Abstract
The crustacean stomatogastric nervous system (STNS) is a well-known model for investigating neuropeptidergic control of rhythmic behavior. Among the peptides known to modulate the STNS are the C-type allatostatins (AST-Cs). In the lobster, Homarus americanus, three AST-Cs are known. Two of these, pQIRYHQCYFNPISCF (AST-C I) and GNGDGRLYWRCYFNAVSCF (AST-C III), have non-amidated C-termini, while the third, SYWKQCAFNAVSCFamide (AST-C II), is C-terminally amidated. Here, antibodies were generated against one of the non-amidated peptides (AST-C I) and against the amidated isoform (AST-C II). Specificity tests show that the AST-C I antibody cross-reacts with both AST-C I and AST-C III, but not AST-C II; the AST-C II antibody does not cross-react with either non-amidated peptide. Wholemount immunohistochemistry shows that both subclasses (non-amidated and amidated) of AST-C are distributed throughout the lobster STNS. Specifically, the antibody that cross-reacts with the two non-amidated peptides labels neuropil in the CoGs and the stomatogastric ganglion (STG), axons in the superior esophageal (son) and stomatogastric (stn) nerves, and ~ 14 somata in each commissural ganglion (CoG). The AST-C II-specific antibody labels neuropil in the CoGs, STG and at the junction of the sons and stn, axons in the sons and stn, ~ 42 somata in each CoG, and two somata in the STG. Double immunolabeling shows that, except for one soma in each CoG, the non-amidated and amidated peptides are present in distinct sets of neuronal profiles. The differential distributions of the two AST-C subclasses suggest that the two peptide groups are likely to serve different modulatory roles in the lobster STNS.
Keywords: Immunohistochemistry, Neuropeptide, Neurohormone, Crustacea, Decapoda
Introduction
The portion of the decapod crustacean nervous system that controls the rhythmic movements of the foregut, i.e., the stomatogastric nervous system (STNS), is a model for understanding the basic principles governing the generation, maintenance and modulation of all rhythmic motor behaviors, including walking, chewing and breathing in humans (Blitz and Nusbaum 2011; Christie 2011; Christie et al. 2010; Fénelon et al. 2003; Harris-Warrick et al. 1992; Hooper and DiCaprio 2004; Marder and Bucher 2007; Marder et al. 1995; Nusbaum et al. 2001; Selverston 2005; Selverston and Ayers 2006; Selverston and Moulins 1987; Selverston et al. 1998; Skiebe 2001; Stein 2009). A major contribution to the field of neuroscience that has come from work on the decapod STNS is the demonstration that neurochemical modulation of intrinsic circuit elements allows numerically simple, “hardwired,” neural networks to produce an essentially infinite array of distinct motor outputs. While many classes of chemicals contribute to the complement of locally released and circulating neuromodulators that influence the neural networks contained within the STNS, peptides are by far the largest and most diverse single group (e.g., Christie 2011; Christie et al. 2010; Skiebe 2001).
To date, approximately 45 distinct neuropeptide families have been identified in decapod crustaceans (e.g., Christie et al. 2010). One species for which a large neuropeptidome has been described is the American lobster, Homarus americanus. In Homarus, nearly 300 distinct neuropeptides encompassing approximately 40 families have been identified, largely via mass spectral analyses and/or in silico transcriptome mining (e.g., Christie et al. 2015, 2017; Ma et al. 2008). Among the neuropeptides that have been identified from H. americanus neural tissues are multiple members of the C-type allatostatin (AST-C) family, a group of peptides that is broadly conserved in crustaceans. Isoforms of the AST-C family are currently known from one or more members of the Remipedia (e.g., Christie 2014a), Branchiopoda (e.g., Gard et al. 2009), Maxillopoda (e.g., Christie 2014b, c, 2015; Christie et al. 2013; Yan et al. 2012) and Malacostraca (e.g., Christie 2014d, e, 2016a, b, 2017; Christie et al. 2017; Christie and Chi 2015; Christie and Pascual 2016; Dickinson et al. 2009; Stemmler et al. 2010; Toullec et al. 2013; Ventura et al. 2014). The AST-Cs are characterized by the carboxyl (C)-terminal motif –PISCF, or a close approximation thereof, as well as a disulfide bridge between cysteine residues located at positions 2 and 9 from the C-terminus (e.g., Audsley et al. 2008; Christie et al. 2010; Stay and Tobe 2007; Verlinden et al. 2015). In the lobster, three AST-Cs, each encoded by a different gene, have thus far been identified: pQIRYHQCYFNPISCF (AST-C I; Stemmler et al. 2010), SYWKQCAFNAVSCFamide (AST-C II; Dickinson et al. 2009) and GNGDGRLYWRCYFNAVSCF (AST-C III; Christie et al. 2017); each of these peptides has a –PISCF C-terminus (or a conservatively substituted variant) and a disulfide bridge between its two cysteines (Fig. 1). A notable difference between these three AST-Cs is that AST-C I and AST-C III are non-amidated, while AST-C II has a C-terminal amide group (Fig. 1b). While very limited in number, the physiological studies that have examined the effects of multiple AST-Cs in decapod nervous tissues suggest that they have similar, though not necessarily identical, bioactivities (Ma et al. 2009; Dickinson et al. 2018). However, all investigations to date have employed bath application of peptide, which is regarded as a good proxy for hormonal delivery, but does necessarily mimic local release. If the three AST-Cs are present in different neurons in a given portion of the nervous system, e.g., the lobster STNS, targeted local release could result in distinct behavioral outputs being elicited by the differentially distributed isoforms. At present, the cellular distributions of AST-C peptides in the H. americanus are unknown. In fact, the distribution of AST-C peptides in crustaceans has thus far been examined in only one species, the copepod crustacean Calanus finmarchicus, in a study that used an antibody generated against an insect isoform of AST-C (Wilson and Christie 2010).
Fig. 1.
