Abstract
Progress in research and developing therapeutics to prevent diabetic kidney disease (DKD) is limited by a lack of animal models exhibiting progressive kidney disease. Chronic hypertension, a driving factor of disease progression in human patients, is lacking in most available models of diabetes. We hypothesized that superimposition of hypertension on diabetic mouse models would accelerate DKD. To test this possibility, we induced persistent hypertension in three mouse models of type 1 diabetes and two models of type 2 diabetes by adeno-associated virus delivery of renin (ReninAAV). Compared with LacZAAV-treated counterparts, ReninAAV-treated type 1 diabetic Akita/129 mice exhibited a substantial increase in albumin-to-creatinine ratio (ACR) and serum creatinine level and more severe renal lesions. In type 2 models of diabetes (C57BKLS db/db and BTBR ob/ob mice), compared with LacZAAV, ReninAAV induced significant elevations in ACR and increased the incidence and severity of histopathologic findings, with increased serum creatinine detected only in the ReninAAV-treated db/db mice. The uninephrectomized ReninAAV db/db model was the most progressive model examined and further characterized. In this model, separate treatment of hyperglycemia with rosiglitazone or hypertension with lisinopril partially reduced ACR, consistent with independent contributions of these disorders to renal disease. Microarray analysis and comparison with human DKD showed common pathways affected in human disease and this model. These results identify novel models of progressive DKD that provide researchers with a facile and reliable method to study disease pathogenesis and support the development of therapeutics.
Keywords: chronic diabetic complications, diabetic nephropathy, diabetic glomerulopathy, electron microscopy, hypertension
Diabetic kidney disease (DKD) is a clinical syndrome composed of proteinuria, hypertension, cardiovascular event risk, and progression to ESRD. Despite growing prevalence and burden on the health care system, there have been few improvements in the treatment of DKD over 25 years aside from recent data indicating that SGLT2 inhibitors play a beneficial role in DKD.1 Discovery of new therapies has been hindered by the lack of animal models that reproduce human disease and progress to renal failure, with most available models developing only early manifestation of DKD.
Hypertension is an independent risk factor for ESRD in patients with diabetes and a major factor driving progressive DKD.2–4 Elevated arterial pressure (AP) increases glomerular pressure, which is critical for initiation and progression of DKD.5,6 Hypertension promotes renal inflammation, a feature of human disease not observed in many DKD models.7,8 The role of hypertension in driving DKD progression is supported by the C57BLKS eNOS−/−db/db model, a hypertensive DKD model that exhibits reduced GFR.9,10 The utility of the eNOS−/−db/db model is constrained by costly breeding requirements and apparent presence of renal infarcts.11 Introduction of additional knockouts or transgenes on this already complex breeding scheme further increases cost and complexity of experimentation. Overactivation of the renin-angiotensin system also has been used to induce hypertension in db/db mice via AngII osmotic minipumps. This resulted in transient increases in albumin-to-creatinine ratio (ACR), which declined after a few weeks, possibly due to loss of drug stability and/or reaching the lifespan of the minipump.12 These observations suggest that alternative approaches to establish chronic hypertension are needed to facilitate study of disease progression in DKD. To address this need, the effects of adeno-associated virus delivery of renin (ReninAAV)–induced hypertension on kidney disease in diverse models of murine diabetes mellitus were examined.
Results
ReninAAV in Type 1 Diabetic Akita Models
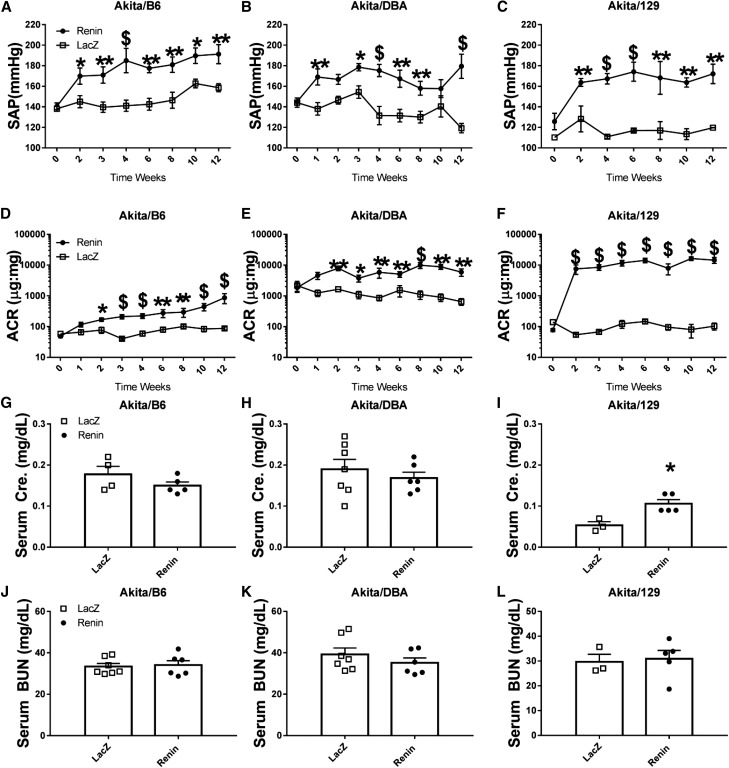
The effects of a single injection of ReninAAV (1×1010 GC) were evaluated in mice with the Akita (Ins2+/C96Y) model introgressed onto three different genetic backgrounds: C57BL/6, DBA/2J, and 129/S6. ReninAAV elevated (P<0.05) systolic arterial pressure (SAP) in all three backgrounds, which persisted in the 12-week study compared with respective LacZAAV controls (Figure 1, A–C). A significant increase in ACR (P<0.05) was detected within 2 weeks of ReninAAV and remained elevated in Akita/DBA (5964±1601) and Akita/129 (14,531±3555), with relatively modest but significant elevations (P<0.05) in Akita/B6 (868±311) compared with respective LacZ controls (652±162, 104±27, and 88±10, respectively) (Figure 1, D–F, Table 1). Serum creatinine was significantly elevated (P<0.01) only in ReninAAV Akita/129 mice (0.11±0.01 mg/dl versus LacZ 0.05±0.01 mg/dl) (Figure 1, G–I, Table 1). ReninAAV did not affect BUN in any background tested (Figure 1, J–L, Table 1).
Figure 1.
ReninAAV in type 1 diabetic Akita models accelerates renal disease. ReninAAV (1×1010 GC) elevated SAP, with effects that were detected within 1–2 weeks of injection and persisted for 12 weeks in Akita mice of three different genetic backgrounds (A) Akita/B6, (B) Akita/DBA, and (C) Akita/129 compared with respective LacZAAV controls. (D–F) ReninAAV increased ACR in all three genetic backgrounds within 2 weeks postinjection and remained elevated for the 12-week duration, with modest effects detected in Akita/B6 mice and most significant changes observed in Akita/129 mice compared with respective LacZ controls. Serum creatinine was unchanged in (G) ReninAAV Akita/B6 and (H) Akita/DBA mice, but it was statistically elevated in (I) ReninAAV Akita/129 mice compared with respective LacZAAV controls. (J–L) ReninAAV did not have a significant effect on serum BUN in any Akita strain tested. n=3–10 per group. *P<0.05; **P<0.01; $P<0.001.
Table 1.
Summary of physiologic readouts of diabetic Akita, db/db, and BTBR-ob/ob models injected with ReninAAV and compared with LacZ controls
| Model and Injection | Sex | N | Duration of AAV Exposure, wk | Age at Necropsy, wk | ACR | SAP, mm Hg | Serum Creatinine, mg/dl | Serum BUN, mg/dl |
|---|---|---|---|---|---|---|---|---|
| Akita/B6 | ||||||||
| LacZ | M | 5 | 12 | 24 | 88±10 | 159±4 | 0.18±0.02 | 31.9±0.9 |
| Renin | M | 5 | 12 | 24 | 868±311a | 191±9b | 0.15±0.01 | 33.6±2.0 |
| Akita/DBA | ||||||||
| LacZ | M | 5 | 12 | 24 | 652±162 | 119±5 | 0.21±0.02 | 40.0±3.9 |
| Renin | M | 10 | 12 | 24 | 5964±1601b | 180±12b | 0.17±0.02 | 35.2±2.6 |
| Akita/129 | ||||||||
| LacZ | M | 3 | 12 | 24 | 104±27 | 120±0.1 | 0.05±0.01 | 29.6±3.7 |
| Renin | M | 6 | 12 | 24 | 14,531±3555b | 172±12b | 0.11±0.01b | 30.9±3.9 |
| db/+ | ||||||||
| LacZ | F | 5 | 12 | 24 | 22.07±9 | NA | 0.19±0.02 | 24.0±1.6 |
| Renin | F | 5 | 12 | 24 | 179.0±42.1b | NA | 0.22±0.04 | 29.3±3.0 |
| db/db | ||||||||
| LacZ | F | 5 | 12 | 24 | 525±121 | 132±4 | 0.09±0.01 | 19.9±2.2 |
| Renin | F | 10 | 12 | 24 | 8977±1620b | 192±9b | 0.16±0.01b | 23.0±0.09c |
| db/db uNx | ||||||||
| LacZ | F | 6 | 12 | 24 | 1868±751 | 114±5 | 0.08±0.01 | 26.0±2.5 |
| Renin | F | 11 | 12 | 24 | 68,890±6868b | 173±6b | 0.20±0.03b | 45.8±3.8b |
| BTBR ob/ob | ||||||||
| LacZ | F | 10 | 8 | 20 | 1042±287 | NA | 0.14±0.01 | 22.6±0.7 |
| Renin | F | 12 | 8 | 20 | 6236±843b | NA | 0.15±0.01 | 23.0±1.0 |
| eNOS−/− db/db | ||||||||
| NA | F | 9 | NA | 24 | 36,989±8304d | NA | 0.14±0.01e | 39±2.5 |
In addition, nondiabetic db/m mice were included to confirm that diabetes and hypertension promote progressive DKD. The progressive DKD model, eNOS−/−db/db mice, was used for comparison of severity of disease progression (n=3+ mice per group). All statistical analysis was performed by comparing with respective LacZ controls. AAV, adeno-associated virus; M, male; F, female; NA, not assessed.
