Abstract
It has become evident that nonlymphoid tissues are populated by distinct subsets of innate and adaptive lymphocytes that are characterized by minimal exchange with recirculating counterparts. Especially at barrier sites, such as the skin, gut, and lung, these tissue-resident lymphocyte populations are ideally positioned to quickly respond to pathogens and other environmental stimuli. The kidney harbors several classes of innate and innate-like lymphocytes that have been described to contribute to this tissue-resident population in other organs, including innate lymphoid cells, natural killer cells, natural killer T cells, mucosal-associated invariant T cells, and γδ T cells. Additionally, a substantial proportion of the adaptive lymphocytes that are found in the kidney displays a surface phenotype suggestive of tissue residency, such as CD69+CD4+ T cells. In this review, we summarize recent advances in the understanding of tissue-resident lymphocyte populations, review the available evidence for the existence of these populations in the kidney, and discuss the potential physiologic and pathophysiologic roles thereof in kidney.
Keywords: lymphocytes, Immunology and pathology, cytokines
The surveillance of peripheral tissues by immune cells serves to identify and eliminate pathogens entering from the environment and is one of the major functions of the immune system. On encounter of foreign antigens in the peripheral tissues, dendritic cells migrate to the secondary lymphoid organs, where they meet adaptive lymphocytes and provide activating signals via antigen-loaded MHC molecules that interact with rearranged antigen receptors on T cells.1 Activated effector T cells can then enter the site of inflammation, direct pathogen defense, and after clearance of the infection, take part in tissue surveillance by recirculating between peripheral tissues and secondary lymphoid organs.2 Similarly, circulating natural killer (NK) cells, which are activated by MHC-independent NK cell receptor ligation, have been known for a long time to survey peripheral tissues in search for damaged, virus-infected, and malignantly transformed cells.3
However, several observations that contradict this view of most lymphocytes being highly mobile cells that recirculate through the body have led to a change of this paradigm in the recent years.4 It has been shown that specialized subsets of “innate-like” or “unconventional” T cells reside, for example, in the skin (dendritic epidermal T cells) and the intestine (intraepithelial lymphocytes), suggesting that specialized lymphocyte subsets can adapt to their microenvironment and may establish local residency.3 Similar observations have been made for certain NK cell populations in peripheral organs, showing a phenotype that greatly differs from their circulating counterparts.4 The recently identified group of innate lymphoid cells (ILCs) has also been shown to be a largely resident population of lymphocytes that shows a tissue-specific phenotype and subtype distribution.5–7
The Concept of Lymphocyte Tissue Residency and Tissue-Specific Memory
The concept of lymphocyte tissue residency has been brought forward by the initial discovery that pathogen-specific “conventional” CD8+ T cells can persist in peripheral tissues, including the kidney, for several months after an infection has resolved.8 Later, it was found that conventional CD4+ T helper cells can also establish tissue residency in peripheral tissues after viral infection.9–12
Although both innate and adaptive lymphocytes can establish a state of residency, there are important differences in the pathways that lead to their retention in the tissue.4,11,12 After initial activation in secondary lymphoid organs, conventional T cells infiltrate the inflamed site as differentiated effector cells in response to local production of chemoattractants and then, persist in the tissue after the initial stimulus has resolved. As elegantly shown in a murine model of viral skin infections, the key advantage of such a persisting pathogen-specific T cell population in the tissue is that it can provide rapid protection from locally recurring infections with the same pathogen.13 It is remarkable that the protection provided by these tissue-resident memory T (Trm) cell populations is much more effective than the protection conferred by recirculating effector memory T cells and central memory T cells that reside in lymphoid organs.10,13 In contrast to adaptive lymphocytes and as indicated by the term “innate,” many innate and innate-like lymphocyte subsets have been shown to populate peripheral tissues in the absence of inflammation during fetal development or in early postnatal life.3,14–16 In the tissues, they can renew locally by homeostatic proliferation or potentially develop from local hematopoietic progenitors.3,16 The early appearance of these innate lymphocyte subsets in nonlymphoid tissues makes it conceivable that some of them play crucial roles for organ homeostasis, development of mucosa-associated lymphoid tissues, and defense against congenital or intrauterine infections.6,7,14 Of note, recent studies indicate that, within nonlymphoid organs, including the kidney, resident lymphocytes might greatly outnumber their recirculating counterparts,17 underlining their critical importance for the local immune response in disease and homeostasis.
Defining Tissue-Resident Lymphocytes
The term “tissue-resident” implies that these lymphocyte populations show a minimum of exchange with their counterparts that recirculate in the bloodstream, lymphoid organs, and the peripheral tissues. Although easy to define, it is indeed complicated to prove tissue residency of an immune cell population of interest in animal experiments or even humans.
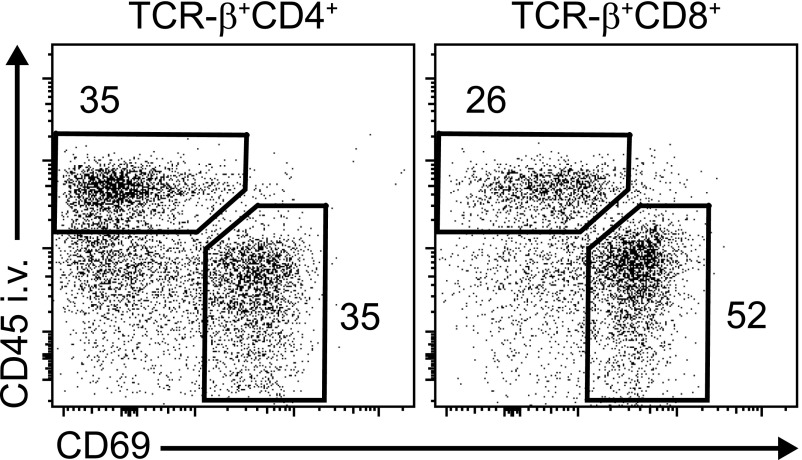
The most important approach to assess potential replenishment from blood-borne cells of a given cell population in a tissue is parabiosis, a technique that was developed in the early 20th century18 and later helped to answer basic questions about the recirculation of immune cells.19 By surgically joining the circulation of two mice with immune cells that can be distinguished by the expression of a congenic surface marker (e.g., CD45.1 versus CD45.2), the contribution of the “donor” circulating cells to an immune cell compartment in a tissue of interest can be defined. After several weeks of parabiosis, analysis of tissue leukocyte populations would exhibit a donor (e.g., CD45.1+) to recipient (e.g., CD45.2+) relation of 1:1 for recirculating populations, whereas tissue-resident populations would typically remain >95% recipient derived.5,10 Another useful tool that has been used to define the anatomic location of an immune cell in the tissue is in vivo staining of intravascular leukocytes combined with flow cytometry or immunohistochemistry. Staining of intravascular leukocytes can be achieved by intravenous injection of a fluorochrome-coupled antibody shortly before euthanasia.20 Interestingly, even after organ perfusion, >30% of the T cells that can be recovered from the kidney of naïve mice are positive for the intravascular staining, indicating that they are located in the kidney vessels. Of the remaining T cells that reside in the kidney tissue, >50% express the marker CD69 (Figure 1).20 CD69, a membrane-bound, type 2 C-lectin receptor, has initially been identified as an early lymphocyte activation marker, but it is also expressed on tissue-resident lymphocytes.21 Together with other markers, such as CD103 and CD49a, it has been shown to correlate with retention of lymphocyte populations in various murine tissues. However, these markers of tissue residency are not exclusive, and their expression pattern might greatly vary between lymphocytes isolated from different organs. In humans, higher expression of CD69 and CD103 has been found on lymphocytes isolated from nonlymphoid tissues and is generally used to discriminate resident from recirculating subsets.22,23 It has to be noted, however, that a detailed characterization of CD69+CD4+ and CD69+CD8+ T cells from various tissues by high-dimensional mass cytometry revealed a multitude of different cell clusters with a tissue-specific distribution, suggesting that the CD69+ Trm cell pool consists of a phenotypically diverse population.24
Figure 1.