Alignment of Homarus americanus AST-C precursor proteins and mature AST-C peptide isoforms. a Alignment of prepro-ASTC I, II and III. The sequence of prepro-AST-C I is that reported in Christie et al. 2015, while those of prepro-AST-C II and prepro-ASTC III are those reported in Christie et al. 2017. b Alignment of the structures of mature AST-C I, II and III peptides. In the line immediately below each sequence grouping, “*” indicates identical amino acid residues, while “:” and “.” denote amino acids that are similar in structure between sequences. In a, the portion of each preprohormone that gives rise to the AST-C peptide has been highlighted in black. In b, the “pQ” at the amino-terminus of AST-C I indicates the presence of pyroglutamic acid, while the “a” at the carboxyl-terminus of AST-C II indicates the presence of an amide group; in all three AST-C isoforms, the cysteine residues involved in disulfide bridging have been underlined. All alignments were done using the online program MAFFT version 7 (http://mafft.cbrc.jp/alignment/software/; Katoh and Standley 2013)
In the study presented here, two AST-C antibodies were generated: one against AST-C I, and the other against AST-C II. These antibodies were used to examine the distribution of members of the AST-C family in the H. americanus STNS. Specificity studies showed that the AST-C I antibody crossreacts with both AST-C I and AST-C III, but not with AST-C II, while the AST-C II antibody is specific for the peptide against which it was generated. Thus, the two antibodies generated here provide reagents for mapping and comparing the distributions of the two subclasses of AST-Cs present in the lobster, i.e., non-amidated (AST-C I and III) and amidated (AST-C II). Wholemount immunolabeling using these antibodies shows that the non-amidated and amidated AST-Cs are both distributed throughout the lobster STNS. However, double labeling revealed that co-localization of non-amidated and amidated AST-Cs is restricted to a single neuron in each commissural ganglion (CoG), a finding that strongly suggests that the two AST-C subclasses are differentially distributed in the lobster STNS. Because of their differential distributions, we hypothesize that the non-amidated and amidated AST-Cs are likely to elicit distinct modulatory effects when locally released within the lobster STNS.
Materials and methods
Animals and tissue collection
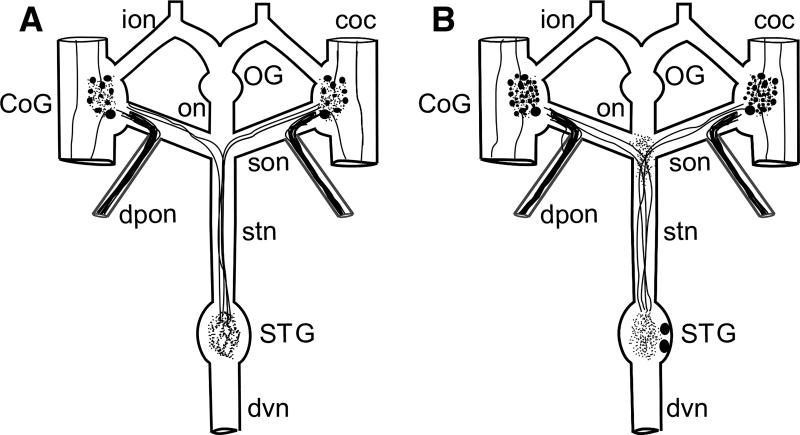
American lobsters, H. americanus, were obtained from commercial suppliers in the greater Brunswick area of Maine (USA) and were housed in recirculating natural seawater aquaria at 10–12 °C. Animals were fed weekly with a diet consisting of chopped shrimp and squid. For tissue collection, lobsters were anesthetized by being packed in ice for 30–60 min, after which the stomach was isolated and the STNS, which consists of the paired CoGs, the single esophageal ganglion (OG) and the single stomatogastric ganglion (STG), as well as a variety of interconnecting nerves (Fig. 2; Harris-Warrick et al. 1992; Selverston and Moulins 1987), was removed from the surface of the stomach via manual microdissection in chilled (approximately 4 °C) physiological saline (composition in mM: NaCl, 479.12; KCl, 12.74; CaCl2, 13.67; MgSO4, 20.00; Na2SO4, 3.91; Trizma base, 11.45; maleic acid, 4.82 [pH, 7.45]).
Fig. 2.
Schematic representation of the Homarus americanus stomatogastric nervous system (STNS) and the distributions of AST-C I/III and AST-C II in the lobster STNS. a Schematic representation of AST-C I/III-like immunoreactivity in the STNS. In this portion of the nervous system, the AST-C I/III antibody labels approximately 14 somata in each commissural ganglion (CoG), neuropil in each CoG and in the stomatogastric ganglion (STG), and axons in the circumesophageal connectives (cocs), superior esophageal (son), dorsal posterior esophageal (dpon) and stomatogastric (stn) nerves. No AST-C I/III-like labeling is present in the OG, nor is immunoreactivity present in the inferior esophageal (ion), esophageal (on) or dorsal ventricular (dvn) nerves. b Schematic representation of AST-C II-like immunoreactivity in the STNS. In the STNS, AST-C II-like labeling is present in approximately 42 somata in each CoG and two somata in the STG. Labeled neuropil is present in each CoG, in the STG, and at the son/stn junction. Labeled axons were seen in the cocs, sons, dpons, and stn. No AST-C II-like labeling was present in the OG, nor was any present in the ions or dvn. In both a and b, filled circles represent immunopositive somata, thick lines within nerves represent immunopositive axons, and tangles of thin lines represent regions of immunopositive neuropil
Generation of AST-C antibodies
Antibody production
To map the distribution of the two non-amidated AST-C peptides, i.e., AST-C I (pQIRYHQCYFNPISCF) and AST-C III (GNGDGRLYWRCYFNAVSCF), we used a custom-produced polyclonal rabbit antibody (Lampire Biological Laboratories, Pipersville, PA, USA). This antibody was generated against the peptide CQIRYHQCYFNPISCF (disulfide bridge present between the second and third cysteine residues) conjugated via the N-terminal cysteine to bovine serum albumen (BSA) using m-maleimidobenzoyl-N-hydroxysuccinimide ester. Our specificity tests (see below) showed that this antibody detected both AST-C I and AST-C III, but does not cross-react with AST-C II. For the detection of AST-C II, we used a custom-produced guinea pig polyclonal antibody generated against the peptide authentic AST-C II (Lampire Biological Laboratories). The immunogen for this antibody was SYWKQCAFNAVSCFamide (disulfide bridging between the two cysteines) conjugated via the amino (N)-terminal serine to BSA using 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide. Both the rabbit anti-AST-C I/III and the guinea pig anti-AST-C II were generated using protocols described in detail in several previous publications (Christie et al. 2006; Dickinson et al. 2015).
