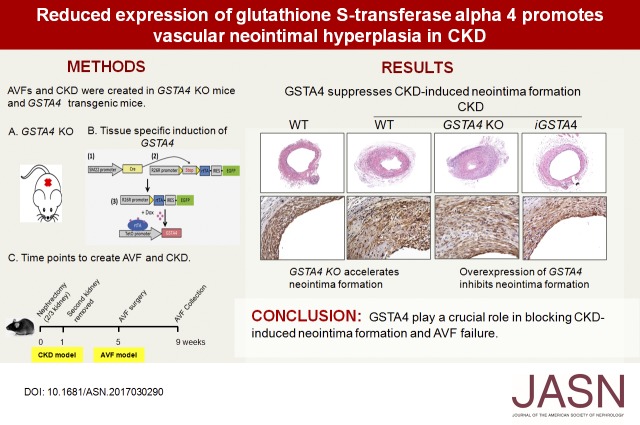
Abstract
Neointima formation is the leading cause of arteriovenous fistula (AVF) failure. We have shown that CKD accelerates this process by transforming the vascular smooth muscle cells (SMCs) lining the AVF from a contractile to the synthetic phenotype. However, the underlying mechanisms affecting this transformation are not clear. Previous studies have shown that the α-class glutathione transferase isozymes have an important role in regulating 4-hydroxynonenal (4-HNE)–mediated proliferative signaling of cells. Here, using both the loss- and gain-of-function approaches, we investigated the role of glutathione S-transferase α4 (GSTA4) in modulating cellular 4-HNE levels for the transformation and proliferation of SMCs. Compared with non-CKD controls, mice with CKD had downregulated expression of GSTA4 at the mRNA and protein levels, with concomitant increase in 4-HNE in arteries and veins. This effect was associated with upregulated phosphorylation of MAPK signaling pathway proteins in proliferating SMCs. Overexpressing GSTA4 blocked 4-HNE–induced SMC proliferation. Additionally, inhibitors of MAPK signaling inhibited the 4-HNE–induced responses. Compared with wild-type mice, mice lacking GSTA4 exhibited increased CKD-induced neointima formation in AVF. Transient expression of an activated form of GSTA4, achieved using a combined Tet-On/Cre induction system in mice, lowered levels of 4-HNE and reduced the proliferation of SMCs. Together, these results demonstrate the critical role of GSTA4 in blocking CKD-induced neointima formation and AVF failure.
Keywords: arteriovenous fistula, glutathione S-transferase A4, neointima, chronic kidney disease
The success of hemodialysis depends on a functioning arteriovenous fistula (AVF), but in the 2 years since its creation, nearly 50% fail, generally due to accumulation of vascular smooth muscle cells (SMCs) and the formation of a neointima.1–4 Thus, AVF is an Achilles heel for patients on hemodialysis.5 It can amount to >$1 billion per year in surgical and radiologic interventions.6 We have shown that dedifferentiation and proliferation of the SMCs plays a major role in neointima formation, and serves as a trigger for the transition from a contractive to the synthetic status.7,8
CKD is recognized as one of the most consistent predictors of cardiovascular disease. We have found that CKD causes an endothelial barrier dysfunction and extracellular matrix deposition around the peripheral vasculature.6,9 There is increasing evidence to show that CKD also increases the production of reactive oxygen species (ROS).10,11 Formation of ROS is a major cause of DNA damage that correlates well with several human diseases. Recent studies have shown that there is an increase in 4-hydroxynonenal (4-HNE) levels in kidney disease.12 4-HNE is a highly reactive but stable aldehyde, generated during oxidative degradation of fatty acids such as arachidonic and linoleic acids.13 It has been shown to stimulate cellular proliferation as well as to block cell terminal differentiation.14,15 In fact, the reported association between levels of 4-HNE and the magnitude of tumor promotion response suggests that 4-HNE–induced oxidative stress may play an important role in the growth of cells.
Normally, cellular 4-HNE is regulated by glutathione S-transferase α4 (GSTA4), a member of the α class of antioxidative enzymes called the glutathione S-transferases (GSTs). The GSTs play a major role in cellular detoxification. By catalyzing the conjugation of 4-HNE with an important cellular thiol-glutathione and facilitating its exclusion out of the cells, GSTs are able to protect cells against oxidative damage. Thus, by neutralizing the reactive electrophilic sites and conjugating 4-HNE with glutathione, GSTA4 is a major regulator of cellular 4-HNE levels.16,17 In its absence, levels of 4-HNE increase and promote disease, such as cancer and kidney disease.18,19
Although it is well agreed that activated SMCs are a major factor in neointima formation, the role of 4-HNE in CKD-induced AVF failure is poorly understood. In this study, we created CKD and AVF mouse models in GSTA4 knockout (GSTA4 KO) and inducible transgenic mice, and investigated the potential role of GSTA4 in SMC activation and neointima formation in AVFs. The downstream signals that mediate these processes were also determined.
Results
CKD Decreases Expression of GSTA4 in AVF
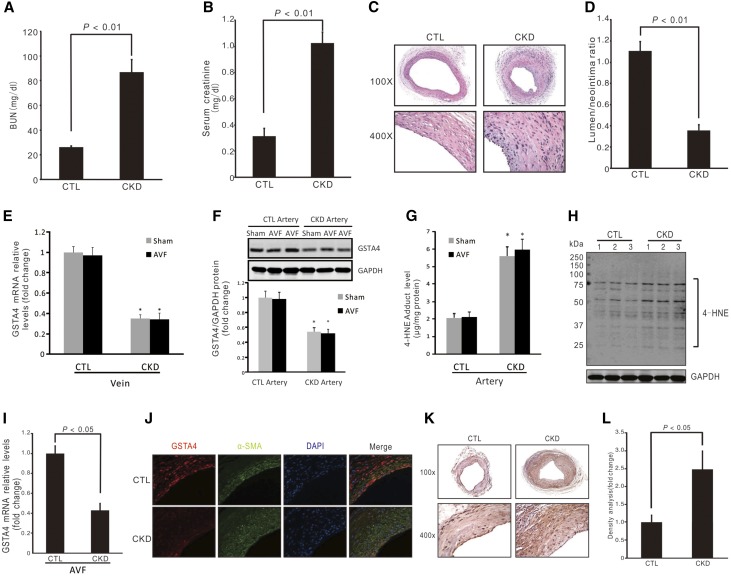
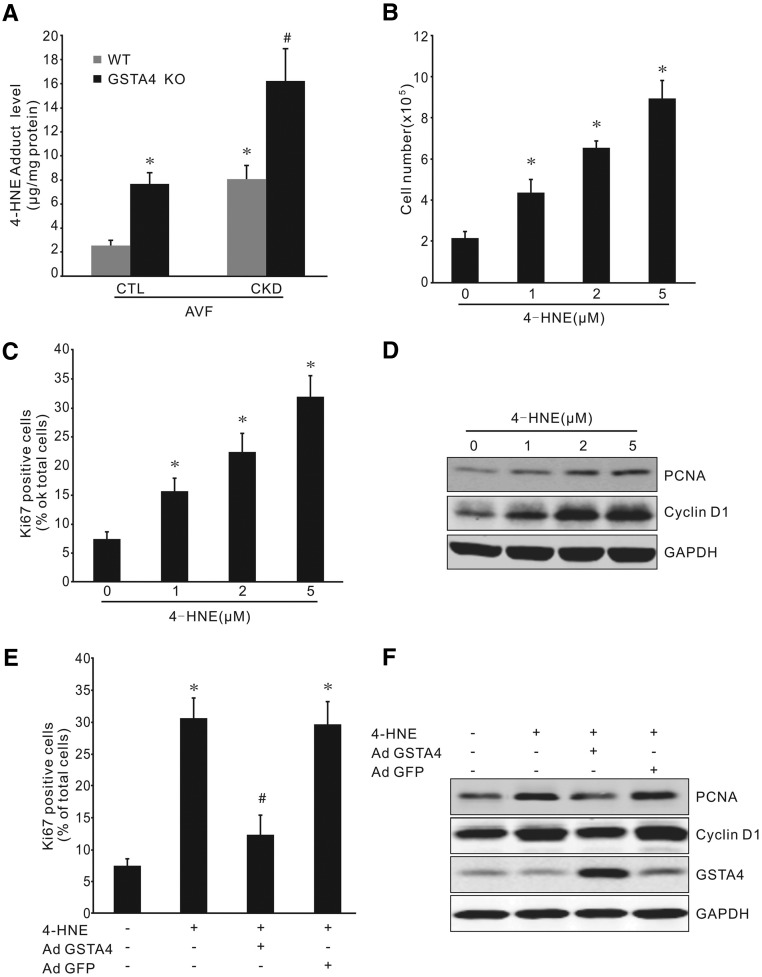
CKD was induced with subtotal nephrectomy. The levels of BUN and serum creatinine were significantly higher in uremic mice than those in control mice (Figure 1, A and B). There was a significant induction of neointima formation and decreased ratio of lumen-to-neointima area in CKD compared with control mice (Figure 1, C and D). GSTA4 is an antioxidative stress protein that plays a role in maintaining cellular homeostasis. We measured levels of GSTA4 in jugular veins of control and CKD mice with or without AVF, and found that CKD decreased GSTA4 mRNA expression whereas AVF surgery had no effect on CKD-mediated downregulation of GSTA4 expression (Figure 1E). In arteries, CKD decreased the GSTA4 protein (Figure 1F), accompanied by an increase in 4-HNE level and proteins bound by 4-HNE (Figure 1, G and H). We also found that CKD decreased the GSTA4 mRNA in AVFs (Figure 1I). Moreover, immunofluorescence analysis indicated that there was a strong GSTA4 expression in endothelial cells and SMCs in control AVFs, whereas GSTA4 level was markedly decreased in AVFs from CKD mice (Figure 1J). More 4-HNE–positive signals were detected in AVFs from mice with CKD (Figure 1, K and L).
