Bardoxolone methyl is a semisynthetic oleanane triterpenoid, and it is thought to act by promoting the resolution of inflammation via activation of Nrf-2 and inhibition of NF κ–light-chain enhancer of activated B cells.1 Initial phase 1 studies showed, as an incidental finding, that bardoxolone increased eGFR,2 which generated interest in its potential as a drug to delay the progression of CKD. Subsequently, the 52-week Bardoxolone Methyl Treatment: Renal Function in CKD/Type 2 Diabetes (BEAM) Study, a phase 2, randomized trial of 227 patients with moderate to severe CKD (eGFR=20–45 ml/min per 1.73 m2) and type 2 diabetes mellitus confirmed that bardoxolone produced dose-dependent elevations in eGFR: for example, participants allocated to a dose of 25 mg daily achieved an 8.2±15-ml/min per 1.73 m2 increase in eGFR at 24 weeks.3 However, the subsequent phase 3 trial, the Bardoxolone Methyl Evaluation in Patients with CKD and Type 2 Diabetes Mellitus: The Occurrence of Renal Events (BEACON) Trial, with around 2000 patients with eGFR from 15 to <30 ml/min per 1.73 m2 and diabetes mellitus was terminated prematurely by the independent data and safety monitoring committee due to an excess risk of hospitalization or death from heart failure (relative risk, 1.83; 95% confidence interval, 1.32 to 2.55; P<0.001).4
Since the termination of the BEACON Trial, the drug company developing bardoxolone, Reata, has revised its development strategy. Although inflammation is a known feature of many primary renal diseases (of which diabetic nephropathy is the most prevalent), Reata has sought and obtained orphan designation for bardoxolone as a treatment for a single rare renal disease—Alport syndrome. A dominant feature of Alport syndrome is primary glomerular disease caused by mutations in COL4A3, COL4A4, or COL4A5, which encode the type IV collagen α3,4,5 network. Mutations in this network weaken the glomerular basement membrane, making it vulnerable to mechanical forces and hyperfiltration. There are <200,000 individuals with Alport syndrome in the United States, and therefore, the condition meets the definition of the Food and Drug Administration (FDA) of a rare disease under the 1983 Orphan Drug Act (ODA). This allows Reata to take advantage of a shortened approval timeline, financial incentives, and—if approved—a period of market exclusivity for its use in Alport syndrome.
Aside from increasing the risk of cardiac disease in the BEACON4 Trial, bardoxolone also increased the risk of elevations in transaminases, hypomagnesaemia, and muscle cramps. More worryingly, bardoxolone increased urinary albumin excretion, a sensitive biomarker of glomerular damage. In epidemiologic studies, albuminuria is strongly positively associated with an increased risk of progression to ESRD.5 The changes in albuminuria associated with bardoxolone treatment are in the opposite direction as those associated with inhibitors of the renin-angiotensin-aldosterone system pathway, which reduce intraglomerular pressure (and hence, reduce both eGFR and albuminuria) and in the longer term, slow the rate of progression of CKD.6 The precise mechanism of action of bardoxolone in the kidney is unknown, but the possibility remains that some or all of the increase in eGFR and albuminuria results from raised intraglomerular pressure. If this is so, then bardoxolone may ultimately accelerate the decline in renal function (Figure 1). Although no trial of bardoxolone has assessed this possibility reliably to date, such a concern is reinforced by the fact that, in the BEAM Study, after an initial increase, eGFR declined over 24 weeks among patients allocated to bardoxolone 25 mg.3
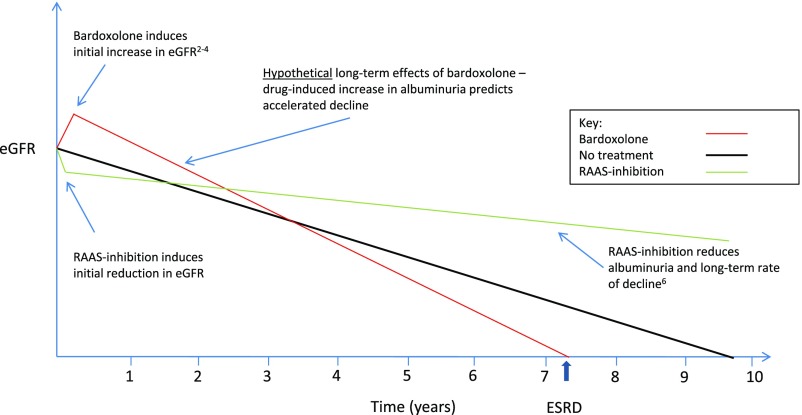
Figure 1.
