Abstract
Excitatory glutamatergic neurotransmission via N-methyl-d-aspartate receptor (NMDAR) is critical for synaptic plasticity and survival of neurons. However, excessive NMDAR activity causes excitotoxicity and promotes cell death, underlying a potential mechanism of neurodegeneration occurred in Alzheimer’s disease (AD). Studies indicate that the distinct outcomes of NMDAR-mediated responses are induced by regionalized receptor activities, followed by different downstream signaling pathways. The activation of synaptic NMDARs initiates plasticity and stimulates cell survival. In contrast, the activation of extrasynaptic NMDARs promotes cell death and thus contributes to the etiology of AD, which can be blocked by an AD drug - memantine, an NMDAR antagonist that selectively blocks the function of extrasynaptic NMDARs.
Alzheimer’s disease
According to World Health Organization, Alzheimer’s disease is the major cause of dementia, accounting for 60–70% of cases. The symptom of this chronic neurodegenerative disease deteriorates over time - from early forgetfulness to gradual worsening in language, orientation and behavior and late severe loss of memory and some bodily function until the ultimate death.
The etiology of AD seems to be complex and multi-factorial. Early onset familial AD is caused by genetic mutation(s) in presenilin (PS1, PS2) and amyloid precursor protein (APP) genes, affecting a common pathogenic pathway in APP synthesis and proteolysis and causing excessive production of amyloid β (Aβ) (Wu, Rosa-Neto et al. 2012). The cause of late onset sporadic AD, however, remains poorly understood. It is believed that the major risk factor is genetics with multiple genes involved. Other risk factors include aging, apolipoprotein (Apo) E4 genotype, head trauma, vascular conditions (Burns and Iliffe 2009). The major deterministic and risk genes of AD are listed in Table 1.
Table 1.
The major deterministic and risk genes involved in Alzheimer’s disease.
| Genes | Chromosomal location | Normal function | Pathology in AD |
|---|---|---|---|
| Presenilin-1 (PS-1) | Chr. 14 | Processing amyloid precursor protein and forming Aβ | Cause early-onset AD (Schellenberg, Bird et al. 1992) |
| Presenilin-2 (PS-2) | Chr. 1 | Processing amyloid precursor protein and forming Aβ | Cause early-onset AD (Levy-Lahad, Wasco et al. 1995) |
| Amyloid precursor protein (APP) | Chr. 21 | Regulating synaptic function | Cause early-onset AD (Chartier-Harlin, Crawford et al. 1991, Goate, Chartier-Harlin et al. 1991, Murrell, Farlow et al. 1991) |
| Apolipoprotein E4 (ApoE4) | Chr. 19 | Transporting cholesterol | Risk factor of AD (Corder, Saunders et al. 1993, Strittmatter, Saunders et al. 1993) |
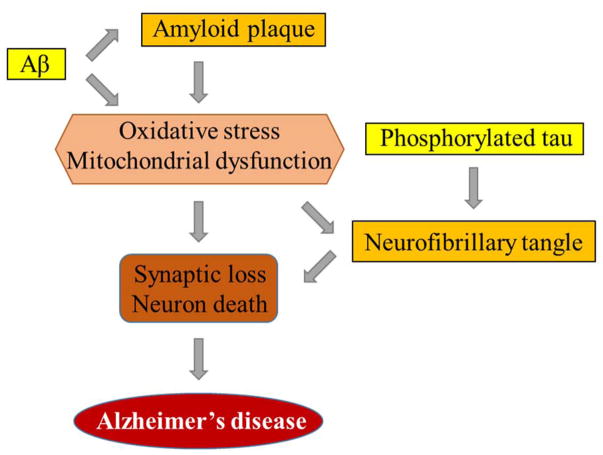
The pathophysiology of AD includes both structural and functional abnormalities. As AD progresses, multiple anatomical lesions occur in the brain, including the appearance of senile plaques consisting of Aβ and neurofibrillary tangles containing phosphorylated tau, the substantial loss of synaptic profiles (Perl 2010). In AD, there are significant oxidative stress and mitochondrial abnormalities. Also, severe synaptic damage and neuronal death can be observed. The association of AD with these pathological changes is illustrated in Figure 1.
Figure 1.
The association of Alzheimer’s disease with Aβ, phosphorylated tau, mitochondria dysfunction and neuronal degeneration. Amyloid plaques and neurofibrillary tangles are commonly seen pathological changes in AD, which are formed from Aβ and phosphorylated tau, respectively. Aβ and amyloid plaques triggers oxidative stress and mitochondrial dysfunction, which damages synapses and promotes neuron degeneration. Neurofibrillary tangles aggravates these processes. The significant synaptic loss and neuronal death manifests the symptoms of Alzheimer’s disease.
There is no cure for AD until now. A few treatments exist though. According to the Alzheimer’s disease Medications Fact Sheet published by National Institute on Aging, current FDA-approved prescription drugs for the treatment of AD patients contain two categories. One is cholinesterase inhibitors for mild to moderate AD. The other is used for treatment of moderate to severe AD and contains memantine, an antagonist against N-methyl-D-aspartate receptor (NMDAR), a receptor gated by the neurotransmitter - glutamate.
The purpose of this review is to discuss the involvement of glutamatergic neurotransmission in Alzheimer’s disease. In particular, we will center on the contribution of NMDAR signaling to AD.
