Abstract
The surface of ZnWO4 nanorods was decorated with Cu2O nanoparticles (Cu2O/ZnWO4) prepared through a precipitation method. The Cu2O nanoparticles were tightly deposited on the ZnWO4 surface and had average diameters of 20 nm. The nanoparticles not only promoted the absorption and utilization of visible light but also facilitated the separation of photogenerated charge carriers. This brought an improvement of the photocatalytic activity. The 5 wt % Cu2O/ZnWO4 photocatalyst displayed the highest degrade efficiency for methylene blue (MB) degradation under visible light, which was 7.8 and 2 times higher than pure ZnWO4 and Cu2O, respectively. Meanwhile, the Cu2O/ZnWO4 composite photocatalyst was able to go through phenol degradation under visible light. The results of photoluminescence (PL), photocurrent, and electrochemical impedance spectra (EIS) measurements were consistent and prove the rapid separation of charge, which originated from the match level structure and the close contact with the interface. The radical and hole trapping experiments were carried out to detect the main active substances in the photodegradation process. The holes and ·O2− radicals were predicted to dominate the photocatalytic process. Based on the characterization analysis and experiment results, a possible photocatalytic mechanism for enhancing photocatalytic activity was proposed.
Keywords: nanoparticles, ZnWO4, degradation, photocatalysis
1. Introduction
Photocatalytic technology can directly use solar energy to degrade organic pollutants using super oxidation capacity, mild reaction conditions, and no secondary pollution. The development of an efficient semiconductor photocatalyst plays an important role in the practical application of photocatalytic technology [1]. Recently, ZnWO4 received widespread attention due to its excellent performance in degrading organic pollutants under ultraviolet light [2,3]. Meanwhile, the ZnWO4 photocatalyst also has the advantages of stable chemical properties and is easy to develop. However, the pure ZnWO4 photocatalyst suffers from a higher photogenerated charge recombination rate and a low utilization rate of sunlight, which severely limits its practical application ability [4,5]. Therefore, the key problem for improving the photocatalytic performance of ZnWO4 is to boost the rapid separation of photo-generated electron-hole pairs on the basis of efficient utilization of solar energy.
Noble metal decorated ZnWO4 can promote the rapid separation of photogenerated charge through the schottky barrier since the Fermi level of noble metal is more positive than ZnWO4 [5,6]. Additionally, ion doping, such as seen in fluorine and chlorine [7,8,9,10], could greatly enhance photocatalytic performance since the doped atoms can act as electron traps and promote photogenerated charge separation. In addition, construction of heterojunction photocatalyst by combining ZnWO4 with other semiconductors, such as Bi2WO6/ZnWO4 [11], ZnWO4/BiOI [12], and BiOBr/ZnWO4 [13], provides a feasible route to facilitate the effective transmission of the charge through interfacial interfaces. Additionally, by combining ZnWO4 with material that has a π–π conjugated structure like C3N4 [14,15] and graphene [16] could effectively inhibit the combination of photo-generated carriers due to the special conductivity of the conjugate material. Recently, Niu [17] and Li [18] prepared Ag/AgCl and Ag@AgBr nanoparticles decorate ZnWO4 nanorods, respectively, to obtain more visible light utilization efficiency for the surface plasmon resonance of Ag nanoparticles. In addition, surface modification of ZnWO4 with narrow band-gap and matching energy level semiconductors not only improved the visible light response but promoted the separation of the photogenerated charges.
As commonly known, Cu2O can absorb the most visible light for the band gap at about 2.2 eV [19] when using Cu2O nanoparticles modified for the wide bandgap semiconductor. Modifications such as TiO2 [20], BiVO4 [21], and BiOBr [22] can greatly enhance the absorption of visible light on the composite. Moreover, based on the morphology, the grain size of the catalyst has an important influence on the activity. Various morphologies of Cu2O can be prepared through multiple methods [23] and exhibit excellent photocatalytic performance. Some studies have pointed out that Cu2O nanoparticles were recently modified on the TiO2 nanosheets or multi-walled carbon nanotubes not to enhance the light absorption but to improve the separation of the photogenerated carriers. This generates a higher photocatalytic performance [24,25]. Considering the criteria, this action loaded the Cu2O nanoparticles onto the surface of the ZnWO4 nanorods to further improve the utilization rate of visible light and then to stimulate production of more electron–holes. Meanwhile, the match between the level structure and the intimately contacted interface of ZnWO4 and Cu2O are beneficial for accelerating the separation of the photogenerated charge carriers, which improves the photocatalytic activity. In fact, there are no previous studies about using Cu2O nanoparticles to decorate ZnWO4 nanorods.
Herein, we modified Cu2O nanoparticles onto the surface of ZnWO4 nanorods. The Cu2O nanoparticles were tightly deposited on the ZnWO4 surface with average diameters of 20 nm. Our results proved that the Cu2O nanoparticles greatly promoted the absorption and utilization of visible light. Meanwhile, the introduction of Cu2O nanoparticles provides a new channel for charge transfer. This greatly inhibited photo-generated carrier recombination and accelerated the migration of interfacial charge carriers. The effects of different Cu2O nanoparticles content on the visible light absorption and charge separation of the composites were investigated. The 5 wt % Cu2O/ZnWO4 photocatalyst displays the highest degrade efficiency for methylene blue (MB) degradation, which was 7.8 and 2 times more than seen in pure ZnWO4 and Cu2O. Based on the characterization analysis and experiment results, a possible photocatalytic mechanism on enhancement of photocatalytic activity was proposed.
2. Experimental
2.1. Synthesis of Photocatalysts
The catalyst is prepared with deionized water. The activity test process uses ultrapure water. All of the reagents are analytically graded. Initially, the synthesis of ZnWO4 was carried out using the hydrothermal method based on previous work [26]. Typically, Na2WO4·2H2O (3 mmol) and Zn(NO3)2·6H2O (3 mmol) were dispersed in 25 mL distilled water with magnetic stirring for 15 min. Then, the two above aqueous solutions were mixed together through magnetic stirring at room temperature for 30 min. Afterward, the mixture was transferred into a Teflon-lined steel autoclave and the autoclaves were heated in a convection oven at 180 °C for 12 h. The resulting precipitates were collected and washed with deionized water and absolute ethanol. The samples were then dried at 80 °C for 10 h.
