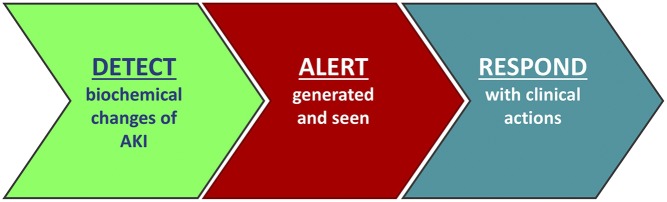
The path toward new, effective treatments for AKI has been difficult, with a frustrating lack of progress and a litany of negative clinical trials.1–4 Faced with large numbers of patients with AKI who display startlingly poor outcomes, it is not surprising that clinicians and professional organizations have sought parallel ways to address this, including the International Society of Nephrology “0by25” campaign and the “Think Kidneys” national program in England. Current guidelines recommend various elements of supportive AKI care, but reports spanning different health care systems tell us that the delivery of these relatively simple measures in “real-life” clinical settings is often suboptimal.5 A number of factors may contribute to this: the silent nature of AKI coupled to competing priorities of coexisting conditions; time pressures of busy clinical staff; or a lack of awareness, training, or knowledge of AKI outside of specialty nephrology or critical care areas where the majority of cases occur. Conceptually, there is an obvious appeal to technologic solutions to improve AKI recognition (Figure 1), with increasing interest in this area over the last few years.6 This has been tempered by valid concerns around “technology overload” arising from poorly designed clinical decision support (CDS) that may detract from the intended aim, or examples of ineffective alerts that do not alter processes of care.7 There are also complexities in evaluating such strategies for AKI. To be effective, proposed interventions must change the behavior of care providers across the hospital, the effect must be sustained, and the effect must translate into improved patient outcomes. Moreover, an effective intervention in one hospital may not work so well elsewhere. Studies should take account of these different elements.
Figure 1.
An electronic alert or computer decision support for AKI must not only alert the health care provider to the presence of AKI but also result in a clinical response if it is to be effective in changing patient outcomes. In many cases, the response to an alert should be prompt review of the patient, which includes making a clinical diagnosis of AKI and determining its etiology. Evaluation of this type of intervention should ideally assess its effect on care processes in tandem with an assessment of patient outcomes and report on how the intervention is implemented.
In this issue of the Journal of the American Society of Nephrology, Al-Jaghbeer et al.8 report an important study that examines the effect of a CDS system for AKI on patient outcomes. The study is notable for its large sample size (>64,000 patients with AKI), its multicenter approach (14 hospitals in Pennsylvania), and its inclusion of a comparator group (463,000 patients without AKI), all of which differentiate this from previous work. CDS consisted of an AKI alert message within the electronic medical record that was automatically triggered when a serum creatinine measurement was 50% higher than a baseline value. The alert message was accompanied by instructions to clinically evaluate the patient, information about AKI staging, and contact details for specialist referral. Outcomes, assessed before and after CDS introduction in patients with a coded diagnosis for AKI, showed reductions in mortality, hospital length of stay, and rates of dialysis. These findings held true in sensitivity analyses, did not occur in the non-AKI group, and were sustained over 24 months. Although the observed reductions in mortality and length of stay may be considered relatively modest at a patient level, they become of much greater significance when considering the high incidence of AKI in hospitalized patients. With an estimated annual incidence of 1 million cases of AKI in patients in the United States,9 a reduction in mortality from 10.2% to 9.4% could translate into 8000 lives saved per year, and potentially significant health economic benefits may result from saved hospital bed days and lower dialysis costs. Although such estimates are overly simplistic and probably overoptimistic (for example, ignoring potential attenuation of effect that comes with wider dissemination), they serve to emphasize the potential impact of the study’s findings.