Glutamate and glutamatergic signaling
Glutamate is the most abundant excitatory neurotransmitter in the mammalian Central Nervous System (CNS). It is extensively distributed in the CNS whereas it is almost exclusively located intracellularly. Glutamate can be synthesized through a number of metabolic pathways (Fonnum 1984). In wet tissue, glutamate is measured at concentrations of 5–15μmol/g (Perry, Yong et al. 1987, Erecinska and Silver 1990). Its concentration in the synaptic cleft at resting conditions is about 0.6μM (Bouvier, Szatkowski et al. 1992). During synaptic transmission glutamate concentration can go above 10μM at spatially localized extracellular regions (Clements, Lester et al. 1992). The residual glutamate is removed by glutamate uptake/transporter system (Danbolt 2001). The amount of available extracellular glutamate is subject to strict regulation to allow appropriate level of signaling.
The vast majority of the excitatory neurotransmission in the mammalian CNS is mediated by glutamate and its receptors, mainly ligand-gated ionotropic glutamate receptors (iGluRs). These receptors also play fundamental roles in synaptic plasticity, the underlying molecular mechanism of learning and memory (Riedel, Platt et al. 2003). Owing to their pivotal roles in excitatory neurotransmission, the disruption of the normal signaling via iGluRs is implicated in a wide range of neuropathological disorders and diseases, such as epilepsy and brain damage, Parkinson’s, Alzheimer’s, Huntington’s, multiple sclerosis, thereby making iGluRs important drug targets for therapeutic purposes (Bleich, Romer et al. 2003).
NMDAR-mediated glutamatergic signaling in synaptic plasticity and neuronal survival
One subgroup of iGluRs is selectively gated by specific agonists N-methyl-d-aspartate (NMDA), thus named as NMDA receptor (NMDAR) (Collingridge, Olsen et al. 2009). NMDAR is distinct than other iGluRs in its voltage-dependent activation via removal of Mg2+ blockade, its high Ca2+ permeability and relatively slow ligand-gated kinetics. These unique features render NMDAR unique and essential for its crucial role in synaptic function and plasticity (Cotman, Monaghan et al. 1988, Collingridge and Singer 1990). Basically, at resting membrane potential of about −70mV, the Ca2+ channel of NMDAR is blocked by Mg2+. During the induction of Long Term Potentiation (LTP), however, the strong and prolonged release of glutamate from the presynaptic terminal activates AMPARs and the subsequent depolarization removes the Mg2+ blockade of the NMDAR channel and allows the influx of Ca2+ ion. This strong activation of NMDARs triggers a Ca2+/calmodulin-dependent protein kinase II (CaMKII) - mediated signaling cascade that eventually leads to an enhanced synaptic strength. On the contrary, a modest activation of NMDARs causes a modest increase in postsynaptic Ca2+ and triggers phosphatases - mediated Long Term Depression (LTD) (Luscher and Malenka 2012).
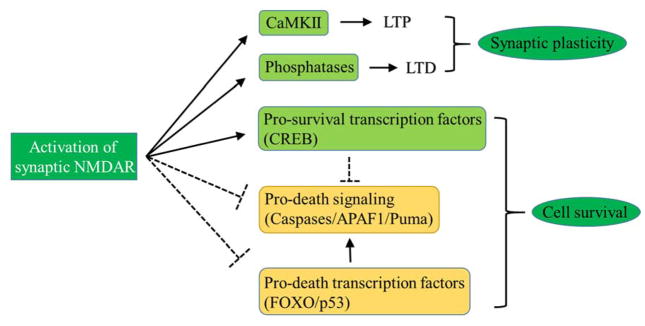
In addition to their crucial roles in synaptic transmission and plasticity, NMDARs seem to be critical for the survival of neurons by activating neuronal survival pathway (Hardingham 2006, Hetman and Kharebava 2006). To support this, studies indicate that the blockade of NMDAR function leads to neuronal apoptosis and degeneration (Ikonomidou, Bosch et al. 1999, Monti and Contestabile 2000). This NMDAR-dependent neuroprotective functions mainly involves the activation of pro-survival transcription factors and the inhibition of apoptosis (Hardingham and Bading 2010, Parsons and Raymond 2014). The activation of synaptic NMDAR promotes survival gene expression by activating Ca2+-dependent transcription factors such as cyclic-AMP response element binding protein (CREB) and suppresses caspases and apoptotic pathway such as Puma (pro-apoptotic Bcl2 homology domain 3 (BH3)-only member gene) - activated signaling cascade and transcription factor FOXO (forkhead box protein O) - induced pro-death gene expression (Hardingham and Bading 2010, Parsons and Raymond 2014). The NMDAR-dependent synaptic plasticity and activation of cell survival signaling is illustrated in Figure 2.
Figure 2.
Activation of synaptic NMDAR mediates survival pathway and induces LTP and LTD. In classical synaptic plasticity model, synaptic NMDAR activity activates CaMKII and phosphatases, which triggers LTP and LTD, respectively. Moreover, the activation of synaptic NMDARs suppresses the pro-apoptotic signaling molecules and pathways such as caspases, APAF1 and Puma occurred in cytoplasm; and it also promotes the expression of the pro-survival transcriptional factors such as CREB and suppresses the expression of pro-apoptotic transcription factors such as FOXO and p53, which, in turn, inhibits apoptosis and promotes cell survival.
