Abstract
AKI after cardiac surgery is associated with mortality, prolonged hospital length of stay, use of dialysis, and subsequent CKD. We evaluated the effects of THR-184, a bone morphogenetic protein-7 agonist, in patients at high risk for AKI after cardiac surgery. We conducted a randomized, double-blind, placebo-controlled, multidose comparison of the safety and efficacy of perioperative THR-184 using a two-stage seamless adaptive design in 452 patients between 18 and 85 years of age who were scheduled for nonemergent cardiac surgery requiring cardiopulmonary bypass and had recognized risk factors for AKI. The primary efficacy end point was the proportion of patients who developed AKI according to Kidney Disease Improving Global Outcomes (KDIGO) criteria. The proportion of patients who developed AKI within 7 days of surgery was similar in THR-184 treatment groups and placebo groups (range, 74%–79%; P=0.43). Prespecified secondary end point analysis did not show significant differences in the severity of AKI stage (P=0.53) or the total duration of AKI (P=0.44). A composite of death, dialysis, or sustained impaired renal function by day 30 after surgery did not differ between groups (range, 11%–20%; P=0.46). Safety-related outcomes were similar across all treatment groups. In conclusion, compared with placebo, administration of perioperative THR-184 through a range of dose exposures failed to reduce the incidence, severity, or duration of AKI after cardiac surgery in high-risk patients.
Keywords: acute renal failure, bone morphogenetic protein, randomized controlled trials
AKI affects up to 10% of all hospitalized patients in the United States, and it is associated with mortality, prolonged hospital length of stay, and subsequent development or progression of CKD.1–3 Cardiac surgery–associated AKI (CSA-AKI) occurs commonly,4 and a multinational, multicenter study reported cardiac surgery to be the second most common etiology for AKI.5 In case series, the incidence of AKI after cardiac surgery has generally varied between approximately 8% and 30%, but it has been reported to be as high as over 50%6; even small postoperative increases in serum creatinine concentration are associated with a significant increase in myocardial infarction, short-term mortality, length of stay, and costs.7–10 A recent nationwide study of AKI after cardiac surgery in Sweden found an association between postoperative AKI and the eventual development of ESRD.11 Despite advances in surgical technique and anesthesia, there has been no reduction in the rate of AKI. These findings collectively underscore the need to establish treatment interventions that can reduce the incidence and severity of AKI after cardiac surgery.
One promising therapeutic approach is to target the TGF-β and bone morphogenetic protein-7 (BMP-7) signaling pathways.12 TGF-β is considered a critical mediator contributing to renal fibrosis, inflammation, and apoptosis after kidney injury, whereas BMP-7 can inhibit TGF-β signaling, thereby protecting the kidney from TGF-mediated propagation of injury. The BMP pathway also has important direct roles in numerous physiologic processes, including cell proliferation, differentiation, and apoptosis, and activation of the pathway yields anti-inflammatory and antifibrotic effects. In animal models of both AKI and CKD, BMP-7 has been found to be nephroprotective and promote kidney regeneration.13 In preclinical studies, administration of BMP-7 in rodent kidney ischemia models has been reported to increase survival.14 BMP-7 attenuates kidney damage by reducing the release of proinflammatory cytokines and chemokines and helping to maintain renal blood flow.15 THR-184 is a member of a synthetic peptide family16,17 that acts as a positive modulator of the BMP-7 signaling pathway. In preclinical studies, BMP-7 agonists exert anti-inflammatory and antiapoptotic actions in the kidney.18,19
We performed a randomized clinical trial to assess the effects of THR-184 in the prevention of CSA-AKI with the primary end point on the basis of Kidney Disease Improving Global Outcomes (KDIGO) criteria. We used a two-stage seamless adaptive trial design (thus allowing for midtrial dosing adjustments), with an enrichment strategy for entry criteria designed to ensure a high rate of AKI.
Results
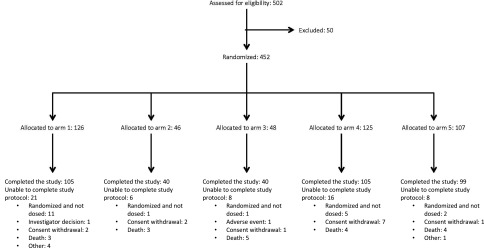
Enrollment of patients began on July 24, 2013 and was completed on September 24, 2015. Over the course of the trial, 452 patients were randomized; 431 patients received at least one dose of study agent (safety population), 401 patients received at least one dose of study agent and had at least one postoperative visit in which the incidence of AKI could be assessed (full analysis set population; Table 1), and 308 patients had assessments up to discharge or day 7 or died within 7 days and did not have any important protocol deviations (per protocol population). The safety population was used for all safety analyses and describing baseline patient characteristics. Full analysis (for the primary analysis) and per protocol analysis set populations were used for the primary and secondary efficacy end points (Table 2).
Table 1.
