Abstract
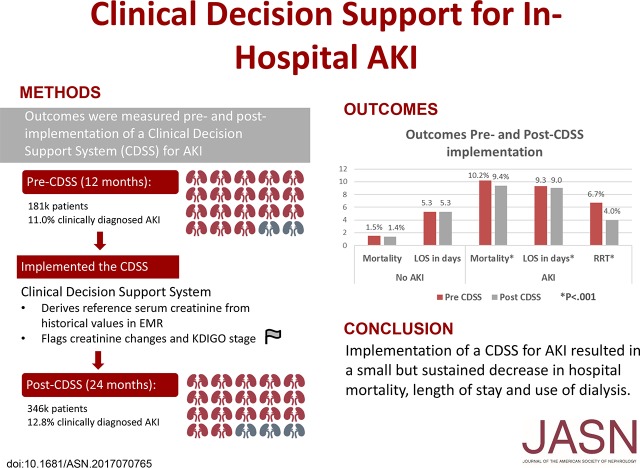
AKI carries a significant mortality and morbidity risk. Use of a clinical decision support system (CDSS) might improve outcomes. We conducted a multicenter, sequential period analysis of 528,108 patients without ESRD before admission, from October of 2012 to September of 2015, to determine whether use of a CDSS reduces hospital length of stay and in-hospital mortality for patients with AKI. We compared patients treated 12 months before (181,696) and 24 months after (346,412) implementation of the CDSS. Coprimary outcomes were hospital mortality and length of stay adjusted by demographics and comorbidities. AKI was diagnosed in 64,512 patients (12.2%). Crude mortality rate fell from 10.2% before to 9.4% after CDSS implementation (odds ratio, 0.91; 95% confidence interval [95% CI], 0.86 to 0.96; P=0.001) for patients with AKI but did not change in patients without AKI (from 1.5% to 1.4%). Mean hospital duration decreased from 9.3 to 9.0 days (P<0.001) for patients with AKI, with no change for patients without AKI. In multivariate mixed-effects models, the adjusted odds ratio (95% CI) was 0.76 (0.70 to 0.83) for mortality and 0.66 (0.61 to 0.72) for dialysis (P<0.001). Change in adjusted hospital length of stay was also significant (incidence rate ratio, 0.91; 95% CI, 0.89 to 0.92), decreasing from 7.2 to 6.0 days for patients with AKI. Results were robust to sensitivity analyses and were sustained for the duration of follow-up. Hence, implementation of a CDSS for AKI resulted in a small but sustained decrease in hospital mortality, dialysis use, and length of stay.
Keywords: Electronic medical record, electronic health record, electronic alerts, radio contrast, nephrotoxicity, acute renal failure
AKI is common in patients admitted to hospitals and intensive care units.1–3 It has been shown to confer a significant increase risk for mortality and morbidity, with even a modest increase in creatinine showing an effect on hospital mortality, length of stay (LOS), and health care costs.4,5 This has led to recommendations from societies for early detection of AKI by the treating physician as it may lead to interventions like drug dose adjustment, avoiding nephrotoxins, and intravenous fluids management.6,7 Early detection of AKI could trigger an early consultation to subspecialists (nephrologists, intensivists) or pharmacists, which might improve outcomes.8 Unfortunately, AKI is often missed by health care providers,9,10 and this can be associated with increased mortality.11
Clinical decision support systems (CDSS) within the electronic medical record (EMR) have been shown to help in some aspects of the clinical decision-making for hospitalized patients.12 However these systems have not shown a consistent effect on patient-centered outcomes like mortality, LOS, or on health care cost.13 CDSS have been developed to automate detection of AKI in the hospital setting chiefly by providing help in ascertaining baseline renal function from the EMR. Although the use of these systems has consistently shown improved detection of AKI,14–18 evidence that CDSS can improve outcomes for patient with AKI is lacking. Indeed, a recent randomized, control trial showed no improvement in patient-centered outcomes.18
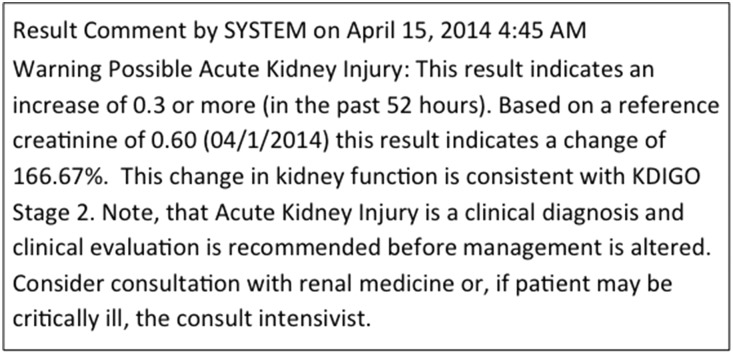
There are multiple limitations of existing CDSS for AKI.19 Chief among these are a lack of specific therapies tied to the AKI alerts. However, limitations also exist in available studies evaluating CDSS effectiveness, and studies have generally sought implausible effect sizes. We implemented a CDSS for AKI in 2013 in a regional health care system that cares for >150,000 inpatients per year. An example alert is shown in Figure 1. In this analysis, we sought to examine what effect implementing our CDSS had on hospital mortality, use of dialysis, and LOS.
