Abstract
The precise mechanism by which propofol enhances GABAergic transmission remains unclear, but much progress has been made regarding the underlying structural and dynamic mechanisms. Furthermore, it is now clear that propofol has additional molecular targets, many of which are functionally influenced at concentrations achieved clinically. Focusing primarily on molecular targets, this brief review attempts to summarize some of this recent progress while pointing out knowledge gaps and controversies. It is not intended to be comprehensive but rather to stimulate further thought, discussion, and study on the mechanisms by which propofol produces its pleiotropic effects.
Keywords: propofol, ligand-gated ion channels, GABA, voltage-gated ion channels
Introduction
Propofol is now the most commonly used general anesthesia induction agent in the world and is being used for total intravenous general anesthesia and sedation with increasing frequency. It is well tolerated and may be associated with fewer post-anesthesia effects than many of our other general anesthetics, factors that have undoubtedly contributed to its entrenched position in various clinical applications. However, propofol has adverse effects, including pain on injection, hypotension, hypoventilation, bradycardia, and hyperlipidemia 1. Aside from clinical usage, propofol is being used extensively in basic neuroscience research to better understand consciousness, memory, and learning 2– 4. For example, a recent study reveals a previously unknown mechanism of unconscious memory under propofol anesthesia, suggesting that general anesthesia acts at stages beyond cellular coding to disrupt sensory integration for higher-order association 2.
The success of this drug is all the more remarkable given how little we know of its full spectrum of molecular targets and actions. Although it has long been considered to be dominantly γ-aminobutyric acid-ergic (GABAergic) 5, 6, recent research has indicated that it is not exclusively so and in fact may rely on other molecular targets to produce even its principal desired effect: unconsciousness or hypnosis. This review will briefly cover recent research on how propofol functions on ligand-gated ion channels and then cover other recently revealed molecular targets.
Ligand-gated ion channels
Pentameric ligand-gated ion channels (pLGICs), particularly GABA type A receptors (GABA ARs), have been extensively investigated as molecular targets for propofol in the past 7– 9. Propofol typically inhibits cation-selective pLGICs, including the nicotinic acetylcholine receptor (nAChR) 10– 13 and prokaryotic homologues from Gloeobacter violaceus (GLIC) 14, 15 and Erwinia chrysanthemi (ELIC) 16, 17. In contrast, propofol at clinical concentrations potentiates agonist-evoked currents of the anion-selective GABA ARs and glycine receptors (GlyRs) and increases the frequency of channel opening 5, 6, 18, 19. Propofol can also directly activate GABA ARs at intermediate concentrations, but then inhibit conductance at high, supra-clinical concentrations 6, 19. Exploration of the structural basis of propofol action on pLGICs is still at an early stage. Only a few crystal structures or cryo-electron microscopy structures of eukaryotic pLGICs have been determined 20– 24, and none of these structures includes anesthetics. In contrast, propofol and other general anesthetics as well as alcohols have been successfully co-crystallized with the prokaryotic GLIC 25, 26 and ELIC 17, 27– 29.
The crystal structure of GLIC in a presumed open/desensitized state shows propofol localized to an intra-subunit pocket at the extracellular end of the transmembrane domain α-helices 25. Photoaffinity labeling of a propofol analogue to purified GLIC in solution leads to the same propofol location as observed in the crystal structure 30. This intra-subunit transmembrane pocket is probably a common anesthetic-binding site for inhibition of pLGICs. For example, desflurane binds close to this location in GLIC 25, and propofol inhibits ELIC via binding to the equivalent site 31. Similarly, propofol 32, etomidate 33, 34, and halothane 35 also bind to an intra-subunit site within the δ subunit helix bundle of the eukaryotic nAChR, another pLGIC that is inhibited by anesthetics.
A significant role of β3GABA AR in behavioral effects of propofol and etomidate has been demonstrated by the reduced sensitivity of β3(N265M) mice 36, and considerable progress has been made in revealing the underlying mechanisms 36– 43. Two recent articles 42, 43 have described sites for propofol in expressed αβGABA AR, albeit with somewhat different locations. In one case, ortho-propofol diazirine ( o-PD) was found to adduct to a β3 residue at the transmembrane/extracellular domain interface 43 while azi-propofol meta (aziP m) formed an adduct with residues deeper in the α1β3 transmembrane domain interface 42. Differences are likely due to different photochemistry of o-PD and aziP m. The less thermally stable o-PD is thought to undergo more complex chemistry on illumination and produce a longer-lived reactive intermediate than the carbene generated by illumination of aziP m. This more stable and likely more hydrophilic o-PD photoproduct then has more time to seek preferential photochemistry partners, such as the β3 histidine267 residue that may line the pore. A means of distinguishing such photochemical artifacts from specific binding is an ability of the parent drug to inhibit or “protect” the candidate site from photoadduction. Thus, aziP m photoadduction was indeed inhibited by propofol, but it is not reported whether o-PD was. Another important difference is the milieu in which the receptor resides during photolabeling. For the aziP m studies, the expressed αβ GABA AR resides in a detergent/lipid mixture, but the o-PD studies were performed with the recombinant receptor in Spodoptera frugiperda (Sf9) cell lipid membranes. Neither milieu is perfectly matched to the native neuronal membranes in which GABA AR normally resides. Thus, further studies will be required to give confidence that these sites are indeed physiologically relevant.
