ABSTRACT
The amino terminus of the human papillomavirus (HPV) minor capsid protein L2 contains a major cross-neutralization epitope which provides the basis for the development of a broadly protecting HPV vaccine. A wide range of protection against different HPV types would eliminate one of the major drawbacks of the commercial, L1-based prophylactic vaccines. Previously, we have reported that insertion of the L2 epitope into a scaffold composed of bacterial thioredoxin protein generates a potent antigen inducing comprehensive protection against different animal and human papillomaviruses. We also reported, however, that although protection is broad, some oncogenic HPV types escape the neutralizing antibody response, if L2 epitopes from single HPV types are used as immunogen. We were able to compensate for this by applying a mix of thioredoxin proteins carrying L2 epitopes from HPV16, -31, and -51. As the development of a cost-efficient HPV prophylactic vaccines is one of our objectives, this approach is not feasible as it requires the development of multiple good manufacturing production processes in combination with a complex vaccine formulation. Here, we report the development of a thermostable thioredoxin-based single-peptide vaccine carrying an L2 polytope of up to 11 different HPV types. The L2 polytope antigens have excellent abilities in respect to broadness of protection and robustness of induced immune responses. To further increase immunogenicity, we fused the thioredoxin L2 polytope antigen with a heptamerization domain. In the final vaccine design, we achieve protective responses against all 14 oncogenic HPV types that we have analyzed plus the low-risk HPVs 6 and 11 and a number of cutaneous HPVs.
IMPORTANCE Infections by a large number of human papillomaviruses lead to malignant and nonmalignant disease. Current commercial vaccines based on virus-like particles (VLPs) effectively protect against some HPV types but fail to do so for most others. Further, only about a third of all countries have access to the VLP vaccines. The minor capsid protein L2 has been shown to contain so-called neutralization epitopes within its N terminus. We designed polytopes comprising the L2 epitope amino acids 20 to 38 of up to 11 different mucosal HPV types and inserted them into the scaffold of thioredoxin derived from a thermophile archaebacterium. The antigen induced neutralizing antibody responses in mice and guinea pigs against 26 mucosal and cutaneous HPV types. Further, addition of a heptamerization domain significantly increased the immunogenicity. The final vaccine design comprising a heptamerized L2 8-mer thioredoxin single-peptide antigen with excellent thermal stability might overcome some of the limitations of the current VLP vaccines.
KEYWORDS: HPV, vaccine, virus-like particle, thioredoxin, L2, minor capsid protein, thermostability, neutralization, epitope, thermostable
INTRODUCTION
At least 15 human papillomavirus (HPV) types are associated with cancer of not only the anogenital but also the oral epithelium (1–3). Clearly, infection with these types is common, and the malignant process requires additional contributing factors (4–6). Infection with oncogenic HPV occurs with the onset of sexual activity, and the majority (i.e., >60 to 80%) of sexually active women become exposed during their lifetime, primarily in their 20s and 30s (7, 8). While most of the women are able to eliminate the infection, about 10% develop a persistent infection accompanied by low- and high-grade intraepithelial lesions (9, 10). This type of infection and the lesions are considered the main risk factor for development of cervical cancer, and detection and treatment of lesions have been the main factor in reducing the incidence of cancer (2). The process of cancer development usually takes 10 to 30 years from initial infection, but lesions occur much earlier. It is widely accepted that for some of the cancers, in particular cervical carcinomas, infection with one of the so-called high-risk HPV types is an almost absolute requirement for the malignant transformation process. During the last 3 decades, a vast amount of epidemiological and molecular evidence has accumulated to support a causal role of HPV in the development of cervical cancer (11). This research drove the development of initially two prophylactic vaccines in the early 1990s. The vaccines have been licensed and introduced to the market in 2006 and 2007, respectively, and ever since, millions of doses have been administered to young female adolescents or adults. Countries with vaccination programs against HPV such as Australia and the United Kingdom achieved high vaccine coverage and meanwhile reported reduction in low-grade lesions and genital warts (12). Recently, an expanded-spectrum vaccine, comprised of a mixture of virus-like particles (VLPs) from 7 oncogenic HPVs plus VLPs from HPV6 and -11 has been licensed to a number of countries (13). All three commercial VLP vaccines are considered a great success for the prevention of HPV-associated malignancies, as they are safe and efficacious. However, despite these facts, the VLP-based vaccines come with a number of limitations. First, the neutralization epitopes are composed of L1 loops on the surface of VLPs that are poorly conserved among different HPV types, and consequently, the protection is highly HPV type restricted with very limited cross-protection. Therefore, the vaccine provides protection against only some of the oncogenic HPV types. Second, VLP vaccines require uninterrupted cooling chains for their distribution because they lack stability at elevated temperatures. Third, all three VLP vaccines comprise a rather sophisticated formulation, and production is complex. Both manufacturers established production and purification protocols, but the complexity of VLP vaccines might prevent the rise of cost-effective biosimilars.
Today, there are >200 different PVs known to infect human mucosa and skin (14). Most of the infections occur unnoticed; however, in immunocompromised individuals HPV-induced skin lesions are very frequent, and they pose a significant burden in, e.g., organ transplant patients. Some of the cutaneous HPV types have been associated with nonmelanoma skin cancer, but despite molecular evidence, a causal role in the transformation process has not been established yet (15–17). This is also because, unlike genital cancer, the HPV-positive tumors show a very low viral load with less than one viral genome copy per cell.
The minor capsid protein L2 was shown to harbor a number of neutralization epitopes, which are, in contrast to the known L1 epitope, of linear nature, meaning that they can be presented outside the VLP context on various heterologous scaffolds (18–20). In particular, the outermost N-terminal region comprising L2 amino acids (aa) 17 to 38 harbors the major cross-neutralization epitope. Antibodies against this epitope of HPV16 have been shown to neutralize a number of different human and bovine PVs. Interestingly, the neutralization epitopes of L2 are buried in mature virions but become exposed and thereby accessible to antibodies during the process of virus entry (21). This is a comparatively slow process in vitro and in vivo in which the virions become susceptible to L2-directed immunity. Still, no natural neutralizing anti-L2 humoral responses have been demonstrated, and immunization with L1 plus L2 VLPs does not induce such responses either. To induce L2-specific neutralizing antibodies, the epitopes have, therefore, been inserted into various scaffolds for presentation. In the past several years, we have developed bacterial thioredoxin (Trx) protein as a carrier for HPV L2 neutralization epitopes (19, 22–26). Initially, our antigens were based on Escherichia coli Trx, but we recently reported that Trx derived from the thermophile archaebacterium Pyrococcus furiosus (PfTrx) is superior in many respects (26). PfTrx is extremely heat stable, which is a major advantage not only during the purification process but also for vaccine distribution strategies. In our studies, however, we also observed that protection against different HPV types when using L2 epitopes from a single HPV, e.g., HPV16, is incomplete. By using mixtures of Trx antigens each carrying an L2 epitope from a different type, we could largely bridge the gaps in protection, but we realized that only a single-peptide vaccine would be a competitive vaccine candidate in aiming to generate cost-effective production and distribution (24). The commercial HPV vaccines are still a product for medium- or high-income societies and are not introduced in the majority of countries (27). Here, we show development of a thermostable single-antigen HPV L2-based vaccine which offers simple means of production and high stability. More importantly, the vaccine induces neutralizing responses against all 14 oncogenic HPV types tested. Note that currently we are not able to test against HPV82 for technical reasons. Protection is also achieved against low-risk mucosal and some cutaneous HPV types. We also demonstrate that the use of the OVX313 heptamerization domain (positively charged version of IMX313, developed by Osivax [28]) derived from a complement binding factor boosts both antibody and T-helper responses.
RESULTS
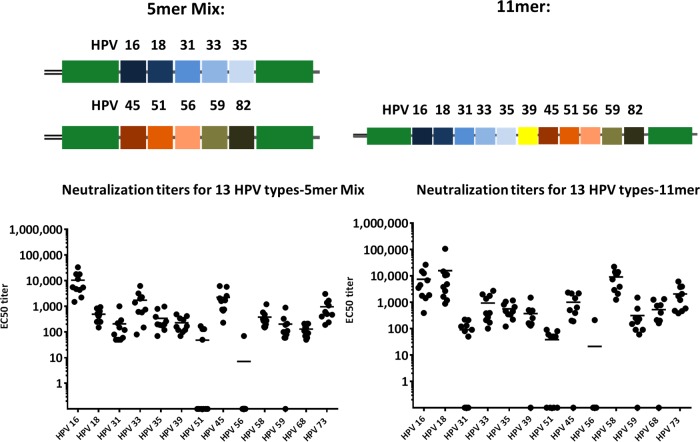
Different HPV L2 epitopes can be presented as a polytope inserted into PfTrx.
Previously, we have shown that the L2 neutralization epitope comprising aa 20 to 38 derived from a single HPV, such as HPV16, -31, or -51, induces neutralizing antibody responses against a variety of different HPVs or even bovine papillomavirus 1 (BPV1). However, immunization with epitopes of single HPVs leaves gaps in the protection breadth which can be overcome by using mixtures of different antigens. This approach, however, somewhat contradicts the attempt to develop a broadly protective HPV vaccine using a simple vaccine design and thereby creates additional hurdles for clinical development. To reduce the complexity of thioredoxin-L2 vaccine formulation, we aimed to combine L2 epitopes from different HPV types on two or fewer antigens. In earlier studies, we have shown that thioredoxin accepts a large insert and still folds into a soluble active antigen, although this very much depends on the composition of the insert. In a first approach, we designed three Trx-L2 antigens: two 5-mers and one 11-mer, comprising 10 and 11 different L2 epitopes, respectively. We immunized 10 mice with a mixture of the two 5-mers and the 11-mer formulated in Montanide adjuvants, respectively, and determined the neutralizing responses against 13 different oncogenic HPV types. Note that the same total dose of antigen was used for the two experimental groups, leading to a higher L2 epitope dose for the 11-mer. Results show that all 13 HPV types tested can be neutralized, albeit with different efficiencies (Fig. 1). In particular, not all mice developed neutralizing antibodies against HPV51 (7 of 10 negative in the 5-mer mix group and 4 of 10 negative in the 11-mer group) or HPV56 (9 of 10 mice negative for both antigens). Overall, there were no large differences between the responses against the two 5-mer epitopes or the 11-mer epitope (HPV16 and HPV33, P = 0.3; HPV45 and HPV73, P = 0.1), but the 11-mer induced about 10-fold-higher titers against HPV18 (P < 0.0001). Notably, both polytope formulations outperformed the previously reported trimeric mix of Trx-16L2 plus Trx31L2 plus Trx51L2 (data not shown).
