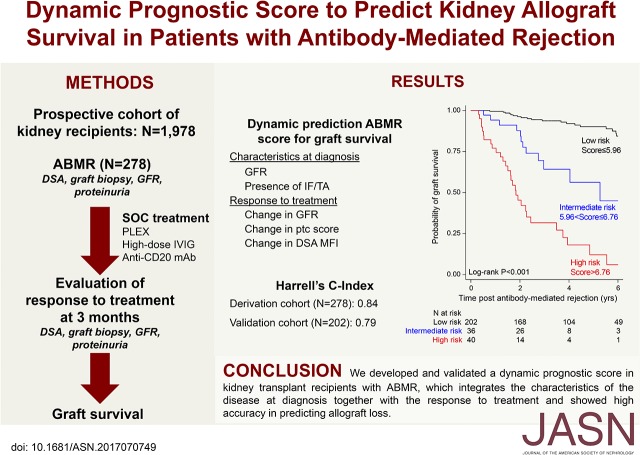
Abstract
No tool is available for the early assessment of response to antibody-mediated rejection (ABMR) therapies in kidney allograft recipients. This study was designed to define a dynamic composite prognostic ABMR score to predict kidney allograft survival, integrating the disease characteristics at diagnosis and the response to treatment. Among 1978 kidney recipients who underwent transplant between 2008 and 2014, we included 278 patients diagnosed with active ABMR and receiving standard treatment, including plasma exchange, intravenous Ig, and rituximab. Patients were prospectively assessed at diagnosis and after treatment for clinical data, histologic characteristics (allograft biopsy specimen), and donor-specific anti-HLA antibodies (DSA). The dynamic ABMR prediction model included GFR (P<0.001) and presence of interstitial fibrosis/tubular atrophy (P=0.003) at diagnosis and changes in GFR (P<0.001), peritubular capillaritis Banff score (P=0.002), and DSA mean fluorescence intensity (P<0.001) after treatment. Overall, this model showed good calibration and discrimination (C-statistic=0.84). The ABMR prognostic score derived from the prediction model identified three risk strata with 6-year kidney allograft survival rates of 6.0% (high-risk group, n=40), 44.9% (intermediate-risk group, n=36), and 84.4% (low-risk group, n=202), and it provided greater net clinical benefit to patients than did considering them all to have the same level of risk of allograft loss. The performance of the ABMR prognostic score was validated in an independent cohort of 202 kidney recipients with ABMR (C-statistic=0.79). The ABMR prognostic score could be used to inform therapeutic decisions in clinical practice and for the design of clinical trials.
Keywords: kidney transplantation, outcomes, rejection
Despite advances in diagnosis and treatment made over the last decade, anti-HLA antibody-mediated rejection (ABMR) remains the main reason for the failure of kidney transplants and the return of kidney transplant recipients to dialysis,1 an event that considerably increases their mortality.2 From the patients’ perspective, preventing allograft loss due to rejection appears to be more important than life itself and the drugs’ side effects,3 thus forcing the transplant community to define better approaches to the therapeutic management of kidney transplant recipients with ABMR.
The current standard of care for the treatment of kidney allograft ABMR was defined by expert consensus, and it is on the basis of antibody-targeting therapies including plasma exchange and intravenous immune globulin, as recommended by the US Food and Drug Administration (FDA).4 This therapeutic combination is the one most frequently used for treating ABMR in clinical practice worldwide5,6 and is considered as standard of care treatment by most of the clinical trials investigating new agents in patients with ABMR.7–10 However, the effect of these therapies on long-term kidney allograft survival has never been determined. The evidence provided by therapeutic trials is low6 because of the lack of measurements defining the early response to ABMR therapies with regard to their long-term effects on allograft survival.4 The important role of early measurements of the response to treatment that surrogate long-term effects on hard outcomes for therapeutics approval has been demonstrated in many fields, such as cancer, infectious diseases, cardiovascular diseases, diabetes, and dyslipidemia.11 Among the 188 novel therapeutics approved for 206 indications by the FDA between 2005 and 2012, pivotal trials using surrogate end points as their primary outcome formed the exclusive basis for approval for 91 (45.3%) indications, none of which were in the transplant field.11 The field of transplantation should integrate these broader medical efforts and define new, earlier, and more sensitive end points that offer the opportunity for trials with acceptable durations, sample sizes, and costs to prove efficacy and safety of therapeutic strategies in ABMR, as highlighted at the FDA meeting held in Arlington in 2015. The identification of relevant end points to assess the biologic response in patients with ABMR receiving therapeutic interventions may also help to resolve the challenge of therapeutic decision making for transplant physicians delivering care to kidney transplant patients with ABMR by identifying which patients have responded to treatment and should not undergo futile additional interventions, and in which patients a more intensive strategy would be necessary to improve outcome.
Although there is substantial heterogeneity in the outcomes of patients with ABMR, with wide variations being observed in the rates and patterns of progression to allograft failure, little is known about which aspects of the disease are meaningful to efficiently delineate patient prognosis and might also be affected by current therapies.4 Small studies mostly focusing on a single diagnostic marker of ABMR have suggested that time since transplantation,12,13 allograft function,14–16 donor-specific anti-HLA antibody (anti-HLA DSA) characteristics,14,16–18 and allograft histology14,19,20 may provide clinically relevant information with respect to allograft outcomes. However, no end point on the basis of a stand-alone ABMR diagnostic marker currently reaches a sufficient level of confidence to be used in clinical practice and clinical trials, supporting the need for developing a composite prognostic system combining clinical, histologic, and immunologic dimensions to encompass the heterogeneity of ABMR and the increasing polymorphism of its clinical expression.21 Furthermore, the continuous nature of ABMR characterized by fluctuations in the level of disease activity requires dynamic prognostic evaluation on the basis of the kinetics of these parameters.22
The objective of this study was to define a dynamic and composite scoring system, integrating the characteristics of the disease at diagnosis and the response to treatment to predict kidney allograft survival in a prospective cohort of unselected patients receiving standard of care therapy for ABMR. We considered all of the contemporary methods for ABMR assessment currently used in routine clinical practice, including clinical parameters, allograft histology, and anti-HLA DSA characteristics defined at the time of diagnosis, as well as their kinetics under therapy to reflect the response to treatment. We also externally validated the scoring system in an independent cohort of kidney transplant patients.
Results
Patient Characteristics
We included 278 kidney transplant recipients diagnosed with biopsy-proven ABMR who received standardized treatment (Supplemental Figure 1). Their baseline recipient, donor, and transplant characteristics are summarized in Table 1. The median time between transplantation and ABMR diagnosis was 9.2 months (interquartile range [IQR], 6.7–21.4).
Table 1.
Characteristics of the development cohort at the time of transplantation
| Recipient Characteristics | Value | N |
|---|---|---|
| Age (yr), mean±SD | 278 | 47.3±14.7 |
| Male sex, n (%) | 278 | 149 (53.6) |
| Retransplantation, n (%) | 278 | 89 (32.0) |
| Pre-emptive transplantation, n (%) | 278 | 34 (12.2) |
| Time since dialysis (yr), mean±SD | 244 | 5.4±5.3 |
| Blood type, n (%) | 278 | |
| A | 121 (43.5) | |
| B | 34 (12.2) | |
| O | 112 (40.3) | |
| AB | 11 (4.0) | |
| CKD, n (%) | 278 | |
| Glomerulopathy | 83 (29.8) | |
| Vascular nephropathy | 22 (7.9) | |
| Chronic interstitial nephropathy | 30 (10.8) | |
| Malformative uropathy | 16 (5.8) | |
| Polycystic kidney disease | 19 (6.8) | |
| Diabetes | 27 (9.7) | |
| Other | 16 (5.8) | |
| Not determined | 65 (23.4) | |
| Donor characteristics | ||
| Age (yr), mean±SD | 278 | 50.7±17.8 |
| Male sex, n (%) | 278 | 150 (54.0) |
| Type, n (%) | 278 | |
| Living | 42 (15.1) | |
| Cerebrovascular death | 108 (38.9) | |
| Other cause of death | 128 (46.0) | |
| Terminal serum creatinine (µmol/L), mean±SD | 278 | 91.5±54.0 |
| Transplant characteristics | ||
| Cold ischemia time (h), mean±SD | 278 | 17.0±9.4 |
| HLA mismatch, mean±SD | 278 | |
| A | 1.1±0.6 | |
| B | 1.4±0.7 | |
| DR | 1.1±0.6 | |
| Anti-HLA DSA at the time of transplantation, n (%) | 278 | 102 (36.7) |
At the time of diagnosis, the mean eGFR in patients with ABMR was 34.9±18.4 ml/min per 1.73 m2, and their mean proteinuria level was 0.86±1.23 g/g. One hundred and thirty-two (47.5%) patients had multiple anti-HLA DSAs, and the mean number of anti-HLA DSAs was 2.1±1.6. Seventy-six (27.3%) patients had class I anti-HLA DSA; 127 (45.7%) patients had class II anti-HLA DSA; and 75 (27.0%) patients had class I and II anti-HLA DSAs. One hundred and seventy-six (63.3%) patients had de novo anti-HLA DSA, and 102 (36.7%) patients had preformed anti-HLA DSA. The immunodominant anti-HLA DSAs were class I in 99 (35.6%) patients and class II in 179 (64.4%) patients with a mean fluorescence intensity (MFI) of 5222.5±317.9. All patients showed microvascular inflammation on kidney allograft biopsy (glomerulitis Banff score of 1.7±0.9 and peritubular capillaritis Banff score of 1.9±0.8). Concurrent interstitial inflammation was observed in 99 (35.6%) patients. A total of 164 (59.0%) patients had complement deposition in allograft peritubular capillaries, and 50 (18.0%) patients showed chronic allograft glomerulopathy.
Changes in Clinical, Histologic, and Immunologic Characteristics after ABMR Therapy
The post-treatment evaluation was performed at a median time of 2.8 months (IQR, 2.4–3.6) after the diagnosis of ABMR.