P<0.05 as compared with respective LacZ controls.
P<0.01 as compared with respective LacZ controls.
P<0.05 compared with respective LacZ control, however only statistically relevant by t test and not more stringent analysis.
For eNOS−/−db/db mice, P<0.01 compared with ReninAAV db/db uNx mice.
For eNOS−/−db/db mice, P<0.05 compared with ReninAAV db/db uNx mice.
Renal injury scores in ReninAAV Akita/B6 were indistinguishable from those in LacZ controls (Table 2). ReninAAV Akita/DBA and Akita/129 mice exhibited marked renal histopathologic changes compared with respective controls. More severe lesions were observed in ReninAAV Akita/129 mice, which exhibited increased proteinaceous casts that expanded the tubular lumen, causing tubular degeneration/regeneration (tubular basophilia) and leading to marginalized tubular epithelium. The interstitium contained inflammatory cells, predominantly lymphocytes. Glomerulopathy was characterized by increased mesangial matrix, fibrosis, increased eosinophilia associated with matrix deposition, and a decrease in the number of mesangial or podocyte nuclei within the glomerular tuft (Table 2).
Table 2.
Summary of renal pathologic changes of Akita/129, db/db intact, db/db uNx, and BTBR-ob/ob models injected with ReninAAV and compared with LacZ controls
| Model and Injection | Tubular Protein | Tubular Basophilia | Interstitial Inflammation | Interstitial Fibrosis | Glomeruli | Renal Arteriopathy |
|---|---|---|---|---|---|---|
| Akita/B6 | ||||||
| LacZ | 0±0 | 0.4±0.27 | 0.4±0.27 | 0±0 | 0±0 | 0±0 |
| Renin | 0±0 | 0.6±0.27 | 0.6±0.27 | 0±0 | 0.4±0.27 | 0±0 |
| Akita/DBA | ||||||
| LacZ | 2.3±1.1 | 0.3±0.41 | 0.3±0.41 | 0±0 | 0±0 | 0±0 |
| Renin | 2±0.5 | 1.8±0.42a | 0.8±0.22 | 0.4±0.27 | 1.6±0.27a | 0±0 |
| Akita/129 | ||||||
| LacZ | 0±0 | 0.3±0.41 | 0.3±0.41 | 0±0 | 1±0 | 0±0 |
| Renin | 2±0a | 2±0a | 1.6±0.27b | 1±0a | 3±0a | 0.2±0.22 |
| db/m | ||||||
| LacZ | 0.2±0.22 | 1±0.4 | 0.6±0.3 | 0.8±0.4 | 0±0 | 0±0 |
| Renin | 1±0a | 0.4±0.3 | 0.2±0.2 | 0.8±0.2 | 0.8±0.2a | 0±0 |
| db/db | ||||||
| LacZ | 0.2±0.22 | 0±0 | 0±0 | 0.4±0.27 | 1.4±0.22 | 0±0 |
| Renin | 2.4±0.27a | 0±0 | 0±0 | 2±0.35a | 2.6±0.22a | 0±0 |
| db/db uNx | ||||||
| LacZ | 0.2±0.22 | 0±0 | 0±0 | 0±0 | 1.6±0.27 | 0±0 |
| Renin | 3±0.30a | 2.9±0.17a | 2.9±0.10a | 2.8±0.24a | 4±0.28a | 1.7±0.15a |
| BTBRob/ob | ||||||
| LacZ | 0.1±0.10 | 0.1±0.11 | 0±0 | 0.6±0.23 | 2.1±0.10 | 0±0 |
| Renin | 1±0a | 0.9±0.11a | 0±0 | 0.8±0.16 | 2.77±0.16a | 0±0 |
| eNOS−/− db/db | ||||||
| NA | 3.1±0.18 | NA | 2.1±0.25c | 3.1±0.30 | 3.2±0.29d | NA |
Nondiabetic db/m mice were included to confirm that diabetes and hypertension synergistically promote progressive DKD. The progressive DKD model, eNOS−/−db/db mice, was used for comparison of severity of disease progression (n=5+ mice per group). NA, not assessed.
P<0.01 compared with respective LacZ controls.
P<0.05 compared with respective LacZ controls.
For eNOS−/−db/db mice, P<0.01 compared with ReninAAV db/db uNx mice.
For eNOS−/−db/db mice, P<0.05 compared with ReninAAV db/db uNx mice.
ReninAAV in Type 2 Diabetic Models
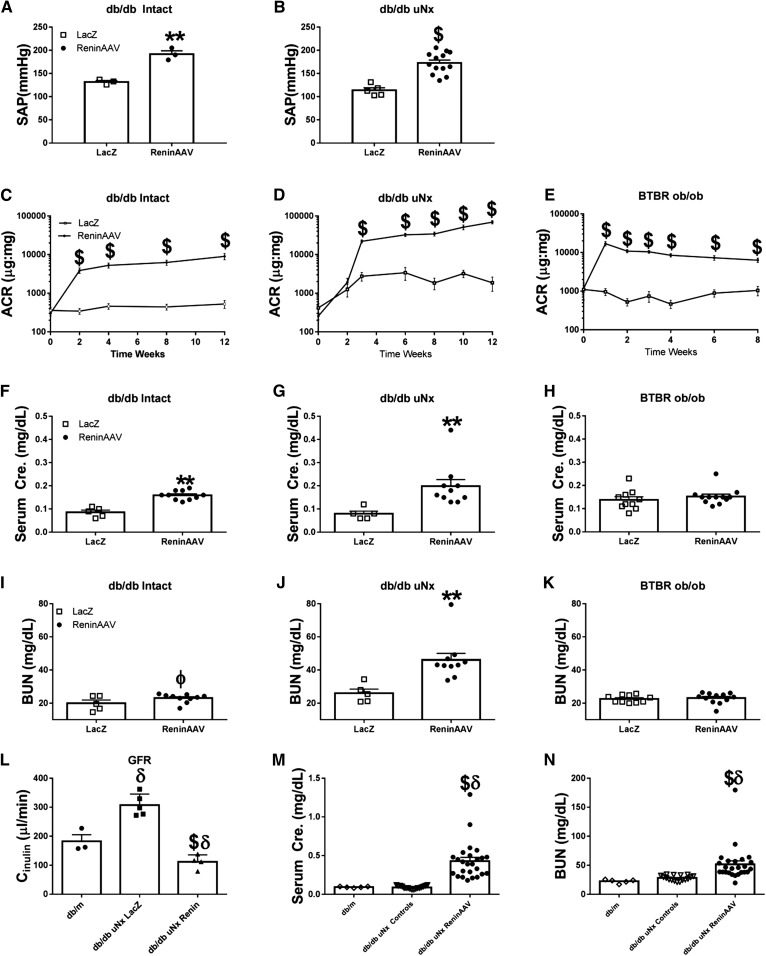
ReninAAV (5×109 GC) was given to db/db intact, db/db uninephrectomized (uNx), and BTBR-ob/ob mice. ReninAAV elevated SAP in db/db intact and uNx mice compared with controls (+60 and +59 mm Hg, respectively; P<0.01) (Figure 2, A and B). AP measurements by tail cuff were not feasible in BTBR-ob/ob mice (possibly a result of their autism spectrum disorder behavior, rendering these mice oversensitive to restraint procedures13). ReninAAV BTBR-ob/ob and db/db uNx mice had similar elevations of plasma renin activity with 2.8- and 2.7-fold increases, respectively, (P<0.01) compared with controls, indicating transgene expression.
Figure 2.
ReninAAV in type 2 diabetic models accelerates renal disease. (A and B) ReninAAV (5×109 GC) significantly elevated SAP of db/db intact and uNx mice compared with respective LacZAAV controls. ReninAAV elevated ACR in (C) db/db intact, (D) db/db uNx, and (E) BTBR-ob/ob mice, with effects typically detected within 1 week postinjection and persisting for the duration of the study. ReninAAV led to statistically significant elevations in serum creatinine in (F) db/db intact and (G) db/db uNx mice but not the (H) BTBR-ob/ob. (I–K) ReninAAV elevated serum BUN only in db/db uNx mice with no detectable changes in db/db intact or BTBR-ob/ob mice. (L) GFR was measured by FITC-inulin clearance in ReninAAV db/db uNx mice with an approximate doubling of serum creatinine and compared with db/m leans and LacZ db/db uNx controls. (L) GFR was significantly reduced in ReninAAV db/db uNx mice compared with both db/m and LacZ db/db uNx controls, whereas LacZ db/db uNx controls were significantly elevated compared the db/m mice. (M and N) Compiled data of serum creatinine and BUN from ReninAAV db/db uNx mice after 11–12 weeks of ReninAAV shows that over 55% of mice have advanced renal disease as shown by over fourfold increases in serum creatinine and further elevations in BUN. n=5+ mice per group. **P<0.01 by t test only and not more stringent analysis versus LacZ db/db uNx controls; $P<0.001 by t test only and not more stringent analysis versus LacZ db/db uNx controls; ϕP=0.05 by t test only and not more stringent analysis versus LacZ db/db uNx controls; δP<0.01 versus db/m.
