Abstract
The modern immunosuppression regimen has greatly improved short-term allograft outcomes but not long-term allograft survival. Complications associated with immunosuppression, specifically nephrotoxicity and infection risk, significantly affect graft and patient survival. Inducing and understanding pathways underlying clinical tolerance after transplantation are, therefore, necessary. We previously showed full donor chimerism and immunosuppression withdrawal in highly mismatched allograft recipients using a bioengineered stem cell product (FCRx). Here, we evaluated the gene expression and microRNA expression profiles in renal biopsy samples from tolerance-induced FCRx recipients, paired donor organs before implant, and subjects under standard immunosuppression (SIS) without rejection and with acute rejection. Unlike allograft samples showing acute rejection, samples from FCRx recipients did not show upregulation of T cell– and B cell–mediated rejection pathways. Gene expression pathways differed slightly between FCRx samples and the paired preimplantation donor organ samples, but most of the functional gene networks overlapped. Notably, compared with SIS samples, FCRx samples showed upregulation of genes involved in pathways, like B cell receptor signaling. Additionally, prediction analysis showed inhibition of proinflammatory regulators and activation of anti-inflammatory pathways in FCRx samples. Furthermore, integrative analyses (microRNA and gene expression profiling from the same biopsy sample) identified the induction of regulators with demonstrated roles in the downregulation of inflammatory pathways and maintenance of tissue homeostasis in tolerance-induced FCRx samples compared with SIS samples. This pilot study highlights the utility of molecular intragraft evaluation of pathways related to FCRx-induced tolerance and the use of integrative analyses for identifying upstream regulators of the affected downstream molecular pathways.
Keywords: kidney transplantation, tolerance, kidney biopsy, transcriptional profiling, Immunology and pathology
Utilization of more effective immunosuppression (IS) has been successful at inhibiting acute rejection and improved the short-term outcome of allogeneic organ transplantation.1 However, the required lifelong use of IS leads to significant morbidities, including nephrotoxicity, infection, malignancies, and cardiovascular disease, thus adversely affecting the long-term survival of both graft and recipient.2–5 Ten-year survival of living donor kidney allografts is only about 48%,6 and attempts at reducing the burden of IS have not been particularly fruitful to date.5 Therefore, a safe and reliable approach to induce allograft tolerance remains an important objective for organ transplantation.
It has been known for over 60 years that hematopoietic stem cell chimerism is associated with tolerance to transplanted tissues and cells.7 Application of this approach has recently been reported by a number of transplant centers.8–18 Except for the few patients who had received HLA-identical bone marrow transplants, became fully chimeric, and subsequently received kidney transplants,2,19,20 no fully chimeric and tolerant patients from HLA disparate and unrelated donors have been reported until recently.
We have shown that full donor chimerism and total IS withdrawal can be attained with minimal toxicity in highly mismatched related and unrelated kidney allograft recipients.12–14,17,18 This was achieved through the use of a bioengineered stem cell product containing donor hematopoietic stem cells and a unique population of cells, termed facilitating cells (the total product termed FCRx), accompanied by reduced intensity conditioning. Aside from this, only a limited number of kidney transplant recipients have been reported over the past two decades as being operationally tolerant (i.e., off all IS), mostly through nonadherence, and retaining allograft function. Therefore, it is paramount that the mechanisms by which these patients have developed donor-specific tolerance be analyzed. In this report, we have evaluated the characteristic molecular signatures that portray the graft with FCRx-induced tolerance.
Results
Patient Demographics and Samples
Three groups of patients with kidney transplants distributed into those who were under FCRx-induced tolerant protocol (FCRx; n=7), diagnosed with rejection (R; n=10), and without acute rejection but under standard immunosuppression (SIS; n=10; six samples were used in microarray and four were used in quantitative RT-PCR [qRT-PCR] to compare in both assays against FCRx) were evaluated. Additional information on the patients is given in Table 1 and Supplemental Material. The biopsy samples were also compared against paired normal kidney preimplantation allograft biopsy samples from FCRx (preimplantation donor [D]; n=5) and SIS (SISD; n=2).
Table 1.
Patient characteristics at baseline
| Patient ID | Age at Tx, yr | Sex | Race | Cause of ESRD | Type of Tx | HLA Mismatch | PRA before Tx | Induction | Time to Biopsy, mo after Tx | Rejection in Biopsy | IS at Time of Biopsy | Creatinine at Biopsy |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R | ||||||||||||
| R1 | 36 | M | Hisp | DM | SPK | 3/6 | 34, 0 | Al | 11 | 1B ACR | FK/MMF | 1.25 |
| R2 | 35 | M | Hisp | MPGN | LRD | 4/6 | 15, 53 | Al | 4 | 1B ACR | FK/MMF | 2.1 |
| R4 | 22 | M | Cau | MCD | LRD | 3/6 | 0, 0 | Al | 6 | 2B ACR | FK/MMF | 1.44 |
| R8 | 72 | F | AA | HTN | CKT | 5/6 | 68, 60 | Al | 0.4 | 2A ACR | FK/MMF | 0.98 |
| R9 | 51 | M | AA | IgAN | LURD | 6/6 | 0, 0 | Simulect | 0.2 | 2A ACR | FK/MMF/Pred | 7.2 |
| R11 | 45 | M | AA | HTN | LRD | 2/6 | 0, 0 | Al | 9 | 1B ACR | FK/MMF | 1.1 |
| R12 | 39 | M | Cau | PKD | CKT | 3/6 | 67, 16 | Al | 3 | 1B ACR | FK/MMF | 0.9 |
| R13 | 41 | F | Cau | DM | LRD | 4/6 | 14, 0 | Al | 10 | 2B ACR | FK/MMF | 0.7 |
| R14 | 43 | F | AA | HTN/DM | CKT | 5/6 | 24, 1 | Al | 6 | 2B ACR | FK/MMF | 1.56 |
| R15 | 53 | M | Cau | HTN | CKT | 4/6 | 45, 12 | Al | 5 | 2A ACR | FK/MMF | 0.76 |
| SIS | ||||||||||||
| SIS1 | 39 | F | Hisp | PKD | LRD | 5/6 | 0, 0 | Al | 12 | None | FK/MMF | 0.75 |
| SIS2 | 56 | F | Cau | PKD | LRD | 3/6 | 0, 0 | Al | 12 | None | FK/MMF | 1.08 |