Characteristics of the subjects at baseline (full analysis set)
| Characteristic | Arm 1, n=113 | Arm 2, n=34 | Arm 3, n=34 | Arm 4, n=116 | Arm 5, n=104 |
|---|---|---|---|---|---|
| Age, yr | |||||
| Median | 74.0 | 71.0 | 75.0 | 75.0 | 71.5 |
| Interquartile range | 65–80 | 62–77 | 67–79 | 67–78 | 64–77 |
| Women, no. (%) | 44 (38.9) | 15 (41.1) | 12 (35.3) | 38 (32.8) | 33 (31.7) |
| Race,a no. (%) | |||||
| White | 103 (91.2) | 30 (88.2) | 32 (94.1) | 107 (92.2) | 94 (90.4) |
| Black | 5 (4.4) | 3 (8.8) | 0 | 5 (4.3) | 3 (2.9) |
| Other | 5 (4.5) | 1 (2.9) | 2 (5.8) | 4 (3.5) | 7 (6.7) |
| Body mass indexb | |||||
| Median | 28.3 | 30.4 | 28.7 | 28.1 | 28.3 |
| Interquartile range | 26–33 | 24–35 | 26–33 | 26–32 | 26–33 |
| GFR,c ml/min per 1.73 m2 | |||||
| Median | 59.5 | 62.8 | 61.2 | 61.3 | 63.6 |
| Interquartile range | 44–79 | 47–79 | 48–75 | 48–76 | 48–83 |
| Medical history,d no. (%) | |||||
| CKD | 42 (37.2) | 19 (55.9) | 15 (44.1) | 44 (37.9) | 40 (38.5) |
| AKI | 23 (20.4) | 7 (20.6) | 10 (29.4) | 20 (17.2) | 28 (26.9) |
| Hypertension | 83 (73.5) | 24 (70.6) | 24 (70.6) | 105 (90.5) | 88 (84.6) |
| Cardiac failure | 49 (43.4) | 17 (50.0) | 18 (52.9) | 47 (40.5) | 55 (52.9) |
| Ischemic heart disease | 86 (76.1) | 28 (82.4) | 26 (76.5) | 91 (78.4) | 80 (76.9) |
| Cardiac arrhythmia | 48 (42.5) | 17 (50.0) | 9 (26.5) | 50 (43.1) | 39 (37.5) |
| Cerebrovascular disorder | 19 (16.8) | 7 (20.6) | 5 (14.7) | 26 (22.4) | 23 (22.1) |
| Diabetes mellitus | 60 (53.1) | 18 (52.9) | 16 (47.1) | 55 (47.4) | 51 (49.0) |
Arm 1 indicates placebo. Arm 2 =0.02-mg/kg preoperative and postoperative doses. Arm 3 =0.12-mg/kg preoperative and 0.02-mg/kg postoperative doses. Arm 4 =0.46-mg/kg preoperative and 0.02-mg/kg postoperative doses. Arm 5 =0.46-mg/kg preoperative and postoperative doses.
Race was self-reported.
The body mass index is the weight in kilograms divided by the square of the height in meters.
The GFR was estimated using the four-variable Modification of Diet in Renal Disease formula.
These data were derived from the clinical database using narrow Standardized Medical Dictionary for Regulatory Activities Queries. AKI was collected using the renal failure query, cerebrovascular disorders were collected using the cerebrovascular nervous system disorders query, and diabetes mellitus was collected using the hyperglycemia/new-onset diabetes mellitus query.
Table 2.
Prespecified end points
| Primary end point |
| Development of AKI within 7 d of surgery according to KDIGO criteria |
| Secondary end points |
| Development of AKI within 7 d of surgery according to serum creatinine–based KDIGO criteria |
| Severity of AKI per KDIGO criteria |
| Duration of AKI within the first 7 d |
| Death, dialysis, or eGFR decline >30% at day 30 |
| Exploratory end points |
| Serum creatinine over time and maximum change from baseline |
| eGFR over time and maximum change from baseline |
| AUC for serum creatinine and serum creatinine change from baseline through days 7 and 90 |
| AKI or death during the first 7 d |
| Time to doubling of serum creatinine, dialysis, or death |
| At least doubling of serum creatinine, dialysis, or death |
| Death, dialysis, or eGFR decline >30% at day 30 |
| Time to dialysis or death |
| Time to death of any cause |
| Urine volume <0.5 ml/kg per hour for 6 h consecutively during the first 48 h |
| Development of AKI within 7 d of surgery according to the RIFLE criteria |
| Development of AKI within 7 d of surgery according to the AKIN criteria |
| Time on dialysis through day 90 |
| Time in ICU, CCU, or step-down unit |
| Time in hospital |
| Readmission in hospital within 30 d of surgery |
| Time to readmission to hospital |
| Creatinine kinase MB isoenzyme fraction, troponin I, and troponin T over time |
| Albumin-to-creatinine ratio, KIM-1–to-creatinine ratio, and NGAL-to-creatinine ratio over time |
AKIN, Acute Kidney Injury Network; ICU, intensive care unit; CCU, cardiac care unit; KIM-1, kidney injury molecule-1; NGAL, neutrophil gelatinase–associated lipocalin.
Efficacy End Points
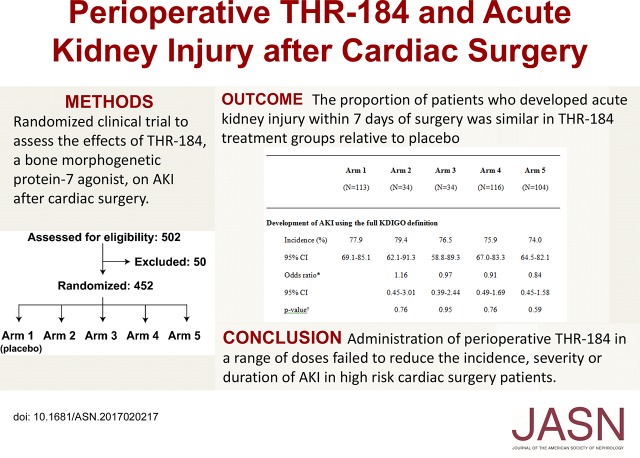
In the full analysis set population, the proportions of patients who developed AKI within 7 days of surgery were 78% in arm 1 (placebo), 79% in arm 2, 77% in arm 3, 76% in arm 4, and 74% in arm 5 (Table 3). The proportion of patients who developed AKI was not statistically significantly different in THR-184 treatment groups relative to placebo (P=0.43). Additional analyses of prespecified secondary end points were generally consistent with the primary analysis; the resulting data did not indicate significant differences in the proportion of patients who developed AKI within 7 days of surgery using the serum creatinine–based KDIGO criteria (P=0.63), the severity of AKI stage using KDIGO criteria during the first 7 days or discharge after surgery (P=0.53), or the total duration of AKI within the first 7 days for patients who had AKI in the first 3 days after surgery (P=0.44). There were no significant differences in a composite of death, dialysis, or sustained impaired renal function by day 30 after surgery (P=0.46) (Table 3). In the per protocol population, the proportion of patients who developed AKI was also not significantly different in THR-184 treatment groups relative to placebo: arm 1 (placebo), 79%; arm 2, 75%; arm 3, 76%; arm 4, 78%; and arm 5, 70% (P=0.47) (Supplemental Table 1). There were no significant differences in postoperative serum concentrations of creatine kinase MB fraction (P=0.22) or highly sensitive troponin (P=0.14 for troponin I and P=0.28 for troponin T) on the basis of treatment assignment.
Table 3.
