Abstract
The phospholipase A2 receptor (PLA2R1) is the major autoantigen in primary membranous nephropathy. Several PLA2R1 epitopes have been characterized, and a retrospective study identified PLA2R1 epitope spreading as a potential indicator of poor prognosis. Here, we analyzed the predictive value of anti-PLA2R1 antibody (PLA2R1-Ab) titers and epitope spreading in a prospective cohort of 58 patients positive for PLA2R1-Ab randomly allocated to rituximab (n=29) or antiproteinuric therapy alone (n=29). At baseline, the epitope profile (CysR, CysRC1, CysRC7, or CysRC1C7) did not correlate with age, sex, time from diagnosis, proteinuria, or serum albumin, but epitope spreading strongly correlated with PLA2R1-Ab titer (P<0.001). Ten (58.8%) of the 17 patients who had epitope spreading at baseline and were treated with rituximab showed reversal of epitope spreading at month 6. In adjusted analysis, epitope spreading at baseline was associated with a decreased remission rate at month 6 (odds ratio, 0.16; 95% confidence interval, 0.04 to 0.72; P=0.02) and last follow-up (median, 23 months; odds ratio, 0.14; 95% confidence interval, 0.03 to 0.64; P=0.01), independently from age, sex, baseline PLA2R1-Ab level, and treatment group. We propose that epitope spreading at baseline be considered in the decision for early therapeutic intervention in patients with primary membranous nephropathy.
Keywords: clinical immunology, membranous nephropathy, Immunology and pathology, immunosuppression, randomized controlled trials
Primary membranous nephropathy is an autoimmune disease against a podocyte antigen. Antibodies to the phospholipase A2 receptor 1 (PLA2R1-Ab) and thrombospondin type 1 domain containing 7A (THSD7A) are associated with 70%–80% and about 2%–3% of cases, respectively.1,2 Although the pathogenic role of anti-THSD7A has been established,3 that of PLA2R1-Ab is not yet proven. However, it is strongly suggested by the observation that PLA2R1-Ab titers usually rise during clinically active phases and decrease before normalization of proteinuria.4 Spontaneous remission occurs in up to 50% of patients, and ESRD occurs in about 30%.5 High titers of PLA2R1-Ab at presentation and their persistence predict poor clinical outcome.6,7 Therefore, reducing PLA2R1-Ab level has become an important goal of therapy. Although the identification of PLA2R1-Ab has been paradigm shifting in the diagnosis and management of patients, there are cases that call for additional biomarkers. Indeed, antibodies may persist during apparent clinical remission, and conversely, a drop in antibody titer may not be associated with a clinical remission.7,8
PLA2R1 is a 180-kDa membrane receptor with a large extracellular region comprising ten distinct globular domains, including a cysteine-rich domain (CysR), a fibronectin type II domain, and eight distinct C-type lectin domains (CTLD1–8).9 Each domain is separated by a small linker sequence of less than ten amino acids. An immunodominant epitope was first identified in a region spanning the Cystein rich domain (CysR)-fibronectin type II-C-type lectin domain 1 (CTLD1) domains,10 which was further restricted to the CysR domain alone.11 In a previous study,12 we became interested in epitope spreading, which is a common process of immune response to infectious agents and self-antigens. It usually first involves the so-called immunodominant epitope recognized by most antibodies (CysR for PLA2R1), then extends to noncrossreactive epitopes on the same protein (intramolecular epitope spreading, CTLD1 and/or CTLD7 for PLA2R1) or to dominant epitopes on neighboring molecules (intermolecular epitope spreading). The result is an increase in the antibody repertoire diversity, responsible for an enhanced overall immune response. Epitope spreading is a primary immunopathogenic event in autoimmune diseases.13–17 In this previous study, we identified reactive epitopes in the CysR, CTLD1, and CTLD7 domains, and we further showed that patients with anti-CysR–restricted activity were younger, had lower proteinuria, and exhibited a higher rate of spontaneous remission and a lower rate of renal failure progression.12 Conversely, high PLA2R1-Ab activity and epitope spreading beyond the CysR epitope were independent risk factors for poor renal prognosis in a collection of 69 sera from five French nephrology centers. Because these patients were analyzed retrospectively, they differed by the severity of the nephrotic syndrome, duration of follow-up, management of antiproteinuric therapy, and indications for immunosuppressive treatment.
The first aim of this study was to test the reactivity of PLA2R1-Ab against the three previously characterized PLA2R1 epitope domains (CysR, CTLD1, and CTLD7) in a well defined prospective cohort (Evaluate Rituximab Treatment for Idiopathic Membranous Nephropathy [GEMRITUX]) of patients with severe nephrotic syndrome at treatment onset. The GEMRITUX cohort is part of a French multicenter, randomized, controlled trial (Clinicaltrial.gov identifier: NCT01508468) testing rituximab added to antiproteinuric therapy (RITUX) against antiproteinuric therapy alone (NIAT), (Supplemental Appendix).18 Among the 75 patients (38 in the NIAT group and 37 in the RITUX group) who were enrolled in the GEMRITUX study,18 58 patients (29 in each group) showed PLA2R1-Ab reactivity by IFTA and/or ELISA and had available serum at baseline for analysis of epitope reactivity.