ReninAAV increased ACR in all type 2 models tested, with effects typically detected at 1 week postinjection and persisting for the entire study duration. Intact ReninAAV db/db had significant (P<0.001) elevations in ACR (8977±1620) compared with LacZ controls (525±121) (Figure 2C, Table 1). ReninAAV db/db uNx exhibited robust elevations in ACR (68,890±6868; P<0.001) versus LacZ db/db uNx (1868±751) (Figure 2D, Table 1). ReninAAV in nondiabetic (db/m) mice led to significant yet modest elevations in ACR (Table 1). ReninAAV BTBR-ob/ob exhibited significantly (P<0.001) increased ACR (6236±843) compared with controls (1042±287) (Figure 2E). ReninAAV did not affect serum glucose levels in any type 2 model tested (Supplemental Figure 1).
ReninAAV db/db intact mice exhibited progressively increased serum creatinine (0.12±0.009 mg/dl versus LacZ 0.09±0.007 mg/dl after 8 weeks [P<0.05] and 0.16±0.01 mg/dl versus LacZ 0.09±0.01 mg/dl after 12 weeks of ReninAAV [P<0.01]), with a modest elevation (P=0.05) in BUN (23.0±0.9 mg/dl) observed after 12 weeks of ReninAAV compared with controls (19.9±2.2 mg/dl) (Figure 2, F and I, Table 1). ReninAAV db/db uNx exhibited elevations in serum creatinine after 8 weeks of ReninAAV (0.16±0.01 versus LacZ 0.12±0.01 mg/dl; P<0.05), with doubling of serum creatinine (0.20±0.03 mg/dl) and elevation in BUN (45.8±4.0 mg/dl) after 12 weeks compared with controls (creatinine =0.08±0.01 and BUN=26.0±2.5 mg/dl; P<0.01) (Figure 2, G and J, Table 1). Elevations in serum creatinine or BUN were not observed in ReninAAV BTBR-ob/ob (Figure 2, H and K).
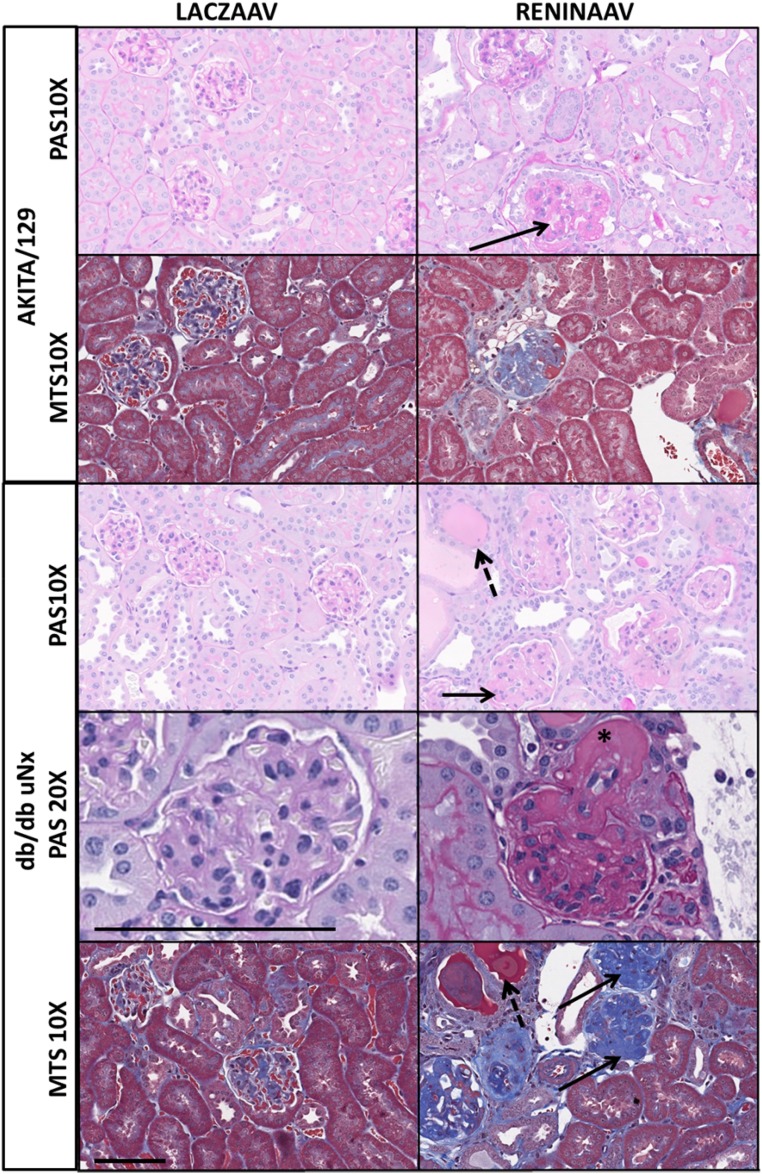
Histopathologic evaluation indicated that ReninAAV increased the incidence and severity of tubular protein, tubular basophilia, and glomerulopathy in db/db intact, db/db uNx, and BTBR-ob/ob mice compared with respective controls (Table 2). ReninAAV db/db uNx exhibited severe pathologic changes, with robust changes in the glomeruli (increased mesangial matrix, intraglomerular fibrosis, periglomerular fibrosis/inflammation, and a decrease in mesangial nuclei within the glomerular tuft) compared with LacZ db/db uNx (Figure 3, Supplemental Figures 2 and 3). Mesangial matrix expansion was corroborated with significant (P<0.001) elevations in COL4A1 and COL4A2 gene expression and increased fibrosis supported by increased fibronectin and TGF-β expression (Supplemental Figure 4A). Nodular mesangial profiles suggestive of Kimmelstiel–Wilson nodules were evident in a significant number of ReninAAV db/db uNx glomeruli, a phenotype not observed in ReninAAV-BTBR-ob/ob and rarely observed in ReninAAV db/db intact. Changes within the renal parenchyma consisted of tubular dilation, proteinaceous casts, tubular regeneration, interstitial inflammation, and fibrosis. Renal arterioles had hypertrophy of tunica media, hyalinization, and degeneration of the tunica media (Figure 3, Table 2).
Figure 3.
ReninAAV leads to renal pathologic changes in most strains tested, with the most pronounced effect observed in db/db uNx and Akita/129 mice. Representative photomicrographs of kidney sections stained with periodic acid–Schiff (PAS) or Masson trichrome (MTS) from mice 12 weeks postinjection of LacZAAV or ReninAAV in Akita/129 or db/db uNx mice. Solid arrows highlight mesangial matrix expansion, dashed arrows indicate tubular protein, and asterisk highlights arterial hyalinosis. Images were taken at ×10 or ×20 magnification. Scale bar, 100 μm.
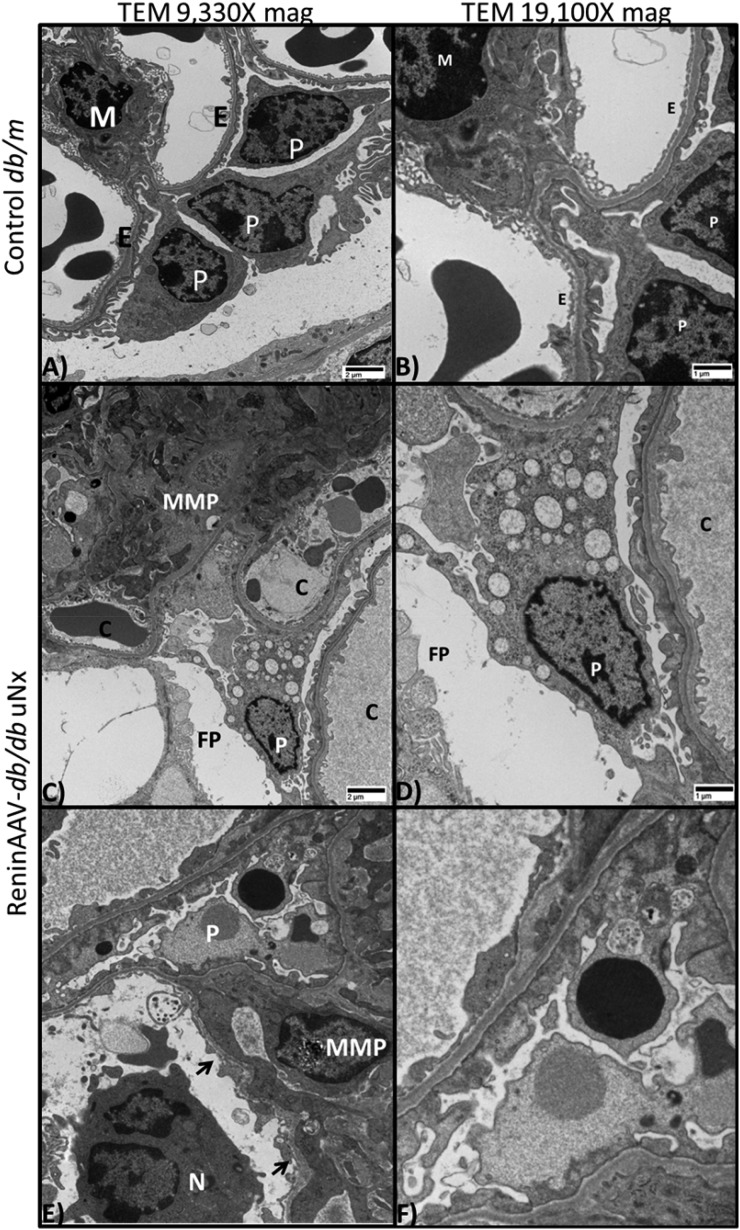
Ultrastructural evaluation of ReninAAV db/db uNx kidneys confirmed histopathologic changes. Increased mesangial matrix was detected compared with controls (Figure 4, A–D). Glomerular basement membrane splitting and reduplication generally occurred concurrently with increased mesangial matrix, a feature not observed in LacZ db/db uNx or db/m (Figure 4, A and B, Supplemental Figure 3B). Glomerular basement membrane thickening was observed with a significant (P<0.01) increase in ReninAAV db/db uNx compared with LacZAAV db/db uNx and db/m (293 versus 192 and 189 nm, respectively) (Supplemental Figure 3A). Increased vacuolar degeneration of podocytes was characterized by numerous intracytoplasmic dilated spaces, often membrane bound, which occasionally contained a flocculent, mildly electron-dense material or occasional membranous whorls (consistent with dilated endoplasmic reticulum) (Figure 4, C–F). Podocyte foot processes were usually fused and/or absent with concurrent loss of slit diaphragms, which was supported by gene expression data showing a loss of podocyte-specific markers (Supplemental Figure 4B). The opposing endothelium had loss and fenestrations, and it was thickened. Periglomerular mononuclear cell infiltrate and fibrosis occurred with slight severity (Figure 4E). The glomerular capillaries included reactive endothelium lining the capillary lumen along with amorphous subendothelial matrix deposits (Figure 4E).