Primary end point, components of the primary end point, and secondary end points (full analysis set)
| Endpoints | Arm 1, n=113 | Arm 2, n=34 | Arm 3, n=34 | Arm 4, n=116 | Arm 5, n=104 |
|---|---|---|---|---|---|
| Development of AKI using the full KDIGO definition | |||||
| Incidence, % | 77.9 | 79.4 | 76.5 | 75.9 | 74.0 |
| 95% CI | 69.1 to 85.1 | 62.1 to 91.3 | 58.8 to 89.3 | 67.0 to 83.3 | 64.5 to 82.1 |
| Odds ratioa | 1.16 | 0.97 | 0.91 | 0.84 | |
| 95% CI | 0.45 to 3.01 | 0.39 to 2.44 | 0.49 to 1.69 | 0.45 to 1.58 | |
| P valueb | 0.76 | 0.95 | 0.76 | 0.59 | |
| Increase in serum creatinine ≥0.3 mg/dl within 48 h | |||||
| Incidence, % | 53.1 | 58.8 | 55.9 | 55.2 | 51.9 |
| 95% CI | 43.5 to 62.5 | 40.7 to 75.4 | 37.9 to 72.8 | 45.7 to 64.4 | 41.9 to 61.8 |
| Odds ratio | 1.37 | 1.21 | 1.12 | 1.01 | |
| 95% CI | 0.61 to 3.06 | 0.54 to 2.68 | 0.65 to 1.92 | 0.58 to 1.74 | |
| Increase in serum creatinine ≥50% within 7 d | |||||
| Incidence, % | 20.4 | 32.4 | 23.5 | 20.7 | 22.1 |
| 95% CI | 13.4 to 29.0 | 17.4 to 50.5 | 10.7 to 41.2 | 13.7 to 29.2 | 14.6 to 31.3 |
| Odds ratio | 1.87 | 1.20 | 1.02 | 1.11 | |
| 95% CI | 0.80 to 4.38 | 0.48 to 3.00 | 0.54 to 1.94 | 0.58 to 2.13 | |
| Urine output <0.5 ml/kg per hour for ≥6 h consecutively | |||||
| Incidence, % | 60.2 | 58.8 | 55.9 | 51.7 | 61.5 |
| 95% CI | 50.5 to 69.3 | 40.7 to 75.4 | 37.9 to 72.8 | 42.3 to 61.1 | 51.5 to 70.9 |
| Odds ratio | 0.95 | 0.85 | 0.71 | 1.09 | |
| 95% CI | 0.44 to 2.08 | 0.39 to 1.84 | 0.42 to 1.20 | 0.63 to 1.89 | |
| Development of AKI using the serum creatinine–based KDIGO definition | |||||
| Incidence, % | 54.9 | 58.8 | 58.8 | 56.0 | 51.9 |
| 95% CI | 45.2 to 64.2 | 40.7 to 75.4 | 40.7 to 75.4 | 46.5 to 65.2 | 41.9 to 61.8 |
| Odds ratio | 1.26 | 1.26 | 1.07 | 0.93 | |
| 95% CI | 0.57 to 2.80 | 0.57 to 2.80 | 0.63 to 1.83 | 0.54 to 1.61 | |
| Composite of death, dialysis, or ≥30% decline in GFR at day 30 | |||||
| Incidence, % | 11.3 | 20.0 | 20.0 | 13.2 | 18.3 |
| 95% CI | 6.0 to 18.9 | 7.7 to 38.6 | 7.7 to 38.6 | 7.4 to 21.2 | 10.6 to 28.4 |
| Odds ratio | 2.00 | 2.01 | 1.19 | 1.74 | |
| 95% CI | 0.68 to 5.88 | 0.68 to 5.94 | 0.52 to 2.72 | 0.76 to 3.96 | |
| Severity of AKI,c no. (%) | |||||
| AKI stage 1 | 53 (46.9) | 15 (44.1) | 14 (41.2) | 59 (50.9) | 39 (37.5) |
| AKI stage 2 | 32 (28.3) | 10 (29.4) | 11 (32.4) | 26 (22.4) | 33 (31.7) |
| AKI stage 3 | 3 (2.7) | 5 (5.9) | 1 (2.9) | 3 (2.6) | 5 (4.8) |
| Duration of AKI within the first 7 d | |||||
| Median | 3.0 | 2.0 | 2.0 | 2.5 | 3.0 |
| Interquartile range | 1–6 | 1–5 | 1–4 | 1–7 | 2–7 |
Arm 1 indicates placebo. Arm 2 =0.02-mg/kg preoperative and postoperative doses. Arm 3 =0.12-mg/kg preoperative and 0.02-mg/kg postoperative doses. Arm 4 =0.46-mg/kg preoperative and 0.02-mg/kg postoperative doses. Arm 5 =0.46-mg/kg preoperative and postoperative doses. 95% CI, 95% confidence interval.
Compared with arm 1 (placebo).
Unadjusted P value (two sided) from logistic regression with baseline eGFR as a covariate, comparing the active arm with arm 1 (placebo).
Using the KDIGO classification and the full KDIGO definition of AKI.
Safety End Points
A full account of safety related outcomes is presented in Supplemental Tables 2 and 3 and Table 4. Adverse events (AEs) were reported in a total of 418 (97%) patients; in 50 (12%) patients, the AE was considered to be treatment related by the investigator. Serious AEs occurred in 190 (44%) patients. The rates of AEs and serious AEs were similar across all treatment groups. There was a nominally higher rate of postoperative wound infections in the highest-dose arm (11%) compared with placebo (4%) that was not observed with other THR-184 dose formulations.
Table 4.
