Abstract
Pathogenetic markers of diabetic kidney disease (DKD) progression to ESRD are lacking. We characterized the prognostic value of histologic findings in DKD for time to ESRD in native kidney specimens from biopsies performed from 1995 to 2011 with diabetic glomerulosclerosis as the only glomerular disease diagnosis (n=109). Biopsy specimens were analyzed according to standard methods, including determination of diabetic nephropathy class, as defined by the Renal Pathology Society. Clinical data were extracted from electronic medical records. We used competing risk models, with death as the competing risk, to estimate subdistribution hazard ratios (HRs) for ESRD. All multivariable models included age, sex, black race, baseline eGFR, and baseline proteinuria. Pathologic characteristics achieving P<0.1 were added into successively complex models. ESRD occurred in 56% of patients, and 26% of patients died before reaching ESRD. In univariate analyses, diabetic nephropathy class was not statistically significant in predicting time to ESRD. The final multivariable model (n=106) showed a borderline association between mild mesangial expansion and decreased risk for ESRD (subdistribution HR, 0.64; 95% confidence interval, 0.40 to 1.00). Poor prognostic factors in the final model included segmental sclerosis and extracapillary hypercellularity (subdistribution HR, 2.04; 95% confidence interval, 1.36 to 3.05; and subdistribution HR, 2.21; 95% confidence interval, 1.19 to 4.11, respectively). In conclusion, we identified segmental sclerosis and extracapillary hypercellularity as novel, poor prognostic indicators of time from DKD to ESRD. Whether these indicators represent a distinct pathogenetic phenotype of DKD will require a large study with a broad spectrum of disease severity.
Keywords: diabetic glomerulosclerosis, pathology, extracapillary hypercellularity, end stage, renal disease, segmental sclerosis
Diabetic kidney disease (DKD) is the most common cause of ESRD in the United States.1 It carries a 10-year mortality rate of 31% among people with diabetes and either microalbuminuria or decreased eGFR<60 ml/min per 1.73 m2.2 The vast majority of people with diabetes and early stages of kidney disease die before the onset of ESRD3; however, those with later stages of kidney disease are more likely to progress to ESRD.4 Methods to predict decline to ESRD and death currently rely upon clinical factors including diabetes duration, hemoglobin A1c (HbA1c), BP, eGFR, and proteinuria.5–9 The quest for alternative pathogenetic markers of DKD progression has been ongoing for decades, with few potential candidates identified.10–12
Nephropathologic markers have proven to be of clinical and prognostic value in nondiabetic kidney diseases such as IgA nephropathy and lupus GN.13,14 Patients with DKD do not generally undergo biopsy unless there is suspicion of an alternative cause of kidney disease that would warrant specific therapy. As a result, data are limited regarding pathologic phenotypes of DKD and whether there are pathologic changes of clinical prognostic value. The presence of Kimmelstiel–Wilson nodules may carry increased risk for subsequent ESRD and death.15 Others have postulated they may represent a distinct pathogenesis, because they are more commonly associated with diabetic retinopathy.16 An issue that previously limited the ability to analyze these variations was the lack of a uniform approach to scoring pathologic lesions in DKD.
To deal with this limitation, the Renal Pathology Society (RPS) devised a DKD classification scheme in 2010 to standardize the identification and scoring of diabetic kidney lesions.17 It provides a single score, or class, in each of the three renal compartments: the glomeruli, tubulointerstitium, and vasculature. Whereas interstitial fibrosis and tubular atrophy (IFTA), arteriolosclerosis, and arteriosclerosis are scored on the basis of the extent of a single parameter, the glomeruli have numerous potential findings, and “diabetic nephropathy class” is on the basis of the most “severe” finding. Moreover, there are several characteristics common to DKD that are not included in the RPS classification, including the presence and/or extent of mesangiolysis and glomerular hyalinosis.18 Additional features not included in the RPS scoring are segmental sclerosis (SS) and extracapillary hypercellularity (EXHC), which have been noted in patients with diabetes and may have prognostic importance.19–21
We postulated that the RPS Diabetic Nephropathy Classification does not include several glomerular parameters that could be useful for phenotyping and prognosticating the clinical course for DKD. The objectives of these analyses were to: (1) provide a detailed characterization of light microscopic pathologic lesions in clinical biopsy specimens with diabetic glomerulosclerosis; and (2) quantify the risk for diabetic ESRD according to individual nephropathologic lesions and determine which are independently associated with the risk for ESRD.
Results
We studied 155 clinical, native kidney biopsy specimens with a pathologic diagnosis of diabetic glomerulosclerosis and excluded those with evidence for any other glomerular disease. The following exclusions were made: 23 due to inadequate tissue, two were repeat biopsies on the same individual, 19 due to an additional glomerular disease, and two due to a positive serology for ANCA. A total of 109 biopsy specimens were available for analysis with a mean number of glomeruli per biopsy of 22 (±9). The pathologic characteristics are tabulated in Table 1 and illustrated in Figures 1 and 2.
Table 1.
