Abstract
Mesenchymal stromal cells have emerged as potential candidates for cell-based therapies to modulate the immune response in organ transplantation and repair tissues after acute or chronic injury. Preclinical studies have shown convincingly in rodent models that mesenchymal stromal cells can prolong solid organ graft survival and that they can induce immune tolerance, accelerate recovery from AKI, and promote functional improvement in chronic nephropathies. Multiple complex properties of the cells, including immunomodulatory, anti-inflammatory, and proregenerative effects, seem to contribute. The promising preclinical studies have encouraged investigators to explore the safety, tolerability, and efficacy of mesenchymal stromal cell–based therapy in pilot clinical trials, including those for bone marrow and solid organ transplantation, autoimmune diseases, and tissue and organ repair. Here, we review the available data on culture-expanded mesenchymal stromal cells tested in renal transplantation, AKI, and CKD. We also briefly discuss the relevant issues that must be addressed to ensure rigorous assessment of the safety and efficacy of mesenchymal stromal cell therapies to allow the translation of this research into the practice of clinical nephrology.
Keywords: mesenchymal stromal cells, kidney disease, acute kidney injury
The last decade has witnessed an explosion of preclinical studies suggesting extraordinary therapeutic potential of multipotent mesenchymal stromal cells (MSCs) as an innovative cell therapy for numerous diseases, including kidney diseases. The administration of exogenous MSCs to rodents induces long-term graft survival in solid organ transplant models1–7; accelerates recovery from AKI induced by toxic agents, including cisplatin8 and glycerol,9 or by sepsis10 or ischemia-reperfusion11–13; and promotes functional improvement in models of CKDs,14–16 including diabetic kidney disease (DKD)17–20 and lupus nephritis.21–23
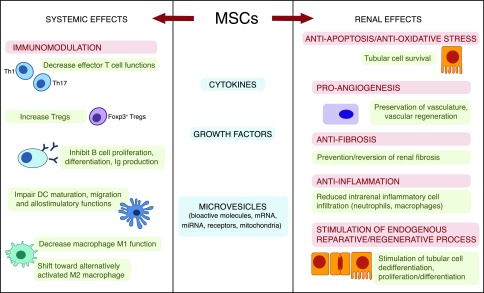
These beneficial effects are the results of multiple and complex actions linked to a wide spectrum of immunomodulatory, anti-inflammatory, and proregenerative properties of the cells. MSCs modulate both the adaptive and innate host immune systems by preventing T1,24–26 and B27 cell activation and proliferation, stimulating the production/expansion of CD4+FOXP3+ regulatory T cells (Tregs),28 and impairing dendritic cell maturation29,30 as well as by reprogramming monocytes and macrophages from a proinflammatory to an anti-inflammatory state.31,32 At the local renal tissue level, MSCs decrease tubular cell apoptosis11 and reduce oxidative stress.11,33 Moreover, these cells promote angiogenesis (favoring vascular supply),34,35 reduce inflammatory cell infiltration,36 and exert antifibrosis actions.15,37 Altogether, these beneficial effects contribute to repairing kidney damage.8–16 MSCs and their secretome also recruit resident progenitor cells and stimulate proliferation/differentiation of injured tubular cells, accelerating renal repair33,38 (Figure 1).
Figure 1.
MSCs exert their potential beneficial effects both systemically and at the renal level. MSCs, either constitutively or through crosstalk with target cells, release a multitude of cytokines, growth factors, and microvesicles that can inhibit the inflammatory functions of adaptive and innate immune cells and promote the development of regulatory cell populations, such as CD4+Foxp3+ Tregs, M2 macrophages, and myeloid-derived suppressor cells (MDSCs), with their own renoprotective effects. MSC secretome protects tubular cells from apoptosis and oxidative stress, favors angiogenesis, prevents/reverses renal fibrosis, inhibits inflammatory cell infiltration, and stimulates the endogenous process of renal repair. DC, dendritic cell; miRNA, micro RNA; Th, CD4+ T helper cell.
Most evidence suggests that MSCs act through the secretion of a sizable number of bioactive paracrine factors and/or release of microvesicles with immunomodulatory and proregenerative properties (Figure 1). This possibility is supported by preclinical studies showing that, after in vivo injection, exogenous MSCs can migrate into the injured kidney, where the MSC secretome generates an environment that limits kidney injury and promotes tissue repair and regeneration.8–16
Promising preclinical studies have encouraged investigators to translate this novel therapeutic approach into clinical application to explore the safety and efficacy of MSC-based therapy. Pilot clinical trials have explored MSC administration in conditions ranging from bone marrow (BM) and solid organ transplantation to autoimmune diseases and tissue and organ regeneration.7,39–42 In this review, we focus on the available safety and preliminary efficacy data on culture-expanded MSCs tested (Table 1) or currently being tested (Table 2) in kidney transplantation, AKI, and CKDs, specifically DKD, renovascular disease, and lupus nephritis. We also discuss the critical issues that will need to be addressed to definitively determine the risks and benefits of MSCs in clinical nephrology.
Table 1.
Results from MSC therapy in kidney diseases
| Studies | No. of Patients (Follow-Up) | MSC (Source, Dose, and Timing) | Main Results |
|---|---|---|---|
| Kidney transplantation | |||
| Protolerance | |||
| Perico et al.56 NCT00752479 | Living donor kidney tx recipients; n=2 (1 yr) | Autologous BM MSCs, single iv infusion of 1.7–2×106/kg, day +7 post-tx | MSC infusion was safe and well tolerated. Transient renal insufficiency associated with MSC infusion. Increased Treg-to-memory CD8+ T cell ratio and donor-specific CD8+ T cell unresponsiveness |
| Perico et al.57 NCT02012153a | Living donor kidney tx recipients; n=2 (1 yr) | Autologous BM MSCs, single iv infusion of 2×106/kg, day −1 pre-tx | MSC infusion was safe and well tolerated. No MSC-associated renal insufficiency. Increased Treg-to-memory CD8+ T cell ratio and donor-specific CD8+ T cell unresponsiveness (CML assay) |
| Mudrabettu et al.60 NCT02409940a | Living donor kidney tx recipients; n=4 (6 mo) | Autologous BM MSCs, two iv infusions of 0.2–0.8×106/kg, day −1 and day +30 post-tx | MSC infusion was safe and well tolerated. Trend of increased peripheral Treg percentages, reduced CD4+ T cell proliferation |
| Reduction of immunosuppressive drugs | |||
| Tan et al.58 NCT00658073 | Living donor kidney tx recipients; n=53 MSC + std. dose CNI; n=52 MSC 80% std. CNI dose; n=51 basiliximab + std. CNI (1 yr) | Autologous BM MSCs, two iv infusions of 1–2×106/kg, day 0 and day +14 post-tx | MSC infusion was safe and well tolerated. MSC-treated patients showed reduced incidence of acute graft rejection and opportunistic infections and had faster renal function recovery compared with controls. MSCs may replace basiliximab and allow reduction of CNI maintenance dose |
| Pan et al.61 | Living donor kidney tx recipients; n=16 MSC +50% std. tacrolimus dose; n=16 std. tacrolimus dose controls (2 yr) | Donor-derived BMs MSCs, two infusions: 5×106 into renal artery at day 0 and 2×106/kg iv at day +30 post-transplant | MSC infusion was safe and well tolerated. Comparable incidence of acute rejection and similar graft function and survival between patient groups. MSCs allow 50% reduction of CNI maintenance immunosuppression |
| Prokidney repair | |||
| Reinders et al.59 NCT00734396 | Living donor kidney tx recipients; n=6 (6 mo) | Autologous BM MSCs, two iv injections of 0.1–1×106/kg, 7 d apart at 6 mo post-transplant | MSC infusion was safe and well tolerated. Increased incidence of opportunistic infections. Resolution of tubulitis and interstitial fibrosis/tubular atrophy in two patients |
| Safety | |||
| Detry et al.62 NCT01429038a | Deceased donor kidney tx recipients; n=5 (6 mo) | Third party MSCs, single iv injection of 1.5–3×106/kg, 3–5 d post-tx | MSC infusion was safe. Three patients experienced nonsevere opportunistic infections. Two patients developed antibodies against shared kidney/MSC HLA mismatches, one patient developed DSAs, one patient developed two specific antibodies against MSCs |
| AKI | |||
| Tögel and Westenfelder68 and Gooch et al.69 NCT00733876 | Patients needing on-pump cardiac surgery at high risk of postoperative AKI; n=16 (16 mo) | Allogeneic BM MSCs (Allocure) dose escalating (doses not specified), intra-aortic infusion | Initial observations showed safety of MSCs at all tested doses and suggested early and late protection of kidney function and reduction of length of hospital stay and need for readmission |
| Swaminathan et al.71 NCT01602328 | Patients experiencing kidney injury within 48 h of their cardiac surgery; n=77 treated with MSCs, n=79 placebo (90 d) | Allogeneic BM MSCs (Allocure), single intra-arterial injection of 2×106/kg | MSC infusion was safe and well tolerated. Time to renal function recovery, need for dialysis, and mortality not different compared with placebo |
| CKDs | |||
| Packham et al.78 NCT01843387 | Patients with moderate to severe diabetic nephropathy; n=10 lower MPC dose, n=10 higher MPC dose, n=10 placebo (60 wk) | Allogeneic BM MPCs (rexlemestrocel-L), single iv injection of 150×106 or 300×106 | MPC infusion was safe and well tolerated. Trend to stabilized or improved eGFR in rexlemestrocel-L 150×106 group |
| Makhlough et al.81 NCT02166489 | Patients with ADPKD; n=6 (12 mo) | Autologous BM MSCs, single iv injection of 1–2×106/kg | MSC infusion was safe and well tolerated. MSC infusion did not associate with any significant changes in eGFR, SCr, or kidney length |
| Saad et al.82 NCT01840540 | Patients with atherosclerotic renal vascular disease; n=7 higher MSC dose, n=7 lower MSC dose, n=14 placebo (3 mo) | Autologous adipose tissue, single intra-arterial infusion of 1×105 or 2.5×105/kg | MSC infusion was safe and well tolerated. Increase in cortical perfusion and renal blood flow in both poststenotic and contralateral kidneys, reduction in tissue fractional hypoxia and GFR stabilization |
| Liang et al.83 NCT00698191 | Patients with refractory SLE; n=13 (>12 mo) | Allogeneic BM MSCs, single iv injection of 1×106/kg | MSC infusion was safe and well tolerated. Decrease in SLEDAI score and 24-h proteinuria. Two patients relapsed |
| Wang et al.86 NCT01741857 | Patients with refractory SLE; n=40 (12 mo) | UC MSCs, two iv injections of 1×106/kg, 7 d apart | MSC infusion was safe and well tolerated; 32% patients achieved major clinical response, 28% achieved partial clinical response, 12% and 16% patients relapsed at 9 and 12 mo, respectively. Significant decrease in SLEDAI and BILAG scores |
| Deng et al.88 NCT01539902 | Patients with lupus nephritis; n=12 MSC, n=6 placebo (12 mo) | UC MSCs, two iv injections of 2×108 cells, 7 d apart | MSC infusion was safe and well tolerated. No difference in achievement of remission of nephritis in MSC-treated and placebo groups |
tx, Transplantation; iv, intravenous; CML, cell-mediated lympholysis; std., standard; DSA, donor-specific antibody; MPC, mesenchymal precursor cell; SCr, serum creatinine level; SLEDAI, SLE Disease Activity Index; BILAG, British Isles Lupus Activity Group.