Figure 1.
CKD-induced neointima formation is associated with decreased GSTA4 expression in AVFs. (A and B) BUN and serum creatinine were detected in control (CTL) and CKD mice (n=6). (C) AVFs were created in control and CKD mice. The morphology of AVFs was analyzed by hematoxylin and eosin staining. Representative images from AVFs are presented. (D) The area of lumen and neointima were measured and the ratio of lumen-to-neointima was calculated (n=6). (E) Contralateral jugular veins were collected from control or CKD mice that had sham or AVF surgeries, and the mRNA levels of GSTA4 were determined by real-time RT-PCR (*P<0.05 versus control mice, n=6). (F) Arteries were collected from control or CKD mice that had sham or AVF surgeries, and the protein levels of GSTA4 were determined by Western blotting (*P<0.05 versus control mice, n=6). (G and H) The 4-HNE adduct levels in the artery were detected by ELISA (G) (*, P<0.05 versus control mice, n=6) and Western blotting (H). (I) RNAs were collected from AVFs created in control and CKD mice and the expression of GSTA4 was detected by real-time RT-PCR. (J) The expression of GSTA4 protein was determined by immunofluorescence staining in AVFs. GSTA4 was colocalized with α-SMA in neointima cells in AVFs. (K and L) 4-HNE levels were detected by immunostaining in AVFs from control and CKD mice (K), and the density of the positive signal was analyzed (L) (n=6).
Deficiency of GSTA4 Leads to Loss of Quiescent Status of SMCs
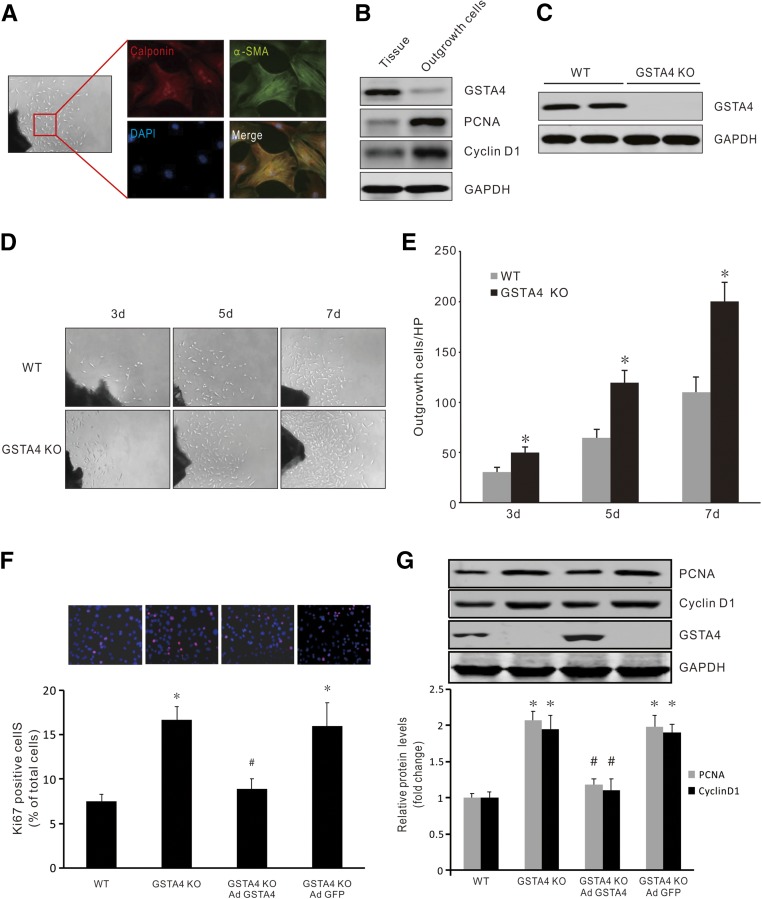
The above results indicate that CKD-induced SMC accumulation in the AVF could be associated with a decreased expression of GSTA4. We then studied the effect of GSTA4 expression on SMC phenotype transition and proliferation. The segments from common carotid arteries and jugular vein were seeded onto plates, and cells outgrown from the artery or vein showed similar morphology and proliferation rates (Supplemental Figure 1). These outgrowing cells were positive for SMC markers calponin and SMA-α (Figure 2A). Compared with the quiescent SMCs in the artery, expression of GSTA4 was dramatically ablated in the outgrowth SMCs. In contrast, proliferative markers PCNA and cyclin D1 were significantly increased in the outgrowth SMCs (Figure 2B). SMCs from GSTA4 KO mice grew faster in ex vivo cultures compared with cells from wild-type (WT) mice, as measured at days 3 and 7 (Figure 2, C–E).
Figure 2.
Loss of GSTA4 promoted SMC phenotype switch from quiescent to synthetic status. (A) Artery segments were cultured in DMEM complete medium for 7 days ex vivo; the outgrowth cells were characterized and stained positively with the SMC markers α-SMA and calponin (original magnification, ×600). (B) Common carotid arteries were dissected and half of the arteries were cultured and half were kept for later use. After 7 days, proteins from the artery and outgrowth SMCs were isolated and Western blotting was performed (n=3), depicting loss of GSTA4 expression and increasing expression of PCNA and Cyclin D1 in the outgrowth SMCs. (C) No GSTA4 protein was detected in arteries from GSTA4 KO mice. (D) Artery segments from WT and GSTA4 KO mice were seeded onto 24-well plates and photographs were taken at indicated time points. Representative pictures are presented. (E) The cell numbers in (D) were counted and summarized (*P<0.05 versus WT, n=5). (F and G) Mouse venous SMCs from WT and GSTA4 KO mice were transfected with AdGSTA4 or GFP adenovirus. (F) Ki67 expression was detected by immunostaining (upper panel), and quantitation analysis was performed of Ki67-positive cells from three experiments (lower panel). (G) Inhibition of the proliferation markers PCNA and Cyclin D1 in GSTA4 expressing SMCs was determined by Western blotting. Representative data from three experiments are shown. *P<0.05 versus WT; #P<0.05 versus GSTA4 KO.
Further, GSTA4 KO in venous SMCs resulted in increased Ki67-positive SMCs, and this progrowth response was blocked by overexpressing GSTA4 (Figure 2F). Finally, GSTA4 KO-induced expression of proliferation markers was found to be inhibited in GSTA4-expressing SMCs (Figure 2G). Consistently, GSTA4 KO in artery SMCs also increased the proliferating cell number (Ki67+) and the expression of proliferation markers (PCNA and cyclin D1), whereas overexpression of GSTA4 blocked these responses (Supplemental Figure 2, A and B). Together, these results indicate that loss of GSTA4 expression is associated with gain of the synthetic phenotype in SMCs.