Pathologic parameter scoring of kidney biopsy specimens from 109 patients with biopsy-proven diabetic glomerulosclerosis between 1995 and 2011
| Pathologic Parameters and Scores | Pathologic Scoring Definitions | Prevalence, n (%) |
|---|---|---|
| RPS DKD classa | ||
| I | Mild, nonspecific LM changes with GBM thickening | 0 (0.0) |
| IIa | Mild mesangial expansion in >25% of mesangium | 6 (5.5) |
| IIb | Severe mesangial expansion in >25% of mesangium | 30 (27.5) |
| III | At least one KW nodule | 56 (51.4) |
| IV | Advanced diabetic glomerulosclerosis | 17 (15.6) |
| Mild mesangial expansionb | ||
| 0 | None | 89 (81.7) |
| 1 | 1%–25% | 3 (2.8) |
| 2 | 26%–50% | 8 (7.3) |
| 3 | 51%–75% | 5 (4.6) |
| 4 | >75% | 4 (3.7) |
| Severe mesangial expansion | ||
| 0 | None | 15 (13.8) |
| 1 | 1%–25% | 5 (4.6) |
| 2 | 26%–50% | 24 (22.0) |
| 3 | 51%–75% | 39 (35.8) |
| 4 | >75% | 26 (23.9) |
| Global glomerulosclerosis | ||
| 0 | None | 6 (5.5) |
| 1 | 1%–25% | 47 (43.1) |
| 2 | 26%–50% | 38 (34.9) |
| 3 | 51%–75% | 15 (13.8) |
| 4 | >75% | 3 (2.8) |
| Presence of KW nodules | ||
| 0 | None | 44 (40.4) |
| 1 | 1%–25% | 24 (22.0) |
| 2 | 26%–50% | 23 (21.1) |
| 3 | 51%–75% | 12 (11.0) |
| 4 | >75% | 6 (5.5) |
| SS | ||
| 0 | None | 17 (15.6) |
| 1 | 1%–25% | 43 (39.5) |
| 2 | 26%–50% | 41 (37.6) |
| 3 | 51%–75% | 8 (7.3) |
| 4 | >75% | 0 (0.0) |
| Mesangiolysis | ||
| 0 | None | 30 (27.5) |
| 1 | 1%–25% | 28 (25.7) |
| 2 | 26%–50% | 36 (33.0) |
| 3 | 50%–75% | 15 (13.8) |
| 4 | >75% | 0 (0.00) |
| Glomerular hyalinosis | ||
| 0 | None | 75 (68.8) |
| 1 | Present | 34 (31.2) |
| EXHC | ||
| 0 | None | 93 (85.3) |
| 1 | 1%–25% | 14 (12.8) |
| 2 | 26%–50% | 2 (1.8) |
| 3 | >50% | 0 (0.0) |
| Arteriolosclerosis | ||
| 0 | None | 0 (0.0) |
| 1 | No thickening of wall | 15 (13.8) |
| 2 | Thickened wall with patent lumen | 45 (41.3) |
| 3 | Severely or completely narrowed lumen | 49 (45.0) |
| Arteriosclerosis | ||
| 0 | None | 4 (3.7) |
| 1 | Intimal fibrosis <25% media thickness | 15 (13.8) |
| 2 | Intimal fibrosis 25%–49% media thickness | 45 (41.3) |
| 3 | Intimal fibrosis ≥50% media thickness | 40 (36.7) |
| Missing | No arteries sampled | 5 (4.6) |
| Interstitial fibrosis | ||
| 0 | None | 2 (1.8) |
| 1 | 1%–25% cortex | 18 (16.5) |
| 2 | 26%–50% cortex | 43 (39.5) |
| 3 | 51%–75% cortex | 46 (42.2) |
| 4 | >75% cortex | 0 (0.00) |
| Interstitial inflammation | ||
| 0 | None | 10 (9.2) |
| 1 | <25% of interstitial fibrotic regions | 94 (86.2) |
| 2 | ≥25% of interstitial fibrotic regions | 5 (4.6) |
For analyses, scores of 3 and 4 were collapsed together as 3 for mild mesangial expansion, severe mesangial expansion, global glomerulosclerosis, KW nodules, SS, and mesangiolysis. Scores of 0 and 1 were collapsed together for interstitial fibrosis. LM, light microscopy; GBM, glomerular basement membrane; KW, Kimmelstiel–Wilson.
Scores as defined by the RPS Diabetic Nephropathy Classification.
Glomerular lesions were scored as % of glomeruli.
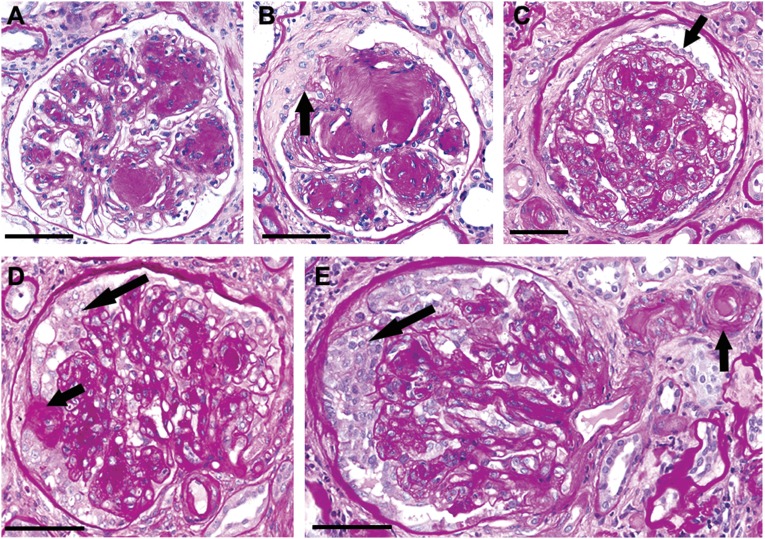
Figure 1.
Diabetic glomerulosclerosis can manifest with SS and EXHC, which correlate with worse outcome. (A) Classic nodular glomerulosclerosis with well defined Kimmelstiel–Wilson nodules with patent capillary lumens and no complicating SS or epithelial hyperplasia. (B) Diabetic nodular glomerulosclerosis complicated by segmental sclerotic obliteration of capillary lumens and an adhesion (arrow) to a broad capsular scar. (C) Diabetic glomerulosclerosis with increased mesangial matrix, extensive obliteration of capillary lumens, and mild visceral epithelial hypertrophy and hyperplasia (arrow) without multilayering. Note also the localized parietal epithelial injury with fibrosis. (D) Diabetic glomerulosclerosis with a small focus of SS with an adhesion to Bowman’s capsule (short arrow), and epithelial hyperplasia in Bowman’s space (long arrow). (E) Diabetic glomerulosclerosis with confluent epithelial hyperplasia in Bowman’s space (long arrow), and extensive obliterative hyalinosis of the afferent arteriole (short arrow). PAS stain; bar=50 µm.