These studies are ongoing (Table 2).
Table 2.
Ongoing National Institutes of Health–registered clinical trials of MSCs in kidney diseases
| NCT | Sponsor | Patient Population | Cell Source | Route, Dose | Timing | Primary Study End Point | Phase, Status |
|---|---|---|---|---|---|---|---|
| Kidney transplantation | |||||||
| NCT02012153a | Mario Negri Institute for Pharmacological Research, Italy | Living donor kidney transplant recipients | Autologous BM | Single iv injection, 1–2×106/kg | Day −1 pre-tx | Safety and biologic/mechanistic study | Phase 1 recruiting |
| NCT02057965 (Triton Study) | Leiden University Medical Center, The Netherlands | Deceased or living donor kidney transplant recipients | Autologous BM | Double iv injections, 1–2×106/kg/each | Post-tx weeks 6 and 7 | To evaluate whether MSCs in combination with everolimus facilitate tacrolimus withdrawal, reduce fibrosis, and decrease the incidence of opportunistic infections | Phase 2 recruiting |
| NCT02387151 (Neptune Study) | Leiden University Medical Center, The Netherlands | Living donor kidney transplant recipients | Allogeneic BM (not sharing HLA mismatches with the donor) | Double iv injections, 1–2×106/kg per each | Post-tx weeks 25 and 26 | Safety (by the composite end point biopsy-proven acute rejection/graft loss at 12 mo) | Phase 1 recruiting |
| NCT02490020 | Third Affiliated Hospital, Sun Yat-Sen University, Guangzhou, China | DCD donor kidney transplant recipients | BM, whether autologous or allogeneic not specified | Single iv injection, 2×106/kg combined or not with intra-arterial injection of 5×106 MSCs | 48 h before tx | Incidence of biopsy-proven acute rejection and delayed graft function | Phase 1 enrolling by invitation |
| NCT02492308 | Fuzhou General Hospital, Fujian, China | Living donor kidney transplant recipients | Autologous adipose tissue | Four doses, cell dose and route not specified | Post-tx days 0, 7, 14, and 21 | To evaluate whether MSC therapy allows reducing 30% the dose of CNI | Phase 1/2 recruiting |
| NCT02409940a | Postgraduate Institute of Medical Education and Research, Chandigarh, India | Living donor kidney transplant recipients | Either autologous or allogeneic (donor-derived) BM | Double iv injections, 1–2×106/kg per each | Days −1 pre-tx and day +30 post-tx | Expansion of Tregs | Phase 1 ongoing, not recruiting |
| NCT02561767 | First Affiliated Hospital, Sun Yat-Sen University, Guangzhou, China | DCD donor kidney transplant recipients | Allogeneic BM | Four iv injections, 1×106/kg per each | Days 0, 7, 14, and 21 post-tx | Safety and efficacy (renal allograft function, rejection, patient/graft survival) | Phase 1/2 not yet open |
| NCT02563366 | First Affiliated Hospital, Sun Yat-Sen University, Guangzhou, China | DCD donor kidney transplant recipients with poor early graft function | Allogeneic BM | Four consecutive weekly iv injections, 1×106/kg per each | At manifestation of early poor graft function | Graft function recovery (eGFR at 1 mo post-tx) | Phase 1/2 not yet open |
| NCT02563340 | First Affiliated Hospital, Sun Yat-Sen University, Guangzhou, China | Kidney transplant recipients with chronic antibody-mediated rejection | Allogeneic BM | Four iv injections, 1×106/kg per each | Every 2 wk for four consecutive doses at diagnosis of AMR | Safety and efficacy (renal allograft function, DSA levels, pathologic features, patient/graft survival) | Phase 1/2 not yet open |
| NCT02565459 | Mario Negri Institute for Pharmacological Research, Italy | Deceased donor kidney transplant recipients | Allogeneic BM | Single iv injection, 1–2×106/kg | Day 0 | Safety and biologic/mechanistic study | Phase 1 recruiting |
| NCT01429038a | University Hospital of Liege, Liege, Belgium | Patients with renal or liver transplant | Allogeneic BM (no HLA matching with recipients and donors) | Single iv injection, 1.5–3×106/kg | Day 3±2 post-transplant | Safety and tolerability | Phase 1/2 unknown |
| NCT00659620 | Fuzhou General Hospital, Fujian, China | Kidney transplant recipients with chronic allograft nephropathy | Not specified | Not specified | Day 0 | To evaluate whether MSC therapy prevents rejection and maintenance of graft function | Phase 1/2 unknown |
| AKI | |||||||
| NCT01275612 | Mario Negri Institute for Pharmacological Research, Italy | Patients with solid organ cancers who developed acute renal failure after chemotherapy with cisplatin | Allogeneic BM | Single iv injection, dose escalation: 1×106, 2×106, and 5×106 MSCs per kilogram | n.a. | Safety and feasibility to improve kidney function | Phase 1 recruiting |
| NCT03015623 | Sentien Biotechnology Inc. | Subjects with AKI receiving continuous RRT | Allogeneic MSC device (SBI-101) | Extracorporeal therapy with device containing 250 or 750 million MSCs | n.a. | Safety and tolerability | Phase 1/2 not yet open |
| CKDs | |||||||
| NCT02195323 | Royan Institute, Teheran, Iran | Patients with CKDs | Autologous BM | Single iv injection, 2×106/kg | n.a. | Safety | Phase 1 completed, no results available |
| NCT02585622 (NEPHSTROM Study) | Mario Negri Institute for Pharmacological Research, Italy | Subjects with T2D and progressive DKD | Allogeneic BM (ORBCEL-M) | Single iv injection, dose escalating 80×106, 160×106, 240×106 | n.a. | Safety, feasibility, and tolerability | Phase 1/2 not yet open |
| NCT02266394 | Mayo Clinic in Rochester | Patients with advanced renovascular disease | Autologous adipose tissue | Single intra-arterial injection, dose not specified | n.a. | To evaluate whether MSCs before percutaneous transluminal renal angioplasty with stenting enhance changes in single kidney blood flow and restoration of kidney function | Phase 1 recruiting |
| NCT00659217 | Organ Transplant Institute, Fujian, China | Patients with lupus nephritis | Autologous BM | Not specified | n.a. | Improvement of lupus disease | Phase 1/2 unknown |
| NCT02633163 (MsciSLE Study) | Medical University of South Carolina | Patients with SLE | Allogeneic, UC | Single iv injection, 1×106 or 5×106/kg, | n.a. | Safety and efficacy in inducing clinical response | Phase 2 not yet open |
NCT, ClinicalTrials.gov identifier; iv, intravenous; tx, transplantation; DCD, donation after cardiac death; AMR, antibody-mediated rejection; DSA, donor-specific antibody; n.a., not applicable; NEPHSTROM, Novel Stromal Cell Therapy for Diabetic Kidney Disease; MsciSLE, MSCs in SLE Trial.