Table 1 depicts the characteristics of the whole population versus randomization groups at baseline (M0) and at month 3 (M3) and month 6 (M6) after treatment onset. Epitope reactivity toward CysR, CTLD1, and CTLD7 domains was measured by specific ELISAs that defined four epitope groups (CysR, CysRC1, CysRC7, and CysRC1C7).12 All patients reactive with CTLD1 and/or CTLD7 were also reactive with CysR. Spreading of the immune response, as defined by addition of CTLD1 and/or CTLD7 reactivity to CysR, was identified in 38 out of 58 (65.5%) patients at baseline. There was no association between the epitope group (CysR, CysRC1, CysRC7, or CysRC1C7) and age, sex, time from diagnosis (kidney biopsy) to inclusion, proteinuria, or serum albumin. Conversely, there was a strong correlation between PLA2R1-Ab titer and epitope group (P<0.001), with the higher titers being associated with reactivity beyond CysR (Supplemental Table 1). Similar results were observed when patients were categorized according to the presence or absence of spreading (P<0.001 for PLA2R1-Ab titer) (Supplemental Table 2). Spreading was constant for a PLA2R1-Ab titer >369.5 RU/ml (Supplemental Figure 1), but because of the small size of the cohort, this threshold will need confirmation in future studies. The lack of association of epitope group and spreading with age and proteinuria is at odds with the observations made by Seitz-Polski et al.12 who found that patients from the CysR group were significantly younger and had lower proteinuria. These discrepancies may be explained by a more advanced disease in the GEMRITUX cohort, where all patients had persistent nephrotic syndrome (mean value of 8.2 g/g for the whole cohort; Table 1) after a 6-month run-in period of optimized antiproteinuric therapy, whereas proteinuria was <3 g/g in 22 out of 69 (32%) patients in the study by Seitz-Polski et al.12,18
Table 1.
Demographic and immunopathologic characteristics of the patients of the GEMRITUX cohort, according to therapeutic intervention at M0, M3, and M6
| Characteristics | Whole Cohort, n=58 | NIAT, n=29 | RITUX, n=29 | P Value |
|---|---|---|---|---|
| Age | 56.5 [42.0–63.0] | 59.0 [44.0–63.0] | 52.0 [41.0–63.0] | 0.5 |
| Sex | 0.4 | |||
| Men | 42 (72.4) | 19 | 23 | |
| Women | 16 (27.6) | 10 | 6 | |
| Proteinuria at M0, g/g of serum creatinine | 8.2 [4.8–10.0] | 7.4 [6.2–9.0] | 8.4 [4.4–11.0] | 0.51 |
| Serum albumin at M0, g/dl | 2.2 [1.9–2.5] | 2.2 [2.0–2.5] | 2.2 [1.8–2.5] | 0.52 |
| Serum creatinine at M0, mg/L | 10.8 [9.0–13.8] | 10.4 [8.7–13.8] | 11.5 [9.4–13.1] | 0.48 |
| PLA2R1-Ab at M0, RU/ml | 101.5 [31.2–481.2] | 199.5 [24.2–480.0] | 100.5 [35.4–481.2] | 0.85 |
| CysR at M0, RU/ml | 1423.5 [369.0–3240.0] | 1572.0 [494.0–3240.0] | 1256.0 [270.0–3114.0] | 0.52 |
| CTLD1 at M0, RU/ml | 157.0 [0.0–2334.0] | 12.0 [0.0–1190.0] | 471.0 [0.0–2334.0] | 0.33 |
| CTLD7 at M0, RU/ml | 220.5 [23.0–609.0] | 136.0 [36.0–662.0] | 223.0 [13.0–604.0] | 0.65 |
| Group M0 | 0.74 | |||
| CysR | 20 (34.48) | 11 (37.93) | 9 (31.03) | |
| CysRC1 | 8 (13.79) | 4 (13.79) | 4 (13.79) | |
| CysRC7 | 10 (17.24) | 6 (20.69) | 4 (13.79) | |
| CysRC1C7 | 20 (34.48) | 8 (27.59) | 12 (41.38) | |