Then, Cu2O/ZnWO4 composite was prepared using a simple reductive solution chemistry route based on a previous report [22]. First, measured amounts of cetyl trimethyl ammonium bromide (CTAB) and ethylene diamine tetra acetic acid (EDTA) were dissolved in 50 mL deionized water and stirred until a stable suspension was obtained. Then, 0.5 g ZnWO4 was added into the uniform suspension and stirred for 30 min, followed by the addition 0.06 g Cu(Ac)2. Thirty minutes later, 25 mL NaOH solution (0.45 mol·L−1) was added dropwise to the mixed solution. The color of the solution became blue, demonstrating the formation of Cu(OH)2. After 30 min, the solution color slowly turned to orange by adding 25 mL of ascorbic acid (AA) (0.3 mol·L−1) solution dropwise. Finally, composites were washed with ethanol and water and dried at 60 °C for 6 h to obtain Cu2O/ZnWO4.
2.2. Characterization
The crystal structure and phase analysis of the Cu2O/ZnWO4 composite was analyzed through X-ray diffraction (XRD) using a Rigaku D/MAX2500 PC diffractometer (D/MAX2500 PC, Rigaku Corporation, Tokyo, Japan) with Cu Kα radiation, with an operating voltage of 40 kV and an operating current of 100 mA. The morphology and particle size of the catalyst were observed through transmission electron microscopy (TEM, JEM-2010, JEOL Ltd., Akishima, Japan). The UV-visible light (UV-Vis) was used to determine the light absorption properties of the catalyst (Puxi, UV1901, Beijing, China). The surface chemical state was analyzed by an X-ray photoelectron spectroscope (XPS, Shimadzu Kratos, AXIS-Ultra DLD, Tokyo, Japan) with a monochromatized Al Kα radiation source (1486.6 eV). We performed electrochemical and photoelectrochemical measurements using a three-electrode quartz (CHI-660E, Chen Hua Instruments, Shanghai, China). Photoluminescence (PL, Hitachi F-7000, Tokyo, Japan) Spectra were collected to explore the recombination of photogenerated carriers.
2.3. Photocurrent and Electrochemical Impedance Spectra Measurements
The photocurrent and electrochemical impedance spectra (EIS) measurements were conducted using an electrochemical analyzer (CHI660E, Chen Hua Instruments, Shanghai, China) with a standard three-electrode configuration. A standard three-electrode cell was used in the photoelectric studies including a working electrode (as-prepared photocatalyst), a platinum wire as the counter electrode, and a standard calomel electrode (SCE) as the reference electrode. An amount of 0.1 M Na2SO4 was used as the electrolyte solution. The visible light irradiation was obtained from a 500 W Xe lamp with a 420 nm cutoff filter.
2.4. Photocatalytic Activity
The photocatalytic gradation of organic pollutants was carried out in a tube photoreactor. In order to eliminate the effect of temperature on the rate of photocatalytic degradation, the photochemical reaction apparatus was connected to a circulating cooling device (Bilon, Xi’an, China) to control the temperature of the reaction solution in the test tube at 25 ± 2 °C. The irradiation light source was a 400 W metal halide lamp. Adding the filter glass sheets between the metal halide lamp and the reaction tube filtered wavelengths <420 nm. The catalyst activity was analyzed primarily by degrading 10 ppm MB by 0.1 g of catalyst. After stirring for 30 min under dark, 1 mL of sample was taken every 15 min. The collected supernatant solutions were analyzed by the spectrometer at the characteristic absorption peak of 664 nm. The samples obtained during the photocatalytic test were filtered through a filter. Additionally, the degradation of phenol solution was detected by using high performance liquid chromatography (HPLC).
3. Results and Discussion
The crystallographic structures of ZnWO4 and Cu2O/ZnWO4 samples were confirmed by XRD measurements. As seen in Figure 1, the diffraction peaks were observed at 18.91°, 23.84°, 24.58°, 30.72°, 36.31°, 41.15°, and 53.63°, corresponding to the (110), (200), (210), (211), (220), (310), (222), (320), (321), (400) and (421) crystal surfaces of ZnWO4, respectively. This aligns with the standard card (JCPDS 15-0774) [27]. The standard card and the characteristic diffraction peaks were narrow and sharp, indicating that the composites possessed of high crystallinity. Meanwhile, the composites showed high purity since there were no traces of other phases examined. Compared with pure ZnWO4, the XRD patterns of the Cu2O/ZnWO4 composites did not vary in shapes or peaks and it was presumed that the addition of Cu2O did not change the crystal form of ZnWO4. Additionally, no characteristic diffraction peaks attributed to Cu2O were detected in the Cu2O/ZnWO4 composites (as seen the Cu2O JCPDS 65-3288) [22], which was caused by the small amounts and the particle size of Cu2O nanoparticles (max 7 wt %) as well as the high dispersion of Cu2O nanoparticles.
Figure 1.
X-Ray Diffraction (XRD) patterns of ZnWO4 and Cu2O/ZnWO4 photocatalysts.
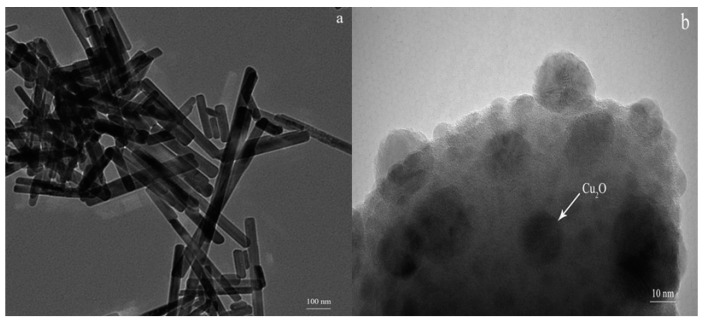
The TEM was performed on the ZnWO4 and 5 wt % Cu2O/ZnWO4 composite to investigate the morphology and detail structural information of the Cu2O/ZnWO4 composite catalyst. As seen in Figure 2a, the pure ZnWO4 nanorods have a smooth surface with a length of 200–500 nm and a width of 20–30 nm. In comparison, as seen in Figure 2b, the Cu2O nanoparticles, with average diameters of 20 nm, were coated on the surface of ZnWO4 nanorods uniformly. The Cu2O nanoparticles did not significantly change its morphology or the size of ZnWO4 photocatalysts, which was consistent with the XRD results. Meanwhile, the Cu2O nanoparticles were beneficial for separating the photo-generated carriers and improving the degradation performance of composites.