There are often multiple anesthetic-binding sites for a given anesthetic in a particular protein 31, 34, 42, 44– 46. The existence of multiple anesthetic sites has introduced a more challenging question: which specific site or sites among all those identified are responsible for the functional modulation? An effective way to answer this question is to use chimeras containing domains from different channels that have opposite responses to anesthetics. For example, photolabeling of ELIC with the light-activated derivative of propofol, aziP m 47, identified multiple aziP m-binding sites in the extracellular domain and one intra-subunit site in the transmembrane domain 31. To determine the functionally relevant propofol-binding site(s), we constructed an ELIC–GABA AR chimera 16, 28, 31 that contains the ELIC extracellular domain and the transmembrane domain of α1β3GABA AR. In contrast to inhibiting ELIC, propofol potentiates the ELIC–GABA AR chimera as it does on α1β3GABA AR. These results support a functionally dominant propofol-binding site in the transmembrane domain of ELIC 16, 31. In heteromeric GABA ARs, propofol binds to multiple allosteric sites at the transmembrane β–α and α–β interfaces 37, 42. Both potentiation and direct activation of GABA ARs are mediated by the same propofol-binding sites 48, but it is not clear whether one site plays a more critical role than others. It is also important to realize that propofol binding affects, not only channel activity but also receptor assembly and trafficking 49, 50.
A homomeric pLGIC, such as GLIC, has five identical subunits and five equivalent binding sites of each type; the crystal structure shows five propofol molecules bound symmetrically in the transmembrane domain 25. Is full occupancy of all five sites necessary for functional modulation? Molecular dynamic simulations suggest that asymmetric binding of propofol to only one, two, or three GLIC sites accelerated channel dehydration, increased conformational heterogeneity of the pore-lining helices, and shifted GLIC toward a closed-channel conformation 51 as compared with symmetrical (five-site) occupancy. Similarly, in homo-pentameric pLGICs, occupancy of from one to three sites by anesthetics has been found to be sufficient to potentiate channel currents 52– 55. Thus, it appears that maximum functional effects are produced by asymmetric occupancy of these channels.
The effect of propofol binding on channel function is largely determined by the intrinsic dynamics of the channel. The cation-conducting GLIC is inhibited by clinically relevant concentrations of general anesthetics 14, 15. The introduction of three mutations at the selectivity filter and one at the hydrophobic gate converted wild-type (wt) GLIC into GLIC4, an anion channel 15. None of the mutated residues is within the propofol-binding pocket, so propofol binding is probably the same in both GLIC4 and wtGLIC 25, 30, 31. Nevertheless, propofol is unable to inhibit GLIC4 as it does wtGLIC 15. Molecular dynamic simulations revealed that, compared with wtGLIC’s pore, GLIC4’s pore was more resistant to perturbation from propofol binding 15. These results underscore the importance of pore dynamics and conformation in ligand modulation of channel functions.
Other molecular targets of propofol
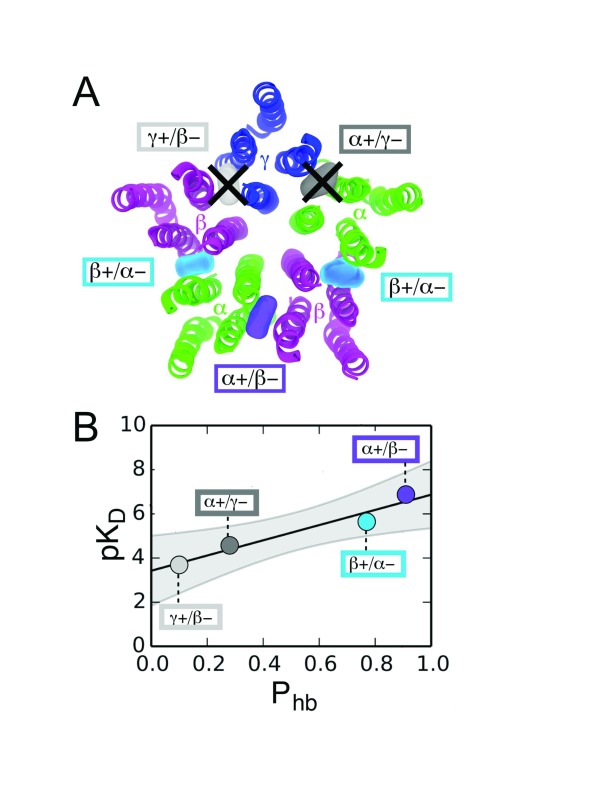
The search for other molecular targets has occurred in three principal ways. The first approach examined the transcriptomic, proteomic, or metabolomic response to propofol exposure in cells or intact animals. Depending on the analysis of the data, this approach has led to hundreds of potential molecular substrates 56. Because it is rarely clear whether, or how many of, these substrates are actually direct molecular binding targets of propofol, additional studies are required; some have been reported. In the second approach, propofol was altered to allow it to covalently bind to its targets on exposure to ultraviolet light (photolabeling), thereby serving as a tag. Tagged proteins then are discovered by using a variety of other technologies. In some cases, the tag is a radiolabel on the altered anesthetic, and scintillation counting and mass spectrometry (MS) are used to detect and identify adducted proteins. More commonly today, the tag is simply the additional mass of the adduct, and tagged proteins can be identified with shotgun MS approaches. The Achilles heel of this approach is that the identified targets are heavily biased toward the most abundant proteins, such as mitochondrial complexes, tubulin, and voltage-dependent anion channels 57. In fact, known targets of propofol, such as GABA AR (above), were not detected and this is presumably because of their low abundance. Thus, in the third approach, photolabeling approaches were refined to include another functional group on the aziP m molecule, in this case an alkyne (in place of the remaining isopropyl), in order to attach subsequent groups after photolabeling by using “click” chemistry 58. Remarkably, this heavily modified clickable photoactive derivative of propofol was still a potent general anesthetic and was still GABAergic 59. Deploying this “aziP m-click” compound involved first photolabeling in the presence of synaptosomes and then using click chemistry to attach a biotin moiety to the propofol analogue now covalently bound to its direct binding targets. An avidin column captured all of these biotin-decorated proteins and then in-column proteolysis released peptides, which were identified by using MS. The results were startling. From a total of about 4,500 proteins identified by MS in crude synaptosomes, about 12% (540) were captured by using the click strategy, and, of these, about 200 were deemed to be “propofol specific” on the basis of propofol protection assays. Although many captured proteins may be simply associated with propofol targets and not themselves true propofol-binding targets, it is clear that propofol is a promiscuous drug, and dozens and perhaps hundreds of molecular targets are influenced at clinical concentrations. In contrast to the results of the simple “shotgun” approach, several of the captured proteins were low-abundance ion channels and, gratifyingly, several synaptic GABA AR subunits. This is the first demonstration of direct propofol binding to GABA AR when still in its native synaptic environment. Curiously, only α and β subunits were captured, despite the relative abundance of the γ subunit in synapses. Subsequent molecular dynamic simulations provided a plausible explanation. The γ–β and γ–α interfaces were overly hydrated as compared with the α–β ones, indicating that water was competing with binding of the propofol analogue. This aligns with results discussed above in that the resulting asymmetric binding pattern around the heteropentamer ( Figure 1) should be more functionally provocative than a symmetric one where all interfaces are occupied 51. Moreover, this implies the importance of H-bonding within the interfacial sites, a hypothesis directly tested with a propofol variant that replaced the hydroxyl with an isosteric fluorine. The resulting “fropofol” molecule has physicochemical properties very similar to those of propofol but no anesthetic or sedative properties whatsoever. This highlights the importance of the hydroxyl in functionally relevant targets 60 like the GABA AR. Fropofol should be distinguished from other non-immobilizers (for example, the cyclobutanes 61), as those molecules had such pronounced physicochemical differences that a lack of activity was almost certainly due to very low water solubility. Fropofol, however, still has significant effects on myocardial (but not skeletal) contractility, indicating that these targets lack an important H-bond in their sites 60. This is one of the first demonstrations that primary and side effects are separable through fairly minor changes to the alkylphenol molecule.