AEs (safety analysis set)
| Arm 1, n=115 | Arm 2, n=45 | Arm 3, n=47 | Arm 4, n=119 | Arm 5, n=105 | |
|---|---|---|---|---|---|
| Subjects with, no. (%) | |||||
| ≥1 AE | 112 (97.4) | 41 (91.1) | 46 (97.9) | 116 (97.5) | 103 (98.1) |
| ≥1 Serious AE | 48 (41.7) | 21 (46.7) | 28 (59.6) | 50 (42.0) | 43 (41.0) |
| ≥1 AE leading to death | 3 (2.6) | 4 (8.9) | 5 (10.6) | 4 (3.4) | 5 (4.8) |
| ≥1 Treatment-related AE | 9 (7.8) | 5 (11.1) | 6 (12.8) | 18 (15.1) | 12 (11.4) |
| ≥1 AE leading to study drug discontinuation | 1 (0.9) | 1 (2.2) | 0 | 0 | 2 (1.9) |
| Patients with a predefined surgical complication, no. (%) | |||||
| Stroke | 0 | 0 | 0 | 2 (1.7) | 2 (1.9) |
| Myocardial infarction | 4 (3.5) | 2 (4.4) | 0 | 2 (1.7) | 4 (3.8) |
| Surgical site infection | 0 | 0 | 0 | 1 (0.8) | 1 (1.0) |
| Nonsurgical site infection | 2 (1.7) | 0 | 3 (6.4) | 4 (3.4) | 2 (1.9) |
| Prolonged ventilation | 6 (5.2) | 6 (13.3) | 3 (6.4) | 9 (7.6) | 6 (5.7) |
| Dialysis | 2 (1.7) | 1 (2.2) | 2 (4.3) | 1 (0.8) | 4 (3.8) |
| Low-cardiac output syndrome | 14 (12.2) | 4 (8.9) | 7 (14.9) | 13 (10.9) | 13 (12.4) |
Arm 1 indicates placebo. Arm 2 =0.02-mg/kg preoperative and postoperative doses. Arm 3 =0.12-mg/kg preoperative and 0.02-mg/kg postoperative doses. Arm 4 =0.46-mg/kg preoperative and 0.02-mg/kg postoperative doses. Arm 5 =0.46-mg/kg preoperative and postoperative doses.
Exploratory End Points
The odds of developing AKI within 7 days for patients in arm 5 (highest cumulative dose) relative to placebo were 0.84 (95% confidence interval, 0.45 to 1.58) in the full analysis set and 0.68 in the per protocol analysis (95% confidence interval, 0.33 to 1.39). Results in the eGFR<60 ml/min per 1.73 m2 subgroup are shown in Supplemental Table 4. Pharmacokinetic (PK) analyses showed that exposure to THR-184 increased in parallel with dose in a nearly proportional manner (Supplemental Figure 1).
Discussion
We found that THR-184 given intravenously through a range of exposures failed to reduce the incidence of AKI, despite the expected rates of the outcome. Given the internal consistency of the findings, we believe that the inferences of a neutral effect in humans in this model are valid and must be interpreted in the light of encouraging animal and early human trial data.
There is no accepted therapeutic approach to either prevention or amelioration of the course of AKI after cardiac surgery.4 Recently completed randomized clinical trials have examined whether high-dose dexamethasone,20 perioperative atorvastatin,21 administration of mesenchymal stem cells,22 a novel α-melanocyte stimulating hormone analog,6 or use of remote ischemic preconditioning23–26 are of benefit in lowering rates of AKI after cardiac surgery. Earlier studies examined a variety of clinical strategies, including optimizing glucose control in patients with diabetes27 and lowering the threshold for red blood cell transfusion.28 Results of these studies either showed no effect or produced mixed or conflicting data. The rationale that the BMP-7 agonist THR-184 could plausibly improve AKI outcomes after cardiac surgery is on the basis of preclinical studies showing that BMP-7 expression is highest in the kidney, that renal tubular epithelial cells have high-affinity receptors for BMP-7, and that BMP-7 is a key regulator of anti-inflammatory, antifibrotic, and antiapoptotic actions in the kidney.14,15
We found no major safety risks related to THR-184. No clinical or statistically significant benefits were observed for THR-184 relative to placebo in the primary or secondary efficacy end points, and there was no demonstrable dose-response relation between preoperative THR-184 dose and outcomes compared with placebo. The observed rates of postoperative AKI were higher than anticipated (>70% in all treatment arms compared with the prestudy estimate of approximately 50% in the placebo group.) Of note, a recently published study using similar entry criteria in an effort to enroll high-risk subjects also reported a high rate (approximately 65%) of AKI in all treatment groups.6
We used an adaptive trial design to evaluate the effects of THR-184, a small molecule agonist of BMP-7, on the incidence of AKI after cardiac surgery in high-risk patients. There is growing interest in the use of adaptive trial designs,27,29,30 and to our knowledge, this is the first use of this approach in a randomized trial of a therapeutic agent for AKI. Although traditional randomized, controlled trials are designed to evaluate a single treatment strategy defined a priori, adaptive designs use accumulating information to allow modifications to an ongoing trial and/or statistical procedures without undermining the validity or integrity of the trial. This can lead to greater flexibility and efficiency for identifying signals or trends for clinical benefit, thereby mitigating uncertainties that exist when a clinical trial is originally designed. Although there are many differing approaches for adaptive designs, in this trial, a two-stage adaptive design was constructed for dose optimization using a “drop the loser” or “pick the winner” strategy. This strategy allowed for an additional arm to be added after the interim analysis, which incorporated a higher postoperative THR-184 dose.31 Analysis of this overall higher-dose arm showed lower nominal incidence of postsurgery AKI relative to placebo; the difference, although not statistically different, was more pronounced among patients with preexisting CKD in the per protocol dataset. Further prospective testing will be required to determine if there is a role for higher doses and/or longer periods of administration of THR-184 to reduce the incidence of AKI after cardiac surgery and potentially mitigate associated complications, particularly in patients with CKD.
This study had numerous strengths. First, to our knowledge, this is the largest randomized clinical trial in a population chosen to be at high risk for AKI after undergoing cardiac surgery. Second, the study used standardized, well accepted definitions of AKI; had excellent follow-up; and assessed 90-day outcomes in addition to short-term outcomes. Third, the adaptive trial design allowed assessment of multiple doses of THR-184.