The marker CD69 identifies most tissue-resident conventional T cells in the kidney. For staining of intravascular leukocytes, naïve C57BL/6 mice were injected intravenously with a fluorochrome-coupled antibody against the panleukocyte marker CD45 5 minutes before euthanasia (CD45 i.v.). After enzymatic digestion and mechanic dissociation of the kidney tissue, leukocytes were isolated by Percoll density gradient centrifugation as previously described35 and stained with a combination of antibodies to identify CD4+ and CD8+ T cells as well as the expression of the tissue retention marker CD69. Flow cytometry plots are representative for three independent animals with similar results and show that, consistent with a Trm phenotype, a majority of CD45 intravenous negative T cells were CD69 positive. TCR, T cell receptor.
The problem of functionally defining immune cell tissue residency in humans was elegantly addressed in two recent studies that analyzed blood and skin samples of patients with cutaneous T cell malignancies before and after treatment with the T cell–depleting antibody alemtuzumab (αCD52).25,26 The authors could show that alemtuzumab selectively depleted malignant and normal T cells from the circulation but did not affect noncirculating T cells with a Trm phenotype that reside in the skin.25 Further characterization of these skin-resident T cells in patients depleted of recirculating T cells and a human skin–engrafted mouse model revealed that CD69 is indeed a valid marker for Trm cells in the human skin. In contrast to the mouse skin, however, where CD8+CD69+CD103+ T cells represent the most prominent Trm population, CD4+CD69+CD103− Trm cells seem to be more frequent in humans.26 These first functional studies in humans clearly show that the concept of long-term residency of certain T cell subsets in the skin and most likely, other organs as well is also valid in human immunology.
Innate, Innate-Like, and Adaptive Lymphocyte Populations
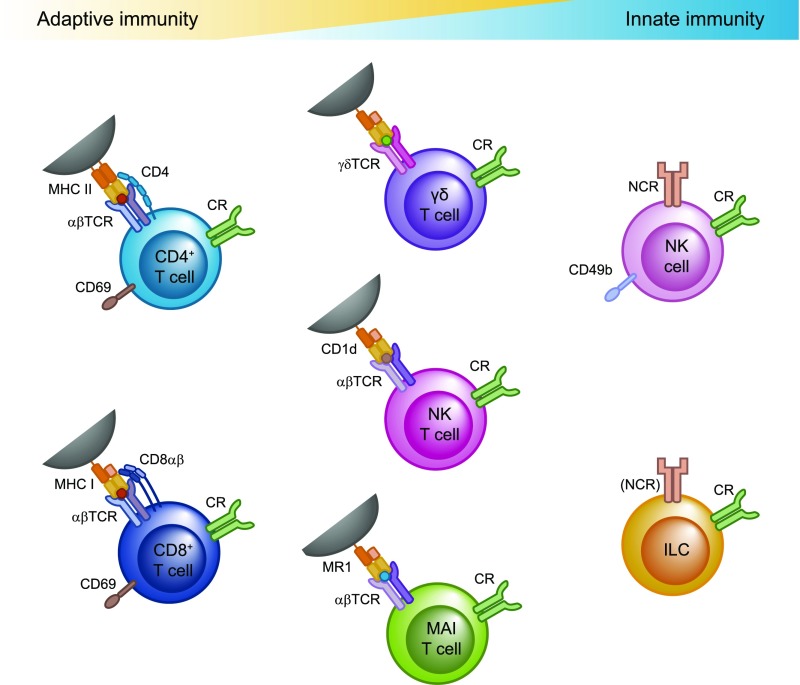
Diverse innate, innate-like, and adaptive subsets have been shown to contribute to the tissue-resident pool of lymphocytes in different organs (Figure 2). A few years ago, conventional CD8+ and CD4+ T cells that show a memory phenotype and establish long-term residency in nonlymphoid tissues were identified and termed Trm cells.11,12 These Trm cells differ in phenotype, gene expression profile, and function from other T cell subsets. There is indication that CD4+ and CD8+ Trm cells can be further subdivided into distinct populations and that the composition of these Trm cell populations varies between different peripheral tissues.24 The origin of these subpopulations and their relation to “conventional” T effector or T memory cell subsets (i.e., TH1, TH2, TH17, and Treg cells) are still unresolved questions.
Figure 2.
Innate, innate-like and adaptive lymphocytes contribute to the tissue-resident population in different organs. CD4+ and CD8+ T cells can be activated by ligation of their T cell receptor by peptide-loaded MHC proteins on the surface of antigen-presenting cells in conjunction with costimulatory molecules and cytokine signals. The γδ T cells, NKT cells, and MAIT cells have a T cell receptor with a restricted specificity. As innate-like T cells, they display a “preactivated” phenotype and can be activated via T cell receptor ligation or directly by cytokine signals. Conventional NK cells and “helper-like” ILCs are grouped together as ILCs and can be activated by direct recognition of damaged cells via natural killer cell receptors (NCRs) and/or cytokine signals (cytokine receptors [CRs]). TCR, T cell receptor.
The γδ T cells, invariant natural killer T (iNKT) cells, and mucosal-associated invariant T (MAIT) cells belong to the innate-like T cell subsets that display features of both innate and adaptive immunity. They express T cell receptors with a restricted variance that can recognize nonpeptide antigens, such as phosphoantigens (γδ T cells), lipids (iNKT cells),14 or vitamin B2 (riboflavin) metabolites (MAIT cells),27 that can be endogenous or microbe-derived metabolic products and are presented in the context of specific MHC-related proteins.14 However, these T cell subsets show a preactivated state and can be activated independently of T cell receptor (TCR) ligation directly by cytokine signals or pattern recognition receptors.
Lymphocytes of the innate immune system are devoid of rearranged antigen receptors and comprise classic NK cells and the more recently discovered family of ILCs. According to a recently proposed nomenclature,28 NK cells are now grouped together with ILCs. NK cells (or “killer” ILCs) are characterized by strong cytotoxic activity, whereas the “helper-like” ILC subtypes have minimal cytotoxicity and exert their effector functions mainly by producing cytokines or modulating T cell responses via cell contact–dependent mechanisms that involve expression of MHCII and/or costimulatory/inhibitory molecules on their surface.6,7 Within the NK cell populations, there are tissue-resident and recirculating subsets, whereas helper-like ILCs have been shown to be almost exclusively tissue-resident in the steady state.5
Tissue-Resident Lymphocytes in the Kidney and Their Role in Renal Diseases
Although a limited number of studies formally addressed kidney-residing lymphocyte populations (Table 1), several reports showed the existence in the kidney of innate and adaptive lymphocytes that show properties consistent with tissue residency. In this section, we will summarize the current knowledge of tissue-residing lymphocyte subsets in the kidney and relate the studies conducted in experimental mouse models of kidney disease to the available data from healthy human kidney tissue and kidney biopsy samples of patients with renal diseases. Characterization of tissue-resident leukocytes is currently based on multicolor flow cytometry. Because classic immunohistochemical staining is often not sufficient to identify tissue-resident leukocyte subsets, there is only limited information on the precise localization of cells within the kidney The establishment of new techniques, such as multicolor histocytometry29,30 and flow cytometry of selected parts of the kidney (e.g., medulla versus cortex), might allow us to study the localization of tissue-resident leukocytes within the kidney and their interaction with the local environment in the future.
Table 1.