| Spreading M0 | 38/58 (65.6) | 18 (62.1) | 20 (68.9) | 0.78 |
| Proteinuria at M3, g/g of serum creatininea | 5.1 [3.2–7.6] | 5.0 [3.0–8.5] | 5.1 [3.5–7.4] | 0.86 |
| Serum albumin at M3, g/dlb | 2.6 [2.0–2.9] | 2.3 [1.8–2.7] | 2.7 [2.1–3.1] | 0.12 |
| Serum creatinine at M3, mg/La | 11.5 [9.4–13.6] | 11.5 [9.7–13.9] | 11.8 [9.0–13.6] | 0.99 |
| PLA2R1-Ab at M3, RU/mlc | 43.1 [0–102.5] | 70.5 [30.3–325.9] | 0.0 [0.0–60.5] | 0.002 |
| Group M3d | 0.002 | |||
| Negative | 12 (24.5) | 0 (0.0) | 12 (46.2) | |
| CysR | 10 (20.4) | 7 (30.4) | 3 (11.5) | |
| CysRC1 | 9 (18.4) | 6 (26.1) | 3 (11.5) | |
| CysRC7 | 6 (12.2) | 3 (13.1) | 3 (11.5) | |
| CysRC1C7 | 12 (24.5) | 7 (30.4) | 5 (19.2) | |
| Spreading M3d | 27/49 (55.1) | 16 (69.6) | 11 (42.3) | 0.09 |
| Proteinuria at M6, g/g of serum creatininee | 4.4 [2.0–7.3] | 6.2 [2.2–7.5] | 3.7 [1.8–6.5] | 0.21 |
| Serum albumin at M6, g/dle | 2.6 [2.1–3.3] | 2.4 [2.0–2.9] | 2.9 [2.5–3.4] | 0.07 |
| Serum creatinine at M6, mg/Le | 11.9 [9.4–14.8] | 12.0 [9.4–14.4] | 11.9 [9.8–14.8] | 0.71 |
| PLA2R1-Ab at M6, RU/mlf | 30.1 [0–98.5] | 57.2 [16.5–298.0] | 0 [0–57.0] | 0.01 |
| Group M6c | ||||
| Negative | 17 (34.0) | 0 (0.0) | 17 (65.4) | |
| CysR | 14 (28.0) | 12 (50.0) | 2 (7.7) | <0.001 |
| CysRC1 | 5(10.0) | 4 (16.7) | 1 (3.9) | |
| CysRC7 | 2 (4.0) | 2 (8.3) | 0 (0.0) | |
| CysRC1C7 | 12 (24.0) | 6 (25.0) | 6 (23.1) | |
| Spreading M6c | 19/50 (38.0) | 12/24 (50.0) | 7/26 (26.9) | 0.15 |
Data are shown as n (%) or median [interquartile range].
One missing value.
Two missing values.
Eight missing values.
Nine missing values.
Four missing values.
Five missing values.
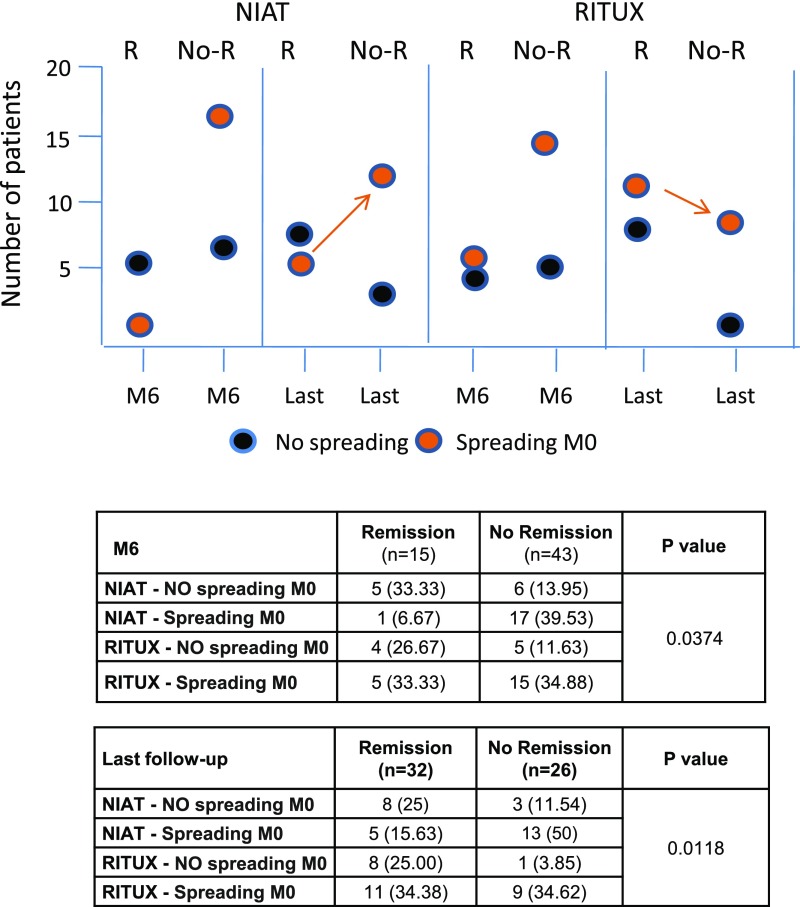
Our second objective was to investigate the effect of rituximab on epitope profiles and spreading. No difference at baseline was observed between randomization groups (NIAT versus RITUX) regarding age, sex, serum creatinine, serum albumin, proteinuria, PLA2R1-Ab titer, and epitope reactivity or spreading (Table 1). Epitope spreading was observed at baseline in 18 out of 29 (62.1%) and 20 out of 29 (68.9%) patients treated with NIAT and rituximab, respectively. At M3, there was a trend of lower rate of spreading in the rituximab group as compared with the NIAT group (42.3% versus 69.6%). This trend was maintained at M6 (26.9% versus 50%). There was a significant effect of rituximab on epitope profile at M3 and M6, being characterized by a drop in the number of patients with CysRC1C7 reactivity concomitant with a dramatic increase in those with CysR reactivity only and in those negative for all epitope domains (P<0.001 and P<0.001, respectively) (Supplemental Table 3). When individually considering the patients who spread at baseline, ten out of 17 (58.8%) patients treated with rituximab and only four out of 16 (25%) patients treated with NIAT showed a “reverse” spreading (CysRC1 and/or CysRC7 to CysR only or negative for all epitope domains) at M6. These results indicate that rituximab favors reversal of epitope spreading in parallel with a decrease in PLA2R1-Ab titers. Among spreaders at baseline treated with rituximab, results at last follow-up showed a significant gradient of response (P=0.01), with