Figure 1.
Example AKI alert posted by the computer CDSS.
Results
During the 12 months before the implementation of the CDSS, 181,696 patients were admitted across the 14 hospitals in the health care system. In the 24 months after CDSS implementation, 346,412 patients were admitted for a total of 528,108 patients form October of 2012 to September of 2015. The cohorts were compatible in demographic characteristics and comorbidities. The mean±SD age was 59±20 years, 57% of the cohort were women, and 84% were white. Baseline characteristics and comorbid conditions for patients in the pre-CDSS and post-CDSS cohorts are presented in Table 1.
Table 1.
Population characteristics by time period and AKI status
| Characteristic | Pre-CDSS, October 2012–September 2013, n=181,696 | Post-CDSS October 2013–September 2015, n=346,412 | ||||
|---|---|---|---|---|---|---|
| No AKI, n=161,661 | AKI, n=20,035 | Total, n=181,696 | No AKI, n=301,935 | AKI, n=44,477 | Total, n=346,412 | |
| Age, yr, mean±SD | 58±20 | 69±16 | 59±20 | 57±20 | 69±16 | 59±20 |
| Women, n (%) | 94,434 (58.4) | 9350 (46.7) | 103,784 (57.1) | 175,457 (58.1) | 20,281 (45.6) | 195,738 (56.5) |
| Race, n (%) | ||||||
| Black | 20,090 (12.4) | 2742 (13.7) | 22,832 (12.6) | 37,657 (12.5) | 6445 (14.5) | 44,102 (12.7) |
| White | 136,376 (84.4) | 16,586 (82.8) | 152,962 (84.2) | 252,968 (83.8) | 36,424 (81.9) | 289,392 (83.5) |
| Other | 5195 (3.2) | 707 (3.5) | 5902 (3.3) | 11,310 (3.8) | 1608 (3.6) | 12,918 (3.7) |
| Charlson Index | ||||||
| Mean±SD | 1.7±2.2 | 3.3±2.4 | 1.8±2.3 | 1.7±2.2 | 3.4±2.4 | 1.9±2.3 |
| Median (IQR) | 1 (0–2) | 3 (1–5) | 1 (0–3) | 1 (0–2) | 3 (2–5) | 1 (0–3) |
| Comorbidities, n (%) | ||||||
| AMI | 14,881 (9) | 3440 (17) | 18,321 (10) | 26,886 (9) | 7334 (16) | 34,220 (10) |
| Diabetes | 30,005 (19) | 6135 (31) | 36,140 (20) | 55,386 (18) | 13,184 (30) | 68,570 (20) |
| Diabetes + complications | 5771 (4) | 2056 (10) | 7827 (4) | 11,799 (4) | 4973 (11) | 16,772 (5) |
| Hemiplegia or paraplegia | 2219 (1) | 351 (2) | 2570 (1) | 4211 (1) | 710 (2) | 4921 (1) |
| Renal disease | 13,309 (8) | 8282 (41) | 21,591 (12) | 25,247 (8) | 19,294 (43) | 44,541 (13) |
| Cancer | 16,915 (10) | 2396 (12) | 19,311 (11) | 32,329 (11) | 5672 (13) | 38,001 (11) |
| Mild LD | 3565 (2) | 1141 (6) | 4706 (3) | 6712 (2) | 2649 (6) | 9361 (3) |
| Moderate/severe LD | 2029 (1) | 1026 (5) | 3055 (2) | 4057 (1) | 2389 (5) | 6446 (2) |
| Metastatic cancer | 7663 (5) | 1046 (5) | 8709 (5) | 13,166 (4) | 2342 (5) | 15,508 (4) |
| AIDS | 308 (0) | 68 (0) | 376 (0) | 652 (0) | 144 (0) | 796 (0) |
| CHF | 20,249 (13) | 6969 (35) | 27,218 (15) | 38,975 (13) | 16,246 (37) | 55,221 (16) |
| PVD | 9807 (6) | 2309 (12) | 12,116 (7) | 18,389 (6) | 5710 (13) | 24,099 (7) |
| CEVD | 11,989 (7) | 1883 (9) | 13,872 (8) | 22,933 (8) | 4491 (10) | 27,424 (8) |
| Dementia | 589 (0) | 164 (1) | 753 (0) | 1115 (0) | 362 (1) | 1477 (0) |
| COPD | 38,440 (24) | 5892 (29) | 44,332 (24) | 73,606 (24) | 12,931 (29) | 86,537 (25) |
| LOS | ||||||
| Mean±SD | 5.3±8.5 | 9.3±11.5 | 5.7±9.0 | 5.3±8.7 | 9.0±10.8 | 5.7±9.1 |
| Median (IQR) | 3 (2–6) | 6 (4–11) | 4 (2–6) | 3 (2–6) | 6 (3–11) | 4 (2–6) |
| Mortality, n (%) | 2349 (1.5) | 2044 (10.2) | 4393 (2.4) | 4236 (1.4) | 4174 (9.4) | 8410 (2.4) |
IQR, interquartile range; AMI, acute myocardial infarction; LD, liver disease; CHF, congestive heart failure; PVD, peripheral vascular disease; CEVD, cerebrovascular disease; COPD, chronic obstructive pulmonary disease.