FIG 1.
A mix of two 5-mers or a single 11-mer Trx-L2 polytope antigen induces antibodies against a wide range of oncogenic HPV types.
OVX313 heptamerization domain increases Trx-L2 immunogenicity.
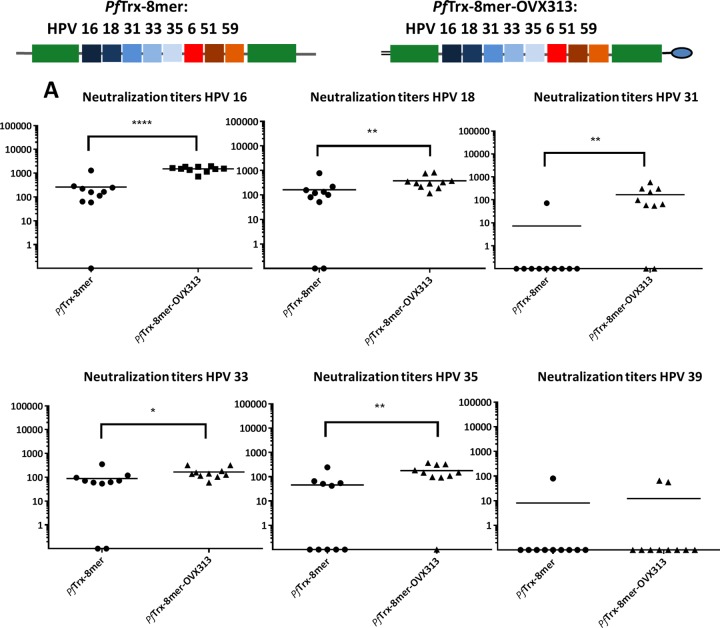
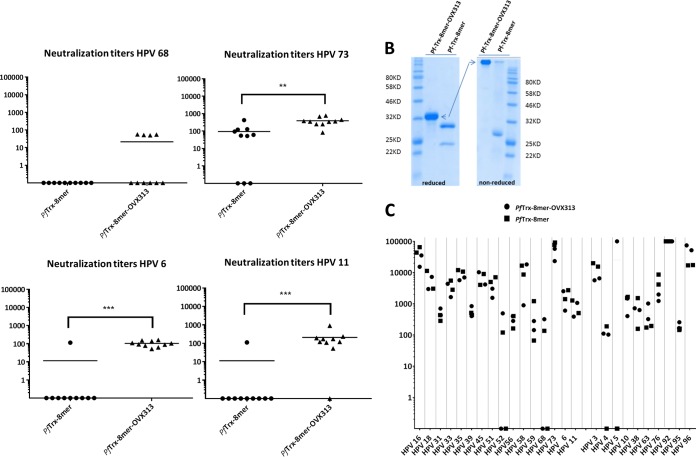
The exceptionally high immunogenicity of virus-like particle vaccines has been attributed to the repetitive epitope display, which presumably favors a hyperactivation of the B-cell receptors. We asked whether structured multimerization of the Trx-L2 antigen would lead to an increase in humoral immunogenicity. To answer this, we started with a simple Trx-L2 antigen and then fused the PfTrx HPV16 L2(20–38)3 antigen with the heptamerization domain OVX313. This domain is a derivative of the C4 complement binding protein (C4bp) but has been sequence modified to eliminate similarity to human C4bp (28). OVX313 has been used previously to boost the immunogenicity of therapeutic and prophylactic vaccine antigens (29, 30). Functional multimerization provided by the OVX313 domain was confirmed by analyzing the proteins by SDS-PAGE using reducing and nonreducing conditions (example shown in Fig. 4B). Only the OVX313-containing antigens were able to form high-molecular-weight assemblies.
FIG 4.
L1-PBNA of mouse and guinea pig sera immunized with PfTrx–8-mer–OVX313 and PfTrx–8-mer antigens. (A) Sera collected from mice (10 per group) 1 month after the last immunization with different antigens (PfTrx–8-mer–OVX313 and PfTrx–8-mer) and analyzed against 16 mucosal and 2 cutaneous HPV type pseudovirions using the L1-PBNA Each symbol represents a single mouse, and geometric means of the titers for each group are indicated by horizontal lines. The y axis displays EC50 titers. A P value of ≤0.05 was considered significant. *, P value < 0.05; **, P value < 0.01; ***, P value < 0.001; ****, P value < 0.0001. (B) The OVX heptamerization domain leads to stable multimerization of the PfTrx–8-mer antigen. Proteins were analyzed by SDS-PAGE under reducing and nonreducing conditions. (C) Neutralization titer of sera collected from guinea pigs immunized with either the PfTrx–8-mer–OVX313 or the PfTrx–8-mer antigen. Note that for HPV92 and -96 titers of >100,000 were observed.
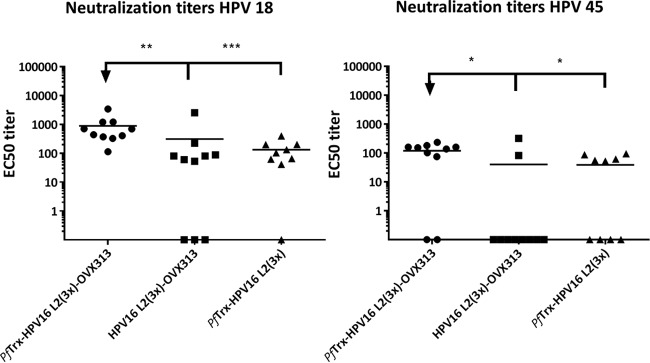
To measure the neutralizing antibody responses induced by PfTrx HPV16 L2(20–38)3-OVX313, the protein was formulated with alum-monophosphoryl lipid A (MPLA) and injected intramuscularly. To determine the role of the thioredoxin scaffold, we also tested HPV16 L2(20–38)3-OVX313, in which the HPV16 epitope trimer was directly fused to the heptamerization domain. Both antigens assemble into higher-molecular-weight forms which are stabilized by intramolecular disulfide bonds bridging cysteines within the OVX313 domain (data not shown). The cross-neutralizing antibody responses against HPV18 and -45 were measured in the standard L1 pseudovirion (PsV)-based neutralization assay (PBNA) (Fig. 2). The data demonstrate that the presence of OVX313 in the vaccine construct confers significantly stronger immunogenicity on the Trx-L2 peptide compared to the corresponding controls. Moreover, the PfTrx scaffold is essential in obtaining the higher immunogenicity (Fig. 2).
FIG 2.
Inclusion of the OVX313 heptamerization domain in the vaccine formulation increases immunogenicity of Trx-L2 antigens. HPV18 and HPV45 L1-PBNA with sera collected 1 month after the last immunization of mice with PfTrx HPV16 L2(20–38)3 (-OVX313) and HPV16 L2(20–38)3 (-OVX313) antigens formulated with alum-MPLA. PfTrx HPV16 L2(20–38)3 was used as a control. Symbols represent individual neutralization titers of immune sera; geometric means of the titers for each group are indicated by horizontal lines. A P value of ≤0.05 was considered significant. *, P value < 0.05; **, P value < 0.01; ***, P value < 0.001.
Combination of the OVX313 heptamerization domain with L2 polytopes generates a potent and broadly protective vaccine antigen.
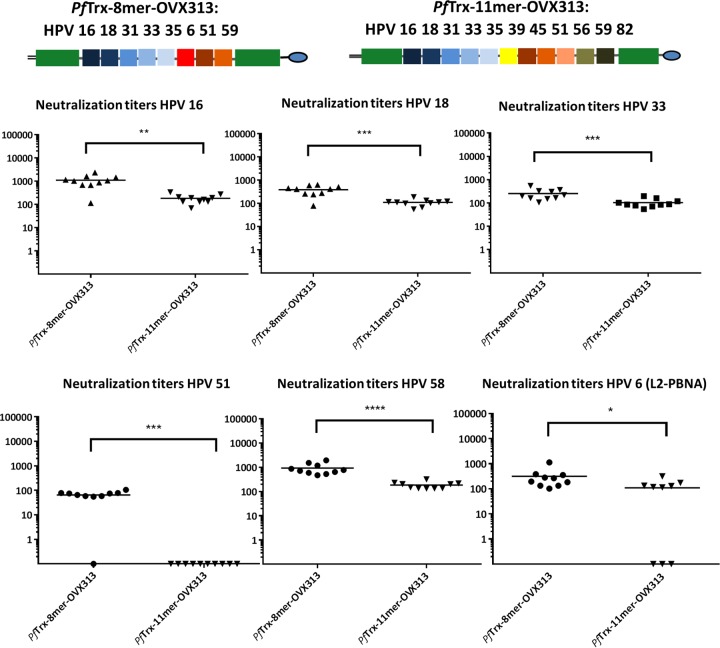
The next step was the combination of the polytope design and the heptamerization domain. The Trx-L2 11-mer induces a broad response against an array of different oncogenic HPV types (Fig. 1) but is not designed to target the two low-risk types HPV6 and -11. Based on sequence analysis and the performance of the two 5-mer antigens when used separately, we designed an additional vaccine antigen comprising 7 epitopes of oncogenic HPV plus the L2 epitope of HPV6 (PfTrx-L2 8-mer).