Preliminary data are available (Table 1); for the NCT01429038 trial, data in patients with liver transplants have been published.65
MSCs for Clinical Use
MSCs are most commonly isolated from the BM.43 On seeding onto cell culture dishes, MSCs readily adhere to the plastic surfaces and can be expanded to yield clinically useful cell numbers. Although final MSC preparations appear morphologically uniform, they consist of heterogeneous subpopulations. So far, however, no specific marker or quantitative assay has been found to be helpful in the identification of MSCs in a mixed population. In an effort to better characterize MSCs, the International Society of Cell Therapy proposed the following minimal criteria for MSC definition: adherence to plastic; positive for CD105, CD73, and CD90 expression on the cell surface; negative for CD45, CD34, CD19, and CD79 marker expression; and exhibiting the capacity to differentiate into chondrocytes, osteoblasts, and adipocytes under controlled in vitro culture conditions.44 Nevertheless, a very high variability in MSC preparations still remains among laboratories depending on different isolation and expansion methods, culture conditions, and initial cell source.45 Specifically, cells exhibiting characteristics of MSCs also have been isolated from multiple fetal and adult tissues, such as umbilical cord (UC) and adipose tissue,46,47 which are more easily accessible than BM. In addition, the frequency of MSCs in primary sources, the cell expansion potential, and the MSC secretome vary considerably from donor to donor and depend on donor age and disease condition.48 These shortcomings coupled with the evidence that MSCs are low-immunogenic and immune-evasive cells49 have stimulated the development of allogeneic MSC products obtained from young, healthy donors and manufactured in large scale in compliance with Good Manufacturing Practice standards to ensure safety, purity, and potency. Off the shelf allogeneic MSC therapy is being exploited for commercial development by small and medium enterprises that are investing in the preparation of a high-quality affordable allogeneic MSC product, starting from more characterized progenitor cells,50 to meet future regulatory guidelines.51
Accordingly, the MSC populations that have been tested or are currently being tested in clinical trials in patients with kidney diseases vary widely in tissue source (BM, UC, or adipose tissue), whether they are of autologous or allogeneic origin, and whether they come from academic facilities or commercial manufacturers (Tables 1 and 2). These differences may underlie at least some of the inconsistencies observed in the results from clinical trials conducted to date.
Kidney Transplantation
Lifelong, nonspecific immunosuppressive drugs, although essential to preventing allograft rejection, impose a substantial risk of morbidity and mortality and hinder tumor immunosurveillance.52–55 Given the immune-regulatory properties of MSCs, these cells have been administered to transplant recipients with the hope of tipping the balance between effector and regulatory pathways and eventually promoting the host potential to control the immune response to the allograft without the use or with minimal use of immunosuppressive drugs. To date, this possibility has mainly been explored in kidney transplantation.7 Results from MSC-based therapy in kidney transplant recipients are, however, available from only six phase 1 clinical studies (four using autologous56–60 and two using allogeneic MSCs61,62) (Table 1), all with cells prepared by academic laboratories. Twelve studies in kidney transplant recipients are still ongoing, with no outcomes publicly available yet (Table 2).
In 2011, we first reported the initial results of a pilot safety and feasibility study with autologous BM MSCs in two recipients of a kidney allograft from a living related donor.7,56 Cells (1.7–2.0×106/kg body wt) were intravenously infused 7 days after kidney transplant at the end of induction therapy with basiliximab/low-dose rabbit antithymocyte globulin (RATG) to avoid possible RATG-mediated MSC lysis and when the patients were on maintenance immunosuppression with low-dose cyclosporin A and mycophenolate mofetil (MMF).7,56 Unexpectedly, transient renal insufficiency (engraftment syndrome) was observed in both patients 7–10 days after the single MSC infusion.7,56
The translation of the post-transplant MSC infusion protocol back to a kidney transplant model in mice confirmed the development of acute renal insufficiency and showed that it could be avoided by performing cell infusion before the transplant.6,7 The model also helped to clarify that, in the setting of post-transplant MSC infusion, the postsurgery subclinical inflammatory environment promoted the recruitment of MSCs into the graft, where the cells amplified the inflammatory process through complement activation and proinflammatory cytokine release.6 The clinical protocol was amended accordingly, and two subsequent patients who received MSCs before transplant surgery did not experience engraftment syndrome.7,57 All MSC-treated patients are still being followed 5–8 years after transplant, and they have stable graft function and no major adverse effects. Extensive longitudinal immunomonitoring of these patients has also revealed that MSC infusion is associated with an increased ratio of circulating CD4+CD25+FOXP3+ Tregs to memory CD8+ T cells, long-lasting donor-specific hyporesponsiveness of CD8+ T cells in ex vivo cell-mediated lympholysis tests,7 and development of the B cell signature reported to be associated with spontaneous and induced immune tolerance.63 Notably, none of the MSC-treated patients developed donor-specific antibodies, at variance with historical matched control kidney transplant recipients given only the same immunosuppressive therapy. This protolerogenic environment was remarkably highlighted in one patient, prompting us to successfully withdraw cyclosporin A and start tapering the already low-dose MMF maintenance immunosuppression (G. Remuzzi, personal communication).
Similar findings have been obtained in a more recent study in India in patients undergoing living donor renal transplant given RATG as induction therapy and two intravenous infusions of autologous BM MSCs (0.21–2.80×106/kg): one infusion the day before and one at 1 month after transplant.60 With the limitations of a wide range of MSC doses in a very small number of patients and the short 6-month follow-up period, the results showed that the cell infusion was safe, and patients had excellent graft function and normal graft histology at 1- and 3-month protocol biopsies. An increase in circulating CD4+CD25+FOXP3+ Tregs and a reduction in ex vivo polyclonal proliferation of CD4+ T cells at day 90 after transplant compared with baseline were also found, suggesting that a protolerogenic environment had developed, possibly induced by MSCs60 (Table 1).
Other investigators took a different approach and examined use of MSC treatment to avoid induction therapy and minimize conventional maintenance pharmacologic immunosuppression. In a large randomized clinical trial in China, patients undergoing kidney transplants with allografts from living related donors received a double intravenous infusion of autologous BM MSCs (1–2×106/kg) at kidney reperfusion and 2 weeks later in combination with standard dose (n=53 patients) or 80% standard dose (n=52 patients) calcineurin inhibitors (CNIs).58 Patients (n=51) in the control group were given the anti–IL-2 receptor antibody basiliximab plus standard dose CNIs. Patients given autologous MSCs did not experience early adverse events (as assessed by monitoring vital signs, hematologic and laboratory parameters, wound healing, and formation of lymphocele) and showed a lower incidence of opportunistic infections than controls exhibited over 1-year follow-up. MSC treatment resulted in a lower incidence of acute rejection at 6 months but not at the 1-year follow-up and better estimated renal function during the 1-year follow-up compared with the control group, suggesting that MSC-based therapy could replace basiliximab induction therapy and enable the use of lower CNI maintenance doses without compromising patient safety and graft outcome58 (Table 1). Unfortunately, this study did not provide any indication regarding the immunologic mechanisms underlying the favorable clinical outcomes of MSC infusion in this large cohort.
It was also proposed that allogeneic MSCs minimize maintenance immunosuppressive therapy. Indeed, in a non-National Institutes of Health–registered Chinese trial,61 16 kidney transplant recipients were given allogeneic MSCs isolated from the BM of living related kidney donors. MSCs, delivered into the renal allograft artery during surgery (5.0×106) and given intravenously 1 month later (2.0×106/kg), were combined with low-dose tacrolimus (0.04–0.05 mg/kg per day) maintenance therapy. Sixteen control kidney transplant recipients received a standard dose of tacrolimus (0.07–0.08 mg/kg per day). All patients underwent induction therapy with cyclophosphamide (Cytoxan). During the 24-month follow-up, a similar incidence of adverse events (fever, diabetes hyperlipidemia, anemia, lung infection, and liver dysfunction) was observed in the two groups of patients. MSC-treated patients showed stable graft function, with no significant differences in the rate of acute rejection episodes or serum creatinine and eGFR values compared with those in the control group61 (Table 1).
Autologous MSC infusion has also been used to treat subclinical rejection and graft tubulointerstitial injury. In a small clinical study, six living donor kidney transplant recipients whose 6-month protocol biopsy specimen showed signs of subclinical rejection and/or increase in interstitial fibrosis/tubular atrophy received two intravenous infusions (7 days apart) of autologous BM MSCs (0.1–1.0×106/kg).59 Cell infusion was associated with the resolution of rejection and interstitial fibrosis/tubular atrophy in two patients undergoing surveillance biopsies after treatment. The ex vivo immunologic test showed reduced lymphocyte proliferation in response to donor antigens without significant changes in total numbers and subsets of circulating T cells, B cells, natural killer cells, and monocytes. However, opportunistic viral infections occurred in three patients, raising some concerns about possible MSC-induced overimmunosuppression59 (Table 1).