Glutamate excitotoxicity and abnormal NMDAR activity in Alzheimer’s disease
Insufficient synaptic NMDAR signaling compromises neuronal cell survival. Excessive stimulation of glutamatergic signaling, however, results in excitotoxicity, in which nerve cells are damaged or killed, or neurological trauma such as stroke (Rothman and Olney 1986). Besides acute effects, many studies indicate a role for glutamate excitotoxicity in delayed slowly-evolving neurodegeneration (Choi 1988, Lipton and Rosenberg 1994). Accumulating evidence demonstrates that the toxicity is principally mediated by excessive Ca2+ entry, primarily through NMDARs (Choi 1987, Choi, Koh et al. 1988, Koh and Choi 1991, Tymianski, Charlton et al. 1993), since NMDARs have a much higher permeability for calcium ions compared to other iGluRs (Choi 1992). In this regard, the modest depolarization of the postsynaptic membrane and other factors that relieve the Mg2+ blockade can activate NMDARs in a mild and chronic way, which causes the prolonged Ca2+ influx into the postsynaptic neuron. The pathological level of Ca2+ signaling leads to gradual loss of synaptic function and ultimate neuronal cell death, which correlates clinically with the progressive decline in cognition/memory and the development of pathological neural anatomy seen in AD patients, and this, in turn, rationalizes the clinical trial of memantine, an NMDAR antagonist, as a symptomatological and neuroprotective treatment for AD (Danysz, Parsons et al. 2000, Danysz and Parsons 2003, Wenk 2006). Thus, given the fact that NMDARs are also important for cell survival, the level of NMDAR signaling must be maintained at a proper level so that it is enough to promote neuronal survival but not harmful to cause neurodegeneration as occurred in AD. The major factors that affect NMDAR signaling in AD include glutamate availability and the modulation of NMDAR channel functions.
Glutamate uptake and recycling system is an important factor that determines the availability of glutamate for signaling processes. Unfortunately, in AD, this system can be severely weakened. In AD patient, a decrease in the glutamate transporter capacity and protein expression and a selective loss of vesicular glutamate transporter (VGluT) were seen (Masliah, Alford et al. 1996, Li, Mallory et al. 1997, Kirvell, Esiri et al. 2006). Moreover, excitatory amino acid transporter 2 (EAAT2) that is primarily located in perisynaptic astrocytes, was reported to have impaired function in AD (Scott, Gebhardt et al. 2011). Studies using various species of Aβ peptides in neuronal cell culture seem to support the same idea that toxic Aβ may allow more glutamate availability by impairing glutamate uptake/recycling mechanisms (Arias, Arrieta et al. 1995, Parpura-Gill, Beitz et al. 1997, Fernandez-Tome, Brera et al. 2004). This enhanced glutamate supply is likely to contribute to AD-associated excitotoxicity and neurodegeneration.
The integrity of the presynaptic neurotransmitter release machinery may also contribute to glutamate availability. It was reported that Aβ can significantly reduce the expression of presynaptic protein such as synaptophysin, syntaxin, and synaptotagmin, many of which are active components of the neurotransmitter release machinery (Jang, In et al. 2014). Other research pointed out that endogenous Aβ played a key part in the regulation of activity-dependent synaptic vesicle release (Abramov, Dolev et al. 2009). The deficits in the presynaptic vesicle release machinery supposedly compromises glutamate availability, making it less possible to initiate an excitotoxicity effect. However, it is consistent with the pathological synaptic loss observed in AD and it is likely to be a relatively later effect occurred in ongoing degenerating neurons.
In addition to the elevated level of glutamate, AD may enhance NMDAR signaling through the modulation of the receptor itself.
Numerous studies have demonstrated that Aβ directly modulates the electrophysiological function of NMDARs. In general, species of Aβ causes elevated NMDAR-mediated synaptic currents and collateral toxicity, which can be attenuated or blocked by NMDAR antagonists, such as MK-801 (Le, Colom et al. 1995, Domingues, Almeida et al. 2007, Kawamoto, Lepsch et al. 2008), D-APV or memantine (Kamenetz, Tomita et al. 2003, Ye, Walsh et al. 2004, Alberdi, Sanchez-Gomez et al. 2010, Texido, Martin-Satue et al. 2011). The structural effects of Aβ, such as synaptic loss, can also be prevented by NMDAR antagonists (Hsieh, Boehm et al. 2006, Shankar, Bloodgood et al. 2007). Aβ may even physically interact with NMDARs, either directly or via synaptic proteins such as PSD95 (De Felice, Velasco et al. 2007, Lacor, Buniel et al. 2007, Venkitaramani, Chin et al. 2007).
AD may also affect the level of NMDAR coagonists. The complete activation of NMDARs by glutamate requires the continuous binding of coagonist D-serine or glycine, and therefore these coagonists play important modulatory role in NMDAR function. In AD hippocampus or Aβ-treated cultured microglia, both D-serine and the expression of serine racemase, which generates D-serine, are reported to be increased (Wu, Bodles et al. 2004). Furthermore, the knockout of serine racemase, which significantly reduce the forebrain D-serine content, ameliorated the NMDA or Aβ caused neurotoxicity (Inoue, Hashimoto et al. 2008).
Taken together, it is widely accepted that Aβ-induced changes in the availability of glutamate and the function of NMDAR channels correlate with the neurotoxicity and degeneration observed in Alzheimer’s disease.
Regionalized NMDAR signaling and Alzheimer’s disease
It seems that Ca2+ level via NMDAR signaling is critical in determining cell fate - insufficient signaling leads to failure in cell survival while too much signaling causes excitotoxicity and neurodegeneration. However, emerging evidence indicates that this is only part of the story. The membranous location and regionalized signaling of NMDARs may be key to AD-associated pathophysiology.