Figure 2.
Transmission Electron Microscopy (TEM) images of (a) ZnWO4; (b) Cu2O/ZnWO4.
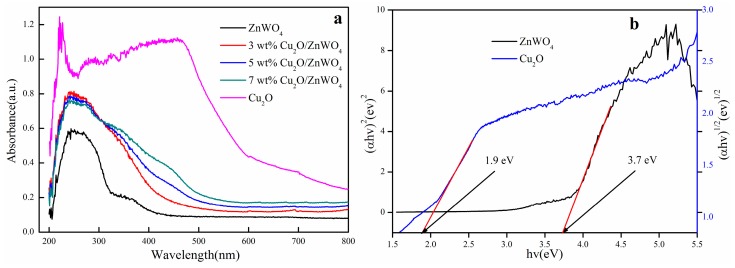
Figure 3a shows the UV-visible diffuse reflectance pattern of ZnWO4, Cu2O and Cu2O/ZnWO4 samples. As seen in Figure 3a, the pure ZnWO4 shows its fundamental absorption edge at 320 nm due to its large band-gap energy while the Cu2O exhibited strong absorption in the λ < 600 nm region. When Cu2O nanoparticles were modified on the ZnWO4 surface, it brought more visible light absorption for all of the Cu2O/ZnWO4 composites, and red shifted into the visible light region because of the photosensitizing effect of the incorporated Cu2O nanoparticles. Additionally, the visible light absorption edge increased with rising Cu2O content. The forbidden band width of a semiconductor catalyst can be calculated using the Kubelka-Munk equation [28]. The result is shown in Figure 4b. According to the literature [21,29], the Cu2O was a direct transition semiconductor and the ZnWO4 was an indirect transition. As such, the band gap of pure Cu2O and ZnWO4 was calculated to be 1.9 and 3.7 eV (as seen in Figure 3b), respectively.
Figure 3.
(a) UV-visible light (UV-vis) diffuses reflection spectra of pure ZnWO4, Cu2O and Cu2O/ZnWO4 samples; (b) the band gap energies of ZnWO4 and Cu2O.
Figure 4.
X-Ray Photoelectron Spectroscopy (XPS) spectra of Cu2O/ZnWO4 sample: (a) Survey of the sample; (b) Zn 2p; (c) Cu 2p and (d) W 4f.
So as to demonstrate the interaction between Cu2O and ZnWO4 in Cu2O/ZnWO4 composites, the valence state and the binding energy in Cu2O/ZnWO4 were determined by using XPS. The results show that the element of Cu, O, Zn, W and C all appear in the full XPS spectrum of Cu2O/ZnWO4 and the corresponding high resolution spectra, which are noted in Figure 4b–d. Zn 2p binding energy position was shown in Figure 4b. In Figure 4b, we can observe that the characteristic peaks appear at 1022.1 and 1044.5 eV, respectively, which corresponds to the peak position of Zn 2p3/2 and Zn 2p1/2 [14]. Figure 4c is the spectrum result of Cu 2p. There are two peaks shown at 931.9 and 951.9 eV, which corresponds to Cu 2p3/2 and Cu 2p1/2, respectively. The experimental results are consistent with reports in the literature [22]. The XPS spectrum of W 4f (as seen in Figure 4d) shows that two binding energy peaks appear at 35.0 eV and at 37.4 eV, respectively for W 4f7/2 and W 4f5/2, which determines the existence of W [30]. After the analysis of XRD and XPS, it can be concluded that Cu2O and ZnWO4 coexist in the Cu2O/ZnWO4 sample.
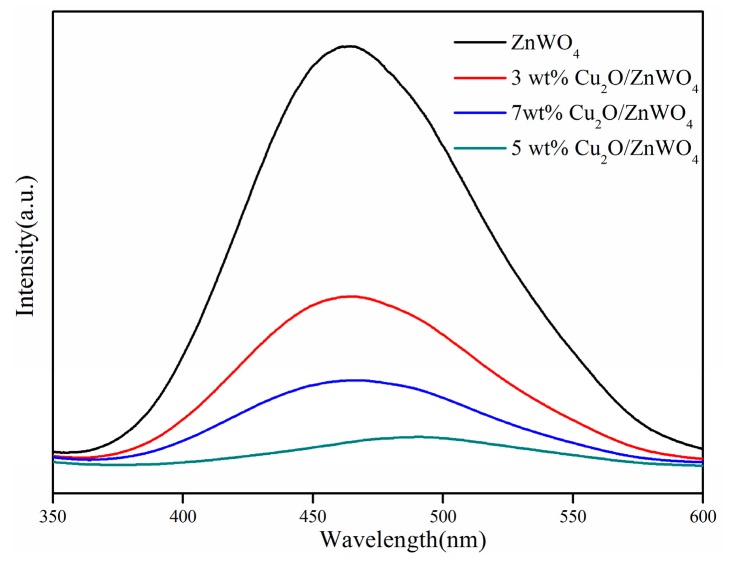
The photoluminescence (PL) spectra can evaluate the transfer and recombination processes of photogenerated e−/h+ pairs in semiconductors. It is widely accepted that the lower PL intensity, the lower photo-carrier recombination rate and the higher photocatalytic performance [31]. The excitation wavelength ZnWO4 and Cu2O in the Cu2O/ZnWO4 architectures was 315 nm. The comparison of PL spectra is shown in Figure 5. The main emission peak is centered at 465 nm for the ZnWO4 sample due to the recombination of photogenerated carriers in the ZnWO4. Once Cu2O nanoparticles were added, the photoluminescence dropped markedly and the 5 wt % Cu2O/ZnWO4 composites displayed the lowest PL intensity, which indicates that the photogenerated carriers were effectively separated in the composite semiconductors. This shows that the process was conducive to promoting the photocatalytic properties of the composite material.