| SIS3 | 66 | F | Cau | Sclero-derma | LURD | 4/6 | 32, 41 | Al | 12 | None | FK/MMF/Pred | 0.98 |
| SIS4 | 55 | M | Cau | HTN | LURD | 2/6 | 0, 0 | Al | 12 | None | FK/MMF | 1.44 |
| SIS5 | 68 | F | Hisp | Unknown | LURD | 6/6 | 0, 39 | Al | 12 | None | FK/MMF | 0.86 |
| SIS8 | 62 | F | Cau | DM | CKT | 0/6 | 94, 31 | Simulect | 12 | None | FK/MMF | 1.0 |
| SIS6a | 23 | F | AA | DM | SPK | 6/6 | 60, 0 | Al | 13 | None | FK/MMF | 1.55 |
| SIS7a | 67 | M | Cau | HTN | LURD | 3/6 | 12, 0 | Al | 12 | None | FK/MMF | 1.4 |
| SIS9a | 62 | M | Cau | PKD | LURD | 5/6 | 0, 0 | Al | 11 | None | FK/MMF | 1.3 |
| SIS10a | 55 | M | Hisp | DM | CKT | 3/6 | 0, 0 | Al | 12 | None | FK/MMF | 1.2 |
| Stem cell | ||||||||||||
| FCRx1 | 46 | F | Cau | PKD | LURD | 5/6 | 15, 0 | None | 12 | None | None | 0.95 |
| FCRx2 | 42 | M | Cau | PKD | LURD | 5/6 | 23, 0 | None | 25 | None | None | 1.35 |
| FCRx3 | 39 | F | Cau | Reflux | LURD | 2/6 | 9, 0 | None | 12 | None | None | 0.87 |
| FCRx4 | 40 | M | Cau | Chronic GN | LURD | 5/6 | 0, 0 | None | 24 | None | None | 1.35 |
| FCRx5 | 44 | M | AA | IgAN | LRD | 3/6 | 0, 0 | None | 25 | None | None | 1.48 |
| FCRx6 | 46 | M | Cau | PKD | LRD | 4/6 | 4, 4 | None | 13 | None | None | 1.53 |
| FCRx7 | 19 | F | Asian | MPGN | LRD | 2/6 | 0, 0 | None | 12 | None | None | 1 |
ID, identification; Tx, transplant; PRA, panel reactive antibody; M, man; Hisp, Hispanic; DM, diabetes mellitus; SPK, simultaneous kidney pancreas transplant; Al, alemtuzumab (campath-1h); ACR, acute cellular rejection; FK, tacrolimus; MMF, mycophenolate mofetil or mycophenolic acid; MPGN, membranoproliferative GN; LRD, living related donor kidney transplant; Cau, white; MCD, medullary cystic disease; F, woman; AA, black; HTN, hypertension; CKT, cadaveric kidney transplant; IgAN, IgA nephropathy; LURD, living unrelated donor kidney transplant; Pred, prednisone; PKD, polycystic kidney disease.
Samples used for independent validation.
Validation of FFPE versus Fresh Frozen Samples
We validated our microarray assays using paired FFPE and frozen biopsies and obtained comparable results (Supplemental Figures 1–3). Hence, subsequent studies of differentially expressed genes (DEGs) were performed with FFPE samples.
DEGs in Tolerant (FCRx) versus Acutely Rejecting Transplants
From the comparison of the two sample sets (FCRx versus R), a total of 1713 differentially expressed probe sets (619 upregulated and 1094 downregulated) were observed (false discovery rate <0.05 and fold change [FC] ≥1.5). The top canonical pathways downregulated in FCRx and upregulated in R were associated with immune response spanning cellular differentiation, antigen presentation, cytokine signaling, and effector functions (Table 2). Specifically, principal pathways downregulated (FC=−11.3 to −2.7) in the FCRx group included antigen presentation pathway (i.e., HLA-A, HLA-B, B2M, CD74, CIITA, HLA-DRB1, TAP1, and TAP2) (Supplemental Figure 4A), graft-versus-host disease signaling (i.e., CD86, multiple HLA genes, PRF1), IFN signaling (IFIT2, IFNGR1, IRF1, and OAS1), allograft rejection signaling (CD40, CD86, PRF1, TRGV9, and FAS) (Supplemental Figure 4B), CD28 signaling in T helper cells (CD4, FCEGR1G, LCP2, CD3E, and TRGV9), and dendritic cell maturation (FCER1G and HLA-DR) among others. Top downregulated genes included those for immunologic diseases (P value range 8.89E-72 to 2.80E-18; including 650 DEGs), inflammatory response (P value range 43.42E-71 to 46E-18; including 575 DEGs), and inflammatory disease (P value range 3.37E-60 to 1.64E-18; with 423 DEGs). Conversely, a small number of signaling pathways were upregulated in FCRx samples, with PPARα/RXRα activation, ketogenesis, and glutathione-mediated detoxification being the top ones, suggestive of active immune regulation and cellular homeostasis.
Table 2.
Downregulated canonical pathways associated with the FCRx group against rejection
| Ingenuity Canonical Pathways | Present Molecules (%) | Downregulated | Upregulated | P Value |
|---|---|---|---|---|
| Antigen presentation pathway | 25/38 (66) | 25 | 0 | <0.001 |
| Type 1 diabetes mellitus signaling | 40/110 (37) | 36 | 4 | <0.001 |
| Graft-versus-host disease signaling | 19/48 (40) | 19 | 0 | <0.001 |
| Autoimmune thyroid disease signaling | 19/47 (40) | 19 | 0 | <0.001 |
| Allograft rejection signaling | 22/84 (26) | 22 | 0 | <0.001 |
| Cdc42 signaling | 37/167 (22) | 35 | 2 | <0.001 |
| IFN signaling | 12/36 (33) | 12 | 0 | <0.001 |
| Dendritic cell maturation | 45/190 (24) | 41 | 4 | <0.001 |
| Crosstalk between dendritic cells and natural killer cells | 30/89 (34) | 30 | 0 | <0.001 |
| OX40 signaling pathway | 24/89 (27) | 24 | 0 | <0.001 |
| CD28 signaling in T helper cells | 19/118 (16) | 19 | 0 | <0.001 |
| T helper cell differentiation | 15/71 (21) | 15 | 0 | <0.001 |
| Fcγ receptor–mediated phagocytosis in macrophages and monocytes | 14/93 (15) | 14 | 0 | <0.001 |
| Remodeling of epithelial adherence junctions | 12/68 (18) | 12 | 0 | <0.001 |
| Regulation of actin-based motility by Rho | 13/91 (14) | 13 | 0 | <0.001 |
Present molecules for each canonical pathway are broken down as downregulated and upregulated. Representative percentages of present versus total molecules per canonical pathways are shown between parentheses.
DEGs in Biopsies from FCRx versus SIS Recipients
A comparison analysis was performed between FCRx and stable SIS renal allograft biopsies to identify specific molecular pathways associated with induced tolerance in the kidney graft. From this analysis, a molecular profile of 1509 probe sets representing 1372 DEGs (529 downregulated and 843 upregulated) was identified (P≤0.05; FC≥1.5). The top upregulated genes (FC>2.0) in FCRx samples include LFNG, APBA3, PARP12, HK1, and NR2F2, whereas downregulated genes (FC>3.5) include ENPEP, GATM, SLC5A12, KL, and ITM2B. Top network functions associated with these genes included cellular assembly and organization, nervous system development and function, and cell death and survival (score =44); molecular transport, RNA trafficking, and RNA post-transcriptional modification (score =42); and cell death and survival, embryonic development, and cellular development (score =42).