Interestingly, the expected increase in specialty referrals after CDS introduction was not seen. This suggests that any effect on outcomes resulted from better care delivered by the parent medical team rather than from nephrology or intensivist input. Although a reduction in iodinated contrast use after CDS introduction may hint at this, other prescribing (e.g., renin-angiotensin-aldosterone system blockers) did not change. One weakness of the study is that processes of care were not studied with granularity, and therefore, mechanisms to explain effects of CDS on patient outcomes could not be established. Other minor limitations include omission of other relevant outcome measures, such as renal recovery, and that it was not clear if activities to support the intervention were performed (e.g., education or publicity). Most important, however, is that the findings are interpreted within the context of a nonrandomized time series design as acknowledged appropriately by the authors. This means that confounding effects on outcomes, in particular, temporal trends, cannot be excluded. Convention would dictate that the next step would be to suggest a randomized, controlled trial (RCT), but this point is worthy of debate. The authors correctly state that an appropriately powered RCT to detect a similar effect size is unlikely to be feasible. In addition, there are coherent arguments that the traditional RCT is not the optimal design to evaluate complex interventions targeted at changing behavior of health care professionals.10 Instead, future work should consider study methodologies that are able to evaluate interventions accounting for their context, content, and application as well as their effect on outcomes; the Tackling AKI Study, which is due to report in 2018, is one example of this.11
In summary, the work by Al-Jaghbeer et al.8 provides some much needed optimism for the use of CDS as a pragmatic tool to help improve short-term outcomes for patients with AKI. Whilst this is not a panacea, it does give a message that in parallel to vital research that pursues novel pharmacotherapeutics, quality improvement strategies focusing on care processes for AKI should not be overlooked.
Disclosures
None.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related article, “Clinical Decision Support for In-hospital AKI,” on pages 654–660.
References
- 1.McCullough PA, Bennett-Guerrero E, Chawla LS, Beaver T, Mehta RL, Molitoris BA, et al.: ABT-719 for the prevention of acute kidney injury in patients undergoing high-risk cardiac surgery: A randomized phase 2b clinical trial. J Am Heart Assoc 5: e003549, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mitaka C, Ohnuma T, Murayama T, Kunimoto F, Nagashima M, Takei T, et al.; JAPAN Investigators: Effects of low-dose atrial natriuretic peptide infusion on cardiac surgery-associated acute kidney injury: A multicenter randomized controlled trial. J Crit Care 38: 253–258, 2017 [DOI] [PubMed] [Google Scholar]
- 3.Bellomo R, Chapman M, Finfer S, Hickling K, Myburgh J; Australian and New Zealand Intensive Care Society (ANZICS) Clinical Trials Group: Low-dose dopamine in patients with early renal dysfunction: A placebo-controlled randomised trial. Lancet 356: 2139–2143, 2000 [DOI] [PubMed] [Google Scholar]
- 4.Gillies MA, Kakar V, Parker RJ, Honoré PM, Ostermann M: Fenoldopam to prevent acute kidney injury after major surgery-a systematic review and meta-analysis. Crit Care 19: 449, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aitken E, Carruthers C, Gall L, Kerr L, Geddes C, Kingsmore D: Acute kidney injury: Outcomes and quality of care. QJM 106: 323–332, 2013 [DOI] [PubMed] [Google Scholar]
- 6.Horne KL, Selby NM: Recent developments in electronic alerts for acute kidney injury. Curr Opin Crit Care 21: 479–484, 2015 [DOI] [PubMed] [Google Scholar]
- 7.Wilson FP, Shashaty M, Testani J, Aqeel I, Borovskiy Y, Ellenberg SS, et al.: Automated, electronic alerts for acute kidney injury: A single-blind, parallel-group, randomised controlled trial. Lancet 385: 1966–1974, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Al-Jaghbeer M, Dealmeida D, Bilderback A, Ambrosino R, Kellum JA: Clinical decision support for in-hospital AKI. J Am Soc Nephrol, 29: 654–660, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goldstein SL, Jaber BL, Faubel S, Chawla LS; Acute Kidney Injury Advisory Group of American Society of Nephrology: AKI transition of care: A potential opportunity to detect and prevent CKD. Clin J Am Soc Nephrol 8: 476–483, 2013 [DOI] [PubMed] [Google Scholar]
- 10.Walshe K: Understanding what works--and why--in quality improvement: The need for theory-driven evaluation. Int J Qual Health Care 19: 57–59, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Selby NM, Casula A, Lamming L, Mohammed M, Caskey F; Tackling AKI Investigators: Design and rationale of ‘Tackling Acute Kidney Injury,’ a Multicentre Quality Improvement Study. Nephron 134: 200–204, 2016 [DOI] [PubMed] [Google Scholar]