Specificity testing
To determine the specificity of the antibodies just described, preadsorption controls were conducted. Specifically, each antibody was incubated with AST-C I, AST-C II or AST-C III peptide (n ≥ 3 for each antibody and each AST-C isoform) at a concentration of 10−3 M for 2 h at room temperature (~ 18–20 °C) prior to its use for immunohistochemistry. Because the STNS of each animal includes two CoGs, we used one as a control (antibody held at room temperature for 2 h, but with no peptide), and the second for the preadsorption.
Wholemount immunohistochemistry
Wholemount immunohistochemistry was conducted using standard methods (e.g., Christie et al. 2006; Dickinson et al. 2015). Specifically, STNSs were fixed in a solution of 4% paraformaldehyde (EM grade; Electron Microscopy Sciences; Hatfield, PA, USA; catalog #15710) in 0.1 M sodium phosphate buffer (P) (pH 7.4) for 12–24 h at 4 °C. At the end of the fixation period, tissues were rinsed five times at ~ 1-h intervals at room temperature in P containing 0.3% Triton-X 100 (P-Triton). Tissues were then incubated for ~ 72 h at 4 °C in primary antibody (or antibodies in the case of double-labeled preparations) diluted to final working concentration (1:5000 for rabbit anti-AST-C I/III and 1:8000 for guinea pig anti-AST-C II) in P-Triton containing 10% normal donkey serum (NDS; Jackson ImmunoResearch Laboratories, West Grove, PA, USA; catalog #017-000-121). After incubation in primary antibody, tissues were rinsed five times at ~ 1-h intervals at room temperature in P-Triton; they were then incubated at 4 °C for 12–24 h in an appropriate secondary antibody (or antibodies in the case of double-labels) diluted 1:300 in P-Triton containing 10% NDS; the secondary antibodies used individually or in combination were Alexa Fluor 488-conjugated AffiniPure donkey anti-rabbit IgG (Jackson ImmunoResearch Laboratories; catalog # 711-545-152), Alexa Fluor 594-conjugated AffiniPure donkey anti-rabbit IgG (Jackson ImmunoResearch Laboratories; catalog # 711-585-152), Alexa Fluor 647-conjugated AffiniPure donkey Anti-rabbit IgG (Jackson ImmunoResearch Laboratories; catalog # 711-605-152), Alexa Fluor 488-conjugated AffiniPure donkey anti-guinea pig IgG (Jackson ImmunoResearch Laboratories; catalog # 706-545-148), Alexa Fluor 594-conjugated AffiniPure donkey anti-guinea pig IgG (Jackson ImmunoResearch Laboratories; catalog # 706-585-148), Alexa Fluor 647-conjugated AffiniPure donkey anti-guinea pig IgG (Jackson ImmunoResearch Laboratories; catalog # 706-605-148), Alexa Fluor 488-conjugated highly cross-adsorbed goat anti-guinea pig IgG (ThermoFisher Scientific, Carlsbad, CA, USA; catalog #A-11073) and Alexa Fluor 568-conjugated highly cross-adsorbed goat anti-guinea pig IgG (ThermoFisher Scientific; catalog #A-11075). After incubation in secondary antibody, tissues were again rinsed five times at ~ 1-h intervals at room temperature in P, and then mounted between a glass microscope slide and coverslip in Vectashield Mounting Medium (Vector Laboratories, Burlingame, CA, USA; catalog #H-1000). Tissues were kept in the dark during the incubation in secondary antibody and in all subsequent processing steps; mounted preparations were stored at 4 °C in the dark until examined for labeling.
Nerve backfilling
To determine whether the two AST-C II immunopositive somata in the STG (see below) project axons into the stomatogastric nerve (stn), nerve backfilling was performed prior to immunolabeling. Specifically, the STNS was pinned down in a Sylgard-lined petri dish filled with physiological saline, and a petroleum jelly well was built around a portion of the stn. After testing the well for leakage, the saline within the well was removed and the well washed several times with deionized water. After these washes, the deionized water in the well was replaced with a solution of either 4% NEUROBIOTIN Tracer (Vector Laboratories; catalog # SP-1120) in 50 mM Tris, 0.5 M KCl in deionized water or 100 mM Lucifer Yellow CH dilithium salt (Sigma-Aldrich, St. Louis, MO, USA; catalog # L-0259) in deionized water, and the nerve within the well was cut using microdissecting scissors. After nerve transection, preparations were incubated overnight at 4 °C and then fixed and immunoprocessed as described above. Lucifer Yellow was visualized directly, while visualization of NEUROBIOTIN was achieved using a 1:500 dilution of Alexa Fluor 488-conjugated streptavidin (ThermoFisher Scientific; catalog # S11223), which was added to the secondary antibody solution (see above).
Imaging
Data were collected, and digital images were generated using an Olympus BX51 fluorescence microscope (Olympus America, Center Valley, PA) or a Leica TCS SP8 laser scanning confocal systems mounted on a Leica DM6 CS upright digital research microscope (Leica Microsystems Inc., Buffalo Grove, IL). Imaging on the Olympus BX51 microscope was done using an UPlanFI 10× or UPlanFL N 40× objective lens and manufacturer supplied GFP or Texas Red filter sets. Imaging on the Leica TCS SP8 system was done using either a HC PL APO 10× or HC PL APO 20× objective lens, with excitation/detection parameters adjusted/optimized using the system’s manufacturer supplied hardware for the specific fluorochrome(s) present in a given piece of tissue.
Figure production
For the production of figures, digital images were exported from the Leica confocal system as TIF files, and composite figures produced using CorelDraw software (Corel, Inc., Mountain View, CA, USA). The contrast and brightness of the final figures were adjusted using Adobe Photoshop CS6 (Adobe Systems, San Jose, CA, USA) to optimize the clarity of the printed images.