However, a recent report in abstract format, showing results of the 6-month safety interim analysis of third party MSC infusion in kidney transplant recipients,62,64 revealed some degree of immunization after treatment with allogeneic MSCs. In this study, five patients with kidney transplants from deceased donors treated with basiliximab induction, tacrolimus, MMF, and steroid received third party MSCs (1.5–3.0×106/kg) 3–5 days after transplant. Each patient had HLA mismatches with kidney and MSC donors. Three patients experienced nonsevere opportunistic infections. A prospective screening for anti-HLA antibody development showed that, during the 6-month follow-up, one patient developed de novo donor-specific antibodies, two patients developed anti-HLA antibodies against shared kidney/MSC mismatches, and one patient developed two specific antibodies against MSCs.62 To assess the clinical relevance of these anti-HLA antibodies, it will be important to monitor whether they persist at high levels in the peripheral blood. Of note, the same group recently published the results of third party MSC therapy in patients with liver transplants,65 showing an excellent safety profile. Ten liver transplant recipients treated with tacrolimus, MMF, and steroids were given 1.9–2.7×106 third party MSCs per kilogram on day 3 after transplant and compared with a control group of ten patients with liver transplants given the immunosuppressive therapy alone. During the 1-year follow-up, the rates of opportunistic infections were comparable in the two groups. Unfortunately, no data on anti-HLA antibody development after allogeneic MSC infusion were reported.65
Overall, the preliminary experience with MSC in kidney transplantation indicates that this cell therapy can be used safely if combined with an adequate immunosuppressive regimen and is capable of dampening alloimmune responses. MSCs have enabled minimization of the dose of current immunosuppressants, at least in low-risk kidney transplant recipients, and in anecdotal cases in patients, MSCs have enabled the almost complete withdrawal of maintenance immunosuppressive drugs. These results allow optimism that MSC therapy may be valuable in reducing adverse effects observed with current immunosuppressive drugs and eventually, that it may prolong patient and graft survival. In kidney transplantation, the final aim of MSC therapy should be to promote donor-specific immune tolerance, eliminating all maintenance immunosuppressive drugs by a certain time after transplant, with an approach that avoids the need for the pre- or peritransplant toxic multidrug conditioning regimens required by other strategies with hematopoietic stem cells or so-called “facilitating cells” currently used to induce graft tolerance.66,67 Our initial experience indicates that this could be achievable, especially by adopting T cell–depleting biologics as an induction therapy that promotes lymphopenia-induced homeostatic proliferation, which is deemed relevant for the later development of an effective protolerogenic environment in transplant recipients.
Ongoing clinical trials (Table 2) will provide more insights into and possibly further support these achievements with MSCs, and eventually, they may verify whether these findings can also be replicated in the setting of a deceased donor kidney transplantation program to benefit the largest number of transplant recipients.
AKI
AKI is a great challenge for nephrologists, because disease-specific treatments are lacking, and the mainstay is supportive care. The evidence from preclinical studies that MSCs can promote tissue repair suggests a new therapeutic possibility for AKI.8–13 However, the available clinical data on the use of MSCs in an AKI setting are still meager.
A recently completed phase 1 exploratory study evaluated the safety and efficacy of allogeneic BM MSCs in preventing AKI in 16 patients undergoing on-pump cardiac surgery who were at high risk of postoperative AKI due to underlying CKD, advanced age, diabetes mellitus, congestive heart failure, or chronic obstructive lung disease.68,69 MSCs were infused at low, intermediate, or high doses into the suprarenal aorta through a femoral catheter after completion of surgery. A preliminary report indicated the safety of the treatment at all tested doses. Compared with results in matched historical control patients, MSCs appeared to protect early and late postsurgery kidney function against AKI development and reduce the length of hospital stays and the need for readmission68,69 (Table 1).
These positive results were not confirmed in a similar cohort of patients when MSCs were administered to treat established AKI. In a randomized, double-blind, phase 2 study (NCT01602328) (Table 1), patients experiencing AKI within 48 hours of cardiac surgery received a single intra-aortic administration of 2×106 allogeneic MSCs per kilogram (AC607; Allocure) or placebo. The trial was terminated prematurely for futility,70 because after the enrollment of 156 patients, time to renal function recovery, the need for dialysis, and mortality were not significantly different between groups. The MSC infusion was, however, safe and well tolerated71 (Table 1).
Although very preliminary, these studies confirm the safety of MSC treatment and suggest that, at least in postcardiac surgery AKI, MSCs could be somewhat helpful in preventing the development of kidney injury but may be of no value as therapy to recover renal function in established AKI. There are no other ongoing registered clinical trials for the treatment of postcardiac surgery AKI, thus leaving unexplored the possibility of a potential beneficial effect of MSC therapy at doses higher than those reported so far (Table 2).
CKDs
Most chronic nephropathies progress relentlessly to ESRD.72 Current therapeutic strategies to prevent or reverse renal disease progression focus on reducing urinary protein excretion and controlling BP through blockade of the renin-angiotensin system with angiotensin-converting enzyme inhibitors and/or angiotensin II type 1 receptor blockers.73–76 Nevertheless, nephroprotection through renin-angiotensin system blockade and BP control is only partial, and loss of renal function is often progressive.75,76 In chronic nonproteinuric nephropathies, such as autosomal dominant polycystic kidney disease (ADPKD), inflammation and injury of the tubulointerstitium are prominent factors in renal disease progression, and disease-modifying treatments are also inadequate. For both cohorts of patients with CKD, BM MSCs have been or are being tested in early stages of clinical translation to explore the safety, tolerability, and preliminary efficacy of this cell therapy on the basis of the anti-inflammatory and tissue regenerative properties.14–23
DKD
Two randomized, placebo-controlled clinical trials in patients with type 2 diabetes (T2D) have recently been published. In the first study, 61 patients with T2D (mean glycated hemoglobin, 8.3%) but no renal involvement were enrolled in 18 centers in the United States and randomized to one of the three doses (0.3, 1.0, or 2.0×106 cells per kilogram) of mesenchymal precursor cells (rexlemestrocel-L; produced by Mesoblast) or placebo.77 Cell infusion was safe and also showed a good safety profile over the 12-week follow-up. Explorative efficacy analysis focusing on glycemic control showed that the glycated hemoglobin target of <7% at week 12 was achieved by 33% of patients given the highest dose of rexlemestrocel-L compared with 0% of patients in the placebo group.77
A more recent trial focused on patients with advanced DKD (eGFR=20–50 ml/min per 1.73 m2) enrolled in three Australian centers; the primary outcome of this study was the safety of rexlemestrocel-L cell therapy, although it included a preliminary look at the effect on disease progression as a secondary outcome.78 Patients received a single intravenous infusion of rexlemestrocel-L at two different fixed doses (150×106 or 300×106 cells) or placebo and were observed for 60 weeks.78 Cell treatment was safe and well tolerated, and none of the patients developed sustained antibodies against allogeneic cell donor HLA. The improvement in glycemic control observed in the United States study,77 however, was not confirmed in the Australian patients with DKD cohort, possibly due to the large range of glycated hemoglobin (5.1%–11.2%) at baseline in a small number of patients enrolled. Interestingly, cell treatment associated with a trend toward stabilized or improved eGFR over the 60-week follow-up, with a more pronounced effect for patients with a baseline eGFR >30 ml/min per 1.73 m278 (Table 1).
A third randomized phase 1b/2a clinical trial with allogeneic BM MSC preparation (ORBCEL-M; Orbsen Therapeutics; 80×106, 160×106, and 240×106 cells) or placebo is ongoing with patients with T2D and progressive DKD with mild to moderate renal insufficiency (the Novel Stromal Cell Therapy for Diabetic Kidney Disease Study; NCT 02585622). In addition to addressing the safety of cell treatment, the add-on value of this multinational study rests in its use of a well characterized and standardized cell product for all participating centers, longer study period (up to 18 months), and gold standard methods to measure GFR79 (Table 2).
Should these initial carefully designed studies consistently confirm the early and long-term safety of MSC therapy, the next efficacy trials should consider starting cell treatment in an early phase of DKD before the structural integrity of the glomerular filter and tubulointerstitium is irreversibly compromised,80 drawing from the lessons of recent clinical trials with conventional and novel drugs.76
ADPKD
The safety and tolerability of autologous BM MSCs have recently been tested in a single-arm phase 1 clinical trial in six patients with ADPKD and eGFR ranging from 25 to 32 ml/min per 1.73 m281 (Table 1), and no cell-related adverse events were observed in MSC-treated patients during the 12-month follow-up. However, a single-cell infusion (2×106 cells per kilogram intravenously) did not significantly affect renal function decline or kidney length, an unusual surrogate index of kidney growth.81 These negative findings, albeit in a very small number of patients, are not surprising considering the patients’ advanced stage of kidney disease when MSC treatment was started and the unexplored possible intrinsic dysfunction in terms of anti-inflammatory and repairing properties of MSCs derived from patients with ADPKD, a genetic disorder.
Renovascular Disease
Very recently, results of a dose-escalating phase 1/2a study assessing safety and efficacy of autologous adipose tissue–derived MSCs in patients with atherosclerotic renovascular disease have been published82 (Table 1). Compared with standardized medical treatment, the infusion of MSCs into the renal artery at a low dose (1.0×105 cells per kilogram; n=7 patients) or higher dose (2.5×105 cells per kilogram; n=7 patients) was well tolerated and induced a significant increase in cortical perfusion and renal blood flow, a reduction of tissue fractional hypoxia, and stabilization of measured GFR at 3-month follow-up.82 This successful study suggests that MSCs, through proangiogenic and immunomodulatory effects, may limit vascular insufficiency and inflammatory injury in the ischemic kidney environment. Another phase 1 study of intrarenal delivery of autologous adipose tissue MSCs in patients with large vessel renovascular disease is currently recruiting patients (Table 2). This study is designed to assess whether MSC infusion before percutaneous transluminal renal angioplasty with stenting further enhances renal blood flow and restores kidney function.