Experimental evidence for tissue-resident lymphocyte subsets in the kidney
| Cell Subset | Model System and Methods | Main Finding Regarding Lymphocyte Tissue Residency in the Kidney | Ref. |
|---|---|---|---|
| NK cells | Mouse, IRI | CD49a+DX5− NK cells are kidney resident | 32 |
| Parabiosis, antibody-mediated depletion of circulating NK cells | Kidney-resident NK cells promote IRI | ||
| NK cells | Renal biopsies of patients showing different degrees of kidney fibrosis | CD69+ NK cells produce IFN-γ and accumulate in fibrotic kidney tissue | 34 |
| CD69 expression by flow cytometry | |||
| CD8+ T cells | Mouse, VSV, and listeria infection | Pathogen-specific CD8+ T cells show long-term persistence in the kidney tissue | 8 |
| Ag-loaded tetramer staining | |||
| CD8+ T cells | Mouse, LCMV infection | Tissue residence of renal LCMV-specific CD8+ T cells | 72 |
| Parabiosis, intravascular leukocyte labeling | S1P1 expression prevents renal Trm cell accumulation | ||
| Continuous TCR signaling is not required for Trm cell persistence | |||
| CD8+ and CD4+ T cells | Mouse, LCMV infection, tumor | i.v. mAb-negative CD69+ CD103± CD4+ and CD8+ Trm cells are found in the kidney tissue | 20 |
| Intravascular leukocyte labeling | |||
| CD8+ T cells | Mouse, LCMV, and VSV infection | Standard protocols for T cell isolation are inefficient | 17 |
| Quantitative immunofluorescence microscopy, intravascular leukocyte labeling | Resident T cells greatly outnumber recirculating cells in nonlymphoid tissues | ||
| CD8+ T cells | Mouse, LCMV infection | The transcription factors Blimp1 and Hobit are required for renal Trm development | 78 |
| Ag-loaded tetramer staining | |||
| CD8+ T cells | Mouse, LCMV infection | TGF-β is required for transendothelial migration of Trm cells into the kidney | 71 |
| Intravascular leukocyte labeling |
VSV, varicella zoster virus; Ag, antigen; LCMV, lymphocytic choriomeningitis virus; S1P1, sphingosine phosphate 1 receptor 1, TCR, T cell receptor.
INNATE LYMPHOID CELLS: NK Cells
NK cells are major effectors of the innate immune system that kills target cells by releasing cytotoxic mediators, such as perforin and granzyme, but they can also produce proinflammatory cytokines, such as TNF-α and IFN-γ. NK cell function is tightly controlled by an interplay of activating receptors, detecting “stress” molecules upregulated on damaged, virally infected, or malignant cells and inhibitory receptors that identify “normal” cells by recognizing their intact MHC class 1 expression, referred to as the recognition of “missing self.”3
It was shown a while ago that the murine kidney harbors a substantial number of NK cells,31 but until recently, it has been unclear if residential NK cells contribute to this population. A detailed study by Victorino et al.32 used parabiosis experiments to prove that around 20% of the NK cells that can be recovered from the mouse kidney belong to a CD49a+DX5− tissue-resident NK cell population that has been described in other organs, such as the liver, uterus, and pancreas. These tissue-resident NK cells showed a more activated phenotype than their circulating counterparts and contributed to renal injury in the ischemia-reperfusion model, which was nicely shown by the use of antibodies that deplete either total NK cells (αNK1.1) or preferentially target circulating NK cells (αAsGM1) (Figure 3).32
Figure 3.
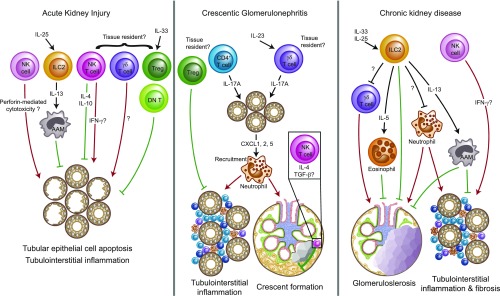
Tissue-resident lymphocytes are likely to play important roles in renal disease. Graphic summary of the so far identified functions of potentially tissue-resident innate, innate-like, and adaptive lymphocyte populations in AKI induced by ischemia and reperfusion,32,36,40,45,65,66,73 crescentic GN,44,48,51,57–59 and chronic progression of kidney disease.34,35 The tissue residency of a subset of NK cells32 and the presence of conventional Trm cells8,17,72 in the murine kidney have been clearly shown (Table 1). Given the results from other organs, the existence of kidney-resident subsets among renal γδ T cells, ILCs, NKT cells, and CD4+ T cells can be assumed. However, the formal experimental proof of this hypothesis is still lacking. Red arrows depict proinflammatory mechanisms that promote disease progression, whereas green symbols represent anti-inflammatory effects that dampen tissue damage. AAM, alternatively activated macrophages; DN T, double-negative T cell, Treg, regulatory T cell.
Importantly, also leukocytes isolated from the healthy human kidney contain a large fraction (up to approximately 25% total of lymphocytes) of NK cells identified as CD3negCD56+CD94+, indicating that the mechanisms used by mouse kidney–residing NK cells in AKI might be translatable to human disease.33 Another very recent investigation of kidney biopsies from patients with different forms of renal diseases revealed that a large fraction of intrarenal NK cells expresses the tissue residency maker CD69 and produces IFN-γ. Moreover, NK cells accumulated in the tubulointerstitial compartment of fibrotic kidneys and were correlated with degree of fibrosis and loss of kidney function in these patients, suggesting that NK cells might play a role in progression of kidney fibrosis.34 Especially in the face of these new results in patients with renal disease, a potential role of tissue-resident NK cells in inflammatory and fibrotic kidney diseases warrants additional study.
Innate Lymphoid Cells: Helper-Like ILCs
The current nomenclature defines three major subsets of helper-like ILCs by their distinct profiles of transcription factor usage and cytokine production: T-bet+IFN-γ+ ILC1s, GATA3+IL-5+IL-13+ ILC2s, and ROR-γt+IL-17A+ and/or IL-22+ ILC3s.28 Studies from the last years have shown that helper-like ILCs are a largely tissue-resident cell population in barrier organs, such as the gut and lung,5 and have characterized important functions of ILCs in these tissues, where they promote tissue homeostasis, mediate defense against pathogens, and contribute to allergic and autoimmune diseases in mice and humans.6,7
Recently, our group performed a comprehensive characterization of helper-like ILC populations in the healthy murine and human kidney, showing that IL-13– and IL-5–producing type ILC2s are a major kidney-residing ILC population in both species.35 However, long-term tissue residency of ILCs in the kidney has not yet been formally addressed. As shown before in other organs,6,7 expansion and activation of ILC2s in the kidney can be induced by application of the epithelial cell–derived cytokines IL-2536 or IL-33.35 In ischemic AKI, the preemptive therapy with IL-25 protected mice from renal injury by ILC2-mediated induction of alternatively activated macrophages. In vitro experiments showed that this “M2 activation” of macrophages depends on IL-4 and IL-13 secreted by ILC2s and that these macrophages can directly promote the survival of tubular epithelial cells.36 In Adriamycin-induced glomerulosclerosis, a model for chronic proteinuric kidney disease, the treatment of mice with IL-33 improved the clinical course of the disease by ILC2-mediated induction of a protective type 2 response.35 In addition to promoting alternative activation of macrophages, as described in the AKI model, the IL-33–expanded ILC2s produced large amounts of IL-5, which induced the accumulation of eosinophils in the kidney, ameliorating glomerulosclerosis. Via a yet unknown mechanism, ILC2 expansion also reduced the infiltration of pathogenic neutrophils and γδ T cells into the kidney, resulting in less tissue injury.35 In this context, it is important to mention that IL-33 is a pleiotropic cytokine37 that can also influence T helper cell and regulatory T cell responses in the kidney,38–40 but if these IL-33–responsive CD4+ T cell subsets show characteristics of tissue-resident cells has not been addressed so far.
Together, this suggests a protective role for tissue-resident ILC2s in the kidney. A potential function of helper-like ILC subtypes in other animal models of renal disease and human kidney pathology remains to be elucidated.