the lower response rate being observed in patients with persistent reactivity versus two or three epitopes (one out of seven), and the higher response rate in patients with no reactivity (seven out of eight) or those with CysR reactivity only (two out of two). Although persistence of spreading is associated with a much lower rate of remission, it does not seem necessary to become nonreactive to all three epitopes for clinical remission to occur. Among the seven spreaders treated with rituximab who did not respond clinically at last follow-up, only one had lost epitope reactivity, and six remained spreaders. Among the 13 patients in the NIAT group who initially showed epitope spreading and did not respond clinically at last follow-up, ten remained spreaders. Therefore, the spreaders who did not respond clinically remained spreaders, except for three. In the NIAT group, among the 11 patients with CysR-only reactivity at baseline, five achieved spontaneous remission at M6 (83.3% of all remissions) and eight (61.5%) achieved spontaneous remission at last follow-up; among the 18 spreaders at baseline, only one (16.7% of all remissions) achieved spontaneous remission at M6, and five achieved spontaneous remission (38.5%) at last follow-up. Although these findings should be taken with caution, given the small size of the cohort, and would require confirmation in future studies, physicians should consider delaying immunosuppressive treatment in patients with CysR-only reactivity.
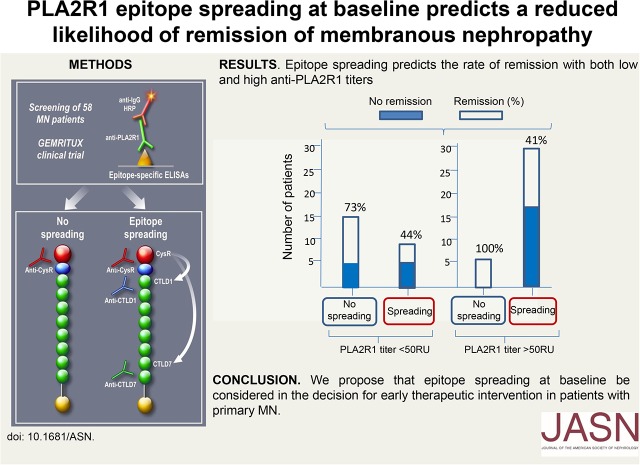
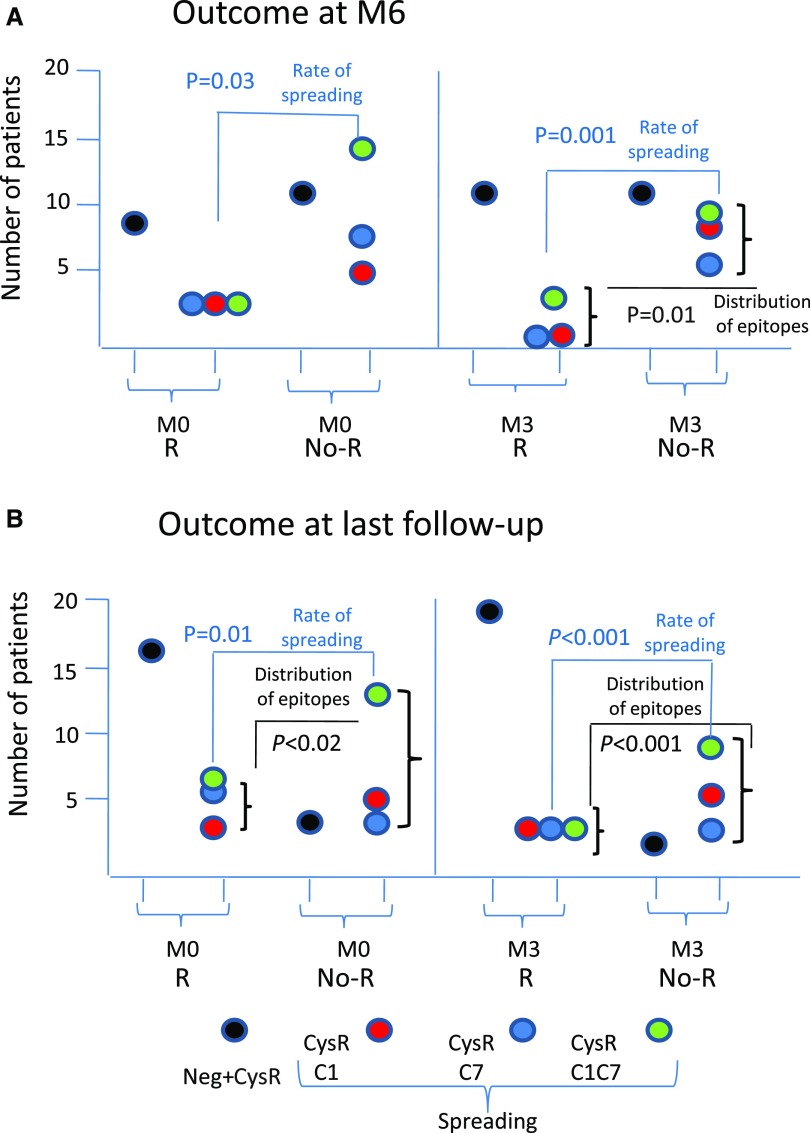
Our third aim was to establish whether epitope spreading was a more potent predictor of remission than PLA2R1-Ab titer, in the context of a randomized trial with strict criteria of eligibility (for definition of remissions, see Supplemental Appendix). At M6, 15 patients had reached clinical remission with (n=9) or without (n=6) rituximab, whereas 43 patients had not. Lower levels of CTLD7 reactivity (P<0.01) and lower rate of epitope spreading (P=0.03), (Figure 1A, Supplemental Table 4) were the only factors at baseline associated with higher remission rates at M6, whereas PLA2R1-Ab titers did not reach statistical significance. At M3, lower titers of PLA2R1-Ab (P<0.01) and lower rate of epitope spreading (P=0.001) were associated with higher remission rates (Figure 1A). Patients with CysR-only reactivity at baseline accounted for 60.0% of remissions and 25.6% of no remissions at M6. Distribution of remissions was different according to treatment group combined with spreading at baseline (Figure 2). In adjusted analysis (Table 2), spreading at baseline was associated with a decreased rate of remission (odds ratio, 0.16; 95% confidence interval, 0.04 to 0.72; P=0.02) independently from age, sex, treatment group, and baseline PLA2R1-Ab titer. We then assessed whether these results could be confirmed during the extended period of follow-up (median, 23 months; interquartile range, 22–25) before any treatment modification. At the end of that period, 32 patients had entered remission whereas 26 patients had not. The level of CysR (P=0.04), CTLD1 (P<0.01), and CTLD7 reactivity (P=0.01), the