GSTA4 KO Accelerates Neointima Formation
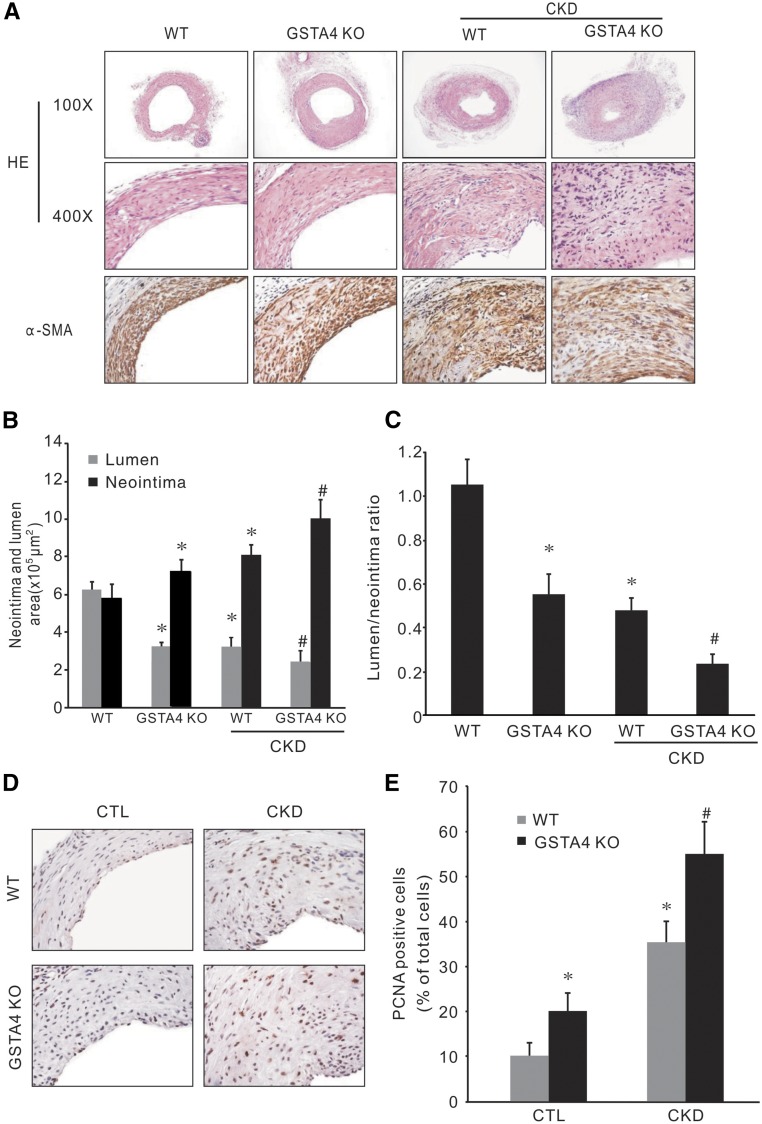
AVFs were performed in WT and GSTA4 KO mice with or without CKD. GSTA4 KO alone induced neointima formation; there was more neointima formation and accumulation of SMA-α–positive cells in AVFs created in GSTA4 KO mice (Figure 3A). The lumen area and the ratio of lumen-to-neointima area were significantly decreased in AVFs from GSTA4 KO mice with CKD compared with that from WT mice with CKD (Figure 3, B and C). PCNA-positive cells were found in the neointima area. Statistical analysis revealed that although CKD increased the PCNA-positive cell numbers in the neointima area of AVFs, there were more proliferating cells (PCNA positive) in AVFs created in GSTA4 KO mice (Figure 3, D and E).
Figure 3.
Knocking out GSTA4 accelerated CKD-induced neointima formation. (A) AVFs were created in WT and GSTA4 KO mice, with and without CKD. The morphology (hematoxylin and eosin) and α-SMA immunostaining were performed in the 1-month-old AVFs. (B and C) The area of lumen and neointima were measured and the ratio of lumen-to-neointima was calculated (*P<0.05 versus WT mice; #P<0.05 versus WT with CKD mice, n=6). (D and E) The proliferation marker PCNA was detected by immunostaining in AVFs (D) (original magnification, ×400). (E) Cells depicting positive staining were counted and summarized (*P<0.05 versus WT mice; #P<0.05 versus WT with CKD mice, n=6).
4-HNE Mediated Proliferation of SMCs
4-HNE is the major substrate of GSTA4.20 We found that GSTA4 KO mice have increased 4-HNE adduct levels in AVFs (Figure 4A). Although the level of 4-HNE adducts increased in the AVFs of uremic mice when compared with sham control mice, 4-HNE levels were further upregulated in GSTA4 KO mice (Figure 4A). In cultured cells, treatment with 4-HNE induced an increase in total cell number of SMCs and Ki67-positive cells in a dose-dependent manner (Figure 4, B and C). 4-HNE at doses of 1–5 µM also stimulated the expression of PCNA and cyclin D1 in SMCs (Figure 4D). These responses were blocked by overexpression of GSTA4, but not by the vector control in SMCs (Figure 4, E and F).
Figure 4.
Low dose of 4-HNE treatment stimulates SMC proliferation. (A) Increased 4-HNE adduct level in AVFs was detected by ELISA in GSTA4 KO mice, with or without CKD (*P<0.05 versus WT mice; #P<0.05 versus WT with CKD mice, n=6). (B and C) Treatment with 4-HNE at indicated dose increased SMC numbers (B) and Ki67-positive cells (C) (*P<0.05 versus no treatment, n=3). (D) 4-HNE induced a dose-dependent expression of PCNA and Cyclin D1 in mouse SMCs (n=3). (E and F) Overexpression of GSTA4 abolishes 4-HNE–induced cell proliferation. SMCs were infected with AdGSTA4 or GFP adenovirus before 4-HNE treatment, and the Ki67-positive cell number (E) and proliferation proteins (F) were detected by immunostaining and Western blotting, respectively. Data shown was from three repeat experiments (*P<0.05 versus no treatment; #P<0.05 versus 4-HNE treatment only).
It has been reported that 4-HNE induces cell death. To determine the effect of 4-HNE on apoptosis, mouse vein SMCs were treated with different doses of 4-HNE. Flow cytometry analysis showed that a high concentration of 4-HNE (20 µM) induces the annexin V exposure in SMCs (Supplemental Figure 3, A and B). Increased expression of Bax and activated caspase 3 were only observed in cells that were treated with 4-HNE at ≥20 µM for 24 hours (Supplemental Figure 3C). These data indicate that exposure to different doses of 4-HNE leads to different responses in SMCs.
4-HNE–Induced SMC Proliferation Is Regulated through the MAPK Signaling Pathway
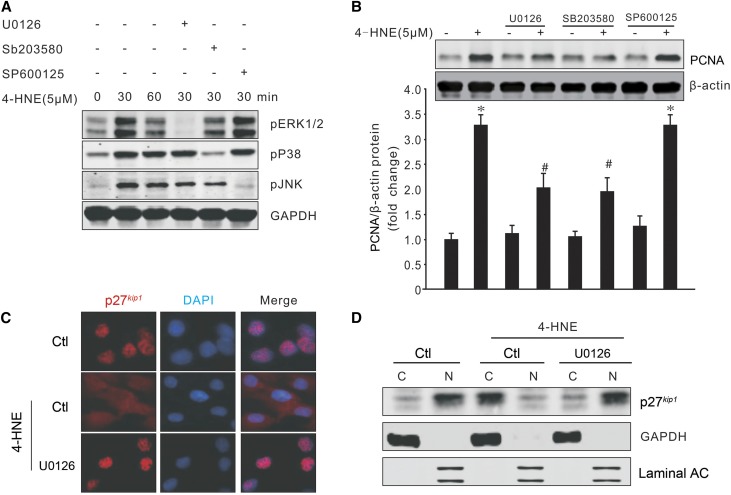
MAPK signaling is one of the important factors in neointima formation in arteriovenous grafts or vascular injury.21–23 It can be upregulated under various stimuli.24 The MAPK pathway was investigated to explain the mechanism of 4-HNE–induced SMC proliferation. Treatment with 4-HNE stimulated the phosphorylation of ERK1/2, p38, and JNK, which were specifically blocked by inhibitors U0126, SB202580, and SP600125, respectively (Figure 5A). Because 4-HNE induces MAPK activation, we next tested whether MAPK mediates 4-HNE–induced SMC proliferation. We found that pretreatment with ERK and p38 inhibitors reduced 4-HNE–induced PCNA expression in SMCs, whereas the JNK inhibitor had no effect on 4-HNE–induced SMC proliferation (Figure 5B). p27kip1 blocks cell cycle progression and is located in the nucleus.25,26 In quiescent SMCs, treatment with 4-HNE led to a cytoplasmic translocation of p27kip1. However, treatment with an MEK inhibitor kept p27kip1 in the nuclei even in the presence of 4-HNE (Figure 5C). Similar results were observed in Western blot analysis of the proteins isolated from the cytosol and nucleus (Figure 5D). These data indicate that 4-HNE–induced p27kip1 cytoplasmic translocation and proliferation are regulated through the MAPK/p27kip1 signaling pathway.
Figure 5.
MAPK signaling pathway mediates 4-HNE-induced SMC proliferation. (A) SMCs were pretreated with U0126 (5 µM), SB203580 (10 µM), or SP600125 (5 µM) for 30 minutes before exposure to 4-HNE. The phosphorylation of MAPKs was detected by Western blotting. (B) SMCs were treated as above and the PCNA expression after 4-HNE treatment (24 hours later) was determined by Western blotting (*P<0.05 versus no treatment; #P<0.05 versus 4-HNE treatment only, n=3). (C and D) SMCs were seeded onto coverslips and treated as above, and the p27kip1 expression and localization were determined by immunofluorescence staining (C) (original magnification, ×600) and Western blotting (D). Representative data were shown from three repeated experiments. C, cytoplasm; N, nuclei.