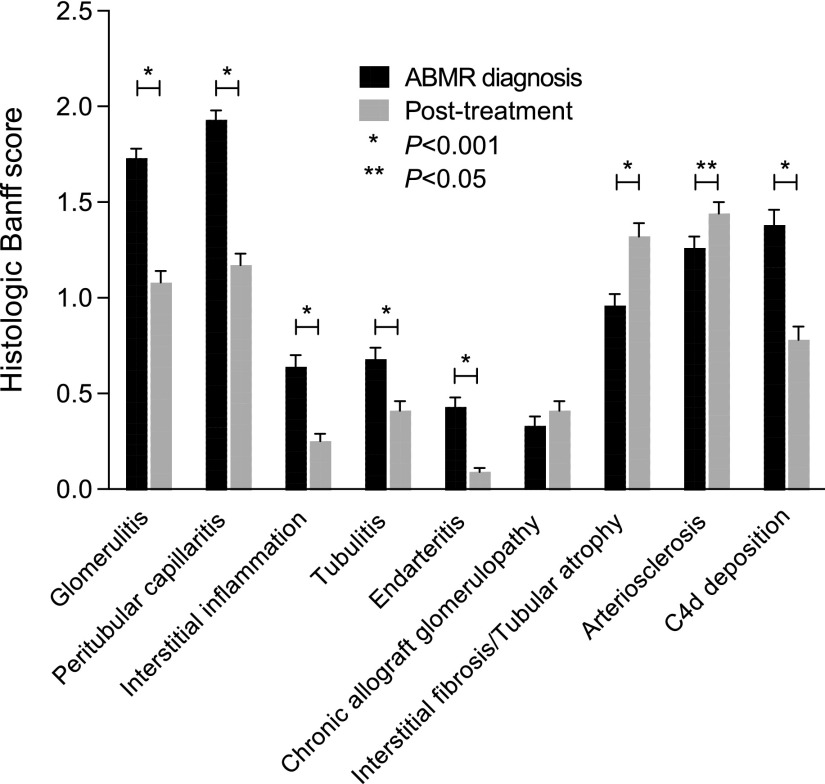
After treatment, the mean eGFR was higher than at the time of ABMR diagnosis (40.7±19.2 ml/min per 1.73 m2 versus 34.9±18.4 ml/min per 1.73 m2, respectively; P<0.001), whereas the proteinuria level was decreased (0.77±1.5 versus 0.86±1.2, respectively; P=0.002). Immunodominant anti-HLA DSA MFI decreased from 5222.5±317.9 to 3610.9±279.6 (P<0.001). Regarding histologic lesions after treatment compared with those observed at the time of ABMR diagnosis, all acute inflammation scores were reduced, including glomerulitis (P<0.001), peritubular capillaritis (P<0.001), interstitial inflammation (P<0.001), tubulitis (P<0.001), and intimal arteritis (P<0.001), whereas chronic lesion scores were increased, including interstitial fibrosis and tubular atrophy (P<0.001), vascular fibrous intimal thickening (P=0.003), and chronic allograft glomerulopathy (P=0.05). The prevalence of complement deposition was 36.7% after treatment compared with 59.0% at the time of ABMR diagnosis (P<0.001). Figure 1 depicts the changes in allograft histologic lesions between ABMR diagnosis and the post-treatment assessment.
Figure 1.
All acute histologic lesion scores were signficantly reduced between the time of ABMR diagnosis and the post-treatment evaluation, whereas chronic lesions scores were increased.
Dynamic Prediction Model for Kidney Allograft Loss in Patients with ABMR
The 6-year death-censored kidney allograft survival after the diagnosis of ABMR was 69.2% (95% confidence interval [95% CI], 61.3 to 75.9). The univariate analysis of the candidate predictors of allograft loss measured at the time of transplantation, at the time of the diagnosis of ABMR, and after treatment is shown in Table 2.
Table 2.
Determinants of kidney allograft loss at the time of transplantation, at the time of ABMR diagnosis, and at the time of the post-treatment evaluation
| Characteristic | Number of Patients | Number of Events | HR | 95% CI | P |
|---|---|---|---|---|---|
| Baseline characteristics | |||||
| Recipient age, yr | 0.28 | ||||
| <30 | 35 | 7 | 1 | ||
| 30–59 | 184 | 38 | 0.79 | (0.35 to 1.78) | |
| ≥60 | 59 | 15 | 1.29 | (0.52 to 3.16) | |
| Recipient sex | |||||
| Female | 129 | 24 | 1 | ||
| Male | 149 | 36 | 1.62 | (0.96 to 2.72) | 0.07 |
| Retransplantation | |||||
| No | 189 | 35 | 1 | ||
| Yes | 89 | 25 | 1.21 | (0.72 to 2.04) | 0.46 |
| Pre-emptive transplantation | |||||
| No | 244 | 54 | 1 | ||
| Yes | 34 | 6 | 0.82 | (0.35 to 1.91) | 0.65 |
| Time since onset of dialysis (per 1-yr increment) | 244 | 54 | 0.94 | (0.89 to 1.01) | 0.07 |
| Donor age (per 1-yr increment) | 278 | 60 | 1.01 | (1.00 to 1.03) | 0.19 |
| Donor sex | |||||
| Female | 128 | 26 | 1 | ||
| Male | 150 | 34 | 1.01 | (0.60 to 1.68) | 0.97 |
| Donor type | 0.26 | ||||
| Living | 42 | 5 | 1 | ||
| Cerebrovascular death | 108 | 27 | 2.19 | (0.84 to 5.69) | |
| Other cause of death | 128 | 28 | 1.76 | (0.68 to 4.56) | |
| Donor SCr (per 1-µmol/L increment) | 278 | 60 | 1.00 | (0.99 to 1.00) | 0.83 |
| Cold ischemia time (per 1-h increment) | 278 | 60 | 1.01 | (0.99 to 1.04) | 0.37 |
| HLA-A mismatch | 0.71 | ||||
| 0 | 45 | 11 | 1 | ||
| 1 | 163 | 33 | 0.88 | (0.45 to 1.75) | |
| 2 | 70 | 16 | 1.13 | (0.52 to 2.45) | |
| HLA-B mismatch | 0.57 | ||||
| 0 | 27 | 8 | 1 | ||
| 1 | 116 | 24 | 0.65 | (0.29 to 1.45) | |
| 2 | 135 | 28 | 0.76 | (0.34 to 1.67) | |
| HLA-DR mismatch | 0.76 | ||||
| 0 | 45 | 10 | 1 | ||
| 1 | 166 | 34 | 0.87 | (0.43 to 1.75) | |
| 2 | 67 | 16 | 1.07 | (0.49 to 2.37) | |
| Clinical characteristics at ABMR diagnosis | |||||
| Recipient age (per 1-yr increment) | 278 | 60 | 1.00 | (0.98 to 1.02) | 0.90 |
| Time since transplantation (mo, square root value, continuous) | 278 | 60 | 1.20 | (1.12 to 1.29) | <0.001 |
| eGFR (per 1-ml/min per 1.73 m2 increment) | 278 | 60 | 0.97 | (0.96 to 99) | 0.001 |
| Proteinuria (g/g, log10 value) | 278 | 60 | 1.18 | (0.96 to 1.46) | 0.12 |
| Histologic characteristics at ABMR diagnosis | |||||
| Glomerulitis | 0.95 | ||||
| Banff score=0 or 1 | 109 | 24 | 1 | ||
| Banff score=2 | 110 | 21 | 0.96 | (0.53 to 1.72) | |
| Banff score=3 | 59 | 15 | 1.06 | (0.56 to 2.03) | |
| Peritubular capillaritis | 0.90 | ||||
| Banff score=0 or 1 | 76 | 18 | 1 | ||
| Banff score=2 | 133 | 27 | 0.90 | (0.50 to 1.63) | |
| Banff score=3 | 69 | 15 | 1.02 | (0.51 to 2.03) | |
| Interstitial inflammation | |||||
| Banff score=0 | 179 | 31 | 1 | ||
| Banff score>0 | 99 | 29 | 2.03 | (1.22 to 3.38) | <0.01 |
| Tubulitis | |||||
| Banff score=0 | 176 | 37 | 1 | ||
| Banff score>0 | 102 | 23 | 1.15 | (0.68 to 1.93) | 0.61 |
| Endarteritis | |||||
| Banff score=0 | 204 | 42 | 1 | ||
| Banff score>0 | 66 | 18 | 1.37 | (0.79 to 2.39) | 0.26 |
| Chronic allograft glomerulopathy | |||||
| Banff score=0 | 228 | 40 | 1 | ||
| Banff score>0 | 50 | 20 | 2.95 | (1.72 to 5.06) | <0.001 |
| Interstitial fibrosis/tubular atrophy | |||||
| Banff score=0 | 130 | 16 | 1 | ||
| Banff score>0 | 148 | 44 | 3.30 | (1.86 to 5.86) | <0.001 |
| Arteriosclerosis | |||||
| Banff score=0 | 82 | 12 | 1 | ||
| Banff score>0 | 188 | 48 | 1.98 | (1.05 to 3.73) | 0.03 |
| C4d deposition in peritubular capillaries | |||||
| Banff score=0 | 114 | 19 | 1 | ||
| Banff score>0 | 164 | 41 | 1.72 | (1.00 to 2.97) | 0.05 |
| Immunodominant anti-HLA DSA characteristics at ABMR diagnosis | |||||
| Anti-HLA DSA status | |||||
| Preformed | 102 | 15 | 1 | ||
| De novo | 176 | 45 | 2.30 | (1.28 to 4.13) | <0.01 |
| HLA class | |||||
| HLA class I | 99 | 19 | 1 | ||
| HLA class II | 179 | 41 | 1.24 | (0.72 to 2.14) | 0.43 |
| MFI (per 1-unit increment) | 278 | 60 | 1.00 | (1.00 to 1.00) | 0.09 |
| Changes in clinical characteristics after treatment | |||||
| eGFR relative change (log10[value+0.7], continuous) | 278 | 60 | 0.40 | (0.29 to 0.57) | <0.001 |
| Proteinuria relative change (log10[value+1.1], continuous) | 278 | 60 | 1.65 | (1.33 to 2.04) | <0.001 |
| Changes in histologic characteristics after treatment | |||||
| ΔGlomerulitis Banff score after treatment | 278 | 60 | 1.20 | (0.97 to 1.48) | 0.09 |
| ΔPeritubular capillaritis Banff score after treatment | 278 | 60 | 1.53 | (1.20 to 1.95) | 0.001 |
| ΔInterstitial inflammation Banff score after treatment | 278 | 60 | 0.99 | (0.77 to 1.28) | 0.93 |
| ΔTubulitis Banff score after treatment | 278 | 60 | 1.13 | (0.91 to 1.39) | 0.28 |
| ΔEndarteritis Banff score after treatment | 270 | 60 | 0.85 | (0.66 to 1.11) | 0.23 |
| ΔChronic allograft glomerulopathy Banff score after treatment | 278 | 60 | 1.17 | (0.82 to 1.67) | 0.39 |
| ΔInterstitial fibrosis/tubular atrophy Banff score after treatment | 278 | 60 | 0.91 | (0.70 to 1.18) | 0.46 |
| ΔArteriosclerosis Banff score after treatment | 270 | 60 | 0.87 | (0.67 to 1.13) | 0.30 |
| ΔC4d Banff score after treatment | 278 | 60 | 1.14 | (0.97 to 1.35) | 0.11 |
| Change in anti-HLA DSA after treatment | |||||
| MFI relative change (continuous) | 278 | 60 | 1.41 | (1.21 to 1.65) | <0.001 |
Relative change= . Δ=value after treatment–value at ABMR diagnosis. SCr, serum creatinine.