Soma counts
To determine the number of labeled somata in each of the three ganglia of the STNS, i.e., the CoG, OG and STG, ganglia were imaged at 40× using the Olympus BX51 fluorescence microscope, and somata were manually counted. A profile was counted as a soma, rather than a release profile, if a nucleus could clearly be identified within it. Labeling for neuropeptides is typically cytoplasmic and the nucleus appears as an area devoid of labeling in a soma, whereas in release profiles, staining appears uniform; this criterion was key for distinguishing between very small somata and very large release terminals. Means for labeled somata were calculated using Microsoft Excel (Redmond, WA, USA) and are reported as mean ± standard error. Cell counts for the paired commissural ganglia of each preparation were averaged, and the average value was used for statistical analysis.
Results
Antibody specificities
Antibodies were generated against two of the three native H. americanus AST-Cs: one, a rabbit polyclonal generated against the non-amidated AST-C I, and the other, a guinea pig polyclonal generated against the amidated AST-C II. To determine the cross-reactivity of these antibodies to the three native lobster AST-Cs, a series of preadsorption controls was conducted (n ≥ 3 for each antibody pre-adsorbed with each peptide). Preadsorption of the AST-C I antibody with either AST-C I or AST-C III abolished all STNS labeling, whereas labeling by this antibody was unaffected by preadsorption with AST-C II. Similarly, preadsorption of the AST-C II antibody with AST-C II abolished all labeling in the STNS, with no effects seen when it was incubated with AST-C I or AST-C III. Collectively, these results suggest that the AST-C I antibody is specific for the non-amidated AST-C isoforms, i.e., AST-C I and AST-C III, while the AST-C II antibody only recognizes the amidated member of this peptide family. Throughout the remainder of this article, the antibody generated against AST-C I, but which also recognizes AST-C III, will be referred to as the anti-AST-C I/III antibody.
Distribution of non-amidated AST-Cs in the STNS
As stated earlier, the STNS consists of the paired CoGs, the single OG, the single STG and a number of interconnecting and motor nerves (Fig. 2). AST-C I/III-like immunoreactivity was distributed throughout each of the eight STNSs for which a systematic count of labeled profiles was conducted. Specifically, the AST-C I/III antibody labeled approximately 14 somata in each CoG (mean = 14.00 ± 0.85 somata/ganglion; range 10–20 somata/ganglion; n = 8 STNSs), as well as a centrally located neuropil (Fig. 3a). Neuropilar labeling in the CoG is likely derived both from the intrinsic immunopositive somata and from AST-C I/III-immunopositive axons projecting to the ganglion via the circumesophageal connectives (cocs), which link the CoG to both the more anteriorly located supraesophageal ganglion (brain) and the more posteriorly located thoracic/abdominal ganglionic chains. In all preparations, a pair of immunopositive axons exited each CoG via the superior esophageal nerve (son), traveling through the son to the stn, ultimately projecting to the STG, where the four axons (two from each CoG) ramified to produce a large region of flocculent neuropil (Fig. 3b). In addition, in all preparations, a fascicle of small diameter, intensely labeled axons was present in each dorsal posterior esophageal nerve (dpon), projecting to/from the CoG via the son. No AST-C I/III-like labeling was seen in any of the eight OGs examined, nor was any immunoreactivity evident in either the inferior esophageal nerve (ion) or esophageal nerve (on). A summary of the AST-C I/III-like labeling is schematized in Fig. 2a.
Fig. 3.
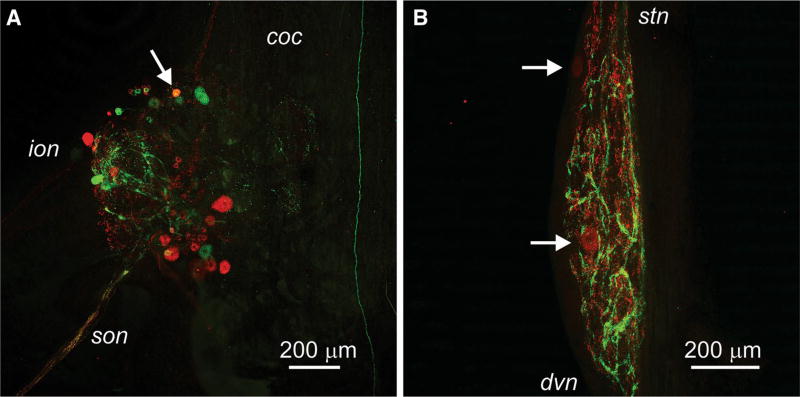
Co-localization of AST-C I/III- and AST-C II-like immunoreactivities in the Homarus americanus stomatogastric nervous system. a AST-C I/III- and AST-C II-like labeling in the commissural ganglion (CoG). AST-C I/III-like immunoreactivity is shown in green, while AST-C II-like immunoreactivity is shown in red. Co-localization of the two immunoreactivities in the CoG is restricted to a single soma (arrow). This confocal image is a maximum projection of 48 optical sections taken at 2 µM intervals. coc circumesophageal connective; ion inferior esophageal nerve; son superior esophageal nerve. b AST-C I/III- and AST-C II-like labeling in the stomatogastric ganglion (STG). AST-C I/III-like immunoreactivity is shown in green, while AST-C II-like immunoreactivity is shown in red. No co-localization of the two immunolabels is present in the STG. Two intrinsic STG somata that are immunopositive for AST-C II are indicated by arrows. This confocal image is a maximum projection of 66 optical sections taken at 2 µM intervals. dvn dorsal ventricular nerve; stn stomatogastric nerve (color figure online).