Consequently, the PfTrx–11-mer–OVX313 and PfTrx–8-mer–OVX313 antigens were compared in mouse immunization experiments. Groups of 10 mice were immunized intramuscularly with PfTrx–11-mer–OVX313 and PfTrx–8-mer–OVX313 adjuvanted with AddaVax. PfTrx HPV16 L2(20–38)3-OVX313 antigen was included as a control. Sera collected 1 month after the last immunization were analyzed in L1-PBNA and L2-PBNA (HPV6 only) to evaluate neutralizing antibody titers against six HPV types (Fig. 3). The results show that PfTrx–8-mer–OVX313 outperforms PfTrx–11-mer–OVX313 in terms of resulting in significantly higher antibody responses against all HPV types tested in L1-PBNA. Specifically, the 11-mer antigen failed to induce HPV51 neutralizing antibodies whereas all mice immunized with the 8-mer were positive in the L1-PBNA. Interestingly, both antigens contain the HPV51 L2 epitope. The PfTrx–8-mer–OVX313 antigen also conferred a consistent antibody response against HPV6 evaluated by L2-PBNA (Fig. 3), while only partial protection was seen for the PfTrx–11-mer–OVX313 antigen.
FIG 3.
PfTrx–8-mer–OVX313 vaccine induces higher neutralizing antibody responses against 5 different high-risk and one low-risk HPV type (HPV6) than the 11-mer antigen. Sera were collected from mice (10 per group) 1 month after the last immunization with different antigens (PfTrx–8-mer–OVX313 and PfTrx–11-mer–OVX313) and analyzed for their ability to neutralize HPV16, -18, -33, -51, and -58 pseudovirions using L1-PBNA (in the case of HPV6, the L2-PBNA was performed). Each symbol represents a single mouse, and geometric means of the titers for each group are indicated by horizontal lines. A P value of ≤0.05 was considered significant. The y axis displays EC50 titers. *, P value < 0.05; **, P value < 0.01; ***, P value < 0.001; ****, P value < 0.0001.
The PfTrx–8-mer–OVX313 antigen induces broadly neutralizing antibody responses against a large panel of high- and low-risk HPV types in mice and guinea pigs.
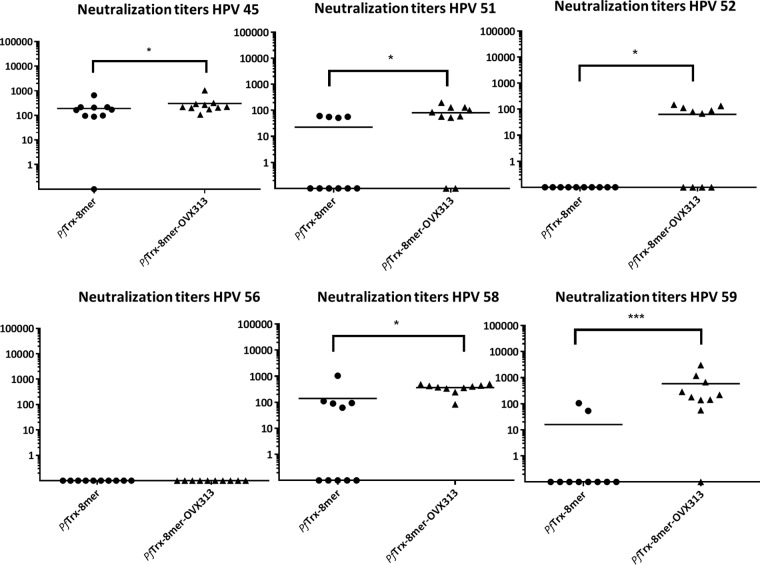
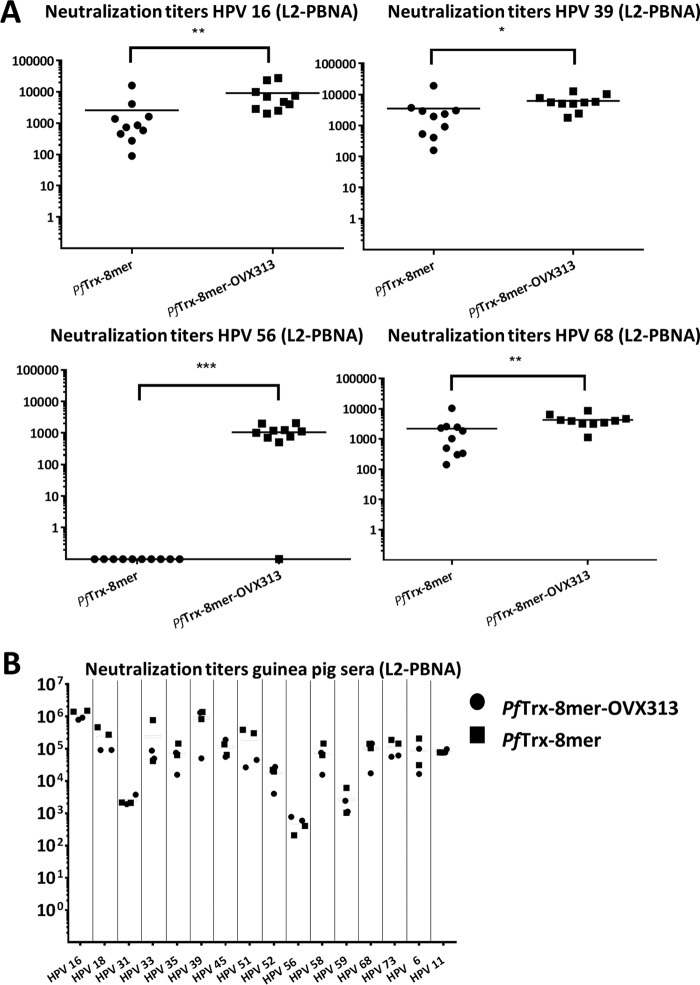
After identifying the 8-mer polytope as a suitable target structure and an easy-to-produce vaccine antigen, we wanted to determine in more detail (i) the broadness of protection and (ii) the influence of the OVX313 heptamerization domain on the immunogenicity. To allow a side-by-side comparison, groups of mice and guinea pigs were immunized as before, using the PfTrx–8-mer polytope antigen with and without the OVX313 domain. For 15 out of the 16 pseudovirions tested (14 human recombinant HPVs [hrHPVs] plus HPV6 and -11), the OVX313-containing 8-mer polytope antigen outperformed the monomeric antigen (Fig. 4A). In most cases, a robust neutralizing response was observed using the low-sensitivity L1-PBNA. In our hands, achieving neutralization of some HPV types such as HPV31 and -51 cannot be reached by using L2 epitopes from single, heterologous HPV types (24). The data indicate that these types can be effectively neutralized by the heptameric 8-mer polytope antigen, which contains the epitopes of both types. Using the low-sensitivity L1-PBNA, in the case of HPV56, however, neither of the two antigens induced measurable responses, and a weak response was observed for HPV39. For guinea pigs, which generally showed stronger responses against Trx-L2 than did BALB/c mice, neutralization was observed for all 16 HPV types tested, although two of the four animals did not respond to HPV68 (Fig. 4B). Particularly high titers (2 × 104 to 1 × 105) were obtained against HPV16 and -73 in the guinea pigs even when using the low-sensitivity L1-PBNA. Furthermore, the 8-mer polytope, although designed against mucosal HPV, induces neutralizing antibodies against all 10 cutaneous HPVs tested. In particular, HPV92 and -96 were neutralized very efficiently. The 8-mer, however, induces only an incomplete response for HPV5.
We then tested selected pseudovirion types in the sensitive L2-PBNA (Fig. 5A [mouse sera] and B [guinea pigs]). As expected, significantly higher titers were determined by this assay. Here, nine out of the 10 mice immunized with the PfTrx–8-mer–OVX313 antigen showed robust neutralizing titers against HPV56, while all mice immunized with the monomeric PfTrx–8-mer antigen were still negative. Also, a clear neutralizing response was observed in mice for HPV39 and -68. The boosting effect of the OVX313 domain was also visible for the other three HPV types tested. Analyzing the guinea pig sera by the L2-PBNA revealed very high 50% effective concentration (EC50) titers for most of the HPV types of up to 106.
FIG 5.
L2-PBNA of mouse and guinea pig sera immunized with PfTrx–8-mer and PfTrx–8-mer–OVX313 antigens. (A) Sera collected from mice (10 per group) 1 month after the last immunization with different antigens (PfTrx–8-mer and PfTrx–8-mer–OVX313) and analyzed against 4 HPV pseudovirions using L2-PBNA. Each symbol represents a single mouse, and geometric means of the titers for each group are indicated by horizontal lines. A P value of ≤0.05 was considered significant. The y axis displays EC50 titers. *, P value < 0.05; **, P value < 0.01; ***, P value < 0.001. (B) Neutralization titer of sera collected from guinea pigs immunized with either the PfTrx–8-mer–OVX313 or the PfTrx–8-mer antigen.
PfTrx–8-mer–OVX313 immunogenicity is adjuvant dependent.
To test different human-compatible adjuvants in combination with the candidate vaccine, the PfTrx–8-mer–OVX313 antigen was adjuvanted with alum-monophosphoryl lipid A (MPLA), glycopyranosyl lipid A (GLA-SE and GLA-AF), and Army liposome formulation-alum (ALFA) (31–33) and administered to BALB/c mice. Sera were analyzed by the L1-PBNA to evaluate neutralizing antibody titers against HPV16 PsV. As shown in Fig. 6, the neutralizing antibody responses elicited by the OVX313 antigen are adjuvant dependent; using the antigen alone results in significantly compromised immune responses induced by PfTrx–8-mer–OVX313.
FIG 6.