. Δ=value after treatment–value at ABMR diagnosis. SCr, serum creatinine.
Predictors of Kidney Allograft Loss at Diagnosis in Patients with ABMR
In multivariable analysis, the independent predictors of allograft loss identified at the time of the diagnosis of ABMR were eGFR (hazard ratio [HR], 0.97; 95% CI, 0.95 to 0.98; P<0.001), the presence of chronic allograft glomerulopathy (HR, 2.25; 95% CI, 1.29 to 3.92; P=0.004), the presence of interstitial fibrosis and tubular atrophy (HR, 2.93; 95% CI, 1.62 to 5.29; P<0.001), and anti-HLA DSA de novo status (HR, 2.45; 95% CI, 1.34 to 4.47; P=0.004) (Table 3).
Table 3.
Independent predictors of time to allograft loss at ABMR diagnosis: multivariable Cox model
| Variable | Number of Patients | Number of Events | HR | 95% CI | P |
|---|---|---|---|---|---|
| eGFR (per 1-ml/min per 1.73 m2 increment) | 278 | 60 | 0.97 | (0.95 to 0.98) | <0.001 |
| Chronic allograft glomerulopathy | |||||
| Banff score=0 | 228 | 40 | 1 | ||
| Banff score>0 | 50 | 20 | 2.25 | (1.29 to 3.92) | 0.004 |
| Interstitial fibrosis/tubular atrophy | |||||
| Banff score=0 | 130 | 16 | 1 | ||
| Banff score>0 | 148 | 44 | 2.93 | (1.62 to 5.29) | <0.001 |
| Anti-HLA DSA status | |||||
| Preformed | 102 | 15 | 1 | ||
| De novo | 176 | 45 | 2.45 | (1.34 to 4.47) | 0.004 |
Dynamic ABMR Prediction Model for Kidney Allograft Loss
The dynamic multivariable ABMR prediction model was built in order to integrate the independent predictors of allograft loss identified at the diagnosis of ABMR, as well as the kinetics of the clinical, histologic, and immunologic parameters after treatment. The dynamic ABMR prediction model included eGFR at diagnosis (HR, 0.93; 95% CI, 0.90 to 0.95; P<0.001), presence of interstitial fibrosis and tubular atrophy at diagnosis (HR, 2.44; 95% CI, 1.36 to 4.37; P=0.003), change in eGFR (HR, 0.24; 95% CI, 0.16 to 0.35; P<0.001), change in peritubular capillaritis Banff score (HR, 1.50; 95% CI, 1.16 to 1.93; P=0.002), and change in immunodominant anti-HLA DSA MFI (HR, 1.30; 95% CI, 1.11 to 1.52; P<0.001) after treatment (Table 4). When coding the difference in peritubular capillaritis Banff score between the post-treatment evaluation and ABMR diagnosis as a categoric variable instead of a continuous variable in a sensitivity analysis, the set of risk predictors identified in the multivariable ABMR prediction Cox model remained unchanged (Supplemental Table 1).
Table 4.
Multivariable dynamic ABMR prediction Cox model integrating the independent predictors of time to allograft loss identified at transplantation, at ABMR diagnosis, and at the post-treatment evaluation
| Variable | Number of Patients | Number of Events | HR | 95% CI | P |
|---|---|---|---|---|---|
| eGFR at ABMR diagnosis (per 1-ml/min per 1.73 m2 increment) | 278 | 60 | 0.93 | (0.90 to 0.95) | <0.001 |
| Interstitial fibrosis/tubular atrophy at ABMR diagnosis | |||||
| Banff score=0 | 130 | 16 | 1 | ||
| Banff score>0 | 148 | 44 | 2.44 | (1.36 to 4.37) | 0.003 |
| eGFR relative change after treatment (log10[value+0.7], continuous) | 278 | 60 | 0.24 | (0.16 to 0.35) | <0.001 |
| ΔPeritubular capillaritis Banff score after treatment (continuous) | 278 | 60 | 1.50 | (1.16 to 1.93) | 0.002 |
| Anti-HLA DSA MFI relative change after treatment (continuous) | 278 | 60 | 1.30 | (1.11 to 1.52) | <0.001 |
Relative change=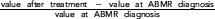 . Δ=value after treatment– value at ABMR diagnosis.
. Δ=value after treatment– value at ABMR diagnosis.
Performance of the Dynamic ABMR Prediction Model for Kidney Allograft Loss
Discrimination
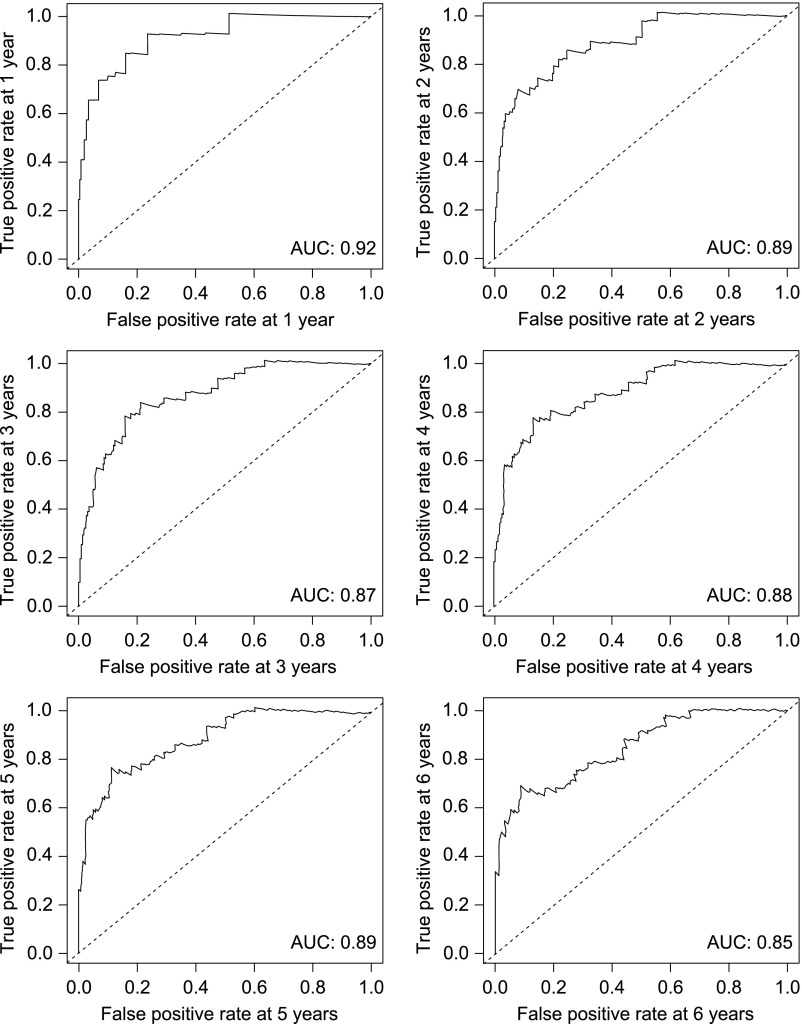
The dynamic ABMR prediction model for kidney allograft loss exhibited good discrimination ability with Harrell’s concordance index [C-statistic] of 0.84 (95% bootstrap percentile CI, 0.80 to 0.89). Time-dependent receiver operator characteristic curves showed areas under the curve of 0.92, 0.89, 0.87, 0.88, 0.89, and 0.85 at 1, 2, 3, 4, 5, and 6 years after the diagnosis of ABMR, respectively (Figure 2).
Figure 2.
The multivariable dynamic ABMR prediction Cox model showed good discimination capacity for kidney allograft loss, as demonstrated by time dependent receiver operator characteristic curves at 1 to 6 years after ABMR diagnosis. AUC, area under the curve.