Distribution of amidated AST-C in the STNS
AST II-like immunoreactivity was also found throughout the lobster STNS (n = 8 preparations for which labeled profiles were systematic analyzed). In each CoG, approximately 42 somata exhibited AST-C II-like labeling (mean = 42.37 ± 3.30 somata/ganglion; range 22–57 somata/ganglion; n = 8 STNSs), as did a centrally located neuropil (Fig. 3a). AST-C II-like immunoreactivity in the CoG neuropil, like that for AST-C I/III, is likely to originate from the intrinsic immunopositive CoG somata, as well as from immunopositive axons that project to the ganglion via the cocs. In all eight preparations, a pair of immunopositive axons exited each CoG via the son, travel through the son to the stn and then on the STG, where they ramified to produce a large region of neuropil (Fig. 3b). In contrast to the flocculent appearance of the neuropil labeled by the AST-C I/III antibody, neuropil that exhibited AST-C II-like labeling was distinctly punctate. In addition, the AST-C II antibody routinely labeled two somata in the STG (mean = 2.00 ± 0.33 somata/ganglion; range 0–3 somata/ganglion; n = 8 ganglia) (Fig. 3b). Labeling in these two cell bodies was always weak in intensity, and no obvious axons could be seen projecting from them in any preparation. Backfills of the stn (n = 3 preparations) show that both cells project up the stn toward the anterior ganglia (i.e., the CoGs and OG). Based on size, the two intrinsic STG immunopositive somata are unlikely to be among the neurons that participate in the STG’s pyloric or gastric mill neural circuits, i.e., the anterior burster (AB) or interneuron 1 (Int1), both of which are considerably smaller than the two AST-C II-labeled cell bodies (e.g., Harris-Warrick et al. 1992; Selverston and Moulins 1987). As was the case for AST-C I/III, a fascicle of small diameter, intensely labeled AST-C II immunopositive axons was present in each dpon, projecting to or from the CoG via the son in all preparations. No AST-C II-like labeling was present in the OG, nor was any evident in the ions. While no axons were seen in the on, patchy regions of neuropil were noted at the junction of the on, sons and stn in all eight of the examined STNSs, staining that is likely derived from the axons that project from the CoGs through the sons and stn to the STG. A summary of the AST-C II-like labeling is schematized in Fig. 2b.
Co-localization of non-amidated and amidated AST-Cs in the STNS
As described in the two preceding sections, both AST-C I/III- and AST-C II-like immunoreactivities are broadly distributed in the lobster STNS. To determine the extent of co-localization of the two classes of AST-C, i.e., nonamidated (AST-C I and AST-C III) or amidated (AST-C II), in the STNS, double immunolabeling was conducted on three preparations. In these STNSs, co-localization of the AST-C I/III- and AST-C II-like labels was limited to a single soma in each of the paired CoGs (Fig. 3a). None of the other structures described in “Distribution of non-amidated ASTCs in the STNS” or “Distribution of amidated AST-C in the STNS” sections, e.g., the STG neuropil (Fig. 3b), exhibited any convincing evidence of co-localization. Thus, it appears that the non-amidated and amidated AST-C subclasses are largely contained in different populations of neuronal profiles in the STNS of H. americanus.
Discussion
Anti-AST-C I/III and anti-AST-C II appear to be differentially selective for the amidation state of the native lobster AST-Cs
In this study, two antibodies were developed to map the distribution of native AST-C peptides in the STNS of H. americanus, a species for which three isoforms are currently known (e.g., Christie et al. 2015; Dickinson et al. 2009; Stemmler et al. 2010). One antibody, a rabbit polyclonal, was generated against AST-C I (pQIRYHQCYFNPISCF), while the other, a guinea pig polyclonal, was generated against AST-C II (SYWKQCAFNAVSCFamide); for both antibodies, the AST-C isoform used for antibody production was conjugated to the carrier protein via its N-terminus. Specificity tests conducted on the two antibodies showed that the rabbit polyclonal cross-reacts with both AST-C I and AST-C III (GNGDGRLYWRCYFNAVSCF), while the guinea pig polyclonal is specific for AST-C II. Interestingly, while both AST-C II and III share an identical set of C-terminal amino acids, i.e., the sequence –FNAVSCF, the rabbit and guinea pig antibodies differentiate between the two peptides. In contrast, the C-terminal amino acids of AST-C I vary at two positions from those present in AST-C II and III, i.e., proline for alanine and isoleucine for valine substitutions at positions 5 and 4 from the C-terminus, respectively, yet both AST-C I and III are recognized by the rabbit antibody. Given the respective selectivity of the antibodies, it appears that selectivity is a reflection of amidation state at the C-terminus: AST-C I and III are non-amidated peptides, while AST-C II has a C-terminal amide group. As many arthropod species have multiple AST-Cs, with one or more non-amidated and one amidated isoform, the antibodies developed here may well provide a means for differentiating the distributions of non-amidated versus amidated AST-Cs in the nervous system and/or other tissues broadly within the Arthropoda. If this is the case, it will be interesting to see if the two subclasses are largely differentially distributed (as is the case for the lobster STNS) or if in some species, or tissues, they are more broadly co-localized.
Non-amidated and amidated AST-Cs are broadly distributed in the STNS and likely serve as local-released modulators in the STG
The distribution of non-amidated and amidated AST-Cs in the STNS of H. americanus suggests that both AST-C subclasses are likely to function as locally released neuromodulators of the neural circuits present in the STNS, i.e., there is immunopositive neuropil for both AST-C I/III and AST-C II in both the CoGs and STG. In a previous study (Dickinson et al. 2009), the effects of AST-C II were assessed on the lobster pyloric motor pattern, which is produced by a neural network largely contained within the STG. Here, a 10−6 M concentration of AST-C II elicited a reliable decrease in the pyloric cycle frequency in preparations in which other local sources of modulation were removed (Dickinson et al. 2009). In similar experiments conducted on the crab Cancer borealis STNS using both AST-C I and AST-C II at 10−6 M, the pyloric cycle frequency was similarly decreased, especially in preparations with slower baseline cycle frequencies (Ma et al. 2009). The effects of the non-amidated and amidated peptides were indistinguishable in the crab STNS (Ma et al. 2009). The concentrations of peptides used in the lobster and crab experiments are typical of what are considered physiologically relevant for locally released peptides; the data presented here show that local release of both the non-amidated and amidated peptides is likely to occur in at least the lobster STG. The effects of AST-C III on the pyloric motor pattern have not yet been assessed in any species.