The study also had several limitations. The rate of AKI events in all groups was higher than anticipated and may have reduced the likelihood of observing a treatment effect. It is plausible that the dose, timing, frequency, and/or duration of THR-184 administered were insufficient for therapeutic benefit. There was a relatively high proportion of participants in whom the study was not conducted per protocol, including 4.6% of patients who were randomized but not dosed. Before the trial, there were limited human PK data available on THR-184, and the PK data generated during the trial showed a large interindividual variation in plasma THR-184 concentrations after administration, which may have reduced therapeutic efficacy. Our findings, however, show a cohesive null effect given a fair opportunity to observe clinical benefit. It is also possible that the KDIGO definition of AKI is not sufficiently sensitive to capture clinically relevant treatment effects in patients undergoing cardiac surgery. Transient hemodynamic and blood volume shifts are common, and as such, changes in serum creatinine and urine output may not be reflective of organ injury in all instances. We recognize that the field of AKI and its definitions are in evolution, and with more precise measures of renal parenchymal damage, THR-184 or other attempted novel therapeutics could show benefit with novel, more specific primary end point(s).
In conclusion, in comparison with placebo, the administration of THR-184 through a range of dose exposures failed to reduce the incidence, severity, or duration of AKI after cardiac surgery in high-risk patients.
Concise Methods
Study Participants
A total of 42 centers in the United States and Canada participated in this trial (Supplemental Appendices 1 and 2 have lists of investigators). Institutional review board approval and written informed consent were obtained before any study procedures commenced. The full list of inclusion and exclusion criteria is found in the trial protocol included in Supplemental Appendix. Briefly, eligible patients were men and women between 18 and 85 years of age who were scheduled for nonemergent cardiac surgery requiring cardiopulmonary bypass with additional recognized risk factors for AKI as determined by one or more of the following parameters: eGFR<60 ml/min per 1.73 m2, age ≥75 years old, higher-risk surgery type (e.g., heart valve plus CABG), New York Heart Association class 3 or 4, left ventricular ejection fraction ≤35%, history of diabetes mellitus with insulin use or proteinuria, or hemoglobin <10 mg/dl. Randomization was stratified on the basis of eGFR (<60 or ≥60 ml/min per 1.73 m2). Patients who had AKI at the time of screening, eGFR<20 ml/min per 1.73 m2, prior organ transplantation, or dialysis dependence were excluded from the trial. Demographic and clinical characteristics of enrolled patients are presented in Table 1.
Adaptive Study Design
The trial uses a two-stage seamless adaptive design methodology. Stage 1 consists of 140 patients randomized in a 1:1:1:1 ratio (approximately 35 patients per treatment arm) to placebo (arm 1) or one of three THR-184 presurgery dose arms: 0.02 (arm 2), 0.12 (arm 3), or 0.46 mg/kg (arm 4). In all three active treatment arms, the initial presurgery dose is followed by three daily postsurgery doses of 0.02 mg/kg each of THR-184. At the end of stage 1 enrollment, data from the 140 patients are used to select the THR-184 dose arms for stage 2. When the last patient enrolled in stage 1 completed 7 days of follow-up, terminated the study early, or was discharged before 7 days, enabling a comparison across the four arms, an interim analysis is performed by the Independent Statistical Center (Cytel, Inc.) and presented to the Independent Data Monitoring Committee (IDMC). Recruitment continues in a 1:1:1:1 ratio during the 7-day follow-up of the 140th patient and during the interim analysis period.
We assumed an umbrella-shaped dose-response, with an incidence of AKI of 50% in the placebo arm and an incidence of AKI of 30% in the most efficacious arm, and that the best arm would be selected at the time of the interim analysis. We further estimated that 280 patients provided 82% power to test for superiority of THR-184 over placebo at the one-sided type 1 error rate of 0.025. On the basis of the predefined enrollment criteria and assuming that the sample size is not increased at the time of the planned interim analysis, approximately 380 patients were to be enrolled and randomized to THR-184 or placebo (140 patients in stage 1 and approximately 210 patients in stage 2 plus approximately 30 “over-run” patients from the time period between stages 1 and 2). If the study was not stopped for safety or futility at the interim analysis, the planned number of patients to be enrolled could be increased by a predefined number up to 600 patients in total, while ensuring that type 1 error was protected. Full details of the methodology and a simulation study to show the control of the type 1 error rate and investigate the influence of the adaptive design on the type 2 error rate are provided in Supplemental Appendix.
After the interim analysis, the IDMC recommended continuing with arm 1 (placebo) and arm 4 (0.46 mg/kg followed by up to three doses of 0.02 mg/kg each) and discontinuing arm 2 and arm 3. Stage 2 was then modified by the sponsor and the trial steering committee by the addition of a fifth treatment arm to study higher drug postoperative exposure (arm 5; initial presurgery THR-184 dose of 0.46 mg/kg followed by three daily postsurgery doses of 0.36 mg/kg each); the proposed modification was endorsed by the IDMC. The modified stage 2 consisted of patients randomized in a 1:1:2 ratio per treatment arm to arm 1 (placebo), arm 4, or arm 5, respectively. With the addition of the new dosing arm, the sample size was increased to a total of approximately 450 patients, including approximately 270 patients in stage 2 (Figure 1).
Figure 1.
Flow of subjects through the trial.
Interventions
Doses of THR-184 were administered as 10-ml intravenous infusions over 60 minutes. Placebo consisted of 10-ml 0.9% sodium chloride solution administered according to the same schedule. Enrolled patients received study drug (THR-184 or placebo) on day 0 (the day of cardiac surgery), day 1, and day 2. Study drug was administered between 75 and 120 minutes before the patient was placed on cardiopulmonary bypass, with three additional doses administered at 8±2 hours after the initiation of the first 60-minute intravenous infusion, 24±2 hours after the initiation of the second 60-minute intravenous infusion, and 24±2 hours after the initiation of the third infusion.
Study Follow-Up
The total duration of the study was approximately 90 days for each patient. Postoperatively, patients had assessments at least once daily until day 7 postsurgery or discharge from the hospital. Patients returned for a follow-up visit on day 30 and day 90, and follow-up phone calls were conducted on day 14 and day 60.
Outcome Measures
The primary efficacy end point was the proportion of patients who develop AKI according to KDIGO criteria.32 The KDIGO criteria were on the basis of a positive result for any of three measures: (1) increase in serum creatinine by ≥0.3 mg/dl (≥26.5 μmol/L) within any 48 hours 7 days after surgery, (2) increase in serum creatinine to ≥1.5 times baseline within 7 days after surgery, or (3) urine volume <0.5 ml/kg per hour for 6 consecutive hours. The secondary efficacy end points were (1) incidence of AKI during the first 7 days or up to discharge after surgery according to the serum creatinine–based component of the KDIGO definition of AKI, (2) severity of AKI stage using KDIGO criteria during the first 7 days or up to discharge after surgery, (3) total duration of AKI within the first 7 days for subjects who had AKI in the first 3 days after surgery, and (4) a composite of death, dialysis, or sustained impaired kidney function by day 30 after surgery. Prespecified exploratory end points are defined in the protocol and described in Supplemental Table 2.