Innate-Like T Cell Subsets: γδ T Cells
The γδ T cells develop in the thymus during the early embryonic stage, and a subset of them migrates directly from the thymus to nonlymphoid tissues, where they are sustained throughout life. Recent experimental studies in the gut and skin have clearly shown that these tissue-resident γδ T cells play a critical role in protective and pathogenic immune responses.41,42 In particular, their rapid capacity to produce cytokines, such as IFN-γ or IL-17A, qualifies γδ T cells to orchestrate a first-line inflammatory response to pathogen-derived as well as immune-mediated signals.43
Although long-term tissue residency of γδ T cells in the kidney has never been formally addressed, there have been a number of studies showing an important role for this potentially tissue-resident T cell subset in kidney pathology (Figure 3). Rosenkranz et al.44 provided the first evidence for a functional role of γδ T cells in kidney disease. Using a murine model of crescentic GN (nephrotoxic nephritis), they showed that TCRδ-deficient mice display reduced neutrophil and macrophage infiltration, develop less severe histopathologic kidney injury, and show less impairment of renal function. The same group subsequently showed that γδ T cells also play a pathogenic role in renal ischemia-reperfusion injury (IRI), potentially by promoting the infiltration of αβ T cells into the kidney.45 In contrast, Wu et al.46 reported that the depletion of γδ T cells with the anti-γδ TCR antibody UC7–13D5 worsens the course of a murine model of progressive glomerulosclerosis (Adriamycin nephropathy). A potential explanation for these controversial findings might be that the antibody used was later found to not entirely deplete γδ T cells but rather, that it led to TCR internalization, thereby generating “undetectable” but activated γδ T cells.47
More recently, our group identified γδ T cells as the major cellular source of IL-17A in the early phase of a murine model of crescentic GN.48 Interestingly, the rapid upregulation of IL-17A expression by γδ T cells was detectable before a marked infiltration of T cells into the kidney and before the development of an adaptive CD4+ TH17 cell response, suggesting the presence of tissue-resident γδ T cells in the kidney that can rapidly respond to tissue-derived stimuli. The stimulus for upregulation of IL-17A production in kidney-residing γδ T cells was found to be IL-23 produced by renal dendritic cells. IL-17A then induced local chemokine expression, which drives renal neutrophil recruitment and thereby, significantly contributes to the pathogenesis of crescentic GN.48 Later, it was shown that γδ T cell–derived IL-17A is also important for renal chemokine expression (e.g., CCL5) and leukocyte infiltration in the ureteral obstruction model of kidney fibrosis.49
An intriguing recent study by Benakis et al.50 suggested that IL-17A–producing γδ T cells originate in the intestine and traffic to the site of inflammation in a model of acute brain injury. However, our own studies using photoconvertible Kaede mice to track intestinal T cell trafficking after GN induction failed to show the migration of γδ T cells into the inflamed kidney, supporting the idea that IL-17A–producing γδ T cells in the kidney are predominantly tissue-resident.51
With respect to human renal disease, a pioneer study reported the presence of significant numbers of CD3+ γδ T cells in renal biopsies from patients with IgA nephropathy, and their accumulation in the kidney was associated with a progressive course of the disease.52 Moreover, it was shown that renal γδ T cells have a restricted γδ TCR repertoire in IgA nephropathy, indicating a clonal expansion of γδ T cells in the kidneys of these patients.53 As a potential indicator for a physiologic role of γδ T cells in the defense against viral infections, it was shown that CMV infection leads to a massive expansion of γδ T cells in the peripheral blood of renal transplant recipients.54 More importantly, the same group of investigators recently provided experimental evidence that CMV-induced circulating and potentially kidney-residing or infiltrating γδ T cells are involved in antibody-mediated microvascular lesions of kidney allografts.55
Taken together, experimental and human studies support a biologic function of γδ T cells in the pathogenesis of several inflammatory kidney diseases. However, the contribution of tissue-resident γδ T cells in this context, their potential protective role against invading pathogens, and the role of different γδ T cells subsets are so far unknown and require further study.
Innate-Like T Cell Subsets: Natural Killer T and MAIT Cells
Natural killer T (NKT) cells and MAIT cells represent two distinct subsets of T cells that recognize nonpeptide antigens and account for a significant fraction of tissue-resident lymphocytes in various organs.56 However, whether subsets of NKT and MAIT cells establish long-term tissue residency in the kidney has not been studied so far.
NKT cells can be classified into two major groups on the basis of differences in T cell receptor usage. NKT cells expressing semi-invariant TCRs restricted to CD1d and stimulated by the α-galactosylceramide (α-GalCer) glycolipid are referred to as iNKT cells or type 1 NKT cells. NKT cells using variable TCRs restricted to CD1d and not stimulated by α-GalCer are termed type 2 NKT cells. Both populations rapidly produce an array of cytokines on activation and play a critical role in regulating various immune responses in health and disease.56
So far, most studies on NKT cells in the field of nephrology focused on their role in autoimmune kidney diseases (Figure 3). In experimental models of crescentic GN, three studies showed that lack or reduction of iNKT cells aggravates the clinical course of the disease, whereas activation of iNKT might ameliorate GN.57–59 The mechanisms by which “regulatory” iNKT cells protect against autoimmune diseases, such as GN, remain to be fully elucidated but likely involve the local (and systemic) production of immunomodulatory cytokines, such as IL-4, IL-10, and TGF-β, all of which have been shown to be protective in immune-mediated kidney diseases.57–59 In addition, abnormalities in the number and function of NKT cells have been detected in patients with SLE and lupus-prone mouse strains (MRL/lpr, NZB/W F1).60 iNKT cell activation by α-GalCer treatment alleviated inflammatory dermatitis without affecting kidney disease in MRL/lpr mice.61 In young NZB/W F1 mice, brief α-GalCer treatment protected against systemic autoimmunity and proteinuria,62 and in line, the lack of functional NKT cells in CD1d−/− mice exacerbated lupus nephritis induced by the hydrocarbon oil pristane.63 However, the repetitive application of α-GalCer in adult NZB/W F1 animals exacerbated TH1 responses and autoantibody secretion that contribute to lupus development,64 suggesting that continuous NKT cell activation can promote autoimmune inflammation.
In a model of renal IRI, Okusa and coworkers65 reported that absence of NKT cells achieved by application of depleting antibodies or in mice deficient of iNKT cells (Jα18−/−) markedly attenuated renal damage (Figure 3). These effects were associated with a significant decrease in renal leukocyte infiltration and a reduction in IFN-γ–producing neutrophils, suggesting that iNKT cells mediate tissue damage in this model.65 In contrast, Yang et al.66 showed that the severity of IRI in mice deficient in iNKT cells (Jα18−/−) and particularly, mice with nonfunctional types 1 and 2 NKT cells (CD1d−/−) was aggravated. In support of a renoprotective function, the number of NKT cells in the renal tissue of patients with acute tubular necrosis tended to be negatively correlated with disease severity.66 The severity of IRI was quite different between the two studies (i.e., in the former report,65 the peak serum creatinine at 24 hours was 0.7 mg/dl compared with 1.59 mg/dl in the study by Yang et al.66), suggesting that the severity of injury might influence iNKT cell function in IRI. Moreover, the renoprotective effect in the latter study was mainly induced by sulfatide-reactive type 2 NKT cells, which were not analyzed by Li et al.,65 indicating that different NKT cell types might have contrasting immune regulatory function in the setting of ischemic injury.
In summary, although experimental studies strongly support that iNKT cells play a regulatory role in immune-mediated kidney disorders by systemic and local production of cytokines (e.g., IFN-γ, IL-10, and IL-4), there is still some controversy regarding the question of whether iNKT cells can directly dampen T cell autoimmunity or under certain circumstances, if they might even promote renal inflammation. Moreover, the contribution of circulating versus kidney-residing iNKT cells in this process is largely unknown. Finally, the recent discovery of distinct iNKT cell subsets, named iNKT1, iNKT2, and iNKT17 cells (analogous to TH1, TH2, and TH17 conventional CD4+ T cells), with different immune regulatory function makes the role of iNKT cells in the pathogenesis of autoimmune diseases more complex than previously thought.67
MAIT cells represent another innate-like T cell subset characterized by the recognition of riboflavin metabolites in context with the MHC-related molecule 1. On activation by TCR ligation and/or cytokine signals, they can rapidly produce a range of proinflammatory cytokines, suggesting that they might be linked to chronic inflammatory disorders in various tissues.68 Although abundant in mucosal tissue, liver, and the peripheral blood in humans, they are relatively rare in mice. A first indication that MAIT cells can be found in the human kidney was provided by a study that showed the presence of MAIT cell–specific invariant Vα7.2-Jα33 TCR sequences in eight of 11 kidney tumors.69 Later, a quantification of MAIT TCR transcript levels among various tissues revealed that the kidney shows a substantial expression of these transcripts. Indeed, in the kidney, MAIT TCR sequence levels were comparable with the liver and much higher than in the peripheral blood.70 However, the location of MAIT cells in the kidney, their functional profile, and a potential role of these cells in kidney diseases await further study.