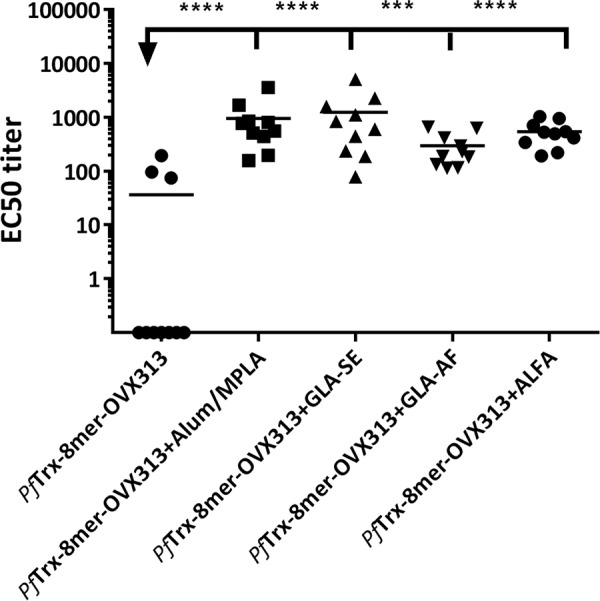
Robust immune responses induced by the PfTrx–8-mer–OVX313 require use of adjuvants. Mice were immunized with PfTrx–8-mer–OVX313 adjuvanted with alum-MPLA, GLA-SE, GLA-AF, and ALFA, and sera were analyzed by L1-PBNA 4 weeks after the final immunization. Symbols represent individual neutralization titers for HPV16; geometric means of the titers for each group are indicated by horizontal lines. A P value of ≤0.05 was considered significant. *, P value < 0.05; **, P value < 0.01; ***, P value < 0.001; ****, P value < 0.0001.
Passive transfer of anti-PfTrx–8-mer–OVX313 immune sera provides robust in vivo protection.
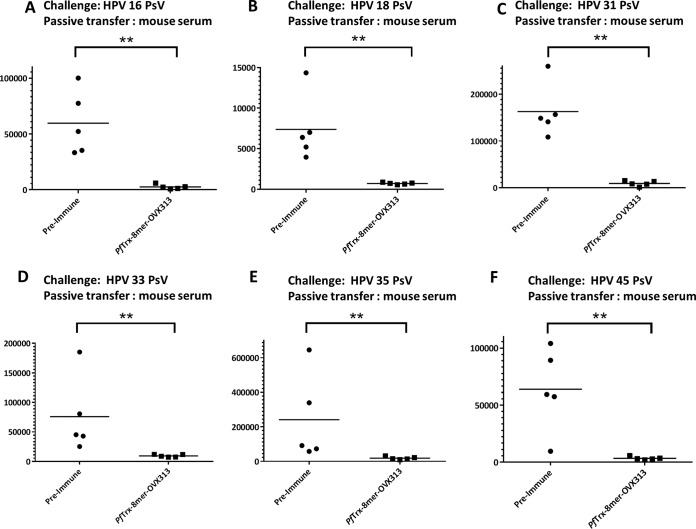
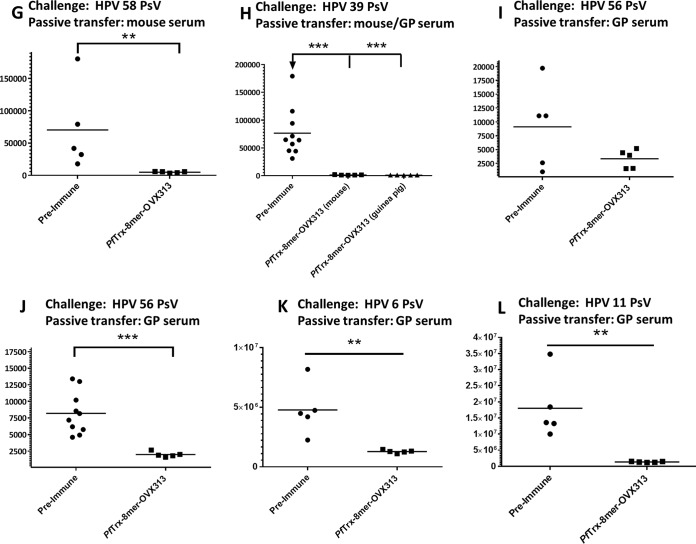
In order to evaluate the in vivo efficacy of the candidate vaccine PfTrx–8-mer–OVX313, passive transfer of immune sera was performed. After final immunization with the PfTrx–8-mer–OVX313 antigen, sera from 10 vaccinated mice were pooled and then passively transferred intraperitoneally (i.p.) into groups of animals, which were subsequently challenged with HPV6, -11, -16, -18, -31, -33, -35, -39, -45, -56, or -58 pseudovirions, respectively. Preimmune sera were used as control. Luciferase activity as indicator for the degree of infection was determined 2 days after pseudovirion instillation. In vivo measured luminescence signals are reported in Fig. 7A to L; a representative image of the vaginal in vivo challenge with HPV6, -11, and -31 is shown in Fig. 7M. Passively transferred sera (corresponding to an approximately 1:40 dilution, serum volume ratio) from PfTrx–8-mer–OVX313-immunized mice provided clear protection against all HPV infections.
FIG 7.
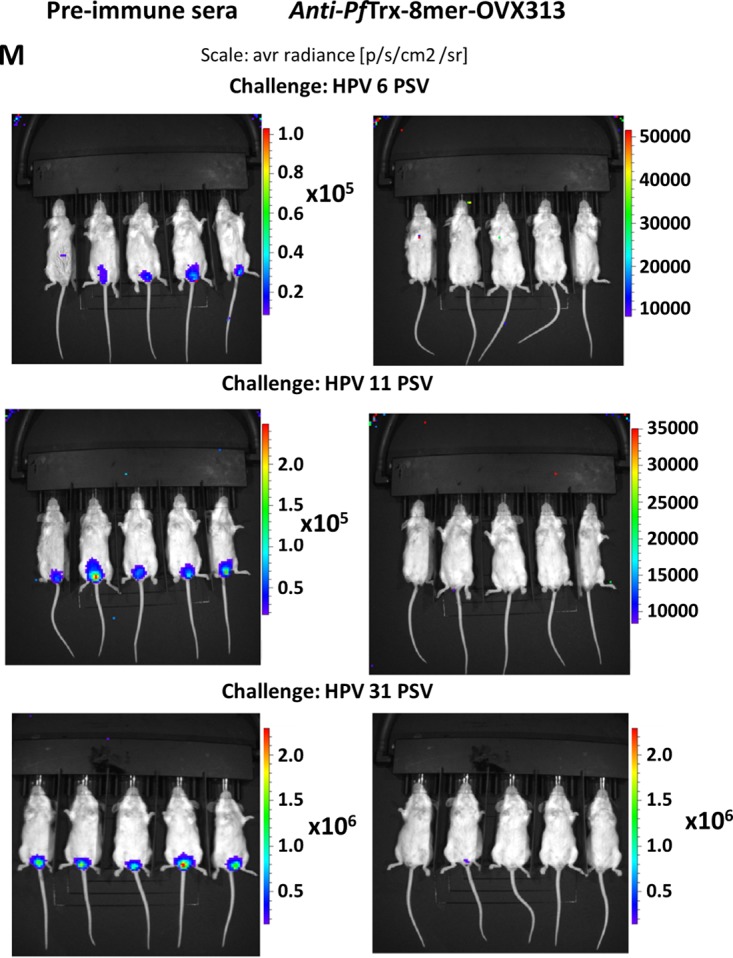
In vivo protection afforded by anti-PfTrx–8-mer–OVX313 sera compared with sera from nonimmunized animals. Sera from either 10 mice (A to I) or 2 guinea pigs (GP) (J) immunized with the PfTrx–8-mer–OVX313 antigen were pooled and passively transferred into naive mice. Recipient animals were then transduced with HPV16 (A), HPV18 (B), HPV31 (C), HPV33 (D), HPV35 (E), HPV45 (F), HPV58 (G), HPV39 (H), HPV56 (I and J), HPV6 (K), and HPV11 (L) pseudovirions. The y axis displays the average radiance measured as photons per second per square centimeter per steradian. The broad protection afforded by PfTrx–8-mer–OVX313-elicited antibodies compared with preimmune sera is apparent in panels A to I; representative images showing the magnitude of vaginal infection by HPV6, -11, and -31 PsVs are shown in panel M. The colors (scale shown on the right) indicate the intensity of luciferase expression; an ROI analysis for average radiance (photons per second per square centimeter per steradian) was performed with Living Image 2.50.1 software. Note that due to the different in vivo transduction activities of the various HPV pseudovirion types, different scales were used. A P value of ≤0.05 was considered significant. *, P value < 0.05; **, P value < 0.01; ***, P value < 0.001.
The OVX313 heptamerization domain results in enhanced T-helper responses.
With the exception of some complex antigens showing highly repetitive arrays of epitopes, the induction of humoral immune responses requires T-helper cell activation. To evaluate the effect of OVX313 on T-cell responses, two groups of mice were immunized with the PfTrx–8-mer and PfTrx–8-mer–OVX313 antigens, respectively. The splenocytes were isolated and stimulated in vitro with recently identified H2d restricted T-helper peptides (PfTrx-C3, OVX313-I5, OVX313-I8, and OVX313-I9 [data not shown]) derived from PfTrx and OVX313 proteins. Consistent with the neutralizing antibody responses, the presence of OVX313 in the vaccine formulation could significantly increase the helper response directed against the Trx-specific epitopes in the group of mice immunized with PfTrx–8-mer–OVX313 compared to the group of mice immunized with the PfTrx–8-mer vaccine (Fig. 8). A T-helper response was also observed for the OVX313-I5 epitope; although this epitope was not showing a dominant response in the enzyme-linked immunosorbent spot (ELISpot) assay, it might additionally contribute to the L2-directed immune response.