Figure 4.
ReninAAV db/db uNx mice exhibit ultrastructural changes observed in DKD. (A and B) Transmission electron micrograph of glomerulus from a healthy db/m control kidney with numerous intact podocytes (P) and basement membranes lined by intact endothelial cells (Es) with normal fenestrations. (A and B) A single mesangial cell (M) appears in the upper left quadrant. (C–F) Transmission electron micrograph of glomerulus from a ReninAAV db/db uNx mouse. (C and D) Extensive mesangial matrix proliferation (MMP) composed of amorphous and occasional fibrillary densities; three capillary profiles (Cs) are shown with thickened endothelium and irregular basement membranes with fused and expanded P foot processes (FPs). (C–F) Ps undergoing vacuolar degeneration; reactive endothelium lining capillaries (arrows) and neutrophil (N) infiltration were detected in the ReninAAV db/db uNx mice. n=6 mice evaluated per group. Scale bar, 1 or 2 μM.
ReninAAV db/db uNx Model Progresses to Renal Failure
To further define renal function in ReninAAV db/db uNx mice, GFR was measured by FITC inulin clearance after 8 weeks of ReninAAV in mice with elevated serum creatinine (0.25±0.03 mg/dl versus LacZ controls 0.12±0.01 mg/dl; P<0.01). ReninAAV db/db uNx exhibited a 64% reduction in GFR compared with LacZ db/db uNx and a 40% reduction compared with db/m (111±14, 308±19, and 182±28 μl/min, respectively) (Figure 2L). Elevated GFR in LacZ db/db uNx suggests hyperfiltration. In all groups, GFR correlated with serum creatinine (R2=0.72; P<0.001) and renal pathology (R2=0.70; P=0.002 for glomerular fibrosis and R2=0.57; P=0.01 for glomerular sclerosis), substantiating that the increased serum creatinine is due to renal impairment. To determine the proportion of mice that develop renal failure as defined by fourfold or greater elevations in serum creatinine compared with controls, serum creatinine and BUN were gathered from noncontemporaneous studies after 11–12 weeks of ReninAAV. Over 55% of ReninAAV db/db uNx mice had fourfold or greater elevation in serum creatinine as well as further elevations in BUN (Figure 2, M and N).
Treatment Attenuates Disease Progression in ReninAAV db/db uNx Mice
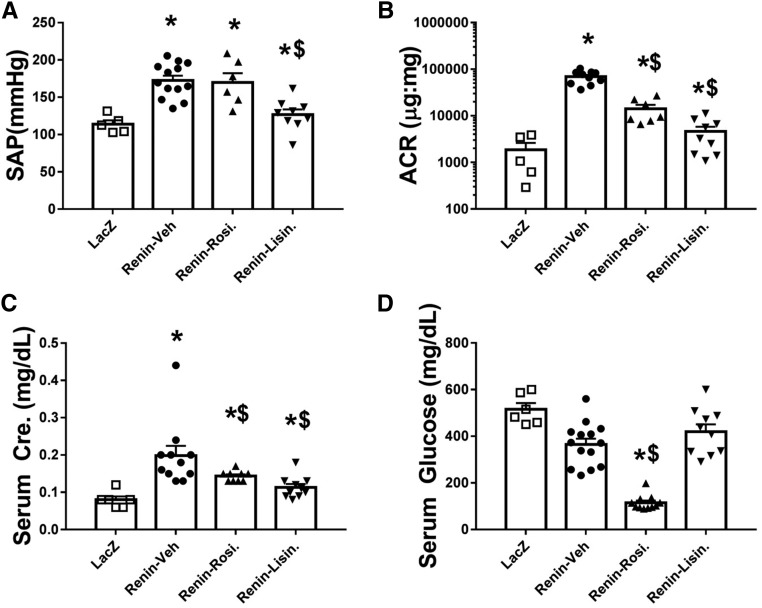
ReninAAV db/db uNx mice were treated with therapies to reduce AP (lisinopril) or glucose (rosiglitazone). Treatments started 3 weeks after adeno-associated virus injection and were provided for an additional 8 weeks. Lisinopril lowered SAP (127±7 versus 168±7 mm Hg Renin-Veh) with residual elevation compared with LacZ controls (110±13 mm Hg; P<0.05) (Figure 5A), whereas rosiglitazone did not alter SAP but significantly reduced serum glucose (Figure 5, A and D). The ReninAAV-Veh group exhibited progressively increasing ACR throughout the treatment period (baseline 22,032±2140 versus 8 weeks of vehicle 68,890±6839) (Figure 5B). Rosiglitazone significantly (P<0.05) lowered ACR (14,164±3068) compared with baseline (22,539±3500). Lisinopril reduced ACR from baseline (4629±1187 versus baseline 18,800±2452; P<0.01) (Figure 5B). Terminal serum creatinine values of ReninAAV-Veh mice were significantly (P<0.01) elevated (0.20±0.03) compared with LacZ controls (0.08±0.01). Rosiglitazone caused a modest (0.14±0.01; P=0.04) reduction in serum creatinine, whereas lisinopril markedly reduced serum creatinine (0.10±0.01; P<0.01) compared with ReninAAV-Veh; however, both groups remained significantly (P<0.04) elevated compared with LacZ controls (Figure 5C).
Figure 5.
ReninAAV db/db uNx mice respond to standard of care treatment. Mice were injected with ReninAAV and allowed to progress until ACR reached approximately 20,000 μg/mg; they were then treated with vehicle (Veh), rosiglitazone (Rosi), or lisinopril (Lisin). (A) ReninAAV-Veh, ReninAAV-Lisin, and ReninAAV-Rosi had significantly elevated AP compared with LacZ controls. ReninAAV-Lisin had significantly lower AP compared with the ReninAAV-Veh group. (B) All ReninAAV-injected groups had ACR values significantly elevated from LacZ controls. Treatment with lisinopril significantly reduced ACR compared with ReninAAV-Veh. Rosiglitazone treatment halted progression, and ACR was lower than Renin-Veh. (C) Serum creatinine was elevated in ReninAAV-Veh and all ReninAAV-treated groups compared with LacZ controls. Treatment with lisinopril and rosiglitazone significantly lowered serum creatinine compared with in ReninAAV-Veh mice. (D) Serum glucose levels were significantly lowered in the Renin-Rosi group only compared with either control group (LacZ or ReninAAV-Veh). n=10–15 mice per group. *P<0.05 compared with LacZ control; $P<0.05 compared with ReninAAV-Veh group.