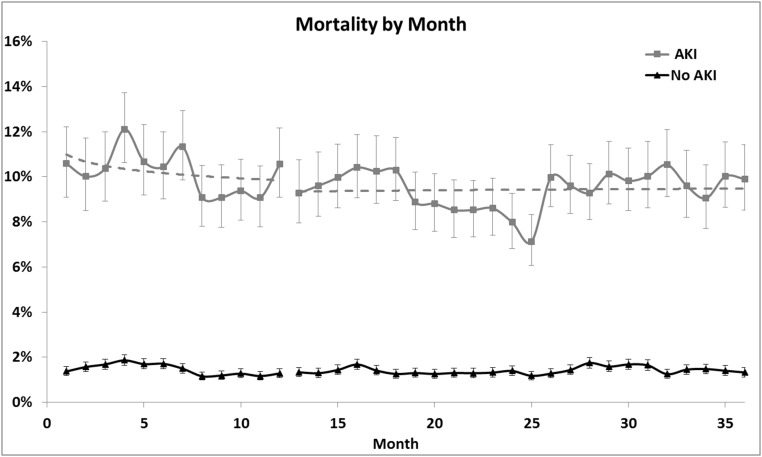
AKI was diagnosed by treating physicians in 64,512 patients (12.2%): 20,035 (11%) pre-CDSS and 44,477 (12.8%) post-CDSS. Crude mortality was 10.2% for patients with AKI and 1.5% for patients without AKI in the pre-CDSS period. Mortality decreased to 9.4% for patients with AKI (odds ratio [OR], 0.91; 95% confidence interval [95% CI], 0.86 to 0.96; P=0.001), whereas no change was observed in patients without AKI (1.4%) (Figure 2). Similarly, mean hospital duration decreased by 0.3 days (9.3 to 9.0 days; P<0.001) for patients with AKI whereas, for patients without AKI, mean duration was 5.3 days during both periods (Table 1). In multivariate models including age, sex, race, and comorbidities (Charlson Index), as well as interactions between AKI diagnosis and CDSS status, the adjusted OR for mortality was 0.76 (95% CI, 0.70 to 0.83) using a mixed-effects model (P<0.001). Adjusted hospital LOS was also significant (incidence rate ratio [IRR], 0.91; 95% CI, 0.89 to 0.92), decreasing about 1.2 days for patients with AKI (Table 2).
Figure 2.
Mortality decreased for patients with AKI, whereas no change was observed in patients without AKI. Unadjusted mortality by month for patients with and without AKI, before and after implementation of the CDSS. Horizontal dashed lines are log regression lines.
Table 2.
Multivariate models for hospital mortality and LOS
| Mortality | Adjusted Mortality Pre-CDSS | Adjusted Mortality Post-CDSS | ||||
|---|---|---|---|---|---|---|
| Model | OR (95% CI) | P Value | No AKI, % | AKI, % | No AKI, % | AKI, % |
| Logistic model with robust standard error accounting for intra-patient clustering | 0.76 (0.70 to 0.83) | <0.001 | 2.1 | 9.1 | 1.4 | 4.9 |
| Mixed effects | 0.76 (0.70 to 0.83) | <0.001 | 1.8 | 8.3 | 1.2 | 4.5 |
| LOS | Adjusted LOS Pre-CDSS | Adjusted LOS Post-CDSS | ||||
| Model | IRR (95% CI) | P Value | No AKI, d | AKI, d | No AKI, d | AKI, d |
| Negative binomial model with robust standard error accounting for intra-patient clustering | 0.87 (0.85 to 0.90) | <0.001 | 5.8 | 9.5 | 5.2 | 7.4 |
| Mixed effects | 0.91 (0.89 to 0.92) | <0.001 | 4.7 | 7.2 | 4.3 | 6.0 |
Our results were robust to sensitivity analyses. The effects on mortality were greater in medical compared with surgical patients with AKI: adjusted OR for mortality was 0.56 (95% CI, 0.48 to 0.66) for medical patients versus 0.72 (95% CI, 0.54 to 0.95) for surgical patients (Supplemental Table 1). However, results in both groups remained significant (P<0.001 and P=0.02, respectively). Results of our analysis, performed by leaving individual centers out one at a time, are shown in Supplemental Table 2. The point estimate for the adjusted OR for mortality remained quite stable between 0.74 and 0.79. The upper limit of the 95% CI never exceeded 0.87 and all iterations remained highly significant (P<0.001). Similar results were seen for hospital LOS. Adjusted IRR was 0.90–0.92 and the upper limit of the 95% CI was never >0.93; all iterations remained highly significant (P<0.001). As shown in Figure 2, the effects on crude mortality in patients with AKI were sustained for the duration of our analysis.