Calibration
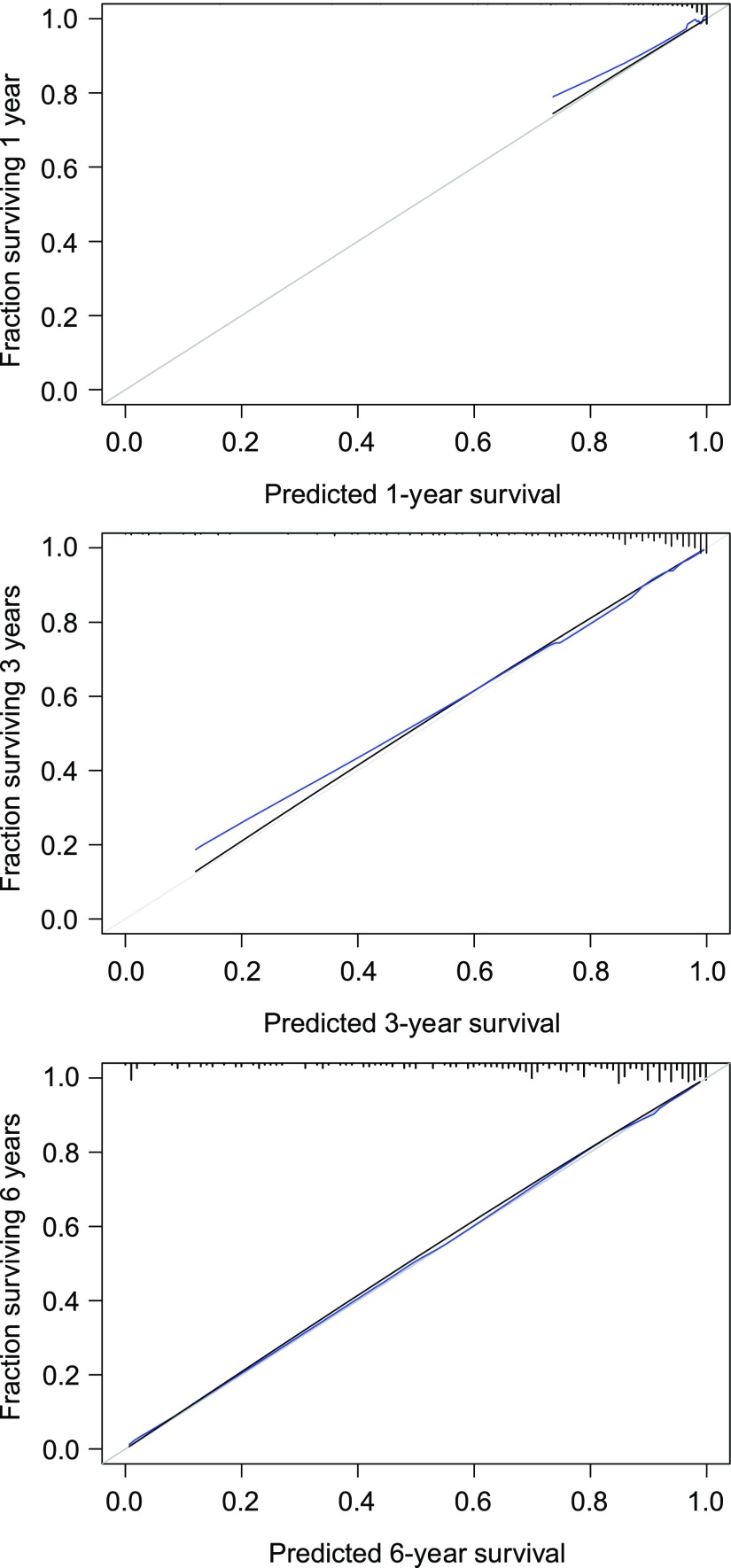
The calibration plots showed an optimal agreement between the probabilities of allograft loss derived from the dynamic ABMR prediction model and actual observations at 1, 2, 3, 4, 5, and 6 years after the diagnosis of ABMR (adapted Hosmer–Lemeshow test, P=0.63 at 6 years) (Figure 3).
Figure 3.
The multivariable dynamic ABMR prediction Cox model showed optimal calibration for predicting kidney allograft loss at 1, 3 and 6 years after ABMR diagnosis. The plots show the concordance between observed probabilities of kidney allograft survival and those predicted by the prediction model. The gray line represents perfect concordance. The black curve represents the concordance observed for the prediction model. The blue curve represents the concordance for the prediction model corrected for optimism by the mean of 1000 bootstrap resamplings.
The Dynamic ABMR Prediction Model Improved Prediction of Allograft Loss over the Initial Assessment at Diagnosis
The dynamic ABMR prediction model showed an increased discrimination capacity for allograft loss compared with the predictors defined at the time of the diagnosis of ABMR (C-statistic of 0.84 and 0.77, respectively) and adequately reclassified patients at lower risk of allograft loss and those at higher risk with a continuous net reclassification improvement (cNRI) of 0.74 (95% CI, 0.47 to 1.00; P<0.001) and an integrated discrimination improvement (IDI) of 0.20 (95% CI, =0.13 to 0.27; P<0.001) (Supplemental Figure 2).
The Dynamic ABMR Prediction Model Improved Prediction of Allograft Loss over Noninvasive Evaluation by eGFR and Anti-HLA DSA MFI
The dynamic ABMR prediction model exhibited a better discrimination capacity (C-statistic of 0.84) for allograft loss compared with the kinetics of eGFR (C-statistic of 0.69) and of anti-HLA DSA MFI (C-statistic of 0.62), and it adequately reclassified the risk of allograft loss predicted by the kinetics of eGFR (cNRI=0.88; 95% CI, 0.63 to 1.13; P<0.001; and IDI=0.23; 95% CI, 0.16 to 0.30; P<0.001) and the risk of allograft loss predicted by the kinetics of anti-HLA DSA MFI (cNRI=1.05; 95% CI, 0.80 to 1.29; P<0.001; and IDI=0.27; 95% CI, 0.20 to 0.35; P<0.001).
ABMR Prognostic Score for Kidney Allograft Loss
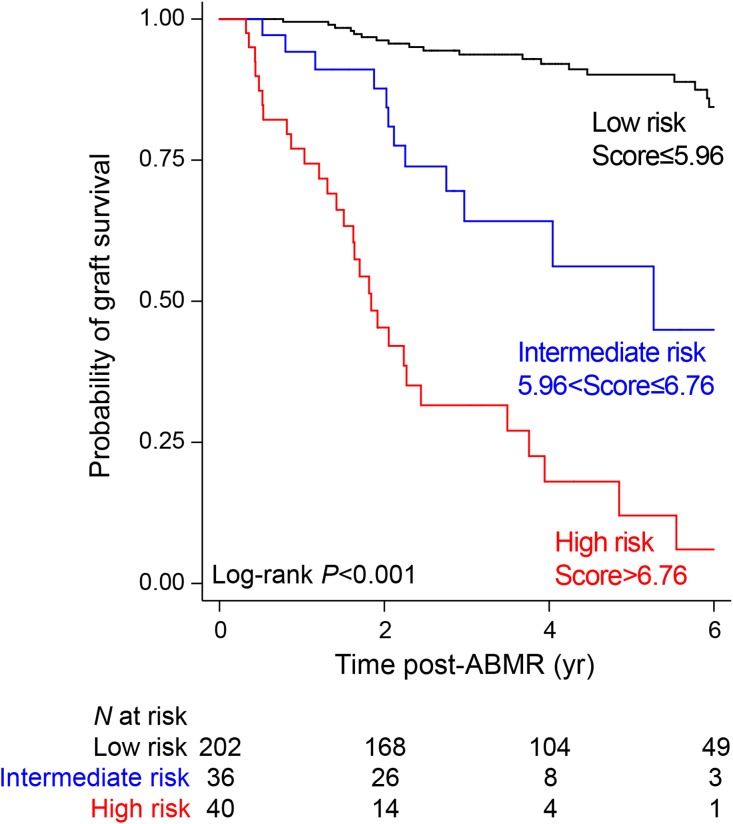
The parameters included in the dynamic ABMR prediction model were used to derive the ABMR prognostic score for kidney allograft loss. All of the factors were weighted according to the regression coefficient estimations issued from the multivariable Cox model. The contribution of each individual factor was summed to generate a raw score that was finally applied to normalization between 0 and 10. The equation of the ABMR prognostic score is provided in the Supplemental Appendix. Using recursive partitioning analysis for censored response for a three-risk group approach, we showed that optimal cut points were equal to 5.956816 and 6.758032. Such grouping achieved a clear separation of the Kaplan–Meier curves (Figure 4). The 6-year kidney allograft survival was 6.0% (95% CI, 0.5 to 22.7) in the high-risk group (n=40), 44.9% (95% CI, 18.9 to 68.1) in the intermediate-risk group (n=36), and 84.4% (95% CI, 75.7 to 90.2) in the low-risk group (n=202) (P<0.001).
Figure 4.
The prognostic ABMR score identified three different risk groups as shown by Kaplan-Meier estimates for death-censored kidney allograft survival.
External Validation of the Discriminatory Ability of the ABMR Prognostic Score
The external validation cohort was composed of 202 patients; their characteristics at transplantation, at the time of the diagnosis of ABMR, and at the post-treatment evaluation are detailed in Supplemental Tables 2 and 3.
The discrimination ability of the ABMR prognostic score developed in the main analysis was externally confirmed with a C-statistic of 0.79 (95% bootstrap percentile CI, 0.72 to 0.86). The 6-year death-censored kidney allograft survival after the diagnosis of ABMR was 8.6% (95% CI, 0.6 to 31.0) in the 27 patients classified in the high-risk group, 50.8% (95% CI, 21.2 to 74.4) in those classified in the intermediate-risk group (n=23), and 71.6% (95% CI, 57.9 to 81.6) in those classified in the low-risk group (n=152) (P<0.001).
Response to Treatment According to the ABMR Prognostic Score Risk Groups
The comparisons of patient characteristics according to the risk groups defined by the ABMR prognostic score at the time of transplantation, at the time of the diagnosis of ABMR, and at the time of the post-treatment evaluation are provided in Table 5.
Table 5.