Safety end points were evaluated by tabulating the incidence of AEs, serious AEs, study withdrawals, mortality, changes in vital signs, electrocardiographic parameters, and incidence of physical examination abnormalities. Additional safety measures were changes in safety laboratory parameters and analysis of selected surgical complications (stroke, myocardial infarction, surgical site infection, nonsurgical site infection, prolonged ventilation, dialysis, and low cardiac output syndrome). In addition to safety and efficacy assessments, the study includes analysis of serum/plasma cardiac injury biomarkers. THR-184 PK assessments were conducted during stage 1 and for a subset of patients in arm 5 during stage 2.
Trial Design, Conduct, and Oversight
This trial was a randomized, double-blinded, placebo-controlled, multicenter, multidose comparison of the safety and efficacy of THR-184 in patients at heightened risk for the development of AKI after cardiac surgery. This trial was conducted in compliance with the protocol and applicable guidelines and regulations, including the International Conference on Harmonization, Guidance for Industry, the US Code of Federal Regulations applicable to clinical studies, and the Canada Food and Drug Regulations applicable to clinical studies. Institutional review board approval and written informed consent were obtained before any study procedures commenced. The study was planned to be conducted at up to 50 centers in North America.
The trial protocol was developed by members of the trial steering committee and the sponsor (Thrasos Therapeutics, Inc.). All data analyses were performed by the Independent Statistical Center (Cytel, Inc.). The first draft of the manuscript was written by academic members of the trial steering committee. All authors contributed to the interpretation of the data, reviewed and edited the manuscript, and made the decision to submit the manuscript for publication. Thrasos Therapeutics, Inc. funded the trial and data analysis, and the Montreal Health Innovations Coordinating Center and CTI Clinical Trial and Consulting Services performed trial oversight and monitoring. The sponsor had no other role in the conduct of the trial. Regulatory oversight was provided by the US Food and Drug Administration and Health Canada.
THR-184 Dose Selection
The selection of doses was on the basis of data from preclinical studies and two phase 1 studies evaluating single ascending doses and multiple ascending doses of THR-184 in adult healthy volunteers as well as PK and pharmacodynamic analyses. The activity of THR-184 was evaluated in several in vitro assays. The activity of THR-184 as an agonist of the BMP pathway was shown in an SMAD phosphorylation and translocation assay. THR-184 interacted with the principal renal BMP receptors but showed no interaction with ALK6, the receptor subtype involved in the osteogenic properties of native BMP-7. In human renal epithelial cells, THR-184 was able to reduce cisplatin-induced apoptosis; TNF-α–stimulated release of IL-6, IL-8, and Intercellular Adhesion Molecule 1; and epithelial to mesenchymal transition. THR-184 was also shown to be effective in lowering serum creatinine levels in three in vivo models of AKI in rats and mice. The median effective dose for this effect was approximately 30 μg/kg in the clamp ischemic model in rats when THR-184 was administered as a 60-minute intravenous infusion. In a cisplatin-induced nephrotoxicity model in mice, the compound significantly lowered terminal deoxynucleotidyl transferase–mediated digoxigenin-deoxyuridine nick-end labeling staining, indicating an antiapoptotic effect. Finally, THR-184 was shown to promote rapid (≤30-minute onset) SMAD phosphorylation in kidney cortex in situ after intravenous administration in rats at doses as low as 1 μg/kg, showing target engagement in the kidney.
In the two phase 1 clinical studies in adult healthy volunteers, single-dose administrations of THR-184 were tested at five dose levels (0.3, 1, 3, 6, and 10 mg/kg) followed by an evaluation of multiple doses (0.3, 1, and 3 mg/kg) administered over 5 consecutive days in the study. No increase in drug exposure was observed on day 5 compared with day 1, when nonsignificant variations of the Cmax and area under the curve (AUC) were generally reported at each dose level. Furthermore, the THR-184 accumulation in plasma remained negligible for the multiple dose regimens. A linear relationship between dose and bioavailability of THR-184 was observed over the 0.3- to 3-mg/kg dose range after one and five once daily dose administrations.
The initial three levels of dosing to be explored in the first part of this protocol were selected on the basis of PK modeling and estimation of the human equivalent doses for the doses that were effective in the preclinical program. The estimated human clearance values for THR-184 that were determined from the phase 1 single-ascending dose study were used to predict the doses that would be expected to result in an exposure area under the plasma concentration-time curve (AUC0−∞), similar to the AUC0−∞ associated with efficacy in the preclinical pharmacology models. The AUC0−∞ obtained after a single 1-hour intravenous administration of THR-184 in healthy rats and humans was used to compare the exposure in rats and humans. The facts that efficacy data from only one species were available and that an apparent U-shaped efficacy dose-response curve was observed were also considered in dose selection. The clinical doses proposed for this study were anticipated to be safe; such as in the phase 1 single-dose and multiple dose studies, there were no serious AEs reported. Similarly, no clinically significant changes in laboratory values, vital signs, ECGs, physical examinations, or neurologic examinations were observed in any subject before this clinical trial.
Randomization
Subjects at increased risk of CSA-AKI were identified, enrolled, and randomized before cardiac surgery. Eligible subjects were randomized to either THR-184 or placebo administered via intravenous infusion presurgery and postsurgery in a 1:1:1:1 ratio. Randomization was stratified on the basis of a subject’s baseline eGFR to one of two subgroups (<60 or ≥60 ml/min per 1.73 m2). Note that the randomization eGFR strata were calculated using the screening visit result from the local laboratory serum creatinine collection, whereas the primary end point and secondary efficacy analyses using serum creatinine collection were primarily on the basis of serum creatinine from the central laboratory.
Also of note, because patients were consented and randomized before surgery, 21 (4.6%) of patients were randomized without receiving study drugs. The most common reasons for randomization without drug dosing were as follows: approximately 38% were due to cancellation of surgery, 24% occurred because the subject no longer met entry criteria before the first dose, 19% were due to site investigator decision, 9.5% occurred because the subject withdrew consent, and 9.5% occurred for other logistic reasons.