distribution of epitope profiles (P=0.02) and the rate of epitope spreading (P=0.01) at baseline were associated with remission (Figure 1B, Supplemental Table 5). The titer of PLA2R1-Ab (P<0.001), the distribution of epitope groups (P<0.001), and the rate of epitope spreading (P<0.001) at M3 were also associated with remission (Supplemental Table 4). Patients with CysR-only reactivity at baseline accounted for 50.0% of remissions and 15.4% of no remissions at last follow-up. Distribution of remissions before any treatment modification was different according to treatment arm combined with spreading at baseline (Figure 2). In adjusted analysis, epitope spreading at baseline was associated with a decreased rate of remission at last follow-up (odds ratio, 0.14; 95% confidence interval, 0.03 to 0.64; P=0.01), independently from age, sex, treatment group, and baseline PLA2R1-Ab titer. To further support the possible preeminence of epitope spreading over PLA2R1-Ab titer, we generated a variable combining PLA2R1-Ab titer <50 RU/ml (considered as low level) versus ≥50 RU/ml and spreading at baseline, and examined clinical response at last follow-up. The results show a significantly different distribution of remissions among the groups (P=0.03). Among the patients with low levels of PLA2R1-Ab (<50 RU/ml), 11 out of 15 (73%) of those with no spreading at baseline achieved clinical remission whereas only four out of nine (44%) of those with spreading did so. Among the patients with higher levels of PLA2R1-Ab (≥50 RU), all patients with no spreading achieved remission whereas only 12 out of 29 (41%) of those with spreading did so. Although these numbers are small, the data suggest that spreading may drive the absence of remission more than PLA2R1-Ab level at baseline (Supplemental Figure 2).
Figure 1.
Clinical outcome is predicted by epitope distribution and spreading at baseline and month 3. Epitope distribution is shown at baseline (M0) and month 3 (M3) according to outcome at (A) M6 (full cohort including both treatment groups) and (B) last follow-up (full cohort). Patients are divided into those who achieved remission (R) and those who did not achieve remission (No-R) at M6 and last follow-up, respectively. Reactivity with the various epitope domains is shown by color codes. “Neg” means lack of reactivity with any epitope domain at M3. Note that the rate of epitope spreading significantly differs from baseline between R and No-R patients.
Figure 2.
Absence of spreading and rituximab treatment are associated with better clinical outcome. Combined effect of treatment group and spreading at baseline on clinical outcome is shown at M6 and last follow-up before modification of treatment (Last). Absence or presence of epitope spreading at baseline (M0) is shown by color codes. Results suggest that rituximab tends to blunt the effect of spreading on clinical remission at last follow-up. Note the higher number of spreaders with no remission (No-R) compared with those with remission (R) in the NIAT group (arrow), contrasting with the lower number of spreaders with No-R versus R in the RITUX group. Exact numbers and percentages are shown in the table.
Table 2.