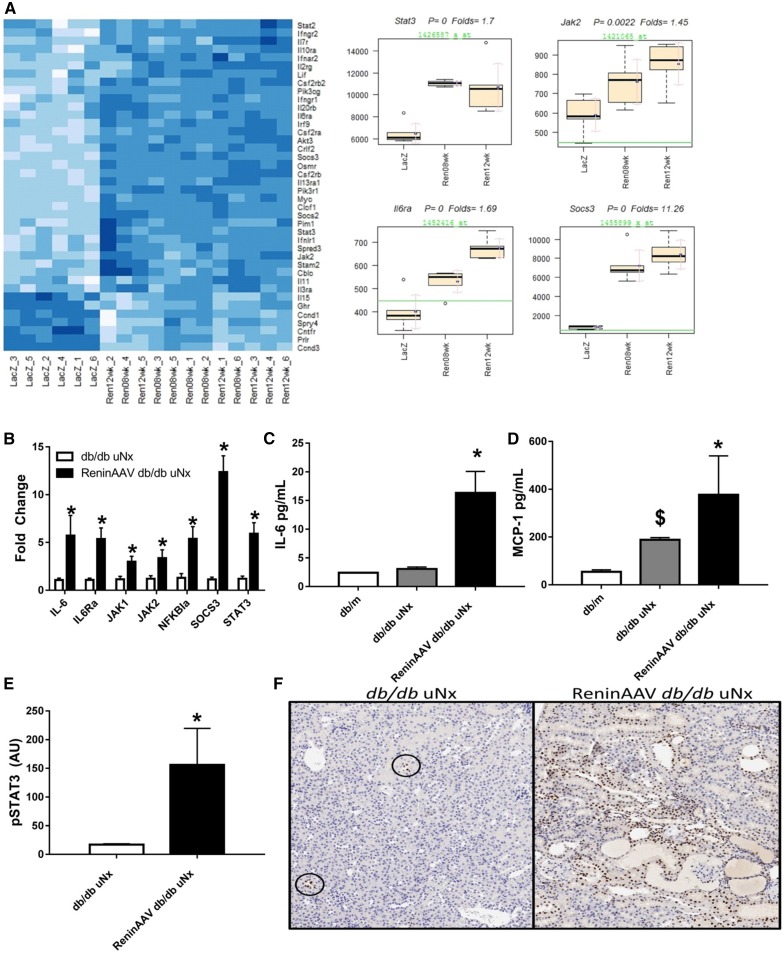
JAK/STAT Pathway Is Upregulated in the ReninAAV db/db uNx Model
Microarray analysis was performed using kidney RNA from ReninAAV db/db uNx and compared with LacZ controls. Analysis of differentially expressed genes identified IL-6/JAK/STAT signaling as a top pathway upregulated in the ReninAAV db/db uNx model (Ingenuity z score =6.670; P=5×10−29) (Figure 6A). Taqman analysis validated microarray results with significant upregulation of SOCS3, IL-6, IL6ra, JAK1, JAK2, STAT3, and NFKB1a (Figure 6B). ReninAAV db/db uNx had elevated circulating cytokines involved in JAK/STAT signaling with increased IL-6 and MCP-1 (Figure 6, C and D). In addition, renal pSTAT3 protein was elevated in ReninAAV db/db uNx compared with controls (Figure 6E). This was further supported by immunostaining, in which increased pSTAT3 staining was detected in the nuclei of tubuli epithelium as well as inflammatory leukocytes of injured nephrons (Figure 6F).
Figure 6.
JAK/STAT pathway is upregulated in the ReninAAV db/db uNx model of DKD. (A) Microarray analysis identified the JAK/STAT pathway as a top pathway upregulated in ReninAAV db/db uNx mice at both 8 and 12 weeks of ReninAAV treatment compared with LacZ db/db uNx controls, including Stat3, Jak2, IL6Ra, and Socs3. (B) Taqman analysis verified microarray data and showed that genes of the JAK/STAT pathway are upregulated in ReninAAV db/db uNx mice compared with LacZ controls. (C and D) Serum IL-6 and MCP-1 were upregulated in ReninAAV db/db uNx mice compared with db/m or db/db uNx controls. (E) Protein expression of pSTAT3 is upregulated in ReninAAV db/db uNx mice compared with LacZ controls. (F) Immunostaining-identified pSTAT3 is increased in renal tubule epithelium and infiltrating leukocytes compared with LacZ controls, with circles highlighting low-level detection in LacZ groups. n=5+ per group. *P<0.05 versus LacZ db/db uNx control; $P<0.05 versus db/m.
Comparison of ReninAAV db/db uNx and eNOS−/−db/db Models
The ReninAAV db/db uNx model appears to progress faster than age-matched eNOS−/−db/db mice, which had significantly lower ACR (P<0.01) compared with ReninAAV db/db uNx (36,989±8304 versus 68,890±6868, respectively) (Table 1). Serum creatinine levels from eNOS−/−db/db were statistically (P<0.05) lower (0.14±0.01) than ReninAAV db/db uNx (0.20±0.03). Histopathology scores of ReninAAV db/db uNx exhibited significantly increased interstitial inflammation and glomerulopathy compared with eNOS−/−db/db (Tables 1 and 2).
The transcriptome changes were used to compare mRNA expression profiles of ReninAAV db/db uNx mice and eNOS−/−db/db mice with similar severity of renal dysfunction as measured by serum creatinine and BUN levels (Supplemental Figure 5). The top ten up- and downregulated genes found in both models were composed of genes implicated in the pathogenesis of DKD, including Lipocalin, a marker of tubular injury, and numerous inflammatory markers (Ighg, H2-DMb2, C3, Cxcl2, Igj, Ighm, and Iglv1), supporting a role of inflammation in the pathogenesis of DKD (Table 3). The ReninAAV db/db uNx model exhibits model specific gene changes that were absent in the eNOS-/- db/db (Supplemental Figure 5, Table 4). Ingenuity analysis identified numerous inflammatory pathways dysregulated in both models (Table 5). Alterations of podocytes in both models are suggested by the downregulation of podocin (Nphs2). Top pathways unique to ReninAAV db/db uNx included those involved in degradation of amino acids, ketogenesis, and apoptosis (Table 5). Many of the pathways unique to the ReninAAV db/db uNx model have also been implicated in human DKD (Table 5).
Table 3.
Top ten up- and downregulated genes identified by microarray analysis of kidney tissue from ReninAAV db/db uNx and eNOS−/−db/db mice with matched serum creatinine values compared with LacZAAV db/db uNx and db/db mice, respectively: top genes changed in both ReninAAV db/db uNx and eNOS−/− db/db models
| Direction | Gene Symbol | FC ReninAAV | P Value | FDR | FC eNOS−/− db/db | P Value | FDR |
|---|---|---|---|---|---|---|---|
| Up | Lcn2 | 18.4 | 0 | 0 | 49.55 | 0 | 0 |
| Up | Ighg | 8.29 | 0 | 0 | 45.56 | 0 | 0.0003 |
| Up | Krt20 | 13.99 | 0 | 0 | 29.84 | 0 | 0 |
| Up | H2-DMb2 | 12.27 | 0 | 0 | 21.6 | 0 | 0 |
| Up | Arg1 | 9.1 | 0 | 0 | 18.79 | 0 | 0.0006 |
| Up | C3 | 7.29 | 0 | 0 | 17.64 | 0 | 0 |
| Up | Cxcl2 | 8.1 | 0 | 0 | 16.41 | <0.001 | 0.0078 |
| Up | Igj | 6.56 | 0 | 0 | 15.94 | 0 | 0.0009 |
| Up | Ighm | 8.28 | 0 | 0 | 15.45 | 0 | 0.0002 |
| Up | Iglv1 | 16.78 | 0 | 0 | 15.28 | 0.002 | 0.0462 |
| Down | Nphs2 | −1.99 | 0 | 0.0001 | −2.59 | <0.001 | 0.0034 |
| Down | Cenpa | 3.48 | 0 | 0 | −2.63 | <0.001 | 0.0049 |
| Down | Cables1 | −1.59 | <0.001 | 0.0002 | −2.74 | 0.002 | 0.0421 |
| Down | Cpne4 | −1.73 | <0.001 | 0.0006 | −2.89 | <0.001 | 0.0071 |
| Down | Slc14a1 | −4.86 | 0 | 0 | −2.91 | 0 | 0.0001 |
| Down | D630029K05Rik | −2.3 | 0 | 0 | −2.93 | 0 | 0.0012 |
| Down | Cyfip2 | −1.51 | 0 | 0 | −3.45 | <0.001 | 0.0105 |
| Down | Sycn | −2.37 | 0.02 | 0.0195 | −3.68 | 0.002 | 0.0463 |
| Down | Fads3 | −1.61 | 0 | 0 | −4.39 | 0 | 0.0004 |
| Down | Dusp15 | −2.69 | <0.001 | 0.0006 | −4.74 | <0.001 | 0.0054 |
Genes were identified that were shared between the two models. Genes were ranked by FC, and only those with a P value and FDR<0.05 were selected. FC, fold change; FDR, false discovery rate.
Table 4.
Top ten up- and downregulated genes identified by microarray analysis of kidney tissue from ReninAAV db/db uNx and eNOS−/−db/db mice with matched serum creatinine values compared with LacZAAV db/db uNx and db/db mice, respectively: genes changed only in the ReninAAV db/db uNx model
| Direction | Gene Symbol | FC ReninAAV | P Value | FDR |
|---|---|---|---|---|
| Up | Areg | 43.92 | 0 | 0 |
| Up | Scn3a | 25.56 | 0 | 0 |
| Up | 4930483C01Rik | 21.95 | 0 | 0 |
| Up | AA467197 | 21.32 | 0 | 0 |
| Up | Wfdc17 | 21.09 | 0 | 0 |
| Up | Sprr2f | 17.74 | 0 | 0 |
| Up | Samd5 | 16.16 | 0 | 0 |
| Up | Maff | 14.36 | 0 | 0 |
| Up | Hp | 13.77 | 0 | 0 |
| Up | Cxcl14 | 11.02 | 0 | 0 |
| Down | Dpys | −3.42 | 0 | 0 |
| Down | Slc22a19 | −3.51 | <0.001 | 0.0002 |
| Down | Slc22a27 | −3.59 | 0.003 | 0.0043 |
| Down | Bhmt | −3.62 | 0 | 0 |
| Down | Grem1 | −3.72 | 0 | 0 |
| Down | Sema4f | −3.75 | 0.001 | 0.0024 |
| Down | Grem2 | −4.42 | 0 | 0 |
| Down | Rhpn1 | −4.48 | 0 | 0 |
| Down | Pvalb | −5.48 | 0 | 0 |
| Down | Rbp2 | −6.2 | 0 | 0 |
Genes were identified that were unique to the ReninAAV db/db uNx model. Genes were ranked by FC, and only those with a P value and FDR<0.05 were selected. FC, fold change; FDR, false discovery rate.
Table 5.