The NMDARs on membrane can be roughly divided into two population groups – synaptic and extrasynaptic. Compared to numerous studies conducted with synaptic NMDARs, few were done to extrasynaptic NMDARs and their function remains largely unknown until recently. A few recent studies contend that extrasynaptic NMDARs may be involved in or responsible for glutamate excitotoxicity and cell death (Sattler, Xiong et al. 2000, Hardingham, Fukunaga et al. 2002, Leveille, El Gaamouch et al. 2008, Stanika, Pivovarova et al. 2009). Although there are still opposing reports, a widely accepted model is that synaptic NMDAR activation promotes cell survival whereas extrasynaptic activation triggers cell death and the tilted balance between synaptic and extrasynaptic NMDAR activity contributes to neuronal dysfunction such as acute cell trauma and chronic neurodegenerative diseases (Hardingham and Bading 2010). Moreover, synaptic and extrasynaptic NMDARs were found to be activated by different endogenous coagonists, D-serine and glycine, respectively (Papouin, Ladepeche et al. 2012). Interestingly, memantine, an NMDAR antagonist, was reported to target preferentially against extrasynaptic NMDAR (Leveille, El Gaamouch et al. 2008).
Extrasynaptic NMDAR-induced responses seem to be tightly related to the physiological changes occurred in AD. A recent study demonstrated that Aβ specifically activated extrasynaptic NMDARs, which caused synaptic loss, and memantine antagonized Aβ induced negative effects (Talantova, Sanz-Blasco et al. 2013). This regionalized NMDAR signaling model can also be used to address Aβ-caused decrease in NMDAR expression (Geddes, Chang-Chui et al. 1986, Procter, Stirling et al. 1989, Hynd, Scott et al. 2001). The decreased number of NMDARs may simply disrupt the balance between synaptic and extrasynaptic NMDARs.
What occurs to the synaptic portion of NMDARs in AD appears to be well established. Numerous studies have shown that soluble Aβ causes the reduction of synaptic glutamatergic transmission and the inhibition of synaptic plasticity. For example, one study demonstrated that application of Aβ1–42 in cultured cortical neurons leads to the internalization of synaptic NMDARs and the depression of NMDAR-mediated currents (Snyder, Nong et al. 2005). Generally speaking, AD-associated synaptic damage or weakening of synaptic function is one of the universal themes of AD pathophysiology.
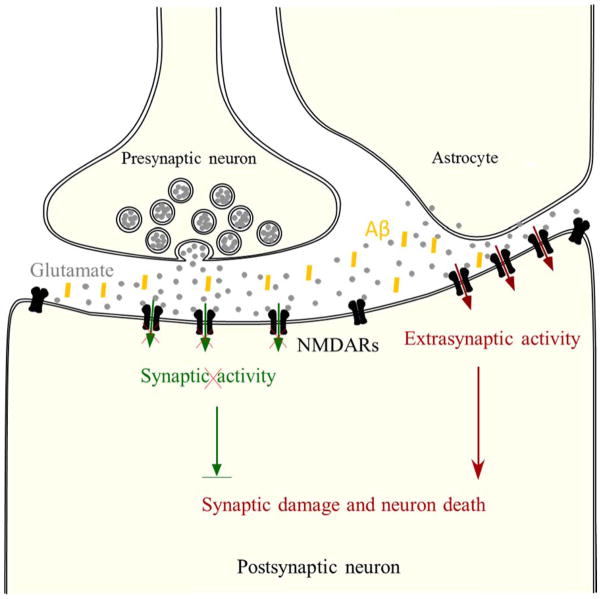
In AD, extrasynaptic NMDAR-mediated signaling pathway antagonizes the cell survival pathway mediated through synaptic NMDARs by inactivating CREB and activating FOXO transcription factors and promoting the related pro-death and oxidative stress signaling (Hardingham and Bading 2010, Parsons and Raymond 2014). Moreover, multiple mechanisms may further undermine the function of synaptic NMDAR function. As a result, the balance of the regionalized NMDAR signaling in synaptic and extrasynaptic regions is tilted towards the downstream signaling pathways that eventually leads to the death of neurons in AD, as illustrated in Figure 3.
Figure 3.
Regionalized NMDAR activity determines cell fate. In principle, extrasynaptic NMDAR-mediated apoptosis antagonizes synaptic NMDAR-mediated survival. In Alzheimer’s disease, glutamate released from astrocytes activates extrasynaptic NMDARs and triggers pro-apoptotic signaling (red) that overcomes synaptic NMDAR-mediated survival signaling (green) that is already undermined by other mechanisms such as the endocytosis of NMDARs, leading to further synaptic damage and eventual neuronal death.
Conclusions and future directions
The involvement of neurotransmitter glutamate and its receptors in the function of synaptic plasticity and the etiology of neurodegenerative diseases such as Alzheimer’s disease has been under investigation for many years. Recent studies reveal that glutamatergic neurotransmission through NMDARs lead to dichotomous results. Synaptic NMDAR signaling is required for the survival of neurons. However, extrasynaptic NMDARs signaling activated by the spillover of astrocyte- or presynaptic terminal-released glutamate plays a key role in antagonizing the synaptic pro-survival signaling pathway and tilt the balance toward excitotoxicity and ultimate neurodegeneration. This is supported by the beneficial clinical effects seen in moderate to severe AD cases by memantine, an FDA-approved NMDAR antagonist, which functions possibly through suppressing the extrasynaptic NMDAR signaling. Further studies on memantine and its derivatives will help elucidate the molecular mechanisms of how glutamate and NMDAR function in the etiology of AD.