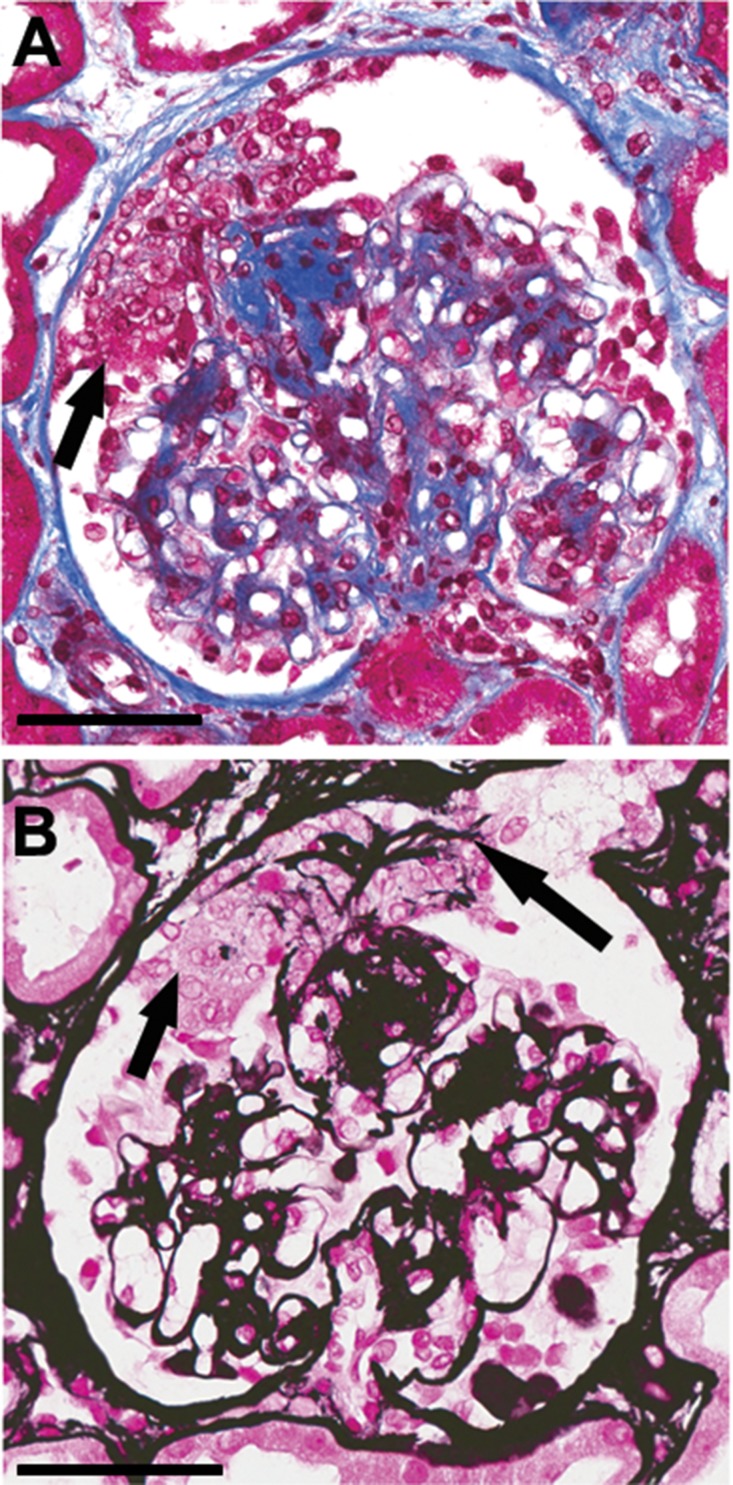
Figure 2.
Segmental EXHC in diabetic glomerulosclerosis occasionally appears to occur at sites of rupture of dilated capillaries caused by mesangiolysis. (A) Segmental EXHC (arrow) overlying a small Kimmelstiel–Wilson nodule. Masson trichrome stain; bar=100 µ. (B) The same glomerulus as in (A) stained with Jones silver stain showing EXHC (short arrow) with embedded fragments of glomerular basement membrane (long arrow). Jones silver stain; bar=50 µm.
Figure 1A shows a glomerulus with very well defined Kimmelstiel–Wilson nodules; however, capillary lumens are patent, podocytes and parietal epithelial cells are unremarkable, and there is no sclerotic obliteration of capillaries. By contrast, Figure 1, B–E, illustrates varying degrees of sclerotic obliteration of the tuft. Figure 1C shows visceral epithelial hyperplasia without multilayering, and Figure 1, D and E, shows extensive EXHC resembling the pseudocrescents of collapsing FSGS.22 Figure 2, A and B, illustrates a less common pattern of EXHC that appears to be associated with breaks in the GBM possibly at a site of mesangiolysis and capillary dilation. This lesion resembles crescents formed at the site of capillary disruption in GN.
No specimens fulfilled Columbia criteria for perihilar variant, tip lesion variant, or cellular variant of FSGS.22 Although no specimens fulfilled Columbia criteria for perihilar variant or tip lesion variant of FSGS, along with other segmental lesions, some glomeruli had SS in the glomerular tip and/or perihilar segments. As noted above, some glomeruli with EXHC had features resembling the collapsing variant of FSGS; however, none had the full complement of classic features; for example, none had numerous large hyaline droplets in the enlarged glomerular epithelial cells, and none had focal microcystic changes in cortical tubules. Although EXHC was found in nearly 15% of biopsy specimens (n=16) (Figure 1), 14 specimens had EXHC in ≤25% of glomeruli, two in 26%–50%, and none in >50%. For this study, EXHC was defined as more than two layers of epithelial cells affecting >10% of the surface of the tuft or Bowman’s capsule and comprising >10% cells. In most instances, the EXCH resembled the EXHC of collapsing FSGS rather than the EXHC of crescents typical for crescentic GN.
Sociodemographic and clinical characteristics at the time of kidney biopsy and follow-up data are shown in aggregate and stratified by black race in Table 2. In total, 59 individuals (56%) reached ESRD and 28 (26%) died before reaching ESRD. Three individuals were lost to follow-up and only had baseline clinical and pathologic data.
Table 2.