Conventional T Cells: CD4+ and CD8+ Trm Cells
Increasing evidence suggests that the small population of conventional CD4+ and CD8+ T cells that can be detected in mouse and human kidney parenchyma under homeostatic conditions largely consists of tissue-resident Trm cells. In the mouse, intravenous antibody-negative CD69+ CD4+ and CD8+ Trm cells can be detected in the kidney20,71,72 (Figure 1). As a proof of tissue residency, parabiosis experiments further revealed that CD8+ T cells, after they are established in the kidney tissue, are excluded from the pool of recirculating T cells.72 It has been shown recently that the healthy kidney also harbors a significant αβ TCR+ CD4 and CD8 double-negative T cell population in human and mice, but the potential tissue residency of these cells has not been addressed so far.73
Importantly, quantitative immunofluorescence microscopy recently revealed that conventional FACS-based assays highly underestimate the T cell numbers in peripheral tissues, including the kidney.17 Using this method, a >20-fold higher number of Trm cells was calculated for the murine kidney.17 It also needs to be considered that mice are usually kept under specific pathogen–free (SPF) conditions and therefore, have an almost virgin immune system. The number of CD4+ and CD8+ T cells that could be isolated from the kidneys of mice bred under germfree conditions was found to be similar to that of SPF mice.74 However, when mice from pet stores or free living populations were compared with SPF mice, significantly higher numbers of T cells could be isolated from the kidney and other peripheral tissues.75 This indicates that the microbiota is not absolutely required to generate kidney-resident T cells, but it clearly shows that the wide array of pathogens that free living mice (and humans) are exposed to contributes to the generation of the tissue-resident T cell population. Therefore, the currently available data for renal Trm cells derived from mice might not fully reflect the situation in the human kidney.
In the mouse, several bacterial and viral infection models show accumulation of pathogen-specific CD8+ Trm cells in the kidney.8,17,71,76 At least some of these pathogens (i.e., Listeria monocytogenes and lymphocytic choriomeningitis virus) transiently or chronically infect the kidney. In these models, CD8+ T cells become activated in secondary lymphoid tissues and acquire the chemokine receptors and adhesion molecules that allow transmigration through the activated endothelium into the infected kidney tissue. Within the tissue, a subset of CD8+ T cells differentiates to long-lived sessile CD8+ Trm cells that remain after clearance of the infection. In the kidney, TGF-β was shown to control the tissue entry of effector T cells and thereby, participate in the development of a kidney-residing Trm cell population.71 Other cytokines, such as tumor TNF-α, IL-33, IL-15, and TGF-β, have been identified as factors that induce CD8+ Trm cell formation in various tissues,72,77 but their exact role for kidney residency of T cells remains to be elucidated. CD8+ Trm cell differentiation in the kidney also depends on expression of the transcription factors Blimp1 and Hobit and downregulation of the transcription factor KLF2.72,78 Mechanistically, Hobit represses Klf2 and S1pr1 expression (coding for KLF2 and sphingosine phosphate 1 receptor 1), and both Blimp1 and Hobit bound target sequences within the S1pr1 and Ccr7 gene.78 KLF2 induces the expression of sphingosine phosphate 1 receptor 1, which together with the chemokine receptor CCR7, coordinates the egress of lymphocytes via lymphatic vessels.72 The factors that allow long-term survival of CD8+ Trm cells in the kidney tissue and especially, the signals that promote CD4+ Trm cell formation and survival in peripheral tissues are still unclear.
Trm cells are also found in kidneys of mice without prior renal disease, which could be the consequence of a subclinical renal inflammation or infection. However, activated CD8+ T cells are capable of trafficking nonlymphoid tissues, even in the absence of local inflammation,8,79 presumably also allowing activated T cells to home to the kidney. Thus, the population of Trm cells in the kidney might be due to prior renal inflammations but could also reflect responses at nonrenal sites. This scenario is in agreement with the large number of renal Trm cells in pet store mice75 and might also apply for human renal Trm cells.
In humans, identification of Trm cells mainly relies on CD69 and CD103 expression. In contrast to other tissues,23,24 there is currently only very limited data available on Trm cells in human kidneys, which are mainly due to the restricted availability of healthy renal tissue. A study on T cells infiltrating human renal cell carcinomas revealed high frequency of CD69+ CD4+ and CD8+ T cells (>50% of CD4+ T cells) compared with T cells from the peripheral blood (approximately 5% of CD4+ T cells).80 Interestingly, similar high frequencies of CD69+ T cells were also detected in renal tissue distant from the tumor.80 Taking into account that, in the mouse, >30% of T cells isolated from the kidney are in the vasculature (Figure 1) (J.-E.T., M.B., H.-W.M., and U.P., unpublished data), this observation indicates that the majority of human renal CD4+ and CD8+ T cells have the phenotype of CD69+ Trm cells, which is in accordance with data on Trm cells in other nonlymphoid human tissues.22–24
In barrier tissues, Trm cells act as sentinels for pathogens previously contacted. On antigen encounter, they rapidly produce inflammatory cytokines, particularly IFN-γ and TNF-α. These cytokines induce a state of alertness in the local environment and cause recruitment of effector T cells, which amplify the response, as well as other inflammatory cells required for pathogen control. As a consequence, a small number of pathogen-specific Trm cells can induce a very rapid and fulminant local response, which ideally causes elimination of the pathogen at the entry site and thereby, prevents dissemination to other tissues.81,82
In nonbarrier tissues and particularly, the kidney, the function of Trm cells is still unclear. In the human kidney, Trm cells might be involved in control of urogenital bacterial infection ascending to the kidney. It is also possible that Trm cells are required to control latent chronic infections (e.g., by polyoma viruses). In sterile inflammatory diseases and autoimmunity, Trm cells could make up a population of autoreactive T cells directed against renal antigens, or by antigen-independent pathways, they could amplify inflammatory responses and thereby, aggravate renal damage. A determination of the TCR repertoire and specificity of renal Trm cells will help to identify the function of these cells in renal disease.
Concluding Remarks
In the last years, it has become increasingly clear that, in addition to circulating lymphocytes and lymphocytes that reside in secondary lymphoid tissues, a major population of lymphocytes persists in nonlymphoid tissues, where it is fundamental for the local immune response. These lymphocytes either constitutively colonize the tissues or accumulate in inflamed and noninflamed tissues during an immune response. The function of tissue-resident lymphocytes in barrier organs is to provide a first line of defense against invading pathogens and amplify and coordinate the local immune response. The fact that these tissue-resident lymphocytes can remain sessile in nonlymphoid tissues long after the infection has resolved implies that our immunologic memory is, in part, determined by these persisting changes in the residential immune cell composition that reflect the “history” of infectious and noninfectious pathologies that the organism is exposed to throughout its life.83–85
From a clinical point of view, the understanding of the pathways that regulate the formation and localization of resident lymphocytes and the tissue-specific factors that control their activation is especially important in vaccine development and designing new strategies for treatment of chronic inflammatory diseases. Given the key features of the tissue-resident lymphocyte populations, it is likely that they play a critical role in tissue-specific inflammatory diseases of both barrier and nonbarrier organs, such as the kidney.
Disclosures
None.