Lupus Nephritis
The results of phase 1/2 clinical trials examining the safety and initial therapeutic benefit of allogeneic BM- or UC-derived MSCs in patients with SLE refractory to current pharmacologic treatment protocols are conflicting. After an initial promising pilot study in four patients,23 the same investigators showed the safety of systemic cell infusion in 13 patients given allogeneic BM MSCs (1×106 cells per kilogram intravenously). Eleven of these patients showed an improvement in the SLE Disease Activity Index disease index score and a reduction in autoreactive antibody serum levels at the 12-month follow-up.83 A significant decrease of proteinuria was also observed in these 11 patients during the entire follow-up, whereas relapse of the disease occurred in the remaining two patients.83 The observed improvement in SLE disease has been attributed to MSC-induced expansion of circulating CD4+Foxp3+ Tregs,83 but the exact mechanism remains ill defined. Similar results have been achieved in patients with severe and refractory SLE given systemic allogeneic UC MSCs (1×106 cells per kilogram intravenously), with improvements in the disease index score, proteinuria, and serologic markers and without any particular adverse effects attributed to cell infusion.84 These studies have also documented that there is an overall survival rate ranging between 92.5% and 95%, with complete or partial clinical remission in approximately 30%–50% of patients with SLE and a rate of relapse of 22%–23% for up to 4 years of follow-up.85–87
In contrast, the recently published multicenter, randomized, double-blind, placebo-controlled clinical trial in 18 patients with World Health Organization class 3 or 4 lupus nephritis failed to show any beneficial effect of UC MSCs.88 Unfortunately, the lack of details about the isolation and expansion procedures and the lack of characterization of UC MSCs preclude proper evaluation of the study. The conflicting results of these studies with very small cohorts of patients with SLE, almost all performed by a single center in China, illustrate the further need of larger, sufficiently powered, randomized studies with appropriate controls before any robust conclusion can be drawn about the therapeutic potential of MSCs in patients with lupus nephritis (Table 1). Overall, none of the trials in >80 patients with SLE reported any adverse events attributable to MSC treatment. Further study will be needed to determine if select patients with refractory SLE could benefit from the immunomodulatory properties of MSCs.
Future Perspectives
Efforts by numerous clinical research groups support the safety and tolerability of MSC treatment (both from academic and commercial entities) in the context of kidney transplantation, AKI, or CKD. The transient renal insufficiency (engraftment syndrome) reported in two kidney transplant recipients when MSCs were infused intravenously a few days after transplant56 can be avoided by giving the cell infusion before transplant. The concern of increasing the risk of opportunistic infections with MSC treatment, as highlighted by anecdotal cases in patients,59 has been tempered by the reassuring safety data from a large kidney transplant program58 and a small liver transplant study.65 The risk of developing anti-HLA antibodies after MSC infusion will need continued assessment but has only been observed in a few patients.62 The reassuring safety profile of MSCs in patients with kidney disease is in line with the overall published information in other conditions,89 but clearly, long-term monitoring of MSC-treated patients with nephropathy should continue, especially for the potential risk of developing anti-HLA antibodies and/or cancers.
The preliminary efficacy data show promise, especially in kidney transplantation and renovascular disease; nevertheless, the road to clinical implementation of this therapeutic approach is hindered by our still limited knowledge of MSC mechanisms of action in vivo. Greater understanding of the mechanism will help define the best administration route, cell source, and dosing. As shown in animal studies9,90–95 and a few human studies,56,96,97 whether MSCs are administered by systemic intravenous infusion or the intrarenal artery affects cell localization and shapes the plasticity of the anti- or proinflammatory phenotype.98–103 Establishing how environmental factors modify MSC phenotype and function is, therefore, crucial to informing the design of large clinical trials to optimize the therapeutic potential of these cells. Thus, efficacy trials of MSC-based therapy should also include long-term mechanistic studies, and as much as possible, methods should be standardized to facilitate comparison and data sharing.
The heterogeneity of MSC preparations represents an important barrier to comparison of clinical trial outcomes and remains problematic for the clinical translation of this therapy. Methods to purify the MSC population are currently being investigated, with the aim of isolating a specific cell subpopulation with efficacy similar or superior to that of the heterogeneous MSCs currently used.104 Although this approach will enable the development of a clinical-grade allogeneic homogeneous cell population to be used for future clinical trials, it is not without challenges.50 Indeed, the lack of specific cell markers for MSCs makes it difficult to isolate an effective cell subpopulation from the initial heterogeneous cell preparation. Furthermore, extensive ex vivo expansion of BM- or UC-derived MSCs is required to generate a sufficient number of cells for treating a given patient cohort. This is a major challenge, because MSCs lose immunosuppressive and trophic potency over time during culture.105,106 The development of robust potency assays to be applied during MSC ex vivo expansion and optimization of culture conditions would provide evidence of persistent key therapeutic effector properties of the cells before storage. This further highlights the importance of using only MSCs that have been characterized and generated as a standard product for clinical studies. Also, the debate regarding the cell dose and whether single or repeated infusions should be used for a given outcome are far from being solved. MSCs have been and are being infused in patients with kidney diseases at the doses of 0.1–5.0×106 cells per kilogram, a range chosen empirically according to previous MSC experiences in patients with hematologic diseases and the expected number of cells achievable by ex vivo MSC expansion. Therefore, well designed dose-finding studies as well as proper cost-effectiveness analyses are still needed before moving this innovative cell therapy to widespread clinical trials.
Even with these unknown factors and much work to be done, we predict that, over the next decade, the translational gap between scientific observation on MSC function and clinical application will be bridged. We see an optimistic future for cell-based therapies of kidney disease.
Disclosures
None.
Acknowledgments
This work was supported by the European Union’s Horizon 2020 Research and Innovation Program under grant 634086 (the Novel Stromal Cell Therapy for Diabetic Kidney Disease Study) and the Fondazione Associazione Ricerca Trapianti (ART) per la Ricerca sui Trapianti (Milan, Italy).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Bartholomew A, Sturgeon C, Siatskas M, Ferrer K, McIntosh K, Patil S, Hardy W, Devine S, Ucker D, Deans R, Moseley A, Hoffman R: Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol 30: 42–48, 2002 [DOI] [PubMed] [Google Scholar]
- 2.Casiraghi F, Azzollini N, Cassis P, Imberti B, Morigi M, Cugini D, Cavinato RA, Todeschini M, Solini S, Sonzogni A, Perico N, Remuzzi G, Noris M: Pretransplant infusion of mesenchymal stem cells prolongs the survival of a semiallogeneic heart transplant through the generation of regulatory T cells. J Immunol 181: 3933–3946, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Ge W, Jiang J, Baroja ML, Arp J, Zassoko R, Liu W, Bartholomew A, Garcia B, Wang H: Infusion of mesenchymal stem cells and rapamycin synergize to attenuate alloimmune responses and promote cardiac allograft tolerance. Am J Transplant 9: 1760–1772, 2009 [DOI] [PubMed] [Google Scholar]
- 4.Popp FC, Eggenhofer E, Renner P, Slowik P, Lang SA, Kaspar H, Geissler EK, Piso P, Schlitt HJ, Dahlke MH: Mesenchymal stem cells can induce long-term acceptance of solid organ allografts in synergy with low-dose mycophenolate. Transpl Immunol 20: 55–60, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Solari MG, Srinivasan S, Boumaza I, Unadkat J, Harb G, Garcia-Ocana A, Feili-Hariri M: Marginal mass islet transplantation with autologous mesenchymal stem cells promotes long-term islet allograft survival and sustained normoglycemia. J Autoimmun 32: 116–124, 2009 [DOI] [PubMed] [Google Scholar]