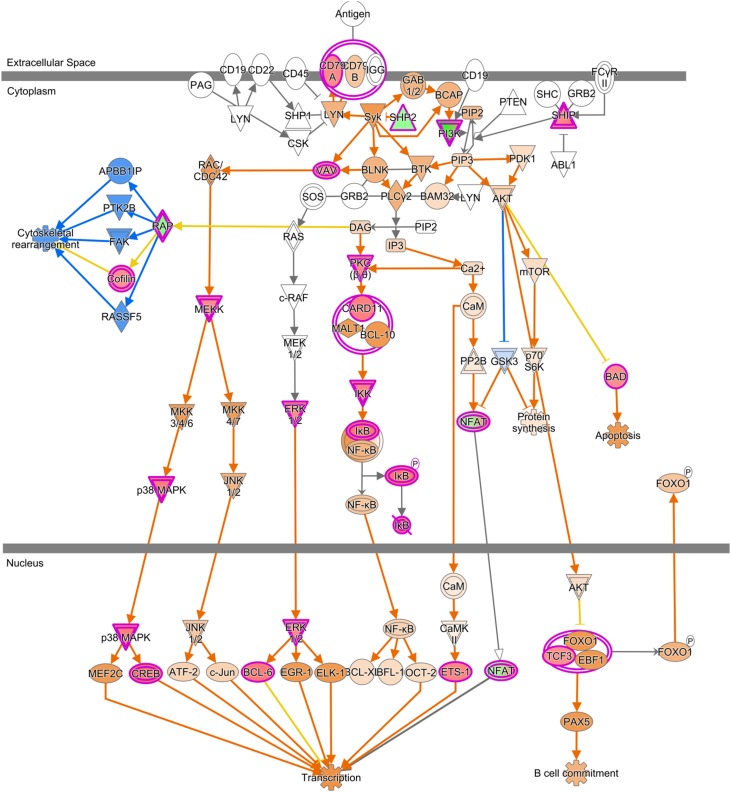
The analysis of canonical pathways associated the DEGs in FCRx versus SIS showed 158 significant signaling cascades (P<0.05). Among these, B cell receptor signaling was identified as one of the top significant canonical pathways with positive predicted activation (P<0.001); the upregulated genes included CD79A, BAD, CFL1, CREB3, ETS1, MAP3K3, MAPK3, MAPK11, MAPK12, NFATC4, NFKBIA, PIK3CD, INPP5J, BCL6, CARD10, IKBKB, PRKCQ, INPPL1, VAV2, FGFR1, and TCF3 (Figure 1). The downstream molecular analysis predicted positive transcriptional activity and therefore, pathway activation in tolerant samples (z score =1.633). The list of the top ten significant canonical pathways (z score >1.6) is shown in Table 3.
Figure 1.
Activation of the B cell receptor signaling canonical pathway in FCRx versus SIS: a schematic representation. Red indicates upregulation, and green indicates downregulation. Prediction of pathway activity is represented in blue (inhibition) and orange (activation). Color gradient intensities indicate magnitudes for expression and activation trends.
Table 3.
Top significant canonical pathways differentially regulated between FCRx and SIS
| Ingenuity Canonical Pathways | Present Molecules (%) | Downregulated | Upregulated | P Value |
|---|---|---|---|---|
| B cell receptor signaling | 25/190 (13.2) | 4 | 21 | <0.001 |
| Protein kinase A signaling | 41/396 (10.3) | 16 | 25 | <0.001 |
| iNOS signaling | 9/45 (20) | 1 | 8 | 0.002 |
| SAPK/JNK signaling | 15/104 (14.5) | 6 | 9 | 0.002 |
| Mouse embryonic stem cell pluripotency | 15/106 (14.1) | 3 | 12 | 0.002 |
| 4–1BB signaling in T lymphocytes | 7/32 (21.9) | 1 | 6 | 0.003 |
| CD27 signaling in lymphocytes | 9/53 (17) | 3 | 6 | 0.005 |
| LPS/IL-1–mediated inhibition of RXR function | 23/221 (10.4) | 15 | 8 | 0.01 |
| Dopamine-DARPP32 feedback in cAMP signaling | 18/163 (11) | 7 | 11 | 0.01 |
| IL-22 signaling | 5/24 (20.8) | 0 | 5 | 0.01 |
Present molecules for each canonical pathway are broken down as downregulated and upregulated. Representative percentages of present versus total molecules per canonical pathways are shown between parentheses. iNOS, inducible nitric oxide synthase; SAPK, stress-activated protein kinases; JNK, jun amino-terminal kinases.
Analysis of the immune-related cellular functions that were upregulated in FCRx revealed predicted activation of immune functions of proliferation, survival, and recruitment in T cells, pro-B cells, and granulocytes (Supplemental Table 1). The top predicted upstream activators with activation z score >2 but significant overlapping (P<0.05) included EIF4E, IFNA2, and TGFB1 (Supplemental Table 2A). Additionally, the analysis of top regulatory networks identified 20 regulator complexes with positive consistency scores in FCRx; they and their functional correlates are listed in Supplemental Table 2B.
Additionally, cell type enrichment analysis21 showed that the DEGs in the FCRx recipients were augmented in kidney cells and immune cells, like B cells, T cells, and BDCA4+ dendritic cells (Supplemental Table 3).
Molecular Profile Associated with Induction of Tolerance—Comparison with Baseline Preimplantation Donor Tissue
A gene expression analysis was performed comparing paired renal biopsy samples collected at preimplantation (D) and then after establishing chimerism and tolerance (FCRx). Twenty of 23 functional networks for DNA replication, recombination, repair, energy production, and nucleic acid metabolism were found to be overlapped between FCRx and D samples (not shown). However, the analysis also identified 355 probe sets representing 327 DEGs, with 143 genes downregulated and 184 genes upregulated in FCRx samples (P<0.001; FCminimal±1.5). Gene ontology and pathway analysis identified eight associated network functions (Table 4). The top canonical pathways associated with tolerance induction included inosine-5′-phosphate biosynthesis II (P<0.001), telomere extension by telomerase (P=0.001), and axonal guidance signaling (P=0.003). The prediction analysis for upstream regulators showed inhibition of a number of effector molecules in FCRx samples (Table 5). Analysis using the database for annotation, visualization, and integrated discovery22 showed that many of the DEGs were involved, especially in glycan biosynthesis and metabolism (Supplemental Figure 5). Cell type enrichment analysis revealed augmentation of CD34+ cells and CD19+ B cells among other immune cell types (Supplemental Table 4).
Table 4.
Associated network functions in tolerance compared with paired donor samples
| Top Associated Network Functions | Score | Focus Molecules |
|---|---|---|
| Carbohydrate metabolism, lipid metabolism, post-translational modification | 50 | 29 |
| Embryonic development, organismal development, tissue morphology | 50 | 29 |
| Cellular assembly and organization, neurologic disease, cell death and survival | 36 | 23 |
| Cellular development, hematologic system development and function, hematopoiesis | 32 | 21 |
| Connective tissue disorders, developmental disorder, respiratory disease | 29 | 20 |
| Cell morphology, developmental disorder, gastrointestinal disease | 29 | 20 |
| Cell death and survival, cellular development, dermatological diseases and conditions | 29 | 20 |
| Cell death and survival, embryonic development, gene expression | 27 | 19 |
Table 5.