In addition to functioning as locally released neuromodulators in the lobster STNS, both the non-amidated and amidated AST-Cs are likely to reach the neural circuits present there as circulating hormones (Dickinson et al. 2018). Immunohistochemistry conducted on the H. americanus pericardial organ (PO), a major neuroendocrine release site in decapod species (Christie 2011), revealed labeling for both AST-C I/III and AST-C II in putative release terminals (Dickinson et al. 2018). In addition, epithelial endocrine cells present in the posterior midgut of the lobster are immunopositive for AST-C I/III (Dickinson et al. 2018). Peptides released from either the PO or the midgut would likely reach the STG through the ophthalmic artery in which it is located, but likely at concentrations lower than are physiologically relevant for local release (e.g., 10−7 M or lower vs. 10−6 M). At present, the threshold concentrations for AST-C bioactivity on the STNS rhythms are unknown, but many peptides likely serve both as locally released and circulating neuromodulators in the STNS, potentially with distinct actions elicited by the two modes of delivery (e.g., Christie et al. 1995).
In addition to being present in the STG neuropil, AST-C II-like immunoreactivity is also present in two somata in the lobster STG. Because these neurons project axons into the stn toward the anterior ganglia and have large cell bodies, they are unlikely to be members of pyloric or gastric mill pattern generating networks present in the STG, i.e., they are not likely to be either AB or Int1, which are key components of these circuits and project axons into the stn, but have much smaller cell bodies (e.g., Harris-Warrick et al. 1992; Selverston and Moulins 1987). While it is known that there are other STG neurons that project axons into the stn, these neurons have not been studied in H. americanus. In other decapods, such as palinuran (spiny and slipper) lobsters, two of these neurons are involved in the control of the rhythmic movements of the cardiac sac and esophagus, i.e., the esophageal dilator 2 (OD2) and cardiac sac dilator 2 (CD2) neurons (e.g., Harris-Warrick et al. 1992; Selverston and Moulins 1987). The functions of others of these neurons, i.e., the “extra” (EX) neurons (e.g., Harris-Warrick et al. 1992; Selverston and Moulins 1987), are not known in any species. Thus, in H. americanus, it is possible that AST-C II may play roles in modulating the cardiac sac and/or esophageal motor patterns via release from one or both of the labeled STG neurons.
Co-localization of I/III and II limited to a single CoG neuron
Prior studies have shown that many neurons in the decapod STNS, including most of the axons that project to the STG, have co-transmitters. For example, in H. americanus, the sole tachykinin-related peptide (TRP) input to the STG also contains the peptide red pigment concentrating hormone (RPCH; Thirumalai and Marder 2002). Similarly, in the crab C. borealis, the sole TRP input to the STG also contains GABA and the peptide proctolin (Blitz et al. 1999). Despite the presence of extensive labeling for AST-C I/III and AST-C II in the STNS, the results of our double-labeling experiments show there to be minimal co-localization of the non-amidated and amidated AST-C isoforms in this portion of the lobster nervous system. We found no evidence for co-localization of AST-C I/III and AST-C II in the input axons to the STG, nor was there any evidence for co-localization of the two peptide groups in the STG neuropil. In fact, the only co-localization that was observed was in a single soma in each CoG. Thus, it is highly unlikely that the non-amidated and amidated AST-C isoforms are co-released within the lobster STNS. Given this situation, the functional roles played by local release of these two AST-C subgroups are likely to be distinct. Because the rabbit polyclonal antibody used here cross-reacts with both AST-C I and AST-C III, the extent to which these two peptides are co-localized cannot be determined.
Summary and implications
The study presented here described the development two AST-C antibodies, one specific for non-amidated isoforms and the other specific for C-terminally amidated peptide. These antibodies were used to map the distribution of the two AST-C subgroups in the STNS of the lobster H. americanus. While extensive immunolabeling was seen in the STNS for each antibody, co-localization of the non-amidated and amidated AST-C subclasses was limited to a single soma in each CoG, a finding that strongly suggests that the two AST-C subgroups are differentially distributed in the lobster STNS. Because they are largely segregated within the STNS, we hypothesize that the non-amidated and amidated AST-Cs are likely to elicit distinct modulatory effects when locally released within this portion of the lobster nervous system.
Acknowledgments
This study was funded by the National Science Foundation (IOS-1353023 and IOS-1354567), the National Institutes of Health (5P20RR016463-12 and 8P20GM103423-12), the MERITS Program of the Maine Space Grant, the Cades Foundation of Honolulu, Hawaii, and a gift from the Henry L. and Grace Doherty Charitable Foundation to Bowdoin College.
Footnotes
Compliance with ethical standards
Conflict of interest The authors declare that they have no conflict of interests.