Acknowledgments
J.-E.T. is supported by Emmy Noether Grant TU316/1-2 of the Deutsche Forschungsgemeinschaft. J.-E.T., H.-W.M., and U.P. are supported by the Collaborative Research Center 1192 “Immune-Mediated Glomerular Diseases” funded by the Deutsche Forschungsgemeinschaft.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Worbs T, Hammerschmidt SI, Förster R: Dendritic cell migration in health and disease. Nat Rev Immunol 17: 30–48, 2017 [DOI] [PubMed] [Google Scholar]
- 2.von Andrian UH, Mackay CR: T-cell function and migration. Two sides of the same coin. N Engl J Med 343: 1020–1034, 2000 [DOI] [PubMed] [Google Scholar]
- 3.Björkström NK, Ljunggren HG, Michaëlsson J: Emerging insights into natural killer cells in human peripheral tissues. Nat Rev Immunol 16: 310–320, 2016 [DOI] [PubMed] [Google Scholar]
- 4.Fan X, Rudensky AY: Hallmarks of tissue-resident lymphocytes. Cell 164: 1198–1211, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gasteiger G, Fan X, Dikiy S, Lee SY, Rudensky AY: Tissue residency of innate lymphoid cells in lymphoid and nonlymphoid organs. Science 350: 981–985, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Artis D, Spits H: The biology of innate lymphoid cells. Nature 517: 293–301, 2015 [DOI] [PubMed] [Google Scholar]
- 7.Eberl G, Colonna M, Di Santo JP, McKenzie AN: Innate lymphoid cells. Innate lymphoid cells: A new paradigm in immunology. Science 348: aaa6566, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Masopust D, Vezys V, Marzo AL, Lefrançois L: Preferential localization of effector memory cells in nonlymphoid tissue. Science 291: 2413–2417, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Gebhardt T, Whitney PG, Zaid A, Mackay LK, Brooks AG, Heath WR, Carbone FR, Mueller SN: Different patterns of peripheral migration by memory CD4+ and CD8+ T cells. Nature 477: 216–219, 2011 [DOI] [PubMed] [Google Scholar]
- 10.Iijima N, Iwasaki A: T cell memory. A local macrophage chemokine network sustains protective tissue-resident memory CD4 T cells. Science 346: 93–98, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park CO, Kupper TS: The emerging role of resident memory T cells in protective immunity and inflammatory disease. Nat Med 21: 688–697, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mueller SN, Mackay LK: Tissue-resident memory T cells: Local specialists in immune defence. Nat Rev Immunol 16: 79–89, 2016 [DOI] [PubMed] [Google Scholar]
- 13.Jiang X, Clark RA, Liu L, Wagers AJ, Fuhlbrigge RC, Kupper TS: Skin infection generates non-migratory memory CD8+ T(RM) cells providing global skin immunity. Nature 483: 227–231, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vermijlen D, Prinz I: Ontogeny of innate T lymphocytes - some innate lymphocytes are more innate than others. Front Immunol 5: 486, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim CH, Hashimoto-Hill S, Kim M: Migration and tissue tropism of innate lymphoid cells. Trends Immunol 37: 68–79, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zook EC, Kee BL: Development of innate lymphoid cells. Nat Immunol 17: 775–782, 2016 [DOI] [PubMed] [Google Scholar]
- 17.Steinert EM, Schenkel JM, Fraser KA, Beura LK, Manlove LS, Igyártó BZ, Southern PJ, Masopust D: Quantifying memory CD8 T cells reveals regionalization of immunosurveillance. Cell 161: 737–749, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rous P: Parabiosis as a test for circulating anti-bodies in cancer: First paper. J Exp Med 11: 810–814, 1909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wright DE, Wagers AJ, Gulati AP, Johnson FL, Weissman IL: Physiological migration of hematopoietic stem and progenitor cells. Science 294: 1933–1936, 2001 [DOI] [PubMed] [Google Scholar]
- 20.Anderson KG, Mayer-Barber K, Sung H, Beura L, James BR, Taylor JJ, Qunaj L, Griffith TS, Vezys V, Barber DL, Masopust D: Intravascular staining for discrimination of vascular and tissue leukocytes. Nat Protoc 9: 209–222, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cibrián D, Sánchez-Madrid F: CD69: From activation marker to metabolic gatekeeper. Eur J Immunol 47: 946–953, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sathaliyawala T, Kubota M, Yudanin N, Turner D, Camp P, Thome JJ, Bickham KL, Lerner H, Goldstein M, Sykes M, Kato T, Farber DL: Distribution and compartmentalization of human circulating and tissue-resident memory T cell subsets. Immunity 38: 187–197, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thome JJ, Yudanin N, Ohmura Y, Kubota M, Grinshpun B, Sathaliyawala T, Kato T, Lerner H, Shen Y, Farber DL: Spatial map of human T cell compartmentalization and maintenance over decades of life. Cell 159: 814–828, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wong MT, Ong DE, Lim FS, Teng KW, McGovern N, Narayanan S, Ho WQ, Cerny D, Tan HK, Anicete R, Tan BK, Lim TK, Chan CY, Cheow PC, Lee SY, Takano A, Tan EH, Tam JK, Tan EY, Chan JK, Fink K, Bertoletti A, Ginhoux F, Curotto de Lafaille MA, Newell EW: A high-dimensional atlas of human T cell diversity reveals tissue-specific trafficking and cytokine signatures. Immunity 45: 442–456, 2016 [DOI] [PubMed] [Google Scholar]
- 25.Clark RA, Watanabe R, Teague JE, Schlapbach C, Tawa MC, Adams N, Dorosario AA, Chaney KS, Cutler CS, Leboeuf NR, Carter JB, Fisher DC, Kupper TS: Skin effector memory T cells do not recirculate and provide immune protection in alemtuzumab-treated CTCL patients. Sci Transl Med 4: 117ra7, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Watanabe R, Gehad A, Yang C, Scott LL, Teague JE, Schlapbach C, Elco CP, Huang V, Matos TR, Kupper TS, Clark RA: Human skin is protected by four functionally and phenotypically discrete populations of resident and recirculating memory T cells. Sci Transl Med 7: 279ra39, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Birkinshaw RW, Kjer-Nielsen L, Eckle SB, McCluskey J, Rossjohn J: MAITs, MR1 and vitamin B metabolites. Curr Opin Immunol 26: 7–13, 2014 [DOI] [PubMed] [Google Scholar]
- 28.Eberl G, Di Santo JP, Vivier E: The brave new world of innate lymphoid cells. Nat Immunol 16: 1–5, 2015 [DOI] [PubMed] [Google Scholar]
- 29.Gerner MY, Kastenmuller W, Ifrim I, Kabat J, Germain RN: Histo-cytometry: A method for highly multiplex quantitative tissue imaging analysis applied to dendritic cell subset microanatomy in lymph nodes. Immunity 37: 364–376, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Winfree S, Khan S, Micanovic R, Eadon MT, Kelly KJ, Sutton TA, Phillips CL, Dunn KW, El-Achkar TM: Quantitative three-dimensional tissue cytometry to study kidney tissue and resident immune cells. J Am Soc Nephrol 28: 2108–2118, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zheng G, Zheng L, Wang Y, Wu H, Kairaitis L, Zhang C, Tay YC, Wang Y, Alexander SI, Harris DC: NK cells do not mediate renal injury in murine adriamycin nephropathy. Kidney Int 69: 1159–1165, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Victorino F, Sojka DK, Brodsky KS, McNamee EN, Masterson JC, Homann D, Yokoyama WM, Eltzschig HK, Clambey ET: Tissue-resident NK cells mediate ischemic kidney injury and are not depleted by anti-asialo-GM1 antibody. J Immunol 195: 4973–4985, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carrega P, Bonaccorsi I, Di Carlo E, Morandi B, Paul P, Rizzello V, Cipollone G, Navarra G, Mingari MC, Moretta L, Ferlazzo G: CD56(bright)perforin(low) noncytotoxic human NK cells are abundant in both healthy and neoplastic solid tissues and recirculate to secondary lymphoid organs via afferent lymph. J Immunol 192: 3805–3815, 2014 [DOI] [PubMed] [Google Scholar]