Figure 1. Asymmetric occupancy of the αβγGABA A receptor by propofol.
( A) Photolabeling has identified interfacial binding sites for propofol (colored blobs) in the transmembrane region (seen from the extracellular view here). Furthermore, several lines of evidence now suggest that asymmetric occupancy of these sites confers a larger change in activity than symmetric occupancy (all five subunits). Click-enabled propofol analogues have confirmed asymmetric occupancy of αβγGABA A receptor sites in their native, unperturbed state in that only α and β subunits were photoadducted. The mechanism in the case of this heteropentamer is differential affinity of the interfaces. ( B) The relationship between H-bond probability (P hb) and affinity (pK D) from molecular dynamic simulations shows where each interfacial binding site lies. The two γ-containing interfaces have a much lower P hb and therefore lower affinity.
Voltage-gated ion channels
Monovalent voltage-gated ion channels (VGICs), which are necessary for setting membrane potential and the initiation and propagation of action potentials, are plausible targets of anesthetics. In general, potassium channels are relatively insensitive to propofol 62, although most inhalational anesthetics and alcohols inhibit them 63. Sodium channels, on the other hand, appear to be inhibited by clinically relevant propofol concentrations 64. The precise mechanisms are not yet clear, but evidence for anesthetic sites on these VGICs exists. Propofol and sevoflurane sites in the simpler Kvx channels have been revealed by photolabeling studies 62, 65, while evidence for propofol sites in the more complex Navx channels has been largely through molecular modeling and mutagenesis, although isoflurane sites have been revealed via nuclear magnetic resonance studies 66. The data suggest at least two general classes of anesthetic sites. First, binding to an important “hinge” site, located between the voltage sensor and the pore domain (S4–5 linker) at the intracellular membrane surface, can alter activity in either direction 63, and, second, the pore domain itself.
The pore is an amphiphilic environment fairly well suited to anesthetics, occupancy of which would be expected to produce inhibition. The direction of modulation in these many examples of Kvx and Navx will then depend on relative affinity, and therefore occupancy, of each of these sites.
HCN-1
The importance of the hyperpolarization-activated cyclic nucleotide-regulated (HCN) channels to propofol actions was demonstrated by a diminished sensitivity to propofol in HCN1-knockout mice 67. Moreover, HCN channels are thought to, in part, underlie the afferent hyperexcitability resulting in neuropathic pain. Propofol and related alkylphenols inhibit the HCN-1 channel at subhypnotic concentrations, making the alkylphenol a reasonable chemotype for further medicinal chemistry to create an analgesic drug with less hypnotic potency 68– 71. This recent work suggests that increasing the bulk and hydrophobicity of the alkylphenol side chains (for example, 2,6-Di-tert-butylphenol) accomplishes this to some degree, lending confidence that an entirely novel class of analgesic, useful for neuropathic pain, may emerge from the alkylphenol chemotype 72. Binding sites and mechanisms by which the alkylphenols inhibit this channel are not yet clear, but the recent publication of a cryo-electron microscopy structure 73 should facilitate work in the area.
Kinesin
Building on work over the previous two decades which implicated actin, tubulin, and other cellular or synaptic cytoskeleton and transport machinery in general anesthetic action 74– 76, investigators recently examined the effects of propofol on the anterograde motor kinesin. Kinesin is like a locomotive that runs along microtubule “tracks” by using its dimeric motor heads to step from β to β tubulin subunit 77. Should these tracks be decorated with bound anesthetic molecules, it is plausible that motor function and ultimately transport would be altered. In single-molecule experiments, where, instead of its usual cargo, kinesin is attached to a fluorescent bead and microtubules are immobilized on a substrate, it was noticed that clinically relevant concentrations of propofol reduced run length by half and had no effect on velocity 78. In other words, the train goes just as fast but derails. This might indicate extensive tubulin binding of propofol, but could also be due to effects within kinesin itself. If the latter, the single-molecule results suggest that the propofol site is allosteric to the nucleotide-binding site and that it is not influencing ATPase activity. Most likely, an allosteric site in kinesin is formed when the motor head binds to β tubulin. In either case, though, the result is the same, and cargo is transported by these anterograde kinesins reaching their destinations more slowly or not at all. Depending on redundancy, this may have important consequences in terms of the critical timing of cellular/neuronal activity. Applying the anesthetic-null variant fropofol (see above) had no effect, lending some credence to the possibility that kinesin modulation underlies propofol-induced unconsciousness. It is also plausible that an interaction between kinesin and propofol underlies a number of propofol’s less desirable actions, such as neurotoxicity at the extremes of age 79.