References
- Audsley N, Matthews HJ, Price NR, Weaver RJ. Allatoregulatory peptides in Lepidoptera, structures, distribution and functions. J Insect Physiol. 2008;54:969–980. doi: 10.1016/j.jinsphys.2008.01.012. [DOI] [PubMed] [Google Scholar]
- Blitz DM, Nusbaum MP. Neural circuit flexibility in a small sensorimotor system. Curr Opin Neurobiol. 2011;21:544–552. doi: 10.1016/j.conb.2011.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blitz DM, Christie AE, Coleman MJ, Norris BJ, Marder E, Nusbaum MP. Different proctolin neurons elicit distinct motor patterns from a multifunctional neuronal network. J Neurosci. 1999;19:5449–5463. doi: 10.1523/JNEUROSCI.19-13-05449.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie AE. Crustacean neuroendocrine systems and their signaling agents. Cell Tissue Res. 2011;345:41–67. doi: 10.1007/s00441-011-1183-9. [DOI] [PubMed] [Google Scholar]
- Christie AE. Prediction of the first neuropeptides from a member of the Remipedia (Arthropoda, Crustacea) Gen Comp Endocrinol. 2014a;201:74–86. doi: 10.1016/j.ygcen.2014.01.017. [DOI] [PubMed] [Google Scholar]
- Christie AE. Prediction of the peptidomes of Tigriopus californicus and Lepeophtheirus salmonis (Copepoda, Crustacea) Gen Comp Endocrinol. 2014b;201:87–106. doi: 10.1016/j.ygcen.2014.02.015. [DOI] [PubMed] [Google Scholar]
- Christie AE. Peptide discovery in the ectoparasitic crustacean Argulus siamensis: identification of the first neuropeptides from a member of the Branchiura. Gen Comp Endocrinol. 2014c;204:114–125. doi: 10.1016/j.ygcen.2014.05.004. [DOI] [PubMed] [Google Scholar]
- Christie AE. Identification of the first neuropeptides from the Amphipoda (Arthropoda, Crustacea) Gen Comp Endocrinol. 2014d;206:96–110. doi: 10.1016/j.ygcen.2014.07.010. [DOI] [PubMed] [Google Scholar]
- Christie AE. Expansion of the Litopenaeus vannamei and Penaeus monodon peptidomes using transcriptome shotgun assembly sequence data. Gen Comp Endocrinol. 2014e;206:235–254. doi: 10.1016/j.ygcen.2014.04.015. [DOI] [PubMed] [Google Scholar]
- Christie AE. Neuropeptide discovery in Eucyclops serrulatus (Crustacea, Copepoda): in silico prediction of the first peptidome for a member of the Cyclopoida. Gen Comp Endocrinol. 2015;211:92–105. doi: 10.1016/j.ygcen.2014.11.002. [DOI] [PubMed] [Google Scholar]
- Christie AE. Prediction of Scylla olivacea (Crustacea; Brachyura) peptide hormones using publicly accessible transcriptome shotgun assembly (TSA) sequences. Gen Comp Endocrinol. 2016a;230–231:1–16. doi: 10.1016/j.ygcen.2016.03.008. [DOI] [PubMed] [Google Scholar]
- Christie AE. Expansion of the neuropeptidome of the globally invasive marine crab Carcinus maenas. Gen Comp Endocrinol. 2016b;235:150–169. doi: 10.1016/j.ygcen.2016.05.013. [DOI] [PubMed] [Google Scholar]
- Christie AE. Neuropeptide discovery in Proasellus cavaticus: prediction of the first large-scale peptidome for a member of the Isopoda using a publicly accessible transcriptome. Peptides. 2017;97:29–45. doi: 10.1016/j.peptides.2017.09.003. [DOI] [PubMed] [Google Scholar]
- Christie AE, Chi M. Prediction of the neuropeptidomes of members of the Astacidea (Crustacea, Decapoda) using publicly accessible transcriptome shotgun assembly (TSA) sequence data. Gen Comp Endocrinol. 2015;224:38–60. doi: 10.1016/j.ygcen.2015.06.001. [DOI] [PubMed] [Google Scholar]
- Christie AE, Pascual MG. Peptidergic signaling in the crab Cancer borealis: tapping the power of transcriptomics for neuropeptidome expansion. Gen Comp Endocrinol. 2016;237:53–67. doi: 10.1016/j.ygcen.2016.08.002. [DOI] [PubMed] [Google Scholar]
- Christie AE, Skiebe P, Marder E. Matrix of neuromodulators in neurosecretory structures of the crab Cancer borealis. J Exp Biol. 1995;198:2431–2439. doi: 10.1242/jeb.198.12.2431. [DOI] [PubMed] [Google Scholar]
- Christie AE, Stemmler EA, Peguero B, Messinger DI, Provencher HL, Scheerlinck P, Hsu YW, Guiney ME, de la Iglesia HO, Dickinson PS. Identification, physiological actions, and distribution of VYRKPPFNGSIFamide (Val1-SIFamide) in the stomatogastric nervous system of the American lobster Homarus americanus. J Comp Neurol. 2006;496:406–421. doi: 10.1002/cne.20932. [DOI] [PubMed] [Google Scholar]
- Christie AE, Stemmler EA, Dickinson PS. Crustacean neuropeptides. Cell Mol Life Sci. 2010;67:4135–4169. doi: 10.1007/s00018-010-0482-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie AE, Roncalli V, Wu LS, Ganote CL, Doak T, Lenz PH. Peptidergic signaling in Calanus finmarchicus (Crustacea, Copepoda): in silico identification of putative peptide hormones and their receptors using a de novo assembled transcriptome. Gen Comp Endocrinol. 2013;187:117–135. doi: 10.1016/j.ygcen.2013.03.018. [DOI] [PubMed] [Google Scholar]
- Christie AE, Chi M, Lameyer TJ, Pascual MG, Shea DN, Stanhope ME, Schulz DJ, Dickinson PS. Neuropeptidergic signaling in the American lobster Homarus americanus: new insights from high-throughput nucleotide sequencing. PLoS ONE. 2015;10:e0145964. doi: 10.1371/journal.pone.0145964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie AE, Roncalli V, Cieslak MC, Pascual MG, Yu A, Lameyer TJ, Stanhope ME, Dickinson PS. Prediction of a neuropeptidome for the eyestalk ganglia of the lobster Homarus americanus using a tissue-specific de novo assembled transcriptome. Gen Comp Endocrinol. 2017;243:96–119. doi: 10.1016/j.ygcen.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson PS, Wiwatpanit T, Gabranski ER, Ackerman RJ, Stevens JS, Cashman CR, Stemmler EA, Christie AE. Identification of SYWKQCAFNAVSCFamide: a broadly conserved crustacean C-type allatostatin-like peptide with both neuromodulatory and cardioactive properties. J Exp Biol. 2009;212:1140–1152. doi: 10.1242/jeb.028621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson PS, Kurland SC, Qu X, Parker BO, Sreekrishnan A, Kwiatkowski MA, Williams AH, Ysasi AB, Christie AE. Distinct or shared actions of peptide family isoforms: II. Multiple pyrokinins exert similar effects in the lobster stomatogastric nervous system. J Exp Biol. 2015;218:2905–2917. doi: 10.1242/jeb.124818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson PS, Armstrong MK, Dickinson ES, Fernandez R, Miller A, Pong S, Powers B, Pupo-Wiss A, Stanhope ME, Walsh PJ, Wiwatpanit T, Christie AE. Three members of a peptide family are differentially distributed and elicit differential state-dependent responses in a pattern generator–effector system. J Neurophysiol. 2018 doi: 10.1152/jn.00850.2017. (submitted) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fénelon V, Le Feuvre Y, Bem T, Meyrand P. Maturation of rhythmic neural network: role of central modulatory inputs. J Physiol Paris. 2003;97:59–68. doi: 10.1016/j.jphysparis.2003.10.007. [DOI] [PubMed] [Google Scholar]