Figure 5.
Photoluminescence (PL) spectra of ZnWO4 and Cu2O/ZnWO4 composites.
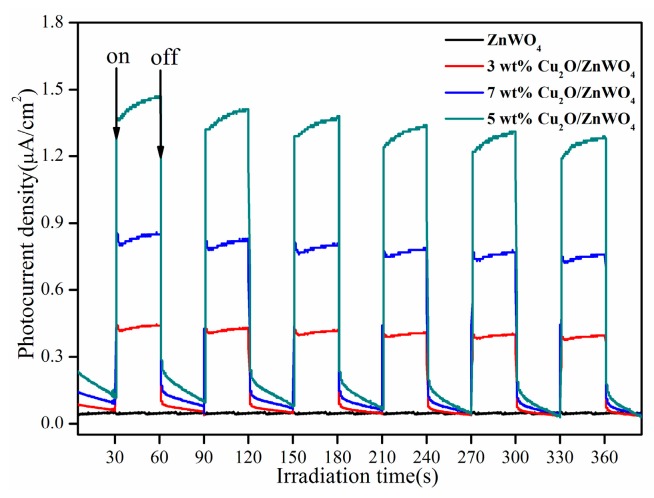
In order to better explain the photogenerated carriers, the Cu2O/ZnWO4 composites should be effectively separated. The transient photocurrent responses of the ZnWO4 and Cu2O/ZnWO4 composites were recorded for several on/off cycles of irradiation. It was generally considered that the higher the peak intensity, the higher the separation efficiency of the photogenerated carriers [32]. As shown in Figure 6, when visible light is irradiated, the photocurrent increases sharply. In contrast, in the dark environment, the photocurrent was immediately reduced to zero. A significant photoelectric response was observed from the on/off photoperiod. In addition, ZnWO4 showed almost no photocurrent response due to its lack of visible light response while the Cu2O/ZnWO4 composites displayed much higher photocurrent intensity, where the 5 wt % Cu2O/ZnWO4 composites exhibited the highest photocurrent density. This shows that the introduction of Cu2O nanoparticles will greatly improve the photocurrent response. The Cu2O/ZnWO4 composites exhibit faster charge separation efficiency, which originates from the ZnWO4 and Cu2O matching energy level structure that rapidly separates photogenerated hole pairs.
Figure 6.
Photocurrent-time curves of bulk ZnWO4 and Cu2O/ZnWO4 composites under visible light (>420 nm) irradiation with 30 s light on/off cycles.
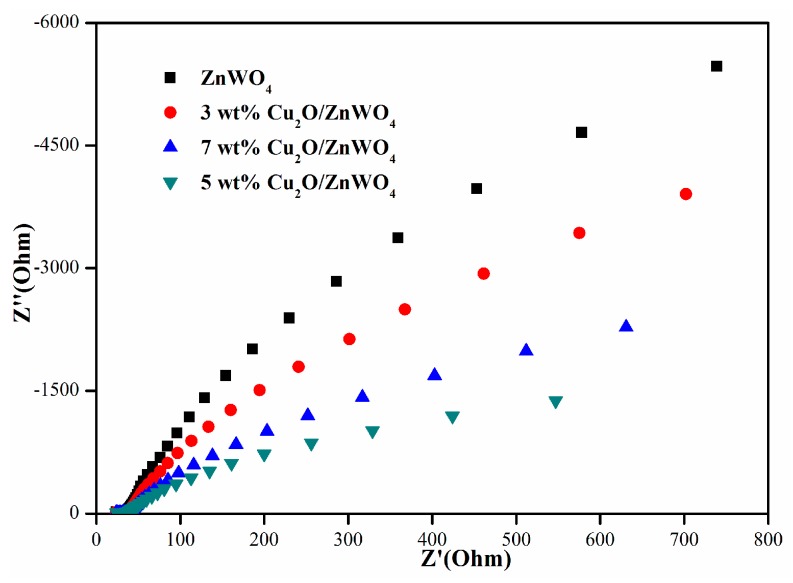
To further test the transport rate of the electrons more intuitively, the electrochemical impedance spectra (EIS) of the prepared materials was carried out. The size of the Nyquist curve reflects the reaction rate and resistance between grain and grain, grain boundaries, and after polarization. The electrode reaction rate decreases due to a larger radius. Small arc radii imply a faster interface electron transfer for better photocatalytic ability [33]. The EIS response of ZnWO4 and Cu2O/ZnWO4 composites in visible light irradiation is shown in Figure 7. Obviously, the semicircular diameter of the composite was smaller than pure ZnWO4, which demonstrates that the photo-generated charge can be effectively separated due to the strong interaction between ZnWO4 and Cu2O. The radius is the smallest when the composite ratio of Cu2O is 5 wt %. All these results of EIS, photocurrent, and PL were consistent and illustrated that the introduction of Cu2O could greatly accelerate the separation and transfer of photoelectron-hole pairs and improve the degradation performance of composites.
Figure 7.
Electrochemical Impedance Spectra (EIS) plots of pure ZnWO4 and as-prepared various Cu2O/ZnWO4 samples irradiated with visible light.