Characteristics of the development cohort according to the risk group defined by the prognostic score at the time of transplantation, at the time of ABMR diagnosis, and at the time of the post-treatment evaluation
| Characteristic | Low-Risk Patients (n=202) | Intermediate-Risk Patients (n=36) | High-Risk Patients (n=40) | P |
|---|---|---|---|---|
| Baseline characteristics | ||||
| Recipient age (yr), mean±SD | 47.1±14.5 | 47.5±15.4 | 47.7±15.9 | 0.95 |
| Recipient sex (male), n (%) | 107 (53.0) | 17 (47.2) | 25 (62.5) | 0.39 |
| Retransplantation, n (%) | 67 (33.2) | 12 (33.3) | 10 (25.0) | 0.59 |
| Pre-emptive transplantation, n (%) | 26 (12.9) | 3 (8.3) | 5 (12.5) | 0.84 |
| Time since dialysis (yr), mean±SD | 5.7±5.5 | 5.0±5.3 | 4.4±4.1 | 0.69 |
| Donor age (yr), mean±SD | 48.9±17.2 | 55.7±10.1 | 55.1±17.3 | 0.04 |
| Donor sex (male), n (%) | 108 (53.5) | 22 (61.1) | 20 (50.0) | 0.60 |
| Donor type, n (%) | 0.03 | |||
| Living | 36 (17.8) | 4 (11.1) | 2 (5.0) | |
| Cerebrovascular death | 68 (33.7) | 17 (47.2) | 23 (57.5) | |
| Other cause of death | 98 (48.5) | 15 (41.7) | 15 (37.5) | |
| Terminal serum creatinine (µmol/L), mean±SD | 92.8±59.6 | 85.6±30.3 | 90.0±39.2 | 0.91 |
| Cold ischemia time (hours), mean±SD | 16.7±9.8 | 16.9±8.8 | 18.9±7.4 | 0.17 |
| HLA mismatch, mean±SD | ||||
| A | 1.1±0.6 | 1.1±0.6 | 1.2±0.7 | 0.63 |
| B | 1.4±0.6 | 1.2±0.8 | 1.4±0.7 | 0.17 |
| DR | 1.1±0.6 | 1.1±0.7 | 1.2±0.6 | 0.73 |
| Anti-HLA DSA at the time of transplantation, n (%) | 87 (43.1) | 9 (25.0) | 6 (15.0) | 0.001 |
| Characteristics at ABMR diagnosis | ||||
| GFR (ml/min per 1.73 m2), mean±SD | 38.0±19.5 | 29.5±9.9 | 22.4±10.5 | <0.001 |
| Proteinuria (g/g), mean±SD | 0.90±1.38 | 0.71±0.81 | 0.79±0.65 | 0.19 |
| Glomerulitis, mean±SD | 1.8±0.9 | 1.7±0.9 | 1.6±0.7 | 0.60 |
| Peritubular capillaritis, mean±SD | 2.0±0.8 | 1.8±0.8 | 1.9±0.6 | 0.29 |
| Interstitial inflammation, mean±SD | 0.6±1.0 | 1.0±1.1 | 0.8±1.0 | 0.03 |
| Tubulitis, mean±SD | 0.6±1.0 | 1.0±1.2 | 0.7±1.0 | 0.21 |
| Endarteritis, mean±SD | 0.4±0.9 | 0.3±0.8 | 0.4±0.9 | 0.53 |
| Chronic allograft glomerulopathy, mean±SD | 0.2±0.6 | 0.5±0.9 | 0.7±1.2 | <0.01 |
| Interstitial fibrosis/tubular atrophy, mean±SD | 0.7±0.9 | 1.8±1.0 | 1.7±1.0 | <0.001 |
| Arteriosclerosis, mean±SD | 1.1±1.0 | 1.6±1.0 | 1.8±1.0 | <0.001 |
| C4d deposition, mean±SD | 1.3±1.3 | 1.8±1.2 | 1.3±1.2 | 0.15 |
| Anti-HLA DSA MFI, mean±SEM | 4870.9±359.3 | 5856.3±914.0 | 6427.5±943.6 | 0.17 |
| Post-treatment characteristics | ||||
| GFR (ml/min per 1.73 m2), mean±SD | 47.7±17.5 | 27.6±7.1 | 17.3±5.0 | <0.001 |
| Proteinuria (g/g), mean±SD | 0.55±1.0 | 0.89±1.70 | 1.74±2.40 | <0.001 |
| Glomerulitis, mean±SD | 1.0±1.0 | 1.3±1.1 | 1.2±1.1 | 0.17 |
| Peritubular capillaritis, mean±SD | 1.1±1.0 | 1.3±1.0 | 1.7±1.0 | 0.002 |
| Interstitial inflammation, mean±SD | 0.2±0.5 | 0.4±0.8 | 0.3±0.7 | 0.54 |
| Tubulitis, mean±SD | 0.4±0.8 | 0.6±1.0 | 0.5±0.8 | 0.33 |
| Endarteritis, mean±SD | 0.1±0.4 | 0.03±0.2 | 0.08±0.3 | 0.53 |
| Chronic allograft glomerulopathy, mean±SD | 0.3±0.8 | 0.4±0.8 | 0.7±1.2 | 0.07 |
| Interstitial fibrosis/tubular atrophy, mean±SD | 1.1±1.0 | 1.9±1.1 | 2.0±1.1 | <0.001 |
| Arteriosclerosis, mean±SD | 1.3±1.0 | 1.7±0.9 | 1.7±0.9 | 0.03 |
| C4d deposition, mean±SD | 0.7±1.0 | 1.1±1.3 | 1.1±1.2 | 0.03 |
| Anti-HLA DSA MFI, mean±SEM | 2708.1±259.8 | 5089.6±975.5 | 6839.5±954.5 | <0.001 |
After ABMR treatment, high-risk patients showed a decrease in GFR (22.4±10.5 ml/min per 1.73 m2 before treatment versus 17.3±5.0 ml/min per 1.73 m2 after treatment, P=0.001), an increase in proteinuria (0.79±0.65 g/g before treatment versus 1.74±2.40 after treatment, P=0.04), persisting peritubular capillaritis (Banff score of 1.9±0.6 before treatment versus 1.7±1.0 after treatment, P=0.18), persisting complement deposition in allograft (C4d Banff score of 1.3±1.2 before treatment versus 1.1±1.2 after treatment, P=0.25), and no reduction in anti-HLA DSA MFI level (6427.5±943.6 before treatment versus 6839.5±954.5 after treatment, P=0.98). Low-risk patients showed an increase in GFR (38.0±19.5 ml/min per 1.73 m2 before treatment versus 47.7±17.5 ml/min per 1.73 m2 after treatment, P<0.001), a decrease in proteinuria (0.90±1.38 g/g before treatment versus 0.55±1.0 after treatment, P<0.001), a decrease in peritubular capillaritis (Banff score of 2.0±0.8 before treatment versus 1.1±1.0 after treatment, P<0.001), a decrease in complement deposition in the allograft (C4d Banff score of 1.3±1.3 before treatment versus 0.7±1.0 after treatment, P<0.001), and a reduction in anti-HLA DSA MFI level (4870.9±359.3 before treatment versus 2708.1±259.8 after treatment, P<0.001).
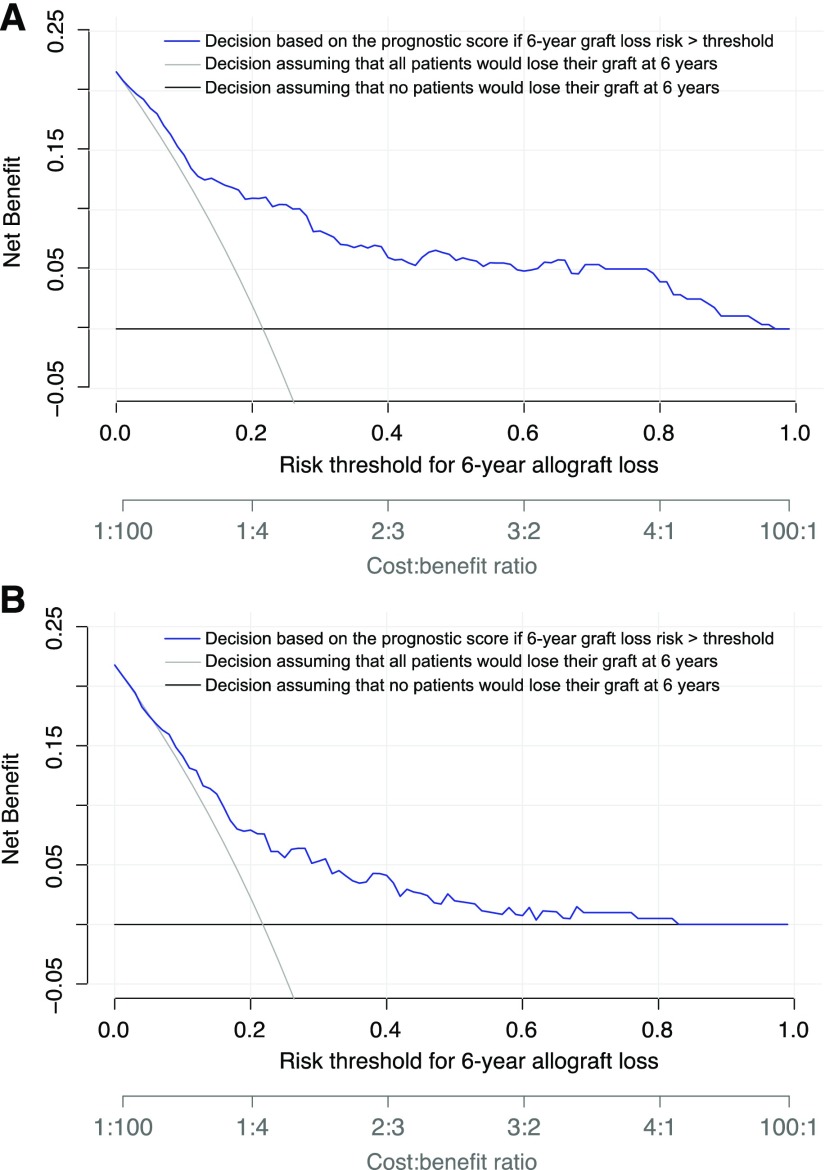
Clinical Benefit of the Prognostic ABMR Score
Across a range of risks from 1% to 96%, decision curve analysis showed that decision making after standard management of ABMR on the basis of the ABMR prognostic score provided greater net clinical benefit to patients than considering all patients to have the same level of risk of allograft loss at 6 years after the diagnosis of ABMR (Figure 5A). The clinical benefit of the prognostic score was confirmed in the external validation cohort (Figure 5B). The net benefit of adding a new intervention to the standard of care treatment according to the ABMR prognostic score is provided for different decision thresholds in Supplemental Table 4. A decision strategy on the basis of the ABMR prognostic score was associated with a significant reduction in avoidable interventions for all decision thresholds (Supplemental Table 4).
Figure 5.