References
- Abramov E, Dolev I, Fogel H, Ciccotosto GD, Ruff E, Slutsky I. Amyloid-beta as a positive endogenous regulator of release probability at hippocampal synapses. Nat Neurosci. 2009;12(12):1567–1576. doi: 10.1038/nn.2433. [DOI] [PubMed] [Google Scholar]
- Alberdi E, Sanchez-Gomez MV, Cavaliere F, Perez-Samartin A, Zugaza JL, Trullas R, Domercq M, Matute C. Amyloid beta oligomers induce Ca2+ dysregulation and neuronal death through activation of ionotropic glutamate receptors. Cell Calcium. 2010;47(3):264–272. doi: 10.1016/j.ceca.2009.12.010. [DOI] [PubMed] [Google Scholar]
- Arias C, Arrieta I, Tapia R. beta-Amyloid peptide fragment 25–35 potentiates the calcium-dependent release of excitatory amino acids from depolarized hippocampal slices. J Neurosci Res. 1995;41(4):561–566. doi: 10.1002/jnr.490410416. [DOI] [PubMed] [Google Scholar]
- Bleich S, Romer K, Wiltfang J, Kornhuber J. Glutamate and the glutamate receptor system: a target for drug action. Int J Geriatr Psychiatry. 2003;18(Suppl 1):S33–40. doi: 10.1002/gps.933. [DOI] [PubMed] [Google Scholar]
- Bouvier M, Szatkowski M, Amato A, Attwell D. The glial cell glutamate uptake carrier countertransports pH-changing anions. Nature. 1992;360(6403):471–474. doi: 10.1038/360471a0. [DOI] [PubMed] [Google Scholar]
- Burns A, Iliffe S. Alzheimer’s disease. BMJ. 2009;338:b158. doi: 10.1136/bmj.b158. [DOI] [PubMed] [Google Scholar]
- Chartier-Harlin MC, Crawford F, Houlden H, Warren A, Hughes D, Fidani L, Goate A, Rossor M, Roques P, Hardy J, et al. Early-onset Alzheimer’s disease caused by mutations at codon 717 of the beta-amyloid precursor protein gene. Nature. 1991;353(6347):844–846. doi: 10.1038/353844a0. [DOI] [PubMed] [Google Scholar]
- Choi DW. Ionic dependence of glutamate neurotoxicity. J Neurosci. 1987;7(2):369–379. doi: 10.1523/JNEUROSCI.07-02-00369.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi DW. Glutamate neurotoxicity and diseases of the nervous system. Neuron. 1988;1(8):623–634. doi: 10.1016/0896-6273(88)90162-6. [DOI] [PubMed] [Google Scholar]
- Choi DW. Excitotoxic cell death. J Neurobiol. 1992;23(9):1261–1276. doi: 10.1002/neu.480230915. [DOI] [PubMed] [Google Scholar]
- Choi DW, Koh JY, Peters S. Pharmacology of glutamate neurotoxicity in cortical cell culture: attenuation by NMDA antagonists. J Neurosci. 1988;8(1):185–196. doi: 10.1523/JNEUROSCI.08-01-00185.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements JD, Lester RA, Tong G, Jahr CE, Westbrook GL. The time course of glutamate in the synaptic cleft. Science. 1992;258(5087):1498–1501. doi: 10.1126/science.1359647. [DOI] [PubMed] [Google Scholar]
- Collingridge GL, Olsen RW, Peters J, Spedding M. A nomenclature for ligand-gated ion channels. Neuropharmacology. 2009;56(1):2–5. doi: 10.1016/j.neuropharm.2008.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collingridge GL, Singer W. Excitatory amino acid receptors and synaptic plasticity. Trends Pharmacol Sci. 1990;11(7):290–296. doi: 10.1016/0165-6147(90)90011-v. [DOI] [PubMed] [Google Scholar]
- Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, Roses AD, Haines JL, Pericak-Vance MA. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 1993;261(5123):921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- Cotman CW, Monaghan DT, Ganong AH. Excitatory amino acid neurotransmission: NMDA receptors and Hebb-type synaptic plasticity. Annu Rev Neurosci. 1988;11:61–80. doi: 10.1146/annurev.ne.11.030188.000425. [DOI] [PubMed] [Google Scholar]
- Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001;65(1):1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- Danysz W, Parsons CG. The NMDA receptor antagonist memantine as a symptomatological and neuroprotective treatment for Alzheimer’s disease: preclinical evidence. Int J Geriatr Psychiatry. 2003;18(Suppl 1):S23–32. doi: 10.1002/gps.938. [DOI] [PubMed] [Google Scholar]
- Danysz W, Parsons CG, Mobius HJ, Stoffler A, Quack G. Neuroprotective and symptomatological action of memantine relevant for Alzheimer’s disease--a unified glutamatergic hypothesis on the mechanism of action. Neurotox Res. 2000;2(2–3):85–97. doi: 10.1007/BF03033787. [DOI] [PubMed] [Google Scholar]
- De Felice FG, Velasco PT, Lambert MP, Viola K, Fernandez SJ, Ferreira ST, Klein WL. Abeta oligomers induce neuronal oxidative stress through an N-methyl-D-aspartate receptor-dependent mechanism that is blocked by the Alzheimer drug memantine. J Biol Chem. 2007;282(15):11590–11601. doi: 10.1074/jbc.M607483200. [DOI] [PubMed] [Google Scholar]