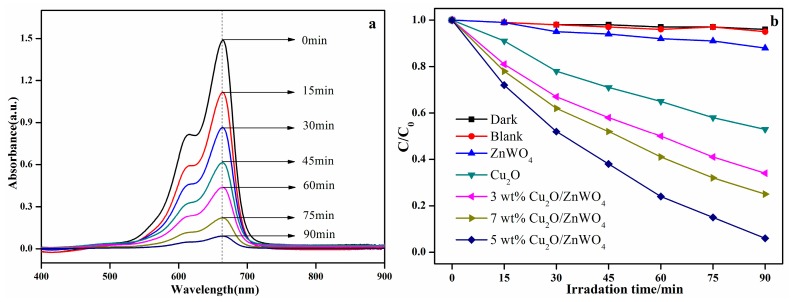
To determine the photocatalytic performance of the catalyst, the activity of degradation MB by ZnWO4, Cu2O, and Cu2O/ZnWO4 were compared under the same experimental conditions. As seen in Figure 8a, the characteristic absorption peak of MB was 664 nm, and the intensity decreased gradually in the absence of 5 wt % Cu2O/ZnWO4 composite under visible light irradiation, and finally disappears at 90 min. Figure 8b displays the change of MB concentration (C/C0) against photodegradation time for the prepared catalyst samples. The experimental results show that MB was not degraded in the absence of a photocatalyst or light irradiation. The MB degradation is a photocatalytic process, in which the photocatalysts generate photo-generated electron-hole pairs under illumination and further act on the target degradation product. Pure ZnWO4 has almost no photocatalytic activity because it does not respond to visible light. At the same time, the degradation rate of Cu2O to MB was only 47%, which was due to the high recombination rate of excited electron-hole pairs [34]. As a comparison, the 5 wt % Cu2O/ZnWO4 composites could be degraded at about 90% MB solution, which was derived from the synergic effect of the Cu2O/ZnWO4 composites, and achieved the rapid separation of photogenerated carriers at the heterojunction. Meanwhile, it was observed that a higher amount of Cu2O would decrease the photocatalytic activities. At higher content, the Cu2O nanoparticles may agglomerate and reduce the specific surface area of the composites. Additionally, extra Cu2O particles may become the new photogenerated recombination centers and, thereby, reduce the effective photogenerated pairs. Then the particles may inhibit the composite photocatalytic performance. All these results were consistent with the PL, photocurrent, and EIS test results of the composites. Therefore, the 5 wt % Cu2O/ZnWO4 composite exhibited better photocatalytic activity.
Figure 8.
(a) UV-Vis spectral changes of methylene blue (MB) aqueous solution in the presence of 5 wt % Cu2O/ZnWO4 photocatalyst; (b) the activity of different catalysts to degrade MB in visible light.
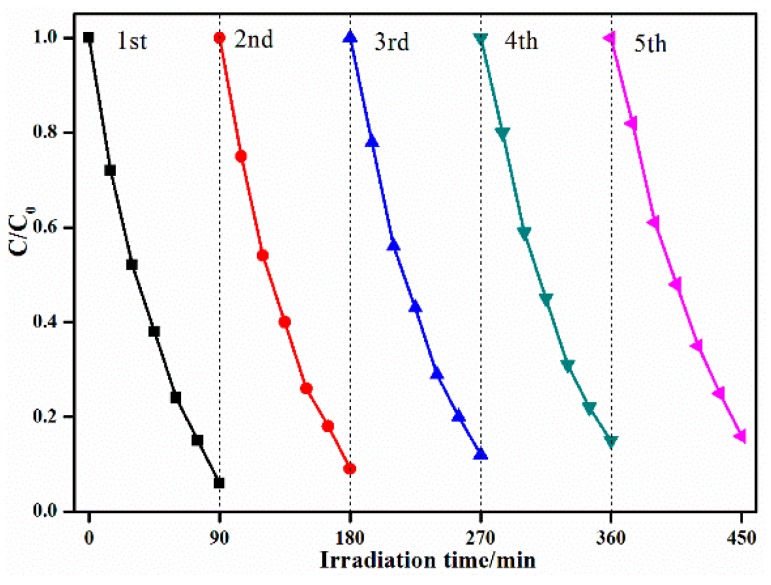
Catalyst stability and reusability are also very important from the viewpoint of the catalyst’s practical applications. The photocatalytic activity of the 5 wt % Cu2O/ZnWO4 composites in the degradation of MB was studied in consecutive cycles under the same conditions (as seen in Figure 9). The degradation efficiency was slightly decreased after five cycles and the final degrade rate was approximately 83%, which indicates that the Cu2O nanoparticles modified in ZnWO4 surface preparation of composite photocatalyst could improve the recycling performance of the catalyst and the practical application value. It could act as a potential photocatalyst for water pollution.
Figure 9.
Stability investigation of MB photocatalytic degradation over Cu2O/ZnWO4.
In order to further study the photocatalytic degradation performance of the composites, phenol was used as the degradation object, and the same conditions were tested. It can be seen from Figure 10 that the photolysis of the phenol solution in the blank condition is negligible, and the adsorption capacity of the composite material to the phenol in the dark environment is also very limited. In contrast, the Cu2O/ZnWO4 photocatalysts exhibit higher catalytic degradation properties for phenol solution. The 5 wt % Cu2O/ZnWO4 composite photocatalyst exhibits better photocatalytic performance, which was much higher than pure Cu2O and ZnWO4. This is due to the composite having a bigger advantage than Cu2O and ZnWO4. More visible light responses can be excited to produce more photogenerated pairs and the photogenerated pairs can be effectively separated between the two composite interfaces, which ultimately promote the photocatalytic performance.
Figure 10.
Linear relationship between ln(C0/C) and light time of phenol degradation.