The effect of CDSS on hospital LOS was stronger in surgical patients (IRR, 0.77; 95% CI, 0.74 to 0.81; P<0.001) compared with medical patients (IRR, 0.95; 95% CI, 0.93 to 0.98; P<0.001), with P=0.02 for the interaction. However, no differences were seen for CDSS by age or Charlson. Conversely, the effect of CDSS on mortality was strongly affected by age (P<0.01) such that patients aged 60 years or greater benefitted (OR, 0.75; 95% CI, 0.68 to 0.82; P<0.001), whereas patients aged <60 years did not (OR, 0.87; 95% CI, 0.75 to 1.04; P=0.13). The effect of mortality was not different by Charlson or by medical versus surgical admissions (see Supplemental Table 3).
The distribution of AKI, AKI treated with dialysis, and CKD are shown in Table 3. Despite an increase in AKI rates from 11% to 12.8%, and CKD rates from 5.0% to 5.7%, dialysis for AKI decreased from 6.7% of patients with AKI to 4.0% (P<0.001). We further analyzed the use of dialysis for AKI using a mixed-effects model. The adjusted OR for dialysis was 0.66 (95% CI, 0.61 to 0.72), for post-CDSS compared with pre-CDSS (P<0.001).
Table 3.
Rates of AKI, dialysis, and CKD by time period
| n (%) | Pre-CDSS, October 2012–September 2013, n=181,696 | Post-CDSS, October 2013–September 2015, n=346,412 | ||||
|---|---|---|---|---|---|---|
| No AKI, n=161,661 | AKI, n=20,035 | Total, n=181,696 | No AKI, n=301,935 | AKI, n=44,477 | Total, n=346,412 | |
| AKI | 0 (0) | 20,035 (100) | 20,035 (11.0) | 0 (0) | 44,477 (100) | 44,477 (12.8) |
| AKI with dialysis | 0 (0) | 1334 (6.7) | 1334 (0.7) | 0 (0) | 1770 (4.0) | 1770 (0.5) |
| CKD | 5251 (3.3) | 3758 (18.8) | 9009 (5.0) | 10,202 (3.4) | 9698 (21.8) | 19,900 (5.7) |
The mean number of days patients received common nephrotoxic medications, before and after CDSS, with and without AKI, are shown in Table 4. Days of angiotensin-converting enzyme inhibitor exposure dropped slightly in the cohort overall (1.34 days per patient to 1.25; P<0.001) but the only significant differences in patients with AKI were a decrease in the use of intravascular radio contrast agents (a 45% reduction; P<0.001) and an increase in days of nonsteroidal any inflammatory drug exposure (by 2%; P<0.01). Subspecialty consults actually decreased after CDSS implementation. Nephrology consults deceased for patients with AKI from 30.5% pre-CDSS to 26.9% post-CDSS (P=0.001). Similarly, there was a decrease in critical care consults for these patients from 1.5% to 0.8% (P<0.001).
Table 4.
Mean medication days for patients pre- and post-CDSS, with and without AKI
| Medication | No AKI, n=82,474 | AKI, n=15,229 | ||||
|---|---|---|---|---|---|---|
| Pre-CDSS, n=29,367 | Post-CDSS, n=53,107 | P Value | Pre-CDSS, n=4655 | Post-CDSS, n=10,574 | P Value | |
| ACEI/ARB | 1.34±3.85 | 1.25±3.60 | <0.001 | 2.20±7.33 | 2.18±7.04 | 0.38 |
| NSAID | 1.82±4.68 | 1.86±4.75 | <0.01 | 4.55±9.52 | 4.83±10.13 | 0.01 |
| Aminoglycoside | 0.22±2.34 | 0.20±2.42 | 0.05 | 0.84±5.98 | 0.82±5.97 | 0.26 |
| Contrasta | 0.30±1.29 | 0.21±1.04 | <0.001 | 0.55±1.95 | 0.30±1.47 | <0.001 |
| Vancomycin | 1.23±4.34 | 1.31±4.48 | <0.001 | 4.56±10.38 | 4.58±10.41 | 0.47 |
ACEI/ARB, angiotensin-converting enzyme inhibitors/angiotensin receptor blockers; NSAID, nonsteroidal anti-inflammatory drugs.