FIG 8.
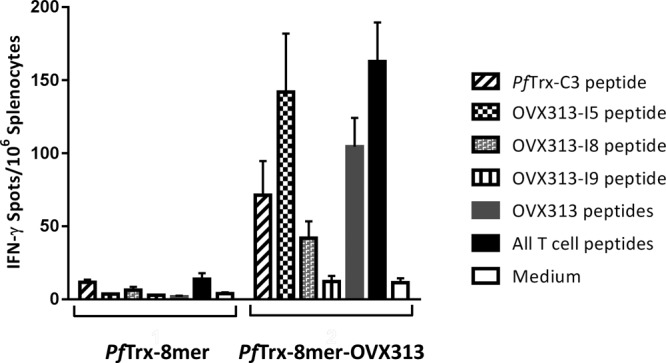
OVX313 augments IFN-γ production by T cells. Numbers of IFN-γ spots per 106 splenocytes are compared in two groups of mice immunized with PfTrx–8-mer and PfTrx–8-mer–OVX313. The splenocytes were stimulated in vitro with T-helper peptides (PfTrx-C3, OVX313-I5, OVX313-I8, and OVX313-I9). Data are shown as the mean ± standard error of the mean for 5 mice per group.
DISCUSSION
In the present study, we exploited a novel strategy to improve the antigenic properties of the L2-based HPV vaccine and generated notable neutralization antibody titers against a comprehensive range of high- and low-risk HPV types by fusing the L2 polytope to an OVX313 multimerization platform (PfTrx–8-mer–OVX313). Also, the vaccine-elicited antibodies protected mice against cervicovaginal HPV pseudovirion challenge in passive transfer experiments. Results are summarized in Table 1.
TABLE 1.
Summary of neutralization observed for the PfTrx–8-mer–OVX313 antigen in mice and/or guinea pigs
| HPV type(s) | Assay or model with animalc: |
Contribution (%) to: |
|||||
|---|---|---|---|---|---|---|---|
|
In vitro PBNA |
In vivo passive transfer mouse model | ||||||
| Mouse |
Guinea pig |
||||||
| L1 | L2 | L1 | L2 | Cervical carcinomaa | Genital wartsb | ||
| 16 | +++ | +++ | +++ | +++ | +++ | 53.5 | 0 |
| 18 | +++ | NT | +++ | +++ | +++ | 17.2 | 0 |
| 45 | +++ | NT | +++ | +++ | +++ | 6.7 | 0 |
| 31 | +++ | NT | +++ | +++ | +++ | 2.9 | 0 |
| 33 | +++ | NT | +++ | +++ | +++ | 2.6 | 0 |
| 52 | ++ | NT | ++ | +++ | NT | 2.3 | 0 |
| 58 | +++ | NT | +++ | +++ | +++ | 2.2 | 0 |
| 35 | +++ | NT | +++ | +++ | +++ | 1.4 | 0 |
| 59 | +++ | NT | +++ | +++ | NT | 1.3 | 0 |
| 56 | − | +++ | +++ | +++ | ++ | 1.2 | 0 |
| 51 | +++ | NT | +++ | +++ | NT | 1.0 | 0 |
| 39 | + | +++ | +++ | +++ | +++ | 0.7 | 0 |
| 68 | ++ | +++ | ++ | +++ | NT | 0.6 | 0 |
| 73 | +++ | NT | +++ | +++ | NT | 0.5 | 0 |
| 82 | NT | NT | NT | NT | NT | 0.3 | 0 |
| 40, 42, 53, 54, 55, 83, 84 | NT | NT | NT | NT | NT | 1.2 | 0 |
| Untyped HPV | NT | NT | NT | NT | NT | 4.4 | 0 |
| 6 | +++ | NT | +++ | +++ | ++ | 0 | 74 |
| 11 | +++ | NT | +++ | +++ | ++ | 0 | 14 |
Two commercial HPV vaccines were licensed in 2006 and 2007, respectively, and hundreds of millions doses have been distributed worldwide ever since. In countries with high vaccine coverage, the reduction in clinical HPV-associated lesions is visible and effects on reduction of invasive tumors are expected to be observed in the coming years. With Gardasil 9, an expanded-spectrum HPV vaccine has been introduced, targeting two low-risk and seven high-risk HPV types (34). Despite the success of the commercial HPV vaccines, the incomplete protection against different HPV types and the requirement for intact cooling chains comprise significant limitations. The latter is, next to vaccine costs per se, one major factor for the rather poor global use of HPV prophylactic vaccination. Consequently, about two-thirds of countries worldwide have not introduced vaccination against HPV into their health care systems (27).
To breach some of the limitations of VLP vaccines, we and others have developed HPV L2-based strategies to induce broadly protective antibody responses. Moving toward clinical development in an academically funded setting, we faced the requirement of simplifying the antigen composition by developing a single-peptide vaccine. As we have shown previously, the major cross-neutralization epitope of HPV16 fails to induce robust responses against certain HPV types, in particular HPV31 and -51. Instead of bridging this gap by the use of antigen cocktails, we developed single-antigen L2 polytopes inserted into the scaffold of bacterial thioredoxin.
Earlier, we presented data that Trx can, depending on the sequence, accept insertions of up to 360 amino acids. In the present study, we inserted L2 epitopes of up to 11 different high-risk HPV types which, including linker sequences, add up to about 240 amino acids, compared to 100 amino acids of PfTrx. By reevaluating the 11-mer sequences based on results of vaccination and homology among different HPV types, we were able to reduce the epitopes to eight, including the epitope of low-risk HPV6 to provide protection against genital warts. Interestingly, we observed different strengths of neutralization against the HPV types tested, and this was not necessarily correlated with the presence or absence of the corresponding L2 epitope in the antigen formulation. For example, the mouse sera failed to neutralize HPV56 PsV in the low-sensitivity L1-PBNA, although neutralization was observed in the L2-PBNA, for guinea pig sera in the L1-PBNA, and also in the in vivo challenge model. This could demonstrate a limitation of the standard neutralization assay for particular HPV pseudovirions. Along this line, we have observed previously that HPV51 L2 antigens induce higher neutralization against HPV16 than do HPV51 pseudovirions (24). The final improvement in our preclinical antigen development was the fusion with the 55-amino-acid-long OVX313 heptamerization domain.
OVX313 is a synthetic hybrid domain derived from complement inhibitor C4 binding protein (C4bp), which acts as a natural inhibitor of classical and lectin pathways of the complement system (35). The hybrid isoform of C4bp consists of a homoheptamer of alpha chains in which the last 54 amino acids do not belong to the complement regulatory proteins. This domain is necessary and sufficient for oligomerization of C4bp or any other fused proteins (36). The hybrid C4bp domain shows only 20% sequence similarity to either the murine or the human domain (28), and it does not induce antibodies against murine and human C4bp. We could demonstrate that Trx-L2-OVX antigens form multimers which are stabilized by covalent disulfide bonds.
OVX313 has been used previously in clinical trials to boost cellular and humoral immunogenicity of tuberculosis antigens, although the mode of action of the heptamerization domain in this context has not been fully revealed (29). Highly oligomeric epitope display has been proposed to be the essence of the immunogenicity observed for VLP-based vaccines, by their intrinsic ability to hyperstimulate B cells (37), although the simplified model of B-cell receptor cross-linking by antigens has been challenged recently (38). The higher humoral immunogenicity observed for the OVX antigens was accompanied with stronger T-helper responses as well. In addition, the mice recognized an additional helper epitope located within the OVX sequences which, however, was not immunodominant. Taken together, the data presented in this study do not allow drawing conclusions whether the OVX effect is directly acting on B cells or whether it is mediated through the T-helper response. Further, the data also do not allow us to conclude that heptamerization of the antigen per se is responsible for the effect. To demonstrate this, mutant OVX peptides would need to be generated, with the caveat that this also requires modification of the primary OVX sequence.
We observed that the presence of T-helper epitopes can be a limiting factor in induction of anti-L2 responses using the Trx antigens, for example, in C57/BL6 mice (data not shown). While we did not observe this for BALB/c mice used in the present study, the observation that guinea pigs are responding much more strongly against the Trx-L2 antigens could also point to the importance of T-helper responses. The immunoglobulin gene reservoir in the inbred BALB/c mice is unlikely to account for this, as we previously isolated high-affinity and broadly neutralizing monoclonal antibodies from this strain.
Further, our data indicate that OVX cannot fully substitute for Trx, as fusion of L2 epitopes in the context of the Trx scaffold yields significantly higher immunogenicity than a direct fusion of L2 to OVX, which is consistent with our previous findings that Trx is well suited for the presentation of L2 neutralization epitopes.
Due to the lower immunogenicity of L2-based vaccines than of VLP vaccines, it is likely that they will require the use of an adjuvant system. There are only a very limited number of adjuvants available for use in humans, and the number shrinks further if limited to those adjuvants for which a license can be obtained, as most commonly used adjuvant systems are proprietary. In our mouse model, we observed the strongest responses using the squalene-based adjuvant AddaVax, a homolog to clinically used MF59, which is proprietary to Novartis. Besides AddaVax, the Trx-L2-OVX antigens can be combined well with alum-MPLA or the synthetic Toll-like receptor 4 (TLR4) agonist GLA formulated in stable oil-in-water emulsion or as an aqueous formulation, at least for the animal species tested so far. GLA is available for licensing as a clinical-grade adjuvant.
Testing immune sera raised against the 8-mer polytope, comprising epitopes of high-risk PV, against a limited number of cutaneous HPV pseudovirions revealed that the cross-protection exceeds the Alphapapillomavirus genus. Despite all the in vitro and in vivo models to assess neutralizing antibody responses, there is yet no formal proof that anti-L2 antibodies will protect against natural HPV infections. Further, our knowledge about protection against natural skin infections by anti-L1 antibodies is limited to a few animal models such as Mastomys coucha, in which transmission of skin PV between animals can be prevented by vaccination (39).