Predicted activity of upstream regulators in tolerant FCRx samples versus D biopsies
| Upstream Regulator | Predicted Activity | Activation z Score | P Value of Overlap |
|---|---|---|---|
| MYD88 | Inhibited | −2.952 | <0.001 |
| EGF | Inhibited | −2.930 | <0.001 |
| TLR3 | Inhibited | −2.574 | <0.001 |
| TNF | Inhibited | −2.244 | <0.001 |
| IL13 | Inhibited | −2.003 | 0.002 |
| TLR9 | Inhibited | −2.397 | 0.002 |
| PTGS2 | Inhibited | −2.540 | 0.003 |
| NF-kB (complex) | Inhibited | −2.132 | 0.003 |
| GDNF | Inhibited | −2.182 | <0.01 |
| PDGF BB | Inhibited | −2.397 | <0.01 |
| SFTPA1 | Activated | 2.236 | <0.01 |
| CD24 | Activated | 2.000 | <0.01 |
| Tlr | Inhibited | −2.408 | 0.01 |
| TLR4 | Inhibited | −2.236 | 0.01 |
| ERK | Inhibited | −2.275 | 0.01 |
| IGF1 | Inhibited | −3.333 | 0.02 |
| FOSL1 | Activated | 2.000 | 0.02 |
| Cg | Inhibited | −2.227 | 0.03 |
| Creb | Inhibited | −2.156 | 0.03 |
| TLR2 | Inhibited | −2.195 | 0.03 |
| EGFR | Inhibited | −2.746 | 0.04 |
| PI3K (complex) | Inhibited | −2.014 | 0.04 |
| IRF8 | Inhibited | −2.195 | 0.05 |
S-score analyses of two SIS grafts and their paired preimplantation donor biopsies (SIS1 versus D6 and SIS2 versus D7) showed that 50 genes (70 probe sets) and 65 genes (84 probe sets) were significantly differentially expressed in SIS1 versus D6 and SIS2 versus D7, respectively. Twenty-one of these DEGs were common in both individual comparisons (Supplemental Table 5), whereas 29 and 44 genes were unique for SIS1 versus D6 and SIS2 versus D7, respectively (Supplemental Figure 6).
DEGs in FCRx: Multifactorial Comparison with D, SIS, and R
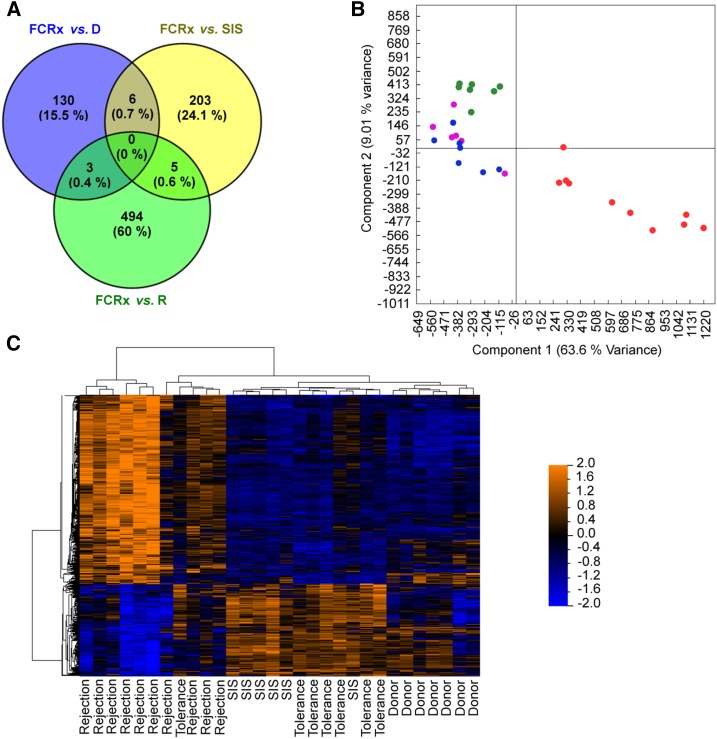
A comparison of the gene expression profiles of the FCRx against paired donors (D), SIS, and R sample groups using a ±2.0 (P value =0.001) FC cutoff resulted in the Venn diagram shown in Figure 2A. Additionally, using DEGs between FCRx and R (because R represents the extreme negative signature opposite to tolerance), a principal component analysis was used for classifying samples (Figure 2B). Figure 2B shows that (1) there was high homogeneity among R samples, (2) there were some levels of heterogeneity among D and FCRx samples, and (3) FCRx samples have more alike with D than R samples. Similar findings were observed when the same set of DEGs was used in a supervised cluster analysis to evaluate how samples (including SIS samples) associate together (Figure 2C).
Figure 2.
Comparison analyses among DEGs stratifying FCRx as being in between D and SIS, and distinctly different from rejection (R) samples. (A) Venn diagram including differential probe sets from each pairwise comparison. (B) Principal component analysis using DEGs from FCRx versus R comparison. (C) Supervised hierarchical clustering analysis.
Confirmation of DEGs Identified by SensationPlus with RT-qPCR Assays
We further validated the results from Sensation Plus assays described above by performing Taqman qRT-PCR assays for the relative levels of the top three DEGs (Supplemental Figure 7).
Distinct Sets of MicroRNAs Are Highly Expressed in FCRx Biopsies
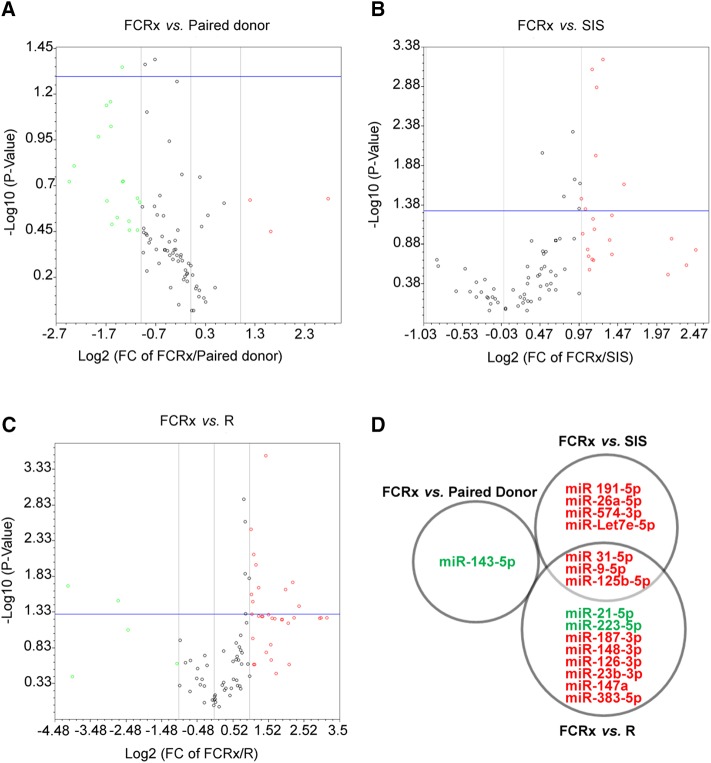
Because microRNAs (miRNAs) are important regulatory elements in gene expression, their profiles were tested in renal biopsies from FCRx, SIS, and R as well as in paired D (Figure 3). Compared with all other groups, the biopsies from the patients on FCRx showed unique upregulation of three miRNAs: miR-31–5p, miR-9–5p, and miR-125b-5p (Figure 3D). In addition, FCRx biopsies, when compared with SIS and R groups, showed upregulation of four (miR-5p, miR-26a-5p, miR574–3p, and miR-Let-7e-5p) and six (miR-187–3p, miR-148–3p, miR-126–3p, miR-23b-3p, miR-147a, and miR-383–5p) miRNAs, respectively. miR-21–5p and miR-223–5p were downregulated in FCRx in comparison with R and in miR-143–5p in comparison with paired D biopsies. The significance of these miRNA expressions was established by integrative network analysis (below).