Excludes patients receiving more than 12 contrast exposures in a single admission.
Discussion
In this study, we sought to assess what effect, if any, our implementation of a CDSS for AKI had on hospital mortality and LOS. Our results indicate a small but significant and sustained effect on both. However, given that AKI is common and can be lethal, small changes could have very large effects on the health of the population. In the United States alone, nearly 18 million people incur at least one hospital admission per year.20 Thus, a rate of AKI per admission of 12% would translate into about 2.2 million new cases of AKI annually. A 0.8% absolute decrease in mortality would equate to 17,600 fewer deaths. Similarly, at an average cost of over $1800 a day,21 a 0.3 day decrease in hospital days would equate to about $1.2 billion.
The use of CDSS to automate the detection of AKI in the hospital setting is not new.14–18 However, studies attempting to determine if CDSS can improve patient outcomes have generally been disappointing. Colpaert et al.17 showed that a CDSS increased the timeliness of interventions (mainly fluids or diuretics) but did not improve outcomes in a study of 1079 admissions, whereas Wilson et al.18 found neither any change in practice nor outcomes in 2393 patients. Similarly, McCoy et al.22 found that overlaying pharmacy surveillance on top of an electronic AKI alert had no effect on adverse drug events in 396 patients. However, Kolhe et al.23 found that implementing an AKI care bundle with an interruptive alert early in the course of AKI can reduce mortality and the progression of AKI stage. A major limitation in all of these studies concerns the small sample sizes and therefore unrealistic hypothesized effect size. For mortality, even the largest study had adequate power only to detect an effect size of 2.3% absolute risk reduction or greater. By comparison, we found only a 0.8% difference in mortality.
Recognizing this limitation, prior studies have looked at a number of nonmortal outcomes, such as rates of dialysis and maximum change in creatinine. However, the relationship between CDSS-enhanced recognition of AKI and changes in these outcomes is complex. Early detection of AKI might actually increase the use of dialysis for very ill patients, and maximum changes in creatinine often reflect the extent of initial injury to the kidney. Because roughly two thirds of AKI cases are community acquired,24 the maximum creatinine may be determined before medical attention even if it manifests while under medical care. For these reasons, we limited our primary analysis to the nonspecific outcomes of hospital mortality and LOS because they could both plausibly be reduced by early AKI detection. We did examine dialysis rates within AKI patients, however, as a secondary end point and did find a large reduction in both crude rates (2.7% absolute difference) and the covariate-adjusted odds (OR, 0.66; 95% CI, 0.61–0.72).
The mechanisms by which hospital mortality, dialysis, and LOS were reduced after implementation of our CDSS are unclear. We expected to see a reduction in nephrotoxic medication and intravascular radio contrast exposure, as well as an increase in consultations for nephrology and critical care medicine. Although days of angiotensin-converting enzyme inhibitor exposure dropped slightly overall, there was no effect in patients with AKI. Only radio contrast exposure decreased in these patients. Although the size of the change in radio contrast exposure (45% relative change) was certainly clinically significant, it is not directly apparent how this would have led to a change in mortality or LOS. Instead, it seems more likely that reduced radio contrast exposure was a surrogate for other unmeasured changes in care. Radio contrast use as a potential nephrotoxin has been the subject of significant investigation over recent years, and contrast-associated AKI may be decreasing because of this increased attention.25 Wilson et al.18 also failed to detect a difference between control and CDSS groups in terms of patients receiving aminoglycosides or non-steroidal anti-inflammatory medications. However, these investigators also did not detect any change in radio contrast use. Failure to reduce exposure to nonsteroidal anti-inflammatory medications may have been a lost opportunity because patients developing AKI had more than twice the days of exposure to these drugs (Table 4).
Like Wilson et al.18, we did not observe any increase in subspecialty consultations with implementation of the CDSS. Because consults are most often reserved for severe cases (and indeed, are mandatory for the most severe cases, such as RRT and cardiac arrest) this result might actually imply that early intervention by primary providers resulted in less progression of AKI. This would be supported by the observed decreases in RRT and LOS.
CDSS appeared to have a greater effect on LOS in surgical patients. This might reflect the fact that AKI is often a complication of surgery in addition to being a complication of the underlying disease condition. For patients undergoing elective surgery, LOS is already closely monitored by hospitals and an AKI event might be expected to have larger effect on hospital stays in this subgroup. Similarly, the effect of CDSS on mortality was similar for medical and surgical patients and for patients with and without comorbid conditions. However, CDSS did not influence mortality in younger patients, whereas a large effect was observed in patients aged 60 years and older. We have previously reported that AKI is harder to predict in older patients26 and that a CDSS might have greater effect in these patients. Of course, hospital mortality is already higher in older patients, providing greater opportunity to see an effect of the intervention.