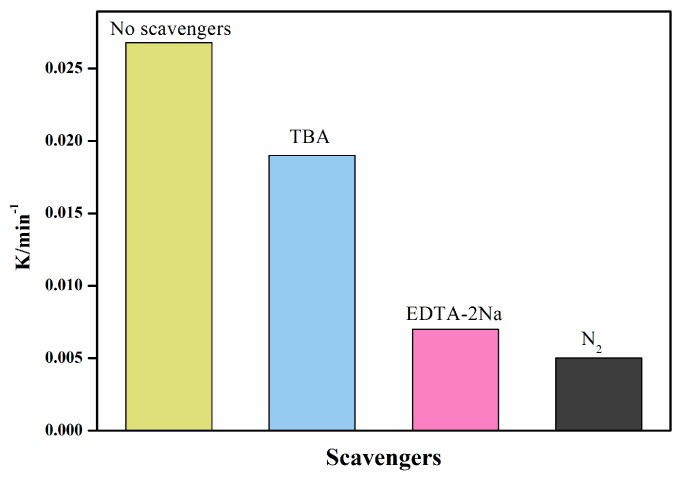
Quenching experiments explain the photodegradation process. In the free radical capture experiments of photodegradation, tert-butanol (TBA) and EDTA-2Na were used as capture agents for ·OH and h+, respectively [30]. Meanwhile, during the course of the experiment, nitrogen was used to remove oxygen to test whether the superoxide free radicals were active species during the degradation process. As seen in Figure 11, the photodegradation efficiency was significantly suppressed when the EDTA-2Na and in the N2-saturated experiment condition was added, which indicates that the h+ and O2− were likely the dominant active species in this process. In contrast, the dissolved ·OH was not the main active species due to the degradation rate constant showing only a slight decline when added to the TBA solution. Therefore, the photodegradation of MB over Cu2O/ZnWO4 photocatalyst can be mainly associated with direct holes and O2− radicals for the photocatalytic degradation.
Figure 11.
First-order rate constant values of photogenerated active species trapped in the system of photocatalytic degradation of MB by Cu2O/ZnWO4 under visible light.
The photocatalytic degradation kinetic curve was investigated by the first-order simplification of Langmuir-Hinshelwood (L-H) kinetics, which is well established for photocatalysis at low initial pollutant concentrations [31]. The relevant equation is as follows:
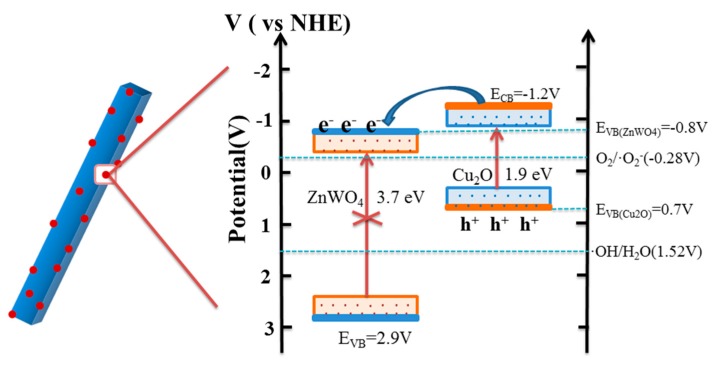
where C0 and C are the concentrations of dye in solution at times 0 and t, respectively, and kapp is the apparent first-order rate constant (min−1). The k value is obtained from the gradient of the graph of ln(C/C0) versus time (t). Based on the characterization analysis and the experimental data, the mechanism of the high photocatalytic activities of Cu2O/ZnWO4 composites is discussed. Since the light absorption extends from ultraviolet light to visible light, there is no doubt that more visible light absorption can stimulate the generation of more photo-generated carriers, which could be effectively separated between the two composite interfaces because of the match level structure and ultimately promote the photocatalytic performance. According to the conduction band (CB) and valence band (VB) potentials of Cu2O (−1.2 and 0.7 eV, respectively [22]) and those of ZnWO4 (−0.8 and 2.9 eV, respectively [10]), it can be seen that Cu2O/ZnWO4 composites have matching energy levels. The staggered energy level structure contributes to the fast separation of photo-generated carriers.
The possible mechanism of improved photocatalytic performance of Cu2O/ZnWO4 composite formed by Cu2O nanoparticles loading on ZnWO4 was deduced based on above experimental results, as seen in Figure 12. This can be explained as follows: Under visible irradiation, Cu2O could stimulate the generation of photogenerated hole pairs while the electrons on the conduction band can quickly migrate to the conduction band of ZnWO4 at the close interface of the composites since the conduction band of Cu2O is more negative than the conduction band of ZnWO4. Then the electrons transferred to the ZnWO4 react with the adsorbed oxygen molecules to produce O2− radicals and participate in the photocatalytic oxidation degradation and the organic pollution process photocatalytic reaction. Simultaneously, after the transition stay in the Cu2O valence band hole, another active species can directly oxidize organic pollutants involved in the reaction. Furthermore, this photo-assisted electron transfer method can effectively avoid the photo-generated carrier recombination, improve the utilization of the quantum pair, and promote the photocatalytic activity of the composite. This is consistent with the PL, photoelectric experiment results, and the active species of the quenching experiment.
Figure 12.
Schematic illustration of photo-generated carriers’ transportation for Cu2O/ZnWO4 composite.
4. Conclusions
The surface of ZnWO4 nanorods was decorated with Cu2O nanoparticles (Cu2O/ZnWO4) prepared through the precipitation method. The 5 wt % Cu2O/ZnWO4 composites displayed the best photocatalytic performance under visible light irradiation and the degradation rate of MB solution was 91%. The improvement in the photocatalytic performance of Cu2O/ZnWO4 composites due to the Cu2O nanoparticles not only promoted the absorption and utilization of visible light, but also facilitated the migration of photogenerated charge carriers due to the matched level structure and the intimately contacted interface. In addition, the O2− and holes were predicted to be the main active species in the photocatalytic degradation process based on free-radical scavenging experiments. In conclusion, the Cu2O/ZnWO4 composite is a highly efficient and stable photocatalyst that can effectively degrade organic pollutants in order to protect the environment.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (No. 51672081), Hebei Natural Science Funds for the Joint Research of Iron and Steel (B2016209348).