- Domingues A, Almeida S, da Cruz e Silva EF, Oliveira CR, Rego AC. Toxicity of beta-amyloid in HEK293 cells expressing NR1/NR2A or NR1/NR2B N-methyl-D-aspartate receptor subunits. Neurochem Int. 2007;50(6):872–880. doi: 10.1016/j.neuint.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Erecinska M, I, Silver A. Metabolism and role of glutamate in mammalian brain. Prog Neurobiol. 1990;35(4):245–296. doi: 10.1016/0301-0082(90)90013-7. [DOI] [PubMed] [Google Scholar]
- Fernandez-Tome P, Brera B, Arevalo MA, de Ceballos ML. Beta-amyloid25–35 inhibits glutamate uptake in cultured neurons and astrocytes: modulation of uptake as a survival mechanism. Neurobiol Dis. 2004;15(3):580–589. doi: 10.1016/j.nbd.2003.12.006. [DOI] [PubMed] [Google Scholar]
- Fonnum F. Glutamate: a neurotransmitter in mammalian brain. J Neurochem. 1984;42(1):1–11. doi: 10.1111/j.1471-4159.1984.tb09689.x. [DOI] [PubMed] [Google Scholar]
- Geddes JW, Chang-Chui H, Cooper SM, Lott IT, Cotman CW. Density and distribution of NMDA receptors in the human hippocampus in Alzheimer’s disease. Brain Res. 1986;399(1):156–161. doi: 10.1016/0006-8993(86)90611-6. [DOI] [PubMed] [Google Scholar]
- Goate A, Chartier-Harlin MC, Mullan M, Brown J, Crawford F, Fidani L, Giuffra L, Haynes A, Irving N, James L, et al. Segregation of a missense mutation in the amyloid precursor protein gene with familial Alzheimer’s disease. Nature. 1991;349(6311):704–706. doi: 10.1038/349704a0. [DOI] [PubMed] [Google Scholar]
- Hardingham GE. Pro-survival signalling from the NMDA receptor. Biochem Soc Trans. 2006;34(Pt 5):936–938. doi: 10.1042/BST0340936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham GE, Bading H. Synaptic versus extrasynaptic NMDA receptor signalling: implications for neurodegenerative disorders. Nat Rev Neurosci. 2010;11(10):682–696. doi: 10.1038/nrn2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham GE, Fukunaga Y, Bading H. Extrasynaptic NMDARs oppose synaptic NMDARs by triggering CREB shut-off and cell death pathways. Nat Neurosci. 2002;5(5):405–414. doi: 10.1038/nn835. [DOI] [PubMed] [Google Scholar]
- Hetman M, Kharebava G. Survival signaling pathways activated by NMDA receptors. Curr Top Med Chem. 2006;6(8):787–799. doi: 10.2174/156802606777057553. [DOI] [PubMed] [Google Scholar]
- Hsieh H, Boehm J, Sato C, Iwatsubo T, Tomita T, Sisodia S, Malinow R. AMPAR removal underlies Abeta-induced synaptic depression and dendritic spine loss. Neuron. 2006;52(5):831–843. doi: 10.1016/j.neuron.2006.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynd MR, Scott HL, Dodd PR. Glutamate(NMDA) receptor NR1 subunit mRNA expression in Alzheimer’s disease. J Neurochem. 2001;78(1):175–182. doi: 10.1046/j.1471-4159.2001.00409.x. [DOI] [PubMed] [Google Scholar]
- Ikonomidou C, Bosch F, Miksa M, Bittigau P, Vockler J, Dikranian K, Tenkova TI, Stefovska V, Turski L, Olney JW. Blockade of NMDA receptors and apoptotic neurodegeneration in the developing brain. Science. 1999;283(5398):70–74. doi: 10.1126/science.283.5398.70. [DOI] [PubMed] [Google Scholar]
- Inoue R, Hashimoto K, Harai T, Mori H. NMDA- and beta-amyloid1–42-induced neurotoxicity is attenuated in serine racemase knock-out mice. J Neurosci. 2008;28(53):14486–14491. doi: 10.1523/JNEUROSCI.5034-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang BG, In S, Choi B, Kim MJ. Beta-amyloid oligomers induce early loss of presynaptic proteins in primary neurons by caspase-dependent and proteasome-dependent mechanisms. Neuroreport. 2014;25(16):1281–1288. doi: 10.1097/WNR.0000000000000260. [DOI] [PubMed] [Google Scholar]
- Kamenetz F, Tomita T, Hsieh H, Seabrook G, Borchelt D, Iwatsubo T, Sisodia S, Malinow R. APP processing and synaptic function. Neuron. 2003;37(6):925–937. doi: 10.1016/s0896-6273(03)00124-7. [DOI] [PubMed] [Google Scholar]
- Kawamoto EM, Lepsch LB, Boaventura MF, Munhoz CD, Lima LS, Yshii LM, Avellar MC, Curi R, Mattson MP, Scavone C. Amyloid beta-peptide activates nuclear factor-kappaB through an N-methyl-D-aspartate signaling pathway in cultured cerebellar cells. J Neurosci Res. 2008;86(4):845–860. doi: 10.1002/jnr.21548. [DOI] [PubMed] [Google Scholar]
- Kirvell SL, Esiri M, Francis PT. Down-regulation of vesicular glutamate transporters precedes cell loss and pathology in Alzheimer’s disease. J Neurochem. 2006;98(3):939–950. doi: 10.1111/j.1471-4159.2006.03935.x. [DOI] [PubMed] [Google Scholar]