Our study has important limitations. First, because this was not a randomized trial, we could only control for secular trends using statistical analysis. The fact that our results revealed differences both in rates of mortality and LOS after adjusting for demographics and comorbidities is reassuring, but cannot exclude unknown sources of bias. The fact that there was no effect of our CDSS in patients without AKI provides further evidence of a likely causal relationship, but one that we cannot directly test for. Furthermore, although the Kidney Disease Improving Global Outcomes (KDIGO) clinical practice guidelines6 had been published a full 18 months before the implementation of the CDSS, and implementation was not paired with any other system-wide quality improvement or educational efforts directed at AKI, we cannot exclude possible effects of increasing awareness of AKI on outcomes. However, we do note that a randomized trial of this size would be unprecedented in AKI research, and thus we have likely generated the strongest evidence possible for this type of “passive” CDSS. Future trials will need to focus on more active CDSS-based approaches in order to generate larger effect sizes.19 Nevertheless, the evidence presented in this study provides proof of concept for CDSS focused on AKI. Second, we could not analyze specific clinician behavior in response to the CDSS. For example, clinicians might have changed drug dosing, increased monitoring, or altered fluid management in response to an AKI alert. We know that they did not increase subspecialty consultation or reduce days of nephrotoxin exposure other than radio contrast, but we could not assess whether they changed other aspects of care.
In conclusion, implementation of a CDSS for AKI was associated with a small but sustained decrease in hospital mortality and LOS. Dialysis for AKI also decreased. These results support the development of CDSS to enhance early AKI detection but demonstrate that passive alerting will have only a limited effect on patient outcomes and more action-based CDSS will likely be needed to increase effect.
Concise Methods
Study Design and Participants
We implemented a CDSS for AKI on the basis of the KDIGO clinical practice guidelines.6 The alert was implemented across all adult hospitals within the University of Pittsburgh Medical Center (UPMC) system in October of 2013. As part of the quality improvement project, we collected data from the EMR for 12 months (from October of 2012 to September of 2013) before the alert (prealert) and for 24 months after the alert (from October of 2013 to September of 2015), after AKI alert implementation (postalert). The project was approved by the UPMC Quality Improvement committee. Our analysis included adult participants (aged >18 years) admitted to UPMC floors or intensive care units with at least one serum creatinine value recorded in the EMR. The sole exclusion criterion was ESRD before admission. Patients with functioning renal transplants were not excluded.
CDSS Procedures
The CDSS was designed to alert clinicians of “possible AKI” according to changes in the serum creatinine concentration. Because the CDSS included patients outside the intensive care unit where urine output could not be reliably obtained, only the serum creatinine concentration was considered. The system determined a reference serum creatinine value, as per the KDIGO guidelines,6 according to the lowest value between the baseline (see below) and admission serum creatinine. The baseline serum creatinine was defined as the lowest value for the patient in the EMR for the previous 12 months before admission. This approach is known to be over-sensitive compared with median values.27 However, our intent was to maximize sensitivity. If no baseline was available in the EMR and the patient did not have a history of CKD, we estimated the baseline on the basis of back-calculation using the Modification of Diet in Renal Disease equation as previously described.27,28 If a history of CKD was recorded and no baseline creatinine was available, the admission serum creatinine was used as the reference serum creatinine.
Next, the CDSS compared the serum creatinine from the first encounter to the reference serum creatinine. If an increase of 50% or more was detected, an AKI alert was returned. For subsequent creatinine values, an increase of 0.3 mg/dl or more from a previous creatinine within 52 hours was used to trigger the alert (on the basis of a 48-hour rule with a 4-hour buffer for reporting). For each AKI alert, the CDSS posted possible AKI next to the corresponding serum creatinine value in the hospital’s EMR (Cerner PowerChart; Cerner Corporation, Kansas City, MO). Clicking on the possible AKI result provided the following information to the clinician: (1) the reference creatinine used for the alert and how it was derived, (2) the stage of AKI according to the KDIGO classification system (see Supplemental Table 4), and (3) a prompt to consult renal medicine or intensive care (with corresponding pager numbers provided). An example of the alert text is shown in Figure 1.
Statistical Analyses
Our primary outcomes were hospital mortality and LOS in days. Secondary outcomes were use of dialysis, days of nephrotoxic drug exposures, and consults for nephrology and critical care medicine. We abstracted data from the EMR on patient characteristics, including demographics, admission location, dates of admission and discharge, and vital status at discharge. In bivariate analyses, generalized linear regression models were used, with clustered sandwich estimators to account for intragroup correlation within patients. In multivariable analyses, linear trends for LOS and mortality were modeled using negative binomial mixed-effects regression and logistic mixed-effects regression, respectively. All multivariable models adjusted for time in quarters, alert status, the interaction between the postalert indicator and AKI diagnosis (International Classification of Diseases Revision 9 codes 584.5, 584.6, 584.7, 584.8, and 584.9), the Charlson Comorbidity Index,29 age, race, and sex.
