, Ying L, Xiang M, Meng G, Ding C. 2016. Newcastle disease virus infection induces activation of the NLRP3 inflammasome. Virology 496:90–96. doi: 10.1016/j.virol.2016.05.023. [DOI] [PubMed] [Google Scholar]
- 69.Komune N, Ichinohe T, Ito M, Yanagi Y. 2011. Measles virus V protein inhibits NLRP3 inflammasome-mediated interleukin-1beta secretion. J Virol 85:13019–13026. doi: 10.1128/JVI.05942-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pfaller CK, Conzelmann K-K. 2008. Measles virus V protein is a decoy substrate for IκB kinase α and prevents Toll-like receptor 7/9-mediated interferon induction. J Virol 82:12365–12373. doi: 10.1128/JVI.01321-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kitagawa Y, Yamaguchi M, Zhou M, Komatsu T, Nishio M, Sugiyama T, Takeuchi K, Itoh M, Gotoh B. 2011. A tryptophan-rich motif in the human parainfluenza virus type 2 V protein is critical for the blockade of Toll-like receptor 7 (TLR7)- and TLR9-dependent signaling. J Virol 85:4606–4611. doi: 10.1128/JVI.02012-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lin Y, Sun M, Fuentes SM, Keim CD, Rothermel T, He B. 2007. Inhibition of interleukin-6 expression by the V protein of parainfluenza virus 5. Virology 368:262–272. doi: 10.1016/j.virol.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Toth AM, Devaux P, Cattaneo R, Samuel CE. 2009. Protein kinase PKR mediates the apoptosis induction and growth restriction phenotypes of C protein-deficient measles virus. J Virol 83:961–968. doi: 10.1128/JVI.01669-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yamaguchi M, Kitagawa Y, Zhou M, Itoh M, Gotoh B. 2014. An anti-interferon activity shared by paramyxovirus C proteins: inhibition of Toll-like receptor 7/9-dependent alpha interferon induction. FEBS Lett 588:28–34. doi: 10.1016/j.febslet.2013.11.015. [DOI] [PubMed] [Google Scholar]
- 75.Sleeman K, Bankamp B, Hummel KB, Lo MK, Bellini WJ, Rota PA. 2008. The C, V and W proteins of Nipah virus inhibit minigenome replication. J Gen Virol 89:1300–1308. doi: 10.1099/vir.0.83582-0. [DOI] [PubMed] [Google Scholar]
- 76.Bose S, Basu M, Banerjee AK. 2004. Role of nucleolin in human parainfluenza virus type 3 infection of human lung epithelial cells. J Virol 78:8146–8158. doi: 10.1128/JVI.78.15.8146-8158.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]