Figure 3.
Differential expression of miRNA demonstrating unique profile in FCRx, when compared to D, SIS and R groups. (A) Volcano plot showing differential expression of miRNA between FCRx and paired donor groups. (B) Volcano plot showing differential expression of miRNA between FCRx and SIS. (C) Volcano plot showing differential expression of miRNA between FCRx and R. (D) Figure showing miRNAs that are differentially expressed in FCRx biopsies versus other indicated groups (i.e., miR-31–5p, miR-9–5p, and miR125b-5p are uniquely upregulated in FCRx); other miRNAs shown in red are upregulated and those shown in green are downregulated in FCRx when compared with indicated groups.
Integration of miRNA and Gene Expression Profiles in FCRx Group
Integration analysis of the differentially expressed miRNAs showed a corresponding differential expression of their target genes in the biopsies. The upregulated miRNAs in FCRx in comparison with SIS group (Supplemental Figure 8) had their target genes involved in (1) inflammatory pathways (NFATC3, MAF, PIK3C2A, and ITGAV), (2) metabolism (GATM, SUCLG2, and GCLM), and (3) mitochondrial fatty acid oxidation (ACADM and ALDH1B1). Similarly, the miRNAs upregulated in FCRx versus R biopsies (Supplemental Figure 9) inhibited their target genes involved in various inflammatory pathways, like antigen presentation, cytotoxic T cell–mediated apoptosis of target cells, NF-kB signaling, dendritic cell maturation, and various cytokine signaling pathways. A few of the genes from these pathways that were downregulated in FCRx and part of integrated dataset included HLA-DOA, TAP2, STAT3, CASP8, PYCARD, PRF-1, and TNFRSF1B.
Discussion
Development of tolerance protocols requires assays or biomarkers that distinguish tolerant from nontolerant recipients to be established. Also, understanding the plausible mechanisms of tolerance by identifying key associated molecular pathways is necessary for progression in the field. Herein, molecular pathways in the allografts of a unique set of renal transplant recipients who display induced tolerance (with high level of donor chimerism) after FCRx treatment protocol and successful weaning of IS are shown. DEGs in biopsies from subjects under SIS without rejection and with R as well as from D biopsies were compared using microarray-based gene expression assay on FFPE tissue RNA (Sensation Plus). This method was previously used exclusively in cancer-related studies23–25 and showed a significant analytic performance correlation with classic gene expression microarray assays. Significant differences in gene expression profiles were observed among the three patient groups, with the FCRx biopsies showing a distinct profile. We also observed a distinct miRNA profile among the same renal biopsies and used integrative approaches to show their possible role as upstream regulators of differential gene expression.
Our first comparison between FCRx and R samples, as expected, showed major differences in immune pathways with activated antigen presentation, immune cell trafficking, and inflammatory responses in the R samples as described previously.26,27 Although these differences were anticipated, it could be concluded from this analysis that, in addition to absence of clinical acute rejection as reflected by normal histologic findings, there was no molecular evidence of subclinical alloactivation after weaning from IS in the FCRx tolerance–induced samples.
The comparison of FCRx with SIS samples showed a comparatively smaller set of DEGs indicative of slightly different mechanisms by which tolerance is achieved through active immune regulation. Enrichment analysis indicated that the downregulated genes, mostly of metabolic function, were within the FCRx kidney cells, indicating use of tissue-specific protective mechanisms. For instance, the downregulated genes included SLC5A12 and ENPEP, which were reported to be associated with kidney injury.28,29 SLC5A12 had been shown to increase reabsorption of monocarboxylates, which have utilization that is involved renal acidosis and calcineurin inhibitor toxicity.28 Conversely, enrichment analysis of upregulated genes in FCRx versus SIS showed augmentation in immune cell subsets, particularly of B cell–related pathways. Upregulation of signaling pathways mediated by B cell receptor, APRIL, PI3k, and B cell activating factor was suggestive of active immune regulation in B cells, supporting a role of development, survival (antiapoptosis), and commitment of B cells in the FCRx samples. Similarly, FLT3 signaling pathway upregulation is indicative of activation of BDC4A+ plasmacytoid dendritic cells,30 which were also identified as enriched in FCRx recipients. These plasmacytoid dendritic cells are known to induce regulatory T and B cells critical for immune homeostasis.31,32 Also, the predicted activation of anti-inflammatory pathways (RAR activation) and the inhibition of proinflammatory upstream regulators (NF-kB complex) were observed in FCRx versus SIS group. Integration of ex vivo miRNA and gene expression profiles also supported their mechanistic role in achieving metabolic and immune homeostasis in patients on FCRx in the absence of IS regimen.