Baseline (at the time of kidney biopsy) and longitudinal clinical characteristics of 109 patients with biopsy proven diabetic glomerulosclerosis (1995–2011), stratified by black race
| Clinical Characteristics | Missing Data | Total Sample | Black Race | Other Races | P Value |
|---|---|---|---|---|---|
| n=109 | n=53 | n=56 | |||
| Median age (IQR), yr | 0 | 52 (46, 62) | 49 (44, 59) | 54 (46, 64) | 0.1 |
| Male sex, n (%) | 0 | 62 (56.9%) | 25 (47.2%) | 37 (66.1%) | 0.05 |
| Type 2 diabetes, n (%) | 3 | 92 (86.8%) | 46 (90.2%) | 46 (83.6%) | 0.4 |
| Median diabetes duration (IQR), yr | 11 | 14 (10, 17) | 16 (10, 17) | 13 (9, 19) | 0.7 |
| HbA1c (IQR), % | 33 | 6.8 (6.2, 9.0) | 6.7 (6.0, 9.1) | 7.0 (6.2, 8.9) | 0.8 |
| Systolic BP (IQR), mmHg | 9 | 151 (134, 165) | 157 (137, 170) | 145 (133, 161) | 0.09 |
| Median follow-up time (IQR), mo | 3 | 18 (6, 42) | 18 (6, 31) | 18 (5, 59) | 0.3 |
| Median eGFR (IQR), ml/min per 1.73 m2 | 0 | 22 (15, 37) | 20 (13, 33) | 24 (17, 56) | 0.1 |
| Median urine proteina (IQR), g/g | 13 | 4.5 (2.0, 8.1) | 7.3 (2.6, 9.1) | 3.0 (0.8, 6.5) | 0.003 |
| Median eGFR slope (IQR), ml/min per 1.73 m2 per yr | 9 | –3.3 (–14.2, 0.0) | –7.1 (–20.1, 0.0) | –1.0 (–6.1, 0.0) | 0.09 |
| ESRD during follow up, n (%) | 3 | 59 (56%) | 37 (73%) | 22 (40%) | <0.001 |
| Median time to ESRD (IQR), mo | 50 | 9 (3, 30) | 15 (4, 31) | 7 (3, 19) | 0.2 |
| Death during follow up, n (%) | 3 | 28 (26%) | 11 (21%) | 17 (30%) | 0.3 |
| Median time to death (IQR), mo | 81 | 23 (12, 44) | 26 (12, 40) | 18 (11, 48) | 0.9 |
IQR, interquartile range.
Urine protein on the basis of either 24 h urine protein collection or spot urine protein-to-creatinine ratio.
Results from univariate and multivariable Cox proportional subdistribution hazards models of ESRD with death as a competing risk are displayed in Table 3. The only pathologic lesions to remain significant in the fully adjusted model were mild mesangial expansion, SS, and EXHC.
Table 3.
Univariate and multivariable competing risk analyses for time to ESRD using death as a competing risk from 106 people with biopsy-proven diabetic glomerulosclerosis (1995–2011)
| Characteristics | Univariate Models SHR (95% CI) | P Value | Glomerular Model SHR (95% CI) | P Value | Fully Adjusted Model SHR (95% CI) | P Value |
|---|---|---|---|---|---|---|
| Clinical characteristics | ||||||
| Age, per 10 yr | 0.88 (0.72 and 1.06) | 0.2 | 0.71 (0.56 and 0.89) | <0.01 | 0.71 (0.56 and 0.90) | <0.01 |
| Female sex | 0.90 (0.54 and 1.49) | 0.7 | 0.77 (0.44 and 1.33) | 0.4 | 0.76 (0.44 and 1.33) | 0.3 |
| Black race | 2.18 (1.27 and 3.72) | <0.01 | 1.46 (0.79 and 2.68) | 0.2 | 1.47 (0.79 and 2.70) | 0.2 |
| Baseline eGFR, per 10 ml/min per 1.73 m2 | 0.81 (0.69 and 0.94) | 0.01 | 0.72 (0.57 and 0.92) | <0.01 | 0.75 (0.62 and 0.92) | <0.01 |
| Baseline proteinuria, per 2 g/g | 1.05 (0.99 and 1.12) | 0.1 | 0.96 (0.85 and 1.08) | 0.5 | 0.95 (0.83 and 1.08) | 0.5 |
| Glomerular characteristics | ||||||
| RPS diabetic nephropathy class | 1.17 (0.82 and 1.66) | 0.4 | – | – | – | – |
| Mild mesangial expansion | 0.69 (0.53 and 0.91) | 0.01 | 0.61 (0.40 and 0.95) | 0.03 | 0.64 (0.40 and 1.00) | 0.05 |
| Mod-severe mesangial expansion | 1.02 (0.84 and 1.24) | 0.8 | – | – | – | – |
| Global glomerulosclerosis | 1.40 (1.03 and 1.90) | 0.03 | 0.95 (0.70 and 1.29) | 0.8 | – | – |
| Kimmelstiel–Wilson nodules | 1.22 (0.98 and 1.52) | 0.08 | 1.02 (0.78 and 1.32) | 0.9 | – | – |
| SS | 1.98 (1.48 and 2.65) | <0.0001 | 2.17 (1.42 and 3.35) | <0.001 | 2.04 (1.36 and 3.05) | <0.001 |
| Mesangiolysis | 1.13 (0.89 and 1.43) | 0.3 | – | – | – | – |
| Glomerular hyalinosis | 1.54 (0.93 and 2.57) | 0.09 | 0.73 (0.36 and 1.48) | 0.4 | – | – |
| EXHC | 1.88 (0.93 and 3.75) | 0.08 | 2.17 (1.16 and 4.05) | 0.02 | 2.21 (1.19 and 4.11) | 0.01 |
| Interstitial characteristics | ||||||
| Interstitial fibrosis | 2.10 (1.37 and 3.21) | <0.001 | – | – | 1.01 (0.58 and 1.77) | 0.9 |
| Interstitial Inflammation | 0.81 (0.37 and 1.74) | 0.6 | – | – | – | – |
| Vascular characteristics | ||||||
| Arteriolosclerosis | 1.67 (1.10 and 2.52) | 0.02 | – | – | 1.01 (0.64 and 1.61) | >0.99 |
| Arteriosclerosis | 1.30 (0.92 and 1.83) | 0.1 | – | – | – | – |
The Glomerular Model=clinical covariates (regardless of P value) and glomerular variables significant to P<0.1 in univariate models. The Fully Adjusted Model=clinical covariates (regardless of P value), glomerular variables significant to P<0.1 in the Glomerular Model, and interstitial and vascular variables significant to P<0.10 in univariate models. SHR, subdistribution hazards ratio; –, not included, Mod-severe, moderate to severe.