We performed two sensitivity analyses. First, we repeated our primary analysis stratifying the cohort by medical and surgical admission. Next, in order to ensure that results were not caused by an effect of a single hospital, we performed the primary analysis repeatedly, leaving one center out each time. We also explored whether CDSS improved outcomes differently by age, underlying comorbidity or medical/surgical admission by fitting models with interaction terms for these variables. We used age ≥60 years versus <60 years and Charlson Index >0 versus 0 for these analyses as they were essentially the medians of the respective distributions. Finally, in the largest facility, we explored differences in care before and after the CDSS was implemented and stratified our analysis by AKI diagnosis. We examined days of nephrotoxic medication exposure and doses of intravenous radio contrast during the hospital stay. We also looked at differences in consults for nephrology and critical care medicine.
We report changes in hospital mortality and use of dialysis using ORs, and LOS using IRRs. A P value <0.05 was considered significant. Analyses were generated using Stata version 14.0 (College Station, TX).
Institutional Review Board Approval
We did not seek institutional review board approval, in accordance with the policy of our institution regarding quality improvement projects.
Disclosures
None.
Supplementary Material
Acknowledgments
The authors wish to thank Martha Stambaugh and Lisa Weber for building and testing the alert, Madhuri Vemulapalli for programming the alert, and Tom Kramer for his advice and assistance with data analysis.
Funding was provided by University of Pittsburgh Medical Center and the University of Pittsburgh.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Making the Right Decision: Do Clinical Decision Support Systems for AKI Improve Patient Outcomes?” on pages 352–354.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2017070765/-/DCSupplemental.
References
- 1.Hoste EAJ, Bagshaw SM, Bellomo R, Cely CM, Colman R, Cruz DN, Edipidis K, Forni LG, Gomersall CD, Govil D, Honoré PM, Joannes-Boyau O, Joannidis M, Korhonen A-M, Lavrentieva A, Mehta RL, Palevsky P, Roessler E, Ronco C, Uchino S, Vazquez JA, Vidal Andrade E, Webb S, Kellum JA: Epidemiology of acute kidney injury in critically ill patients: The multinational AKI-EPI study. Intensive Care Med 41: 1411–1423, 2015 [DOI] [PubMed] [Google Scholar]
- 2.Wang HE, Muntner P, Chertow GM, Warnock DG: Acute kidney injury and mortality in hospitalized patients. Am J Nephrol 35: 349–355, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Uchino S, Bellomo R, Goldsmith D, Bates S, Ronco C: An assessment of the RIFLE criteria for acute renal failure in hospitalized patients. Crit Care Med 34: 1913–1917, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW: Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol 16: 3365–3370, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Kellum JA, Sileanu FE, Murugan R, Lucko N, Shaw AD, Clermont G: Classifying AKI by urine output versus serum creatinine level. J Am Soc Nephrol 26: 2231–2238, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.KDIGO AKIWG : Kidney Disease: Improving Global Outcomes (KDIGO) clinical practice guideline for acute kidney injury. Kidney Inter Suppl 2: 1.–, 2012 [Google Scholar]
- 7.Brochard L, Abroug F, Brenner M, Broccard AF, Danner RL, Ferrer M, Laghi F, Magder S, Papazian L, Pelosi P, Polderman KH: ATS/ERS/ESICM/SCCM/SRLF Ad Hoc Committee on Acute Renal Failure: An official ATS/ERS/ESICM/SCCM/SRLF statement: Prevention and management of acute renal failure in the ICU patient: An international consensus conference in intensive care medicine. Am J Respir Crit Care Med 181: 1128–1155, 2010 [DOI] [PubMed] [Google Scholar]
- 8.Balasubramanian G, Al-Aly Z, Moiz A, Rauchman M, Zhang Z, Gopalakrishnan R, Balasubramanian S, El-Achkar TM: Early nephrologist involvement in hospital-acquired acute kidney injury: A pilot study. Am J Kidney Dis 57: 228–234, 2011 [DOI] [PubMed] [Google Scholar]
- 9.Aitken E, Carruthers C, Gall L, Kerr L, Geddes C, Kingsmore D: Acute kidney injury: Outcomes and quality of care. QJM 106: 323–332, 2013 [DOI] [PubMed] [Google Scholar]
- 10.MacLeod A: NCEPOD report on acute kidney injury-must do better. Lancet 374: 1405–1406, 2009 [DOI] [PubMed] [Google Scholar]