Author Contributions
Lingyu Tian, Yulan Rui and Weijia An conceived and designed the experiments; Lingyu Tian, Kelei Sun and Wenquan Cui analyzed the data; Lingyu Tian, Yulan Rui and Weijia An wrote the paper. All authors reviewed and approved the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Patnaik S., Martha S., Parida K.M. An overview of the structural, textural and morphological modulations of g-C3N4 towards photocatalytic hydrogen production. RSC Adv. 2016;6:46929–46951. doi: 10.1039/C5RA26702A. [DOI] [Google Scholar]
- 2.Feng K.L., Huang S.Q., Lou Z.Y., Zhu N.W., Yuan H.P. Enhanced photocatalytic activities of the heterostructured upconversion photocatalysts with cotton mediated on TiO2/ZnWO4:Yb3+, Tm3+ Dalton Trans. 2015;44:13681–13687. doi: 10.1039/C5DT01761K. [DOI] [PubMed] [Google Scholar]
- 3.Lin J., Lin J., Zhu Y.F. Controlled Synthesis of the ZnWO4 Nanostructure and Effects on the Photocatalytic Performance. Inorg. Chem. 2007;46:8372–8378. doi: 10.1021/ic701036k. [DOI] [PubMed] [Google Scholar]
- 4.Sun L.M., Zhao X., Cheng X.F., Sun H.G., Li Y.L., Li P., Fan W.L. Evaluating the C, N, and F Pairwise Codoping Effect on the Enhanced Photoactivity of ZnWO4: The Charge Compensation Mechanism in Donor Acceptor Pairs. J. Phys. Chem. C. 2011;115:15516–15524. doi: 10.1021/jp204324n. [DOI] [Google Scholar]
- 5.Yu C.L., Yu J.C. Sonochemical fabrication, characterization and photocatalytic properties of Ag/ZnWO4 nanorod catalyst. Mater. Sci. Eng. B. 2009;164:16–22. doi: 10.1016/j.mseb.2009.06.008. [DOI] [Google Scholar]
- 6.Song X.C., Zheng Y.F., Yang E., Liu G., Zhang Y., Chen H.F., Zhang Y.Y. Photocatalytic activities of Cd-doped ZnWO4 nanorods prepared by a hydrothermal process. J. Hazard. Mater. 2010;179:1122–1127. doi: 10.1016/j.jhazmat.2010.03.123. [DOI] [PubMed] [Google Scholar]
- 7.Huang G.L., Zhu Y.F. Enhanced Photocatalytic Activity of ZnWO4 Catalyst via Fluorine Doping. J. Phys. Chem. C. 2007;111:11952–11958. doi: 10.1021/jp071987v. [DOI] [Google Scholar]
- 8.Huang G.L., Zhang S.C., Xu T.G., Zhu Y.F. Fluorination of ZnWO4 Photocatalyst and Influence on the Degradation Mechanism for 4-Chlorophenol. Environ. Sci. Technol. 2008;42:8516–8521. doi: 10.1021/es801672a. [DOI] [PubMed] [Google Scholar]
- 9.Chen S.H., Sun S.X., Sun H.G., Fan W.L., Zhao X., Sun X. Experimental and Theoretical Studies on the Enhanced Photocatalytic Activity of ZnWO4 Nanorods by Fluorine Doping. J. Phys. Chem. C. 2010;114:7680–7688. doi: 10.1021/jp911952v. [DOI] [Google Scholar]
- 10.Huang G.L., Zhu Y.F. Synthesis and photoactivity enhancement of ZnWO4 photocatalysts doped with chlorine. CrystEngComm. 2012;14:8076–8082. doi: 10.1039/c2ce26005k. [DOI] [Google Scholar]
- 11.He D.Q., Wang L.L., Xu D.D., Zhai J.L., Wang D.J., Xie T.F. Investigation of Photocatalytic Activities over Bi2WO6/ZnWO4 Composite under UV Light and Its Photoinduced Charge Transfer Properties. ACS Appl. Mater. Interfaces. 2011;3:3167–3171. doi: 10.1021/am200664y. [DOI] [PubMed] [Google Scholar]
- 12.Li P., Zhao X., Jia C.J., Sun H.G., Sun L.M., Cheng X.F., Liu L., Fan W.L. ZnWO4/BiOI heterostructures with highly efficient visible light photocatalytic activity: the case of interface lattice and energy level match. J. Mater. Chem. A. 2013;1:3421–3429. doi: 10.1039/c3ta00442b. [DOI] [Google Scholar]
- 13.Song X.C., Li W.T., Huang W.Z., Zhou H., Zheng Y.F., Yin H.Y. A novel pen heterojunction BiOBr/ZnWO4: Preparation and its improved visible light photocatalytic activity. Mater. Chem. Phys. 2015;160:251–256. doi: 10.1016/j.matchemphys.2015.04.033. [DOI] [Google Scholar]
- 14.Sun L.M., Zhao X., Jia C.J., Zhou Y.X., Cheng X.F., Li P., Liu L., Fan W.L. Enhanced visible-light photocatalytic activity of g-C3N4-ZnWO4 by fabricating a heterojunction: investigation based on experimental and theoretical studies. J. Mater. Chem. 2012;22:23428–23438. doi: 10.1039/c2jm34965e. [DOI] [Google Scholar]
- 15.Wang Y.J., Wang Z.X., Muhammad S., He J. Graphite-like C3N4 hybridized ZnWO4 nanorods: Synthesis and its enhanced photocatalysis in visible light. CrystEngComm. 2012;14:5065–5070. doi: 10.1039/c2ce25517k. [DOI] [Google Scholar]
- 16.Bai X.J., Wang L., Zhu Y.F. Visible Photocatalytic Activity Enhancement of ZnWO4 by Graphene Hybridization. ACS Catal. 2012;2:2769–2778. doi: 10.1021/cs3005852. [DOI] [Google Scholar]
- 17.Ke J., Niu C.G., Zhang J., Zeng G.M. Significantly enhanced visible light photocatalytic activity and surfaceplasmon resonance mechanism of Ag/AgCl/ZnWO4 composite. J. Mol. Catal. A Chem. 2014;395:276–282. doi: 10.1016/j.molcata.2014.08.039. [DOI] [Google Scholar]