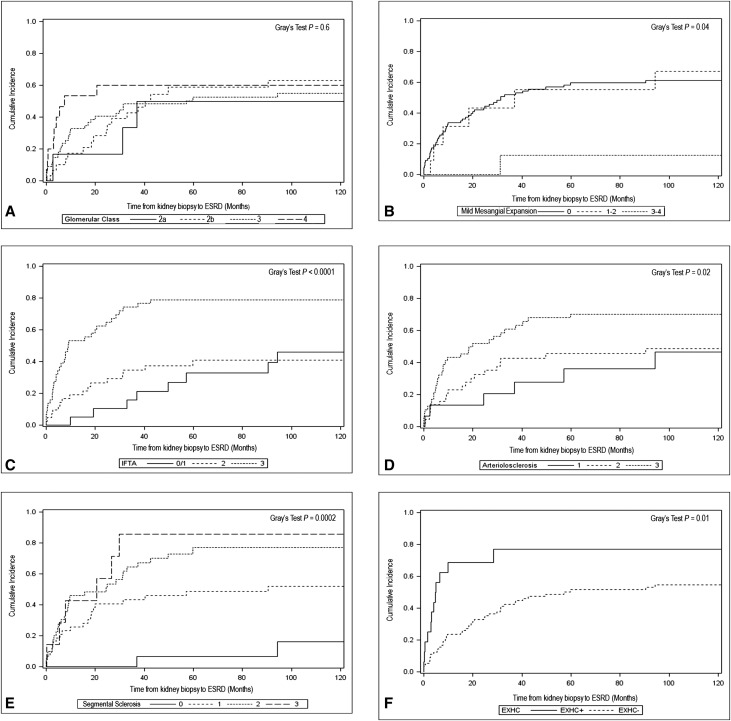
Figure 3, A–F, displays the cumulative incidence function curves for glomerular class, mild mesangial expansion, IFTA, arteriolosclerosis, SS, and EXHC. RPS Diabetic Nephropathy Class remained nonsignificant, whereas arteriolosclerosis gained significance for predicting time to ESRD. IFTA, SS, and EXHC maintained univariate significance for their ability to predict clinical prognosis.
Figure 3.
Cumulative incidence plots for ESRD according to nephropathologic characteristics. (A) Nephropathy class; (B) mild mesangial expansion; (C) IFTA; (D) arteriolosclerosis; (E) SS; (F) EXHC.
We conducted several post hoc analyses. Incorporating diabetes type into the fully adjusted model, we found the significance of EXHC was no longer statistically significant (subdistribution hazard ratio [SHR]=1.72; 95% confidence interval [95% CI], 0.66 to 4.49; P=0.3). When we restricted our fully adjusted model to those with type 2 diabetes, the significance of EXHC was greater than in the full cohort (SHR=3.25; 95% CI, 1.41 to 7.50; P<0.01), signifying a possible interaction between diabetes type and the prognostic significance of EXHC. Given the small number of subjects with type 1 diabetes, we did not have the power to detect significance so did not perform the analysis in this group. We also performed a post hoc competing risk model of ESRD with death (adjusted for age, sex, race, baseline eGFR, and urine protein) incorporating both SS and EXHC into a single composite exposure and modeled as a categoric variable. Results yielded SHR=7.84 (95% CI, 2.22 to 27.68) when comparing SS+/EXHC− versus SS−/EXHC− and SHR=17.73 (95% CI, 4.63 to 67.89) when comparing SS+/EXHC+ versus SS−/EXHC−.
Discussion
This is a detailed study of the association of pathologic findings from clinical biopsies of DKD and clinical outcome. The most interesting and potentially impactful conclusion is that the degree of SS and presence of EXHC are independent predictors of clinical progression to ESRD. We hypothesize that disturbances in glomerular visceral and parietal epithelial cells, as well as SS, are manifestations of more severe glomerular injury, including more severe podocyte injury. Epithelial hyperplasia resulting in EXHC has been identified in other forms of progressive glomerular sclerosis, including FSGS, and there is evidence that it is related to the progression of glomerular sclerosis.22–24 As is true in the collapsing variant of FSGS, the origin of the EXHC from podocytes versus parietal epithelial cells versus both is not known; thus, we have referred to this as EXHC rather than podocyte hyperplasia. We also avoided the term crescent, because we do not know if the EXHC arises from epithelial dedifferentiation and hyperplasia or as a result of rupture of capillaries at sites of mesangiolysis and capillary aneurysm formation. The former would be similar to pseudocrescents of collapsing FSGS and the latter to the crescents of GN. Most of the EXHC lesions we observed resembled the lesions of collapsing FSGS (Figure 1, D and E); however, a few appeared to be related to capillary rupture (Figure 2, A and B).
This is the first report that the presence of EXHC in DKD is predictive of time to ESRD. Moreover, post hoc analyses demonstrate there may be effect modification by diabetes type, given that EXHC was NS if diabetes type was included, but was highly significant when analyses were isolated to those with type 2 diabetes. This study was primarily of people with advanced type 2 diabetes, however, and the lack of significance in type 1 diabetes could have been due to a lack of power. Further testing of effect modification by diabetes type is necessary. Before our study, there have been occasional case reports of “crescents” in DKD,25–27 one of which had rapid progression of renal failure.26 There are two case series of crescentic GN due to diabetes, but both reported a lower prevalence of EXHC (3%–5%) than in our cohort.19,28 In 1975, Elfenbein and Reyes19 proposed that the rare occurrence of crescent formation in diabetes is related to “exudative lesions,” or “fibrin caps” (hyaline caps), because they are more highly correlated with the severity of diabetic vascular disease than the degree of mesangial expansion. Theoretically, eventual rupture of the glomerular basement membrane stems from the sheer stress placed on the capillary wall of a hyaline cap rather than inflammatory damage as occurs in immune-mediated glomerulonephritides. Hyaline caps occur at sites of mesangiolysis and capillary aneurysm formation. We did not observe an association between mesangiolysis or hyalinosis with EXHC (data not shown).
Salvatore et al.20 described a series (n=26) of HIV-negative patients with diabetes and collapsing glomerulopathy with epithelial proliferation in Bowman’s space (pseudocrescents), virtually identical to the EXHC observed in our study. They propose that severe arteriolosclerosis leads to hypoxic injury to podocytes and resultant collapse of the glomerular tuft. We observed marked arteriolosclerosis with hyalinosis in arterioles in our patients (Figure 1E); however, this did not correlate with EXHC (data not shown).