Decision making after standard management of ABMR on the basis of the ABMR prognostic score provided greater net clinical benefit to patients than considering all patients to have the same level of risk of allograft loss at 6 years after the diagnosis of ABMR. Decision curve analysis shows the net benefit achieved by making clinical decisions on the basis of the predictions at 6 years after ABMR diagnosis computed by the prognostic score (A) in the development cohort and (B) in the external validation cohort. The decision curve analysis shows the net benefit of the prognostic score in regard to decision making for potential intervention across all of the thresholds of risk of allograft loss at 6 years as defined by clinician judgement and patient preference (blue), compared with considering patients with ABMR at the same level of risk of allograft loss: all patients at risk (gray) or no patients at risk (black). If the decision is defined by the performance of additional therapy to standard treatment, the expected benefit is represented by the number of patients who would lose their allograft and who would undergo clinical intervention (true positives) using the proposed decision rule. The expected harm is represented by the number of patients without allograft loss who would undergo clinical intervention in error (false positives) multiplied by a weighting factor on the basis of the risk threshold. The highest curve at any given risk threshold is the optimal strategy for decision-making in order to maximize net benefit. Overall, the net benefit for decisions on the basis of the prognostic score is higher than that for decisions on the basis of the same level of risk for thresholds from 1% to 96%.
Discussion
In this prospective study performed in an unselected population of 278 kidney transplant recipients with active ABMR receiving standard therapy, we developed a dynamic, prognostic score for kidney allograft loss that exhibited high accuracy, and we defined a composite assessment of the response to treatment. We showed that the kinetics of clinical, pathologic, and immunologic markers of ABMR under treatment carried a strong prognostic value for allograft outcomes and improved the predictive performance of the evaluation performed at the time of diagnosis. A decrease in peritubular capillaritis intensity, a decrease in anti-HLA DSA MFI level, and an increase in GFR were predictive of good long-term outcome, independent of the initial ABMR characteristics. We also showed that the dynamic prognostic score improved decision making in ABMR compared with considering patients on the same level of risk of allograft loss. The performance of the score for the prediction of allograft loss was externally validated in an independent cohort of 202 kidney transplant patients with active ABMR.
To date, small studies have suggested that allograft function, proteinuria, anti-HLA DSA characteristics, and allograft histology may be associated with allograft loss in kidney transplant recipients with ABMR; however, no study has integrated simultaneously all of the disease characteristics and their kinetics under therapy using current diagnostic and therapeutic standards. According to a PubMed database request for reports in any language published before June of 2017, 23 studies reporting on ABMR prognosis were published (median n=48; IQR, 26–70). Most of these studies investigated a single diagnostic marker of ABMR, including time since transplantation,12,13 acute versus chronic ABMR,19 mixed rejection versus pure ABMR,12,23 C4d positivity,24 presence of intimal arteritis,20 proteinuria level,25,26 anti-HLA DSA level,18,27–30 and persistent versus de novo antibodies.17,31 Two studies considered the simultaneous assessment of allograft function, histologic lesions, and anti-HLA DSA, which was only performed at diagnosis.14,16
By integrating the whole spectrum of ABMR markers in a large cohort, our study identified the independent predictors of kidney allograft loss that we can measure at the time of ABMR, including GFR, chronic allograft glomerulopathy, interstitial fibrosis and tubular atrophy, and de novo status of anti-HLA DSA. We also demonstrated that the performance of these predictors defined at the time of the diagnosis of ABMR was significantly improved by adding the kinetics of GFR, peritubular capillaritis intensity, and anti-HLA DSA level under treatment. The consistency of the predictors we identified to characterize the response to ABMR treatment is supported by several small studies showing that the decrease of anti-HLA DSA level under treatment is associated with good outcomes17,18,28–30; one study (n=21) showed that a decline in GFR after treatment was associated with allograft loss,15 and one study (n=21) showed that increase in the peritubular capillaritis level was associated with poor outcomes.32 By combining the predictors of allograft loss derived from all of the contemporary clinical, histologic, and immunologic ABMR markers assessed in routine clinical practice, we defined a composite, dynamic, prognostic score that exhibited high accuracy to predict kidney allograft failure up to 6 years. Discrimination, calibration, and external validation in an independent cohort demonstrated good performance and validity of the ABMR prediction model.
The ABMR prognostic score allowed for the early identification (after ABMR treatment) of homogeneous subsets of patients according to their long-term allograft survival projected by the score: 73% of patients with good outcomes showed a biologic response to treatment, 13% of patients with intermediate outcomes showed partial response to treatment, and 14% of patients with poor outcomes were nonresponders to treatment. After treatment, nonresponders were characterized by worsening allograft function and proteinuria, persistent antibody-mediated injury with microcirculation inflammation and C4d deposition in the allograft, and no reduction of the anti-HLA DSA level, whereas responders to treatment showed improvements in allograft function, proteinuria, microcirculation inflammation, C4d deposition in the allograft, and reduction of the anti-HLA DSA level.
We demonstrated the clinical value of the ABMR prognostic score with regard to decision-making across a wide range of thresholds for potential intervention, as defined by clinician judgment and patient preference, compared with considering all patients with ABMR to be at the same level of risk of allograft loss. After taking into account false-positive predictions of allograft loss, the ABMR prognostic score provided a significant net benefit by identifying nonresponders to standard of care therapy who would be eligible for further interventions. It also allows avoidance of futile additional interventions in responders in whom standard therapy appears sufficient with respect to the expected allograft survival. Thus, the prognostic score has the potential to inform a personalized management of kidney transplant recipients with ABMR. However, few second-line therapies have been investigated in patients with ABMR who are considered nonresponders to standard of care therapy, and there is little evidence supporting additional interventions in these patients because of the lack of tools for patient selection and robust surrogate end points. Preliminary studies have suggested that complement inhibitors,28,29 bortezomib,30 or anti-IL6 agents31 might improve the outcomes in these patients. Finally, the prognostic score has major implications for the design of future interventional trials by helping reduce sample size, costs, and duration. By defining consistent risk strata in patients with ABMR, our prognostic score will also allow the use of prognostic enrichment strategies in clinical trials to increase study power by enrolling high-risk patients who are more likely to experience the study outcome, thus reducing sample sizes.33
A significant limitation of our study may be related to the fact that the prognostic ABMR score includes post-treatment histologic evaluation, which is not currently a universal practice. However, our study supports the importance of an early and comprehensive evaluation of the response to ABMR treatment, including not only noninvasive parameters represented by allograft function and anti-HLA DSA MFI, but also allograft histology. We showed that the information provided by both noninvasive ABMR markers and allograft biopsy specimens had substantial performance to early predict the long-term response to therapy and outperformed the current practice on the basis of the monitoring of allograft function or anti-HLA DSA. Although the optimal time point for performing the post-treatment evaluation remains to be determined, we showed the robustness of this approach in the validation cohort, in which the post-treatment evaluation occurred in a larger time frame, between 2 and 9 months after ABMR treatment, and was not performed per protocol, regardless of clinical course. This fact reinforces the generalizability of the prognostic ABMR score to different policies for ABMR management. One may also question the influence of MFI variability across laboratories using single antigen bead assays and of the reproducibility of the assessment of Banff scores in kidney allograft biopsy specimens on the generalizability of the prognostic ABMR score. By validating the predictive performance of the prognostic ABMR score in centers performing local assessments of anti-HLA antibodies and allograft histology, we showed its generalizability, despite the variability in the measurement of the parameters of the score. By considering the individual changes in the MFI values and Banff scores rather than net measures, our study limited the effect of intercenter variability and addressed the real-life management of patients with ABMR in clinical transplant centers. Finally, the prognostic ABMR score was not validated in kidney recipients who had undergone multiple previous lines of treatment for recurrent ABMR episodes and in the specific population of those patients receiving antibody-targeting therapies in intensive desensitization protocols. Further studies are needed to validate this approach in other kidney transplant populations and to evaluate its cost-effectiveness for defining second-line therapeutic strategies.
In conclusion, we developed and validated a prognostic score in kidney allograft ABMR that showed high accuracy in predicting allograft loss. This dynamic and composite prognostic score was on the basis of six independent clinical prognostic factors routinely assessed in current practice, including GFR, allograft histology, and anti-HLA DSA level, and integrated the characteristics of the disease at diagnosis together with the response to treatment. This measurement may provide an early and reliable end point for the assessment of treatment efficacy in kidney allograft ABMR.
Concise Methods
Study Population
This prospective study considered all consecutive patients who underwent kidney transplantation at Necker Hospital and Saint-Louis Hospital (Paris, France) between January of 2008 and January of 2014 (development cohort, n=1978).
We included patients with a first episode of ABMR diagnosed on indication kidney allograft biopsy specimens receiving standard of care treatment (n=312). These patients were screened for clinical parameters, anti-HLA DSA, and allograft histology at the time of ABMR diagnosis and post-treatment (systematic allograft biopsy 3 months after the diagnosis) according to the standard management of patients with ABMR in both centers. Inclusion criteria were: (1) biopsy-proven active (acute or chronic) ABMR according to the most recent international Banff classification criteria on indication allograft biopsy specimens34; (2) at least one circulating donor-specific anti-HLA-A, -B, Cw, -DR, -DQ, or -DP antibody in the serum obtained at the time of allograft biopsy; and (3) standard of care treatment of ABMR, including plasma exchange, high-dose intravenous immune globulin, and rituximab, and triple maintenance immunosuppression, including mycophenolate mofetil, tacrolimus, and steroids after ABMR diagnosis. Exclusion criteria consisted in (1) participation in interventional trials or treatment with nonstandard therapies (anti-C5 mAb, proteasome inhibitor, C1 esterase inhibitor, or splenectomy) (n=8); (2) contraindication or refusal of post-treatment allograft biopsy (n=4); (3) previous episode of ABMR treated with at least one of the following therapies: plasma exchange, high-dose intravenous immune globulin, anti-CD20 mAb, anti-C5 mAb, proteasome inhibitor, or C1 esterase inhibitor (n=10); and (4) contraindication to standard of care ABMR therapies (n=12). A flow diagram of the study population is provided in Supplemental Figure 1.
Patients were followed up to January 1, 2017 to assess kidney allograft survival after ABMR.