TRPx receptors
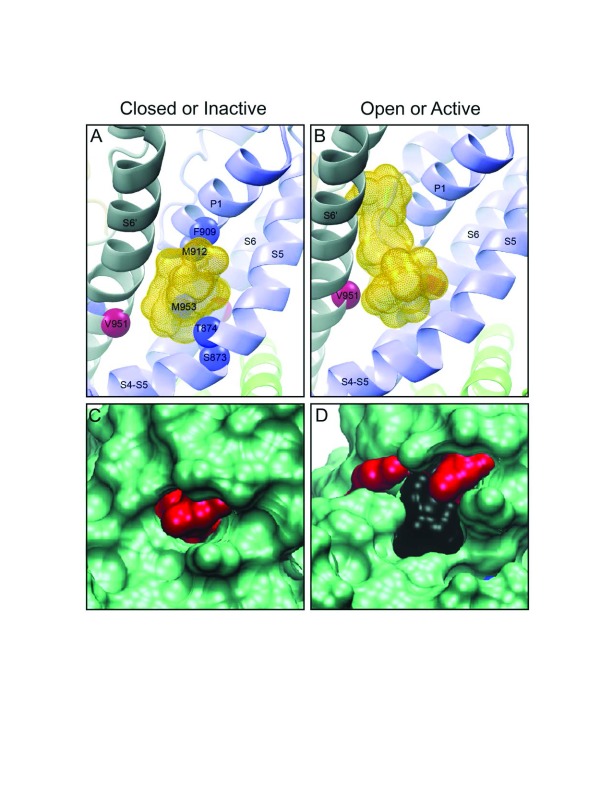
It is well known that propofol both causes pain on injection and sensitizes nociception. The candidate molecular targets for transducing these effects are the widely distributed TRPA and TRPV receptors 80, 81. TRPA-1, in particular, is known to be modulated by propofol in a biphasic manner: activated at low, clinically relevant concentrations and inhibited at higher concentrations. The mechanism or mechanisms by which propofol produces these actions are suggested by recent mutagenesis 82 and photolabeling results with aziP m 83. For example, the canonical “hinge” region of these channels, connecting sensor to pore domains and typically located in and around the cytoplasmic face of the transmembrane region, forms a binding site whereby, when occupied, the open state is stabilized, enhancing current flow ( Figure 2). However, when the channel is in the open state, a lower-affinity pore site is created which becomes progressively occupied as the propofol concentration is raised, reducing current flow. This sequential occupancy of two sites of differing affinity and effect is presumably responsible for the bimodal modulation of the TRPA-1 receptor and may be a general model for actions on other channels. Since activation of a nociceptor is not a desirable attribute of a general anesthetic, it would be important to determine characteristics of the binding site in order to use medicinal chemistry approaches to weaken binding to this activating site. This may not only reduce pain of injection but also increase anesthetic potency, depending on the central nervous system (CNS) distribution of the TRPA-1 receptor.
Figure 2. Recent examples of propofol-binding proteins.
In both the TRPA1 ( A, B) and SIRT2 ( C, D), note that the propofol-enhanced active or open state ( B, D) suggests that the alkylphenol-binding pocket is actually enlarged somewhat as compared with the closed or inactive state ( A, C). We believe that this is an example of enhanced affinity as a result of reduction in the entropic penalties of binding and may be a common feature in conformationally sensitive binding, a form of “induced fit”. The yellow stippled shapes in ( A) and ( B) represent multiple poses of both propofol and azipropofol when docked in the vicinity of the photolabeled residues. In ( C) and ( D), the red shapes are photolabeled residues and the black shape is the surface rendering of the cavity occupied by propofol.
SIRT-2 deacetylase
On evaluation of the propofol-binding proteome of various CNS tissues, it was found that myelin contained a highly specific protein target of propofol that was ultimately determined to be the SIRT-2 deacetylase 84. The acetylase and deacetylase enzymes are key intracellular regulators of a wide variety of events 85; thus, an effect of anesthetics would be expected to have far-reaching consequences. Since a binding target does not necessarily indicate a functional target, a number of experiments were conducted to determine whether propofol had an effect on SIRT-2 deacetylase activity and at a clinically relevant concentration. Indeed, when isolated SIRT-2 was exposed to low-micromolar concentrations of propofol, its activity was significantly inhibited, and the structural mechanism was characterized ( Figure 2) 84. Though unlikely to underlie the primary, desired action of propofol, this interaction may underlie one or more of the many side effects of propofol. Since acetylation and methylation systems are responsible for epigenetic regulation, this interaction could even be responsible for delayed or persistent effects of propofol, such as developmental neuromodulation 79.
Summary
Much progress has been made in understanding the mechanisms of propofol’s actions on its canonical molecular target, the GABA AR, but a role for other molecular targets is gaining momentum. Propofol is undeniably a promiscuous anesthetic ligand, and its diverse molecular targets are integrated in a manner that is still poorly characterized. Finally, evidence suggests that further medicinal chemistry of the alkylphenol chemotype may allow enhancement of selected actions.
Editorial Note on the Review Process
F1000 Faculty Reviews are commissioned from members of the prestigious F1000 Faculty and are edited as a service to readers. In order to make these reviews as comprehensive and accessible as possible, the referees provide input before publication and only the final, revised version is published. The referees who approved the final version are listed with their names and affiliations but without their reports on earlier versions (any comments will already have been addressed in the published version).