Tissue-Specific Induction of GSTA4 via a Combined Tetracycline-On/Cre System
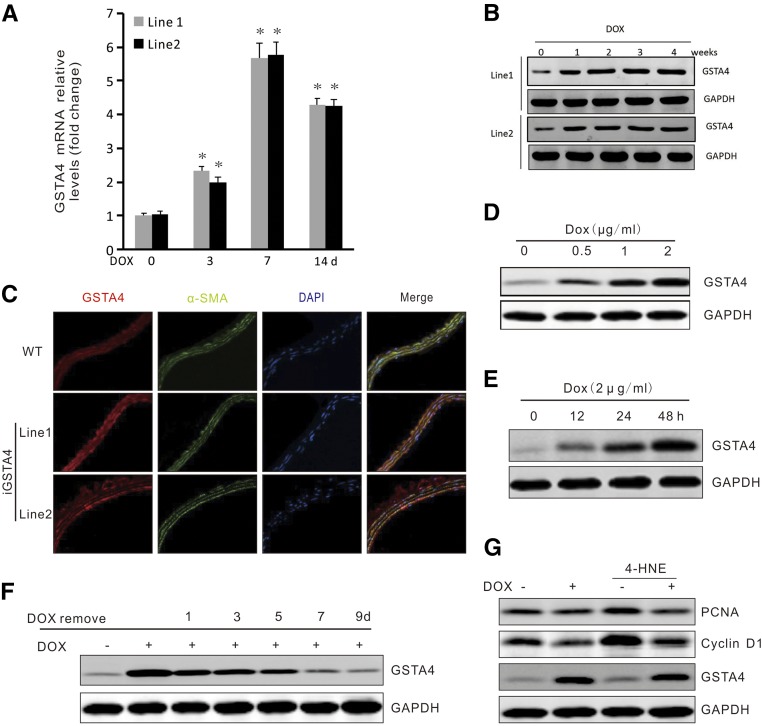
To test whether overexpression of GSTA4 can protect against CKD-induced AVF failure, we used both the tetracycline-on (Tet-On) system and the Cre-LoxP system (Supplemental Figure 4A). A conditional GSTA4 allele can be transactivated in a tissue-specific and doxycycline-dependent manner. We obtained four Tet-On-GSTA4 transgenic founders. Two of the founders transmitted the transgene in a Mendelian fashion. To test the performance of the Tet-On-GSTA4 alleles, we bred founder Tet-On-GSTA4 transgenic mice with rtTA-stop/SM22-Cre transgenic mice proven to be Cre-positive for SMCs.27 The triple transgenic mice in both lines showed an upregulation of GSTA4 expression in multiple organs after administration of doxycycline for 10 days, and the organs containing more SMCs (artery, intestine, and lung) showed a higher level of induced GSTA4 expression (Supplemental Figure 4B). Expression of GSTA4 was gradually induced in veins and arteries from both lines after doxycycline administration (Figure 6, A and B). In veins, the peak in GSTA4 mRNA level was at 7 days, and the GSTA4 protein level in the arteries peaked at 2 weeks after doxycycline induction (Figure 6, A and B). Immunostaining analysis of arteries removed from triple transgenic GSTA4/rtTA-stop/SM22-Cre mice confirmed that the GSTA4 transgene was expressed only in the SMC of the triple transgenic mice treated with doxycycline (Figure 6C). Expression of GSTA4 in SMCs isolated from the triple transgenic mice was induced by doxycycline in a dose- and time-dependent manner (Figure 6, D and E). Induced overexpression of GSTA4 by doxycycline inhibited 4-HNE–induced PCNA and cyclin D1 expression (Figure 6G). However, withdrawing doxycycline from these triple transgenic mice decreased GSTA4 to baseline levels within 2 weeks (Figure 6F).
Figure 6.
Conditional inducible expression of GSTA4 inhibits SMC proliferation. (A and B) Time-dependent expressions of GSTA4 in the veins (RT-PCR) and arteries (Western blot) were detected after doxycycline treatment (*, P <0.05 versus no DOX treatment, n=6). (C) GSTA4 expression was detected after doxycycline induction by double immunostaining of GSTA4 and α-SMA in SMCs in the common carotid artery in WT and iGSTA4 transgenic mice (original magnification, ×400). (D and E) GSTA4 expression was induced in cultured SMCs. SMCs from GSTA4/rtTA/SM22-Cre mice (iGSTA4) were isolated and the expression of GSTA4 was induced by doxycycline in a (D) dose- and (E) time-dependent manner. (F) GSTA4 overexpression was reduced after stop adding doxycycline. (G) Doxycycline treatment blocks 4-HNE–induced expression of the proliferation markers. Representative data were shown from three repeated experiments.
Conditional GSTA4 Transgenic Mice Inhibit CKD-Induced Neointima Formation
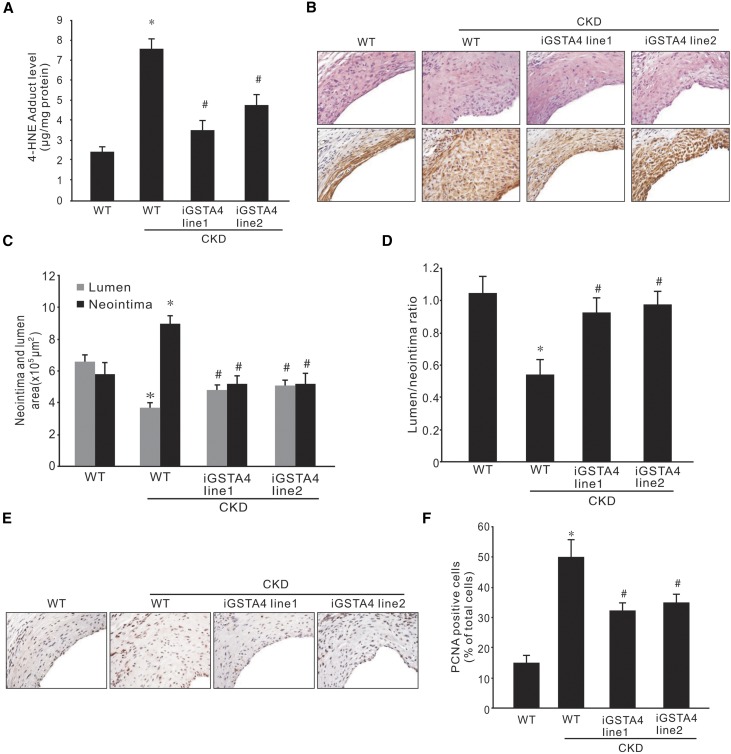
Because the GSTA4 overexpression cassette was randomly inserted into the mouse genome, we used two GSTA4 overexpression mouse lines (line 1 and line 2) to determine the specific effect of GSTA4 on CKD-induced neointima formation in AVFs. AVFs were performed in WT and the two GSTA4 transgenic mice lines with or without CKD. The 4-HNE levels in the AVFs from mice with overexpression of GSTA4 were lower compared with that in controls (Figure 7A). The morphology showed that thicker walls and smaller lumens were found near the venous anastomosis area in AVFs in mice with CKD (Figure 7B). GSTA4 expression was increased in doxycycline-induced triple transgenic mice and costained with SMA-α in neointima cells in AVFs (data not shown). Overexpression of GSTA4 in SMCs ameliorated neointima formation and accumulation of SMA-α–positive cells in AVFs (Figure 7B). The lumen area and the lumen-to-neointima area ratio were increased significantly in mice overexpressing GSTA4 (Figure 7, C and D). Fewer PCNA-positive cells were found in the neointima in AVFs from GSTA4 transgenic mice with CKD (Figure 7, E and F), indicating that a higher GSTA4 level results in lower SMC growth potential.
Figure 7.
Overexpression of GSTA4 in SMCs suppresses neointima formation in AVF. CKD and AVFs were performed in WT or GSTA4 transgenic mouse lines. Doxycycline was applied 7 days before the AVF surgery. (A) The levels of 4-HNE adducts in AVFs were detected in GSTA4 overexpression mice (*P<0.05 versus WT without CKD mice; #P<0.05 versus WT with CKD mice, n=6). (B) The AVFs were collected after 1 month, and hematoxylin and eosin and α-SMA immunostaining were performed (n=6) (original magnification, ×400). (C and D) The area of lumen and neointima were measured and summarized (C), and the ratio of lumen-to-neointima area was calculated (D) (*P<0.05 versus WT without CKD mice; #P<0.05 versus WT with CKD mice, n=6). (E and F) Immunostaining of PCNA was detected and shown in brown color in these AVFs (E) (original magnification, ×400), and the PCNA-positive cells were counted and summarized (F) (*P<0.05 versus WT without CKD mice; #P<0.05 versus WT with CKD mice, n=6).