Unadjusted and adjusted odd ratios for clinical remission at M6 and last follow-up of baseline indicators (M0)
| Unadjusted | Adjusted | |||||||
|---|---|---|---|---|---|---|---|---|
| Effect | Odds Ratio | 95% Wald Confidence Limits | Type 3 Analysis P Value | Odds Ratio | 95% Wald Confidence Limits | Type 3 Analysis P Value | ||
| M6 | ||||||||
| Age, yr | 1.02 | 0.98 | 1.07 | 0.38 | 1.02 | 0.97 | 1.07 | 0.37 |
| Sex | 0.93 | 0.96 | ||||||
| Men | 1 | 1 | ||||||
| Women | 0.94 | 0.25 | 3.53 | 1.04 | 0.24 | 4.54 | ||
| PLA2R1-Ab at M0, RU/ml | 1.00 | 0.99 | 1.00 | 0.67 | 1.00 | 0.99 | 1.00 | 0.44 |
| Treatment | 0.37 | 0.21 | ||||||
| NIAT | 1 | 1 | ||||||
| NIAT and RITUX | 1.73 | 0.52 | 5.69 | 2.37 | 0.62 | 9.06 | ||
| Spreading M0 | 0.02 | 0.02 | ||||||
| No | 1 | 1 | ||||||
| Yes | 0.23 | 0.07 | 0.79 | 0.16 | 0.04 | 0.72 | ||
| Last follow-up | ||||||||
| Age, yr | 1.01 | 0.98 | 1.05 | 0.51 | 1.02 | 0.98 | 1.07 | 0.38 |
| Sex | ||||||||
| Men | 1 | 0.92 | 1 | 0.54 | ||||
| Women | 1.06 | 0.33 | 3.39 | 1.54 | 0.4 | 5.99 | ||
| PLA2R1-Ab at M0, RU/ml | 1.00 | 0.99 | 1.00 | 0.22 | 1.00 | 0.99 | 1.00 | 0.86 |
| Treatment | ||||||||
| NIAT | 1 | 0.12 | 1 | 0.04 | ||||
| NIAT and RITUX | 2.34 | 0.81 | 6.74 | 3.72 | 1.05 | 13.2 | ||
| Spreading M0 | ||||||||
| No | 1 | 0.01 | 1 | 0.01 | ||||
| Yes | 0.18 | 0.05 | 0.65 | 0.15 | 0.03 | 0.64 | ||
Note that spreading at baseline is the only predictor of remission at M6, and it also predicts remission at last follow-up before any treatment modification, independently from PLA2R1-Ab titer.
In our initial GEMRITUX study, we showed that remission was associated with PLA2R1-Ab level <275 RU/ml at baseline.18 We now add an important, independent prognostic factor, epitope spreading at baseline, which is associated with low remission rates both at M6 and at last follow-up in a well phenotyped population of patients with severe clinical presentation. Our data suggest that if a patient has only a low level of anti-PLA2R1 but significant spreading, he/she should be treated with rituximab at the time of diagnosis. If a patient has high anti-PLA2R1 levels but no spreading, he/she could be watched and not treated. However, these potentially important results have to be confirmed in further studies.
This study has several limitations. We did not measure epitope-specific IgG subclasses because in our previous study, we did not observe an anti-CTLD7 antibody different from IgG4, and we rarely detected IgG1, IgG2, and IgG3 anti-CysR and anti-CTLD1 antibodies.12 Because of the sample size of PLA2R1-positive patients in the GEMRITUX cohort, we could not perform a multivariate analysis in the RITUX group only, to identify epitope spreading as a predictor of response to rituximab.
Despite these defects, our study suggests that the absence of epitope spreading at onset is an independent factor of remission at M6 and last follow-up in patients with persisting nephrotic syndrome despite full renin-angiotensin system blockade. We propose that baseline epitope spreading should be carefully considered in the decision for early therapeutic intervention in patients with primary membranous nephropathy. Although treatment may be delayed in patients with CysR-only reactivity, epitope spreading and its persistence after 3 months should be added to the therapeutic algorithm. Additional studies are needed to further define the therapeutic window opportunity on the basis of epitope reactivity. As long as epitope-specific assays for PLA2R1-Ab are not commercially available, a high titer of total PLA2R1-Ab (by ELISA) at baseline is the best surrogate currently available for detection of epitope spreading.
Concise Methods
Study Population
This study presents the results of an ancillary study to the GEMRITUX randomized controlled trial (Clinicaltrial.gov identifier: NCT01508468).18 Eligibility criteria, study treatments and monitoring, and definition of clinical and immunologic remission are given in the Supplemental Appendix and detailed elsewhere.18 The GEMRITUX study was approved by a national ethical committee. In this study, eligible patients were those positive by IFTA or ELISA and with serum available during follow-up for epitope profiling (n=58).
Anti-PLA2R1 Assays with Full-Length Antigen
PLA2R1-Ab (total IgG) toward the full-length PLA2R1 antigen were assessed using IFTA and the quantitative ELISA test commercialized by EuroImmun AG (Lübeck, Germany) as previously described.19 For ELISA, sera were diluted to 1:100 and incubated with PLA2R1 (full extracellular domain of PLA2R1) already coated onto microplates and detected by incubation with anti-human IgG horseradish peroxidase conjugate. The final titer for each sample was calculated from the calibration curve extinction values plotted against the concentration for each calibrator. ELISA cutoff values were established according to the manufacturer’s protocol, and the results were considered as negative for values <14 RU/ml and positive for values ≥14 RU/ml. In our laboratory, the calculated intra- and inter-assay variations are <4% and <9%, respectively.