The referees who approved this article are:
Stuart A. Forman, Dept. of Anesthesia Critical Care & Pain Medicine, Massachusetts General Hospital, Massachusetts, USA
Eintrei Christina, Linköping University, Linköping, Sweden
Tim Hales, University of Dundee, Dundee, UK
Gustav Akk, Department of Anesthesiology, Washington University School of Medicine, St Louis, MO, USA
Funding Statement
The research was supported by funding from the NIH (Pei Tang, R01GM056257 and R01GM066358; Roderic G. Eckenhoff, P01GM55876). The funders had no role in the decision to prepare or publish this review article.
[version 1; referees: 4 approved]
References
- 1. Vuyk J, Sitsen E, Reekers M: Chapter 30 Intravenous Anesthetics. Miller’s Anesthesia. 8th edition. Edited by Miller RD, Cohen, N.H., Eriksson, L.I., Fleisher, L.A., Weiner-Kronish, J.P., Young, W.L. Philadelphia, Elsevier,2015;821–863. Reference Source [Google Scholar]
- 2. Jia L, Tang P, Brandon NR, et al. : Effects of Propofol General Anesthesia on Olfactory Relearning. Sci Rep. 2016;6: 33538. 10.1038/srep33538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Purdon PL, Pierce ET, Mukamel EA, et al. : Electroencephalogram signatures of loss and recovery of consciousness from propofol. Proc Natl Acad Sci USA. 2013;110(12):E1142–51. 10.1073/pnas.1221180110 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 4. Schrouff J, Perlbarg V, Boly M, et al. : Brain functional integration decreases during propofol-induced loss of consciousness. Neuroimage. 2011;57(1):198–205. 10.1016/j.neuroimage.2011.04.020 [DOI] [PubMed] [Google Scholar]
- 5. Hales TG, Lambert JJ: The actions of propofol on inhibitory amino acid receptors of bovine adrenomedullary chromaffin cells and rodent central neurones. Br J Pharmacol. 1991;104(3):619–28. 10.1111/j.1476-5381.1991.tb12479.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Orser BA, Wang LY, Pennefather PS, et al. : Propofol modulates activation and desensitization of GABAA receptors in cultured murine hippocampal neurons. J Neurosci. 1994;14(12):7747–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Franks NP, Lieb WR: Molecular and cellular mechanisms of general anaesthesia. Nature. 1994;367(6464):607–14. 10.1038/367607a0 [DOI] [PubMed] [Google Scholar]
- 8. Hemmings HC , Jr, Akabas MH, Goldstein PA, et al. : Emerging molecular mechanisms of general anesthetic action. Trends Pharmacol Sci. 2005;26(10):503–10. 10.1016/j.tips.2005.08.006 [DOI] [PubMed] [Google Scholar]
- 9. Forman SA, Miller KW: Anesthetic sites and allosteric mechanisms of action on Cys-loop ligand-gated ion channels. Can J Anaesth. 2011;58(2):191–205. 10.1007/s12630-010-9419-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Forman SA, Chiara DC, Miller KW: Anesthetics target interfacial transmembrane sites in nicotinic acetylcholine receptors. Neuropharmacology. 2015;96(Pt B):169–77. 10.1016/j.neuropharm.2014.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Flood P, Ramirez-Latorre J, Role L: Alpha 4 beta 2 neuronal nicotinic acetylcholine receptors in the central nervous system are inhibited by isoflurane and propofol, but alpha 7-type nicotinic acetylcholine receptors are unaffected. Anesthesiology. 1997;86(4):859–65. 10.1097/00000542-199704000-00016 [DOI] [PubMed] [Google Scholar]
- 12. Violet JM, Downie DL, Nakisa RC, et al. : Differential sensitivities of mammalian neuronal and muscle nicotinic acetylcholine receptors to general anesthetics. Anesthesiology. 1997;86(4):866–74. 10.1097/00000542-199704000-00017 [DOI] [PubMed] [Google Scholar]
- 13. Dilger JP, Vidal AM, Mody HI, et al. : Evidence for direct actions of general anesthetics on an ion channel protein. A new look at a unified mechanism of action. Anesthesiology. 1994;81(2):431–42. [DOI] [PubMed] [Google Scholar]
- 14. Weng Y, Yang L, Corringer PJ, et al. : Anesthetic sensitivity of the Gloeobacter violaceus proton-gated ion channel. Anesth Analg. 2010;110(1):59–63. 10.1213/ANE.0b013e3181c4bc69 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 15. Tillman T, Cheng MH, Chen Q, et al. : Reversal of ion-charge selectivity renders the pentameric ligand-gated ion channel GLIC insensitive to anaesthetics. Biochem J. 2013;449(1):61–8. 10.1042/BJ20121072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tillman TS, Seyoum E, Mowrey DD, et al. : ELIC-α7 Nicotinic acetylcholine receptor (α7nAChR) chimeras reveal a prominent role of the extracellular-transmembrane domain interface in allosteric modulation. J Biol Chem. 2014;289(20):13851–7. 10.1074/jbc.M113.524611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chen Q, Kinde MN, Arjunan P, et al. : Direct Pore Binding as a Mechanism for Isoflurane Inhibition of the Pentameric Ligand-gated Ion Channel ELIC. Sci Rep. 2015;5: 13833. 10.1038/srep13833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sanna E, Garau F, Harris RA: Novel properties of homomeric beta 1 gamma-aminobutyric acid type A receptors: actions of the anesthetics propofol and pentobarbital. Mol Pharmacol. 1995;47(2):213–7. [PubMed] [Google Scholar]