Discussion
AVF provides the best access for longevity and the lowest association with morbidity and mortality in patients on hemodialysis.28 However, formation of neointima from proliferation of SMCs is a hallmark of AVF failure.29,30 Among a host of multiple factors, CKD is one of the most important factors that accelerates AVF failure. Hence, understanding the mechanisms leading to SMCs accumulating in the forming neointima is critical for designing preventive therapeutic strategies. By using the loss- and gain-of-function approaches in transgenic mice, we demonstrate for the first time that GSTA4 is critical for the SMC phenotype transition, and can regulate the maturation of an AVF in CKD mice. The major finding from this study is that CKD decreases the expression of GSTA4. Loss of GSTA4 induced an accumulation of 4-HNE that formed 4-HNE adducts with proteins, and thereby interfered with normal protein function. MAPK was identified as a critical signaling pathway of 4-HNE, which triggered the p27kip1 cytoplasmic translocation and release from the cell cycle arrest, resulting in SMC proliferation. Further, the gain in functions of GSTA4 helped to maintain the SMC quiescent status and inhibit their migration and proliferation.
Uremia is a state of increased oxidative stress characterized by circulating tissue proteins altered by oxidative activity.31 CKD is associated with accelerated atherosclerosis and cardiovascular disease, which is also largely mediated by oxidative stress and formation of ROS.32 ROS initiate a complex series of molecular events that may cause neointima formation. Earlier reports indicated that loss of the glutathione peroxidase 1 induced neointima formation through increased ROS production. This, in turn, led to vascular SMC proliferation and decreased endothelial regeneration in an arterial injury model.33 Although the uremic condition is linked to AVF failure, the actual events affecting the AVF during dialysis are complicated.34–37
4-HNE is a highly reactive, unsaturated hydroxyalkenal produced by lipid peroxidation in cells. A uremic toxin, spermine, increases 4-HNE and impairs glucose metabolism through reduction in pyruvate generation and transamination.38 CKD-associated oxidative species (e.g., the advanced glycation end products) enhance lipid peroxidation and, eventually, 4-HNE in tissues.39,40 There are two ways that 4-HNE exerts its effects on macromolecules: firstly, by direct binding with macromolecules to form 4-HNE adducts that interfere with the function of these molecules; and secondly, 4-HNE itself is an active oxidizing molecule. We found that CKD induced formation of 4-HNE adducts in the AVF (Figure 4A). Increased 4-HNE modifies intracellular proteins, causing cytoskeletal disorganization and VE-cadherin dissociation and stimulating SMC growth.41,42 The CKD-induced neointima formation is in parallel with an increased 4-HNE levels in GSTA4 KO mice (Figures 4 and 7). This observation is supported by a previous report, demonstrating higher 4-HNE levels in failed AVFs and expanded polytetrafluoroethylene grafts in patients.43 Increased 4-HNE levels in patients with CKD was also one of the factors that induced neointima formation in the vein itself.44 Taken together, these facts indicate that 4-HNE could be a potential tool for alleviating the vascular complications of CKD.
The α class isozyme GSTA4 exhibits a high catalytic efficiency toward 4-HNE. The normal level of GSTA4 expression in tissues determines the concentration of 4-HNE. GSTA4 is a protein linked to cellular transformation; its expression level has been observed to be altered during podocyte differentiation or cancer cell transformation.15,45 Under normal conditions, the body contains “protectors” including the redox-sensitive thiols, such as glutathione and phase 2 detoxifying proteins (e.g., GSTA4), which function as putative sensors of oxidative stress in SMCs. On the other hand, under uremic conditions, expression of GSTA4 is inhibited. Decreased GSTA4 expression is linked with dysfunction of tubule cells and SMC activation. We found that GSTA4 levels have an inverse relationship with loss of the SMC quiescent status: abundant GSTA4 protein expression was observed in normal vasculature (where the SMCs and ECs are quiescent), however, when the artery or vein was ex vivo cultured to activate the SMCs, the GSTA4 expression was dramatically decreased in the outgrowth SMCs (Figure 2). Thus, overexpression of GSTA4 can inhibit SMC proliferation and ameliorate CKD-induced neointima formation in AVFs created in GSTA4 transgenic mice (Figure 7). Consistent with our findings, overexpression of human GSTA4 in carotid artery has been reported to prevent neointima formation after carotid allograft in rabbits.46 These results confirm that GSTA4 expression suppresses SMC activation.
4-HNE has been reported to phosphorylate EGFR and thereby increase retinal epithelial cell growth.41 We found that that 4-HNE increased MAPK activity and thus mediated the translocation of p27kip1 out of the nucleus and into the cytoplasm, and after its degradation, cell cycle progression in SMCs was promoted.47 Moreover, in studies conducted in GSTA4 KO mice, there was an increased 4-HNE leading to MAPK, SMC proliferation, and neointima formation in AVFs. These results indicate that CKD-mediated alteration of GSTA4 expression is associated with neointima formation.
On the basis of our findings in this study, one of the therapeutic strategies to attenuate CKD-induced neointima formation in AVFs could be the induction of GSTA4 by antioxidants and chemopreventive agents. Several antioxidants, such as butylated hydroxyanisole and butyrate, have been shown to induce GSTA4 in cell culture and animal models.48 Dietary chemopreventive agents, including sulforaphane, benzyl isothiocyanate, and some flavones, have been shown to induce GSTA4.48,49 Additionally, it is also worth mentioning the importance of antioxidant transcription factor Nrf2 in the regulation of phase 2 enzymes, including GSTA4.50 Activation of Nrf2 in tissues can prevent the progression of CKD.51 However, the beneficial effects of antioxidant compounds to prevent the failure of AVF are yet to be evaluated. We believe that treatment with antioxidants before the placement of AVF could suppress SMC activation and neointima formation in AVFs.
This study has several limitations: Firstly, BP data is missing because BP in CKD mice may affect the biologic and histologic changes in neointima formation in AVF. Secondly, the cultured cells do not completely mimic the high flow and shear stress environment in vivo. Thirdly, we found that although GSTA4 overexpression improves the lumen-to-neointima area in CKD mice, the level of lumen-to-neointima area ratio is still lower than that in AVFs from WT control mice, indicating that other factors, such as the changes in sheer stress of dynamic flow, could not be overcome by GSTA4 overexpression.
In summary, GSTA4 is involved in the CKD-induced acceleration of SMC proliferation and neointima formation. Under uremic conditions, GSTA4 is inhibited and its substrate, 4-HNE, is upregulated, which leads to SMC proliferation. Further, MAPK is the mediator of 4-HNE. Upregulated expression and activation of MAPK lead to AVF failure. The proposed 4-HNE/MAPK signaling pathway in GSTA4 KO mice with CKD will advance our understanding on SMC activation and neointima formation, and could be exploited as a target for development of medicines that could help to control CKD-induced AVF failures.
Concise Methods
Reagents
Penicillin, streptomycin, DMEM, and FBS were obtained from Invitrogen Life Technologies (Carlsbad, CA). The protein assay kit was from Bio-Rad (Hercules, CA). The anti-mouse GSTA4 antibody was generated from rabbit.19 The antibodies against pERK1/2, pp38, pJNK, and cyclin D1, MEK inhibitor U0126 were purchased from Cell Signaling Technology (Beverly, MA). The antibodies against α-SMA, caspase 3, and p27kip1 were purchased from Abcam (Cambridge, MA); the antibodies against PCNA, calponin, GAPDH, and the secondary antibodies horseradish peroxidase–linked anti-mouse IgG and anti-rabbit IgG, were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). The anti–Ki67-Alexa Fluor 565 antibody was from BD Bioscience (San Jose, CA). The MAPK p38 inhibitor (SB203580) and JNK inhibitor (SP600125) were obtained from Calbiochem (Gibbstown, NJ). The doxycycline hyclate and Bax antibody were purchased from Sigma-Aldrich (St. Louis, MO). The adenovirus mGSTA4 expression vector was constructed by inserting mGSTA4 cDNA into pTracker-CMV vector as reported previously.19
Flow Cytometry and Apoptosis
Cell apoptosis was assessed using the annexin V-FITC apoptosis detection kit (BestBio, Shanghai, China). Briefly, vein SMCs were collected after treatment with different doses of 4-HNE for 24 hours, and after washing twice in ice-cold PBS, the cells were resuspended with annexin V binding buffer. Then, the cell suspension was mixed with 5 μl annexin V-FITC for 15 minutes at 2–8°C, and counterstained with 10 µl propidium iodide for 5 minutes in the dark. Apoptotic cells (FITC+/propidium iodide−) was determined on flow cytometer and analyzed with CellQuest software (BD Biosciences).