Clinical Data
Clinical data on the donors and recipients in the development cohort (Necker and Saint-Louis Hospitals) were extracted from a prospective national database: Données Informatiques Validées en Transplantation (DIVAT) (official website: https://www.divat.fr). The data are computerized in real time as well as at each transplant anniversary and are submitted for an annual audit. Anonymized data from these registries were prospectively entered at specific time points for each patient (at day 0, 6 months, and 1 year after transplantation) and were updated annually thereafter. Data were retrieved from the databases on January 1, 2017.
Renal function was assessed by eGFR using the abbreviated Modification of Diet in Renal Disease formula35 at the time of ABMR diagnosis and at the time of the post-treatment evaluation. Relative change in eGFR after treatment was defined as follows: (eGFR after treatment−eGFR at ABMR diagnosis)/(eGFR at ABMR diagnosis).
Detection and Characterization of Anti-HLA DSAs
HLA typing of all of the kidney transplant donors and recipients was performed by molecular biology (Innolipa HLA Typing Kit; Innogenetics, Gent, Belgium). All of the study patients were tested for circulating donor-specific anti-HLA-A, -B, -Cw, -DR, -DQ, and -DP antibodies in serum samples obtained at the time of transplantation, at the time of the diagnosis of ABMR, and at the time of the post-treatment evaluation. Single-antigen flow bead assays were used (One Lambda, Inc., Canoga Park, CA) on a Luminex platform. All beads showing a normalized MFI>1000 were considered positive. The highest MFI value toward a donor-specific allele was considered to be the immunodominant anti-HLA DSA, and we calculated the relative change after treatment of immunodominant anti-HLA DSA MFI as follows: (MFI after treatment−MFI at ABMR diagnosis)/(MFI at ABMR diagnosis).
Kidney Allograft Pathology
Renal tissue was fixed in acetic formol absolute alcohol fixative and stained with Masson’s trichrome and periodic acid–Schiff. All of the allograft biopsy specimens were scored and graded from zero to three according to the Banff criteria34 for the following histologic factors: glomerulitis (g), tubulitis (t), mononuclear cell interstitial inflammation, intimal arteritis (v), peritubular capillaritis (ptc), chronic allograft glomerulopathy, interstitial fibrosis/tubular atrophy (IF/TA), arteriolar hyaline thickening, and vascular fibrous intimal thickening (cv). Complement split product C4d staining was performed by immunochemical analysis on paraffin sections using polyclonal human anti-C4d antibodies (Biomedica Gruppe, Vienna, Austria).
Definition of ABMR
The diagnosis of ABMR was made on specimens from indication allograft biopsies performed for a decrease in eGFR>15% as compared with baseline level and/or urine protein-to-creatinine ratio >0.5 g/g.
Acute/active and chronic/active ABMR were defined according to the most recent update of the Banff classification34 by (1) histologic evidence of acute tissue injury, including one or more of the following: microvascular inflammation (g>0 and/or ptc>0), intimal or transmural arteritis (v>0), acute thrombotic microangiopathy in the absence of any other cause, or acute tubular injury and in the absence of any other apparent cause; and (2) evidence of current antibody interaction with vascular endothelium with linear C4d staining in peritubular capillaries (C4d>0) or moderate microvascular inflammation (g+ptc≥2).
We considered patients with histologic diagnosis of ABMR and serologic evidence of anti-HLA DSA at the time of allograft biopsy.
Treatment of ABMR
In the development cohort, the ABMR treatment consisted of four or five plasma exchanges, three courses of high-dose intravenous immune globulins (Privigen; CSL Behring, Marburg, Germany; 2 g/kg, repeated every 3–4 weeks), methylprednisolone pulses (500 mg/d for 3 days), and one dose per week for two to four weeks of rituximab (Mabthera; Roche, Meylan, France; 375 mg/m2 of body-surface area). Thirty-nine (14.0%) patients with HIV, hepatitis C or B virus infection, tuberculosis, or uncontrolled infection did not receive rituximab. The maintenance immunosuppression therapy included mycophenolate mofetil (CellCept; Roche; 750 mg twice a day BID), tacrolimus to maintain a trough level between 6 and 10 ng/ml, and prednisone tapered to 10 mg daily per the centers’ protocols from the time of ABMR diagnosis in all patients.
External Validation Cohort
The external validation cohort comprised kidney transplant recipients diagnosed with ABMR meeting the same inclusion criteria as those used in the development cohort in Foch Hospital (Suresnes, France), Rangueil Hospital (Toulouse, France), and Bellvitge University Hospital (Barcelona, Spain) between January of 2009 and January of 2014, who underwent post-treatment evaluation including a kidney allograft biopsy performed between 2 and 9 months after diagnosis on the basis of the clinician’s decision. In the validation cohort, the ABMR treatment consisted of four to six plasma exchanges, one to three courses of high-dose intravenous immune globulins (2 g/kg, repeated every 3–4 weeks), methylprednisolone pulses (500 mg/d for 3 days), and two weekly doses of rituximab (Mabthera; Roche, Meylan, France; 375 mg/m2 of body-surface area).
Statistical Analyses
Mean±SD values and frequency were provided for the description of continuous and categoric variables, respectively, unless otherwise stated. Means and proportions were compared using the Mann–Whitney t test and Chi-squared test (or Fisher’s exact test, if appropriate), respectively. Paired means and proportions were compared using the Wilcoxon signed-rank test and McNemar’s test.
Kidney allograft survival was calculated from the date of the diagnosis of ABMR to the date of allograft loss as defined by return to dialysis. In the case of death with a functioning allograft, allograft survival was censored at the time of death. Kidney allograft survival was estimated using the Kaplan–Meier method and described using median or rate at specific time points with 95% CIs.
Cox proportional-hazard models were performed to estimate the HRs and 95% CIs for factors associated with time to kidney allograft loss in three sets of predictors: the parameters assessed at the time of transplantation, at the time of ABMR diagnosis, and at the time of the post-treatment evaluation. The association of the parameters with time to kidney allograft loss was first assessed using univariate Cox analyses, and then, those with P<0.10 were entered into a multivariable Cox regression model for each subset of predictors using stepwise backward selection. A final multivariable model was built by integrating the models defined in each subset and using stepwise backward selection. We checked the linearity assumption of the Cox model for continuous variables using a fractional polynomial modeling approach to assess and transform those covariates that showed nonlinear associations with time to allograft loss. Hazard proportionality was checked by plotting log-minus-log survival curves and by the cumulative martingale process plots.
Accuracy of the final model was verified regarding two parameters: discrimination and calibration. The discrimination ability of the final model was evaluated with the C-statistic. One thousand random samples of the population were used to derive 95% CIs for the C-statistic. Calibration was assessed by visual examination of the calibration plot and tested with an extension of the Hosmer–Lemeshow test for survival data.
Improvement in discrimination ability was evaluated with the C-statistic. We used cNRI and IDI to quantify risk reclassification.
The final model was used to derive a prognostic score, which was constructed and weighted with β-coefficient estimations in the final model and normalized between 0 and 10. To give a reasonable spread of risk, we chose to work on three prognostic risk groups. Optimal cut points for discriminating prognostic groups were determined by performing recursive partitioning analysis for censored response.
The prognostic ABMR score discrimination ability was confirmed in an external validation cohort and evaluated with the C-statistic. To identify risk groups and to determine their survival profile, the same development cohort–derived cut points were applied.
Clinical benefit–centered accuracy of the prognostic score was evaluated by a decision curve analysis for the development and validation cohorts.36,37
All analyses were performed using STATA version 14.1 (StataCorp, College Station, TX) and R software version 2.15.2 (R Development Core Team, Vienna, Austria). Values of P<0.05 were considered statistically significant, and all tests were two-sided.
Study Approval
This study was approved by the Institutional Review Boards of Necker and Saint-Louis Hospitals. Each patient included in this study provided written, informed consent to be included in the French national registry agency (Agence de la Biomédecine) database CRISTAL (official website: https://www.sipg.sante.fr/portail/) and DIVAT. The DIVAT and CRISTAL database networks have been approved by the National French Commission for bioinformatics data and patients’ liberty: DIVAT: Commission Nationale de l'Informatique et des Libertés, registration number: 1016618, validated 8th June, 2004; and CRISTAL: CNIL, registration number: 363505, validated 3rd April, 1996.
Disclosures
None.
Supplementary Material
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Any Progress in the Treatment of Antibody-Mediated Rejection?,” on pages 350–352.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2017070749/-/DCSupplemental.