- 19. Adodra S, Hales TG: Potentiation, activation and blockade of GABA A receptors of clonal murine hypothalamic GT1-7 neurones by propofol. Br J Pharmacol. 1995;115(6):953–60. 10.1111/j.1476-5381.1995.tb15903.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Morales-Perez CL, Noviello CM, Hibbs RE: X-ray structure of the human α4β2 nicotinic receptor. Nature. 2016;538(7625):411–5. 10.1038/nature19785 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 21. Hassaine G, Deluz C, Grasso L, et al. : X-ray structure of the mouse serotonin 5-HT 3 receptor. Nature. 2014;512(7514):276–81. 10.1038/nature13552 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 22. Miller PS, Aricescu AR: Crystal structure of a human GABA A receptor. Nature. 2014;512(7514):270–5. 10.1038/nature13293 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 23. Huang X, Chen H, Michelsen K, et al. : Crystal structure of human glycine receptor-α3 bound to antagonist strychnine. Nature. 2015;526(7572):277–80. 10.1038/nature14972 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 24. Du J, Lü W, Wu S, et al. : Glycine receptor mechanism elucidated by electron cryo-microscopy. Nature. 2015;526(7572):224–9. 10.1038/nature14853 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 25. Nury H, Van Renterghem C, Weng Y, et al. : X-ray structures of general anaesthetics bound to a pentameric ligand-gated ion channel. Nature. 2011;469(7330):428–31. 10.1038/nature09647 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 26. Sauguet L, Howard RJ, Malherbe L, et al. : Structural basis for potentiation by alcohols and anaesthetics in a ligand-gated ion channel. Nat Commun. 2013;4:1697. 10.1038/ncomms2682 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 27. Pan J, Chen Q, Willenbring D, et al. : Structure of the pentameric ligand-gated ion channel GLIC bound with anesthetic ketamine. Structure. 2012;20(9):1463–9. 10.1016/j.str.2012.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chen Q, Wells MM, Tillman TS, et al. : Structural Basis of Alcohol Inhibition of the Pentameric Ligand-Gated Ion Channel ELIC. Structure. 2017;25(1):180–7. 10.1016/j.str.2016.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Spurny R, Billen B, Howard RJ, et al. : Multisite binding of a general anesthetic to the prokaryotic pentameric Erwinia chrysanthemi ligand-gated ion channel (ELIC). J Biol Chem. 2013;288(12):8355–64. 10.1074/jbc.M112.424507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chiara DC, Gill JF, Chen Q, et al. : Photoaffinity labeling the propofol binding site in GLIC. Biochemistry. 2014;53(1):135–42. 10.1021/bi401492k [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kinde MN, Bu W, Chen Q, et al. : Common Anesthetic-binding Site for Inhibition of Pentameric Ligand-gated Ion Channels. Anesthesiology. 2016;124(3):664–73. 10.1097/ALN.0000000000001005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jayakar SS, Dailey WP, Eckenhoff RG, et al. : Identification of propofol binding sites in a nicotinic acetylcholine receptor with a photoreactive propofol analog. J Biol Chem. 2013;288(9):6178–89. 10.1074/jbc.M112.435909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chiara DC, Hong FH, Arevalo E, et al. : Time-resolved photolabeling of the nicotinic acetylcholine receptor by [ 3H]azietomidate, an open-state inhibitor. Mol Pharmacol. 2009;75(5):1084–95. 10.1124/mol.108.054353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hamouda AK, Stewart DS, Husain SS, et al. : Multiple transmembrane binding sites for p-trifluoromethyldiazirinyl-etomidate, a photoreactive Torpedo nicotinic acetylcholine receptor allosteric inhibitor. J Biol Chem. 2011;286(23):20466–77. 10.1074/jbc.M111.219071 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 35. Chiara DC, Dangott LJ, Eckenhoff RG, et al. : Identification of nicotinic acetylcholine receptor amino acids photolabeled by the volatile anesthetic halothane. Biochemistry. 2003;42(46):13457–67. 10.1021/bi0351561 [DOI] [PubMed] [Google Scholar]
- 36. Jurd R, Arras M, Lambert S, et al. : General anesthetic actions in vivo strongly attenuated by a point mutation in the GABA A receptor beta3 subunit. FASEB J. 2003;17(2):250–2. 10.1096/fj.02-0611fje [DOI] [PubMed] [Google Scholar]
- 37. Eaton MM, Germann AL, Arora R, et al. : Multiple Non-Equivalent Interfaces Mediate Direct Activation of GABA A Receptors by Propofol. Curr Neuropharmacol. 2016;14(7):772–80. 10.2174/1570159X14666160202121319 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 38. Forman SA, Miller KW: Mapping General Anesthetic Sites in Heteromeric γ-Aminobutyric Acid Type A Receptors Reveals a Potential For Targeting Receptor Subtypes. Anesth Analg. 2016;123(5):1263–73. 10.1213/ANE.0000000000001368 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 39. Nourmahnad A, Stern AT, Hotta M, et al. : Tryptophan and Cysteine Mutations in M1 Helices of α1β3γ2L γ-Aminobutyric Acid Type A Receptors Indicate Distinct Intersubunit Sites for Four Intravenous Anesthetics and One Orphan Site. Anesthesiology. 2016;125(6):1144–58. 10.1097/ALN.0000000000001390 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 40. Chiara DC, Jounaidi Y, Zhou X, et al. : General Anesthetic Binding Sites in Human α4β3δ γ-Aminobutyric Acid Type A Receptors (GABA ARs). J Biol Chem. 2016;291(51):26529–39. 10.1074/jbc.M116.753335 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 41. Eaton MM, Cao LQ, Chen Z, et al. : Mutational Analysis of the Putative High-Affinity Propofol Binding Site in Human β3 Homomeric GABA A Receptors. Mol Pharmacol. 2015;88(4):736–45. 10.1124/mol.115.100347 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 42. Jayakar SS, Zhou X, Chiara DC, et al. : Multiple propofol-binding sites in a γ-aminobutyric acid type A receptor (GABA AR) identified using a photoreactive propofol analog. J Biol Chem. 2014;289(40):27456–68. 