Previously, we have shown that the ability to neutralize a particular pseudovirion via anti-L2 antibodies can be dependent on the primary sequence but also on other factors, possibly exposure and availability of the epitope (24). We know little about the number of L2 molecules that need to be targeted in order to prevent infection, and this might vary for diverse HPV types. For example, the 8-mer polytope described in this study induces titers in the range of 106 in the case of HPV92 even when determined by the low-sensitivity standard PBNA. Interestingly, the L2 epitopes of both HPV92 and HPV96 are very similar to the epitope of HPV35, which is contained within the 8-mer polytope. The neutralization assay adapted for detection of anti-L2 antibodies enforces the exposure of the L2 N terminus and thereby leads to a 10- to 200-fold increase in neutralization titers, simulating the in vivo entry process in which the virus presumably first binds to the extracellular matrix (ECM) of the basement membrane before moving to the target cells (40). It is possible that different titers observed for various HPV types reflect in part differences in L2 exposure in the cell culture-based assay.
The three commercial VLP-based vaccines have demonstrated great success. A number of strategies have been reported in recent years to overcome one important limitation of the L1 antigen, i.e., the highly type-specific humoral antibody response (for a review, see references 41 to 44). All these approaches have in common that they present one or more L2 neutralization epitopes in a scaffold. For the latter, virus-like particles (45–48) or larger portions of the L2 protein itself (49) have been shown to be essential to increase the low intrinsic immunogenicity of the L2 epitopes. Likewise, we have reported that bacterial thioredoxin protein can serve as a robust presentation platform (25). At the same time, we and others observed that a neutralization epitope from a single L2 protein provides protection against some, but not all, relevant HPV types, which can be compensated for by simultaneous insertion of epitopes from different HPV types (24, 48, 49).
In summary, we believe that with the single-peptide vaccine antigen based on a heptamerized thioredoxin scaffold, we have developed a candidate antigen for clinical evaluation with the potential of providing broad protection against oncogenic and nononcogenic HPV types.
MATERIALS AND METHODS
Protein expression and purification.
Synthetic DNA encoding the PfTrx HPV16 L2(20–38)3-OVX313 and HPV16 L2(20–38)3-OVX313 proteins was cloned into the pET26 plasmid in order to express the recombinant protein in E. coli BL21 cells. The recombinant proteins were purified by ion-exchange chromatography aided by an arginine-rich motif at the C terminus of OVX313. The OVX313 heptamerization domain represents a positively charged variant of IMX313, developed by Osivax, and is part of the OligoDOM technology. IMX313 is a derivative of the C4 binding protein (C4bp), which was shown to lack homology to human C4bp. Next, the purified protein was subjected to gel filtration chromatography on a Superdex 200 column to separate the heptameric conformation from the monomeric form and from higher-molecular-weight aggregates. Likewise, two other polypeptides, PfTrx–8-mer–OVX313 (HPV16, -18, -31, -33, -35, -6, -51, and -59) and PfTrx–11-mer–OVX313 (HPV16, -18, -31, -33, -35, -39, -45, -51, -56, -59, and -82), were produced.
For this, synthetic DNAs encoding the 8-mer and 11-mer proteins were cloned into pET26 PfTrx-OVX313 plasmid. The PfTrx–8-mer–OVX313 and PfTrx–11-mer–OVX313 proteins were purified by heparin chromatography. The concentration and quality of the proteins were analyzed by SDS-PAGE–Coomassie blue staining. Prior to immunization, all proteins were detoxified twice by use of Triton X-114 to reduce endotoxin levels below 8 IU per ml. Details of the OVX313 protein purification and characterization will be published elsewhere (53).
Mouse and guinea pig immunization.
Six- to 8-week-old female BALB/c mice were purchased from Charles River Laboratories (Sulzfeld, Germany) and kept at the German Cancer Research Center under specific-pathogen-free conditions (animal permit G19/13, Regierungspräsidium Karlsruhe, Germany). Mice, except for those in the clinical-grade adjuvant experiment, were immunized four times at biweekly intervals with 20 μg of the different detoxified and filter-sterilized antigens adjuvanted with 50% (vol/vol) AddaVax (InvivoGen) (25 μl, intramuscular injection) or 50 μg aluminum hydroxide (Brenntag) and 10 μg synthetic monophosphoryl lipid A (Avanti Lipids) (5 μl aluminum hydroxide, 10 μl monophosphoryl lipid A, intramuscular injection). For the experiment involving a clinical-grade adjuvant, glycopyranosyl lipid A (GLA-SE) composed of 5 μg GLA (5 μl) and 2% oil (SE; 5 μl) was mixed by brief vortexing, and then 5 μg antigen was added, GLA-AF (5 μg AF [5 μl]) and 5 μg antigen were mixed, and for ALFA, aluminum-adsorbed antigen (50 μg alum and 5 μg antigen) was directly added to lyophilized ALF. GLA was sourced from Immune Design, Seattle, WA, USA, and ALFA was kindly provided by the Walter Reed Army Institute of Research. After brief mixing for a minute, the tube was rolled for 1 h at room temperature until a homogenous solution formed. All the injections were performed intramuscularly in a 50-μl volume.
Outbred Hartley (Crl:HA) guinea pigs (350- to 400-g female animals) were obtained from Charles River Laboratories and immunized four times at triweekly intervals with 30 μg of different antigens formulated with 50% (vol/vol) AddaVax (100 μl, subcutaneous [s.c.] injection). Four weeks after the last immunization, blood samples were collected by cardiac puncture.
Pseudovirion preparation.
Different types of pseudovirions (PsVs) were prepared by cotransfection of the human fibroblast cell line 293TT with plasmids carrying humanized HPV L1 and L2 coding sequences plus a reporter plasmid, and purification was performed by iodixanol gradient ultracentrifugation according to a previously described protocol (18, 23).
In vitro standard L1-PBNA.
Unless otherwise stated, the L1 pseudovirion-based neutralization assay (L1-PBNA) was used to detect neutralizing antibodies in sera against human papillomaviruses. L1-PBNAs were performed as described previously (23). Briefly, 50 μl of diluted serum (in Dulbecco modified Eagle medium [DMEM]) was combined with 50 μl of diluted pseudovirion (in DMEM) and incubated at room temperature for 20 min. Next, 50 μl of HeLa T cells (2.5 × 105 cells/ml) was added to the pseudovirion-antibody mixture and incubated for 48 h at 37°C in a humidified incubator. The amount of secreted Gaussia luciferase was determined in 10 μl of cell culture medium using the Gaussia Glow Juice kit according to the manufacturer's instructions (PJK GmbH, Germany). The light emissions of samples were measured 15 min after substrate addition.
In vitro L2-PBNA.
The L2 pseudovirion-based neutralization assay (L2-PBNA) was performed as previously described (40). Briefly, extracellular matrix (ECM) was prepared from overnight culture of MCF10A cells (2 × 105 cells/ml; culture medium composed of DMEM–F-12, 5% horse serum, 2 mM l-glutamine, 100 U/ml streptomycin, 100 μg/ml penicillin, 500 ng/ml hydrocortisone, 10 μg/ml insulin, 20 ng/ml epidermal growth factor [EGF]). After one round of washing and cell lysis (phosphate-buffered saline [PBS] containing 0.5% [vol/vol] Triton X-100–20 mM NH4OH) for 5 min at 37°C, pseudovirion solution prepared in conditioned medium (collected from CHO furin-secreting cells in the presence of 5 μg/ml of heparin; culture medium composed of DMEM, 10% fetal calf serum [FCS], 200 μM proline, 100 U/ml streptomycin, 100 μg/ml penicillin) was added to the ECM-containing wells. The pseudovirion-furin-heparin mixture was incubated overnight at 37°C. The next day, the medium was removed and replaced with an antibody dilution series made in pgsa-745 growth medium (DMEM, 10% FCS, 200 μM proline, 100 U/ml streptomycin, 100 μg/ml penicillin). The plate was then incubated at 37°C for 2 h to allow efficient antibody binding to target epitopes. Following this incubation, pgsa-745 cells were added directly to the antibody-containing wells to a final concentration of 1.6 × 105 cells/ml. For all experiments, 3-fold dilution series of antisera were made starting with an initial dilution of 1:50. The activity of secreted Gaussia luciferase was measured as described above.
Data were analyzed, and 50% inhibitory concentrations (IC50) were determined using the GraphPad Prism software program. All L2-PBNA cell lines, including pgsa-745, CHO furin, and MCF10A, were kindly provided by John Schiller's laboratory (NIH, Bethesda, MD), and authenticity was confirmed periodically by multiplex cell authentication (MCA) assay (50).
ELISpot assay.
Splenocytes (1 × 106) from vaccinated mice were cultured in RPMI 1640 supplemented with 10% fetal bovine serum (FBS), 2 mM l-glutamine, 10 mM HEPES buffer, 100 U/ml streptomycin, and 100 μg/ml penicillin and stimulated with a panel of 20-mer peptides derived from PfTrx and OVX313 protein sequences (PfTrx-C3, DIQIVHINAGKWKNIVDKFN; OVX313-I5, VCGEVAYIQSVVSDCHVPTA; OVX313-I8, LLEIRKLFLEIQKLKVEGRR; OVX313-I9, RKLFLEIQKLKVEGRRRRRS) at a concentration of 10 μg peptide/ml (Mimotopes, Australia). Briefly, Multiscreen IP plates (Merck Millipore, Germany) were coated with anti-mouse gamma interferon (IFN-γ; BD Pharmingen, USA). After 40 h of incubation of peptide-stimulated splenocytes at 37°C, cells were washed and cytokine spots were developed using biotinylated anti-mouse IFN-γ antibody (BD Pharmingen, USA), alkaline phosphatase (AKP) streptavidin (BD Pharmingen, USA), and 1-Step nitroblue tetrazolium (NBT)–5-bromo-4-chloro-3-indolylphosphate (BCIP) substrate (Sigma, USA). Concanavalin A and unstimulated splenocytes were used as positive and negative controls, respectively. Spots were counted using an ELISpot reader (AID, Strassberg, Germany). The number of spots in medium-stimulated splenocytes was subtracted from the number in peptide-stimulated cells to generate the net number of peptide-specific spot-forming units (SFUs).