Salvatore et al.20 observed that cells within these “pseudocrescents” stained positively for cytokeratin, a marker of parietal epithelial cells. Markers of parietal epithelial cells (claudin-1) have also been found within the glomerular tuft in advanced DKD without collapsing glomerulopathy.29 Further, markers of podocytes (WT-1, p57) have been found on Bowman’s capsule, suggesting that parietal epithelial cells may have the capacity to act as progenitor cells for injured podocytes. Our finding that EXHC is only present in the setting of SS, a marker of podocyte injury, would also be supportive of this notion.
We found that RPS Diabetic Nephropathy Class is not predictive of time to ESRD. This finding is counter to the contention that nodular glomerulosclerosis carries a poorer clinical outcome than diffuse mesangial lesions.15,16,30 Moreover, it is contrary to previous studies, which have supported the prognostic utility of the RPS Diabetic Nephropathy Classification.31–34 These data have been generated in Southeast Asian cohorts, in whom the natural history of DKD may be distinct from European- or African-derived cohorts. Moreover, care practices may vary between countries, so the findings of clinical biopsies performed elsewhere may not be applicable in the United States.
Our study also illustrates the trend in the United States to perform kidney biopsies in diabetes at late stages of disease. Further evidence of this practice comes from a large study (n=620) wherein patients with diabetes underwent clinical kidney biopsy at a median eGFR of 29 ml/min per 1.73 m2 and proteinuria of 4.3 g/d.35 Pathologic changes signifying increased clinical risk likely vary depending on the severity of disease under study. A study of Chinese patients with diabetes undergoing clinical biopsy had earlier stage disease, with a mean baseline eGFR of 73±33 ml/min per 1.73 m2 and 24-hour urine protein 1.5 g/d.34 In this study, glomerular and interstitial lesions were independently predictive of the time to ESRD; however, when RPS classes I and IIa were removed from the analyses, RPS class was no longer an independent predictor.
Our study has several limitations, including a small sample size from a single institution. Thus, our findings are hypothesis generating and will require re-examination using larger data sets. There was also extensive missing data on several important clinical prognostic parameters such as HbA1c, SBP, and diabetes duration; although given the late stage of kidney disease in this population, we don’t know how much effect these data would have when added to eGFR, urine protein, and pathologic data. Future, prospective studies will be important to determine this. As stated previously, clinical kidney biopsies are generally performed in people with diabetes who are suspected of having nondiabetic kidney disease. As such, these individuals may not be representative of DKD in the general population. Strengths of our study include the broad ethnic diversity of our population and the well defined hard clinical outcomes. The blinded, detailed pathology evaluation is also a strength of this study.
Our study illustrates the late stage of disease from clinical biopsies performed in patients with predominantly type 2 diabetic glomerulosclerosis. Despite this fact, we were able to demonstrate the utility of biopsy for prognosticating clinical disease course. Our finding of SS and EXHC as novel, independent markers of time to diabetic ESRD needs to be re-examined in other cohorts. The biologic basis for the EXHC will be the subject of future studies. The presence of EXHC may represent a phenotypic variant of diabetic glomerulosclerosis that leads to rapid decline in GFR and ultimately to ESRD.
Concise Methods
Study Design and Population
This is a longitudinal, retrospective study of patients who underwent clinical, native kidney biopsy at the University of North Carolina (UNC) between January 1, 1995 and December 31, 2011, and had a pathologic diagnosis of diabetic glomerulosclerosis. Patients with superimposed glomerular disease or a positive ANCA test were excluded. If patients had more than one kidney biopsy performed during this time period, only the first biopsy was included for analysis. Biopsy cores with inadequate tissue (<10 glomeruli on pre-existing slides from paraffin-embedded cores) were excluded.
Clinical data were abstracted from electronic medical records from the time of kidney biopsy to one of four endpoints: ESRD, death, loss to follow-up, or until December 31, 2015. ESRD was defined as the need for chronic RRT. The eGFR was calculated using the creatinine-based Chronic Kidney Disease Epidemiology Collaboration equation.36 Proteinuria was quantified by either the spot urine protein-to-creatinine ratio or the 24-hour urine protein-to-creatinine ratio, depending on which was available. Slope of eGFR was calculated using both prebiopsy and postbiopsy data, when available. Diabetes type was frequently ambiguous in medical records. In these cases, a physician (A.K.M.) deduced diabetes type on a combination of age of onset, history of oral hypoglycemic use, and/or insulin regimen. The UNC Committee on the Protection of the Rights of Human Subjects approved the study.
Pathology Review of Kidney Biopsy Specimens
All kidney biopsy specimens were processed at UNC and evaluated by: (1) light microscopy on formalin-fixed, paraffin-embedded tissue using hematoxylin and eosin, periodic acid–Schiff, and Masson trichrome staining; (2) immunofluorescence microscopy on frozen tissue using antibodies specific for IgG, IgA, IgM, λ light chains, κ light chains, C3, C1q, and fibrin; and (3) transmission electron microscopy on tissue fixed in 2.5% gluteraldehyde and plastic embedded.
Light microscopy was performed in a similar fashion to usual diagnostic evaluation with ten slides with three to four step sections of tissue each stained with hematoxylin and eosin, periodic acid–Schiff, Masson trichrome, and Jones silver stains. Light microscopy slides were reviewed and scored by two expert nephropathologists (J.C.J. and A.G.). Any scoring discrepancies were resolved by agreement. Renal biopsy review was carried out without the knowledge of clinical outcomes. Pathologic scoring definitions are summarized as categoric variables in Table 1. The glomerular compartment was scored according to the RPS Diabetic Nephropathy Classification.17 Electron micrographs prepared for the original clinical biopsy interpretation and reports of findings by immunofluorescence microscopy were reviewed to confirm the absence of nondiabetic glomerular disease.
Statistical Analyses
Baseline (at the time of kidney biopsy) demographic and clinical characteristics and outcomes were quantified using percentages for categoric variables and median and interquartile ranges for continuous variables. Characteristics were compared between black race versus all other races, with P values for trend calculated by Fisher exact test for categoric variables and Kruskal–Wallis test for continuous variables.