References
- 1.Sellarés J, de Freitas DG, Mengel M, Reeve J, Einecke G, Sis B, Hidalgo LG, Famulski K, Matas A, Halloran PF: Understanding the causes of kidney transplant failure: The dominant role of antibody-mediated rejection and nonadherence. Am J Transplant 12: 388–399, 2012 [DOI] [PubMed] [Google Scholar]
- 2.Kaplan B, Meier-Kriesche HU: Death after graft loss: An important late study endpoint in kidney transplantation. Am J Transplant 2: 970–974, 2002 [DOI] [PubMed] [Google Scholar]
- 3.Howell M, Tong A, Wong G, Craig JC, Howard K: Important outcomes for kidney transplant recipients: A nominal group and qualitative study. Am J Kidney Dis 60: 186–196, 2012 [DOI] [PubMed] [Google Scholar]
- 4.Archdeacon P, Chan M, Neuland C, Velidedeoglu E, Meyer J, Tracy L, Cavaille-Coll M, Bala S, Hernandez A, Albrecht R: Summary of FDA antibody-mediated rejection workshop. Am J Transplant 11: 896–906, 2011 [DOI] [PubMed] [Google Scholar]
- 5.Burton SA, Amir N, Asbury A, Lange A, Hardinger KL: Treatment of antibody-mediated rejection in renal transplant patients: A clinical practice survey. Clin Transplant 29: 118–123, 2015 [DOI] [PubMed] [Google Scholar]
- 6.Roberts DM, Jiang SH, Chadban SJ: The treatment of acute antibody-mediated rejection in kidney transplant recipients-a systematic review. Transplantation 94: 775–783, 2012 [DOI] [PubMed] [Google Scholar]
- 7.Efficacy and Safety of Eculizumab for Treatment of Antibody-mediated Rejection Following Renal Transplantation, 2013. Available at: https://clinicaltrials.gov/ct2/show/NCT01895127. Accessed October 17, 2017
- 8.Bortezomib in Rejection of Kidney Transplants (TRIBUTE), 2014. Available at: https://clinicaltrials.gov/ct2/show/NCT02201576. Accessed October 17, 2017
- 9.Recombinant Human C1 Inhibitor for the Treatment of Early Antibody-Mediated Rejection in Renal Transplantation, 2009. Available at: https://clinicaltrials.gov/ct2/show/NCT01035593. Accessed October 17, 2017
- 10.Sautenet B, Blancho G, Büchler M, Morelon E, Toupance O, Barrou B, Ducloux D, Chatelet V, Moulin B, Freguin C, Hazzan M, Lang P, Legendre C, Merville P, Mourad G, Mousson C, Pouteil-Noble C, Purgus R, Rerolle JP, Sayegh J, Westeel PF, Zaoui P, Boivin H, Le Gouge A, Lebranchu Y: One-year results of the effects of rituximab on acute antibody-mediated rejection in renal transplantation: RITUX ERAH, a multicenter double-blind randomized placebo-controlled trial. Transplantation 100: 391–399, 2016 [DOI] [PubMed] [Google Scholar]
- 11.Downing NS, Aminawung JA, Shah ND, Krumholz HM, Ross JS: Clinical trial evidence supporting FDA approval of novel therapeutic agents, 2005-2012. JAMA 311: 368–377, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krisl JC, Alloway RR, Shield AR, Govil A, Mogilishetty G, Cardi M, Diwan T, Abu Jawdeh BG, Girnita A, Witte D, Woodle ES: Acute rejection clinically defined phenotypes correlate with long-term renal allograft survival. Transplantation 99: 2167–2173, 2015 [DOI] [PubMed] [Google Scholar]
- 13.Dörje C, Midtvedt K, Holdaas H, Naper C, Strøm EH, Øyen O, Leivestad T, Aronsen T, Jenssen T, Flaa-Johnsen L, Lindahl JP, Hartmann A, Reisæter AV: Early versus late acute antibody-mediated rejection in renal transplant recipients. Transplantation 96: 79–84, 2013 [DOI] [PubMed] [Google Scholar]
- 14.Redfield RR, Ellis TM, Zhong W, Scalea JR, Zens TJ, Mandelbrot D, Muth BL, Panzer S, Samaniego M, Kaufman DB, Astor BC, Djamali A: Current outcomes of chronic active antibody mediated rejection - A large single center retrospective review using the updated BANFF 2013 criteria. Hum Immunol 77: 346–352, 2016 [DOI] [PubMed] [Google Scholar]
- 15.Bachelet T, Nodimar C, Taupin JL, Lepreux S, Moreau K, Morel D, Guidicelli G, Couzi L, Merville P: Intravenous immunoglobulins and rituximab therapy for severe transplant glomerulopathy in chronic antibody-mediated rejection: A pilot study. Clin Transplant 29: 439–446, 2015 [DOI] [PubMed] [Google Scholar]
- 16.Willicombe M, Roufosse C, Brookes P, Galliford JW, McLean AG, Dorling A, Warrens AN, Cook TH, Cairns TD, Taube D: Antibody-mediated rejection after alemtuzumab induction: Incidence, risk factors, and predictors of poor outcome. Transplantation 92: 176–182, 2011 [DOI] [PubMed] [Google Scholar]
- 17.Haas M, Mirocha J, Reinsmoen NL, Vo AA, Choi J, Kahwaji JM, Peng A, Villicana R, Jordan SC: Differences in pathologic features and graft outcomes in antibody-mediated rejection of renal allografts due to persistent/recurrent versus de novo donor-specific antibodies. Kidney Int 91: 729–737, 2017 [DOI] [PubMed] [Google Scholar]
- 18.Everly MJ, Rebellato LM, Ozawa M, Briley KP, Catrou PG, Haisch CE, Terasaki PI: Beyond histology: Lowering human leukocyte antigen antibody to improve renal allograft survival in acute rejection. Transplantation 89: 962–967, 2010 [DOI] [PubMed] [Google Scholar]
- 19.Gubensek J, Buturovic-Ponikvar J, Kandus A, Arnol M, Lindic J, Kovac D, Rigler AA, Romozi K, Ponikvar R: Treatment of antibody-mediated rejection after kidney transplantation - 10 years’ experience with apheresis at a single center. Ther Apher Dial 20: 240–245, 2016 [DOI] [PubMed] [Google Scholar]
- 20.Wu K, Budde K, Schmidt D, Neumayer HH, Rudolph B: The relationship of the severity and category of acute rejection with intimal arteritis defined in Banff classification to clinical outcomes. Transplantation 99: e105–e114, 2015 [DOI] [PubMed] [Google Scholar]
- 21.Djamali A, Kaufman DB, Ellis TM, Zhong W, Matas A, Samaniego M: Diagnosis and management of antibody-mediated rejection: Current status and novel approaches. Am J Transplant 14: 255–271, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Loupy A, Hill GS, Jordan SC: The impact of donor-specific anti-HLA antibodies on late kidney allograft failure. Nat Rev Nephrol 8: 348–357, 2012 [DOI] [PubMed] [Google Scholar]
- 23.Matignon M, Muthukumar T, Seshan SV, Suthanthiran M, Hartono C: Concurrent acute cellular rejection is an independent risk factor for renal allograft failure in patients with C4d-positive antibody-mediated rejection. Transplantation 94: 603–611, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Orandi BJ, Alachkar N, Kraus ES, Naqvi F, Lonze BE, Lees L, Van Arendonk KJ, Wickliffe C, Bagnasco SM, Zachary AA, Segev DL, Montgomery RA: Presentation and outcomes of C4d-negative antibody-mediated rejection after kidney transplantation. Am J Transplant 16: 213–220, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chung BH, Kim Y, Jeong HS, Hong YA, Choi BS, Park CW, Choi YJ, Kim YS, Yang CW: Clinical outcome in patients with chronic antibody-mediated rejection treated with and without rituximab and intravenous immunoglobulin combination therapy. Transpl Immunol 31: 140–144, 2014 [DOI] [PubMed] [Google Scholar]
- 26.Oblak M, Kandus A, Mlinšek G, Buturović-Ponikvar J, Arnol M: Increase in proteinuria after acute kidney graft rejection is associated with decreased graft function and survival. Transplant Proc 45: 1453–1457, 2013 [DOI] [PubMed] [Google Scholar]
- 27.Moktefi A, Parisot J, Desvaux D, Canoui-Poitrine F, Brocheriou I, Peltier J, Audard V, Kofman T, Suberbielle C, Lang P, Rondeau E, Grimbert P, Matignon M: C1q binding is not an independent risk factor for kidney allograft loss after an acute antibody-mediated rejection episode: A retrospective cohort study. Transpl Int 30: 277–287, 2017 [DOI] [PubMed] [Google Scholar]
- 28.Ramon DS, Huang Y, Zhao L, Rendulic T, Park JM, Sung RS, Samaniego M: Use of complement binding assays to assess the efficacy of antibody mediated rejection therapy and prediction of graft survival in kidney transplantation. Hum Immunol 78: 57–63, 2017 [DOI] [PubMed] [Google Scholar]
- 29.Everly MJ, Everly JJ, Arend LJ, Brailey P, Susskind B, Govil A, Rike A, Roy-Chaudhury P, Mogilishetty G, Alloway RR, Tevar A, Woodle ES: Reducing de novo donor-specific antibody levels during acute rejection diminishes renal allograft loss. Am J Transplant 9: 1063–1071, 2009 [DOI] [PubMed] [Google Scholar]
- 30.Lefaucheur C, Nochy D, Andrade J, Verine J, Gautreau C, Charron D, Hill GS, Glotz D, Suberbielle-Boissel C: Comparison of combination Plasmapheresis/IVIg/anti-CD20 versus high-dose IVIg in the treatment of antibody-mediated rejection. Am J Transplant 9: 1099–1107, 2009 [DOI] [PubMed] [Google Scholar]
- 31.Aubert O, Loupy A, Hidalgo L, Duong van Huyen JP, Higgins S, Viglietti D, Jouven X, Glotz D, Legendre C, Lefaucheur C, Halloran PF: Antibody-mediated rejection due to preexisting versus de novo donor-specific antibodies in kidney allograft recipients. J Am Soc Nephrol 28: 1912–1923, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lefaucheur C, Nochy D, Hill GS, Suberbielle-Boissel C, Antoine C, Charron D, Glotz D: Determinants of poor graft outcome in patients with antibody-mediated acute rejection. Am J Transplant 7: 832–841, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Food and Drug Administration: Guidance for industry: Enrichment strategies for clinical trials to support approval of human drugs and biological products (draft guidance), 2012. Available at: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM332181.pdf. Accessed May 16, 2017
- 34.Loupy A, Haas M, Solez K, Racusen L, Glotz D, Seron D, Nankivell BJ, Colvin RB, Afrouzian M, Akalin E, Alachkar N, Bagnasco S, Becker JU, Cornell L, Drachenberg C, Dragun D, de Kort H, Gibson IW, Kraus ES, Lefaucheur C, Legendre C, Liapis H, Muthukumar T, Nickeleit V, Orandi B, Park W, Rabant M, Randhawa P, Reed EF, Roufosse C, Seshan SV, Sis B, Singh HK, Schinstock C, Tambur A, Zeevi A, Mengel M: The Banff 2015 kidney meeting report: Current challenges in rejection classification and prospects for adopting molecular pathology. Am J Transplant 17: 28–41, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D; Modification of Diet in Renal Disease Study Group : A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Ann Intern Med 130: 461–470, 1999 [DOI] [PubMed] [Google Scholar]
- 36.Vickers AJ, Elkin EB: Decision curve analysis: A novel method for evaluating prediction models. Med Decis Making 26: 565–574, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vickers AJ, Cronin AM, Elkin EB, Gonen M: Extensions to decision curve analysis, a novel method for evaluating diagnostic tests, prediction models and molecular markers. BMC Med Inform Decis Mak 8: 53, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.