In vivo neutralization in the cervicovaginal mouse model.
The mouse cervicovaginal model experiment was performed as described previously (51) with minor modifications (animal permit number G246/11). The passive transfer assay was performed over the course of 8 days. On day 1, BALB/c male mouse cage bedding was transferred to the cages of female mice to induce hormonal synchronization (Whitten effect). On day 3, 100 μl of 30-μg/ml medroxyprogesterone acetate (Depo-Provera; Pharmacia) was injected subcutaneously (s.c.). One hundred microliters of serum from immunized mice (diluted 1:2 with PBS) was delivered i.p. to each mouse on day 5. On day 6, mice were treated intravaginally with 50 μl of 4% Nonoxynol-9 (N9; Spectrum) in 4% carboxymethyl cellulose (Sigma). Four hours after N9 treatment, HPV pseudovirions encapsidating a firefly luciferase plasmid in 4% carboxymethyl cellulose (Sigma) were instilled intravaginally. Luminescence-based imaging using a Xenogen in vivo imaging system (IVIS) imager (Xenogen Corporation; PerkinElmer) was performed on day 8, and the efficiency of L2-directed immune responses was assessed after intravaginal instillation of 20 μl luciferin substrate (15 mg/ml; Promega). A region-of-interest (ROI) analysis was performed using Living Image 2.50.1 software (Xenogen; PerkinElmer). Background signal was obtained and subtracted by imaging each group of mice prior to installition of luciferin.
Statistical analysis.
Statistical significance was determined with the nonparametric Mann-Whitney test performed with GraphPad Prism 6.00 (GraphPad Software, USA); differences between groups were considered significant at a P value of <0.05.
ACKNOWLEDGMENTS
This work was supported by a grant from Wilhelm-Sander-Stiftung to Somayeh Pouyanfard and Martin Müller (grant number 2013.136.2). Research conducted at the University of Parma was supported by a grant from the Italian Association for Research on Cancer (AIRC; IG 12956) to S.O.
We are grateful to our colleagues Reinhard Kirnbauer, Chris Buck, Richard Roden, and Joakim Dillner for kindly providing pseudovirion expression constructs.
REFERENCES
- 1.Munoz N, Bosch FX, Castellsague X, Diaz M, de Sanjose S, Hammouda D, Shah KV, Meijer CJ. 2004. Against which human papillomavirus types shall we vaccinate and screen? The international perspective. Int J Cancer 111:278–285. doi: 10.1002/ijc.20244. [DOI] [PubMed] [Google Scholar]
- 2.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. 2007. Human papillomaviruses. IARC Monogr Eval Carcinog Risks Hum 90:1–636. [PMC free article] [PubMed] [Google Scholar]
- 3.zur Hausen H. 2009. Papillomaviruses in the causation of human cancers—a brief historical account. Virology 384:260–265. doi: 10.1016/j.virol.2008.11.046. [DOI] [PubMed] [Google Scholar]
- 4.zur Hausen H. 1985. Genital papillomavirus infections. Prog Med Virol 32:15–21. [PubMed] [Google Scholar]
- 5.zur Hausen H. 1994. Disrupted dichotomous intracellular control of human papillomavirus infection in cancer of the cervix. Lancet 343:955–957. doi: 10.1016/S0140-6736(94)90070-1. [DOI] [PubMed] [Google Scholar]
- 6.zur Hausen H. 2001. Cervical carcinoma and human papillomavirus: on the road to preventing a major human cancer. J Natl Cancer Inst 93:252–253. doi: 10.1093/jnci/93.4.252. [DOI] [PubMed] [Google Scholar]
- 7.Winer RL, Lee SK, Hughes JP, Adam DE, Kiviat NB, Koutsky LA. 2003. Genital human papillomavirus infection: incidence and risk factors in a cohort of female university students. Am J Epidemiol 157:218–226. doi: 10.1093/aje/kwf180. [DOI] [PubMed] [Google Scholar]
- 8.Dunne EF, Sternberg M, Markowitz LE, McQuillan G, Swan D, Patel S, Unger ER. 2011. Human papillomavirus (HPV) 6, 11, 16, and 18 prevalence among females in the United States—National Health And Nutrition Examination Survey, 2003-2006: opportunity to measure HPV vaccine impact? J Infect Dis 204:562–565. doi: 10.1093/infdis/jir342. [DOI] [PubMed] [Google Scholar]
- 9.Rodriguez AC, Schiffman M, Herrero R, Wacholder S, Hildesheim A, Castle PE, Solomon D, Burk R, Proyecto Epidemiologico Guanacaste Group. 2008. Rapid clearance of human papillomavirus and implications for clinical focus on persistent infections. J Natl Cancer Inst 100:513–517. doi: 10.1093/jnci/djn044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hildesheim A, Hadjimichael O, Schwartz PE, Wheeler CM, Barnes W, Lowell DM, Willett J, Schiffman M. 1999. Risk factors for rapid-onset cervical cancer. Am J Obstet Gynecol 180:571–577. doi: 10.1016/S0002-9378(99)70256-5. [DOI] [PubMed] [Google Scholar]
- 11.zur Hausen H. 2002. Papillomaviruses and cancer: from basic studies to clinical application. Nat Rev Cancer 2:342–350. doi: 10.1038/nrc798. [DOI] [PubMed] [Google Scholar]
- 12.Hestbech MS, Lynge E, Kragstrup J, Siersma V, Vazquez-Prada Baillet M, Brodersen J. 2015. The impact of HPV vaccination on future cervical screening: a simulation study of two birth cohorts in Denmark. BMJ Open 5:e007921. doi: 10.1136/bmjopen-2015-007921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Petrosky E, Bocchini JA Jr, Hariri S, Chesson H, Curtis CR, Saraiya M, Unger ER, Markowitz LE, Centers for Disease Control and Prevention. 2015. Use of 9-valent human papillomavirus (HPV) vaccine: updated HPV vaccination recommendations of the advisory committee on immunization practices. MMWR Morb Mortal Wkly Rep 64:300–304. [PMC free article] [PubMed] [Google Scholar]
- 14.Bernard HU, Burk RD, Chen Z, van Doorslaer K, zur Hausen H, de Villiers EM. 2010. Classification of papillomaviruses (PVs) based on 189 PV types and proposal of taxonomic amendments. Virology 401:70–79. doi: 10.1016/j.virol.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wieland U, Kreuter A, Pfister H. 2014. Human papillomavirus and immunosuppression. Curr Probl Dermatol 45:154–165. doi: 10.1159/000357907. [DOI] [PubMed] [Google Scholar]
- 16.Tommasino M. 2017. The biology of beta human papillomaviruses. Virus Res 231:128–138. doi: 10.1016/j.virusres.2016.11.013. [DOI] [PubMed] [Google Scholar]
- 17.Ghittoni R, Accardi R, Hasan U, Gheit T, Sylla B, Tommasino M. 2010. The biological properties of E6 and E7 oncoproteins from human papillomaviruses. Virus Genes 40:1–13. doi: 10.1007/s11262-009-0412-8. [DOI] [PubMed] [Google Scholar]
- 18.Gambhira R, Karanam B, Jagu S, Roberts JN, Buck CB, Bossis I, Alphs H, Culp T, Christensen ND, Roden RB. 2007. A protective and broadly cross-neutralizing epitope of human papillomavirus L2. J Virol 81:13927–13931. doi: 10.1128/JVI.00936-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rubio I, Seitz H, Canali E, Sehr P, Bolchi A, Tommasino M, Ottonello S, Muller M. 2011. The N-terminal region of the human papillomavirus L2 protein contains overlapping binding sites for neutralizing, cross-neutralizing and non-neutralizing antibodies. Virology 409:348–359. doi: 10.1016/j.virol.2010.10.017. [DOI] [PubMed] [Google Scholar]
- 20.Kondo K, Ishii Y, Ochi H, Matsumoto T, Yoshikawa H, Kanda T. 2007. Neutralization of HPV16, 18, 31, and 58 pseudovirions with antisera induced by immunizing rabbits with synthetic peptides representing segments of the HPV16 minor capsid protein L2 surface region. Virology 358:266–272. doi: 10.1016/j.virol.2006.08.037. [DOI] [PubMed] [Google Scholar]
- 21.Day PM, Gambhira R, Roden RB, Lowy DR, Schiller JT. 2008. Mechanisms of human papillomavirus type 16 neutralization by l2 cross-neutralizing and L1 type-specific antibodies. J Virol 82:4638–4646. doi: 10.1128/JVI.00143-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seitz H, Ribeiro-Muller L, Canali E, Bolchi A, Tommasino M, Ottonello S, Muller M. 2015. Robust In vitro and in vivo neutralization against multiple high-risk HPV types induced by a thermostable thioredoxin-L2 vaccine. Cancer Prev Res (Phila) 8:932–941. doi: 10.1158/1940-6207.CAPR-15-0164. [DOI] [PubMed] [Google Scholar]
- 23.Seitz H, Dantheny T, Burkart F, Ottonello S, Muller M. 2013. Influence of oxidation and multimerization on the immunogenicity of a thioredoxin-L2 prophylactic papillomavirus vaccine. Clin Vaccine Immunol 20:1061–1069. doi: 10.1128/CVI.00195-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seitz H, Canali E, Ribeiro-Muller L, Palfi A, Bolchi A, Tommasino M, Ottonello S, Muller M. 2014. A three component mix of thioredoxin-L2 antigens elicits broadly neutralizing responses against oncogenic human papillomaviruses. Vaccine 32:2610–2617. doi: 10.1016/j.vaccine.2014.03.033. [DOI] [PubMed] [Google Scholar]
- 25.Rubio I, Bolchi A, Moretto N, Canali E, Gissmann L, Tommasino M, Muller M, Ottonello S. 2009. Potent anti-HPV immune responses induced by tandem repeats of the HPV16 L2 (20-38) peptide displayed on bacterial thioredoxin. Vaccine 27:1949–1956. doi: 10.1016/j.vaccine.2009.01.102. [DOI] [PubMed] [Google Scholar]