Univariate risk models of ESRD with death as a competing risk were constructed to assess the association between individual clinical and pathologic variables and ESRD as the primary event and death as a competing event.37
Data for urine protein was missing in 13 individuals (11 of whom were included in multivariable analyses). We used Rubin multiple imputation methods for multivariable competing risk models. Two multivariable competing risk models were created, both of which included clinical characteristics: age, sex (female versus male), race (black versus other), baseline eGFR, and urinary protein. The first multivariable model (the “Glomerular Model”) incorporated only those glomerular characteristics significant to P<0.1 in the univariate models. The “Fully Adjusted Model” included glomerular characteristics significant to P<0.05 in the Glomerular Model, as well as interstitial and vascular characteristics significant to P<0.1 in univariate models. Because of the number of missing clinical data, additional adjustments for HbA1c, SBP, and diabetes duration were not made.
Cumulative incidence function curves were used for comparing the cumulative incidence of ESRD among the categories of the RPS glomerular class, mild mesangial expansion, interstitial fibrosis, arteriolosclerosis, SS, and EXH. In these analyses, variables were modeled as ordinal measures. Gray’s test was used to evaluate if there was a statistically significant difference for cumulative incidence of ESRD among comparison groups.
A P value for two-sided tests of <0.05 was considered to be significant in the statistical analyses. All analyses were performed using SAS version 9.4 statistical software (SAS Institute, Cary, NC).
Disclosures
None.
Supplementary Material
Acknowledgments
We would like to thank Ms. Caroline Poulton for providing data management.
Research reported in this publication was supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under Award Number K23DK093804.
Part of this work was presented at the 2015 American Society of Nephrology Scientific Sessions in San Diego, California, and at the 2017 International Society of Nephrology Scientific Sessions in Mexico City, Mexico.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.United States Renal Data System : USRDS 2016 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, Bethesda, MD, National Institute of Diabetes and Digestive and Kidney Diseases, 2016 [Google Scholar]
- 2.Afkarian M, Sachs MC, Kestenbaum B, Hirsch IB, Tuttle KR, Himmelfarb J, de Boer IH: Kidney disease and increased mortality risk in type 2 diabetes. J Am Soc Nephrol 24: 302–308, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Retnakaran R, Cull CA, Thorne KI, Adler AI, Holman RR; UKPDS Study Group : Risk factors for renal dysfunction in type 2 diabetes: U.K. Prospective Diabetes study 74. Diabetes 55: 1832–1839, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Packham DK, Alves TP, Dwyer JP, Atkins R, de Zeeuw D, Cooper M, Shahinfar S, Lewis JB, Lambers Heerspink HJ: Relative incidence of ESRD versus cardiovascular mortality in proteinuric type 2 diabetes and nephropathy: Results from the DIAMETRIC (Diabetes Mellitus Treatment for Renal Insufficiency Consortium) database. Am J Kidney Dis 59: 75–83, 2012 [DOI] [PubMed] [Google Scholar]
- 5.Ivory SE, Packham DK, Reutens AT, Wolfe R, Rohde RD, Lewis J, Atkins RC; Collaborative Study Group : Residual proteinuria and eGFR predict progression of renal impairment within 2 years in type 2 diabetic patients with nephropathy who are receiving optimal treatment with angiotensin receptor blockers. Nephrology (Carlton) 18: 516–524, 2013 [DOI] [PubMed] [Google Scholar]
- 6.Zoppini G, Targher G, Chonchol M, Ortalda V, Negri C, Stoico V, Bonora E: Predictors of estimated GFR decline in patients with type 2 diabetes and preserved kidney function. Clin J Am Soc Nephrol 7: 401–408, 2012 [DOI] [PubMed] [Google Scholar]
- 7.Bakris GL, Weir MR, Shanifar S, Zhang Z, Douglas J, van Dijk DJ, Brenner BM; RENAAL Study Group : Effects of blood pressure level on progression of diabetic nephropathy: Results from the RENAAL study. Arch Intern Med 163: 1555–1565, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Pavkov ME, Knowler WC, Hanson RL, Bennett PH, Nelson RG: Predictive power of sequential measures of albuminuria for progression to ESRD or death in Pima Indians with type 2 diabetes. Am J Kidney Dis 51: 759–766, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Astor BC, Hallan SI, Miller ER 3rd, Yeung E, Coresh J: Glomerular filtration rate, albuminuria, and risk of cardiovascular and all-cause mortality in the US population. Am J Epidemiol 167: 1226–1234, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Niewczas MA, Gohda T, Skupien J, Smiles AM, Walker WH, Rosetti F, Cullere X, Eckfeldt JH, Doria A, Mayadas TN, Warram JH, Krolewski AS: Circulating TNF receptors 1 and 2 predict ESRD in type 2 diabetes. J Am Soc Nephrol 23: 507–515, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schanstra JP, Zürbig P, Alkhalaf A, Argiles A, Bakker SJ, Beige J, Bilo HJ, Chatzikyrkou C, Dakna M, Dawson J, Delles C, Haller H, Haubitz M, Husi H, Jankowski J, Jerums G, Kleefstra N, Kuznetsova T, Maahs DM, Menne J, Mullen W, Ortiz A, Persson F, Rossing P, Ruggenenti P, Rychlik I, Serra AL, Siwy J, Snell-Bergeon J, Spasovski G, Staessen JA, Vlahou A, Mischak H, Vanholder R: Diagnosis and prediction of CKD progression by assessment of urinary peptides. J Am Soc Nephrol 26: 1999–2010, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pena MJ, de Zeeuw D, Mischak H, Jankowski J, Oberbauer R, Woloszczuk W, Benner J, Dallmann G, Mayer B, Mayer G, Rossing P, Lambers Heerspink HJ: Prognostic clinical and molecular biomarkers of renal disease in type 2 diabetes. Nephrol Dial Transplant 30[Suppl 4]: iv86–iv95, 2015 [DOI] [PubMed] [Google Scholar]