- 34.Law BMP, Wilkinson R, Wang X, Kildey K, Lindner M, Rist MJ, Beagley K, Healy H, Kassianos AJ: Interferon-γ production by tubulointerstitial human CD56(bright) natural killer cells contributes to renal fibrosis and chronic kidney disease progression. Kidney Int 92: 79–88, 2017 [DOI] [PubMed] [Google Scholar]
- 35.Riedel JH, Becker M, Kopp K, Düster M, Brix SR, Meyer-Schwesinger C, Kluth LA, Gnirck AC, Attar M, Krohn S, Fehse B, Stahl RAK, Panzer U, Turner JE: IL-33-mediated expansion of type 2 innate lymphoid cells protects from progressive glomerulosclerosis. J Am Soc Nephrol 28: 2068–2080, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang Q, Niu Z, Tan J, Yang J, Liu Y, Ma H, Lee VW, Sun S, Song X, Guo M, Wang Y, Cao Q: IL-25 elicits innate lymphoid cells and multipotent progenitor type 2 cells that reduce renal ischemic/reperfusion injury. J Am Soc Nephrol 26: 2199–2211, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Molofsky AB, Savage AK, Locksley RM: Interleukin-33 in tissue homeostasis, injury, and inflammation. Immunity 42: 1005–1019, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Akcay A, Nguyen Q, He Z, Turkmen K, Won Lee D, Hernando AA, Altmann C, Toker A, Pacic A, Ljubanovic DG, Jani A, Faubel S, Edelstein CL: IL-33 exacerbates acute kidney injury. J Am Soc Nephrol 22: 2057–2067, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tran VG, Kim HJ, Kim J, Kang SW, Moon UJ, Cho HR, Kwon B: IL-33 enhances host tolerance to candida albicans kidney infections through induction of IL-13 production by CD4+ T cells. J Immunol 194: 4871–4879, 2015 [DOI] [PubMed] [Google Scholar]
- 40.Stremska ME, Jose S, Sabapathy V, Huang L, Bajwa A, Kinsey GR, Sharma PR, Mohammad S, Rosin DL, Okusa MD, Sharma R: IL233, A novel IL-2 and IL-33 hybrid cytokine, ameliorates renal injury. J Am Soc Nephrol 28: 2681–2693, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Riol-Blanco L, Ordovas-Montanes J, Perro M, Naval E, Thiriot A, Alvarez D, Paust S, Wood JN, von Andrian UH: Nociceptive sensory neurons drive interleukin-23-mediated psoriasiform skin inflammation. Nature 510: 157–161, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ismail AS, Severson KM, Vaishnava S, Behrendt CL, Yu X, Benjamin JL, Ruhn KA, Hou B, DeFranco AL, Yarovinsky F, Hooper LV: Gammadelta intraepithelial lymphocytes are essential mediators of host-microbial homeostasis at the intestinal mucosal surface. Proc Natl Acad Sci U S A 108: 8743–8748, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vantourout P, Hayday A: Six-of-the-best: Unique contributions of γδ T cells to immunology. Nat Rev Immunol 13: 88–100, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rosenkranz AR, Knight S, Sethi S, Alexander SI, Cotran RS, Mayadas TN: Regulatory interactions of alphabeta and gammadelta T cells in glomerulonephritis. Kidney Int 58: 1055–1066, 2000 [DOI] [PubMed] [Google Scholar]
- 45.Hochegger K, Schätz T, Eller P, Tagwerker A, Heininger D, Mayer G, Rosenkranz AR: Role of alpha/beta and gamma/delta T cells in renal ischemia-reperfusion injury. Am J Physiol Renal Physiol 293: F741–F747, 2007 [DOI] [PubMed] [Google Scholar]
- 46.Wu H, Wang YM, Wang Y, Hu M, Zhang GY, Knight JF, Harris DC, Alexander SI: Depletion of gammadelta T cells exacerbates murine adriamycin nephropathy. J Am Soc Nephrol 18: 1180–1189, 2007 [DOI] [PubMed] [Google Scholar]
- 47.Koenecke C, Chennupati V, Schmitz S, Malissen B, Förster R, Prinz I: In vivo application of mAb directed against the gammadelta TCR does not deplete but generates “invisible” gammadelta T cells. Eur J Immunol 39: 372–379, 2009 [DOI] [PubMed] [Google Scholar]
- 48.Turner JE, Krebs C, Tittel AP, Paust HJ, Meyer-Schwesinger C, Bennstein SB, Steinmetz OM, Prinz I, Magnus T, Korn T, Stahl RA, Kurts C, Panzer U: IL-17A production by renal γδ T cells promotes kidney injury in crescentic GN. J Am Soc Nephrol 23: 1486–1495, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Peng X, Xiao Z, Zhang J, Li Y, Dong Y, Du J: IL-17A produced by both γδ T and Th17 cells promotes renal fibrosis via RANTES-mediated leukocyte infiltration after renal obstruction. J Pathol 235: 79–89, 2015 [DOI] [PubMed] [Google Scholar]
- 50.Benakis C, Brea D, Caballero S, Faraco G, Moore J, Murphy M, Sita G, Racchumi G, Ling L, Pamer EG, Iadecola C, Anrather J: Commensal microbiota affects ischemic stroke outcome by regulating intestinal γδ T cells. Nat Med 22: 516–523, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Krebs CF, Paust HJ, Krohn S, Koyro T, Brix SR, Riedel JH, Bartsch P, Wiech T, Meyer-Schwesinger C, Huang J, Fischer N, Busch P, Mittrücker HW, Steinhoff U, Stockinger B, Perez LG, Wenzel UO, Janneck M, Steinmetz OM, Gagliani N, Stahl RA, Huber S, Turner JE, Panzer U: Autoimmune renal disease is exacerbated by S1P-receptor-1-dependent intestinal Th17 cell migration to the kidney. Immunity 45: 1078–1092, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Falk MC, Ng G, Zhang GY, Fanning GC, Roy LP, Bannister KM, Thomas AC, Clarkson AR, Woodroffe AJ, Knight JF: Infiltration of the kidney by alpha beta and gamma delta T cells: Effect on progression in IgA nephropathy. Kidney Int 47: 177–185, 1995 [DOI] [PubMed] [Google Scholar]
- 53.Wu H, Clarkson AR, Knight JF: Restricted gammadelta T-cell receptor repertoire in IgA nephropathy renal biopsies. Kidney Int 60: 1324–1331, 2001 [DOI] [PubMed] [Google Scholar]
- 54.Déchanet J, Merville P, Lim A, Retière C, Pitard V, Lafarge X, Michelson S, Méric C, Hallet MM, Kourilsky P, Potaux L, Bonneville M, Moreau JF: Implication of gammadelta T cells in the human immune response to cytomegalovirus. J Clin Invest 103: 1437–1449, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bachelet T, Couzi L, Pitard V, Sicard X, Rigothier C, Lepreux S, Moreau JF, Taupin JL, Merville P, Déchanet-Merville J: Cytomegalovirus-responsive γδ T cells: Novel effector cells in antibody-mediated kidney allograft microcirculation lesions. J Am Soc Nephrol 25: 2471–2482, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mori L, Lepore M, De Libero G: The immunology of CD1- and MR1-restricted T cells. Annu Rev Immunol 34: 479–510, 2016 [DOI] [PubMed] [Google Scholar]
- 57.Yang SH, Kim SJ, Kim N, Oh JE, Lee JG, Chung NH, Kim S, Kim YS: NKT cells inhibit the development of experimental crescentic glomerulonephritis. J Am Soc Nephrol 19: 1663–1671, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mesnard L, Keller AC, Michel ML, Vandermeersch S, Rafat C, Letavernier E, Tillet Y, Rondeau E, Leite-de-Moraes MC: Invariant natural killer T cells and TGF-beta attenuate anti-GBM glomerulonephritis. J Am Soc Nephrol 20: 1282–1292, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Riedel JH, Paust HJ, Turner JE, Tittel AP, Krebs C, Disteldorf E, Wegscheid C, Tiegs G, Velden J, Mittrücker HW, Garbi N, Stahl RA, Steinmetz OM, Kurts C, Panzer U: Immature renal dendritic cells recruit regulatory CXCR6(+) invariant natural killer T cells to attenuate crescentic GN. J Am Soc Nephrol 23: 1987–2000, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Godó M, Sessler T, Hamar P: Role of invariant natural killer T (iNKT) cells in systemic lupus erythematosus. Curr Med Chem 15: 1778–1787, 2008 [DOI] [PubMed] [Google Scholar]