- 6.Casiraghi F, Azzollini N, Todeschini M, Cavinato RA, Cassis P, Solini S, Rota C, Morigi M, Introna M, Maranta R, Perico N, Remuzzi G, Noris M: Localization of mesenchymal stromal cells dictates their immune or proinflammatory effects in kidney transplantation. Am J Transplant 12: 2373–2383, 2012 [DOI] [PubMed] [Google Scholar]
- 7.Casiraghi F, Perico N, Cortinovis M, Remuzzi G: Mesenchymal stromal cells in renal transplantation: Opportunities and challenges. Nat Rev Nephrol 12: 241–253, 2016 [DOI] [PubMed] [Google Scholar]
- 8.Morigi M, Introna M, Imberti B, Corna D, Abbate M, Rota C, Rottoli D, Benigni A, Perico N, Zoja C, Rambaldi A, Remuzzi A, Remuzzi G: Human bone marrow mesenchymal stem cells accelerate recovery of acute renal injury and prolong survival in mice. Stem Cells 26: 2075–2082, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Herrera MB, Bussolati B, Bruno S, Fonsato V, Romanazzi GM, Camussi G: Mesenchymal stem cells contribute to the renal repair of acute tubular epithelial injury. Int J Mol Med 14: 1035–1041, 2004 [PubMed] [Google Scholar]
- 10.Luo CJ, Zhang FJ, Zhang L, Geng YQ, Li QG, Hong Q, Fu B, Zhu F, Cui SY, Feng Z, Sun XF, Chen XM: Mesenchymal stem cells ameliorate sepsis-associated acute kidney injury in mice. Shock 41: 123–129, 2014 [DOI] [PubMed] [Google Scholar]
- 11.Tögel F, Hu Z, Weiss K, Isaac J, Lange C, Westenfelder C: Administered mesenchymal stem cells protect against ischemic acute renal failure through differentiation-independent mechanisms. Am J Physiol Renal Physiol 289: F31–F42, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Duffield JS, Park KM, Hsiao LL, Kelley VR, Scadden DT, Ichimura T, Bonventre JV: Restoration of tubular epithelial cells during repair of the postischemic kidney occurs independently of bone marrow-derived stem cells. J Clin Invest 115: 1743–1755, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lange C, Tögel F, Ittrich H, Clayton F, Nolte-Ernsting C, Zander AR, Westenfelder C: Administered mesenchymal stem cells enhance recovery from ischemia/reperfusion-induced acute renal failure in rats. Kidney Int 68: 1613–1617, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Lee SR, Lee SH, Moon JY, Park JY, Lee D, Lim SJ, Jeong KH, Park JK, Lee TW, Ihm CG: Repeated administration of bone marrow-derived mesenchymal stem cells improved the protective effects on a remnant kidney model. Ren Fail 32: 840–848, 2010 [DOI] [PubMed] [Google Scholar]
- 15.Semedo P, Correa-Costa M, Antonio Cenedeze M, Maria Avancini Costa Malheiros D, Antonia dos Reis M, Shimizu MH, Seguro AC, Pacheco-Silva A, Saraiva Camara NO: Mesenchymal stem cells attenuate renal fibrosis through immune modulation and remodeling properties in a rat remnant kidney model. Stem Cells 27: 3063–3073, 2009 [DOI] [PubMed] [Google Scholar]
- 16.Zhu XY, Urbieta-Caceres V, Krier JD, Textor SC, Lerman A, Lerman LO: Mesenchymal stem cells and endothelial progenitor cells decrease renal injury in experimental swine renal artery stenosis through different mechanisms. Stem Cells 31: 117–125, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee RH, Seo MJ, Reger RL, Spees JL, Pulin AA, Olson SD, Prockop DJ: Multipotent stromal cells from human marrow home to and promote repair of pancreatic islets and renal glomeruli in diabetic NOD/scid mice. Proc Natl Acad Sci U S A 103: 17438–17443, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ezquer FE, Ezquer ME, Parrau DB, Carpio D, Yañez AJ, Conget PA: Systemic administration of multipotent mesenchymal stromal cells reverts hyperglycemia and prevents nephropathy in type 1 diabetic mice. Biol Blood Marrow Transplant 14: 631–640, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Lv S, Cheng J, Sun A, Li J, Wang W, Guan G, Liu G, Su M: Mesenchymal stem cells transplantation ameliorates glomerular injury in streptozotocin-induced diabetic nephropathy in rats via inhibiting oxidative stress. Diabetes Res Clin Pract 104: 143–154, 2014 [DOI] [PubMed] [Google Scholar]
- 20.Park JH, Hwang I, Hwang SH, Han H, Ha H: Human umbilical cord blood-derived mesenchymal stem cells prevent diabetic renal injury through paracrine action. Diabetes Res Clin Pract 98: 465–473, 2012 [DOI] [PubMed] [Google Scholar]
- 21.Zhou K, Zhang H, Jin O, Feng X, Yao G, Hou Y, Sun L: Transplantation of human bone marrow mesenchymal stem cell ameliorates the autoimmune pathogenesis in MRL/lpr mice. Cell Mol Immunol 5: 417–424, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schena F, Gambini C, Gregorio A, Mosconi M, Reverberi D, Gattorno M, Casazza S, Uccelli A, Moretta L, Martini A, Traggiai E: Interferon-γ-dependent inhibition of B cell activation by bone marrow-derived mesenchymal stem cells in a murine model of systemic lupus erythematosus. Arthritis Rheum 62: 2776–2786, 2010 [DOI] [PubMed] [Google Scholar]
- 23.Sun L, Akiyama K, Zhang H, Yamaza T, Hou Y, Zhao S, Xu T, Le A, Shi S: Mesenchymal stem cell transplantation reverses multiorgan dysfunction in systemic lupus erythematosus mice and humans. Stem Cells 27: 1421–1432, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, Matteucci P, Grisanti S, Gianni AM: Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood 99: 3838–3843, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Glennie S, Soeiro I, Dyson PJ, Lam EW, Dazzi F: Bone marrow mesenchymal stem cells induce division arrest anergy of activated T cells. Blood 105: 2821–2827, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Krampera M, Glennie S, Dyson J, Scott D, Laylor R, Simpson E, Dazzi F: Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen-specific T cells to their cognate peptide. Blood 101: 3722–3729, 2003 [DOI] [PubMed] [Google Scholar]
- 27.Tabera S, Pérez-Simón JA, Díez-Campelo M, Sánchez-Abarca LI, Blanco B, López A, Benito A, Ocio E, Sánchez-Guijo FM, Cañizo C, San Miguel JF: The effect of mesenchymal stem cells on the viability, proliferation and differentiation of B-lymphocytes. Haematologica 93: 1301–1309, 2008 [DOI] [PubMed] [Google Scholar]
- 28.English K, Ryan JM, Tobin L, Murphy MJ, Barry FP, Mahon BP: Cell contact, prostaglandin E(2) and transforming growth factor beta 1 play non-redundant roles in human mesenchymal stem cell induction of CD4+CD25(High) forkhead box P3+ regulatory T cells. Clin Exp Immunol 156: 149–160, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.English K, Barry FP, Mahon BP: Murine mesenchymal stem cells suppress dendritic cell migration, maturation and antigen presentation. Immunol Lett 115: 50–58, 2008 [DOI] [PubMed] [Google Scholar]
- 30.Chiesa S, Morbelli S, Morando S, Massollo M, Marini C, Bertoni A, Frassoni F, Bartolomé ST, Sambuceti G, Traggiai E, Uccelli A: Mesenchymal stem cells impair in vivo T-cell priming by dendritic cells. Proc Natl Acad Sci U S A 108: 17384–17389, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chiossone L, Conte R, Spaggiari GM, Serra M, Romei C, Bellora F, Becchetti F, Andaloro A, Moretta L, Bottino C: Mesenchymal stromal cells induce peculiar alternatively activated macrophages capable of dampening both innate and adaptive immune responses. Stem Cells 34: 1909–1921, 2016 [DOI] [PubMed] [Google Scholar]
- 32.Németh K, Leelahavanichkul A, Yuen PS, Mayer B, Parmelee A, Doi K, Robey PG, Leelahavanichkul K, Koller BH, Brown JM, Hu X, Jelinek I, Star RA, Mezey E: Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med 15: 42–49, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu H, McTaggart SJ, Johnson DW, Gobe GC: Original article anti-oxidant pathways are stimulated by mesenchymal stromal cells in renal repair after ischemic injury. Cytotherapy 14: 162–172, 2012 [DOI] [PubMed] [Google Scholar]
- 34.Tögel F, Zhang P, Hu Z, Westenfelder C: VEGF is a mediator of the renoprotective effects of multipotent marrow stromal cells in acute kidney injury. J Cell Mol Med 13[8B]: 2109–2114, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rubina K, Kalinina N, Efimenko A, Lopatina T, Melikhova V, Tsokolaeva Z, Sysoeva V, Tkachuk V, Parfyonova Y: Adipose stromal cells stimulate angiogenesis via promoting progenitor cell differentiation, secretion of angiogenic factors, and enhancing vessel maturation. Tissue Eng Part A 15: 2039–2050, 2009 [DOI] [PubMed] [Google Scholar]
- 36.Chen YT, Sun CK, Lin YC, Chang LT, Chen YL, Tsai TH, Chung SY, Chua S, Kao YH, Yen CH, Shao PL, Chang KC, Leu S, Yip HK: Adipose-derived mesenchymal stem cell protects kidneys against ischemia-reperfusion injury through suppressing oxidative stress and inflammatory reaction. J Transl Med 9: 51, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alfarano C, Roubeix C, Chaaya R, Ceccaldi C, Calise D, Mias C, Cussac D, Bascands JL, Parini A: Intraparenchymal injection of bone marrow mesenchymal stem cells reduces kidney fibrosis after ischemia-reperfusion in cyclosporine-immunosuppressed rats. Cell Transplant 21: 2009–2019, 2012 [DOI] [PubMed] [Google Scholar]