Animals
All studies were approved by the Institutional Animal Care and Use Committee of Baylor College of Medicine (Houston, TX) and performed in accordance with National Institutes of Health guidelines. Mice were housed in a conventional animal facility with a 12-hour light/dark cycle. WT mice were from Jackson Laboratory (Bar Harbor, Maine) and GSTA4 KO mice were obtained as previously described.19,52
Generation of Transgenic Mice
To construct the GSTA4 inducible expression vector, the cDNA encoding mGSTA4 was amplified by GCGGGGCCCATGGCAGCCAAACCTAAG CTC (ApaI restriction site is underlined) and GCGTGGCCACTACTTATCGTCGTCA TCCTTGTAATCGAACTTCAGGACAGTCCTG (MscI restriction site is underlined). The PCR products were cut with ApaI and MscI, and cloned into pLVX-Tet-On vector (catalog no. 632162; Clontech), where the BamH I site was changed to the ApaI site using the site-directed mutation kit. To test the inducible expression of GSTA4, HEK293 cells were transfected with pLVX-Tet-On/GSTA4 and rtTA plasmids, and the induced expression of GSTA4 was confirmed after doxycycline treatment (0.2 mg/ml) for 24 hours by Western blot.
To generate GSTA4 inducible expression mice, pLVX-Tet-On/GSTA4 was linearized with EcoR1 and MscI and the big fragments were gel-purified for comicroinjection into fertilized C57BL/6 CBA oocytes by the Transgenic Core Facility of Baylor College of Medicine. TetO-GSTA4 vector was created and injected into embryos at the same facility. Among four founders and 23 babies, we obtained two colonies that were confirmed by genotyping (data not shown). The genotype of the offspring was performed by genotyping using the forward and reverse primers (CGGCCAAGTACCCTT GGTTGAAAT and AATGGAGCCACGGCAATCATCATC). The PCR conditions were as follows: 4 minutes at 95°C, followed by 32 cycles of 95°C for 1 minute, 56°C for 1 minute, and 72°C for 1.5 minute, with a final extension step at 72°C for 10 minutes. The positive fragment is 260 bp.
GSTA4 Overexpression Mice
We used the TetOn/rtTA (reverse tetracycline transactivator) inducible system and Flox-Cre recombination techniques to create SMC-specific, conditional, inducible GSTA4 transgenic mice. To achieve this goal, three strains of mice were required (Supplemental Figure 4A):
SM22-Cre mice (Jackson Laboratory) that constitutively expressed Cre recombinase, driven by the tissue-specific promoter in SMCs;
rtTA-EGFP transgenic mice (Jackson Laboratory), in which the expression of floxed-rtTA was under the control of the ubiquitously expressed ROSA26 promoter, and a floxed stop cassette was present between the ROSA26 promoter and rtTA, which confined rtTA expression to the cells in which Cre recombinase was present, and an internal ribosome entry site followed by the EGFP gene was located downstream of rtTA, thereby allowing tracking of the rtTA-expressing cells by EGFP expression; and
the GSTA4 allele in the TetO-GSTA4 transgenic mice, driven by the tetracycline inducible promoter (TetO).
The genotyping was performed according to the protocol provided by the Jackson Laboratory.
Induction of GSTA4 Expression in Transgenic Mice
Transgenic founders were generated by standard techniques, and two independent founder lines were evaluated, each giving equivalent results. The resulting line is hereafter referred to as iGSTA4/SMC. Transgenic littermates were treated with doxycycline-containing water (0.5 mg/ml, with 5% sucrose added; Sigma Chemicals) and control mice were given 5% sucrose solutions. The expression of GSTA4 were determined by Western blotting.
CKD Model
CKD was induced by subtotal nephrectomy in anesthetized mice as previously described.6,9 Briefly, mice were fed 20% protein chow and, after matching for body weight, subtotal nephrectomy was performed in anesthetized mice in a two-step surgery method (Rodent III Combo). First, the left kidney was decapsulated to avoid ureter and adrenal damage, and approximately three quarters of the left kidney was removed. During recovery, mice were given two doses of buprenorphine (0.1–2.5 mg/kg body wt) after surgery and 12 hours later. The diet was changed to 6% Protein Rodent Diet Chow (Harlan Teklad, Madison, WI) ad libitum to reduce mortality and limit hypertrophy of the injured kidney. Second, the right kidney was removed 1 week later, and after 1 week, the mice with CKD were pair-fed 40% protein chow with sham-operated control mice to induce uremia that includes metabolic acidosis. The BUN was measured by the Comparative Pathology Laboratory Center at Baylor College of Medicine. The serum creatinine level was detected by using the QuantiChrom Creatinine Assay Kit (BioAssay Systems, Hayward, CA). After 4 weeks, AVFs were created.
AVF Model
Mouse AVFs were created as previously described.6 Briefly, mice of 12 weeks of age were anesthetized, and the right internal jugular vein was isolated using a dissecting microscope (Leica MZ6). Its distal end was clamped and ligated, the common carotid artery was ligated below its bifurcation, and the proximal end was clamped. An end-to-end anastomosis was created using 12–0 nylon suture with an interrupted stitch. After unclamping, patency was confirmed visually. The mice were kept warm after surgery, and the analgesic (buprenorphine) was given two times, at 12 hours apart. At 4 weeks after surgery, the mice were anesthetized by intraperitoneal injection and euthanized by perfusing the left ventricle with PBS and 10% formalin for 10 minutes (to maintain the endothelium and morphology of the AVF). AVFs were collected, and slides from 0.5 to 1 mm from the venous anastomosis were collected for hematoxylin and eosin staining. All AVF figures in this article were taken from the venous anastomosis because the arterial anastomosis of AVFs has no significant neointima formation in this AVF mouse model.6 The neointima and media were defined as the regions between the lumen and the adventitia. The vessel wall thickness was determined by measuring the difference between the area of the lumen and the neointima, using the NIS-Elements BR 3.0 program (Nikon, Melville, NY). Five cross-section slides were obtained by selecting the first of every ten sections from each AVF. These slides were used to evaluate neointima formation.
Mouse SMC Isolation and Cell Culture
Mouse SMCs were isolated as previously described.53–55 Briefly, the artery or vein were dissected free from adipose tissue. The endothelium was removed gently with a cotton swab and the adventitia was peeled off. The vessels were cut into small pieces (approximately 1 mm2), and seeded on the culture plate and cultured in DMEM supplemented with 20% heat inactivated FBS (Hyclone, Logan, UT), 100 μg/ml penicillin, and 100 μg/ml streptomycin (Invitrogen Life Technologies). The medium was refreshed after 7 days. The total number of outgrown cells from each piece was counted by a blinded observer. The isolated mouse SMCs were positively stained with α-SMA and calponin.
Immunohistochemistry
For histologic analysis, AVFs were perfused through the left ventricle with 10% phosphate-buffered formaldehyde and processed as previously described.53 Sections were blocked with 10% goat serum (Vector Laboratories, Burlingame, CA) for 30 minutes and then incubated with primary antibodies (GSTA4, 1:1000; α-SMA, 1:500; PCNA, 1:300; p27kip1, 1:300; Calponin, 1:300; and Ki67, 1:500). Sections were washed in 0.5% Tween 20 in PBS and incubated at room temperature with a biotinylated secondary antibody (Vector Laboratories). After washing in 0.5% Tween 20 in PBS, tissue sections were incubated with an Elite ABC reagent (Vector Laboratories) in a peroxidase substrate kit, per manufacturer’s instructions (Vector Laboratories). Sections were counterstained by hematoxylin and eosin. For double immunofluorescence staining of samples, fluorescent secondary antibodies were applied to sections; 4′, 6-diamidino-2- phenylindole was used in counterstaining. For cell immunofluorescence staining, cells were fixed in 4% paraformaldehyde in PBS for 10 minutes at room temperature. Cells were permeabilized in PBS containing 0.1% Triton X-100 and blocked by incubating in 5% BSA for 30 minutes. Fixed cells were washed with PBS and incubated for 60 minutes at room temperature or overnight at 4°C with primary antibodies. After washing three times, the cells were stained with Alexa Fluor secondary antibodies. Pictures were recorded using a Nikon Eclipse 80i Fluorescence Microscope (Nikon).
Real-Time RT-PCR
Total RNA from AVFs was isolated using the RNeasy kit (Qiagen, Valencia, CA). Real-time RT-PCR was performed using the Opticon real-time RT-PCR machine (MJ Research, Waltham, MA). GAPDH was used as an internal standard. Primers for mouse GSTA4 and GAPDH were as follows: forward, 5′-CGGCCAAGTACCCTTGGTTGAAAT-3′; reverse, 5′-AATGGAGCCACGGCAATCATC ATC-3′ and forward, 5′-AGTGGGAGTTGCTGTTGAAATC-3′; reverse, 5′-TGCTGAG TATGTCGTGGAGTCTA-3′, respectively.
Western Blot Analysis
Tissues or cell extracts were prepared in RIPA buffer; protein concentrations in the extracts were assayed using the Bradford protein assay kit (Bio-Rad) and 30 μg protein was separated by SDS–PAGE. After transferring to nitrocellulose membranes, immunoblots were probed separately with various primary antibodies after blocking with 5% skimmed milk in tris-buffered saline. Fluorescence-labeled secondary antibodies were used for detection by the Odyssey Infrared Imaging System (LICOR Inc, Lincoln, NE).