- Koh JY, Choi DW. Selective blockade of non-NMDA receptors does not block rapidly triggered glutamate-induced neuronal death. Brain Res. 1991;548(1–2):318–321. doi: 10.1016/0006-8993(91)91140-v. [DOI] [PubMed] [Google Scholar]
- Lacor PN, Buniel MC, Furlow PW, Clemente AS, Velasco PT, Wood M, Viola KL, Klein WL. Abeta oligomer-induced aberrations in synapse composition, shape, and density provide a molecular basis for loss of connectivity in Alzheimer’s disease. J Neurosci. 2007;27(4):796–807. doi: 10.1523/JNEUROSCI.3501-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le WD, Colom LV, Xie WJ, Smith RG, Alexianu M, Appel SH. Cell death induced by beta-amyloid 1–40 in MES 23.5 hybrid clone: the role of nitric oxide and NMDA-gated channel activation leading to apoptosis. Brain Res. 1995;686(1):49–60. doi: 10.1016/0006-8993(95)00450-5. [DOI] [PubMed] [Google Scholar]
- Leveille F, El Gaamouch F, Gouix E, Lecocq M, Lobner D, Nicole O, Buisson A. Neuronal viability is controlled by a functional relation between synaptic and extrasynaptic NMDA receptors. FASEB J. 2008;22(12):4258–4271. doi: 10.1096/fj.08-107268. [DOI] [PubMed] [Google Scholar]
- Levy-Lahad E, Wasco W, Poorkaj P, Romano DM, Oshima J, Pettingell WH, Yu CE, Jondro PD, Schmidt SD, Wang K, et al. Candidate gene for the chromosome 1 familial Alzheimer’s disease locus. Science. 1995;269(5226):973–977. doi: 10.1126/science.7638622. [DOI] [PubMed] [Google Scholar]
- Li S, Mallory M, Alford M, Tanaka S, Masliah E. Glutamate transporter alterations in Alzheimer disease are possibly associated with abnormal APP expression. J Neuropathol Exp Neurol. 1997;56(8):901–911. doi: 10.1097/00005072-199708000-00008. [DOI] [PubMed] [Google Scholar]
- Lipton SA, Rosenberg PA. Excitatory amino acids as a final common pathway for neurologic disorders. N Engl J Med. 1994;330(9):613–622. doi: 10.1056/NEJM199403033300907. [DOI] [PubMed] [Google Scholar]
- Luscher C, Malenka RC. NMDA receptor-dependent long-term potentiation and long-term depression (LTP/LTD) Cold Spring Harb Perspect Biol. 2012;4(6) doi: 10.1101/cshperspect.a005710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masliah E, Alford M, DeTeresa R, Mallory M, Hansen L. Deficient glutamate transport is associated with neurodegeneration in Alzheimer’s disease. Ann Neurol. 1996;40(5):759–766. doi: 10.1002/ana.410400512. [DOI] [PubMed] [Google Scholar]
- Monti B, Contestabile A. Blockade of the NMDA receptor increases developmental apoptotic elimination of granule neurons and activates caspases in the rat cerebellum. Eur J Neurosci. 2000;12(9):3117–3123. doi: 10.1046/j.1460-9568.2000.00189.x. [DOI] [PubMed] [Google Scholar]
- Murrell J, Farlow M, Ghetti B, Benson MD. A mutation in the amyloid precursor protein associated with hereditary Alzheimer’s disease. Science. 1991;254(5028):97–99. doi: 10.1126/science.1925564. [DOI] [PubMed] [Google Scholar]
- Papouin T, Ladepeche L, Ruel J, Sacchi S, Labasque M, Hanini M, Groc L, Pollegioni L, Mothet JP, Oliet SH. Synaptic and extrasynaptic NMDA receptors are gated by different endogenous coagonists. Cell. 2012;150(3):633–646. doi: 10.1016/j.cell.2012.06.029. [DOI] [PubMed] [Google Scholar]
- Parpura-Gill A, Beitz D, Uemura E. The inhibitory effects of beta-amyloid on glutamate and glucose uptakes by cultured astrocytes. Brain Res. 1997;754(1–2):65–71. doi: 10.1016/s0006-8993(97)00043-7. [DOI] [PubMed] [Google Scholar]
- Parsons MP, Raymond LA. Extrasynaptic NMDA receptor involvement in central nervous system disorders. Neuron. 2014;82(2):279–293. doi: 10.1016/j.neuron.2014.03.030. [DOI] [PubMed] [Google Scholar]
- Perl DP. Neuropathology of Alzheimer’s disease. Mt Sinai J Med. 2010;77(1):32–42. doi: 10.1002/msj.20157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry TL, V, Yong W, Bergeron C, Hansen S, Jones K. Amino acids, glutathione, and glutathione transferase activity in the brains of patients with Alzheimer’s disease. Ann Neurol. 1987;21(4):331–336. doi: 10.1002/ana.410210403. [DOI] [PubMed] [Google Scholar]
- Procter AW, Stirling JM, Stratmann GC, Cross AJ, Bowen DM. Loss of glycine-dependent radioligand binding to the N-methyl-D-aspartate-phencyclidine receptor complex in patients with Alzheimer’s disease. Neurosci Lett. 1989;101(1):62–66. doi: 10.1016/0304-3940(89)90441-2. [DOI] [PubMed] [Google Scholar]
- Riedel G, Platt B, Micheau J. Glutamate receptor function in learning and memory. Behav Brain Res. 2003;140(1–2):1–47. doi: 10.1016/s0166-4328(02)00272-3. [DOI] [PubMed] [Google Scholar]
- Rothman SM, Olney JW. Glutamate and the pathophysiology of hypoxic--ischemic brain damage. Ann Neurol. 1986;19(2):105–111. doi: 10.1002/ana.410190202. [DOI] [PubMed] [Google Scholar]