- 38.Bruno S, Grange C, Collino F, Deregibus MC, Cantaluppi V, Biancone L, Tetta C, Camussi G: Microvesicles derived from mesenchymal stem cells enhance survival in a lethal model of acute kidney injury. PLoS One 7: e33115, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stenger EO, Krishnamurti L, Galipeau J: Mesenchymal stromal cells to modulate immune reconstitution early post-hematopoietic cell transplantation. BMC Immunol 16: 74, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Munneke JM, Spruit MJ, Cornelissen AS, van Hoeven V, Voermans C, Hazenberg MD: The potential of mesenchymal stromal cells as treatment for severe steroid-refractory acute graft-versus-host disease: A critical review of the literature. Transplantation 100: 2309–2314, 2016 [DOI] [PubMed] [Google Scholar]
- 41.Pistoia V, Raffaghello L: Mesenchymal stromal cells and autoimmunity. Int Immunol 29: 49–58, 2017 [DOI] [PubMed] [Google Scholar]
- 42.Morigi M, Benigni A: Mesenchymal stem cells and kidney repair. Nephrol Dial Transplant 28: 788–793, 2013 [DOI] [PubMed] [Google Scholar]
- 43.Friedenstein AJ, Chailakhjan RK, Lalykina KS: The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet 3: 393–403, 1970 [DOI] [PubMed] [Google Scholar]
- 44.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Horwitz E: Minimal criteria for defining multipotent mesenchymal stromal cells. The international society for cellular therapy position statement. Cytotherapy 8: 315–317, 2006 [DOI] [PubMed] [Google Scholar]
- 45.Bieback K, Wuchter P, Besser D, Franke W, Becker M, Ott M, Pacher M, Ma N, Stamm C, Klüter H, Müller A, Ho AD; START-MSC consortium : Mesenchymal stromal cells (MSCs): Science and f(r)iction. J Mol Med (Berl) 90: 773–782, 2012 [DOI] [PubMed] [Google Scholar]
- 46.Flynn A, Barry F, O’Brien T: UC blood-derived mesenchymal stromal cells: An overview. Cytotherapy 9: 717–726, 2007 [DOI] [PubMed] [Google Scholar]
- 47.Orbay H, Tobita M, Mizuno H: Mesenchymal stem cells isolated from adipose and other tissues: Basic biological properties and clinical applications. Stem Cells Int 2012: 461718, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dimmeler S, Leri A: Aging and disease as modifiers of efficacy of cell therapy. Circ Res 102: 1319–1330, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ankrum JA, Ong JF, Karp JM: Mesenchymal stem cells: Immune evasive, not immune privileged. Nat Biotechnol 32: 252–260, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Griffin MD, Elliman SJ, Cahill E, English K, Ceredig R, Ritter T: Concise review: Adult mesenchymal stromal cell therapy for inflammatory diseases: How well are we joining the dots? Stem Cells 31: 2033–2041, 2013 [DOI] [PubMed] [Google Scholar]
- 51.Ancans J: Cell therapy medicinal product regulatory framework in Europe and its application for MSC-based therapy development. Front Immunol 3: 253, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kotton CN, Fishman JA: Viral infection in the renal transplant recipient. J Am Soc Nephrol 16: 1758–1774, 2005 [DOI] [PubMed] [Google Scholar]
- 53.Rama I, Grinyó JM: Malignancy after renal transplantation: The role of immunosuppression. Nat Rev Nephrol 6: 511–519, 2010 [DOI] [PubMed] [Google Scholar]
- 54.Stoumpos S, Jardine AG, Mark PB: Cardiovascular morbidity and mortality after kidney transplantation. Transpl Int 28: 10–21, 2015 [DOI] [PubMed] [Google Scholar]
- 55.Tufton N, Ahmad S, Rolfe C, Rajkariar R, Byrne C, Chowdhury TA: New-onset diabetes after renal transplantation. Diabet Med 31: 1284–1292, 2014 [DOI] [PubMed] [Google Scholar]
- 56.Perico N, Casiraghi F, Introna M, Gotti E, Todeschini M, Cavinato RA, Capelli C, Rambaldi A, Cassis P, Rizzo P, Cortinovis M, Marasà M, Golay J, Noris M, Remuzzi G: Autologous mesenchymal stromal cells and kidney transplantation: A pilot study of safety and clinical feasibility. Clin J Am Soc Nephrol 6: 412–422, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Perico N, Casiraghi F, Gotti E, Introna M, Todeschini M, Cavinato RA, Capelli C, Rambaldi A, Cassis P, Rizzo P, Cortinovis M, Noris M, Remuzzi G: Mesenchymal stromal cells and kidney transplantation: Pretransplant infusion protects from graft dysfunction while fostering immunoregulation. Transpl Int 26: 867–878, 2013 [DOI] [PubMed] [Google Scholar]
- 58.Tan J, Wu W, Xu X, Liao L, Zheng F, Messinger S, Sun X, Chen J, Yang S, Cai J, Gao X, Pileggi A, Ricordi C: Induction therapy with autologous mesenchymal stem cells in living-related kidney transplants: A randomized controlled trial. JAMA 307: 1169–1177, 2012 [DOI] [PubMed] [Google Scholar]
- 59.Reinders ME, de Fijter JW, Roelofs H, Bajema IM, de Vries DK, Schaapherder AF, Claas FH, van Miert PP, Roelen DL, van Kooten C, Fibbe WE, Rabelink TJ: Autologous bone marrow-derived mesenchymal stromal cells for the treatment of allograft rejection after renal transplantation: Results of a phase I study. Stem Cells Transl Med 2: 107–111, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mudrabettu C, Kumar V, Rakha A, Yadav AK, Ramachandran R, Kanwar DB, Nada R, Minz M, Sakhuja V, Marwaha N, Jha V: Safety and efficacy of autologous mesenchymal stromal cells transplantation in patients undergoing living donor kidney transplantation: A pilot study. Nephrology (Carlton) 20: 25–33, 2015 [DOI] [PubMed] [Google Scholar]
- 61.Pan GH, Chen Z, Xu L, Zhu JH, Xiang P, Ma JJ, Peng YW, Li GH, Chen XY, Fang JL, Guo YH, Zhang L, Liu LS: Low-dose tacrolimus combined with donor-derived mesenchymal stem cells after renal transplantation: A prospective, non-randomized study. Oncotarget 7: 12089–12101, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Weekers L, Erpicum P, Detry O, Lechanteur C, Baudoux E, Jouret F, Beguin Y: Third party mesenchymal stromal cell infusion in kidney transplant recipient: 6-Month safety interim analysis [abstract], 2015. Available at http://atcmeetingabstracts.com/abstract/third-party-mesenchymal-stromal-cell-infusion-in-kidney-transplant-recipient-6-month-safety-interim-analysis/. Accessed November 17, 2017
- 63.Newell KA, Asare A, Sanz I, Wei C, Rosenberg A, Gao Z, Kanaparthi S, Asare S, Lim N, Stahly M, Howell M, Knechtle S, Kirk A, Marks WH, Kawai T, Spitzer T, Tolkoff-Rubin N, Sykes M, Sachs DH, Cosimi AB, Burlingham WJ, Phippard D, Turka LA: Longitudinal studies of a B cell-derived signature of tolerance in renal transplant recipients. Am J Transplant 15: 2908–2920, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rowart P, Erpicum P, Detry O, Weekers L, Grégoire C, Lechanteur C, Briquet A, Beguin Y, Krzesinski JM, Jouret F: Mesenchymal stromal cell therapy in ischemia/reperfusion injury. J Immunol Res 2015: 602597, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Detry O, Vandermeulen M, Delbouille MH, Somja J, Bletard N, Briquet A, Lechanteur C, Giet O, Baudoux E, Hannon M, Baron F, Beguin Y: Infusion of mesenchymal stromal cells after deceased liver transplantation: A phase I-II, open-label, clinical study. J Hepatol 67: 47–55, 2017 [DOI] [PubMed] [Google Scholar]
- 66.Kawai T, Sachs DH, Sykes M, Cosimi AB; Immune Tolerance Network : HLA-mismatched renal transplantation without maintenance immunosuppression. N Engl J Med 368: 1850–1852, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Leventhal J, Abecassis M, Miller J, Gallon L, Ravindra K, Tollerud DJ, King B, Elliott MJ, Herzig G, Herzig R, Ildstad ST: Chimerism and tolerance without GVHD or engraftment syndrome in HLA-mismatched combined kidney and hematopoietic stem cell transplantation. Sci Transl Med 4: 124ra28, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tögel FE, Westenfelder C: Kidney protection and regeneration following acute injury: Progress through stem cell therapy. Am J Kidney Dis 60: 1012–1022, 2012 [DOI] [PubMed] [Google Scholar]
- 69.Gooch A, Doty J, Flores J: Initial report on a phase I clinical trial: Prevention and treatment of post-operative acute kidney injury with allogeneic mesenchymal stem cells in patients who required on-pump cardiac surgery. Cell Ther Transplant 1: 31–35, 2008 [Google Scholar]
- 70.Bersenev A: Cell Therapy Clinical Trials Failure in 2014, 2014. Available at: http://celltrials.info/2015/01/04/failure-2014. Accessed April 24, 2017
- 71.Swaminathan M, Mazer D, Chertow G, Warnock D, Paragamian V, Brenner R: ACT-AKI: A phase 2 multicenter, randomized, double-blind, placebo-controlled trial of AC607 for the treatment of acute kidney injury in cardiac surgery subjects. Presented at the American Society of Nephrology Kidney Week, Philadelphia, November 11–16, 2014 [Google Scholar]
- 72.Remuzzi G, Benigni A, Remuzzi A: Mechanisms of progression and regression of renal lesions of chronic nephropathies and diabetes. J Clin Invest 116: 288–296, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ruggenenti P, Perticucci E, Cravedi P, Gambara V, Costantini M, Sharma SK, Perna A, Remuzzi G: Role of remission clinics in the longitudinal treatment of CKD. J Am Soc Nephrol 19: 1213–1224, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ruggenenti P, Cravedi P, Remuzzi G: Mechanisms and treatment of CKD. J Am Soc Nephrol 23: 1917–1928, 2012 [DOI] [PubMed] [Google Scholar]