Ingenuity Pathway Analysis of ReninAAV db/db uNx and eNOS−/−db/db models
| Pathways Changed | ReninAAV −log(P Value) | eNOS−/− db/db −log(P Value) |
|---|---|---|
| Pathways shared in ReninAAV db/db uNx and eNOS−/− db/db models | ||
| Hepatic fibrosis/hepatic stellate cell activation | 11.6 | 9 |
| Acute-phase response signaling | 10.1 | 8.46 |
| Granulocyte adhesion and diapedesis | 9.23 | 12.3 |
| Dendritic cell maturation | 8.32 | 10.4 |
| Role of macrophages, fibroblasts, and endothelial cells in rheumatoid arthritis | 7.51 | 2.86 |
| Atherosclerosis signaling | 7.39 | 8.2 |
| Antigen presentation pathway | 7.22 | 6.66 |
| Role of NFAT in regulation of the immune response | 7.13 | 2.34 |
| Agranulocyte adhesion and diapedesis | 7.01 | 8.75 |
| TREM1 signaling | 6.83 | 3.26 |
| Pathways unique to ReninAAV db/db uNx model | ||
| Valine degradation I | 7.54 | 0.31 |
| Tryptophan degradation III (eukaryotic) | 5.01 | 0.29 |
| Isoleucine degradation I | 4.95 | 0.39 |
| PI3K signaling in B lymphocytes | 4.83 | 1.03 |
| Ethanol degradation II | 3.96 | 1.04 |
| Leucine degradation I | 3.95 | 1.38 |
| LPS/IL-1–mediated inhibition of RXR functiona | 3.66 | 0.98 |
| Oxidative ethanol degradation III | 3.6 | 1.57 |
| Ketogenesis | 3.49 | 0.50 |
| Noradrenaline and adrenaline degradation | 3.4 | 0.94 |
| PPARα/RXRα activation | 3.35 | 1.35 |
| Apoptosis signaling | 3.35 | 0.66 |
| Macropinocytosis signaling | 3.32 | 1.57 |
| Ketolysis | 3.29 | 0.58 |
| PPAR signaling | 3.17 | 1.33 |
| STAT3 pathway | 3.1 | 1.74 |
| TWEAK signaling | 3.08 | 1.45 |
| Fcγ receptor–mediated phagocytosis in macrophages and monocytesa | 3.08 | 1.31 |
| Aryl hydrocarbon receptor signalinga | 3.04 | 1.28 |
| Axonal guidance signalinga | 2.94 | 0.38 |
Top pathways shared between the ReninAAV db/db uNx model and the eNOS−/−db/db model and the top 20 pathways unique to the ReninAAV db/db uNx model were identified. Pathways were identified by those with statistical significance (−log[P value]) in both models for shared genes and models for unique genes that were only statistically significant for the ReninAAV db/db uNx model but did not reach statistical significance in the eNOS−/−db/db model.
Pathways unique to the ReninAAV db/db uNx model that were additionally found to be dysregulated in human DKD datasets.
Discussion
Registration of drugs for the treatment of DKD requires showing slowing the loss of GFR and incidence of ESRD, whereas changes in albuminuria alone are insufficient for approval.14–16 The lack of mouse models that reproducibly exhibit albuminuria and decline in GFR has been a significant impediment in the development of therapies for DKD. These studies support the ReninAAV db/db uNx mice as a reliable model of progressive renal dysfunction attributed to both hypertension and hyperglycemia. A feature of this model is the progressive increase in albuminuria and creatinine, features that are absent in many murine models of DKD but that are observed in human DKD followed by the decline in renal function.
Currently, there are very few type 1 models of DKD that show progressive DKD. Streptozotocin (STZ) treatment is an experimental model of insulin-dependent diabetes. STZ treatment results in hyperglycemia with modest kidney disease. The renin transgenic mouse, TTRhRen, treated with STZ is a more progressive DKD model than STZ alone.17 In rats, inducing diabetes with STZ leads to more robust proteinuria in the spontaneously hypertensive rat compared with normotensive controls.18–20 These studies were supported with the hypertensive (mREN-2)27 rat, in which STZ-induced diabetes led to elevations in ACR and decline in renal function.21 One limiting factor in these studies is the possible contribution of STZ-induced renal toxicity in the development of kidney disease. This study shows that ReninAAV Akita 129/S6 mice represent a novel model of progressive type 1 DKD. The data confirm published data on the strain-dependent susceptibility to the development of renal disease in the Akita model.22,23 One limitation of the ReninAAV Akita/129 model is that the Akita/129 mice are currently commercially available only from a cryopreserved stock, thus the low n values in these studies.
ReninAAV also enabled determination of the effect of inducing persistent hypertension on type 2 models of DKD. The BTBR ob/ob model is a documented model of type 2 DKD,24 but similar to recent studies, this study did not observe progressively increased ACR or elevations in serum creatinine. ReninAAV in BTBR ob/ob mice produced increased ACR; however, increased serum creatinine was not observed. A limitation of this model is the short lifespan (22–24 weeks) that prevented examination of long-term effects of ReninAAV.
The most progressive model of type 2 DKD identified was the ReninAAV db/db uNx model. ReninAAV introduced to either db/db intact and db/db uNx mice produced significant elevations in ACR, serum creatinine, and pathologic changes; however, the uNx mice had more advanced and accelerated disease. In addition, pathologic changes closely mimicking advanced human DKD were observed after 12 weeks of ReninAAV, many of which have not been observed in murine models of DKD. The eNOS−/−db/db model, to our knowledge, is the only murine DKD model that exhibits histopathologic features of the ReninAAV db/db uNx; however, their disease develops at a much later age.9 A notable pathologic feature was basement membrane splitting and duplication, a feature of thrombotic microangiopathy (TMA) as well as human DKD.25 This model lacked other ultrastructural features of TMA, such as glomerular capillary or arteriolar acute fibrin thrombi, and therefore, these pathologic changes are not classic TMA. The basement membrane duplication/splitting was not observed in normotensive models of diabetes (db/m or LacZ db/db uNx), and the hyperplastic arteriosclerosis (onion skinning) typically observed in rodent models of hypertension was not seen in the ReninAAV db/db uNx model,26,27 suggesting that these findings are due to a combination of diabetes and hypertension.
Another feature of the ReninAAV db/db uNx is the >50% reduction in GFR, which is not observed in most available DKD models.28,29 SOC treatment improved disease in the model, similar to what is observed in human DKD and eNOS−/−db/db.30 Identification of JAK/STAT, a pathway upregulated in human disease,31,32 as a major upregulated pathway further supports the similarity to human DKD. In addition, pathways unique to the model (compared with eNOS−/−db/db mice), such as LPS/IL-1–mediated inhibition of RXR function and FcγR-mediated phagocytosis in macrophages and monocytes, are also found to be dysregulated in human DKD gene expression studies.33
The DKD mouse model with best characterized kidney disease progression to date is the hypertensive and diabetic eNOS−/−db/db.9,34 Our studies support data on this model,9 with elevations in ACR and serum creatinine and worsening of renal pathology observed. The ReninAAV db/db uNx model had comparable if not greater disease progression compared with eNOS−/−db/db. In addition, ReninAAV db/db uNx is a more expedient and less resource-intensive model, circumventing complex breeding required to generate eNOS−/−db/db mice. Microarray analysis comparing ReninAAV db/db uNx with eNOS−/−db/db identified common genes and pathways dysregulated in both models. These include inflammatory cell processes, such as dendritic cell maturation and granular adhesion, consistent with the well documented presence of inflammation in DKD.7,35 Top pathways unique to the ReninAAV db/db uNx model were involved in apoptosis and amino acid degradation. These data are consistent with studies showing that AngII plays a physiologic role in autophagy and ER stress–induced apoptosis in the kidney.36,37 AngII-mediated increases in AP as opposed to eNOS deficiency may further contribute to the pathogenesis of DKD by activating not only hemodynamic pathways but also, additional AngII-dependent stress pathways that contribute to disease progression.
Effects of AngII-induced hypertension have previously been studied with AngII infusion in intact and uNx db/db mice.12 Increased ACR was detected after 2 weeks; however, a decline in ACR approaching baseline after 4 weeks of AngII infusion was observed,12 suggesting loss of drug stability as a limiting factor of AngII infusion.38 This study highlights the unique advantage of ReninAAV to reliably induce persistent hypertension required for the development of its complications in animal models.
In conclusion, this study substantiates the importance of hypertension and diabetes in progressive DKD. ReninAAV allowed testing of the effects of chronic hypertension in six different diabetic models, resulting in acceleration of DKD in most models tested. ReninAAV db/db uNx mice represent a rapidly progressive DKD model, exhibiting robust elevations in ACR, >50% reduction in GFR, doubling of serum creatinine, and renal pathology characteristic of human DKD. This model responds to treatment similar to human patients with DKD and shares common upregulated molecular pathways with the human disease. This provides researchers with a convenient tool to rapidly and reliably test potential therapeutics as well as a understand disease progression.
Concise Methods
Animals
All animal procedures were performed under approved IACUP. Animals were purchased from the following vendors: Taconic (eNOS−/−db/db mice), Harlan (db/db KS with or without vendor-performed surgical removal of one kidney at 4–5 weeks of age), or Jackson Labs (Akita/B6, Akita/DBA, and Akita/129 mice). For Akita/129 mice, pregnant females were purchased from Jackson Labs and colony bred in house. Mice were fed ad libitum a standard 5008 diet (Lab Diets). In some studies, water was supplemented with lisinopril (100 mg/L; Sigma-Aldrich), or they were fed a custom diet of 0.005% rosiglitazone (Adipogen)-supplemented 5008 diet. Male mice were used for the type 1 models and females were used in the obese type 2 models to prevent risk of pyelonephritis.