- 26.Canali E, Bolchi A, Spagnoli G, Seitz H, Rubio I, Pertinhez TA, Muller M, Ottonello S. 2014. A high-performance thioredoxin-based scaffold for peptide immunogen construction: proof-of-concept testing with a human papillomavirus epitope. Sci Rep 4:4729. doi: 10.1038/srep04729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bruni L, Diaz M, Barrionuevo-Rosas L, Herrero R, Bray F, Bosch FX, de Sanjose S, Castellsague X. 2016. Global estimates of human papillomavirus vaccination coverage by region and income level: a pooled analysis. Lancet Glob Health 4:e453–463. doi: 10.1016/S2214-109X(16)30099-7. [DOI] [PubMed] [Google Scholar]
- 28.Ogun SA, Dumon-Seignovert L, Marchand JB, Holder AA, Hill F. 2008. The oligomerization domain of C4-binding protein (C4bp) acts as an adjuvant, and the fusion protein comprised of the 19-kilodalton merozoite surface protein 1 fused with the murine C4bp domain protects mice against malaria. Infect Immun 76:3817–3823. doi: 10.1128/IAI.01369-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Minhinnick A, Satti I, Harris S, Wilkie M, Sheehan S, Stockdale L, Manjaly Thomas ZR, Lopez-Ramon R, Poulton I, Lawrie A, Vermaak S, Le Vert A, Del Campo J, Hill F, Moss P, McShane H. 2016. A first-in-human phase 1 trial to evaluate the safety and immunogenicity of the candidate tuberculosis vaccine MVA85A-IMX313, administered to BCG-vaccinated adults. Vaccine 34:1412–1421. doi: 10.1016/j.vaccine.2016.01.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spencer AJ, Hill F, Honeycutt JD, Cottingham MG, Bregu M, Rollier CS, Furze J, Draper SJ, Sogaard KC, Gilbert SC, Wyllie DH, Hill AV. 2012. Fusion of the Mycobacterium tuberculosis antigen 85A to an oligomerization domain enhances its immunogenicity in both mice and non-human primates. PLoS One 7:e33555. doi: 10.1371/journal.pone.0033555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Odegard JM, Flynn PA, Campbell DJ, Robbins SH, Dong L, Wang K, Ter Meulen J, Cohen JI, Koelle DM. 2016. A novel HSV-2 subunit vaccine induces GLA-dependent CD4 and CD8 T cell responses and protective immunity in mice and guinea pigs. Vaccine 34:101–109. doi: 10.1016/j.vaccine.2015.10.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beck Z, Matyas GR, Jalah R, Rao M, Polonis VR, Alving CR. 2015. Differential immune responses to HIV-1 envelope protein induced by liposomal adjuvant formulations containing monophosphoryl lipid A with or without QS21. Vaccine 33:5578–5587. doi: 10.1016/j.vaccine.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 33.Coler RN, Baldwin SL, Shaverdian N, Bertholet S, Reed SJ, Raman VS, Lu X, DeVos J, Hancock K, Katz JM, Vedvick TS, Duthie MS, Clegg CH, Van Hoeven N, Reed SG. 2010. A synthetic adjuvant to enhance and expand immune responses to influenza vaccines. PLoS One 5:e13677. doi: 10.1371/journal.pone.0013677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.World Health Organization. 2017. Vaccine in National Immunization Programme update. World Health Organization, Geneva, Switzerland: http://www.who.int/entity/immunization/monitoring_surveillance/VaccineIntroStatus.pptx. [Google Scholar]
- 35.Blom AM, Villoutreix BO, Dahlback B. 2004. Complement inhibitor C4b-binding protein-friend or foe in the innate immune system? Mol Immunol 40:1333–1346. doi: 10.1016/j.molimm.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 36.Kask L, Hillarp A, Ramesh B, Dahlback B, Blom AM. 2002. Structural requirements for the intracellular subunit polymerization of the complement inhibitor C4b-binding protein. Biochemistry 41:9349–9357. doi: 10.1021/bi025980+. [DOI] [PubMed] [Google Scholar]
- 37.Spohn G, Bachmann MF. 2008. Exploiting viral properties for the rational design of modern vaccines. Expert Rev Vaccines 7:43–54. doi: 10.1586/14760584.7.1.43. [DOI] [PubMed] [Google Scholar]
- 38.Yang J, Reth M. 2016. Receptor dissociation and B-cell activation. Curr Top Microbiol Immunol 393:27–43. doi: 10.1007/82_2015_482. [DOI] [PubMed] [Google Scholar]
- 39.Vinzon SE, Braspenning-Wesch I, Muller M, Geissler EK, Nindl I, Grone HJ, Schafer K, Rosl F. 2014. Protective vaccination against papillomavirus-induced skin tumors under immunocompetent and immunosuppressive conditions: a preclinical study using a natural outbred animal model. PLoS Pathog 10:e1003924. doi: 10.1371/journal.ppat.1003924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Day PM, Pang YY, Kines RC, Thompson CD, Lowy DR, Schiller JT. 2012. A human papillomavirus (HPV) in vitro neutralization assay that recapitulates the in vitro process of infection provides a sensitive measure of HPV L2 infection-inhibiting antibodies. Clin Vaccine Immunol 19:1075–1082. doi: 10.1128/CVI.00139-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chackerian B, Frietze KM. 2016. Moving towards a new class of vaccines for non-infectious chronic diseases. Expert Rev Vaccines 15:561–563. doi: 10.1586/14760584.2016.1159136. [DOI] [PubMed] [Google Scholar]
- 42.Schellenbacher C, Roden RB, Kirnbauer R. 2017. Developments in L2-based human papillomavirus (HPV) vaccines. Virus Res 231:166–175. doi: 10.1016/j.virusres.2016.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jiang RT, Schellenbacher C, Chackerian B, Roden RB. 2016. Progress and prospects for L2-based human papillomavirus vaccines. Expert Rev Vaccines 15:853–862. doi: 10.1586/14760584.2016.1157479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pouyanfard S, Muller M. 2017. Human papillomavirus first and second generation vaccines—current status and future directions. Biol Chem 398:871–889. doi: 10.1515/hsz-2017-0105. [DOI] [PubMed] [Google Scholar]
- 45.Caldeira JDC, Medford A, Kines RC, Lino CA, Schiller JT, Chackerian B, Peabody DS. 2010. Immunogenic display of diverse peptides, including a broadly cross-type neutralizing human papillomavirus L2 epitope, on virus-like particles of the RNA bacteriophage PP7. Vaccine 28:4384–4393. doi: 10.1016/j.vaccine.2010.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schellenbacher C, Roden R, Kirnbauer R. 2009. Chimeric L1-L2 virus-like particles as potential broad-spectrum human papillomavirus vaccines. J Virol 83:10085–10095. doi: 10.1128/JVI.01088-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Boxus M, Fochesato M, Miseur A, Mertens E, Dendouga N, Brendle S, Balogh KK, Christensen ND, Giannini SL. 2016. Broad cross-protection is induced in preclinical models by a human papillomavirus vaccine composed of L1/L2 chimeric virus-like particles. J Virol 90:6314–6325. doi: 10.1128/JVI.00449-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nieto K, Weghofer M, Sehr P, Ritter M, Sedlmeier S, Karanam B, Seitz H, Muller M, Kellner M, Horer M, Michaelis U, Roden RB, Gissmann L, Kleinschmidt JA. 2012. Development of AAVLP(HPV16/31L2) particles as broadly protective HPV vaccine candidate. PLoS One 7:e39741. doi: 10.1371/journal.pone.0039741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jagu S, Karanam B, Gambhira R, Chivukula SV, Chaganti RJ, Lowy DR, Schiller JT, Roden RB. 2009. Concatenated multitype L2 fusion proteins as candidate prophylactic pan-human papillomavirus vaccines. J Natl Cancer Inst 101:782–792. doi: 10.1093/jnci/djp106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Castro F, Dirks WG, Fahnrich S, Hotz-Wagenblatt A, Pawlita M, Schmitt M. 2013. High-throughput SNP-based authentication of human cell lines. Int J Cancer 132:308–314. doi: 10.1002/ijc.27675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Roberts JN, Buck CB, Thompson CD, Kines R, Bernardo M, Choyke PL, Lowy DR, Schiller JT. 2007. Genital transmission of HPV in a mouse model is potentiated by nonoxynol-9 and inhibited by carrageenan. Nat Med 13:857–861. doi: 10.1038/nm1598. [DOI] [PubMed] [Google Scholar]
- 52.Garland SM, Steben M, Hernandez-Avila M, Koutsky LA, Wheeler CM, Perez G, Harper DM, Leodolter S, Tang GW, Ferris DG, Esser MT, Vuocolo SC, Nelson M, Railkar R, Sattler C, Barr E. 2007. Noninferiority of antibody response to human papillomavirus type 16 in subjects vaccinated with monovalent and quadrivalent L1 virus-like particle vaccines. Clin Vaccine Immunol 14:792–795. doi: 10.1128/CVI.00478-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Spagnoli G, Pouyanfard S, Cavazzini D, Canali E, Maggi S, Tommasino M, Bolchi A, Müller M, Ottonello S. Broadly neutralizing antiviral responses induced by a single-molecule HPV vaccine based on thermostable thioredoxin-L2 multiepitope nanoparticles. Sci Rep, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]