- 75.Porrini E, Ruggenenti P, Mogensen CE, Barlovic DP, Praga M, Cruzado JM, Hojs R, Abbate M, de Vries AP; ERA-EDTA diabesity working group : Non-proteinuric pathways in loss of renal function in patients with type 2 diabetes. Lancet Diabetes Endocrinol 3: 382–391, 2015 [DOI] [PubMed] [Google Scholar]
- 76.Chan GC, Tang SC: Diabetic nephropathy: Landmark clinical trials and tribulations. Nephrol Dial Transplant 31: 359–368, 2016 [DOI] [PubMed] [Google Scholar]
- 77.Skyler JS, Fonseca VA, Segal KR, Rosenstock J; MSB-DM003 Investigators : Allogeneic mesenchymal precursor cells in type 2 diabetes: A Randomized, Placebo-Controlled, Dose-Escalation Safety and Tolerability Pilot Study. Diabetes Care 38: 1742–1749, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Packham DK, Fraser IR, Kerr PG, Segal KR: Allogeneic mesenchymal precursor cells (MPC) in diabetic nephropathy: A Randomized, Placebo-controlled, Dose Escalation Study. EBioMedicine 12: 263–269, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gaspari F, Ruggenenti P, Porrini E, Motterlini N, Cannata A, Carrara F, Jiménez Sosa A, Cella C, Ferrari S, Stucchi N, Parvanova A, Iliev I, Trevisan R, Bossi A, Zaletel J, Remuzzi G; GFR Study Investigators : The GFR and GFR decline cannot be accurately estimated in type 2 diabetics. Kidney Int 84: 164–173, 2013 [DOI] [PubMed] [Google Scholar]
- 80.Schievink B, Kröpelin T, Mulder S, Parving HH, Remuzzi G, Dwyer J, Vemer P, de Zeeuw D, Lambers Heerspink HJ: Early renin-angiotensin system intervention is more beneficial than late intervention in delaying end-stage renal disease in patients with type 2 diabetes. Diabetes Obes Metab 18: 64–71, 2016 [DOI] [PubMed] [Google Scholar]
- 81.Makhlough A, Shekarchian S, Moghadasali R, Einollahi B, Hosseini SE, Jaroughi N, Bolurieh T, Baharvand H, Aghdami N: Safety and tolerability of autologous bone marrow mesenchymal stromal cells in ADPKD patients. Stem Cell Res Ther 8: 116, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Saad A, Dietz AB, Herrmann SMS, Hickson LJ, Glockner JF, McKusick MA, Misra S, Bjarnason H, Armstrong AS, Gastineau DA, Lerman LO, Textor SC: Autologous mesenchymal stem cells increase cortical perfusion in renovascular disease. J Am Soc Nephrol 28: 2777–2785, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Liang J, Zhang H, Hua B, Wang H, Lu L, Shi S, Hou Y, Zeng X, Gilkeson GS, Sun L: Allogenic mesenchymal stem cells transplantation in refractory systemic lupus erythematosus: A pilot clinical study. Ann Rheum Dis 69: 1423–1429, 2010 [DOI] [PubMed] [Google Scholar]
- 84.Sun L, Wang D, Liang J, Zhang H, Feng X, Wang H, Hua B, Liu B, Ye S, Hu X, Xu W, Zeng X, Hou Y, Gilkeson GS, Silver RM, Lu L, Shi S: Umbilical cord mesenchymal stem cell transplantation in severe and refractory systemic lupus erythematosus. Arthritis Rheum 62: 2467–2475, 2010 [DOI] [PubMed] [Google Scholar]
- 85.Wang D, Zhang H, Liang J, Li X, Feng X, Wang H, Hua B, Liu B, Lu L, Gilkeson GS, Silver RM, Chen W, Shi S, Sun L: Allogeneic mesenchymal stem cell transplantation in severe and refractory systemic lupus erythematosus: 4 Years of experience. Cell Transplant 22: 2267–2277, 2013 [DOI] [PubMed] [Google Scholar]
- 86.Wang D, Li J, Zhang Y, Zhang M, Chen J, Li X, Hu X, Jiang S, Shi S, Sun L: Umbilical cord mesenchymal stem cell transplantation in active and refractory systemic lupus erythematosus: A multicenter clinical study. Arthritis Res Ther 16: R79, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gu F, Wang D, Zhang H, Feng X, Gilkeson GS, Shi S, Sun L: Allogeneic mesenchymal stem cell transplantation for lupus nephritis patients refractory to conventional therapy. Clin Rheumatol 33: 1611–1619, 2014 [DOI] [PubMed] [Google Scholar]
- 88.Deng D, Zhang P, Guo Y, Lim TO: A randomised double-blind, placebo-controlled trial of allogeneic umbilical cord-derived mesenchymal stem cell for lupus nephritis. Ann Rheum Dis 76: 1436–1439, 2017 [DOI] [PubMed] [Google Scholar]
- 89.Casiraghi F, Remuzzi G, Abbate M, Perico N: Multipotent mesenchymal stromal cell therapy and risk of malignancies. Stem Cell Rev 9: 65–79, 2013 [DOI] [PubMed] [Google Scholar]
- 90.Gao J, Dennis JE, Muzic RF, Lundberg M, Caplan AI: The dynamic in vivo distribution of bone marrow-derived mesenchymal stem cells after infusion. Cells Tissues Organs 169: 12–20, 2001 [DOI] [PubMed] [Google Scholar]
- 91.Eggenhofer E, Benseler V, Kroemer A, Popp FC, Geissler EK, Schlitt HJ, Baan CC, Dahlke MH, Hoogduijn MJ: Mesenchymal stem cells are short-lived and do not migrate beyond the lungs after intravenous infusion. Front Immunol 3: 297, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Leibacher J, Henschler R: Biodistribution, migration and homing of systemically applied mesenchymal stem/stromal cells. Stem Cell Res Ther 7: 7, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Morigi M, Imberti B, Zoja C, Corna D, Tomasoni S, Abbate M, Rottoli D, Angioletti S, Benigni A, Perico N, Alison M, Remuzzi G: Mesenchymal stem cells are renotropic, helping to repair the kidney and improve function in acute renal failure. J Am Soc Nephrol 15: 1794–1804, 2004 [DOI] [PubMed] [Google Scholar]
- 94.Humphreys BD, Bonventre JV: Mesenchymal stem cells in acute kidney injury. Annu Rev Med 59: 311–325, 2008 [DOI] [PubMed] [Google Scholar]
- 95.Uccelli A, de Rosbo NK: The immunomodulatory function of mesenchymal stem cells: Mode of action and pathways. Ann N Y Acad Sci 1351: 114–126, 2015 [DOI] [PubMed] [Google Scholar]
- 96.Koç ON, Gerson SL, Cooper BW, Dyhouse SM, Haynesworth SE, Caplan AI, Lazarus HM: Rapid hematopoietic recovery after coinfusion of autologous-blood stem cells and culture-expanded marrow mesenchymal stem cells in advanced breast cancer patients receiving high-dose chemotherapy. J Clin Oncol 18: 307–316, 2000 [DOI] [PubMed] [Google Scholar]
- 97.Gholamrezanezhad A, Mirpour S, Bagheri M, Mohamadnejad M, Alimoghaddam K, Abdolahzadeh L, Saghari M, Malekzadeh R: In vivo tracking of 111In-oxine labeled mesenchymal stem cells following infusion in patients with advanced cirrhosis. Nucl Med Biol 38: 961–967, 2011 [DOI] [PubMed] [Google Scholar]
- 98.Krampera M: Mesenchymal stromal cell ‘licensing’: A multistep process. Leukemia 25: 1408–1414, 2011 [DOI] [PubMed] [Google Scholar]
- 99.Duijvestein M, Wildenberg ME, Welling MM, Hennink S, Molendijk I, van Zuylen VL, Bosse T, Vos AC, de Jonge-Muller ES, Roelofs H, van der Weerd L, Verspaget HW, Fibbe WE, te Velde AA, van den Brink GR, Hommes DW: Pretreatment with interferon-γ enhances the therapeutic activity of mesenchymal stromal cells in animal models of colitis. Stem Cells 29: 1549–1558, 2011 [DOI] [PubMed] [Google Scholar]
- 100.Waterman RS, Tomchuck SL, Henkle SL, Betancourt AM: A new mesenchymal stem cell (MSC) paradigm: Polarization into a pro-inflammatory MSC1 or an Immunosuppressive MSC2 phenotype. PLoS One 5: e10088, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Liotta F, Angeli R, Cosmi L, Filì L, Manuelli C, Frosali F, Mazzinghi B, Maggi L, Pasini A, Lisi V, Santarlasci V, Consoloni L, Angelotti ML, Romagnani P, Parronchi P, Krampera M, Maggi E, Romagnani S, Annunziato F: Toll-like receptors 3 and 4 are expressed by human bone marrow-derived mesenchymal stem cells and can inhibit their T-cell modulatory activity by impairing Notch signaling. Stem Cells 26: 279–289, 2008 [DOI] [PubMed] [Google Scholar]
- 102.Kilpinen L, Impola U, Sankkila L, Ritamo I, Aatonen M, Kilpinen S, Tuimala J, Valmu L, Levijoki J, Finckenberg P, Siljander P, Kankuri E, Mervaala E, Laitinen S: Extracellular membrane vesicles from umbilical cord blood-derived MSC protect against ischemic acute kidney injury, a feature that is lost after inflammatory conditioning [published online ahead of print December 10, 2013]. J Extracell Vesicles doi:10.3402/jev.v2i0.21927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.van de Vyver M: Intrinsic mesenchymal stem cell dysfunction in diabetes mellitus: Implications for autologous cell therapy. Stem Cells Dev 26: 1042–1053, 2017 [DOI] [PubMed] [Google Scholar]
- 104.Prockop DJ: The exciting prospects of new therapies with mesenchymal stromal cells. Cytotherapy 19: 1–8, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.von Bahr L, Sundberg B, Lönnies L, Sander B, Karbach H, Hägglund H, Ljungman P, Gustafsson B, Karlsson H, Le Blanc K, Ringdén O: Long-term complications, immunologic effects, and role of passage for outcome in mesenchymal stromal cell therapy. Biol Blood Marrow Transplant 18: 557–564, 2012 [DOI] [PubMed] [Google Scholar]
- 106.Galipeau J: The mesenchymal stromal cells dilemma--Does a negative phase III trial of random donor mesenchymal stromal cells in steroid-resistant graft-versus-host disease represent a death knell or a bump in the road? Cytotherapy 15: 2–8, 2013 [DOI] [PubMed] [Google Scholar]