ELISA using Isolated PLA2R1 Domains
The three PLA2R1 domains CysR (Ala-26 to Lys-164), CTLD1 (Thr-223 to Asn-359), and CTLD7 (Thr-1102 to Glu-1237) were produced in HEK293 cells as secreted proteins harboring an HA tag.12 The reactivity of sera toward these domains was analyzed essentially as previously described.12 Briefly, plates were coated with anti-HA antibody (Sigma-Aldrich) diluted at 1:5000 in 20 mM Tris (pH 8.0; 100 μl per well) at 4°C overnight. Plates were then blocked for 2 hours with Seramun Block (Seramun Diagnostica). Cell medium from HEK293 cells transfected with the soluble forms of the three PLA2R1 domains (10–100 μl per well, depending on protein expression) were then added and incubated for 1 hour. Plates were washed and patients’ sera, diluted at 1:100 (or higher as needed) in PBS/0.1% dry milk, were added in duplicate (100 μl per well) to the ELISA plates, which also contained a serial dilution of an MN standard serum and a quality control calibrator (between plates). After 2 hours incubation at room temperature on a plate shaker, plates were washed four times with PBS/0.02% Tween 20. Anti-human IgG4 horseradish peroxidase conjugate (#9200–05; Southern Biotech) diluted at 1:7500 in Seramun Stab ST plus was added (100 μl per well; Seramun Diagnostica) and incubated for 1 hour at room temperature on a plate shaker. After four washes, tetramethylbenzidine was added, and the reactions were developed for 15 minutes and then stopped with HCl 1.2 N. The plates were read at 450 nm. The cutoff was optimized by receiver-operating characteristics curve analysis. A highly positive index patient serum was used in each plate to generate a standard curve and a negative control.
An ELISA index value for each antigen was obtained for patients or controls as follows (mean test result−mean domain negative control)/(mean domain positive control−mean domain negative control)×domain correction factor×100. The domain correction factor was determined for each domain as the mean of all of the positive controls for that domain on all plates minus the mean of the negative controls, divided by the cutoff for that domain assay, as described by Warren et al.20 Results were expressed as relative units per milliliter.
Statistical Analyses
Characteristics of patients were described with frequencies and percentages for categorical data, and with medians and interquartile ranges for quantitative data. Categorical data were compared using Fisher exact test, whereas quantitative data were compared using Wilcoxon–Mann–Whitney nonparametric test. Adjusted analysis was performed using logistic regression. Because of the small number of patients analyzed, adjustment was limited to age, sex, treatment group, and PLA2R1 titer. All tests were two-sided and a P value <0.05 indicated statistical significance. Analyses were performed using SAS v.9.3 software (SAS Institute, Cary, NC).
Disclosures
None.
Supplementary Material
Acknowledgments
This research is supported by European Research Council ERC-2012 ADG_20120314 grant 322947 (to P.R.), the Seventh Framework Programme of the European Community contract no. 2012-305608 (European Consortium for High-Throughput Research in Rare Kidney Diseases) (to P.R.), and by grants from CNRS, the National Research Agency to G.L. through the “Investments for the Future” Laboratory of Excellence SIGNALIFE, a network for innovation on signal transduction pathways in life sciences, (programme reference ANR-11-LABX-0028-01), and to G.L. and P.R. (grant MNaims ANR-17-CE17-0012-01) the Fondation pour la Recherche Médicale (ING20140129210 and SPF20150934219), and the Centre Hospitalier Universitaire de Nice and the Direction générale de l’offre de soins of the French Ministry of Health (Phospholipase A2 Receptor (PLA2R1) Autoantibodies in Membranous Nephropathy in Kidney Transplantation Programme, PHRC2011-A01302-39, NCT01897961) to G.L., B.S.-P., and V.L.M.E. Principal Investigator. This work has been developed and supported through the Fédération Hospitalo-Universitaire Oncoage (Nice, France) with V.L.M.E. as PI.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2017070734/-/DCSupplemental.