It is reasonable to expect a steady state of gene expression for functional operations in the normal kidney (i.e., D samples) and a comparable state in the same organ after development of transplant tolerance (i.e., in the FCRx biopsies). However, it is interesting to note that some differences were observed in the gene expression profile between the FCRx samples versus paired kidney D biopsies. Thus, on the one hand, 20 out of 23 cellular functional networks were found to be overlapped, and on the other hand, 143 genes, primarily those associated with innate immunity and inflammatory pathways, were downregulated in the FCRx samples. This would suggest that active regulatory/suppressive mechanisms are occurring in the transplanted and tolerant kidneys, similar to those that we observed in another group of nonchimeric-induced tolerant HLA-identical kidney transplant recipients.11,33 Additionally, the enrichment of CD34+ cell–specific genes is consistent with the notion that the CD34+ cells used for the tolerance induction maybe homing to the allograft. Similar enrichment of genes of glycan biosynthesis pathway is suggestive of their crucial role in cell adhesion, differentiation, cell survival, and signal transduction processes.34
Several studies have shown that tolerance (induced, operational, immunologic, or “prope”) was accomplished through activation and differentiation of B cell subtypes and regulatory T cells.11,35–42 However, these studies were mostly limited to analysis of gene expression profiles in the PBMCs. Specifically, for operationally tolerant kidney transplant recipients, cellular and molecular profiles associated with B and T cells populations were reported exclusively in peripheral blood.11,36,39,43 Brouard et al.44 reported a set of 33 genes preferentially related to T cell activation and the TGF-α signaling pathway. Newell et al.36 proposed a three-gene signature (IGKV1D-13, IGKV4–1, and IGLL1) that may predict tolerance in operationally tolerant kidney transplant recipients and then further corroborated this in an independent study.45 Although the above three gene–based signature was not observed in these gene array studies performed on the renal allografts, induction of tolerance in FCRx recipients was also found to be associated with modulation of molecular mechanisms involved in activation and survival of B cells. Deregulated B cell molecular pathways in peripheral blood were also shown to correlate with increases in total B cells and subpopulations of B cells (mainly naïve and transitional) in most operationally tolerant kidney transplant recipients.33,36,39,46 These changes together with amplified regulatory T cells have been proposed as a crucial component for induction of tolerance.33,42 Interim results from the current cohort of FCRx also showed an increase from baseline in the circulating regulatory T cells and B cells through 5 years of transplantation.13
We have shown for the first time the molecular pathways occurring in the allografts of patients with FCRx-induced tolerance. Although the study is restricted in sample size, a common limitation in transplant tolerance studies, we have used stringent cutoffs for the molecular analysis and well selected patient groups. The extended analysis using various comparisons with preimplant biopsies, biopsies with R, and those on SIS helped better understand the possible molecular pathways in this unique set of patient biopsy samples. Moreover, this is the first study using integration of two biologic layers (mRNA and miRNA) to better understand regulation of pathway’s expression. Although using FFPE samples might entail to loss of certain information when compared with samples stored in RNALater, the insights from this study together with further longitudinal analysis of the patient biopsies will help understanding of molecular pathways and signatures that need to be monitored to track continuation of tolerance in these patients.
Concise Methods
Gene Expression Profiling
Total RNA was isolated from FFPE renal biopsy samples. Briefly, three sections of 10-μm thickness were obtained from each FFPE block, and total RNA was isolated using the High Pure RNA Paraffin Kit (Roche) following the manufacturer’s instructions. Then, the total RNA was labeled with SensationPlus FFPE Amplification and WT Labeling Kit SensationPlus and used for Affymetrix GeneChip HG-U133 2.0 microarray hybridization. Normalized signals were generated using RMA. Pairwise ANOVAs were performed among study groups (including FCRx versus R, FCRx versus SIS, and FCRx versus D) to identify DEGs. S-score method was used for the identification of significantly and differentially expressed probe sets in SISD versus D comparisons (SIS1 versus D6 and SIS2 versus D7) from two individually analyzed renal transplant recipients.47 For statistical significance, a P value ≤0.05 was considered significant after a controlled false discovery rate of <5% and an FC of ≥1.5.
miRNA Profiling and Integration with Gene Expression
Part of the total isolated RNA from the FFPE renal biopsies was used for pathway-directed miRNA profiling. Eighty-four different miRNAs previously reported to be associated with immunopathology were studied using qRT-PCR arrays (n=12; Qiagen) and compared between FCRx and each of the remaining groups (paired donors, rejection, and SIS). The results were then analyzed using a web-based tool from SABiosciences. Volcano plots were obtained to report the significantly and differentially expressed miRNA.
Statistical Analyses
Data were analyzed as the mean±SD. Parametric (paired t tests) and nonparametric (Mann–Whitney U test/Wilcoxon signed rank test) tests were used among compared groups. Significance was established at two-sided α-levels of <0.05 using statistical software (SAS, Inc., Cary, NC).
Biologic Interpretation
Gene ontology and pathways analysis of gene expression profiles, identified as significantly differentially expressed, were performed using the Ingenuity Pathway Analysis (IPA) tool (www.ingenuity.com). Spreadsheets containing probe set identification and FC magnitudes were uploaded to the IPA tool. A P value of <0.05 was considered significant. Molecular and cellular functions activity trends were predicted and analyzed using IPA calculated z scores. Additionally, the database for annotation, visualization, and integrated discovery was used for pathway analysis,22 and CTen software was used for cell type enrichment analysis.21
Data obtained from the renal biopsy samples for miRNAs were analyzed by miRNA target profiling for data integration and to determine their differential effect on ex vivo gene expressions. Furthermore, the pathways and integrative networks in which the gene sets were involved were defined using either IPA or Panther analysis.
Validation of Gene Expression Profiling from FFPE Samples
The methods for paired comparisons of fresh frozen versus FFPE biopsies as well as microarrays versus qRT-PCR are described in Supplemental Material.
Disclosures
S.T.I. has equity interest in and is the chief executive officer of Regenerex, LLC, a startup biotechnology company. J.R.L. receives grant support from Regenerex, LLC and Novartis Pharmaceuticals. All other authors have no conflicts of interest to declare.
Supplementary Material
Acknowledgments
The FCRx subjects were enrolled in a clinical trial (trial registry FDA-IDE 13947; ClinicalTrials.gov identifier NCT00497926) supported, in part, by Regenerex, LLC and Novartis Pharmaceuticals.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2017030348/-/DCSupplemental.