Blinding and Assignment Concealment of Study Drugs
This was a double-blind study. The investigator, patient, clinical staff, and sponsor’s study management team were blinded to treatment assignments. At the investigational site, only the pharmacist or designee is unblinded. There is an unblinded study manager and unblinded monitor at the CRO. The pharmacist at the clinical site or the investigator’s designee is responsible for the preparation and dispensation of study drug according to the randomization schedule provided by the sponsor or designee. Study drug is administered in a blinded fashion. Site personnel who participated in treatment preparation are not allowed to collect or assess any study data during the course of the study or share any unblinded treatment records with the research personnel involved in the assessment of study patients. Patients are assigned to one of the double-blind treatment arms in the order in which they are entered into the randomization system and according to a randomization schedule prepared by the sponsor before the start of the study.
Data Analyses
All descriptive statistical analyses are performed using SAS statistical software (version 9.2). We used logistic regression to examine the treatment effect (THR-184 versus placebo) stratified by eGFR. To obtain the final trial-wide P value for the primary end point, we combined Simes adjusted P values for each dose regimen versus placebo from the first and second stages of the trial using the inverse normal combination function. The components contributing to the stage 1 portion of the P value included data from the four original dose group patients who were enrolled during stage 1 defined as on or before the data snapshot date for the interim evaluation of efficacy. The components contributing to the stage 2 portion of the P value were data from patients from the dose groups that were not discontinued who were enrolled after the date of the data snapshot for the interim evaluation of efficacy. The final P value for the trial’s primary end point was a weighted combination of the two components. We performed prespecified subgroup analyses for primary and secondary end points by eGFR strata <60 or ≥60 ml/min per 1.73 m2, age at randomization (<65, 65 to <75, or ≥75 years old), and sex.
Where hypothesis testing was applicable, arm 5 was compared formally with the placebo group only. Because the arm 5 dose was not prespecified, patients randomized during stage 2 to arm 5 were included in final analyses but excluded from hypothesis testing related to overall treatment effect and hypothesis testing of comparisons with other active dose arms. However, results for arm 5 are presented in Tables 1–4.
Disclosures
None.
Supplementary Material
Acknowledgments
Thrasos Therapeutics, Inc. funded the trial and data analysis, and the Montreal Health Innovations Coordinating Center and CTI Clinical Trial and Consulting Services performed trial oversight and monitoring.
The sponsor had no other role in the conduct of the trial. Regulatory oversight was provided by the US Food and Drug Administration and Health Canada.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2017020217/-/DCSupplemental.
References
- 1.Bellomo R, Kellum JA, Ronco C: Acute kidney injury. Lancet 380: 756–766, 2012 [DOI] [PubMed] [Google Scholar]
- 2.Chawla LS, Eggers PW, Star RA, Kimmel PL: Acute kidney injury and chronic kidney disease as interconnected syndromes. N Engl J Med 371: 58–66, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hsu CY, McCulloch CE, Fan D, Ordoñez JD, Chertow GM, Go AS: Community-based incidence of acute renal failure. Kidney Int 72: 208–212, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thiele RH, Isbell JM, Rosner MH: AKI associated with cardiac surgery. Clin J Am Soc Nephrol 10: 500–514, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Uchino S, Kellum JA, Bellomo R, Doig GS, Morimatsu H, Morgera S, Schetz M, Tan I, Bouman C, Macedo E, Gibney N, Tolwani A, Ronco C; Beginning and Ending Supportive Therapy for the Kidney (BEST Kidney) Investigators : Acute renal failure in critically ill patients: A multinational, multicenter study. JAMA 294: 813–818, 2005 [DOI] [PubMed] [Google Scholar]
- 6.McCullough PA, Bennett-Guerrero E, Chawla LS, Beaver T, Mehta RL, Molitoris BA, Eldred A, Ball G, Lee HJ, Houser MT, Khan S: ABT-719 for the prevention of acute kidney injury in patients undergoing high-risk cardiac surgery: A randomized phase 2b clinical trial. J Am Heart Assoc 5: e003549, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haase M, Bellomo R, Matalanis G, Calzavacca P, Dragun D, Haase-Fielitz A: A comparison of the RIFLE and Acute Kidney Injury Network classifications for cardiac surgery-associated acute kidney injury: A prospective cohort study. J Thorac Cardiovasc Surg 138: 1370–1376, 2009 [DOI] [PubMed] [Google Scholar]
- 8.Kuitunen A, Vento A, Suojaranta-Ylinen R, Pettilä V: Acute renal failure after cardiac surgery: Evaluation of the RIFLE classification. Ann Thorac Surg 81: 542–546, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Robert AM, Kramer RS, Dacey LJ, Charlesworth DC, Leavitt BJ, Helm RE, Hernandez F, Sardella GL, Frumiento C, Likosky DS, Brown JR; Northern New England Cardiovascular Disease Study Group : Cardiac surgery-associated acute kidney injury: A comparison of two consensus criteria. Ann Thorac Surg 90: 1939–1943, 2010 [DOI] [PubMed] [Google Scholar]
- 10.Zanardo G, Michielon P, Paccagnella A, Rosi P, Caló M, Salandin V, Da Ros A, Michieletto F, Simini G: Acute renal failure in the patient undergoing cardiac operation. Prevalence, mortality rate, and main risk factors. J Thorac Cardiovasc Surg 107: 1489–1495, 1994 [PubMed] [Google Scholar]
- 11.Rydén L, Sartipy U, Evans M, Holzmann MJ: Acute kidney injury after coronary artery bypass grafting and long-term risk of end-stage renal disease. Circulation 130: 2005–2011, 2014 [DOI] [PubMed] [Google Scholar]