References
- 1.Beck LH Jr, Bonegio RG, Lambeau G, Beck DM, Powell DW, Cummins TD, Klein JB, Salant DJ: M-type phospholipase A2 receptor as target antigen in idiopathic MN. N Engl J Med 361: 11–21, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tomas NM, Beck LH Jr, Meyer-Schwesinger C, Seitz-Polski B, Ma H, Zahner G, Dolla G, Hoxha E, Helmchen U, Dabert-Gay AS, Debayle D, Merchant M, Klein J, Salant DJ, Stahl RAK, Lambeau G: Thrombospondin type-1 domain-containing 7A in idiopathic membranous nephropathy. N Engl J Med 371: 2277–2287, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tomas NM, Hoxha E, Reinicke AT, Fester L, Helmchen U, Gerth J, Bachmann F, Budde K, Koch-Nolte F, Zahner G, Rune G, Lambeau G, Meyer-Schwesinger C, Stahl RA: Autoantibodies against thrombospondin type 1 domain-containing 7A induce membranous nephropathy. J Clin Invest 126: 2519–2532, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ronco P, Debiec H: Pathophysiological advances in membranous nephropathy: Time for a shift in patient’s care. Lancet 385: 1983–1992, 2015 [DOI] [PubMed] [Google Scholar]
- 5.Glassock RJ: Diagnosis and natural course of membranous nephropathy. Semin Nephrol 23: 324–332, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Kanigicherla D, Gummadova J, McKenzie EA, Roberts SA, Harris S, Nikam M, Poulton K, McWilliam L, Short CD, Venning M, Brenchley PE: Anti-PLA2R antibodies measured by ELISA predict long-term outcome in a prevalent population of patients with idiopathic membranous nephropathy. Kidney Int 83: 940–948, 2013 [DOI] [PubMed] [Google Scholar]
- 7.Hoxha E, Thiele I, Zahner G, Panzer U, Harendza S, Stahl RA: Phospholipase A2 receptor autoantibodies and clinical outcome in patients with primary membranous nephropathy. J Am Soc Nephrol 25: 1357–1366, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seitz-Polski B, Payré C, Ambrosetti D, Albano L, Cassuto-Viguier E, Berguignat M, Jeribi A, Thouret MC, Bernard G, Benzaken S, Lambeau G, Esnault VL: Prediction of membranous nephropathy recurrence after transplantation by monitoring of anti-PLA2R1 (M-type phospholipase A2 receptor) autoantibodies: A case series of 15 patients. Nephrol Dial Transplant 29: 2334–2342, 2014 [DOI] [PubMed] [Google Scholar]
- 9.Ancian P, Lambeau G, Mattéi MG, Lazdunski M: The human 180-kDa receptor for secretory phospholipases A2. Molecular cloning, identification of a secreted soluble form, expression, and chromosomal localization. J Biol Chem 270: 8963–8970, 1995 [DOI] [PubMed] [Google Scholar]
- 10.Kao L, Lam V, Waldman M, Glassock RJ, Zhu Q: Identification of the immunodominant epitope region in phospholipase A2 receptor-mediating autoantibody binding in idiopathic membranous nephropathy. J Am Soc Nephrol 26: 291–301, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fresquet M, Jowitt TA, Gummadova J, Collins R, O’Cualain R, McKenzie EA, Lennon R, Brenchley PE: Identification of a major epitope recognized by PLA2R autoantibodies in primary membranous nephropathy. J Am Soc Nephrol 26: 302–313, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seitz-Polski B, Dolla G, Payré C, Girard CA, Polidori J, Zorzi K, Birgy-Barelli E, Jullien P, Courivaud C, Krummel T, Benzaken S, Bernard G, Burtey S, Mariat C, Esnault VL, Lambeau G: Epitope spreading of autoantibody response to PLA2R associates with poor prognosis in membranous nephropathy. J Am Soc Nephrol 27: 1517–1533, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cornaby C, Gibbons L, Mayhew V, Sloan CS, Welling A, Poole BD: B cell epitope spreading: Mechanisms and contribution to autoimmune diseases. Immunol Lett 163: 56–68, 2015 [DOI] [PubMed] [Google Scholar]
- 14.Chen JL, Hu SY, Jia XY, Zhao J, Yang R, Cui Z, Zhao MH: Association of epitope spreading of antiglomerular basement membrane antibodies and kidney injury. Clin J Am Soc Nephrol 8: 51–58, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li N, Aoki V, Hans-Filho G, Rivitti EA, Diaz LA: The role of intramolecular epitope spreading in the pathogenesis of endemic pemphigus foliaceus (fogo selvagem). J Exp Med 197: 1501–1510, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McRae BL, Vanderlugt CL, Dal Canto MC, Miller SD: Functional evidence for epitope spreading in the relapsing pathology of experimental autoimmune encephalomyelitis. J Exp Med 182: 75–85, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Naserke HE, Ziegler AG, Lampasona V, Bonifacio E: Early development and spreading of autoantibodies to epitopes of IA-2 and their association with progression to type 1 diabetes. J Immunol 161: 6963–6969, 1998 [PubMed] [Google Scholar]
- 18.Dahan K, Debiec H, Plaisier E, Cachanado M, Rousseau A, Wakselman L, Michel PA, Mihout F, Dussol B, Matignon M, Mousson C, Simon T, Ronco P; GEMRITUX Study Group : Rituximab for severe membranous nephropathy: A 6-month trial with extended follow-up. J Am Soc Nephrol 28: 348–358, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ruggenenti P, Debiec H, Ruggiero B, Chianca A, Pellé T, Gaspari F, Suardi F, Gagliardini E, Orisio S, Benigni A, Ronco P, Remuzzi G: Anti-phospholipase A2 receptor antibody titer predicts post-rituximab outcome of membranous nephropathy. J Am Soc Nephrol 26: 2545–2558, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Warren SJ, Arteaga LA, Rivitti EA, Aoki V, Hans-Filho G, Qaqish BF, Lin MS, Giudice GJ, Diaz LA: The role of subclass switching in the pathogenesis of endemic pemphigus foliaceus. J Invest Dermatol 120: 104–108, 2003 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.