References
- 1.Billingham RE, Brent L, Medawar PB: Actively acquired tolerance of foreign cells. Nature 172: 603–606, 1953 [DOI] [PubMed] [Google Scholar]
- 2.Spitzer TR, Delmonico F, Tolkoff-Rubin N, McAfee S, Sackstein R, Saidman S, Colby C, Sykes M, Sachs DH, Cosimi AB: Combined histocompatibility leukocyte antigen-matched donor bone marrow and renal transplantation for multiple myeloma with end stage renal disease: The induction of allograft tolerance through mixed lymphohematopoietic chimerism. Transplantation 68: 480–484, 1999 [DOI] [PubMed] [Google Scholar]
- 3.Kawai T, Cosimi AB, Spitzer TR, Tolkoff-Rubin N, Suthanthiran M, Saidman SL, Shaffer J, Preffer FI, Ding R, Sharma V, Fishman JA, Dey B, Ko DSC, Hertl M, Goes NB, Wong W, Williams WW Jr, Colvin RB, Sykes M, Sachs DH: HLA-mismatched renal transplantation without maintenance immunosuppression. N Engl J Med 358: 353–361, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kawai T, Sachs DH, Sykes M, Cosimi AB; Immune Tolerance Network : HLA-mismatched renal transplantation without maintenance immunosuppression. N Engl J Med 368: 1850–1852, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Millan MT, Shizuru JA, Hoffmann P, Dejbakhsh-Jones S, Scandling JD, Grumet FC, Tan JC, Salvatierra O, Hoppe RT, Strober S: Mixed chimerism and immunosuppressive drug withdrawal after HLA-mismatched kidney and hematopoietic progenitor transplantation. Transplantation 73: 1386–1391, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Gondos A, Döhler B, Brenner H, Opelz G: Kidney graft survival in Europe and the United States: Strikingly different long-term outcomes. Transplantation 95: 267–274, 2013 [DOI] [PubMed] [Google Scholar]
- 7.Scandling JD, Busque S, Dejbakhsh-Jones S, Benike C, Millan MT, Shizuru JA, Hoppe RT, Lowsky R, Engleman EG, Strober S: Tolerance and chimerism after renal and hematopoietic-cell transplantation. N Engl J Med 358: 362–368, 2008 [DOI] [PubMed] [Google Scholar]
- 8.Scandling JD, Busque S, Shizuru JA, Lowsky R, Hoppe R, Dejbakhsh-Jones S, Jensen K, Shori A, Strober JA, Lavori P, Turnbull BB, Engleman EG, Strober S: Chimerism, graft survival, and withdrawal of immunosuppressive drugs in HLA matched and mismatched patients after living donor kidney and hematopoietic cell transplantation. Am J Transplant 15: 695–704, 2015 [DOI] [PubMed] [Google Scholar]
- 9.Scandling JD, Busque S, Dejbakhsh-Jones S, Benike C, Sarwal M, Millan MT, Shizuru JA, Lowsky R, Engleman EG, Strober S: Tolerance and withdrawal of immunosuppressive drugs in patients given kidney and hematopoietic cell transplants. Am J Transplant 12: 1133–1145, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ciancio G, Burke GW, Garcia-Morales R, Suzart K, Rosen A, Ricordi C, Kenyon NS, Mathew JM, Tzakis AG, Esquenazi V, Miller J: Effect of living-related donor bone marrow infusion on chimerism and in vitro immunoregulatory activity in kidney transplant recipients. Transplantation 74: 488–496, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Leventhal JR, Mathew JM, Salomon DR, Kurian SM, Suthanthiran M, Tambur A, Friedewald J, Gallon L, Charette J, Levitsky J, Kanwar Y, Abecassis M, Miller J: Genomic biomarkers correlate with HLA-identical renal transplant tolerance. J Am Soc Nephrol 24: 1376–1385, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leventhal J, Abecassis M, Miller J, Gallon L, Ravindra K, Tollerud DJ, King B, Elliott MJ, Herzig G, Herzig R, Ildstad ST: Chimerism and tolerance without GVHD or engraftment syndrome in HLA-mismatched combined kidney and hematopoietic stem cell transplantation. Sci Transl Med 4: 124ra28, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leventhal JR, Elliott MJ, Yolcu ES, Bozulic LD, Tollerud DJ, Mathew JM, Konieczna I, Ison MG, Galvin J, Mehta J, Badder MD, Abecassis MMI, Miller J, Gallon L, Ildstad ST: Immune reconstitution/immunocompetence in recipients of kidney plus hematopoietic stem/facilitating cell transplants. Transplantation 99: 288–298, 2015 [DOI] [PubMed] [Google Scholar]
- 14.Leventhal J, Abecassis M, Miller J, Gallon L, Tollerud D, Elliott MJ, Bozulic LD, Houston C, Sustento-Reodica N, Ildstad ST: Tolerance induction in HLA disparate living donor kidney transplantation by donor stem cell infusion: Durable chimerism predicts outcome. Transplantation 95: 169–176, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thomas ED: The nobel lectures in immunology. The nobel prize for physiology or medicine, 1990. Bone marrow transplantation--past, present and future. Scand J Immunol 39: 339–345, 1994 [DOI] [PubMed] [Google Scholar]
- 16.Johnsen HE, Beatty PG, Michelson E, Hansen JA, Thomas ED: Donor alloreactivity may predict acute graft-versus-host disease in HLA-matched bone marrow transplantation for leukemia in early remission. Eur J Haematol 48: 249–253, 1992 [DOI] [PubMed] [Google Scholar]
- 17.Leventhal J, Miller J, Abecassis M, Tollerud DJ, Ildstad ST: Evolving approaches of hematopoietic stem cell-based therapies to induce tolerance to organ transplants: The long road to tolerance. Clin Pharmacol Ther 93: 36–45, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leventhal J, Huang Y, Xu H, Goode I, Ildstad ST: Novel regulatory therapies for prevention of Graft-versus-host disease. BMC Med 10: 48, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spitzer TR, Sykes M, Tolkoff-Rubin N, Kawai T, McAfee SL, Dey BR, Ballen K, Delmonico F, Saidman S, Sachs DH, Cosimi AB: Long-term follow-up of recipients of combined human leukocyte antigen-matched bone marrow and kidney transplantation for multiple myeloma with end-stage renal disease. Transplantation 91: 672–676, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fudaba Y, Spitzer TR, Shaffer J, Kawai T, Fehr T, Delmonico F, Preffer F, Tolkoff-Rubin N, Dey BR, Saidman SL, Kraus A, Bonnefoix T, McAfee S, Power K, Kattleman K, Colvin RB, Sachs DH, Cosimi AB, Sykes M: Myeloma responses and tolerance following combined kidney and nonmyeloablative marrow transplantation: In vivo and in vitro analyses. Am J Transplant 6: 2121–2133, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Shoemaker JE, Lopes TJ, Ghosh S, Matsuoka Y, Kawaoka Y, Kitano H: CTen: A web-based platform for identifying enriched cell types from heterogeneous microarray data. BMC Genomics 13: 460, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiao X, Sherman BT, Huang W, Stephens R, Baseler MW, Lane HC, Lempicki RA: DAVID-WS: A stateful web service to facilitate gene/protein list analysis. Bioinformatics 28: 1805–1806, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roberts SS, Leeborg N, Loriaux M, Johnson FL, Huang ML, Stenzel P, Thiede C, Godder KT: Acute graft-versus-host disease of the heart. Pediatr Blood Cancer 47: 624–628, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Pillai R, Deeter R, Rigl CT, Nystrom JS, Miller MH, Buturovic L, Henner WD: Validation and reproducibility of a microarray-based gene expression test for tumor identification in formalin-fixed, paraffin-embedded specimens. J Mol Diagn 13: 48–56, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koh SS, Opel ML, Wei JP, Yau K, Shah R, Gorre ME, Whitman E, Shitabata PK, Tao Y, Cochran AJ, Abrishami P, Binder SW: Molecular classification of melanomas and nevi using gene expression microarray signatures and formalin-fixed and paraffin-embedded tissue. Mod Pathol 22: 538–546, 2009 [DOI] [PubMed] [Google Scholar]