- 12.Meng XM, Chung AC, Lan HY: Role of the TGF-β/BMP-7/Smad pathways in renal diseases. Clin Sci (Lond) 124: 243–254, 2013 [DOI] [PubMed] [Google Scholar]
- 13.Chen D, Zhao M, Mundy GR: Bone morphogenetic proteins. Growth Factors 22: 233–241, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Vukicevic S, Basic V, Rogic D, Basic N, Shih MS, Shepard A, Jin D, Dattatreyamurty B, Jones W, Dorai H, Ryan S, Griffiths D, Maliakal J, Jelic M, Pastorcic M, Stavljenic A, Sampath TK: Osteogenic protein-1 (bone morphogenetic protein-7) reduces severity of injury after ischemic acute renal failure in rat. J Clin Invest 102: 202–214, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gould SE, Day M, Jones SS, Dorai H: BMP-7 regulates chemokine, cytokine, and hemodynamic gene expression in proximal tubule cells. Kidney Int 61: 51–60, 2002 [DOI] [PubMed] [Google Scholar]
- 16.Griffith DL, Keck PC, Sampath TK, Rueger DC, Carlson WD: Three-dimensional structure of recombinant human osteogenic protein 1: Structural paradigm for the transforming growth factor beta superfamily. Proc Natl Acad Sci U S A 93: 878–883, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sugimoto H, LeBleu VS, Bosukonda D, Keck P, Taduri G, Bechtel W, Okada H, Carlson W Jr, Bey P, Rusckowski M, Tampe B, Tampe D, Kanasaki K, Zeisberg M, Kalluri R: Activin-like kinase 3 is important for kidney regeneration and reversal of fibrosis. Nat Med 18: 396–404, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zeisberg M, Bottiglio C, Kumar N, Maeshima Y, Strutz F, Müller GA, Kalluri R: Bone morphogenic protein-7 inhibits progression of chronic renal fibrosis associated with two genetic mouse models. Am J Physiol Renal Physiol 285: F1060–F1067, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Zeisberg M, Hanai J, Sugimoto H, Mammoto T, Charytan D, Strutz F, Kalluri R: BMP-7 counteracts TGF-beta1-induced epithelial-to-mesenchymal transition and reverses chronic renal injury. Nat Med 9: 964–968, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Jacob KA, Leaf DE, Dieleman JM, van Dijk D, Nierich AP, Rosseel PM, van der Maaten JM, Hofland J, Diephuis JC, de Lange F, Boer C, Kluin J, Waikar SS; Dexamethasone for Cardiac Surgery (DECS) Study Group : Intraoperative high-dose dexamethasone and severe AKI after cardiac surgery. J Am Soc Nephrol 26: 2947–2951, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Billings FT IV, Hendricks PA, Schildcrout JS, Shi Y, Petracek MR, Byrne JG, Brown NJ: High-dose perioperative atorvastatin and acute kidney injury following cardiac surgery: A randomized clinical trial. JAMA 315: 877–888, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.ClinicalTrials.gov: A Study to Evaluate the Safety and Efficacy of AC607 for the Treatment of Kidney Injury in Cardiac Surgery Subjects (ACT-AKI). NCT01602328. 2012, 2016. Available at: http://clinicaltrials.gov/ct2/show/NCT01602328. Accessed August 8, 2016
- 23.Choi YS, Shim JK, Kim JC, Kang KS, Seo YH, Ahn KR, Kwak YL: Effect of remote ischemic preconditioning on renal dysfunction after complex valvular heart surgery: A randomized controlled trial. J Thorac Cardiovasc Surg 142: 148–154, 2011 [DOI] [PubMed] [Google Scholar]
- 24.Gallagher SM, Jones DA, Kapur A, Wragg A, Harwood SM, Mathur R, Archbold RA, Uppal R, Yaqoob MM: Remote ischemic preconditioning has a neutral effect on the incidence of kidney injury after coronary artery bypass graft surgery. Kidney Int 87: 473–481, 2015 [DOI] [PubMed] [Google Scholar]
- 25.Zimmerman RF, Ezeanuna PU, Kane JC, Cleland CD, Kempananjappa TJ, Lucas FL, Kramer RS: Ischemic preconditioning at a remote site prevents acute kidney injury in patients following cardiac surgery. Kidney Int 80: 861–867, 2011 [DOI] [PubMed] [Google Scholar]
- 26.Meybohm P, Bein B, Brosteanu O, Cremer J, Gruenewald M, Stoppe C, Coburn M, Schaelte G, Böning A, Niemann B, Roesner J, Kletzin F, Strouhal U, Reyher C, Laufenberg-Feldmann R, Ferner M, Brandes IF, Bauer M, Stehr SN, Kortgen A, Wittmann M, Baumgarten G, Meyer-Treschan T, Kienbaum P, Heringlake M, Schön J, Sander M, Treskatsch S, Smul T, Wolwender E, Schilling T, Fuernau G, Hasenclever D, Zacharowski K; RIPHeart Study Collaborators : A multicenter trial of remote ischemic preconditioning for heart surgery. N Engl J Med 373: 1397–1407, 2015 [DOI] [PubMed] [Google Scholar]
- 27.Gandhi GY, Nuttall GA, Abel MD, Mullany CJ, Schaff HV, O’Brien PC, Johnson MG, Williams AR, Cutshall SM, Mundy LM, Rizza RA, McMahon MM: Intensive intraoperative insulin therapy versus conventional glucose management during cardiac surgery: A randomized trial. Ann Intern Med 146: 233–243, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Hajjar LA, Vincent JL, Galas FR, Nakamura RE, Silva CM, Santos MH, Fukushima J, Kalil Filho R, Sierra DB, Lopes NH, Mauad T, Roquim AC, Sundin MR, Leão WC, Almeida JP, Pomerantzeff PM, Dallan LO, Jatene FB, Stolf NA, Auler JO Jr.: Transfusion requirements after cardiac surgery: The TRACS randomized controlled trial. JAMA 304: 1559–1567, 2010 [DOI] [PubMed] [Google Scholar]
- 29.Chow SC, Corey R: Benefits, challenges and obstacles of adaptive clinical trial designs. Orphanet J Rare Dis 6: 79, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coffey CS, Levin B, Clark C, Timmerman C, Wittes J, Gilbert P, Harris S: Overview, hurdles, and future work in adaptive designs: Perspectives from a National Institutes of Health-funded workshop. Clin Trials 9: 671–680, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bhatt DL, Mehta C: Adaptive designs for clinical trials. N Engl J Med 375: 65–74, 2016 [DOI] [PubMed] [Google Scholar]
- 32.Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group : KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl 2: 1–138, 2012 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.