Contrasting short-term effects of bardoxolone and renin-angiotensin-aldosterone system (RAAS) blockers on eGFR and albumin excretion may lead to contrasting long-term effects on renal function. In progressive CKD (e.g., diabetic nephropathy), eGFR declines in the absence of treatment (black line). Randomized trials have shown that inhibition of the RAAS reduces eGFR in the short term and reduces albuminuria, but then, it slows progressive loss of eGFR. By contrast, bardoxolone increases eGFR (through unknown mechanisms) and also increases albuminuria. Because higher levels of albuminuria are associated with faster decline in eGFR, this raises the hypothesis that bardoxolone may have the opposite effect of RAAS inhibitors and that it may accelerate long-term decline of eGFR.
Against this background, it is reasonable to consider whether the design of the ongoing registration trial of bardoxolone in patients with Alport syndrome (the CARDINAL Trial) will address this concern about its potential for accelerating decline of eGFR in the longer term. Patients with a genetic diagnosis of Alport syndrome are eligible for the CARDINAL Trial if they are ages 12–60 years old, have an eGFR of 30–90 ml/min per 1.73 m2, and are on stable renin-angiotensin-aldosterone system blockade with a urine albumin-to-creatinine ratio of ≤3500 mg/g. Patients at risk for fluid retention (history of cardiovascular disease or BNP>200 pg/ml) are excluded. The open label, phase 2 component of 30 patients, which has now completed recruitment, showed that bardoxolone increased eGFR in patients with Alport syndrome (6.9 ml/min per 1.73 m2 at week 4 [n=19; P<0.001] and 12.7 ml/min per 1.73 m2 at 12 weeks [n=8; P<0.001]; http://investors.reatapharma.com/phoenix.zhtml?c=254306&p=irol-newsArticle&ID=2288113). The double-blind, phase 3 component of this trial is randomizing patients to bardoxolone (titrated up to 30 mg daily) versus placebo for 48 weeks followed by a 4-week discontinuation and then, another 48 weeks taking the original assigned treatment, with a final 4-week discontinuation. Changes in eGFR will be compared at 1 and 2 years before and after discontinuation.7 On the basis of the findings in phase 2 of the CARDINAL Trial, the sample size of the phase 3 trial has now been reduced from 180 to 150 patients.
The CARDINAL Trial will not be able to detect any long-term increase in the rate of CKD progression, because the longest period that patients will be exposed to bardoxolone without interruption will be 48 weeks. There is, therefore, a potential risk that, by using a surrogate outcome (i.e., change in eGFR) that is potentially unreliable as a guide to effects on the relevant clinical outcome (i.e., progression to ESRD), this trial could support the approval of a drug with the opposite effect to the intended one. The potential risks for patients with Alport syndrome are obvious, but the scope for harm from off-label use in the wider community of patients with CKD is even greater. This would not be the first time that safety signals were missed for an orphan drug that was subsequently used widely off label. In the case of epoietin-α (Epogen; Amgen), which was originally approved by the FDA in 1989 as an orphan drug for the treatment of anemia in ESRD, it was nearly a decade before the first large randomized trial (n=1233) raised concerns about an increased risk of death in patients with cardiac disease on hemodialysis,8 by which time the drug was being used very widely off label (e.g., in patients with anemia due to malignancy).