- Gard AL, Lenz PH, Shaw JR, Christie AE. Identification of putative peptide paracrines/hormones in the water flea Daphnia pulex (Crustacea; Branchiopoda; Cladocera) using transcriptomics and immunohistochemistry. Gen Comp Endocrinol. 2009;160:271–287. doi: 10.1016/j.ygcen.2008.12.014. [DOI] [PubMed] [Google Scholar]
- Harris-Warrick RM, Marder E, Selverston AI, Moulins M. Dynamic biological networks: the stomatogastric nervous system. MIT Press; Cambridge: 1992. [Google Scholar]
- Hooper SL, DiCaprio RA. Crustacean motor pattern generator networks. Neurosignals. 2004;13:50–69. doi: 10.1159/000076158. [DOI] [PubMed] [Google Scholar]
- Katoh K, Standley DM. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol. 2013;30:772–780. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma M, Chen R, Sousa GL, Bors EK, Kwiatkowski MA, Goiney CC, Goy MF, Christie AE, Li L. Mass spectral characterization of peptide transmitters/hormones in the nervous system and neuroendocrine organs of the American lobster Homarus americanus. Gen Comp Endocrinol. 2008;156:395–409. doi: 10.1016/j.ygcen.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma M, Szabo TM, Jia C, Marder E, Li L. Mass spectrometric characterization and physiological actions of novel crustacean C-type allatostatins. Peptides. 2009;30:1660–1668. doi: 10.1016/j.peptides.2009.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marder E, Bucher D. Understanding circuit dynamics using the stomatogastric nervous system of lobsters and crabs. Annu Rev Physiol. 2007;69:291–316. doi: 10.1146/annurev.physiol.69.031905.161516. [DOI] [PubMed] [Google Scholar]
- Marder E, Christie AE, Kilman VL. Functional organization of cotransmission systems: lessons from small nervous systems. Invert Neurosci. 1995;1:105–112. doi: 10.1007/BF02331908. [DOI] [PubMed] [Google Scholar]
- Nusbaum MP, Blitz DM, Swensen AM, Wood D, Marder E. The roles of co-transmission in neural network modulation. Trends Neurosci. 2001;24:146–154. doi: 10.1016/s0166-2236(00)01723-9. [DOI] [PubMed] [Google Scholar]
- Selverston AI. A neural infrastructure for rhythmic motor patterns. Cell Mol Neurobiol. 2005;25:223–244. doi: 10.1007/s10571-005-3154-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selverston AI, Ayers J. Oscillations and oscillatory behavior in small neural circuits. Biol Cybern. 2006;95:537–554. doi: 10.1007/s00422-006-0125-1. [DOI] [PubMed] [Google Scholar]
- Selverston AI, Moulins M. The crustacean stomatogastric system. Springer; Berlin Heidelberg: 1987. [Google Scholar]
- Selverston A, Elson R, Rabinovich M, Huerta R, Abarbanel H. Basic principles for generating motor output in the stomatogastric ganglion. Ann NY Acad Sci. 1998;860:35–50. doi: 10.1111/j.1749-6632.1998.tb09037.x. [DOI] [PubMed] [Google Scholar]
- Skiebe P. Neuropeptides are ubiquitous chemical mediators: using the stomatogastric nervous system as a model system. J Exp Biol. 2001;204:2035–2048. doi: 10.1242/jeb.204.12.2035. [DOI] [PubMed] [Google Scholar]
- Stay B, Tobe SS. The role of allatostatins in juvenile hormone synthesis in insects and crustaceans. Annu Rev Entomol. 2007;52:277–299. doi: 10.1146/annurev.ento.51.110104.151050. [DOI] [PubMed] [Google Scholar]
- Stein W. Modulation of stomatogastric rhythms. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2009;195:989–1009. doi: 10.1007/s00359-009-0483-y. [DOI] [PubMed] [Google Scholar]
- Stemmler EA, Bruns EA, Cashman CR, Dickinson PS, Christie AE. Molecular and mass spectral identification of the broadly conserved decapod crustacean neuropeptide pQIRYHQCYFNPISCF: the first PISCF-allatostatin (Manduca sexta- or C-type allatostatin) from a non-insect. Gen Comp Endocrinol. 2010;165:1–10. doi: 10.1016/j.ygcen.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thirumalai V, Marder E. Colocalized neuropeptides activate a central pattern generator by acting on different circuit targets. J Neurosci. 2002;22:1874–1882. doi: 10.1523/JNEUROSCI.22-05-01874.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toullec JY, Corre E, Bernay B, Thorne MA, Cascella K, Ollivaux C, Henry J, Clark MS. Transcriptome and peptidome characterisation of the main neuropeptides and peptidic hormones of a euphausiid: the Ice Krill, Euphausia crystallorophias. PLoS One. 2013;8:e71609. doi: 10.1371/journal.pone.0071609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura T, Cummins SF, Fitzgibbon Q, Battaglene S, Elizur A. Analysis of the central nervous system transcriptome of the eastern rock lobster Sagmariasus verreauxi reveals its putative neuropeptidome. PLoS ONE. 2014;9:e97323. doi: 10.1371/journal.pone.0097323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verlinden H, Gijbels M, Lismont E, Lenaerts C, Vanden Broeck J, Marchal E. The pleiotropic allatoregulatory neuropeptides and their receptors: a mini-review. J Insect Physiol. 2015;80:2–14. doi: 10.1016/j.jinsphys.2015.04.004. [DOI] [PubMed] [Google Scholar]
- Wilson CH, Christie AE. Distribution of C-type allatostatin (C-AST)-like immunoreactivity in the central nervous system of the copepod Calanus finmarchicus. Gen Comp Endocrinol. 2010;167:252–260. doi: 10.1016/j.ygcen.2010.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan XC, Chen ZF, Sun J, Matsumura K, Wu RS, Qian PY. Transcriptomic analysis of neuropeptides and peptide hormones in the barnacle Balanus amphitrite: evidence of roles in larval settlement. PLoS ONE. 2012;7:e46513. doi: 10.1371/journal.pone.0046513. [DOI] [PMC free article] [PubMed] [Google Scholar]