- 26.Einecke G, Melk A, Ramassar V, Zhu LF, Bleackley RC, Famulski KS, Halloran PF: Expression of CTL associated transcripts precedes the development of tubulitis in T-cell mediated kidney graft rejection. Am J Transplant 5: 1827–1836, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Reeve J, Einecke G, Mengel M, Sis B, Kayser N, Kaplan B, Halloran PF: Diagnosing rejection in renal transplants: A comparison of molecular- and histopathology-based approaches. Am J Transplant 9: 1802–1810, 2009 [DOI] [PubMed] [Google Scholar]
- 28.Becker HM, Mohebbi N, Perna A, Ganapathy V, Capasso G, Wagner CA: Localization of members of MCT monocarboxylate transporter family Slc16 in the kidney and regulation during metabolic acidosis. Am J Physiol Renal Physiol 299: F141–F154, 2010 [DOI] [PubMed] [Google Scholar]
- 29.Quesada A, Segarra AB, Montoro-Molina S, de Gracia MD, Osuna A, O’Valle F, Gómez-Guzmán M, Vargas F, Wangensteen R: Glutamyl aminopeptidase in microvesicular and exosomal fractions of urine is related with renal dysfunction in cisplatin-treated rats. PLoS One 12: e0175462, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maraskovsky E, Daro E, Roux E, Teepe M, Maliszewski CR, Hoek J, Caron D, Lebsack ME, McKenna HJ: In vivo generation of human dendritic cell subsets by Flt3 ligand. Blood 96: 878–884, 2000 [PubMed] [Google Scholar]
- 31.Rogers NM, Isenberg JS, Thomson AW: Plasmacytoid dendritic cells: No longer an enigma and now key to transplant tolerance? Am J Transplant 13: 1125–1133, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Swiecki M, Colonna M: The multifaceted biology of plasmacytoid dendritic cells. Nat Rev Immunol 15: 471–485, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leventhal JR, Mathew JM, Salomon DR, Kurian SM, Friedewald JJ, Gallon L, Konieczna I, Tambur AR, Charette J, Levitsky J, Jie C, Kanwar YS, Abecassis MM, Miller J: Nonchimeric HLA-identical renal transplant tolerance: Regulatory immunophenotypic/genomic biomarkers. Am J Transplant 16: 221–234, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li X, Li D, Pang X, Yang G, Deeg HJ, Guan F: Quantitative analysis of glycans, related genes, and proteins in two human bone marrow stromal cell lines using an integrated strategy. Exp Hematol 43: 760–769.e7, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Graca L: Transplantation tolerance: Context matters. Eur J Immunol 45: 1921–1925, 2015 [DOI] [PubMed] [Google Scholar]
- 36.Newell KA, Asare A, Kirk AD, Gisler TD, Bourcier K, Suthanthiran M, Burlingham WJ, Marks WH, Sanz I, Lechler RI, Hernandez-Fuentes MP, Turka LA, Seyfert-Margolis VL; Immune Tolerance Network ST507 Study Group : Identification of a B cell signature associated with renal transplant tolerance in humans. J Clin Invest 120: 1836–1847, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Newell KA, Asare A, Sanz I, Wei C, Rosenberg A, Gao Z, Kanaparthi S, Asare S, Lim N, Stahly M, Howell M, Knechtle S, Kirk A, Marks WH, Kawai T, Spitzer T, Tolkoff-Rubin N, Sykes M, Sachs DH, Cosimi AB, Burlingham WJ, Phippard D, Turka LA: Longitudinal studies of a B cell-derived signature of tolerance in renal transplant recipients. Am J Transplant 15: 2908–2920, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Newell KA, Phippard D, Turka LA: Regulatory cells and cell signatures in clinical transplantation tolerance. Curr Opin Immunol 23: 655–659, 2011 [DOI] [PubMed] [Google Scholar]
- 39.Sagoo P, Perucha E, Sawitzki B, Tomiuk S, Stephens DA, Miqueu P, Chapman S, Craciun L, Sergeant R, Brouard S, Rovis F, Jimenez E, Ballow A, Giral M, Rebollo-Mesa I, Le Moine A, Braudeau C, Hilton R, Gerstmayer B, Bourcier K, Sharif A, Krajewska M, Lord GM, Roberts I, Goldman M, Wood KJ, Newell K, Seyfert-Margolis V, Warrens AN, Janssen U, Volk H-D, Soulillou J-P, Hernandez-Fuentes MP, Lechler RI: Development of a cross-platform biomarker signature to detect renal transplant tolerance in humans. J Clin Invest 120: 1848–1861, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chesneau M, Pallier A, Braza F, Lacombe G, Le Gallou S, Baron D, Giral M, Danger R, Guerif P, Aubert-Wastiaux H, Néel A, Michel L, Laplaud DA, Degauque N, Soulillou JP, Tarte K, Brouard S: Unique B cell differentiation profile in tolerant kidney transplant patients. Am J Transplant 14: 144–155, 2014 [DOI] [PubMed] [Google Scholar]
- 41.Gökmen R, Hernandez-Fuentes MP: Biomarkers of tolerance. Curr Opin Organ Transplant 18: 416–420, 2013 [DOI] [PubMed] [Google Scholar]
- 42.Peters JH, Koenen HJ, Hilbrands LB, Joosten I: Immunotherapy with regulatory T cells in transplantation. Immunotherapy 1: 855–871, 2009 [DOI] [PubMed] [Google Scholar]
- 43.Lozano JJ, Pallier A, Martinez-Llordella M, Danger R, López M, Giral M, Londoño MC, Rimola A, Soulillou JP, Brouard S, Sánchez-Fueyo A: Comparison of transcriptional and blood cell-phenotypic markers between operationally tolerant liver and kidney recipients. Am J Transplant 11: 1916–1926, 2011 [DOI] [PubMed] [Google Scholar]
- 44.Brouard S, Mansfield E, Braud C, Li L, Giral M, Hsieh SC, Baeten D, Zhang M, Ashton-Chess J, Braudeau C, Hsieh F, Dupont A, Pallier A, Moreau A, Louis S, Ruiz C, Salvatierra O, Soulillou JP, Sarwal M: Identification of a peripheral blood transcriptional biomarker panel associated with operational renal allograft tolerance. Proc Natl Acad Sci U S A 104: 15448–15453, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moreso F, Torres IB, Martínez-Gallo M, Benlloch S, Cantarell C, Perelló M, Jimeno J, Pujol-Borrell R, Seron D: Gene expression signature of tolerance and lymphocyte subsets in stable renal transplants: Results of a cross-sectional study. Transpl Immunol 31: 11–16, 2014 [DOI] [PubMed] [Google Scholar]
- 46.Pallier A, Hillion S, Danger R, Giral M, Racapé M, Degauque N, Dugast E, Ashton-Chess J, Pettré S, Lozano JJ, Bataille R, Devys A, Cesbron-Gautier A, Braudeau C, Larrose C, Soulillou JP, Brouard S: Patients with drug-free long-term graft function display increased numbers of peripheral B cells with a memory and inhibitory phenotype. Kidney Int 78: 503–513, 2010 [DOI] [PubMed] [Google Scholar]
- 47.Kennedy RE, Archer KJ, Miles MF: Empirical validation of the S-Score algorithm in the analysis of gene expression data. BMC Bioinformatics 7: 154, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.