- 13.Tanaka S, Ninomiya T, Katafuchi R, Masutani K, Tsuchimoto A, Noguchi H, Hirakata H, Tsuruya K, Kitazono T: Development and validation of a prediction rule using the Oxford classification in IgA nephropathy. Clin J Am Soc Nephrol 8: 2082–2090, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hwang J, Kim HJ, Oh JM, Ahn JK, Lee YS, Lee J, Kim YG, Huh WS, Seo J, Koh EM, Cha HS: Outcome of reclassification of World Health Organization (WHO) class III under International Society of Nephrology-Renal Pathology Society (ISN-RPS) classification: Retrospective observational study. Rheumatol Int 32: 1877–1884, 2012 [DOI] [PubMed] [Google Scholar]
- 15.Heaf JG, Løkkegaard H, Larsen S: The relative prognosis of nodular and diffuse diabetic nephropathy. Scand J Urol Nephrol 35: 233–238, 2001 [DOI] [PubMed] [Google Scholar]
- 16.Schwartz MM, Lewis EJ, Leonard-Martin T, Lewis JB, Batlle D; The Collaborative Study Group : Renal pathology patterns in type II diabetes mellitus: Relationship with retinopathy. Nephrol Dial Transplant 13: 2547–2552, 1998 [DOI] [PubMed] [Google Scholar]
- 17.Tervaert TW, Mooyaart AL, Amann K, Cohen AH, Cook HT, Drachenberg CB, Ferrario F, Fogo AB, Haas M, de Heer E, Joh K, Noël LH, Radhakrishnan J, Seshan SV, Bajema IM, Bruijn JA; Renal Pathology Society : Pathologic classification of diabetic nephropathy. J Am Soc Nephrol 21: 556–563, 2010 [DOI] [PubMed] [Google Scholar]
- 18.Najafian B, Fogo AB, Lusco MA, Alpers CE: AJKD atlas of renal pathology: Diabetic nephropathy. Am J Kidney Dis 66: e37–e38, 2015 [DOI] [PubMed] [Google Scholar]
- 19.Elfenbein IB, Reyes JW: Crescents in diabetic glomerulopathy. Incidence and clinical significance. Lab Invest 33: 687–695, 1975 [PubMed] [Google Scholar]
- 20.Salvatore SP, Reddi AS, Chandran CB, Chevalier JM, Okechukwu CN, Seshan SV: Collapsing glomerulopathy superimposed on diabetic nephropathy: Insights into etiology of an under-recognized, severe pattern of glomerular injury. Nephrol Dial Transplant 29: 392–399, 2014 [DOI] [PubMed] [Google Scholar]
- 21.Fogo AB: Causes and pathogenesis of focal segmental glomerulosclerosis. Nat Rev Nephrol 11: 76–87, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.D’Agati VD, Fogo AB, Bruijn JA, Jennette JC: Pathologic classification of focal segmental glomerulosclerosis: A working proposal. Am J Kidney Dis 43: 368–382, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Appel D, Kershaw DB, Smeets B, Yuan G, Fuss A, Frye B, Elger M, Kriz W, Floege J, Moeller MJ: Recruitment of podocytes from glomerular parietal epithelial cells. J Am Soc Nephrol 20: 333–343, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lim BJ, Yang JW, Do WS, Fogo AB: Pathogenesis of socal segmental glomerulosclerosis. J Pathol Transl Med 50: 405–410, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Otani N, Akimoto T, Yumura W, Matsubara D, Iwazu Y, Numata A, Miki T, Takemoto F, Fukushima N, Muto S, Kusano E: Is there a link between diabetic glomerular injury and crescent formation? A case report and literature review. Diagn Pathol 7: 46, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tóth T: Epithelial crescent in diabetic glomeruli. A case report. Int Urol Nephrol 19: 347–353, 1987 [DOI] [PubMed] [Google Scholar]
- 27.Wakabayashi N, Takeda S, Imai T, Akimoto T, Nagata D: Unexpected observation of glomerular crescents in a patient with diabetes who developed drug-induced acute tubulointerstitial nephritis: A possible feature of diabetic nephropathy? Nephrology (Carlton) 20: 438–439, 2015 [DOI] [PubMed] [Google Scholar]
- 28.Jennette JC: Rapidly progressive crescentic glomerulonephritis. Kidney Int 63: 1164–1177, 2003 [DOI] [PubMed] [Google Scholar]
- 29.Andeen NK, Nguyen TQ, Steegh F, Hudkins KL, Najafian B, Alpers CE: The phenotypes of podocytes and parietal epithelial cells may overlap in diabetic nephropathy. Kidney Int 88: 1099–1107, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao HL, Lai FM, Tong PC, Tomlinson B, Chan JC: Clinicopathologic characteristics of nodular glomerulosclerosis in Chinese patients with type 2 diabetes. Am J Kidney Dis 44: 1039–1049, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Okada T, Nagao T, Matsumoto H, Nagaoka Y, Wada T, Nakao T: Histological predictors for renal prognosis in diabetic nephropathy in diabetes mellitus type 2 patients with overt proteinuria. Nephrology (Carlton) 17: 68–75, 2012 [DOI] [PubMed] [Google Scholar]
- 32.Oh SW, Kim S, Na KY, Chae DW, Kim S, Jin DC, Chin HJ: Clinical implications of pathologic diagnosis and classification for diabetic nephropathy. Diabetes Res Clin Pract 97: 418–424, 2012 [DOI] [PubMed] [Google Scholar]
- 33.Mise K, Hoshino J, Ueno T, Hazue R, Sumida K, Hiramatsu R, Hasegawa E, Yamanouchi M, Hayami N, Suwabe T, Sawa N, Fujii T, Hara S, Ohashi K, Takaichi K, Ubara Y: Clinical and pathological predictors of estimated GFR decline in patients with type 2 diabetes and overt proteinuric diabetic nephropathy. Diabetes Metab Res Rev 31: 572–581, 2015 [DOI] [PubMed] [Google Scholar]
- 34.An Y, Xu F, Le W, Ge Y, Zhou M, Chen H, Zeng C, Zhang H, Liu Z: Renal histologic changes and the outcome in patients with diabetic nephropathy. Nephrol Dial Transplant 30: 257–266, 2015 [DOI] [PubMed] [Google Scholar]
- 35.Sharma SG, Bomback AS, Radhakrishnan J, Herlitz LC, Stokes MB, Markowitz GS, D’Agati VD: The modern spectrum of renal biopsy findings in patients with diabetes. Clin J Am Soc Nephrol 8: 1718–1724, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, Kusek JW, Manzi J, Van Lente F, Zhang YL, Coresh J, Levey AS; CKD-EPI Investigators : Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med 367: 20–29, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fine J, Gray RJ: A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 94: 496–509, 1999 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.