Quantification of 4-HNE
4-HNE in mouse AVFs was determined using the OxiSelect HNE-His Adduct ELISA Kit (catalog no. STA 338; Cell Biolab, San Diego, CA) according to the manufacturer’s protocol. By using this kit, instead of free 4-HNE, the conjugated HNE-His proteins were detected. A series of 4-HNE-BSA standards were prepared for each determination. AVFs from WT and GSTA4 KO mice were rinsed twice with cold PBS and homogenized to a 20% w/v mixture. The homogenates were centrifuged at 13,000×g for 15 minutes and 0.1 ml supernatant was used for each determination, according to the manufacturer’s instructions. Each sample was diluted to 10 μg/ml in 1× PBS. After binding to a 96-well plate at 4°C overnight, the samples were incubated with anti–HNE-His antibody for 1 hour at room temperature. Reaction mixtures were incubated further with the secondary antibody horseradish peroxidase conjugate at room temperature for 1 hour. After halting the enzyme reaction by the addition of the stop solution, the absorbance of the supernatant was determined at 450 nm.
Statistical Analyses
All data are presented as the mean±SD. Comparison among groups was made using one-way ANOVA followed by pairwise comparisons with P value adjustment; P<0.05 was considered to be statistically significant.
Disclosures
None.
Supplementary Material
Acknowledgments
We acknowledge Dr. William E. Mitch for constructive suggestions.
This work was supported by grants from the American Heart Association (115GRNT25700209 and R01 DK095867 to J.C.), the National Institutes of Health (R37DK37175), and a generous grant from Dr. and Mrs. Harold Selzman.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2017030290/-/DCSupplemental.
References
- 1.Allon M, Robbin ML: Increasing arteriovenous fistulas in hemodialysis patients: Problems and solutions. Kidney Int 62: 1109–1124, 2002 [DOI] [PubMed] [Google Scholar]
- 2.Cheung AK, Imrey PB, Alpers CE, Robbin ML, Radeva M, Larive B, Shiu YT, Allon M, Dember LM, Greene T, Himmelfarb J, Roy-Chaudhury P, Terry CM, Vazquez MA, Kusek JW, Feldman HI; Hemodialysis Fistula Maturation Study Group . Intimal hyperplasia, stenosis, and arteriovenous fistula maturation failure in the hemodialysis fistula maturation study. J Am Soc Nephrol 28: 3005–3013, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allon M, Litovsky S, Young CJ, Deierhoi MH, Goodman J, Hanaway M, Lockhart ME, Robbin ML: Medial fibrosis, vascular calcification, intimal hyperplasia, and arteriovenous fistula maturation. Am J Kidney Dis 58: 437–443, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee T, Chauhan V, Krishnamoorthy M, Wang Y, Arend L, Mistry MJ, El-Khatib M, Banerjee R, Munda R, Roy-Chaudhury P: Severe venous neointimal hyperplasia prior to dialysis access surgery. Nephrol Dial Transplant 26: 2264–2270, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Riella MC, Roy-Chaudhury P: Vascular access in haemodialysis: Strengthening the Achilles’ heel. Nat Rev Nephrol 9: 348–357, 2013 [DOI] [PubMed] [Google Scholar]
- 6.Liang A, Wang Y, Han G, Truong L, Cheng J: Chronic kidney disease accelerates endothelial barrier dysfunction in a mouse model of an arteriovenous fistula. Am J Physiol Renal Physiol 304: F1413–F1420, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liang M, Liang A, Wang Y, Jiang J, Cheng J: Smooth muscle cells from the anastomosed artery are the major precursors for neointima formation in both artery and vein grafts. Basic Res Cardiol 109: 431, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liang M, Wang Y, Liang A, Mitch WE, Roy-Chaudhury P, Han G, Cheng J: Migration of smooth muscle cells from the arterial anastomosis of arteriovenous fistulas requires Notch activation to form neointima. Kidney Int 88: 490–502, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Y, Liang A, Luo J, Liang M, Han G, Mitch WE, Cheng J: Blocking notch in endothelial cells prevents arteriovenous fistula failure despite CKD. J Am Soc Nephrol 25: 773–783, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rhyu DY, Yang Y, Ha H, Lee GT, Song JS, Uh ST, Lee HB: Role of reactive oxygen species in TGF-beta1-induced mitogen-activated protein kinase activation and epithelial-mesenchymal transition in renal tubular epithelial cells. J Am Soc Nephrol 16: 667–675, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Sedeek M, Nasrallah R, Touyz RM, Hébert RL: NADPH oxidases, reactive oxygen species, and the kidney: Friend and foe. J Am Soc Nephrol 24: 1512–1518, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yeh CH, Chiang HS, Lai TY, Chien CT: Unilateral ureteral obstruction evokes renal tubular apoptosis via the enhanced oxidative stress and endoplasmic reticulum stress in the rat. Neurourol Urodyn 30: 472–479, 2011 [DOI] [PubMed] [Google Scholar]
- 13.Esterbauer H, Schaur RJ, Zollner H: Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med 11: 81–128, 1991 [DOI] [PubMed] [Google Scholar]
- 14.Chaudhary P, Sharma R, Sahu M, Vishwanatha JK, Awasthi S, Awasthi YC: 4-Hydroxynonenal induces G2/M phase cell cycle arrest by activation of the ataxia telangiectasia mutated and Rad3-related protein (ATR)/checkpoint kinase 1 (Chk1) signaling pathway. J Biol Chem 288: 20532–20546, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abel EL, Angel JM, Riggs PK, Langfield L, Lo HH, Person MD, Awasthi YC, Wang LE, Strom SS, Wei Q, DiGiovanni J: Evidence that Gsta4 modifies susceptibility to skin tumor development in mice and humans. J Natl Cancer Inst 102: 1663–1675, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singh SP, Niemczyk M, Saini D, Awasthi YC, Zimniak L, Zimniak P: Role of the electrophilic lipid peroxidation product 4-hydroxynonenal in the development and maintenance of obesity in mice. Biochemistry 47: 3900–3911, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Xu Y, Gong B, Yang Y, Awasthi YC, Woods M, Boor PJ: Glutathione-S-transferase protects against oxidative injury of endothelial cell tight junctions. Endothelium 14: 333–343, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Zimniak P, Singhal SS, Srivastava SK, Awasthi S, Sharma R, Hayden JB, Awasthi YC: Estimation of genomic complexity, heterologous expression, and enzymatic characterization of mouse glutathione S-transferase mGSTA4-4 (GST 5.7). J Biol Chem 269: 992–1000, 1994 [PubMed] [Google Scholar]
- 19.Liang A, Wang Y, Woodard LE, Wilson MH, Sharma R, Awasthi YC, Du J, Mitch WE, Cheng J: Loss of glutathione S-transferase A4 accelerates obstruction-induced tubule damage and renal fibrosis. J Pathol 228: 448–458, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheng JZ, Yang Y, Singh SP, Singhal SS, Awasthi S, Pan SS, Singh SV, Zimniak P, Awasthi YC: Two distinct 4-hydroxynonenal metabolizing glutathione S-transferase isozymes are differentially expressed in human tissues. Biochem Biophys Res Commun 282: 1268–1274, 2001 [DOI] [PubMed] [Google Scholar]
- 21.Bornfeldt KE, Campbell JS, Koyama H, Argast GM, Leslie CC, Raines EW, Krebs EG, Ross R: The mitogen-activated protein kinase pathway can mediate growth inhibition and proliferation in smooth muscle cells. Dependence on the availability of downstream targets. J Clin Invest 100: 875–885, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pintucci G, Saunders PC, Gulkarov I, Sharony R, Kadian-Dodov DL, Bohmann K, Baumann FG, Galloway AC, Mignatti P: Anti-proliferative and anti-inflammatory effects of topical MAPK inhibition in arterialized vein grafts. FASEB J 20: 398–400, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Jain M, Singh A, Singh V, Maurya P, Barthwal MK: Gingerol inhibits serum-induced vascular smooth muscle cell proliferation and injury-induced neointimal hyperplasia by suppressing p38 MAPK activation. J Cardiovasc Pharmacol Ther 21: 187–200, 2016 [DOI] [PubMed] [Google Scholar]