10.1074/jbc.M114.581728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yip GM, Chen ZW, Edge CJ, et al. : A propofol binding site on mammalian GABA A receptors identified by photolabeling. Nat Chem Biol. 2013;9(11):715–20. 10.1038/nchembio.1340 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 44. Bondarenko V, Mowrey D, Liu LT, et al. : NMR resolved multiple anesthetic binding sites in the TM domains of the α4β2 nAChR. Biochim Biophys Acta. 2013;1828(2):398–404. 10.1016/j.bbamem.2012.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bondarenko V, Mowrey DD, Tillman TS, et al. : NMR structures of the human α7 nAChR transmembrane domain and associated anesthetic binding sites. Biochim Biophys Acta. 2014;1838(5):1389–95. 10.1016/j.bbamem.2013.12.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Chen Q, Cheng MH, Xu Y, et al. : Anesthetic binding in a pentameric ligand-gated ion channel: GLIC. Biophys J. 2010;99(6):1801–9. 10.1016/j.bpj.2010.07.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hall MA, Xi J, Lor C, et al. : m-Azipropofol (AziP m) a photoactive analogue of the intravenous general anesthetic propofol. J Med Chem. 2010;53(15):5667–75. 10.1021/jm1004072 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 48. Ruesch D, Neumann E, Wulf H, et al. : An allosteric coagonist model for propofol effects on α1β2γ2L γ-aminobutyric acid type A receptors. Anesthesiology. 2012;116(1):47–55. 10.1097/ALN.0b013e31823d0c36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Li Y, Wu Y, Li R, et al. : Propofol Regulates the Surface Expression of GABAA Receptors: Implications in Synaptic Inhibition. Anesth Analg. 2015;121(5):1176–83. 10.1213/ANE.0000000000000884 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 50. Speigel I, Bichler EK, García PS: The Influence of Regional Distribution and Pharmacologic Specificity of GABA AR Subtype Expression on Anesthesia and Emergence. Front Syst Neurosci. 2017;11:58. 10.3389/fnsys.2017.00058 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 51. Mowrey D, Cheng MH, Liu LT, et al. : Asymmetric ligand binding facilitates conformational transitions in pentameric ligand-gated ion channels. J Am Chem Soc. 2013;135(6):2172–80. 10.1021/ja307275v [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 52. Roberts MT, Phelan R, Erlichman BS, et al. : Occupancy of a single anesthetic binding pocket is sufficient to enhance glycine receptor function. J Biol Chem. 2006;281(6):3305–11. 10.1074/jbc.M502000200 [DOI] [PubMed] [Google Scholar]
- 53. Rayes D, De Rosa MJ, Sine SM, et al. : Number and locations of agonist binding sites required to activate homomeric Cys-loop receptors. J Neurosci. 2009;29(18):6022–32. 10.1523/JNEUROSCI.0627-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Beato M, Groot-Kormelink PJ, Colquhoun D, et al. : The activation mechanism of alpha1 homomeric glycine receptors. J Neurosci. 2004;24(4):895–906. 10.1523/JNEUROSCI.4420-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Amin J, Weiss DS: Insights into the activation mechanism of rho1 GABA receptors obtained by coexpression of wild type and activation-impaired subunits. Proc Biol Sci. 1996;263(1368):273–82. 10.1098/rspb.1996.0042 [DOI] [PubMed] [Google Scholar]
- 56. Tang J, Xue Q, Ding H, et al. : Proteomic profiling of the phosphoproteins in the rat thalamus, hippocampus and frontal lobe after propofol anesthesia. BMC Anesthesiol. 2014;14:3. 10.1186/1471-2253-14-3 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 57. Weiser BP, Kelz MB, Eckenhoff RG: In vivo activation of azipropofol prolongs anesthesia and reveals synaptic targets. J Biol Chem. 2013;288(2):1279–85. 10.1074/jbc.M112.413989 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 58. Meghani NM, Amin HH, Lee BJ: Mechanistic applications of click chemistry for pharmaceutical drug discovery and drug delivery. Drug Discov Today. 2017;22(11):1604–1619. 10.1016/j.drudis.2017.07.007 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 59. Woll KA, Murlidaran S, Pinch BJ, et al. : A Novel Bifunctional Alkylphenol Anesthetic Allows Characterization of γ-Aminobutyric Acid, Type A (GABA A), Receptor Subunit Binding Selectivity in Synaptosomes. J Biol Chem. 2016;291(39):20473–86. 10.1074/jbc.M116.736975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Woll KA, Weiser BP, Liang Q, et al. : Role for the propofol hydroxyl in anesthetic protein target molecular recognition. ACS Chem Neurosci. 2015;6(6):927–35. 10.1021/acschemneuro.5b00078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Burkat PM, Lor C, Perouansky M, et al. : Enhancement of α5-containing γ-aminobutyric acid type A receptors by the nonimmobilizer 1,2-dichlorohexafluorocyclobutane (F6) is abolished by the β3(N265M) mutation. Anesth Analg. 2014;119(6):1277–84. 10.1213/ANE.0000000000000423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Bu W, Liang Q, Zhi L, et al. : Sites and Functional Consequence of Alkylphenol Anesthetic Binding to Kv1.2 Channels. Mol Neurobiol. 2017;1–11. 10.1007/s12035-017-0437-2 [DOI] [PubMed] [Google Scholar]
- 63. Covarrubias M, Barber AF, Carnevale V, et al. : Mechanistic Insights into the Modulation of Voltage-Gated Ion Channels by Inhalational Anesthetics. Biophys J. 2015;109(10):2003–11. 10.1016/j.bpj.2015.09.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ouyang W, Wang G, Hemmings HC, Jr: Isoflurane and propofol inhibit voltage-gated sodium channels in isolated rat neurohypophysial nerve terminals. Mol Pharmacol. 2003;64(2):373–81. 10.1124/mol.64.2.373 [DOI] [PubMed] [Google Scholar]