Although it is clearly important to develop effective drugs for patients with rare renal diseases and although the ODA has provided an important stimulus to the pharmaceutical industry to support this activity, it is crucial that trials supporting regulatory approval are fit for purpose. Drug safety is a relative concept, and although it may be less of a concern to patients with a rapidly progressive and incurable disease (such as cancer), in the case of Alport syndrome, most patients considering bardoxolone treatment will be children or young adults who are well but seek to delay progression to ESRD. Therefore, patients and nephrologists need to be confident that, notwithstanding its apparently favorable short-term effects on eGFR, bardoxolone does not accelerate the long-term rate of progression (Figure 1).
Providing such reassurance will require trials that are much larger than the CARDINAL Trial.9 Because the drug target of bardoxolone is likely to be present in many primary renal diseases with a higher prevalence (including diabetes, the original target disease in the BEAM Study and the BEACON Trial), efficacy and safety would be most reliably assessed in long-term trials that include a wider range of patients. Given its known hazards, the theoretical potential for adverse long-term effects on renal function, and a target population that is young and relatively healthy, we believe that the model of accelerated drug development of bardoxolone in patients with Alport syndrome is inappropriate.
Disclosures
C.B. and R.L. are both trustees of alport uk, a registered United Kingdom charity supporting patients with Alport syndrome and their families. C.B. has Alport syndrome. C.B. has received grant support from Merck, Pfizer, Novartis, Bayer, and Boehringer Ingelheim.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Ruiz S, Pergola PE, Zager RA, Vaziri ND: Targeting the transcription factor Nrf2 to ameliorate oxidative stress and inflammation in chronic kidney disease. Kidney Int 83: 1029–1041, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hong DS, Kurzrock R, Supko JG, He X, Naing A, Wheler J, et al.: A phase I first-in-human trial of bardoxolone methyl in patients with advanced solid tumors and lymphomas. Clin Cancer Res 18: 3396–3406, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pergola PE, Raskin P, Toto RD, Meyer CJ, Huff JW, Grossman EB, et al.; BEAM Study Investigators: Bardoxolone methyl and kidney function in CKD with type 2 diabetes. N Engl J Med 365: 327–336, 2011 [DOI] [PubMed] [Google Scholar]
- 4.de Zeeuw D, Akizawa T, Audhya P, Bakris GL, Chin M, Christ-Schmidt H, et al.; BEACON Trial Investigators: Bardoxolone methyl in type 2 diabetes and stage 4 chronic kidney disease. N Engl J Med 369: 2492–2503, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gansevoort RT, Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, et al.; Chronic Kidney Disease Prognosis Consortium: Lower estimated GFR and higher albuminuria are associated with adverse kidney outcomes. A collaborative meta-analysis of general and high-risk population cohorts. Kidney Int 80: 93–104, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heerspink HJL, Kröpelin TF, Hoekman J, de Zeeuw D; Reducing Albuminuria as Surrogate Endpoint (REASSURE) Consortium: Drug-induced reduction in albuminuria is associated with subsequent renoprotection: A meta-analysis. J Am Soc Nephrol 26: 2055–2064, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meyer C, Warnock D, Chin M, Goldsberry A, McCullough P, O’Grady M, et al.: A phase 2/3 study of the efficacy and safety of bardoxolone methyl in patients with Alport syndrome. Nephrol Dial Transplant 32[Suppl 3]: iii480, 2017. [Google Scholar]
- 8.Besarab A, Bolton WK, Browne JK, Egrie JC, Nissenson AR, Okamoto DM, Schwab SJ, Goodkin DA: The effects of normal as compared with low hematocrit values in patients with cardiac disease who are receiving hemodialysis and epoetin. N Engl J Med 339: 584–590, 1998 [DOI] [PubMed] [Google Scholar]
- 9.Baigent C, Herrington WG, Coresh J, Landray MJ, Levin A, Perkovic V, Pfeffer MA, Rossing P, Walsh M, Wanner C, Wheeler DC, Winkelmayer WC, McMurray JJV; KDIGO Controversies Conference on Challenges in the Conduct of Clinical Trials in Nephrology Conference Participants: Challenges in conducting clinical trials in nephrology: Conclusions from a Kidney Disease-Improving Global Outcomes (KDIGO) controversies conference. Kidney Int 92: 297–305, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]