- 61.Yang JQ, Saxena V, Xu H, Van Kaer L, Wang CR, Singh RR: Repeated alpha-galactosylceramide administration results in expansion of NK T cells and alleviates inflammatory dermatitis in MRL-lpr/lpr mice. J Immunol 171: 4439–4446, 2003 [DOI] [PubMed] [Google Scholar]
- 62.Yang JQ, Kim PJ, Singh RR: Brief treatment with iNKT cell ligand α-galactosylceramide confers a long-term protection against lupus. J Clin Immunol 32: 106–113, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yang JQ, Singh AK, Wilson MT, Satoh M, Stanic AK, Park JJ, Hong S, Gadola SD, Mizutani A, Kakumanu SR, Reeves WH, Cerundolo V, Joyce S, Van Kaer L, Singh RR: Immunoregulatory role of CD1d in the hydrocarbon oil-induced model of lupus nephritis. J Immunol 171: 2142–2153, 2003 [DOI] [PubMed] [Google Scholar]
- 64.Zeng D, Liu Y, Sidobre S, Kronenberg M, Strober S: Activation of natural killer T cells in NZB/W mice induces Th1-type immune responses exacerbating lupus. J Clin Invest 112: 1211–1222, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li L, Huang L, Sung SS, Lobo PI, Brown MG, Gregg RK, Engelhard VH, Okusa MD: NKT cell activation mediates neutrophil IFN-gamma production and renal ischemia-reperfusion injury. J Immunol 178: 5899–5911, 2007 [DOI] [PubMed] [Google Scholar]
- 66.Yang SH, Lee JP, Jang HR, Cha RH, Han SS, Jeon US, Kim DK, Song J, Lee DS, Kim YS: Sulfatide-reactive natural killer T cells abrogate ischemia-reperfusion injury. J Am Soc Nephrol 22: 1305–1314, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lee YJ, Wang H, Starrett GJ, Phuong V, Jameson SC, Hogquist KA: Tissue-specific distribution of iNKT cells impacts their cytokine response. Immunity 43: 566–578, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hinks TS: Mucosal-associated invariant T cells in autoimmunity, immune-mediated diseases and airways disease. Immunology 148: 1–12, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Peterfalvi A, Gomori E, Magyarlaki T, Pal J, Banati M, Javorhazy A, Szekeres-Bartho J, Szereday L, Illes Z: Invariant Valpha7.2-Jalpha33 TCR is expressed in human kidney and brain tumors indicating infiltration by mucosal-associated invariant T (MAIT) cells. Int Immunol 20: 1517–1525, 2008 [DOI] [PubMed] [Google Scholar]
- 70.Lepore M, Kalinichenko A, Colone A, Paleja B, Singhal A, Tschumi A, Lee B, Poidinger M, Zolezzi F, Quagliata L, Sander P, Newell E, Bertoletti A, Terracciano L, De Libero G, Mori L: Parallel T-cell cloning and deep sequencing of human MAIT cells reveal stable oligoclonal TCRβ repertoire. Nat Commun 5: 3866, 2014 [DOI] [PubMed] [Google Scholar]
- 71.Ma C, Mishra S, Demel EL, Liu Y, Zhang N: TGF-β controls the formation of kidney-resident T cells via promoting effector T cell extravasation. J Immunol 198: 749–756, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Skon CN, Lee JY, Anderson KG, Masopust D, Hogquist KA, Jameson SC: Transcriptional downregulation of S1pr1 is required for the establishment of resident memory CD8+ T cells. Nat Immunol 14: 1285–1293, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Martina MN, Noel S, Saxena A, Bandapalle S, Majithia R, Jie C, Arend LJ, Allaf ME, Rabb H, Hamad AR: Double-negative αβ T cells are early responders to AKI and are found in human kidney. J Am Soc Nephrol 27: 1113–1123, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jang HR, Gandolfo MT, Ko GJ, Satpute S, Racusen L, Rabb H: Early exposure to germs modifies kidney damage and inflammation after experimental ischemia-reperfusion injury. Am J Physiol Renal Physiol 297: F1457–F1465, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Beura LK, Hamilton SE, Bi K, Schenkel JM, Odumade OA, Casey KA, Thompson EA, Fraser KA, Rosato PC, Filali-Mouhim A, Sekaly RP, Jenkins MK, Vezys V, Haining WN, Jameson SC, Masopust D: Normalizing the environment recapitulates adult human immune traits in laboratory mice. Nature 532: 512–516, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Beura LK, Anderson KG, Schenkel JM, Locquiao JJ, Fraser KA, Vezys V, Pepper M, Masopust D: Lymphocytic choriomeningitis virus persistence promotes effector-like memory differentiation and enhances mucosal T cell distribution. J Leukoc Biol 97: 217–225, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mackay LK, Rahimpour A, Ma JZ, Collins N, Stock AT, Hafon ML, Vega-Ramos J, Lauzurica P, Mueller SN, Stefanovic T, Tscharke DC, Heath WR, Inouye M, Carbone FR, Gebhardt T: The developmental pathway for CD103(+)CD8+ tissue-resident memory T cells of skin. Nat Immunol 14: 1294–1301, 2013 [DOI] [PubMed] [Google Scholar]
- 78.Mackay LK, Minnich M, Kragten NA, Liao Y, Nota B, Seillet C, Zaid A, Man K, Preston S, Freestone D, Braun A, Wynne-Jones E, Behr FM, Stark R, Pellicci DG, Godfrey DI, Belz GT, Pellegrini M, Gebhardt T, Busslinger M, Shi W, Carbone FR, van Lier RA, Kallies A, van Gisbergen KP: Hobit and Blimp1 instruct a universal transcriptional program of tissue residency in lymphocytes. Science 352: 459–463, 2016 [DOI] [PubMed] [Google Scholar]
- 79.Masopust D, Vezys V, Usherwood EJ, Cauley LS, Olson S, Marzo AL, Ward RL, Woodland DL, Lefrançois L: Activated primary and memory CD8 T cells migrate to nonlymphoid tissues regardless of site of activation or tissue of origin. J Immunol 172: 4875–4882, 2004 [DOI] [PubMed] [Google Scholar]
- 80.Van den Hove LE, Van Gool SW, Van Poppel H, Baert L, Coorevits L, Van Damme B, Ceuppens JL: Phenotype, cytokine production and cytolytic capacity of fresh (uncultured) tumour-infiltrating T lymphocytes in human renal cell carcinoma. Clin Exp Immunol 109: 501–509, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Schenkel JM, Fraser KA, Beura LK, Pauken KE, Vezys V, Masopust D: T cell memory. Resident memory CD8 T cells trigger protective innate and adaptive immune responses. Science 346: 98–101, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ariotti S, Hogenbirk MA, Dijkgraaf FE, Visser LL, Hoekstra ME, Song JY, Jacobs H, Haanen JB, Schumacher TN: T cell memory. Skin-resident memory CD8+ T cells trigger a state of tissue-wide pathogen alert. Science 346: 101–105, 2014 [DOI] [PubMed] [Google Scholar]
- 83.Chen HD, Fraire AE, Joris I, Welsh RM, Selin LK: Specific history of heterologous virus infections determines anti-viral immunity and immunopathology in the lung. Am J Pathol 163: 1341–1355, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Netea MG, Quintin J, van der Meer JW: Trained immunity: A memory for innate host defense. Cell Host Microbe 9: 355–361, 2011 [DOI] [PubMed] [Google Scholar]
- 85.Hussell T: Heterologous immunity meets tissue-specific training. Nat Rev Immunol 16: 275, 2016 [DOI] [PubMed] [Google Scholar]