- 24.Ma Y, Zhang L, Peng T, Cheng J, Taneja S, Zhang J, Delafontaine P, Du J: Angiotensin II stimulates transcription of insulin-like growth factor I receptor in vascular smooth muscle cells: Role of nuclear factor-kappaB. Endocrinology 147: 1256–1263, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hong F, Larrea MD, Doughty C, Kwiatkowski DJ, Squillace R, Slingerland JM: mTOR-raptor binds and activates SGK1 to regulate p27 phosphorylation. Mol Cell 30: 701–711, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Iacovelli J, Lopera J, Bott M, Baldwin E, Khaled A, Uddin N, Fernandez-Valle C: Serum and forskolin cooperate to promote G1 progression in Schwann cells by differentially regulating cyclin D1, cyclin E1, and p27Kip expression. Glia 55: 1638–1647, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Zhang J, Zhong W, Cui T, Yang M, Hu X, Xu K, Xie C, Xue C, Gibbons GH, Liu C, Li L, Chen YE: Generation of an adult smooth muscle cell-targeted Cre recombinase mouse model. Arterioscler Thromb Vasc Biol 26: e23–e24, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dixon BS: Why don’t fistulas mature? Kidney Int 70: 1413–1422, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Kwon SH, Li L, He Y, Tey CS, Li H, Zhuplatov I, Kim SJ, Terry CM, Blumenthal DK, Shiu YT, Cheung AK: Prevention of venous neointimal hyperplasia by a multitarget receptor tyrosine kinase inhibitor. J Vasc Res 52: 244–256, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dember LM: Fistulas first--but can they last? Clin J Am Soc Nephrol 6: 463–464, 2011 [DOI] [PubMed] [Google Scholar]
- 31.Roselaar SE, Nazhat NB, Winyard PG, Jones P, Cunningham J, Blake DR: Detection of oxidants in uremic plasma by electron spin resonance spectroscopy. Kidney Int 48: 199–206, 1995 [DOI] [PubMed] [Google Scholar]
- 32.Jourde-Chiche N, Dou L, Cerini C, Dignat-George F, Brunet P: Vascular incompetence in dialysis patients--protein-bound uremic toxins and endothelial dysfunction. Semin Dial 24: 327–337, 2011 [DOI] [PubMed] [Google Scholar]
- 33.Ali ZA, de Jesus Perez V, Yuan K, Orcholski M, Pan S, Qi W, Chopra G, Adams C, Kojima Y, Leeper NJ, Qu X, Zaleta-Rivera K, Kato K, Yamada Y, Oguri M, Kuchinsky A, Hazen SL, Jukema JW, Ganesh SK, Nabel EG, Channon K, Leon MB, Charest A, Quertermous T, Ashley EA: Oxido-reductive regulation of vascular remodeling by receptor tyrosine kinase ROS1. J Clin Invest 124: 5159–5174, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kokubo T, Ishikawa N, Uchida H, Chasnoff SE, Xie X, Mathew S, Hruska KA, Choi ET: CKD accelerates development of neointimal hyperplasia in arteriovenous fistulas. J Am Soc Nephrol 20: 1236–1245, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Langer S, Kokozidou M, Heiss C, Kranz J, Kessler T, Paulus N, Krüger T, Jacobs MJ, Lente C, Koeppel TA: Chronic kidney disease aggravates arteriovenous fistula damage in rats. Kidney Int 78: 1312–1321, 2010 [DOI] [PubMed] [Google Scholar]
- 36.Bugnicourt JM, Da Silveira C, Bengrine A, Godefroy O, Baumbach G, Sevestre H, Bode-Boeger SM, Kielstein JT, Massy ZA, Chillon JM: Chronic renal failure alters endothelial function in cerebral circulation in mice. Am J Physiol Heart Circ Physiol 301: H1143–H1152, 2011 [DOI] [PubMed] [Google Scholar]
- 37.Wang L, Zhang D, Zheng J, Feng Y, Zhang Y, Liu W: Actin cytoskeleton-dependent pathways for ADMA-induced NF-κB activation and TGF-β high expression in human renal glomerular endothelial cells. Acta Biochim Biophys Sin (Shanghai) 44: 918–923, 2012 [DOI] [PubMed] [Google Scholar]
- 38.Sinha-Hikim I, Shen R, Paul Lee W-NN, Crum A, Vaziri ND, Norris KC: Effects of a novel cystine-based glutathione precursor on oxidative stress in vascular smooth muscle cells. Am J Physiol Cell Physiol 299: C638–C642, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Karimi J, Goodarzi MT, Tavilani H, Khodadadi I, Amiri I: Relationship between advanced glycation end products and increased lipid peroxidation in semen of diabetic men. Diabetes Res Clin Pract 91: 61–66, 2011 [DOI] [PubMed] [Google Scholar]
- 40.Negre-Salvayre A, Coatrieux C, Ingueneau C, Salvayre R: Advanced lipid peroxidation end products in oxidative damage to proteins. Potential role in diseases and therapeutic prospects for the inhibitors. Br J Pharmacol 153: 6–20, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vatsyayan R, Chaudhary P, Sharma A, Sharma R, Rao Lelsani PC, Awasthi S, Awasthi YC: Role of 4-hydroxynonenal in epidermal growth factor receptor-mediated signaling in retinal pigment epithelial cells. Exp Eye Res 92: 147–154, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Usatyuk PV, Parinandi NL, Natarajan V: Redox regulation of 4-hydroxy-2-nonenal-mediated endothelial barrier dysfunction by focal adhesion, adherens, and tight junction proteins. J Biol Chem 281: 35554–35566, 2006 [DOI] [PubMed] [Google Scholar]
- 43.Weiss MF, Scivittaro V, Anderson JM: Oxidative stress and increased expression of growth factors in lesions of failed hemodialysis access. Am J Kidney Dis 37: 970–980, 2001 [DOI] [PubMed] [Google Scholar]
- 44.Wasse H, Huang R, Naqvi N, Smith E, Wang D, Husain A: Inflammation, oxidation and venous neointimal hyperplasia precede vascular injury from AVF creation in CKD patients. J Vasc Access 13: 168–174, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sharma R, Brown D, Awasthi S, Yang Y, Sharma A, Patrick B, Saini MK, Singh SP, Zimniak P, Singh SV, Awasthi YC: Transfection with 4-hydroxynonenal-metabolizing glutathione S-transferase isozymes leads to phenotypic transformation and immortalization of adherent cells. Eur J Biochem 271: 1690–1701, 2004 [DOI] [PubMed] [Google Scholar]
- 46.Xu Y, Gong B, Yang Y, Awasthi YC, Boor PJ: Adenovirus-mediated overexpression of glutathione-s-transferase mitigates transplant arteriosclerosis in rabbit carotid allografts. Transplantation 89: 409–416, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cheng J, Wang Y, Ma Y, Chan BT, Yang M, Liang A, Zhang L, Li H, Du J: The mechanical stress-activated serum-, glucocorticoid-regulated kinase 1 contributes to neointima formation in vein grafts. Circ Res 107: 1265–1274, 2010 [DOI] [PubMed] [Google Scholar]
- 48.Pool-Zobel BL, Selvaraju V, Sauer J, Kautenburger T, Kiefer J, Richter KK, Soom M, Wölfl S: Butyrate may enhance toxicological defence in primary, adenoma and tumor human colon cells by favourably modulating expression of glutathione S-transferases genes, an approach in nutrigenomics. Carcinogenesis 26: 1064–1076, 2005 [DOI] [PubMed] [Google Scholar]
- 49.Ranganna K, Mathew OP, Yatsu FM, Yousefipour Z, Hayes BE, Milton SG: Involvement of glutathione/glutathione S-transferase antioxidant system in butyrate-inhibited vascular smooth muscle cell proliferation. FEBS J 274: 5962–5978, 2007 [DOI] [PubMed] [Google Scholar]
- 50.Hayes JD, Chanas SA, Henderson CJ, McMahon M, Sun C, Moffat GJ, Wolf CR, Yamamoto M: The Nrf2 transcription factor contributes both to the basal expression of glutathione S-transferases in mouse liver and to their induction by the chemopreventive synthetic antioxidants, butylated hydroxyanisole and ethoxyquin. Biochem Soc Trans 28: 33–41, 2000 [DOI] [PubMed] [Google Scholar]
- 51.Noel S, Hamad AR, Rabb H: Reviving the promise of transcription factor Nrf2-based therapeutics for kidney diseases. Kidney Int 88: 1217–1218, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Engle MR, Singh SP, Czernik PJ, Gaddy D, Montague DC, Ceci JD, Yang Y, Awasthi S, Awasthi YC, Zimniak P: Physiological role of mGSTA4-4, a glutathione S-transferase metabolizing 4-hydroxynonenal: Generation and analysis of mGsta4 null mouse. Toxicol Appl Pharmacol 194: 296–308, 2004 [DOI] [PubMed] [Google Scholar]
- 53.Cheng J, Du J: Mechanical stretch simulates proliferation of venous smooth muscle cells through activation of the insulin-like growth factor-1 receptor. Arterioscler Thromb Vasc Biol 27: 1744–1751, 2007 [DOI] [PubMed] [Google Scholar]
- 54.Cheng J, Wang Y, Liang A, Jia L, Du J: FSP-1 silencing in bone marrow cells suppresses neointima formation in vein graft. Circ Res 110: 230–240, 2012 [DOI] [PubMed] [Google Scholar]
- 55.Wilhelmson AS, Fagman JB, Johansson I, Zou ZV, Andersson AG, Svedlund Eriksson E, Johansson ME, Lindahl P, Fogelstrand P, Tivesten Å: Increased intimal hyperplasia after vascular injury in male androgen receptor-deficient mice. Endocrinology 157: 3915–3923, 2016 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.