- 65. Woll KA, Peng W, Liang Q, et al. : Photoaffinity Ligand for the Inhalational Anesthetic Sevoflurane Allows Mechanistic Insight into Potassium Channel Modulation. ACS Chem Biol. 2017;12(5):1353–62. 10.1021/acschembio.7b00222 [DOI] [PubMed] [Google Scholar]
- 66. Kinde MN, Bondarenko V, Granata D, et al. : Fluorine-19 NMR and computational quantification of isoflurane binding to the voltage-gated sodium channel NaChBac. Proc Natl Acad Sci U S A. 2016;113(48):13762–7. 10.1073/pnas.1609939113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Chen X, Shu S, Bayliss DA: HCN1 channel subunits are a molecular substrate for hypnotic actions of ketamine. J Neurosci. 2009;29(3):600–9. 10.1523/JNEUROSCI.3481-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 68. Funahashi M, Mitoh Y, Matsuo R: The sensitivity of hyperpolarization-activated cation current (Ih) to propofol in rat area postrema neurons. Brain Res. 2004;1015(1–2):198–201. 10.1016/j.brainres.2004.04.043 [DOI] [PubMed] [Google Scholar]
- 69. Cacheaux LP, Topf N, Tibbs GR, et al. : Impairment of hyperpolarization-activated, cyclic nucleotide-gated channel function by the intravenous general anesthetic propofol. J Pharmacol Exp Ther. 2005;315(2):517–25. 10.1124/jpet.105.091801 [DOI] [PubMed] [Google Scholar]
- 70. Ying SW, Abbas SY, Harrison NL, et al. : Propofol block of I h contributes to the suppression of neuronal excitability and rhythmic burst firing in thalamocortical neurons. Eur J Neurosci. 2006;23(2):465–80. 10.1111/j.1460-9568.2005.04587.x [DOI] [PubMed] [Google Scholar]
- 71. Tibbs GR, Rowley TJ, Sanford RL, et al. : HCN1 channels as targets for anesthetic and nonanesthetic propofol analogs in the amelioration of mechanical and thermal hyperalgesia in a mouse model of neuropathic pain. J Pharmacol Exp Ther. 2013;345(3):363–73. 10.1124/jpet.113.203620 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 72. Tibbs GR, Posson DJ, Goldstein PA: Voltage-Gated Ion Channels in the PNS: Novel Therapies for Neuropathic Pain? Trends Pharmacol Sci. 2016;37(7):522–42. 10.1016/j.tips.2016.05.002 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 73. Lee CH, Mackinnon R: Structures of the Human HCN1 Hyperpolarization-Activated Channel. Cell. 2017;168(1–2):111–120.e11. 10.1016/j.cell.2016.12.023 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 74. Emerson DJ, Weiser BP, Psonis J, et al. : Direct modulation of microtubule stability contributes to anthracene general anesthesia. J Am Chem Soc. 2013;135(14):5389–98. 10.1021/ja311171u [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Craddock TJ, St George M, Freedman H, et al. : Computational predictions of volatile anesthetic interactions with the microtubule cytoskeleton: implications for side effects of general anesthesia. PLoS One. 2012;7(6):e37251. 10.1371/journal.pone.0037251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Turina D, Gerhardsson H, Bjornstrom K: Orexin A reverses propofol and thiopental induced cytoskeletal rearrangement in rat neurons. J Physiol Pharmacol. 2014;65(4):531–41. [PubMed] [Google Scholar]; F1000 Recommendation
- 77. Verhey KJ, Kaul N, Soppina V: Kinesin assembly and movement in cells. Annu Rev Biophys. 2011;40:267–88. 10.1146/annurev-biophys-042910-155310 [DOI] [PubMed] [Google Scholar]
- 78. Bensel BM, Guzik-Lendrum S, Masucci EM, et al. : Common general anesthetic propofol impairs kinesin processivity. Proc Natl Acad Sci U S A. 2017;114(21):E4281–E4287. 10.1073/pnas.1701482114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Eckenhoff RG, Jevtovic-Todorovic V: Chapter 15 Perioperative and Anesthesia Neurotoxicity. Miller's AnesthesiaEdited by Miller RD, Cohen, N.H., Eriksson, L.I., Fleisher, L.A., Weiner-Kronish, J.P., Young, W.L. Philadelphia, Elsevier,2015;329–346. Reference Source [Google Scholar]
- 80. Fischer MJ, Leffler A, Niedermirtl F, et al. : The general anesthetic propofol excites nociceptors by activating TRPV1 and TRPA1 rather than GABA A receptors. J Biol Chem. 2010;285(45):34781–92. 10.1074/jbc.M110.143958 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 81. Matta JA, Cornett PM, Miyares RL, et al. : General anesthetics activate a nociceptive ion channel to enhance pain and inflammation. Proc Natl Acad Sci U S A. 2008;105(25):8784–9. 10.1073/pnas.0711038105 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 82. Ton HT, Phan TX, Abramyan AM, et al. : Identification of a putative binding site critical for general anesthetic activation of TRPA1. Proc Natl Acad Sci U S A. 2017;114(14):3762–7. 10.1073/pnas.1618144114 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 83. Woll KA, Skinner KA, Gianti E, et al. : Sites Contributing to TRPA1 Activation by the Anesthetic Propofol Identified by Photoaffinity Labeling. Biophys J. 2017;113(10):2168–2172. 10.1016/j.bpj.2017.08.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Weiser BP, Eckenhoff RG: Propofol inhibits SIRT2 deacetylase through a conformation-specific, allosteric site. J Biol Chem. 2015;290(13):8559–68. 10.1074/jbc.M114.620732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Li L, Yang XJ: Tubulin acetylation: responsible enzymes, biological functions and human diseases. Cell Mol Life Sci. 2015;72(22):4237–55. 10.1007/s00018-015-2000-5 [DOI] [PMC free article] [PubMed] [Google Scholar]