- 11.Wilson FP, Bansal AD, Jasti SK, Lin JJ, Shashaty MGS, Berns JS, Feldman HI, Fuchs BD: The impact of documentation of severe acute kidney injury on mortality. Clin Nephrol 80: 417–425, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dexter PR, Perkins S, Overhage JM, Maharry K, Kohler RB, McDonald CJ: A computerized reminder system to increase the use of preventive care for hospitalized patients. N Engl J Med 345: 965–970, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Thompson G, O’Horo JC, Pickering BW, Herasevich V: Impact of the electronic medical record on mortality, length of stay, and cost in the hospital and ICU: A systematic review and metaanalysis. Crit Care Med 43: 1276–1282, 2015 [DOI] [PubMed] [Google Scholar]
- 14.Porter CJ, Juurlink I, Bisset LH, Bavakunji R, Mehta RL, Devonald MA: A real-time electronic alert to improve detection of acute kidney injury in a large teaching hospital. Nephrol Dial Transplant 29: 1888–1893, 2014 [DOI] [PubMed] [Google Scholar]
- 15.Selby NM, Crowley L, Fluck RJ, McIntyre CW, Monaghan J, Lawson N, Kolhe NV: Use of electronic results reporting to diagnose and monitor AKI in hospitalized patients. Clin J Am Soc Nephrol 7: 533–540, 2012 [DOI] [PubMed] [Google Scholar]
- 16.Sawhney S, Fluck N, Marks A, Prescott G, Simpson W, Tomlinson L, Black C: Acute kidney injury-how does automated detection perform? Nephrol Dial Transplant 30: 1853–1861, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Colpaert K, Hoste EA, Steurbaut K, Benoit D, Van Hoecke S, De Turck F, Decruyenaere J: Impact of real-time electronic alerting of acute kidney injury on therapeutic intervention and progression of RIFLE class. Crit Care Med 40: 1164–1170, 2012 [DOI] [PubMed] [Google Scholar]
- 18.Wilson FP, Shashaty M, Testani J, Aqeel I, Borovskiy Y, Ellenberg SS, Feldman HI, Fernandez H, Gitelman Y, Lin J, Negoianu D, Parikh CR, Reese PP, Urbani R, Fuchs B: Automated, electronic alerts for acute kidney injury: A single-blind, parallel-group, randomised controlled trial. Lancet 385: 1966–1974, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kellum JA, Kane-Gill SL, Handler SM: Can decision support systems work for acute kidney injury? Nephrol Dial Transplant 30: 1786–1789, 2015 [DOI] [PubMed] [Google Scholar]
- 20.Summary Health Statistics: National Health Interview Survey, 2015. Available at https://ftp.cdc.gov/pub/Health_Statistics/NCHS/NHIS/SHS/2015_SHS_Table_P-10.pdf. Accessed September 1, 2017
- 21.Kaiser Family Foundation: Health costs, 2017. Available at: www.kff.org/health-costs/. Accessed September 1, 2017
- 22.McCoy AB, Cox ZL, Neal EB, Waitman LR, Peterson NB, Bhave G, Siew ED, Danciu I, Lewis JB, Peterson JF: Real-time pharmacy surveillance and clinical decision support to reduce adverse drug events in acute kidney injury: A randomized, controlled trial. Appl Clin Inform 3: 221–238, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kolhe NV, Staples D, Reilly T, Merrison D, Mcintyre CW, Fluck RJ, Selby NM, Taal MW: Impact of compliance with a care bundle on acute kidney injury outcomes: A Prospective Observational study. PLoS One 10: e0132279, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hsu C-N, Lee C-T, Su C-H, Wang Y-CL, Chen H-L, Chuang J-H, Tain Y-L: Incidence, outcomes, and risk factors of community-acquired and hospital-acquired acute kidney injury: A Retrospective Cohort study. Medicine (Baltimore) 95: e3674, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Amin AP, Salisbury AC, McCullough PA, Gosch K, Spertus JA, Venkitachalam L, Stolker JM, Parikh CR, Masoudi FA, Jones PG, Kosiborod M: Trends in the incidence of acute kidney injury in patients hospitalized with acute myocardial infarction. Arch Intern Med 172: 246–253, 2012 [DOI] [PubMed] [Google Scholar]
- 26.Kane-Gill SL, Sileanu FE, Trietley G, Murugan R, Handler SM, Kellum JA: Assessing risk for acute kidney injury is more difficult in older adults. Crit Care Med 41: 997, 2013 [Google Scholar]
- 27.Siew ED, Matheny ME, Ikizler TA, Lewis JB, Miller RA, Waitman LR, Go AS, Parikh CR, Peterson JF: Commonly used surrogates for baseline renal function affect the classification and prognosis of acute kidney injury. Kidney Int 77: 536–542, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Závada J, Hoste E, Cartin-Ceba R, Calzavacca P, Gajic O, Clermont G, Bellomo R, Kellum JA; AKI6 investigators : A comparison of three methods to estimate baseline creatinine for RIFLE classification. Nephrol Dial Transplant 25: 3911–3918, 2010 [DOI] [PubMed] [Google Scholar]
- 29.Charlson M, Szatrowski TP, Peterson J, Gold J: Validation of a combined comorbidity index. J Clin Epidemiol 47: 1245–1251, 1994 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.