In Vivo Assessment of ReninAAV on DKD Progression
ReninAAV and LacZAAV were obtained as previously described.38 Mice received a single retro-orbital injection of ReninAAV (1×1010 or 5×109 GC per animal; doses on the basis of previous studies) or LacZAAV at approximately 12 weeks of age.38 The following parameters were monitored weekly for the first 4 weeks postinjection and bimonthly for an additional 4–8 weeks (8–12 weeks total): body weight, ACR, and SAP (tail cuff) as previously described.38 Mice were euthanized at 8–12 weeks postinjection, and kidney were collected and fixed in 10% neutral buffered formalin for histologic processing. Serum and plasma were collected at necropsy for measurement of serum creatinine (enzymatic creatinine using a protocol validated by HPLC), BUN, glucose, and plasma renin activity as previously described.38 Serum cytokine analysis was performed at Myriad RBM using Rodent Map, v.3.1. Renal pSTAT3 expression was measured according to the manufacturer’s directions (MSD Elisa Kit #K150DID-1; Meso Scale Discovery). GFR was measured using FITC-inulin (Sigma-Aldrich) clearance as described.39
Histopathologic Evaluation
Formalin-fixed kidneys were transversely trimmed, routinely processed, paraffin embedded, microtome sectioned at a thickness of 5 μm, and stained with hematoxylin and eosin, Masson trichrome, or periodic acid–Schiff. Tissue sections were examined by light microscopy and graded for tubular regeneration, tubular protein, tubular dilation, and interstitial inflammation by board-certified veterinary pathologists. Periodic acid–Schiff-stained slides were evaluated for glomerular injury, expansion of the mesangium, and tubular basement membranes. Masson trichrome–stained slides were evaluated for interstitial and glomerular fibrosis. To evaluate model/compound effects compared with controls, the histologic changes were given a severity grade that ranged from zero to five (normal to severe, respectively) and referred to the severity and percentage of nephrons affected by the change: within the normal limits (score zero): no changes or changes consistent with spontaneous background finding in the age, sex, and/or strain; minimal (score one): 0%–10% affected; slight (score two): 10%–25% affected; moderate (score three): 25%–50% affected; marked (score four): 50%–75% affected; and severe (score five): >75% affected. Immunostain was performed on paraffin-embedded slides using anti-pSTAT3 (Tyr705; D3A7) antibody (CST9145; Cell Signaling Technology) at 0.5 μg/ml.
Transmission Electron Microscopy
At euthanasia, sections of kidney (cortex) were collected from untreated db/m lean, LacZ db/db uNx control mice and ReninAAV db/db uNx mice at 8 or 12 weeks after ReninAAV. Sections were preserved in 50% strength Karnovsky fixative, and they were subsequently embedded in epoxy resin, sectioned at 1 μm, stained with Toluidine Blue, and examined by light microscopy to identify representative areas of interest. On the basis of review of the Toluidine Blue–stained sections, a minimum of one block was prepared for ultrathin (approximately 50- to 70-nm thickness) sections, placed onto grids, and stained with Reynold lead citrate and uranyl acetate for ultrastructural examination and image capture. Measurement of basement membrane thickening was performed using Harmonic Mean methods as previously described.40
Microarray Submission and Analyses
Kidneys from 8- to 12-week postinjection ReninAAV db/db uNx and LacZ db/db uNx controls were used for analysis. Kidneys from eNOS−/−db/db mice were compared with db/db controls and selected on the basis of their serum creatinine and BUN values to match those from the 12-week ReninAAV db/db uNx mice. Total RNA was extracted using the TRIzol method followed by additional purification using the Qiagen RNeasy Kit (catalog no. 74104) following the manufacturer’s protocol. RNA integrity and concentration was verified using a Nanodrop ND-1000 spectrophotometer. RNA integrity was further verified by obtaining RIN value using the Agilent RNA 6000 Nano Kit (catalog no. 5067–1511) with analysis on the Agilent 2100 Bioanalyzer instrument. Only RNA with RIN values >7 was used. Samples were shipped on dry ice to Assuragen for microarray.
Chip processing, image capturing, and raw data analyses were performed using the Affymetrix GCOS system. Probe set signal intensities of each hybridized gene chip were extracted using MAS5 and normalized using all probe sets to reach the overall 2% trimmed mean of 1500 for each chip. Chip performance of both control and treatment samples met standard quality assurance criteria as recommended by Affymetrix. All of the following analyses were done in the R statistical environment and Bioconductor.41,42 The expression signal intensity data were fitted to an ANOVA model to compare the LacZ group of animals with the ReninAAV group of animals at 8- and 12-week time points. Differentially expressed genes were identified as expressed genes with absolute fold changes ≥1.5, P value <0.05, and false discovery error rate <0.05. Bioconductor KEGG.db package was used to identify all mouse genes in the JAK-STAT signaling pathway. Hypergeometric test, as implemented in GOstat Bioconductor package,43 was used to statistically infer if the JAK-STAT signaling pathway was significantly changed with the disease induction. Clustering analysis of differentially expressed genes in the JAK-STAT signaling pathway was performed using the cluster R package. The results were further validated by analysis using QIAGEN’s Ingenuity Pathway Analysis (QIAGEN; www.qiagen.com/ingenuity). To compare the results from the ReninAAV db/db uNx model with human data, the published dataset GSE30529 was used.33
Validation of a subset of genes identified as upregulated in microarray results was validated by Taqman analysis. RNA was reverse transcribed into cDNA using the Superscript III Kit (Invitrogen). Probes were used at 1:20 dilution with TaqMan Universal PCR Master Mix (Applied Biosystems). Detection and quantification were performed on the ABI 7900HT Sequence Detection System (Applied Biosystems), and data were collected and analyzed with the SDS 2.2.2 software from Applied Biosystems. Data were analyzed by calculating the Ct value, ∆Ct in reference to 18S, and ∆∆Ct, and ultimately, fold change was calculated from the ∆∆Ct value; n=6 for each group in which the QRT for each was run in triplicate for each. Probes used were all purchased from Applied Biosystems, with 18S used as a loading control. Probes used were SOCS3 MM00545913_s1, IL-6 MM00446191_m1, IL6Ra MM00439653_m1, JAK1 MM00600614_m1, JAK2 MM012–8489_m1, STAT3 MM01219775_m1, and NFKB1a MM00477800_g1.
Statistical Analyses
For in vivo studies, mice were first randomized on the basis of baseline body weight, ACR, and in some studies, SAP. Data were analyzed with JMP, v.8.0 software (SAS Institute) using Tukey–Kramer comparison of pairs or control Dunnett, one-way ANOVA, and/or t test. A P value of <0.05 was considered statistically significant. Time course analysis of BP and ACR was performed using R stats package, in which log2 transformation of measurements was obtained and fitted to an ANOVA model with repeated measures. Renin was compared with LacZ at each time point. Raw P values are reported. Values used in text are for 8 weeks after the AAV injection time point for BTBR-ob/ob mice. All data points for the Akita, db/db intact, and db/db uNx studies are for 12 weeks after adeno-associated virus injection unless otherwise noted.
Disclosures
All authors performed their work as employees of Eli Lilly and Company (Indianapolis, IN).
Supplementary Material
Acknowledgments
We would like to thank Brenda Hanssen for assistance with the clinical chemistry and Armando Irizarry and William A. Meier for their expertise in evaluating electron microscopy.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2017040385/-/DCSupplemental.