- Sattler R, Xiong Z, Lu WY, MacDonald JF, Tymianski M. Distinct roles of synaptic and extrasynaptic NMDA receptors in excitotoxicity. J Neurosci. 2000;20(1):22–33. doi: 10.1523/JNEUROSCI.20-01-00022.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schellenberg GD, Bird TD, Wijsman EM, Orr HT, Anderson L, Nemens E, White JA, Bonnycastle L, Weber JL, Alonso ME, et al. Genetic linkage evidence for a familial Alzheimer’s disease locus on chromosome 14. Science. 1992;258(5082):668–671. doi: 10.1126/science.1411576. [DOI] [PubMed] [Google Scholar]
- Scott HA, Gebhardt FM, Mitrovic AD, Vandenberg RJ, Dodd PR. Glutamate transporter variants reduce glutamate uptake in Alzheimer’s disease. Neurobiol Aging. 2011;32(3):553 e551–511. doi: 10.1016/j.neurobiolaging.2010.03.008. [DOI] [PubMed] [Google Scholar]
- Shankar GM, Bloodgood BL, Townsend M, Walsh DM, Selkoe DJ, Sabatini BL. Natural oligomers of the Alzheimer amyloid-beta protein induce reversible synapse loss by modulating an NMDA-type glutamate receptor-dependent signaling pathway. J Neurosci. 2007;27(11):2866–2875. doi: 10.1523/JNEUROSCI.4970-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder EM, Nong Y, Almeida CG, Paul S, Moran T, Choi EY, Nairn AC, Salter MW, Lombroso PJ, Gouras GK, Greengard P. Regulation of NMDA receptor trafficking by amyloid-beta. Nat Neurosci. 2005;8(8):1051–1058. doi: 10.1038/nn1503. [DOI] [PubMed] [Google Scholar]
- Stanika RI, Pivovarova NB, Brantner CA, Watts CA, Winters CA, Andrews SB. Coupling diverse routes of calcium entry to mitochondrial dysfunction and glutamate excitotoxicity. Proc Natl Acad Sci U S A. 2009;106(24):9854–9859. doi: 10.1073/pnas.0903546106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strittmatter WJ, Saunders AM, Schmechel D, Pericak-Vance M, Enghild J, Salvesen GS, Roses AD. Apolipoprotein E: high-avidity binding to beta-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease. Proc Natl Acad Sci U S A. 1993;90(5):1977–1981. doi: 10.1073/pnas.90.5.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talantova M, Sanz-Blasco S, Zhang X, Xia P, Akhtar MW, Okamoto S, Dziewczapolski G, Nakamura T, Cao G, Pratt AE, Kang YJ, Tu S, Molokanova E, McKercher SR, Hires SA, Sason H, Stouffer DG, Buczynski MW, Solomon JP, Michael S, Powers ET, Kelly JW, Roberts A, Tong G, Fang-Newmeyer T, Parker J, Holland EA, Zhang D, Nakanishi N, Chen HS, Wolosker H, Wang Y, Parsons LH, Ambasudhan R, Masliah E, Heinemann SF, Pina-Crespo JC, Lipton SA. Abeta induces astrocytic glutamate release, extrasynaptic NMDA receptor activation, and synaptic loss. Proc Natl Acad Sci U S A. 2013;110(27):E2518–2527. doi: 10.1073/pnas.1306832110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Texido L, Martin-Satue M, Alberdi E, Solsona C, Matute C. Amyloid beta peptide oligomers directly activate NMDA receptors. Cell Calcium. 2011;49(3):184–190. doi: 10.1016/j.ceca.2011.02.001. [DOI] [PubMed] [Google Scholar]
- Tymianski M, Charlton MP, Carlen PL, Tator CH. Source specificity of early calcium neurotoxicity in cultured embryonic spinal neurons. J Neurosci. 1993;13(5):2085–2104. doi: 10.1523/JNEUROSCI.13-05-02085.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkitaramani DV, Chin J, Netzer WJ, Gouras GK, Lesne S, Malinow R, Lombroso PJ. Beta-amyloid modulation of synaptic transmission and plasticity. J Neurosci. 2007;27(44):11832–11837. doi: 10.1523/JNEUROSCI.3478-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenk GL. Neuropathologic changes in Alzheimer’s disease: potential targets for treatment. J Clin Psychiatry. 2006;67(Suppl 3):3–7. quiz 23. [PubMed] [Google Scholar]
- Wu L, Rosa-Neto P, Hsiung GY, Sadovnick AD, Masellis M, Black SE, Jia J, Gauthier S. Early-onset familial Alzheimer’s disease (EOFAD) Can J Neurol Sci. 2012;39(4):436–445. doi: 10.1017/s0317167100013949. [DOI] [PubMed] [Google Scholar]
- Wu SZ, Bodles AM, Porter MM, Griffin WS, Basile AS, Barger SW. Induction of serine racemase expression and D-serine release from microglia by amyloid beta-peptide. J Neuroinflammation. 2004;1(1):2. doi: 10.1186/1742-2094-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye C, Walsh DM, Selkoe DJ, Hartley DM. Amyloid beta-protein induced electrophysiological changes are dependent on aggregation state: N-methyl-D-aspartate (NMDA) versus non-NMDA receptor/channel activation. Neurosci Lett. 2004;366(3):320–325. doi: 10.1016/j.neulet.2004.05.060. [DOI] [PubMed] [Google Scholar]