- 18.Li K.B., Xue J., Zhang Y.H., Wei H., Liu Y.L., Dong C.X. ZnWO4 nanorods decorated with Ag/AgBr nanoparticles as highlyefficient visible-light-responsive photocatalyst for dye AR18 photodegradation. Appl. Surf. Sci. 2014;320:1–9. doi: 10.1016/j.apsusc.2014.09.060. [DOI] [Google Scholar]
- 19.Zhang Z.H., Du R., Zhang L.B., Zhu H.B., Zhang H.N., Wang P. Carbon-Layer-Protected Cuprous Oxide Nanowire Arrays for Efficient Water Reduction. ACS Nano. 2013;7:1709–1717. doi: 10.1021/nn3057092. [DOI] [PubMed] [Google Scholar]
- 20.Wang M.Y., Sun L., Lin Z.Q., Cai J.H., Xie K.P., Lin C.J. P-N heterojunction photoelectrodes composed of Cu2O-loaded TiO2 nanotube arrays with enhanced photoelectrochemical and photoelectrocatalytic activities. Energy Environ. Sci. 2013;6:1211–1220. doi: 10.1039/c3ee24162a. [DOI] [Google Scholar]
- 21.Wang W.Z., Huang X.W., Wu S., Zhou Y.X., Wang L.J., Shi H.L., Liang Y.J., Zou B. Preparation of p-n junction Cu2O/BiVO4 heterogeneous nanostructures withenhanced visible-light photocatalytic activity. Appl. Catal. B. 2013;134–135:293–301. doi: 10.1016/j.apcatb.2013.01.013. [DOI] [Google Scholar]
- 22.Cui W.Q., An W.J., Liu L., Hu J.S., Liang Y.H. Novel Cu2O quantum dots coupled flower-like BiOBr for enhanced photocatalytic degradation of organic contaminant. J. Hazard. Mater. 2014;280:417–427. doi: 10.1016/j.jhazmat.2014.08.032. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Z.L., Che H.W., Wang Y.L., Gao J.J., Zhao L.R., She X.L., Sun J., Poernomo G.W., Zhong Z.Y., Su F.B. Facile Synthesis of Mesoporous Cu2O Microspheres with Improved Catalytic Property for Dimethyldichlorosilane Synthesis. Ind. Eng. Chem. Res. 2012;51:1264–1274. doi: 10.1021/ie2020747. [DOI] [Google Scholar]
- 24.Liu L.C., Gu X.R., Sun C.Z., Li H., Deng Y., Gao F., Dong L. In situ loading of ultra-small Cu2O particles on TiO2 nanosheets to enhance the visible-light photoactivity. Nanoscale. 2012;4:6351–6359. doi: 10.1039/c2nr31859h. [DOI] [PubMed] [Google Scholar]
- 25.Song S.Q., Rao R.C., Yang H.X., Zhang A.M. Cu2O/MWCNTs Prepared by Spontaneous Redox: Growth Mechanism and Superior Catalytic Activity. J. Phys. Chem. C. 2010;114:13998–14003. doi: 10.1021/jp103621q. [DOI] [Google Scholar]
- 26.Yu S.H., Liu B., Mo M.S., Huang J.H., Liu X.M., Qian Y.T. General synthesis of single-crystal tungstate nanorods/nanowires: A facile, low-temperature solution approach. Adv. Funct. Mater. 2003;13:639–647. doi: 10.1002/adfm.200304373. [DOI] [Google Scholar]
- 27.Hojamberdieva M., Katsumata K., Morita K., Bilmes S.A., Matsushita N., Okada K. One-step hydrothermal synthesis and photocatalytic performance of ZnWO4/Bi2WO6 composite photocatalysts for efficient degradation of acetaldehyde under UV light irradiation. Appl. Catal. A Gen. 2013;457:12–20. doi: 10.1016/j.apcata.2013.03.014. [DOI] [Google Scholar]
- 28.Chen Z.H., Bing F., Liu Q., Zhang Z.G., Fang X.M. Novel Z-scheme visible-light-driven Ag3PO4/Ag/SiC photocatalysts with enhanced photocatalytic activity. J. Mater. Chem. A. 2015;3:4652–4658. doi: 10.1039/C4TA06530A. [DOI] [Google Scholar]
- 29.Khyzhuna O.Y., Bekeneva V.L., Atuchinb V.V., Galashovc E.N., Shlegelc V.N. Electronic properties of ZnWO4 based on ab initio FP-LAPW band-structure calculations and X-ray spectroscopy data. Mater. Chem. Phys. 2013;140:588–595. doi: 10.1016/j.matchemphys.2013.04.010. [DOI] [Google Scholar]
- 30.Hu J.S., An W.J., Wang H., Geng J.P., Cui W.Q., Zhan Y. Synthesis of a hierarchical BiOBr nanodots/Bi2WO6 p-n heterostructure with enhanced photoinduced electric and photocatalytic degradation performance. RSC Adv. 2016;6:29554–29562. doi: 10.1039/C6RA00794E. [DOI] [Google Scholar]
- 31.An W.J., Cui W.Q., Liang Y.H., Hu J.S., Liu L. Surface decoration of BiPO4 with BiOBr nanoflakes to build heterostructure photocatalysts with enhanced photocatalytic activity. Appl. Surf. Sci. 2015;351:1131–1139. doi: 10.1016/j.apsusc.2015.06.098. [DOI] [Google Scholar]
- 32.Liu L., Ding L., Liu Y.G., An W.J., Lin S.L., Liang Y.H., Cui W.Q. A stable Ag3PO4@PANI core@shell hybrid: Enrichment photocatalytic degradation with π-π conjugation. Appl. Catal. B. 2017;201:92–104. doi: 10.1016/j.apcatb.2016.08.005. [DOI] [Google Scholar]
- 33.Chen F.Y., An W.J., Liu L., Liang Y.H., Cui W.Q. Highly efficient removal of bisphenol A by a three-dimensional graphene hydrogel-AgBr@rGO exhibiting adsorption/photocatalysis synergy. Appl. Catal. B. 2017;217:65–80. doi: 10.1016/j.apcatb.2017.05.078. [DOI] [Google Scholar]
- 34.Ai Z.H., Xiao H.Y., Mei T., Liu J., Zhang L.Z., Deng K.J., Qiu J.R. Electro-fenton degradation of Rhodamine B based on a composite cathode of Cu2O nanocubes and carbon nanotubes. J. Phys. Chem. C. 2008;112:11929–11935. doi: 10.1021/jp803243t. [DOI] [Google Scholar]