References
- 1.Wanner C, Inzucchi SE, Lachin JM, Fitchett D, von Eynatten M, Mattheus M, Johansen OE, Woerle HJ, Broedl UC, Zinman B; EMPA-REG OUTCOME Investigators : Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med 375: 323–334, 2016 [DOI] [PubMed] [Google Scholar]
- 2.Conway BR, Rennie J, Bailey MA, Dunbar DR, Manning JR, Bellamy CO, Hughes J, Mullins JJ: Hyperglycemia and renin-dependent hypertension synergize to model diabetic nephropathy. J Am Soc Nephrol 23: 405–411, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van Buren PN, Toto R: Hypertension in diabetic nephropathy: Epidemiology, mechanisms, and management. Adv Chronic Kidney Dis 18: 28–41, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Betz B, Conway BR: Recent advances in animal models of diabetic nephropathy. Nephron, Exp Nephrol 126: 191–195, 2014 [DOI] [PubMed] [Google Scholar]
- 5.Giunti S, Barit D, Cooper ME: Mechanisms of diabetic nephropathy: Role of hypertension. Hypertension 48: 519–526, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Hostetter TH, Rennke HG, Brenner BM: The case for intrarenal hypertension in the initiation and progression of diabetic and other glomerulopathies. Am J Med 72: 375–380, 1982 [DOI] [PubMed] [Google Scholar]
- 7.McMaster WG, Kirabo A, Madhur MS, Harrison DG: Inflammation, immunity, and hypertensive end-organ damage. Circ Res 116: 1022–1033, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kirabo A, Fontana V, de Faria AP, Loperena R, Galindo CL, Wu J, Bikineyeva AT, Dikalov S, Xiao L, Chen W, Saleh MA, Trott DW, Itani HA, Vinh A, Amarnath V, Amarnath K, Guzik TJ, Bernstein KE, Shen XZ, Shyr Y, Chen SC, Mernaugh RL, Laffer CL, Elijovich F, Davies SS, Moreno H, Madhur MS, Roberts J 2nd, Harrison DG: DC isoketal-modified proteins activate T cells and promote hypertension. J Clin Invest 124: 4642–4656, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao HJ, Wang S, Cheng H, Zhang MZ, Takahashi T, Fogo AB, Breyer MD, Harris RC: Endothelial nitric oxide synthase deficiency produces accelerated nephropathy in diabetic mice. J Am Soc Nephrol 17: 2664–2669, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brosius FC 3rd, Alpers CE, Bottinger EP, Breyer MD, Coffman TM, Gurley SB, Harris RC, Kakoki M, Kretzler M, Leiter EH, Levi M, McIndoe RA, Sharma K, Smithies O, Susztak K, Takahashi N, Takahashi T; Animal Models of Diabetic Complications Consortium : Mouse models of diabetic nephropathy. J Am Soc Nephrol 20: 2503–2512, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Forbes MS, Thornhill BA, Park MH, Chevalier RL: Lack of endothelial nitric-oxide synthase leads to progressive focal renal injury. Am J Pathol 170: 87–99, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hartono SP, Knudsen BE, Lerman LO, Textor SC, Grande JP: Combined effect of hyperfiltration and renin angiotensin system activation on development of chronic kidney disease in diabetic db/db mice. BMC Nephrol 15: 58, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meyza KZ, Defensor EB, Jensen AL, Corley MJ, Pearson BL, Pobbe RL, Bolivar VJ, Blanchard DC, Blanchard RJ: The BTBR T+ tf/J mouse model for autism spectrum disorders-in search of biomarkers. Behav Brain Res 251: 25–34, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thompson A: Proteinuria as a surrogate end point--more data are needed. Nat Rev Nephrol 8: 306–309, 2012 [DOI] [PubMed] [Google Scholar]
- 15.Inker LA, Lambers Heerspink HJ, Mondal H, Schmid CH, Tighiouart H, Noubary F, Coresh J, Greene T, Levey AS: GFR decline as an alternative end point to kidney failure in clinical trials: A meta-analysis of treatment effects from 37 randomized trials. Am J Kidney Dis 64: 848–859, 2014 [DOI] [PubMed] [Google Scholar]
- 16.Levey AS, Inker LA, Matsushita K, Greene T, Willis K, Lewis E, de Zeeuw D, Cheung AK, Coresh J: GFR decline as an end point for clinical trials in CKD: A scientific workshop sponsored by the National Kidney Foundation and the US Food and Drug Administration. Am J Kidney Dis 64: 821–835, 2014 [DOI] [PubMed] [Google Scholar]
- 17.Thibodeau JF, Holterman CE, Burger D, Read NC, Reudelhuber TL, Kennedy CR: A novel mouse model of advanced diabetic kidney disease. PLoS One 9: e113459, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cooper ME, Allen TJ, Jerums G, Doyle AE: Accelerated progression of diabetic nephropathy in the spontaneously hypertensive streptozotocin diabetic rat. Clin Exp Pharmacol Physiol 13: 655–662, 1986 [DOI] [PubMed] [Google Scholar]
- 19.Cooper ME, Allen TJ, Macmillan P, Bach L, Jerums G, Doyle AE: Genetic hypertension accelerates nephropathy in the streptozotocin diabetic rat. Am J Hypertens 1: 5–10, 1988 [DOI] [PubMed] [Google Scholar]
- 20.Cooper ME, Allen TJ, O’Brien RC, Macmillan PA, Clarke B, Jerums G, Doyle AE: Effects of genetic hypertension on diabetic nephropathy in the rat--functional and structural characteristics. J Hypertens 6: 1009–1016, 1988 [DOI] [PubMed] [Google Scholar]
- 21.Kelly DJ, Wilkinson-Berka JL, Allen TJ, Cooper ME, Skinner SL: A new model of diabetic nephropathy with progressive renal impairment in the transgenic (mRen-2)27 rat (TGR). Kidney Int 54: 343–352, 1998 [DOI] [PubMed] [Google Scholar]
- 22.Gurley SB, Clare SE, Snow KP, Hu A, Meyer TW, Coffman TM: Impact of genetic background on nephropathy in diabetic mice. Am J Physiol Renal Physiol 290: F214–F222, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Gurley SB, Mach CL, Stegbauer J, Yang J, Snow KP, Hu A, Meyer TW, Coffman TM: Influence of genetic background on albuminuria and kidney injury in Ins2(+/C96Y) (Akita) mice. Am J Physiol Renal Physiol 298: F788–F795, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hudkins KL, Pichaiwong W, Wietecha T, Kowalewska J, Banas MC, Spencer MW, Mühlfeld A, Koelling M, Pippin JW, Shankland SJ, Askari B, Rabaglia ME, Keller MP, Attie AD, Alpers CE: BTBR Ob/Ob mutant mice model progressive diabetic nephropathy. J Am Soc Nephrol 21: 1533–1542, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Katz SM: Reduplication of the glomerular basement membrane. A study of 110 cases. Arch Pathol Lab Med 105: 67–70, 1981 [PubMed] [Google Scholar]
- 26.Hsu H, Churg J: The ultrastructure of mucoid “onionskin” intimal lesions in malignant nephrosclerosis. Am J Pathol 99: 67–80, 1980 [PMC free article] [PubMed] [Google Scholar]
- 27.Sethi S, Iida S, Sigmund CD, Heistad DD: Renal thrombotic microangiopathy in a genetic model of hypertension in mice. Exp Biol Med (Maywood) 231: 196–203, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Rosansky SJ, Glassock RJ: Is a decline in estimated GFR an appropriate surrogate end point for renoprotection drug trials? Kidney Int 85: 723–727, 2014 [DOI] [PubMed] [Google Scholar]
- 29.Schnermann J, Oppermann M, Huang Y: Nephron filtration rate and proximal tubular fluid reabsorption in the Akita mouse model of type I diabetes mellitus. F1000 Res 2: 83, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang MZ, Wang S, Yang S, Yang H, Fan X, Takahashi T, Harris RC: Role of blood pressure and the renin-angiotensin system in development of diabetic nephropathy (DN) in eNOS-/- db/db mice. Am J Physiol Renal Physiol 302: F433–F438, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hodgin JB, Nair V, Zhang H, Randolph A, Harris RC, Nelson RG, Weil EJ, Cavalcoli JD, Patel JM, Brosius FC 3rd, Kretzler M: Identification of cross-species shared transcriptional networks of diabetic nephropathy in human and mouse glomeruli. Diabetes 62: 299–308, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Berthier CC, Zhang H, Schin M, Henger A, Nelson RG, Yee B, Boucherot A, Neusser MA, Cohen CD, Carter-Su C, Argetsinger LS, Rastaldi MP, Brosius FC, Kretzler M: Enhanced expression of Janus kinase-signal transducer and activator of transcription pathway members in human diabetic nephropathy. Diabetes 58: 469–477, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Woroniecka KI, Park AS, Mohtat D, Thomas DB, Pullman JM, Susztak K: Transcriptome analysis of human diabetic kidney disease. Diabetes 60: 2354–2369, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grove KJ, Voziyan PA, Spraggins JM, Wang S, Paueksakon P, Harris RC, Hudson BG, Caprioli RM: Diabetic nephropathy induces alterations in the glomerular and tubule lipid profiles. J Lipid Res 55: 1375–1385, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Declèves AE, Sharma K: Novel targets of antifibrotic and anti-inflammatory treatment in CKD. Nat Rev Nephrol 10: 257–267, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang W, Cai J, Tang S, Zhang Y, Gao X, Xie L, Mou Z, Wu Y, Wang L, Zhang J: Sinomenine attenuates angiotensin II-induced autophagy via inhibition of P47-Phox translocation to the membrane and influences reactive oxygen species generation in podocytes. Kidney Blood Press Res 41: 158–167, 2016 [DOI] [PubMed] [Google Scholar]
- 37.Li C, Lin Y, Luo R, Chen S, Wang F, Zheng P, Levi M, Yang T, Wang W: Intrarenal renin-angiotensin system mediates fatty acid-induced ER stress in the kidney. Am J Physiol Renal Physiol 310: F351–F363, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harlan SM, Ostroski RA, Coskun T, Yantis LD, Breyer MD, Heuer JG: Viral transduction of renin rapidly establishes persistent hypertension in diverse murine strains. Am J Physiol Regul Integr Comp Physiol 309: R467–R474, 2015 [DOI] [PubMed] [Google Scholar]
- 39.Qi Z, Whitt I, Mehta A, Jin J, Zhao M, Harris RC, Fogo AB, Breyer MD: Serial determination of glomerular filtration rate in conscious mice using FITC-inulin clearance. Am J Physiol Renal Physiol 286: F590–F596, 2004 [DOI] [PubMed] [Google Scholar]
- 40.Jensen EB, Gundersen HJ, Osterby R: Determination of membrane thickness distribution from orthogonal intercepts. J Microsc 115: 19–33, 1979 [DOI] [PubMed] [Google Scholar]
- 41.Gentleman RC, Carey VJ, Bates DM, Bolstad B, Dettling M, Dudoit S, Ellis B, Gautier L, Ge Y, Gentry J, Hornik K, Hothorn T, Huber W, Iacus S, Irizarry R, Leisch F, Li C, Maechler M, Rossini AJ, Sawitzki G, Smith C, Smyth G, Tierney L, Yang JY, Zhang J: Bioconductor: Open software development for computational biology and bioinformatics. Genome Biol 5: R80, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ihaka R, Gentleman R: R: A language for data analysis and graphics. J Comput Graph Stat 5: 299–314, 1996 [Google Scholar]
- 43.Falcon S, Gentleman R: Using GOstats to test gene lists for GO term association. Bioinformatics 23: 257–258, 2007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.